Abstract

The monoamine oxidase enzyme (MAO), which is bound on the membrane of mitochondria, catalyzes the oxidative deamination of endogenous and exogenous monoamines, including monoamine neurotransmitters such as serotonin, adrenaline, and dopamine. These enzymes have been proven to play a significant role in neurodegeneration; thus, they have recently been researched as prospective therapeutic targets for neurodegenerative illness treatment and management. MAO inhibitors have already been marketed as neurodegeneration illness treatments despite their substantial side effects. Hence, researchers are concentrating on developing novel molecules with selective and reversible inhibitory properties. Piperine, which is a phytochemical component present in black pepper, has been established as a potent MAO inhibitor. Piperine encompasses a piperidine nucleus with antibacterial, anti-inflammatory, antihypertensive, anticonvulsant, antimalarial, antiviral, and anticancer properties. The current Review focuses on the structural changes and structure–activity relationships of piperidine derivatives as MAO inhibitors.
1. Introduction
Neurodegeneration is the primary pathophysiological alteration in most brain illnesses.1 Neurodegenerative diseases (NDDs) can be categorized following their fundamental clinical characteristics (such as dementia, parkinsonism, or motor neuron disease), anatomical illness distribution (such as frontotemporal degenerations, extrapyramidal disorders, or spinocerebellar degenerations), or primary molecular aberrations.2 The most prevalent of these illnesses are Alzheimer’s disease (AD), Parkinson’s disease (PD), amyotrophic lateral sclerosis, and Huntington’s disease. These illnesses are referred to as NDDs, which are associated with the atrophy of the central nervous system (CNS) components.3 A NDD is characterized by memory loss, problem-solving or planning difficulties, mood or personality changes, and declined judgment or decision-making.4 AD is the predominant reason for dementia globally. The number of patients with AD is quickly increasing, and this neuronal impairment has no cure.5 PD is another neurological illness that is characterized by tremors, muscular stiffness, and sluggish movement. This disorder is brought on by the loss of certain dopamine-producing neurons.6 Monoamine oxidase-A (MAO-A) inhibitors cure various types of depression and other nervous system diseases, such as panic disorder, social phobia, and depression with atypical symptoms, in contrast to conventional antidepressants.7
Genetic, environmental, and endogenous aging-related factors are considered to be the principal basic mechanisms causing neurodegeneration. However, their pathogenic significance and fundamental molecular mechanisms remain largely unknown.8 There are numerous uncommon NDDs with known genetic origins. Mutations cause the early onset of severe neurological illnesses by impairing the function of genes essential for neuronal or glial cell functioning.9 Other factors responsible for neurodegeneration include epigenetics, toxins, protein misfolding, impaired protein clearance, altered cell signaling, impaired energy metabolism, oxidative stress, DNA damage, impaired cytoskeleton axonal transport, neuroinflammation, demyelination, and induced cell death.10
Monoamine oxidases (MAOs) are proteins on the mitochondrial outer membrane that catalyze the breakdown of numerous amines in the body. MAO isozymes, namely, MAO-A and MAO-B, are each coded by respective genes on the X chromosome.11 Ammonia, aldehyde, and hydrogen peroxide are the three main intermediates produced through MAO-accelerated oxidative deamination.12,13 MAO-A inhibitors are regarded as efficient therapeutic medicines for the treatment of neurological diseases, including anxiety and depression.14 MAO-B is one of the isozymes of MAO that is associated with neurodegeneration because its activity is markedly elevated in the brains of patients with AD.15 MAO inhibitors, which are involved in the oxidative deamination pathway, might diminish the buildup of oxidative stress mediators, such as hydrogen peroxide, aldehydes, and ammonia, possibly slowing the progression of AD.16 These enzymes catalyze the oxidative deamination of neurochemicals, including dopamine (DA), serotonin, phenylethylamine, adrenaline, and noradrenaline.17,18 MAO-A substrates include serotonin (5-HT), norepinephrine (NE), and DA, whereas MAO-B substrates include phenylethylamine (PEA) and benzylamine.19
The anatomical areas exhibiting neuronal dysfunction, biochemical and conformational changes in protein indicators, and pathologies of neuronal cells such as protein deposition, as well as differences in genetics and epigenetics, can all be used to distinguish between NDDs.20 The diagnosis of NDDs is frequently associated with measurements of their particular receptor binding, cellular metabolism modifications, or anatomical structure alterations.21,22 Structural neuroimaging methods, such as computed tomography and magnetic resonance imaging, are no longer used for diagnosis due to their extremely low specificity. Instead, new methods, such as positron emission tomography and single-photon emission computed tomography, have taken their place. Another more recent method for NDD diagnosis and prognosis is the metabolomic application.23 However, brain mapping techniques may be used to characterize the regional anatomical alterations in pathological states.24
The first MAO antagonist used in the treatment of depression was iproniazid, which was initially used in tuberculosis therapy.25−27 While unsuccessful, it was seen to have “psycho-energizing” effects on patients and also suppressed MAO.28 Several analogs of hydrazine that are MAO inhibitors, including phenelzine, were later developed as antidepressants.29 However, several MAO inhibitors were removed from the clinic after the increased incidences of liver damage, hypertensive crises, hemorrhage, and, in some cases, death.30 Creating nonhydrazine inhibitors, such as tranylcypromine and pargyline, helped prevent liver damage, which was significantly associated with inhibitors generated from hydrazine.31,32 However, hypertensive crises persist as an issue. Tyramine and other adrenergic amines contained within fermented foods, such as cheese, enter circulation and increase sympathetic cardiovascular activity by producing noradrenaline, resulting in the adverse impact known as the “cheese response”.26,33 Selective irreversible MAO-B inhibitors do not have these effects because intestinal MAO successfully metabolizes tyramine and MAO-B is minimal in the gut.34−36 The creation of reversible MAO-A inhibitors, such as moclobemide and lazabemide, also prevented this issue, since these drugs may block enough MAO-A in the brain to have an antidepressant effect. Still, dietary tyramine can displace the inhibitor from peripheral MAO-A, permitting its metabolism.37,38
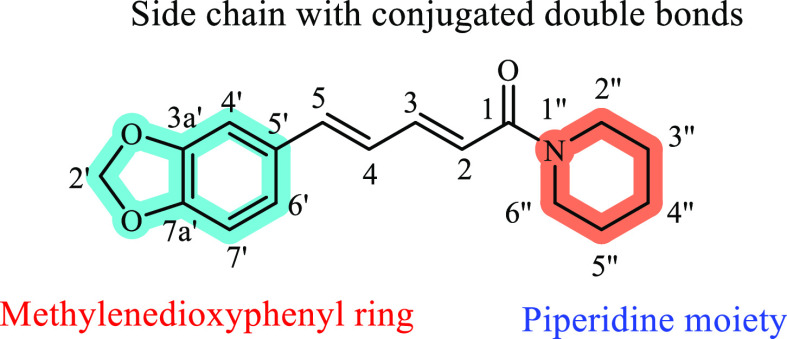
Piperidine is a nonaromatic heterocyclic nucleus with a six-membered ring with five methylene groups (−CH2−) and one secondary amine group (−NH−) (Figure 1). Using a secondary amine, ketones are transformed into enamines, which can then be used in the Stork enamine alkylation procedure.39 Piperidine is a major heterocyclic unit that is present in piperine, which is the active ingredient in black pepper (Psilocaulon absimile [Aizoaceae] and Petrosimonia monandra).11,16
Figure 1.

Structure of piperidine.
Numerous biological actions, including antibacterial, anti-inflammatory, antihypertensive, anticonvulsant, antimalarial, antiviral, and anticancer effects, have been established for piperidine and its derivatives.40 Two piperidine-containing anaplastic lymphoma kinase (ALK) inhibitors, ceritinib and alectinib, were made into a series of radiolabeled fluoroanalogs by Piwnica-Worms et al. They obtained enhanced CNS pharmacokinetic characteristics for all the drugs.41,42 Pyridine is hydrogenated to make piperidine in the industrial setting, typically using a molybdenum disulfide catalyst.43 A modified Birch reduction using sodium in ethanol can also reduce pyridine to piperidine. Figure 2 explains some important reactions for the synthesis of piperidine nuclei from different sources.41,44−46 Hence, numerous efforts have been made over the years to create new techniques for the synthesis of piperidine-containing molecules.47
Figure 2.
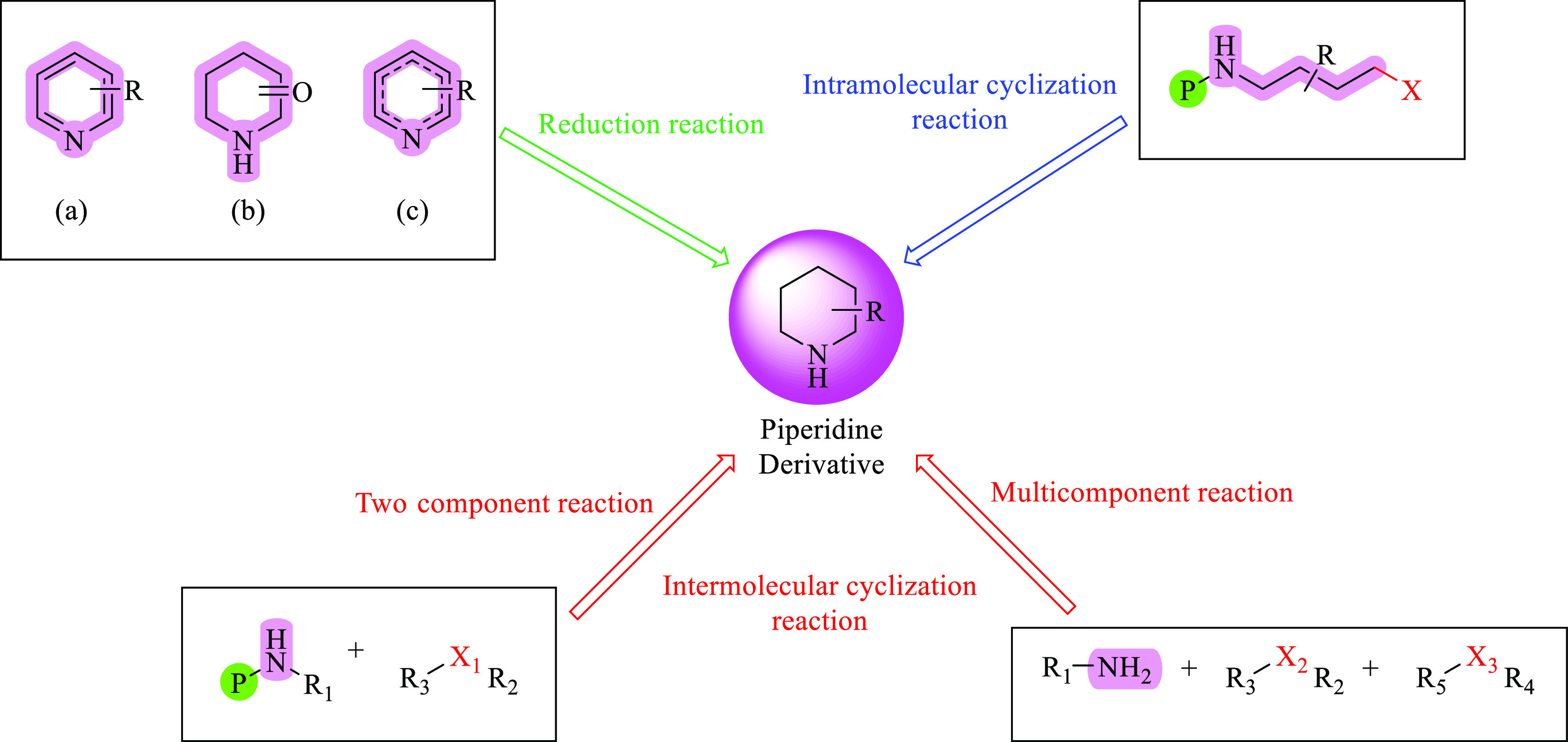
Different synthetic routes adopted for the production of piperidine derivatives.
Currently, Food and Drug Administration (FDA)-approved drugs, such as antipsychotics, include haloperidol,48 benperidol,49 risperidone,50 and thioridazine51 for the symptomatic management of schizophrenia; droperidol, a dopamine antagonist used to prevent and treat postoperative nausea and vomiting;52 and anticholinesterases, including donepezil53 for treating AD (Figure 3).26
Figure 3.
Structures of FDA-approved drugs containing a piperidine nucleus.
Recently, many structural scaffolds, such as chalcones,54 conjugated dienones,55 isatins,56 chromones,57 coumarins,58 pyrazolines,59 quinazolines,60 β-carbolines,61 and benzyloxy-derived molecules,62 are used to develop MAO inhibitors. In search for newer MAO inhibitors, researchers have identified the inevitable role of halogens in selective and potent MAO-B inhibition.63 The introduction of a specific halogen, such as fluorine and bromine, will enable the compounds to optimize the pharmacokinetic properties and thereby improves the biological activities.63 The current Review focuses on piperidine-containing MAO inhibitors and their detailed structure–activity relationships (SARs).
2. Natural Analogs for Piperidine as MAO Inhibitors
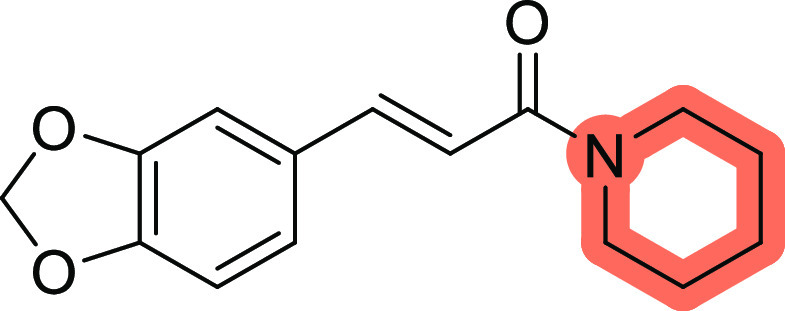
Kong et al. (2004) established the inhibitory activity of piperine (Figure 4) on both types of MAO enzymes isolated from rat brains. They investigated the MAO inhibitory action of 17 naturally occurring compounds, including alkaloids, phenols, and anthraquinones, and revealed that piperine showed dose-dependent MAO inhibitory activity on both types, with half-maximal inhibitory concentration (IC50) values of 49.3 and 91.3 μM for types A and B, respectively. Here, the MAO-A and MAO-B inhibitory activity experiments used clorgyline and deprenyl as positive controls, respectively. Piperine inhibits MAO-A in a mixed-type manner, while MAO-B is inhibited competitively according to the Lineweaver–Burk plot. The pyrroline analogue stachydrine, the monoterpenic indole strychnine, and the benzylisoquinoline derivatives sinomenine and fangchinoline that were also evaluated in the study did not have any inhibitory effects on any MAO subtype. Piperine (1-[5-(1,3-benzodioxol-5-yl)-1-oxo-2,4-pentadienyl]piperidine), which is the sole piperidine derivative, was found to be active against both types of MAO enzymes, with a greater potency for MAO-A compared to the seven examined alkaloids. The compound’s exposed amide group, which may create hydrogen bonds between functional protons, such as −NH–, −OH–, and −SH within the active regions of MAO enzymes, may cause the observed suppression according to a scientific theory.64
Figure 4.
Structure of piperine.
Lee et al. (2005) performed the extraction and activity-guided isolation of piperine from the Piper longum and analyzed its MAO inhibitory activity. They investigated the MAO inhibitory activity using the enzyme that was isolated from mouse brain mitochondrial fractions and iproniazid as the positive control. Piperine was found to have an IC50 threshold of 11.1 μM, with 38.0% MAO activity suppression at a concentration of 8 μM. Additionally, piperine has a stronger inhibitory effect on MAO-B than MAO-A, with IC50 values of 7.0 and 20.9 μM, respectively. Moreover, kinetic studies were conducted to consider the piperine-induced inhibition pattern of MAO, revealing that MAO-A and MAO-B demonstrated competing inhibition, with Ki concentrations of 19.0 ± 0.9 and 3.19 ± 0.5 μM, respectively. The tail suspension test is also resistant to in vivo antidepressant-like effects of piperine.65
A study by Li et al. (2007) used two depression models, including the tail suspension test and the forced swimming test, to examine the antidepressant-like effects of piperine and its derivative, antiepilepsirine (AES) (Figure 5). Additional measurements were made to clarify the mechanisms underlying the mouse brain monoamine concentrations and the functioning of MAO-A and MAO-B. Piperine and AES demonstrated marginally effective MAO-inhibiting actions according to the MAO activity assay. This study revealed that piperine and AES both have marginally effective MAO-suppressing effects.66
Figure 5.
Structure of anti-epilepsirine.
Lee et al. (2008) performed the activity-guided fractionation of methylene chloride-soluble extracts of fruits of P. longum and obtained three piperine derivatives, namely methylpiperate, piperlonguminine, and guineensine (Figure 6). This study was based on their previous work on naturally occurring MAO inhibitors, and the ability of piperine to hinder the MAO-A and MAO-B was already established.67 They evaluated the inhibitory activity of extracted derivatives using mouse-brain-isolated MAO enzymes by fluorometric analysis using kynuramine as substrate. The study revealed that piperlonguminine has no inhibitory activity on MAO, and methylpiperate demonstrated a stronger inhibitory effect against MAO-B than MAO-A. Methylpiperate, guineensine, and piperlonguminine, from the three piperine derivatives, were found to possess potent, intermediate, and no inhibitory activity, respectively.67
Figure 6.
Structures of (a) methylpiperate, (b) piperlonguminine, and (c) guineensine.
The in silico research conducted by Rahman and Rahmatullah (2010) determined the molecular processes of piperine and its related molecules. They selected the MAO-A and MAO-B crystal structures (PDB 2Z5X(68) and 2V5Z,69 respectively), from the Protein Data Bank, for the computational studies. The ligands for the study were obtained from the PubChem database, and the authors performed molecular dynamic simulation (MD) concerning flexible docking using NAMD version 2.7b1 and AutoDock 4.0. Figure 4 explains the different groups that make up the body of piperine. The piperine molecule interacts with Tyr69, Ile180, Asn181, Ile207, Gln215, Cys323, Ile335, Leu337, and Tyr407, as well as the isoalloxazine ring of the FAD residues at the active site of MAO-A. The methylenedioxyphenyl (MDP) ring’s oxygen atoms make hydrogen bonds with water molecules (726th, 746th, and 805th in 2Z5X), while the carbonyl oxygen present in the carbonyl amide binds to the thiol group of Cys323; these hydrogen bonds are essential for keeping the molecule at the active site.70
MAO-B features a precursor interaction space and a single-entrance space, in contrast to MAO-A, and docking studies established that piperine can bind to both sites mainly through hydrophobic interactions. The binding fashion of piperine within those sites was such that the MDP ring interacts with the substrate cavity (Tyr398, Tyr435, Tyr188, Gln206, Gly434, Cys172, and the isoalloxazine ring of FAD) and the piperidine heterocyclic ring binds to the entrance cavity through interacting with Ileu199, Tyr326, Leu171, Thr201, Phe168, Leu164, and Leu167 residues. Two hydrogen bonds are also observed due to the water molecules present in the active site (the water molecule at the 1155th position in 2V5Z to which Cys172 and Tyr188 make hydrogen bonds and the connection of the carbonyl oxygen present in the carbonyl amide of piperine with a different water molecule [1229th position in 2V5Z] to which Ileu199, Tyr326, Thr201, and Gln206 seem to engage in comparable interactions). The piperidine moiety in the structure is what causes piperine to be MAO-B specific, but notably MAO-A-selective action is minimally impacted by its absence. Piperidine alone has some MAO-antagonistic effects, suggesting that the piperidine group in the molecule and its derivatives may be a crucial constitutional component for the overall MAO suppression. The authors investigated molecules that are similar to piperine, such as the shorter counterpart of piperine, antiepilepserine (Figure 5) and concluded that shortening the piperine scaffold can boost MAO-B affinity while maintaining MAO-A potency.70
A study to ascertain the effect of piperine on depressive disorder brought on by pilocarpine-induced status epilepticus in rats was conducted by Pal et al. (2011). The monoaminergic transference of noradrenergic, dopaminergic, and serotonergic signals is improved by piperine, which is an MAO inhibitor. The research established that piperine and its derivative, antiepilepsirine, efficiently trigger serotonin generation. The impact of piperine in MAO and γ-aminobutyric acid is responsible for the antidepressive function of piperine in post-SE rats.71
Huang et al. studied the probable mechanisms underlying the combined antidepressant-like effects of piperine and trans-resveratrol in mice. Results revealed that trans-resveratrol affects serotonergic signaling as well as hypothalamic–pituitary–adrenal axis functioning. The findings further revealed that the compound’s biological availability is poor and its duration of action is short, which hinder its effectiveness in treating neurological illnesses. Piperine seems to have a potent ability to enhance bioavailability with a wide range of structurally and therapeutically diverse drugs due to its contribution to enhancing the systemic absorption of other drugs. Earlier studies have revealed that resveratrol has an antidepressant-like effect on the brain by boosting monoaminergic neurotransmitter levels. These results prompted the researchers to experiment with combining trans-resveratrol and piperine. The findings revealed that the combination of piperine and resveratrol causes highly specific MAO-A inhibition. Simultaneously, MAO-B activity was observed only at a concentration of 20 mg/kg resveratrol with 2.5 mg/kg piperine. The MAO antagonism property of the combination is due to the antagonistic activity of piperine on MAO.71
3. Synthetic Analogs for Piperidine as MAO Inhibitors
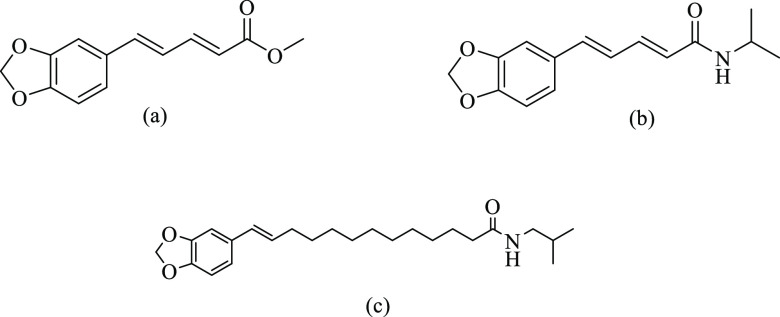
Mu et al. (2012) designed and synthesized 19 piperine derivatives by modifying the amide linkage and evaluated their activity potential against the MAO enzyme (Table 1). The property of MAO-B inhibition was present in all the compounds, and only compound 10 (Figure 7) was observed to be more active for MAO-A, with inhibitory concentrations of 0.8 and 1.57 μM for the A and B isoforms of MAO, respectively, and a selectivity index for MAO-B of 0.5095. The compound 6 (5-(3,4-methylenedioxyphenyl)-2E,4E-pentadienoic acid n-propyl amide) was discovered as particularly effective against MAO-B (IC50 = 0.045 μM and SI value of 81.33 for MAO-B) and more potent than piperine, and the same was observed with comparable activity for MAO-A (IC50 = 3.66 μM). Therefore, piperine derivatives with a small amine moiety are effective toward MAO-B with high selectivity.72
Table 1. rMAO Inhibitory Activity of Piperine Derivatives.
Selectivity index for MAO-B (IC50 for MAO-A/IC50 for MAO-B).
Figure 7.
Structure of compound 10.
Compounds with substituents of −N-propyl and −N-diethyl groups were discovered to be potent derivatives, with activity greater than that of piperine. Substituting the piperidine ring with small amino functional groups yields compounds with comparatively higher activity in the series for MAO-B inhibition. Only compound 10 (Figure 7) exhibited MAO-A inhibitory properties out of all the analogs that were synthesized. It is noted that caution must be paid to the possible inhibition of MAO-A because it depends on which spectrophotometric assay is used. Further, compounds lacking the piperidine ring become less active toward the enzyme, in contrast to the MAO-A-suppressive activity of all the synthesized compounds. The findings indicate the importance of the carbonyl group and conjugation of piperine derivatives for the ability to inhibit MAO.72 The SAR of the derivatives is depicted in Figure 8.
Figure 8.
Structure–activity relationships (SARs) of the piperine derivatives.
In 2012, Al-Baghdadi et al. created and synthesized several substances with structural similarities with piperine and revealed that all the compounds have MAO-antagonistic activity, with comparable selectivity toward MAO-B (Table 2). The selectivity of compounds toward MAO-B increased with the butylamino side chain in the R position, reaching >303.030. Substituting R with a diethylamino group gives a compound with less selectivity than that with an n-butylamino group. The most potent compound was compound 14 with an inhibitory concentration of 0.497 μM toward MAO-B, where the piperidine ring’s fourth position was substituted with a methyl moiety.73
Table 2. Inhibitory Activity of Structurally Similar Piperine Derivatives against hMAO (A and B).

Selectivity index for MAO-B (IC50 for MAO-A/IC50 for MAO-B).
The optimal length of the linker between the MDP ring and the nitrogen-containing heterocyclic ring for the MAO-B suppressive activity is 2–5 carbons, which should be conjugated. The maximum inhibitory activity is observed when the heterocyclic ring is a six-membered one. The inhibitory activity is drastically reduced when the amide linkage is turned into a thioamide link. The most potent derivative of piperine was compound 14 with a three-membered amide linkage and a 4-methyl-substituted piperidine ring, showing high inhibitory activity for MAO-B with a selectivity value greater than 201.207. Compound 12 shows good antagonistic activity against MAO-B, with a SI value greater than 151.515; in 12, the piperidine ring is changed to the cyclohexanamine group, and the activity is reduced significantly whenever oxygen is introduced instead of the 4-position carbon (Figure 9).
Figure 9.
SAR of structurally similar piperine derivatives.
Piperine is an established compound reported with diverse biological benefits, such as analgesic, anti-inflammatory, insecticidal, and antidepressant activity. Prashanth et al. designed a collection of substances derived from the trans-isoform of piperine because the (Z)-form of the compound was discovered to have biological activity. All the synthesized compounds were evaluated for antibacterial, antidepressant, and antioxidant activity, and selected compounds underwent the enzyme inhibitory assay against MAO-A and MAO-B using mouse brain homogenate as a source of MAO. The three tested compounds were found to possess lower inhibitory concentrations than that of the standard drug used (Table 3). Compound 20 was the most potent, and compounds 19 and 21 were more potent than clorgyline, which is the standard drug used in the study.74
Table 3. In Vitro hMAO Inhibition Activity of Derivatives of the trans-Form of Piperine.

Selectivity index for MAO-B (IC50 for MAO-A/IC50 for MAO-B).
The compound with a para-hydroxy piperidine ring substitution demonstrated maximum inhibitory activity, with IC50 values of 0.01446 ± 0.00183 and 0.01572 ± 0.00192 μM for MAO-A and MAO-B, respectively. The findings revealed that the para-substitution of piperidine is preferable to the meta-substitution and the addition of a hydroxyl function increases the MAO inhibitory effect (Figure 10).
Figure 10.
SAR of derivatives of the trans-form of piperine.
By combining Tables 2 and 3, we can draw another SAR (Figure 11). The introduction of a methoxy group on the benzodioxazole ring increases the MAO inhibitory activity. Selecting an allyl group for linking the carbonyl group and benzodioxazole ring increases the MAO inhibition. A 4-methyl piperidine substituent as the R group produces high MAO-B inhibition.
Figure 11.
SAR of benzodioxazole-coupled piperidine derivatives.
Pettersson et al. assessed the impact of a series of 4-phenylpiperidines and 4-phenylpiperazines with substitutions on the para-position of the phenyl ring on antagonist activity against the MAO enzyme. They synthesized a set of para-substituted 4-phenylpiperidines and 4-phenylpiperazines and tested their compatibility with recombinant rat cerebral cortex MAO-A and MAO-B. The pKi value for the synthesized derivatives and the Ki calculations, including confidence intervals from IC50, were calculated using the Cheng Prusoff equation (Ki = IC50/(1 + (L/KD)), where L is the concentration of radioligand in the assay and KD is the affinity of the radioligand for the enzyme (Table 4). Furthermore, they evaluated the SAR for 4-phenylpiperidines and 4-phenylpiperazines with substitutions on the para-position and looked at the equivalent analogs with substitutions at the fourth position of the dopamine receptor stabilizer pridopidine. The presence of methyl sulfone at an aromatic position of pridopidine generated a molecule in which changing the substituent position from meta to para did not affect the striatal DOPAC.75
Table 4. rMAO Inhibitory Activity of para-Substituted 4-Phenylpiperidines and 4-Phenylpiperazines.

| compound | X | R | pKi (MAO-A) (mM) | pKi (MAO-B) (mM) |
|---|---|---|---|---|
| 22 | –CH | –H | 5.01 | NT |
| 23 | –CH | –Cl | 5.82 | 4.42 |
| 24 | –CH | –CF3 | 5.16 | 4.89 |
| 25 | –CH | –morpholine | 5.92 | 4.89 |
| 26 | –CH | –OMe | 6.62 | 3.66 |
| 27 | –CH | –O-n-Bu | 6.43 | 5.80 |
| 28 | –CH | –CN | 4.03 | 3.23 |
| 29 | –CH | –SO2Me | 3.23 | 3.23 |
| 30 | –N | –H | 4.33 | NT |
| 31 | –N | –OMe | 5.85 | 3.23 |
| 32 | –N | –OSO2CF3 | 4.77 | 7.48 |
pK: negative logarithm of binding affinities. NT: not tested.
Methoxy compounds displayed a strong affinity for MAO-A. In contrast, compounds with substituents with high dipole moments, such as the cyano group, yielded molecules with negligible or only minimal MAO-A affinity. The MAO-A affinity slightly decreased for each of the unsubstituted compounds (22 and 30) and the compounds modified with the methoxy group (26 and 31) when the piperidine ring was converted to piperazines, as seen in Figure 12.75
Figure 12.
SAR of para-substituted 4-phenylpiperidines/piperazines.
The substances were better blockers of MAO-A than MAO-B, although those methoxy-substituted analogs were more efficient MAO-B antagonists. The substance with the trifluoromethanesulfonate substitution was discovered as an effective antagonist of MAO-B. Generally, −CH substitution at the X position led to more effective and specific MAO-A inhibitors. The electron-donating groups, such as methoxy and butoxy, were shown to be more effective at inhibiting MAO-A than electron-donating halogens and morpholine. In contrast, the alkoxy compounds in the series followed a somewhat different pattern, with n-butoxy (27) exhibiting a lower affinity than methoxy (26).75
4. Donepezil Hybrids as MAO Inhibitors
Bolea et al. generated a collection of unique hybrid compounds by linking the benzyl piperidine moiety of donepezil and the [(1-methyl-1H-indol-2-yl)methyl]-N-methylprop-2-yn-1-amine moiety of N-[(5-benzyloxy-1-methyl-1H-indol-2-yl)methyl]-N-methylprop-2-yn-1-amine through an oligomethylene bond, hoping that they would be potent against both MAO and AChE enzymes (Figure 13). The benzyl piperidine component of the effective AChE inhibitor donepezil is thought to best interact with the receptor’s catalytic and middle sites, whereas the [(1-methyl-1H-indol-2-yl)methyl]-N-methylprop-2-yn-1-amine functional part in N-[(5-benzyloxy-1-methyl-1H-indol-2-yl)methyl]-N-methylprop-2-yn-1-amine is a promising binder for the MAO enzyme’s substrate binding site.76
Figure 13.
Design strategy of donepezil–N[(5-benzyloxy-1-methyl-1H-indol-2-yl)methyl]-N-methylprop-2-yn-1-amine hybrids.
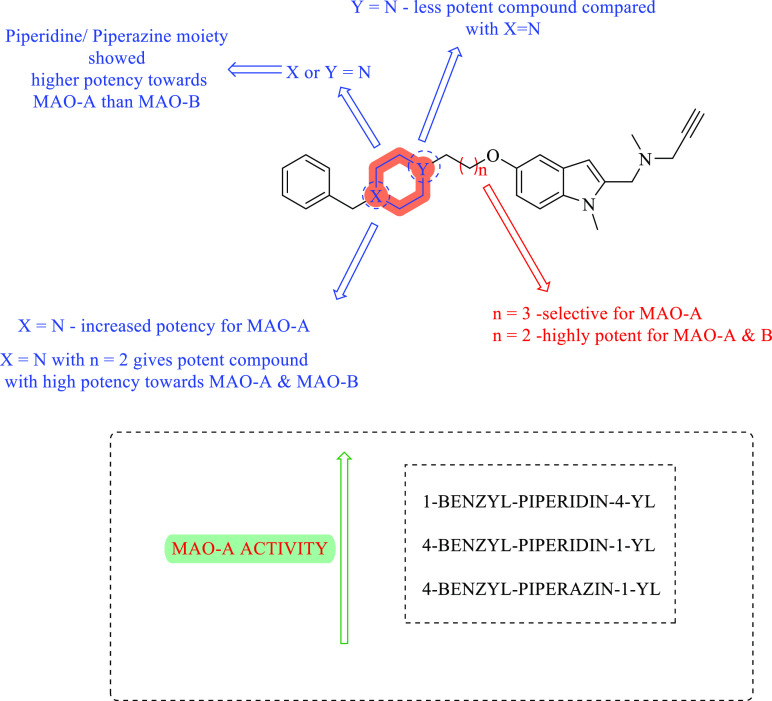
Here, the hybrid molecules were tested for MAO and AChE/BuChE inhibitory action. Each of the molecules has been tested for its antagonism effect on A and B isozymes of MAO that were retrieved from the mitochondrial membrane of rat liver, and the results were then compared to those of the standard donepezil and N-[(5-benzyloxy-1-methyl-1H-indol-2-yl)methyl]-N-methylprop-2-yn-1-amine (Table 5). The investigation revealed that all the 1-benzylpiperidin-4-yl derivatives 33–36 are potent for MAO-A but have lower potency for MAO-B except compound 35, which was the most potent one toward both types of enzymes, with IC50 values of 5.2 ± 1.1 and 43 ± 8.0 nM for MAO-A and MAO-B, respectively.76
Table 5. Rat Liver Mitochondrial MAO (rMAO) Enzyme Inhibitory Activity of Donepezil–N-[(5-Benzyloxy-1-methyl-1H-indol-2-yl)methyl]-N-methylprop-2-yn-1-amine Hybrids.
| compound | X | Y | n | IC50 for MAO-A (μM) | IC50 for MAO-A (μM) | SI valuea |
|---|---|---|---|---|---|---|
| 33 | N | CH | 0 | 0.082 ± 0.003 | 0.750 ± 0.0020 | 0.1093 |
| 34 | N | CH | 1 | 0.0067 ± 0.0018 | 0.130 ± 0.041 | 0.0515 |
| 35 | N | CH | 2 | 0.0052 ± 0.0011 | 0.043 ± 0.008.0 | 0.1209 |
| 36 | N | CH | 3 | 0.010 ± 0.0040 | 2.700 ± 0.110 | 0.00370 |
| 37 | CH | N | 1 | 0.140 ± 0.044 | 1.400 ± 0.500 | 0.1 |
| 38 | CH | N | 2 | 0.065 ± 0.017 | 11.000 ± 2.400 | 0.0059 |
| 39 | N | N | 2 | 0.031 ± 0.014 | 1.600 ± 0.710 | 0.0193 |
Selectivity index for MAO-B (IC50 for MAO-A/IC50 for MAO-B).
The potency of 1-benzylpiperidin-4-yl-substituted compounds is higher than that of 4-benzylpiperidin-1-yl-substituted compounds. The resulting compound 8, which is the most effective for both MAO-A and MAO-B, is created when the 1-benzylpiperidin-4-yl group is combined with [(1-methyl-1H-indol-2-yl)methyl]-N-methylprop-2-yn-1-amine through an alkyl chain with n = 2. Potent compounds against MAO-A are produced when the X group is replaced with a tertiary amine group (Figure 14).
Figure 14.
SAR of donepezil-N–[(5-benzyloxy-1-methyl-1H-indol-2-yl)methyl]-N-methylprop-2-yn-1-amine hybrids.
Previous investigations by Marco-Contelles et al. designed and synthesized 16 donepezil–pyridyl hybrids and evaluated them for their antagonistic activity toward cholinesterase and MAO. Further, the authors incorporated the benzyl piperidine group present in the donepezil and compound ASS234 and the propargylamine moiety of ASS234 in the second and sixth positions of the pyridine nucleus containing a six-membered aromatic ring, respectively(Figure 15).
Figure 15.
Design strategy of donepezil–ASS234 hybrids.
The biological assessment revealed that the majority of the synthesized compounds were almost inactive (49–51 and 54–55) or moderate inhibitors (44, 48, 52, and 53) (Table 6). Compounds 48 and 53 exhibited stronger inhibition of MAO-A and MAO-B, respectively, with IC50 values of 5.7 ± 2.1 and 3.95 ± 0.94 μM. Further, compound 52 demonstrated a moderate selectivity for MAO-B and had an IC50 value of 6.11 ± 1.4 μM. The selective inhibition of MAO-B was demonstrated by compounds with N-methyl propargylamine. Removing the phenyl ring from the fourth position of the pyridine nucleus is directly linked to a change in the discernment of the compounds from MAO-A to MAO-B. Compound 53 was the most appropriate suppressor of MAO, with a MAO-B/MAO-A selectivity index of <0.039.77
Table 6. hMAO Enzyme Inhibitory Activity of Donepezil–ASS234 Hybrids.
| compound | n | R1 | R2 | IC50 for MAO-A (μM) | IC50 for MAO-B (μM) | SI valuea |
|---|---|---|---|---|---|---|
| 40 | 0 | –Ph | –Me | >100 | >100 | 1 |
| 41 | 1 | –Ph | –Me | >100 | >100 | 1 |
| 42 | 2 | –Ph | –Me | >100 | >100 | 1 |
| 43 | 3 | –Ph | –Me | >100 | >100 | 1 |
| 44 | 0 | –Ph | –H | 14.1 ± 3.8 | >100 | 0.141 |
| 45 | 2 | –H | –Me | >100 | >100 | 1 |
| 46 | 0 | –H | –H | >100 | >100 | 1 |
| 47 | 2 | –H | –H | >100 | >100 | 1 |
| 48 | 4 | –Ph | –Me | 5.7 ± 2.1 | >100 | 0.057 |
| 49 | 2 | –Ph | –H | >100 | >100 | 1 |
| 50 | 3 | –Ph | –H | >100 | >100 | 1 |
| 51 | 4 | –Ph | –H | >100 | >100 | 1 |
| 52 | 3 | –H | –Me | >100 | 6.11 ± 1.4 | 16.366 |
| 53 | 4 | –H | –Me | >100 | 3.95 ± 0.94 | 25.316 |
| 54 | 3 | –H | –H | >100 | >100 | 1 |
| 55 | 4 | –H | –H | >50 | >50 | 1 |
Selectivity index for MAO-B (IC50 for MAO-A/IC50 for MAO-B).
Wang et al. developed novel molecules for AD management by clubbing the structural features of donepezil, propargylamine, and 8-hydroxyquinoline. Here, the design of new molecules was based on the structural features of some already reported molecules, including donepezil, M30, M30A, M30B, HLA20A, and ASS234. All seven derivatives they synthesized were evaluated for their inhibitory activity toward MAOs (Table 7). The racemic mixture of compound 61 was the most active one, with an irreversible type of antagonism against both MAO-A and MAO-B. Blood–brain barrier (BBB) permeability, toxicity, and binding ability with both isoforms of MAO were evaluated for the effective compound 61. All the results indicate that the compound has good CNS permeation, low toxicity, and better binding scores for MAO-A and MAO-B.78
Table 7. Rat Liver-Isolated MAO (rMAO) Inhibitory Activity of Donepezil–Propargylamine–8-Hydroxyquinoline Derivatives.

| compound | X | n | IC50 for MAO-A (μM) | IC50 for MAO-B (μM) | SI valuea |
|---|---|---|---|---|---|
| 56 | H | 0 | ≥100 | ≥100 | 1 |
| 57 | CN | 1 | 22.1 ± 0.7 | 39.5 ± 1.4 | 0.559 |
| 58 | H | 1 | 85.4 ± 3.7 | 19.4 ± 3.2 | 4.402 |
| 59 | CN | 2 | 9.7 ± 1.5 | 12.4 ± 2.5 | 0.782 |
| 60 | H | 2 | ≥100 | 50.1 ± 5 | 1.996 |
| 61 | CN | 3 | 6.2 ± 0.7 | 10.2 ± 0.9 | 0.607 |
| 62 | H | 3 | ≥100 | 34.5 ± 3.5 | 2.898 |
Selectivity index for MAO-B (IC50 for MAO-A/IC50 for MAO-B).
Outcomes of the enzyme inhibitory assay indicate that compounds substituted with the cyano group had potent inhibitory activity against both MAO-A and MAO-B when compared with unsubstituted derivatives. Selective and potent MAO-B inhibition was produced by the unsubstituted piperidine derivative with a methylene linker, and the selectivity index was calculated to be 4.402. The number of alkyl groups between the benzyl piperidine moiety and the propargylamine moiety has an important role, i.e., an increase in MAO inhibition was observed with an increase in the number of carbons in the linker (Figure 16).78
Figure 16.
SAR of donepezil–propargylamine–8-hydroxyquinoline derivatives.
In 2016, Li et al. developed several molecules with structural similarities with the donepezil molecule, which is an effective acetylcholinesterase inhibitor, and some MAO inhibitors, including lazabemide, Ro16-6491 (MAO-B inhibitor), and moclobemide (MAO-A inhibitor). They have been designed by preserving the benzyl piperidine moiety in both donepezil and picolinamide, which is a common feature of MAO inhibitors, and these are connected through the alkyl chain (Figure 17). The suppressive potency toward MAO isoenzymes was studied for each of the developed molecules, and a majority of them possess antagonistic activity for MAO-B and MAO-A with high potency.79
Figure 17.
Design strategy of donepezil–picolinamide hybrids.
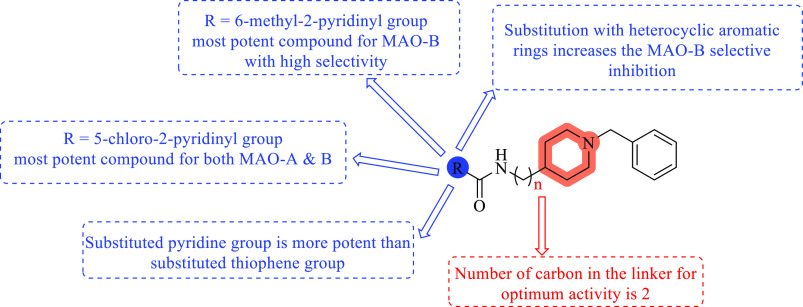
The SAR analysis revealed two carbons in the linker between the benzyl piperidine moiety and the amide functional moiety was optimal for MAO inhibitory activity (Figure 18). Substituting the R group with a pyridine or a thiophene nucleus containing electronegative atoms, such as chlorine and bromine, at the fifth position gives potent compounds with selective inhibition of MAO-B. The strongest compound obtained from the series is compound 69, with inhibitory concentrations of 13.4 ± 0.9 and 3.14 ± 0.27 μM for MAO-A and MAO-B, respectively. From the series, compound 70 was discovered as an effective molecule against MAO-B, with an IC50 value 2.53 ± 1.4 μM, and was found to have greater selectivity toward MAO-B, with a selectivity index of >39.5. Substituted five-membered heterocyclic aromatic rings (such as thiophene) increase the selectivity toward MAO-B inhibition compared to other substituted heterocyclic aromatic rings. The substituted pyridine ring produced potent compounds for MAO inhibition compared to the activity of the substituted thiophene ring. A methyl-substituted aromatic or heteroaromatic ring system gives selective inhibition toward MAO-B.79
Figure 18.
SAR of donepezil–picolinamide hybrids.
Xie et al. designed a series of molecules for targetting ChE and MAO by combining the N-benzyl piperidine moiety of donepezil with the coumarin nucleus. Based on their previous work, the researchers were trying to replace the acridine-based tacrine structure from the molecule that was obtained from their research. In this work, they incorporated the benzyl piperidine nucleus of donepezil (which is more potent than tacrine and has no hepatotoxic effects) instead of the tacrine structure. They synthesized and evaluated 15 derivatives of donepezil–coumarin hybrids. The study revealed that no compounds have an effect on MAO-A at a concentration of 100 μM, and most of the compounds show inhibition toward MAO-B at the same concentration range (Table 9).
Table 9. Inhibitory Activity of hMAO Isoenzymes by Donepezil–Coumarin Hybrids.
| compound | R1 | R2 | n | m | IC50 for MAO-B (μM) |
|---|---|---|---|---|---|
| 73 | H | H | 2 | 0 | 8.39 ± 0.91 |
| 74 | H | H | 3 | 0 | 12.6 ± 1.2 |
| 75 | H | H | 2 | 2 | 30.4 ± 2.5 |
| 76 | H | Me | 2 | 0 | 2.75 ± 0.22 |
| 77 | H | Cl | 2 | 0 | 33.9 ± 2.1 |
| 78 | H | OMe | 2 | 0 | 33.6 ± 0.9 |
| 79 | H | OEt | 2 | 0 | 31.9 ± 1.9 |
| 80 | H | CF3 | 2 | 0 | 28.5 ± 1.7 |
| 81 | H | Ph | 2 | 0 | 45.6 ± 3.6 |
| 82 | Me | H | 2 | 0 | 2.38 ± 0.11 |
| 83 | Me | Me | 2 | 0 | 23.4 ± 1.1 |
| 84 | Cl | Me | 2 | 0 | 14.7 ± 1.5 |
| 85 | –(CH2)4– | 2 | 0 | 2.62 ± 0.81 | |
| 86 | H | Me | 0 | 1.93 ± 0.33 | |
| 87 | H | Me | 2 | 40.5 ± 4.1 | |
All the compounds with an electron-donating group, such as the methyl group at either R1 or R2, produce stronger MAO-B inhibition, whereas substitution with electron-donating groups (such as −Cl, −OCH3, and −OCF3) will largely reduce the MAO-B inhibition activity. A potentially effective and specific MAO-B blocker was obtained by substituting R1 with H and R2 with CH3, and the length of carbons in the alkyl linker between the benzyl piperidinyl ring and the secondary amino group was set at two. The ether linker between the coumarin ring and the benzyl piperidinyl alkylamine is unnecessary for MAO-B inhibition because the inhibitory activity is retained with the removal of the linker (Figure 19). Alkyl substitution of the coumarin ring in the case of compound 85 is where R1 and R2 are connected to form another ring, and 85 exhibited the third most potent inhibitory activity against MAO-B.80 Pisani et al. studied the coumarin derivatives with 1,3- and 1,4-substituted piperidinyl compounds and determined that the position of substituents at the piperidine ring strongly influenced the MAO-B inhibition activity. Compounds with a 1,3-substituted piperidine ring showed better activity, with IC50 values <0.25 μM.81
Figure 19.
SAR of donepezil–coumarin hybrids.
Joubert et al. developed derivatives of coumarin by substituting benzyl, piperidine, N-benzyl piperidine, or p-bromo-N-benzyl piperidine at the seventh position connected by an alkyl ether linkage to evaluate their anti-MAO effect. They performed the biological study using kynuramine as a combination MAO-A/B substrate (Table 10). The inhibitory concentrations of all of the compounds show their higher selectivity toward MAO-B than MAO-A. Compounds 88–95 possessed a higher inhibition on MAO-B than that produced by the reference standard selegiline. Bromobenzyl derivatives (88–91) exhibited better activity than unsubstituted benzyl derivatives (92–95). A remarkable reduction in MAO-B activity was noted when ethylpiperidine was introduced instead of the benzyl group. The results suggest no significant role for the substituent on the coumarin ring (R2 and R3) in MAO inhibition. Compounds with p-bromine substitution, from series 88–100, have effective MAO inhibition. The compounds 92–95 and 108–112 are examples where the authors prove that the incorporation of a heterocyclic ring, such as piperazine between the coumarin and p-bromobenzyl group, will sharply reduce the MAO inhibition property. A piperidine ring produces increased MAO-B inhibition potency and no change in the MAO-A activity. The comparison of compounds 101 and 102 with the 103–107 series suggested that benzyl piperidinyl derivatives have higher inhibitory activity when compared with piperidinyl derivatives (Figure 20).82 Compound 92 was the most dominant, with IC50 values of 0.24 and 0.0005 μM for MAO-A and MAO-B, respectively, and a selectivity of 480 toward MAO-B, followed by compounds 92, 94, and 93 with almost similar IC50 values for MAO-B and lower IC50 values for MAO-A (93, 0.05 and 0.0009 μM; 94, 0.37 and 0.0008 μM, respectively).82
Table 10. In Vitro hMAO Enzyme Inhibitory Activity of Coumarin Conjugates.
| compound | R1 | R2 | R3 | R4 | IC50 for MAO-A (μM) | IC50 for MAO-B (μM) | SI valuea |
|---|---|---|---|---|---|---|---|
| 88 | H | H | H | 3.49 | 0.0038 | 930 | |
| 89 | H | CH3 | H | 2.62 | 0.0020 | 1310 | |
| 90 | H | CH3 | Cl | 2.13 | 0.0021 | 1024 | |
| 91 | H | CH3 | CN | 1.38 | 0.0019 | 700 | |
| 92 | p-Br | H | H | 0.24 | 0.0005 | 480 | |
| 93 | p-Br | CH3 | H | 0.05 | 0.0009 | 56 | |
| 94 | p-Br | CH3 | Cl | 0.37 | 0.0008 | 463 | |
| 95 | p-Br | CH3 | CN | 0.05 | 0.0013 | 38 | |
| 96 | p-Br | H | >10 | 0.104 | >96 | ||
| 97 | p-F | CH3 | CN | 0.60 | 0.0022 | 273 | |
| 98 | p-Cl | CH3 | CN | 1.13 | 0.013 | 87 | |
| 99 | o-Br | CH3 | CN | >10 | 0.073 | >137 | |
| 100 | o,p-Br | CH3 | CN | 0.72 | 0.018 | 40 | |
| 101 | H | H | H | NA | 9.21 | ||
| 102 | H | CH3 | H | NA | 3.09 | ||
| 103 | H | H | H | CH | >10 | 0.47 | >21 |
| 104 | H | CH3 | H | CH | >10 | 0.53 | >19 |
| 105 | H | CH3 | C | CH | >10 | 0.29 | >34 |
| 106 | H | CH3 | CN | CH | >10 | 0.30 | >33 |
| 107 | H | CF3 | H | CH | >10 | 5.33 | >2 |
| 108 | Br | H | H | N | >10 | 1.70 | >6 |
| 109 | Br | CH3 | H | N | >10 | 3.60 | >3 |
| 110 | Br | CH3 | Cl | N | >10 | 1.55 | >7 |
| 111 | Br | CH3 | CN | N | >10 | 1.41 | >7 |
| 112 | Br | CF3 | H | N | >10 | 5.64 | >2 |
Selectivity index for MAO-B (IC50 for MAO-A/IC50 for MAO-B).
Figure 20.
SAR of coumarin conjugates.
Cai et al. designed and developed a group of compounds by combining the structural motifs of trolox and donepezil to evaluate the multitarget effect, such as the anticholinesterase inhibitory and MAO-antagonistic effect, to develop a safer and more effective compound to control AD (Figure 21). Trolox, which is a derivative of vitamin E, is a potent antioxidant compound and has been biologically proven to reduce reactive oxygen species. The substituted benzyl piperidine functional group of donepezil is connected to this chemical structure through an amide linkage, and the ability to inhibit AChE as well as MAO was evaluated by Ellman and fluorescence-based methods, respectively. According to the results published, the compounds with a methyl amide linker (113–125) have a stronger inhibitory potency when compared with propyl amide-linked compounds (126–138) for all the molecules created.83
Figure 21.
Design strategy of donepezil–trolox hybrids.
The biological evaluation and inhibitory assays revealed that compounds with an acetamide linkage between the benzyl piperidine and trolox moiety are more selective toward MAO-B than the MAO-A isoform (Table 11). The compounds with a propyl amide linkage between the benzyl piperidine and trolox moiety were nonselective MAO inhibitors. Compounds with benzyl piperidine substituted with an electronegative functional group have higher inhibitory activity those with other substitutions from all these derivatives. Fluorine group substitution is better for MAO inhibition, and the electron-donating groups have reduced potency compared with other unsubstituted derivatives (Figure 22). A highlyeffective MAO-B suppressor was compound 117, with an IC50 value of 1.6 ± 0.3 μM, and compound 129 was considered the most effective compound of all the synthesized molecules, exhibiting nonselective MAO inhibition with IC50 values of 4.4 ± 0.2 and 4.3 ± 0.2 μM for MAO-A and MAO-B, respectively. Both the best compounds possess a fluorine on the aromatic ring of the benzyl piperidine moiety, which confirms its importance in MAO enzyme inhibition.83
Table 11. hMAO Inhibitory Activity of Donepezil–Trolox Hybrids.
| compound | n | R | IC50 for MAO-A (μM) | IC50 for MAO-B (μM) | SI valuea |
|---|---|---|---|---|---|
| 113 | 0 | 4-OCH3 | 15.3 ± 0.2 | 2.5 ± 0.1 | 6.12 |
| 114 | 0 | 2-CH3 | 13.1 ± 0.3 | 3.1 ± 0.2 | 4.225 |
| 115 | 0 | 4-CH3 | 12.7 ± 0.3 | 3.3 ± 0.2 | 3.848 |
| 116 | 0 | 2-F | 8.9 ± 0.1 | 1.7 ± 0.2 | 5.235 |
| 117 | 0 | 3-F | 9.3 ± 0.2 | 1.6 ± 0.3 | 5.812 |
| 118 | 0 | 4-F | 11.4 ± 0.1 | 1.8 ± 0.3 | 6.333 |
| 119 | 0 | 2,4-2F | 11.8 ± 1.2 | 1.9 ± 0.2 | 6.210 |
| 120 | 0 | 3,4-2F | 12.5 ± 1.5 | 2.3 ± 0.5 | 5.434 |
| 121 | 0 | 2-Cl | 12.7 ± 2.1 | 1.7 ± 0.3 | 7.470 |
| 122 | 0 | 3-Cl | 13.8 ± 1.5 | 3.1 ± 0.1 | 4.451 |
| 123 | 0 | 4-Br | 12.6 ± 0.3 | 2.7 ± 0.9 | 4.666 |
| 124 | 0 | 4-NO2 | 13.2 ± 0.5 | 1.9 ± 0.3 | 6.947 |
| 125 | 0 | H | 11.1 ± 0.4 | 3.2 ± 0.3 | 3.468 |
| 126 | 2 | 4-OCH3 | 7.8 ± 0.6 | 7.3 ± 0.2 | 1.068 |
| 127 | 2 | 2-CH3 | 8.4 ± 0.1 | 6.9 ± 0.1 | 1.217 |
| 128 | 2 | 4-CH3 | 8.9 ± 0.1 | 7.5 ± 0.1 | 1.186 |
| 129 | 2 | 2-F | 4.4 ± 0.2 | 4.3 ± 0.2 | 1.023 |
| 130 | 2 | 3-F | 5.3 ± 0.5 | 4.6 ± 0.2 | 1.152 |
| 131 | 2 | 4-F | 4.8 ± 0.1 | 4.5 ± 0.3 | 1.066 |
| 132 | 2 | 2,4-2F | 5.7 ± 0.3 | 5.8 ± 1.0 | 0.982 |
| 133 | 2 | 3,4-2F | 8.1 ± 0.2 | 5.3 ± 0.7 | 1.528 |
| 134 | 2 | 2-Cl | 7.2 ± 0.4 | 6.1 ± 0.4 | 1.180 |
| 135 | 2 | 3-Cl | 5.8 ± 0.5 | 4.8 ± 0.3 | 1.208 |
| 136 | 2 | 4-Br | 6.3 ± 0.2 | 5.7 ± 0.2 | 1.105 |
| 137 | 2 | 4-NO2 | 5.4 ± 0.2 | 4.9 ± 0.1 | 1.102 |
| 138 | 2 | H | 5.6 ± 0.2 | 5.1 ± 0.2 | 1.098 |
Selectivity index for MAO-B (IC50 for MAO-A/IC50 for MAO-B).
Figure 22.
SAR of donepezil–trolox hybrids.
In 2018, Cai et al. discovered novel hybrid compounds by combining donepezil and butylated hydroxytoluene (BHT). They evaluated the MAO inhibitory activity using a fluorescence-based method with kynuramine as a reference molecule, which is a nonspecific MAO inhibitor. They incorporated the benzyl piperidine of donepezil with BHT and designed a series of compounds for optimizing the linker. the results that the compound with an alkene bridged amide with two methylene linkage between BHT and the piperidine ring (139) possessed the highest MAO inhibitory activity (Figure 23). The authors designed 13 derivatives of donepezil–BHT hybrids, each containing different substitutions on the aromatic ring of the benzyl piperidine moiety. All 13 derivatives were evaluated for inhibition activity against both the isoenzymes of MAO, revealing that all have a higher potency toward MAO-B compared with MAO-A.84
Figure 23.
Structure of compound 139
The evaluation of enzyme inhibition revealed compound 144 as the best molecule with specific MAO-B inhibition, with an IC50 value of 6.5 ± 0.2 μM, and the same molecule has a maximal inhibitory concentration of 58.4 ± 0.5 μM against MAO-A (Table 12). The analysis of the structures and inhibitory activity of the derivatives suggested that an electron-donating group on the aromatic ring of benzyl piperidine may enhance the MAO inhibition; simultaneously, we can say that an electron-withdrawing group can reduce the inhibition property. However, the most potent molecule 144 has a fluorine atom at the second position of the aromatic ring on the benzyl piperidine moiety (Figure 24).84
Table 12. hMAO Inhibition Property of Donepezil–Butylated Hydroxytoluene (BHT) Hybrids.
| compound | R | IC50 for MAO-A (μM) | IC50 for MAO-B (μM) | SI valuea |
|---|---|---|---|---|
| 139 | H | 62.3 ± 3.1 | 8.5 ± 0.3 | 7.329 |
| 140 | 4-OCH3 | 72.5 ± 5.1 | 6.7 ± 1.2 | 10.820 |
| 141 | 2-CH3 | 69.3 ± 3.2 | 7.2 ± 0.2 | 9.625 |
| 142 | 3-CH3 | 70.1 ± 0.1 | 8.9 ± 0.3 | 7.876 |
| 143 | 2-F | 60.2 ± 0.4 | 7.4 ± 0.2 | 8.135 |
| 144 | 3-F | 58.4 ± 0.5 | 6.5 ± 0.2 | 8.984 |
| 145 | 4-F | 59.6 ± 0.4 | 11.1 ± 0.1 | 5.369 |
| 146 | 2,4–2F | 70.6 ± 0.3 | 12.5 ± 1.1 | 5.648 |
| 147 | 3,4–2F | 64.5 ± 1.2 | 9.3 ± 0.2 | 6.935 |
| 148 | 2-Cl | 72.1 ± 2.2 | 9.8 ± 0.2 | 7.357 |
| 149 | 3-Cl | 65.1 ± 2.3 | 8.7 ± 0.1 | 7.482 |
| 150 | 4-Br | 73.2 ± 1.1 | 13.2 ± 1.2 | 5.545 |
| 151 | 3-NO2 | 70.9 ± 1.4 | 12.5 ± 1.1 | 5.672 |
| 152 | 4-CN | 78.2 ± 1.3 | 13.9 ± 0.3 | 5.625 |
Selectivity index for MAO-B (IC50 for MAO-A/IC50 for MAO-B).
Figure 24.
SAR of donepezil-BHT hybrids.
5. Summary of Effective Piperidine Hybrids as MAO Inhibitors
Any substitutions that make the benzyl piperidine more electron deficient, such as a halogen substitution on the benzyl group, are observed to lead to potent nonselective MAO inhibition. Piperidine analogs substituted with hydrophilic groups like hydroxyl groups were also found to be effective molecules for MAO inhibition. The highly potent hybrid compounds of piperidine with effective MAO inhibitory activity are summarized in Table 13.
Table 13. Summary of Piperidine Hybrids with Effective MAO Inhibition.
6. Conclusion and Future Perspectives
MAO enzymes were the most significant pharmacological targets for managing and treating NDDs. Several FDA-approved drugs available for CNS activity possess the piperidine nucleus. The piperidine moiety has MAO inhibitory activity, and the piperidine in piperine is responsible for its MAO-B selective inhibition property. The in silico studies of the piperine molecule revealed that the piperidine nucleus interacts with the amino acid residues present in the entrance cavity of MAO. The piperidine nucleus has secondary nitrogen at its first position. By combining the whole reports, SARs of piperidine derivatives as human and rat MAO enzyme inhibitors (hMAO and rMAO, respectively) are summarized in Figures 25 and 26, respectively.
Figure 25.
SAR of piperidine derivatives as hMAO inhibitors.
Figure 26.
SAR of piperidine derivatives as rMAO inhibitors.
SAR of piperidine derivatives as hMAO inhibitors:
Substitution of piperidine ring with an electron-withdrawing group at the third or fourth position may produce a nonselective hMAO inhibitor.
For selective inhibition of hMAO-B inhibition, electron-donating groups such as methoxy and methyl groups can be incorporated into the piperidine ring at its third or fourth position.
An electron-withdrawing substituent linked to the first position of piperidine was observed as an important structural feature for hMAO inhibition.
A benzyl group at the first position of piperidine nucleus may produce nonselective hMAO inhibition.
Any electron-withdrawing group (such as halogens) substituent at the phenyl ring of a 1-benzyl piperidine derivative may enhance selective inhibition of the hMAO-B isoenzyme.
Selective hMAO-B inhibition may be achieved by connecting a heterocyclic ring such as coumarin or quinoline at the fourth position of piperidine ring through an amide linkage.
SAR of piperidine derivatives as rMAO inhibitors:
An electron-withdrawing group at the first position may enhance the nonselective rMAO inhibition property of the compound.
An electron-donating group (an alkyl group) substituent at the first position of piperidine derivative may produce selective rMAO-B inhibition.
A phenyl ring at the fourth position of piperidine gives a rMAO-A inhibitor.
Substituting the fourth position of the piperidine nucleus with a phenyl group with an electron-releasing group at its fourth position may enhance selective rMAO-B inhibition
A benzyl substitution at the fourth position of piperidine ring may produce nonselective rMAO inhibition.
The rMAO-A inhibition property can be enhanced by the incorporation of an unsaturated alkyl chain to the secondary nitrogen with an amide linkage, whereas reducing the unsaturated chain to a saturated one will reduce the activity. The insertion of a benzyl group at the secondary nitrogen of the piperidine ring improves nonselective MAO inhibition in both human and rat MAO enzymes. Considering all of these facts, novel molecules can be developed in the future with potent MAO inhibition properties.
Researchers can conduct 2D- and 3D-QSAR in the future to build novel pharmacophores with substantial activity for MAO enzyme inhibition using the compounds listed in Tables 8, 11, and 12. 3D-QSAR modeling can be performed to predict the affinity and pharmacological activity of the molecules using CoMFA and CoMSIA methods. All these in silco methods can be utilized as tools for developing novel molecular designs for MAO inhibition activity. The essential objective of this Review is to give insight into emerging tactics that may be utilized to create novel compounds of powerful MAO inhibitors for creating medications to treat neurodegenerative diseases.
Table 8. hMAO Enzyme Inhibition Activity of Donepezil–Picolinamide Hybrids.
| compound | R | n | IC50 FOR MAO-A (μM) | IC50 FOR MAO-B (μM) | SI valuea |
|---|---|---|---|---|---|
| 63 | 2-Br-Ph-CH2 | 2 | NA | 46.8 ± 2.2 | |
| 64 | 3-NO2–Ph | 2 | 92.4 ± 7.1 | 26.2 ± 1.5 | 3.526 |
| 65 | 4-NO2–Ph | 2 | 22.6 ± 1.8 | 68.3 ± 4 | 0.330 |
| 66 | 4-CH3–Ph | 2 | NA | 94.1 ± 4.9 | |
| 67 | 4-Cl-Ph | 2 | 23.2 ± 1.8 | 9.27 ± 1.3 | 2.502 |
| 68 | 2-quinoline | 2 | 24.5 ± 2.3 | 48.6 ± 3.3 | 0.504 |
| 69 | 5-Cl-2-pyridine | 2 | 13.4 ± 0.9 | 3.14 ± 0.27 | 4.267 |
| 70 | 6-CH3-2-pyridine | 2 | NA | 2.53 ± 1.4 | |
| 71 | 5-Cl-2-thiophene | 2 | 76.4 ± 5.4 | 11.5 ± 0.9 | 6.643 |
| 72 | 5-Br-2-thiophene | 2 | 96.2 ± 4.8 | 9.47 ± 0.54 | 10.158 |
Selectivity index for MAO-B (IC50 for MAO-A/IC50 for MAO-B).
Acknowledgments
We sincerely thanks to the library facility of Amrita Vishwa Vidyapeetham University for providing a digital library for the completion for this work.
Author Contributions
Conceptualization: S.M.Z., H.K., and B.M. Writing, original draft preparation: J.J., N.C., A.C.T., M.A.A., M.M.G., P.U., and F.B. Writing, review and editing: S.M.Z. Supervision: H.K. and B.M. All authors have read and agreed to the published version of the manuscript.
The authors declare no competing financial interest.
References
- Lamptey R. N. L.; Chaulagain B.; Trivedi R.; Gothwal A.; Layek B.; Singh J. A Review of the Common Neurodegenerative Disorders: Current Therapeutic Approaches and the Potential Role of Nanotherapeutics. Int. J. Mol. Sci. 2022, 23, 1851. 10.3390/ijms23031851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugger B. N.; Dickson D. W. Pathology of Neurodegenerative Diseases. Cold Spring Harbor Perspect. Biol. 2017, 9, a028035. 10.1101/cshperspect.a028035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choonara Y. E.; Pillay V.; Du Toit L. C.; Modi G.; Naidoo D.; Ndesendo V. M. K.; Sibambo S. R. Trends in the Molecular Pathogenesis and Clinical Therapeutics of Common Neurodegenerative Disorders. Int. J. Mol. Sci. 2009, 10, 2510–2557. 10.3390/ijms10062510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kareem R. T.; Abedinifar F.; Mahmood E. A.; Ebadi A. G.; Rajabi F.; Vessally E. The Recent Development of Donepezil Structure-Based Hybrids as Potential Multifunctional Anti-Alzheimer’s Agents: Highlights from 2010 to 2020. RSC Adv. 2021, 11, 30781–30797. 10.1039/D1RA03718H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabir M. T.; Uddin M. S.; Mamun A. A.; Jeandet P.; Aleya L.; Mansouri R. A.; Ashraf G. M.; Mathew B.; Bin-Jumah M. N.; Abdel-Daim M. M. Combination Drug Therapy for the Management of Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 3272. 10.3390/ijms21093272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A.; Sudevan S. T.; Nair A. S.; Singh A. K.; Kumar S.; Jose J.; Behl T.; Mangalathillam S.; Mathew B.; Kim H. Current and Future Nano-Carrier-Based Approaches in the Treatment of Alzheimer’s Disease. Brain Sci. 2023, 13, 213. 10.3390/brainsci13020213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culpepper L. Reducing the Burden of Difficult-to-Treat Major Depressive Disorder. Prim. Care Companion CNS Discord. 2013, 10.4088/PCC.13r01515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jellinger K. A. Basic Mechanisms of Neurodegeneration: A Critical Update. J. Cell Mol. Med. 2010, 14 (3), 457–487. 10.1111/j.1582-4934.2010.01010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao C. C.; Cheng K. M.; Ma Y. L.; Hsu W. L.; Chen Y. C.; Fuh J. L.; Lee W. J.; Chao C. C.; Lee E. H. Y. Galectin-3 Promotes Aβ Oligomerization and Aβ Toxicity in a Mouse Model of Alzheimer’s Disease. Cell Death Differ. 2020, 27 (1), 192–209. 10.1038/s41418-019-0348-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertens J.; Herdy J. R.; Traxler L.; Schafer S. T.; Schlachetzki J. C. M.; Böhnke L.; Reid D. A.; Lee H.; Zangwill D.; Fernandes D. P.; Agarwal R. K.; Lucciola R.; Zhou-Yang L.; Karbacher L.; Edenhofer F.; Stern S.; Horvath S.; Paquola A. C. M.; Glass C. K.; Yuan S. H.; Ku M.; Szücs A.; Goldstein L. S. B.; Galasko D.; Gage F. H. Age-Dependent Instability of Mature Neuronal Fate in Induced Neurons from Alzheimer’s Patients. Cell Stem Cell 2021, 28 (9), 1533–1548.e6. 10.1016/j.stem.2021.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bari A.; Iqbal A.; Khan Z. A.; Shahzad S. A.; Yar M. Synthetic Approaches toward Piperidine Related Structures: A Review. Synth. Commun. 2020, 50, 2572–2589. 10.1080/00397911.2020.1776878. [DOI] [Google Scholar]
- Nair A. S.; Oh J. M.; Koyiparambath V. P.; Kumar S.; Sudevan S. T.; Soremekun O.; Soliman M. E.; Khames A.; Abdelgawad M. A.; Pappachen L. K.; Mathew B.; Kim H. Development of Halogenated Pyrazolines as Selective Monoamine Oxidase-B Inhibitors: Deciphering via Molecular Dynamics Approach. Molecules 2021, 26 (11), 3264. 10.3390/molecules26113264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay R. R. Inhibitor Design for Monoamine Oxidases. Curr. Pharm. Des. 2013, 19, 2529–2539. 10.2174/1381612811319140004. [DOI] [PubMed] [Google Scholar]
- Benny F.; Kumar S.; Jayan J.; Abdelgawad M. A.; Ghoneim M. M.; Kumar A.; Manoharan A.; Susan R.; Sudevan S. T.; Mathew B. Review of Β-carboline and Its Derivatives as Selective MAO-A Inhibitors. Arch Pharm. 2023, 2300091. 10.1002/ardp.202300091. [DOI] [PubMed] [Google Scholar]
- Kumar S.; Manoharan A.; J J.; Abdelgawad M. A.; Mahdi W. A.; Alshehri S.; Ghoneim M. M.; Pappachen L. K.; Zachariah S. M.; Aneesh T. P.; Mathew B. Exploiting Butyrylcholinesterase Inhibitors through a Combined 3-D Pharmacophore Modeling, QSAR, Molecular Docking, and Molecular Dynamics Investigation. RSC Adv. 2023, 13 (14), 9513–9529. 10.1039/D3RA00526G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G. Q.; Opatz T. Recent Advances in the Synthesis of Piperidines: Functionalization of Preexisting Ring Systems. Adv. Heterocycl. Chem. 2018, 125, 107–234. 10.1016/bs.aihch.2017.10.001. [DOI] [Google Scholar]
- Shih J. C.; Chen K.; Ridd M. J. MONOAMINE OXIDASE: From Genes to Behavior. Ann. Rev. Neurosci. 1999, 22, 197–217. 10.1146/annurev.neuro.22.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youdim M. B. H.Monoamine Oxidases and Their Inhibitors. In Encyclopedia of Molecular Pharmacology; Offermanns S., Rosenthal W., Eds.; Springer, 2008; pp 783–791. 10.1007/978-3-540-38918-7_191. [DOI] [Google Scholar]
- Koyiparambath V. P.; Prayaga Rajappan K.; Rangarajan T. M.; Al-Sehemi A. G.; Pannipara M.; Bhaskar V.; Nair A. S.; Sudevan S. T.; Kumar S.; Mathew B. Deciphering the Detailed Structure–Activity Relationship of Coumarins as Monoamine Oxidase Enzyme Inhibitors—An Updated Review. Chem. Biol. Drug Des. 2021, 98, 655–673. 10.1111/cbdd.13919. [DOI] [PubMed] [Google Scholar]
- Kovacs G. G.; Budka H. Molecular Pathology of Human Prion Diseases. Int. J. Mol. Sci. 2009, 10, 976–999. 10.3390/ijms10030976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou S.-H. I.; Wu F.; Harrich D.; Leó L.; Garciá L. F.; Garciá-Martińez G.; Martińez M.; Gaynor R. B. Cloning and Characterization of a Novel Cellular Protein, TDP-43, That Binds to Human Immunodeficiency Virus Type 1 TAR DNA Sequence Motifs 1995, 69 (6), 3584–3596. 10.1128/jvi.69.6.3584-3596.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercruysse P.; Vieau D.; Blum D.; Petersén Å.; Dupuis L. Hypothalamic Alterations in Neurodegenerative Diseases and Their Relation to Abnormal Energy Metabolism. Front. Mol. Neurosci. 2018, 11, 2 10.3389/fnmol.2018.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duyckaerts C.; Delatour B.; Potier M. C. Classification and Basic Pathology of Alzheimer Disease. Acta Neuropathologica 2009, 118, 5–36. 10.1007/s00401-009-0532-1. [DOI] [PubMed] [Google Scholar]
- Dehay B.; Bourdenx M.; Gorry P.; Przedborski S.; Vila M.; Hunot S.; Singleton A.; Olanow C. W.; Merchant K. M.; Bezard E.; Petsko G. A.; Meissner W. G. Targeting α-Synuclein for Treatment of Parkinson’s Disease: Mechanistic and Therapeutic Considerations. Lancet Neurol. 2015, 14, 855–866. 10.1016/S1474-4422(15)00006-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane G. E. Iproniazid (Marsilid) Phosphate, a Therapeutic Agent for Mental Disorders and Debilitating Diseases. Psychiatr. Res. Rep. Am. Psychiatr. Assoc. 1957, 8, 142–152. [PubMed] [Google Scholar]
- Youdim M. B. H.; Weinstock M. Therapeutic Applications of Selective and Non-Selective Inhibitors of Monoamine Oxidase A and B That Do Not Cause Significant Tyramine Potentiation. Neurotoxicology 2004, 25 (1–2), 243–250. 10.1016/S0161-813X(03)00103-7. [DOI] [PubMed] [Google Scholar]
- Hillhouse T. M.; Porter J. H. A Brief History of the Development of Antidepressant Drugs: From Monoamines to Glutamate. Exp Clin Psychopharmacol 2015, 23 (1), 1–21. 10.1037/a0038550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West E. D.; Dally P. J. Effects of Iproniazid in Depressive Syndromes. BMJ. 1959, 1 (5136), 1491–1494. 10.1136/bmj.1.5136.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHILDKRAUT J. J. THE CATECHOLAMINE HYPOTHESIS OF AFFECTIVE DISORDERS: A REVIEW OF SUPPORTING EVIDENCE. American Journal of Psychiatry 1965, 122 (5), 509–522. 10.1176/ajp.122.5.509. [DOI] [PubMed] [Google Scholar]
- Edinoff A. N.; Swinford C. R.; Odisho A. S.; Burroughs C. R.; Stark C. W.; Raslan W. A.; Cornett E. M.; Kaye A. M.; Kaye A. D. Clinically Relevant Drug Interactions with Monoamine Oxidase Inhibitors. Health Psychol Res. 2022, 10 (4), 39576 10.52965/001c.39576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Prada M.; Zürcher G.; Wüthrich I.; Haefely W. E. On Tyramine, Food, Beverages and the Reversible MAO Inhibitor Moclobemide. J. Neural Transm. Suppl. 1988, 26, 31–56. [PubMed] [Google Scholar]
- Chaurasiya N.; Leon F.; Muhammad I.; Tekwani B. Natural Products Inhibitors of Monoamine Oxidases—Potential New Drug Leads for Neuroprotection, Neurological Disorders, and Neuroblastoma. Molecules 2022, 27 (13), 4297. 10.3390/molecules27134297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoll J. (−)Deprenyl (Selegiline): Past, Present and Future. Neurobiology (Bp) 2000, 8 (2), 179–199. [PubMed] [Google Scholar]
- Hasan F.; McCrodden J. M.; Kennedy N. P.; Tipton K. F. The Involvement of Intestinal Monoamine Oxidase in the Transport and Metabolism of Tyramine. J. Neural. Transm. Suppl. 1988, 26, 1–9. [PubMed] [Google Scholar]
- Da Prada M.; Kettler R.; Keller H. H.; Cesura A. M.; Richards J. G.; Saura Marti J.; Muggli-Maniglio D.; Wyss P. C.; Kyburz E.; Imhof R. From Moclobemide to Ro 19–6327 and Ro 41–1049: The Development of a New Class of Reversible, Selective MAO-A and MAO-B Inhibitors. Nerutotransm. Act. Interact. 1990, 29, 279–292. 10.1007/978-3-7091-9050-0_27. [DOI] [PubMed] [Google Scholar]
- Ostadkarampour M.; Putnins E. E. Monoamine Oxidase Inhibitors: A Review of Their Anti-Inflammatory Therapeutic Potential and Mechanisms of Action. Front. Pharmacol. 2021, 12, 676239 10.3389/fphar.2021.676239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson M. C.; Hasan F.; Mccrodden J. M.; Tipton K. F. Monoamine Oxidase Inhibitors and the Cheese Effect. Neurochem. Res. 1993, 18, 1145–1149. 10.1007/BF00978365. [DOI] [PubMed] [Google Scholar]
- Florvall L.; Fagerval1 I.; Ask A.-L.; Ross S. B. Selective Monoamine Oxidase Inhibitors. 4. 4-Aminophenethylamine Derivatives with Neuron-Selective Action. J. Med. Chem. 1986, 29, 2250–2256. 10.1021/jm00161a020. [DOI] [PubMed] [Google Scholar]
- Goel P.; Alam O.; Naim M. J.; Nawaz F.; Iqbal M.; Alam M. I. Recent Advancement of Piperidine Moiety in Treatment of Cancer- A Review. Eur. J. Med. Chem. 2018, 157, 480–502. 10.1016/j.ejmech.2018.08.017. [DOI] [PubMed] [Google Scholar]
- Edmondson D. E.; Binda C.; Mattevi A. Structural Insights into the Mechanism of Amine Oxidation by Monoamine Oxidases A and B. Arch. Biochem. Biophys. 2007, 464, 269–276. 10.1016/j.abb.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frolov N. A.; Vereshchagin A. N. Piperidine Derivatives: Recent Advances in Synthesis and Pharmacological Applications. Int. J. Mol. Sci. 2023, 24, 2937. 10.3390/ijms24032937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radaram B.; Pisaneschi F.; Rao Y.; Yang P.; Piwnica-Worms D.; Alauddin M. M. Novel Derivatives of Anaplastic Lymphoma Kinase Inhibitors: Synthesis, Radiolabeling, and Preliminary Biological Studies of Fluoroethyl Analogues of Crizotinib, Alectinib, and Ceritinib. Eur. J. Med. Chem. 2019, 182, 111571 10.1016/j.ejmech.2019.111571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F.; Li W.; Sahoo B.; Kreyenschulte C.; Agostini G.; Lund H.; Junge K.; Beller M. Hydrogenation of Pyridines Using a Nitrogen-Modified Titania-Supported Cobalt Catalyst. Angew. Chem., Int. Ed. 2018, 57 (44), 14488–14492. 10.1002/anie.201803426. [DOI] [PubMed] [Google Scholar]
- Bourriquen F.; Hervochon J.; Qu R.; Bartling S.; Rockstroh N.; Junge K.; Fischmeister C.; Beller M. Diastereoselective Hydrogenation of Arenes and Pyridines Using Supported Ruthenium Nanoparticles under Mild Conditions. Chem. Commun. 2022, 58 (63), 8842–8845. 10.1039/D2CC02928F. [DOI] [PubMed] [Google Scholar]
- Kamesu K.; Mohan G. V. K.; Rajasekhar K. SYNTHESIS OF 4-CHLORO-PIPERIDINE DERIVATIVES VIA NbCl5MEDIATED AZA-PRINS TYPE CYCLIZATION OF EPOXIDES AND HOMOALLYLIC AMINES. Rasayan Journal of chemistry 2020, 13 (01), 494–498. 10.31788/RJC.2020.1315392. [DOI] [Google Scholar]
- Chithanna S.; Roy A.; Yang D.-Y. Acid-Catalyzed, Regioselective [3 + 3] Annulation of Enaminones and α-Substituted Cinnamic Acids: Access to 3,4-Dihydropyridones and 2-Piperidinones. Org. Biomol Chem. 2021, 19 (45), 9897–9905. 10.1039/D1OB01115D. [DOI] [PubMed] [Google Scholar]
- Vitaku E.; Smith D. T.; Njardarson J. T. Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals. J. Med. Chem. 2014, 57 (24), 10257–10274. 10.1021/jm501100b. [DOI] [PubMed] [Google Scholar]
- Ulrich S.; Wurthmann C.; Brosz M.; Meyer F. P. The Relationship Between Serum Concentration and Therapeutic Effect of Haloperidol in Patients with Acute Schizophrenia. Clin Pharmacokinet 1998, 34 (3), 227–263. 10.2165/00003088-199834030-00005. [DOI] [PubMed] [Google Scholar]
- Schwarz C.; Hartung B.; Leucht S. Benperidol for Schizophrenia. Cochrane Database Syst. Rev. 2005, CD003083 10.1002/14651858.CD003083.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder S. R.; Meibach R. C. Risperidone in the Treatment of Schizophrenia. Am. J. Psychiatry 1994, 151, 825–835. 10.1176/ajp.151.6.825. [DOI] [PubMed] [Google Scholar]
- Fenton M.; Rathbone J.; Reilly J. Thioridazine for Schizophrenia. Cochrane Database Syst. Rev. 2007, CD001944 10.1002/14651858.CD001944.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhart L. H. J.; Morin A. M.; Bothner U.; Georgieff M. Droperidol and 5-HT 3-Receptor Antagonists, Alone or in Combination, for Prophylaxis of Postoperative Nausea and Vomiting A Meta-Analysis of Randomised Controlled Trials. Acta Anaesthesiol Scand 2000, 44, 1252–1257. 10.1034/j.1399-6576.2000.441011.x. [DOI] [PubMed] [Google Scholar]
- Seltzer B. Donepezil: A Review. Expert Opin. Drug Metab. Toxicol. 2005, 1, 527–536. 10.1517/17425255.1.3.527. [DOI] [PubMed] [Google Scholar]
- Mathew B.; Haridas A.; Suresh J.; Mathew G. E.; Uçar G.; Jayaprakash V. Monoamine Oxidase Inhibitory Action of Chalcones: A Mini Review. Cent. Nerv. Syst. Agents Med. Chem. 2016, 16 (2), 120–136. 10.2174/1871524915666151002124443. [DOI] [PubMed] [Google Scholar]
- Mathew B.; Oh J. M.; Abdelgawad M. A.; Khames A.; Ghoneim M. M.; Kumar S.; Nath L. R.; Sudevan S. T.; Parambi D. G. T.; Agoni C.; Soliman M. E. S.; Kim H. Conjugated Dienones from Differently Substituted Cinnamaldehyde as Highly Potent Monoamine Oxidase-B Inhibitors: Synthesis, Biochemistry, and Computational Chemistry. ACS Omega 2022, 7 (9), 8184–8197. 10.1021/acsomega.2c00397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S.; Nair A. S.; Abdelgawad M. A.; Mathew B. Exploration of the Detailed Structure–Activity Relationships of Isatin and Their Isomers As Monoamine Oxidase Inhibitors. ACS Omega 2022, 7 (19), 16244–16259. 10.1021/acsomega.2c01470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew B.; Mathew G. E.; Petzer J. P.; Petzer A. Structural Exploration of Synthetic Chromones as Selective MAO-B Inhibitors: A Mini Review. Comb. Chem. High Throughput Screen. 2017, 20 (6), 522–532. 10.2174/1386207320666170227155517. [DOI] [PubMed] [Google Scholar]
- Koyiparambath V. P.; Prayaga Rajappan K.; Rangarajan T. M.; Al-Sehemi A. G.; Pannipara M.; Bhaskar V.; Nair A. S.; Sudevan S. T.; Kumar S.; Mathew B. Deciphering the Detailed Structure–Activity Relationship of Coumarins as Monoamine Oxidase Enzyme Inhibitors—An Updated Review. Chem. Biol. Drug Des. 2021, 98, 655–673. 10.1111/cbdd.13919. [DOI] [PubMed] [Google Scholar]
- Mathew B.; Suresh J.; Anbazhagan S.; Mathew G. Pyrazoline: A Promising Scaffold for the Inhibition of Monoamine Oxidase. Cent Nerv Syst. Agents Med. Chem. 2014, 13 (3), 195–206. 10.2174/1871524914666140129122632. [DOI] [PubMed] [Google Scholar]
- Rehuman N. A.; Al-Sehemi A. G.; Parambi D. G. T.; Rangarajan T. M.; Nicolotti O.; Kim H.; Mathew B. Current Progress in Quinazoline Derivatives as Acetylcholinesterase and Monoamine Oxidase Inhibitors. ChemistrySelect 2021, 6 (28), 7162–7182. 10.1002/slct.202101077. [DOI] [Google Scholar]
- Benny F.; Kumar S.; Jayan J.; Abdelgawad M. A.; Ghoneim M. M.; Kumar A.; Manoharan A.; Susan R.; Sudevan S. T.; Mathew B. Review of Β-carboline and Its Derivatives as Selective MAO-A Inhibitors. Arch Pharm. 2023, 2300091 10.1002/ardp.202300091. [DOI] [PubMed] [Google Scholar]
- Sudevan S. T.; Rangarajan T. M.; Al-Sehemi A. G.; Nair A. S.; Koyiparambath V. P.; Mathew B. Revealing the Role of the Benzyloxy Pharmacophore in the Design of a New Class of Monoamine Oxidase-B Inhibitors. Arch Pharm. (Weinheim) 2022, 355 (8), 2200084 10.1002/ardp.202200084. [DOI] [PubMed] [Google Scholar]
- Mathew B.; Carradori S.; Guglielmi P.; Uddin Md. S.; Kim H. New Aspects of Monoamine Oxidase B Inhibitors: The Key Role of Halogens to Open the Golden Door. Curr. Med. Chem. 2020, 28 (2), 266–283. 10.2174/0929867327666200121165931. [DOI] [PubMed] [Google Scholar]
- Kong L. D.; Cheng C. H. K.; Tan R. X. Inhibition of MAO a and B by Some Plant-Derived Alkaloids, Phenols and Anthraquinones. J. Ethnopharmacol 2004, 91 (2–3), 351–355. 10.1016/j.jep.2004.01.013. [DOI] [PubMed] [Google Scholar]
- Lee S. A.; Hong S. S.; Han X. H.; Hwang J. S.; Oh G. J.; Lee K. S.; Lee M. K.; Hwang B. Y.; Ro J. S. Piperine from the Fruits of Piper Longum with Inhibitory Effect on Monoamine Oxidase and Antidepressant-Like Activity. Chem. Pharm. Bull. (Tokyo) 2005, 53 (7), 832–835. 10.1248/cpb.53.832. [DOI] [PubMed] [Google Scholar]
- Li S.; Wang C.; Li W.; Koike K.; Nikaido T.; Wang M. W. Antidepressant-like Effects of Piperine and Its Derivative, Antiepilepsirine. J. Asian Nat. Prod Res. 2007, 9 (5), 421–430. 10.1080/10286020500384302. [DOI] [PubMed] [Google Scholar]
- Lee S. A.; Hwang J. S.; Han X. H.; Lee C.; Lee M. H.; Choe S. G.; Hong S. S.; Lee D.; Lee M. K.; Hwang B. Y. Methylpiperate Derivatives from Piper Longum and Their Inhibition of Monoamine Oxidase. Arch Pharm. Res. 2008, 31 (6), 679–683. 10.1007/s12272-001-1212-7. [DOI] [PubMed] [Google Scholar]
- Son S.-Y.; Ma J.; Kondou Y.; Yoshimura M.; Yamashita E.; Tsukihara T. Structure of Human Monoamine Oxidase A at 2. 2-Å Resolution: The Control of Opening the Entry for Substrates/Inhibitors. Proc. Natl. Acad. Sci. U. S. A. 2008, 105 (15), 5739–5744. 10.1073/pnas.0710626105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binda C.; Wang J.; Pisani L.; Caccia C.; Carotti A.; Salvati P.; Edmondson D. E.; Mattevi A. Structures of Human Monoamine Oxidase B Complexes with Selective Noncovalent Inhibitors: Safinamide and Coumarin Analogs. J. Med. Chem. 2007, 50 (23), 5848–5852. 10.1021/jm070677y. [DOI] [PubMed] [Google Scholar]
- Rahman T.; Rahmatullah M. Proposed Structural Basis of Interaction of Piperine and Related Compounds with Monoamine Oxidases. Bioorg. Med. Chem. Lett. 2010, 20 (2), 537–540. 10.1016/j.bmcl.2009.11.106. [DOI] [PubMed] [Google Scholar]
- Pal A.; Nayak S.; Sahu P. K.; Swain T. Piperine Protects Epilepsy Associated Depression: A Study on Role of Monoamines. Eur. Rev. Med. Pharmacol Sci. 2011, 15 (11), 1288–1295. [PubMed] [Google Scholar]
- Mu L. H.; Wang B.; Ren H. Y.; Liu P.; Guo D. H.; Wang F. M.; Bai L.; Guo Y. S. Synthesis and Inhibitory Effect of Piperine Derivates on Monoamine Oxidase. Bioorg. Med. Chem. Lett. 2012, 22 (9), 3343–3348. 10.1016/j.bmcl.2012.02.090. [DOI] [PubMed] [Google Scholar]
- Al-Baghdadi O. B.; Prater N. I.; Van Der Schyf C. J.; Geldenhuys W. J. Inhibition of Monoamine Oxidase by Derivatives of Piperine, an Alkaloid from the Pepper Plant Piper Nigrum, for Possible Use in Parkinson’s Disease. Bioorg. Med. Chem. Lett. 2012, 22 (23), 7183–7188. 10.1016/j.bmcl.2012.09.056. [DOI] [PubMed] [Google Scholar]
- Prashanth M. K.; Revanasiddappa H. D.; Lokanatha Rai K. M.; Veeresh B. Synthesis, Characterization, Antidepressant and Antioxidant Activity of Novel Piperamides Bearing Piperidine and Piperazine Analogues. Bioorg. Med. Chem. Lett. 2012, 22 (23), 7065–7070. 10.1016/j.bmcl.2012.09.089. [DOI] [PubMed] [Google Scholar]
- Pettersson F.; Svensson P.; Waters S.; Waters N.; Sonesson C. Synthesis and Evaluation of a Set of Para-Substituted 4-Phenylpiperidines and 4-Phenylpiperazines as Monoamine Oxidase (MAO) Inhibitors. J. Med. Chem. 2012, 55 (7), 3242–3249. 10.1021/jm201692d. [DOI] [PubMed] [Google Scholar]
- Bolea I.; Juárez-Jiménez J.; De Los Ríos C.; Chioua M.; Pouplana R.; Luque F. J.; Unzeta M.; Marco-Contelles J.; Samadi A. Synthesis, Biological Evaluation, and Molecular Modeling of Donepezil and N-[(5-(Benzyloxy)-1-Methyl-1H-Indol-2-Yl)Methyl]-N-Methylprop-2-Yn-1-Amine Hybrids as New Multipotent Cholinesterase/Monoamine Oxidase Inhibitors for the Treatment of Alzheimer’s Disease. J. Med. Chem. 2011, 54 (24), 8251–8270. 10.1021/jm200853t. [DOI] [PubMed] [Google Scholar]
- Bautista-Aguilera O. M.; Esteban G.; Chioua M.; Nikolic K.; Agbaba D.; Moraleda I.; Iriepa I.; Soriano E.; Samadi A.; Unzeta M.; Marco-Contelles J. Multipotent Cholinesterase/Monoamine Oxidase Inhibitors for the Treatment of Alzheimer’s Disease: Design, Synthesis, Biochemical Evaluation, ADMET, Molecular Modeling, and QSAR Analysis of Novel Donepezil-Pyridyl Hybrids. Drug Des Devel Ther 2014, 8, 1893–1910. 10.2147/DDDT.S69258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L.; Esteban G.; Ojima M.; Bautista-Aguilera O. M.; Inokuchi T.; Moraleda I.; Iriepa I.; Samadi A.; Youdim M. B. H.; Romero A.; Soriano E.; Herrero R.; Fernández Fernández A. P.; Ricardo-Martínez-Murillo; Marco-Contelles J.; Unzeta M. Donepezil + Propargylamine + 8-Hydroxyquinoline Hybrids as New Multifunctional Metal-Chelators, ChE and MAO Inhibitors for the Potential Treatment of Alzheimer’s Disease. Eur. J. Med. Chem. 2014, 80, 543–561. 10.1016/j.ejmech.2014.04.078. [DOI] [PubMed] [Google Scholar]
- Li F.; Wang Z. M.; Wu J. J.; Wang J.; Xie S. S.; Lan J. S.; Xu W.; Kong L. Y.; Wang X. B. Synthesis and Pharmacological Evaluation of Donepezil-Based Agents as New Cholinesterase/Monoamine Oxidase Inhibitors for the Potential Application against Alzheimer’s Disease. J. Enzyme Inhib Med. Chem. 2016, 31, 41–53. 10.1080/14756366.2016.1201814. [DOI] [PubMed] [Google Scholar]
- Xie S. S.; Lan J. S.; Wang X.; Wang Z. M.; Jiang N.; Li F.; Wu J. J.; Wang J.; Kong L. Y. Design, Synthesis and Biological Evaluation of Novel Donepezil-Coumarin Hybrids as Multi-Target Agents for the Treatment of Alzheimer’s Disease. Bioorg. Med. Chem. 2016, 24 (7), 1528–1539. 10.1016/j.bmc.2016.02.023. [DOI] [PubMed] [Google Scholar]
- Pisani L.; Farina R.; Catto M.; Iacobazzi R. M.; Nicolotti O.; Cellamare S.; Mangiatordi G. F.; Denora N.; Soto-Otero R.; Siragusa L.; Altomare C. D.; Carotti A. Exploring Basic Tail Modifications of Coumarin-Based Dual Acetylcholinesterase-Monoamine Oxidase B Inhibitors: Identification of Water-Soluble, Brain-Permeant Neuroprotective Multitarget Agents. J. Med. Chem. 2016, 59 (14), 6791–6806. 10.1021/acs.jmedchem.6b00562. [DOI] [PubMed] [Google Scholar]
- Joubert J.; Foka G. B.; Repsold B. P.; Oliver D. W.; Kapp E.; Malan S. F. Synthesis and Evaluation of 7-Substituted Coumarin Derivatives as Multimodal Monoamine Oxidase-B and Cholinesterase Inhibitors for the Treatment of Alzheimer’s Disease. Eur. J. Med. Chem. 2017, 125, 853–864. 10.1016/j.ejmech.2016.09.041. [DOI] [PubMed] [Google Scholar]
- Cai P.; Fang S. Q.; Yang X. L.; Wu J. J.; Liu Q. H.; Hong H.; Wang X. B.; Kong L. Y. Rational Design and Multibiological Profiling of Novel Donepezil-Trolox Hybrids against Alzheimer’s Disease, with Cholinergic, Antioxidant, Neuroprotective, and Cognition Enhancing Properties. ACS Chem. Neurosci. 2017, 8 (11), 2496–2511. 10.1021/acschemneuro.7b00257. [DOI] [PubMed] [Google Scholar]
- Cai P.; Fang S. Q.; Yang H. L.; Yang X. L.; Liu Q. H.; Kong L. Y.; Wang X. B. Donepezil-Butylated Hydroxytoluene (BHT) Hybrids as Anti-Alzheimer’s Disease Agents with Cholinergic, Antioxidant, and Neuroprotective Properties. Eur. J. Med. Chem. 2018, 157, 161–176. 10.1016/j.ejmech.2018.08.005. [DOI] [PubMed] [Google Scholar]