Abstract
Background
Although thought of as a multimodal-acting antidepressant targeting the serotonin system, more molecules are being shown to participate in the antidepressant mechanism of vortioxetine. A previous report has shown that vortioxetine administration enhanced the expression of rapamycin complex 1 (mTORC1) in neurons. It has been well demonstrated that mTORC1 participates in not only the pathogenesis of depression but also the pharmacological mechanisms of many antidepressants. Therefore, we speculate that the antidepressant mechanism of vortioxetine may require mTORC1.
Methods
Two mouse models of depression (chronic social defeat stress and chronic unpredictable mild stress) and western blotting were first used together to examine whether vortioxetine administration produced reversal effects against the chronic stress–induced downregulation in the whole mTORC1 signaling cascade in both the hippocampus and medial prefrontal cortex (mPFC). Then, LY294002, U0126, and rapamycin were used together to explore whether the antidepressant effects of vortioxetine in mouse models of depression were attenuated by pharmacological blockade of the mTORC1 system. Furthermore, lentiviral-mTORC1-short hairpin RNA-enhanced green fluorescence protein (LV-mTORC1-shRNA-EGFP) was adopted to examine if genetic blockade of mTORC1 also abolished the antidepressant actions of vortioxetine in mice.
Results
Vortioxetine administration produced significant reversal effects against the chronic stress–induced downregulation in the whole mTORC1 signaling cascade in both the hippocampus and mPFC. Both pharmacological and genetic blockade of the mTORC1 system notably attenuated the antidepressant effects of vortioxetine in mice.
Conclusions
Activation of the mTORC1 system in the hippocampus and mPFC is required for the antidepressant actions of vortioxetine in mice.
Keywords: Chronic social defeat stress, chronic unpredictable mild stress, depression, mammalian target of rapamycin complex 1, vortioxetine
Significance Statement.
Exploring novel antidepressant targets beyond the monoaminergic serotonin (5-HT)/norepinephrine (NA) system is now popular in depression research, and one such is mTORC1. Vortioxetine is developed as a multimodal-acting antidepressant targeting at the 5-HT transporter and several 5-HT receptors. However, more molecules are being reported to participate in the antidepressant mechanism of vortioxetine. Our study is the first comprehensive in vivo evidence, to our knowledge, showing that the antidepressant effects of vortioxetine in mice were accompanied with a significant enhancement on the whole mTORC1 signaling cascade in both the hippocampus and mPFC. Our study further shows that the antidepressant actions of vortioxetine in mice require the mTORC1 system. These findings not only extend the knowledge of vortioxetine’s pharmacologic targets but also further prove that mTORC1 could be a novel antidepressant target.
INTRODUCTION
Despite decades of study, the exact neurobiology of depression remains elusive (Krishnan and Nestler, 2008; Dean and Keshavan, 2017). The monoaminergic hypothesis of depression was widely accepted. However, the focus of recent depression research has been at a level beyond the monoamingeric system to the intracellular signaling cascades that regulate various neuronal functions such as neurogenesis, neuronal survival, and synaptic plasticity (Berton and Nestler, 2006; Shelton, 2007; Krishnan and Nestler, 2008). It is now thought that a requirement for intracellular signaling cascade adaptations could account for the time delay (weeks to months) in the therapeutic responses to clinical antidepressants such as serotonin reuptake inhibitors and serotonin-noradrenaline reuptake inhibitors (Berton and Nestler, 2006). According to this opinion, more novel targets for antidepressant therapies have been reported (Chen et al., 2022; Jiang et al., 2019; Wang et al., 2020a; Guan et al., 2021).
As a novel monoamine-based antidepressant approved by the Food and Drug Administration for the treatment of major depressive disorder in 2013, vortioxetine is thought to be multimodal acting (Pehrson et al., 2016). It has been demonstrated that vortioxetine functions as a blocker for serotonin (5-HT) transporter, an antagonist for 5-HT3, 5-HT7, and 5-HT1D receptors, and also an agonist for 5-HT1A and 5-HT1B receptors (Pehrson et al., 2016). Recently, more pharmacological targets beyond 5-HT have been reported to participate in the antidepressant mechanism of vortioxetine, such as peroxisome proliferator activated receptor α (PPARα), F3/Contactin, the brain-derived neurotrophic factor (BDNF)-tyrosine kinase B (TrkB) system, and so on (Yu et al., 2017; Lu et al., 2018; Sun et al., 2020; Wang et al., 2020a, 2021). Interestingly, in 2016, du Jardin et al. reported that a single dose of vortioxetine significantly enhanced the expression of several plasticity-related genes (mammalian target of rapamycin complex 1 [mTORC1], Mglur-1, PKCα, Homer-3, Spinophilin, and Synapsin-3) in the frontal cortex of rats (du Jardin et al., 2016). Among these genes, mTORC1 has been fully demonstrated to be closely involved in the pathogenesis of depression. For example, both chronic unpredictable mild stress (CUMS) and chronic social defeat stress (CSDS) significantly downregulated the central mTORC1 signaling pathway expression in rodents (Yang et al., 2018; Chen et al., 2021). Knockout/knockdown of mTORC1 made rodents susceptible to chronic stress (Abelaira et al., 2014). Moreover, some clinical antidepressants (fluoxetine, paroxetine, fluvoxamine, venlafaxine, etc.) have already been reported to produce antidepressant efficacy by enhancing the mTORC1 system (Liu et al., 2015; Xu et al., 2018, 2020; Wang et al., 2020b) in the hippocampus and medial prefrontal cortex (mPFC). It is widely known that mTORC1 has 2 downstream molecules (p70 S6 kinase 1 [S6K1] and eukaryotic initiation factor 4E-binding protein 1 [4E-BP-1]) and 2 upstream molecules (protein kinase B [AKT] and extracellular regulated protein kinase 1/2 [ERK1/2]) (Asati et al., 2016; Popova and Jücker, 2021).
Activation of the mTORC1 signaling cascade is closely implicated in many physiological processes in the brain, including neurogenesis, axonal sprouting, and dendritic spine growth (Switon et al., 2017; Liu and Sabatini, 2020). These physiological processes regulated by mTORC1 underlie higher nervous system functions such as neuronal development and synaptic plasticity (Switon et al., 2017; Liu and Sabatini, 2020). Previous studies have already shown that activation of the mTORC1 system culminates in behavioral responses critical to antidepressant treatments (Abelaira et al., 2014; Ignácio et al., 2016). Therefore, here we speculated that like PPARα and F3/Contactin, activation of the mTORC1 system may also underlie the pharmacological effects of vortioxetine. This assumption may extend the knowledge of vortioxetine’s pharmacological actions and further highlights the importance of mTORC1 in depression research, and it was thoroughly investigated in the present study using mouse models of depression.
MATERIALS AND METHODS
Animals
Male C57BL/6J mice (8 weeks old) and retired CD1 mice (over 50 weeks old) were used in the present study. These animals were obtained from the experimental animal center of Nantong University and kept in a climate-controlled environment at a consistent temperature (22°C ± 2°C) and humidity (40%–55%) on a 12-hour-light/-dark cycle (light off at 8:00 am and light on at 8:00 pm). Before the experiments, all mice were habituated to animal facilities for 1 week with free access to food and water. All animal procedures were approved by the Institutional Animal Care and Use Committee at Nantong University and were carried out in accordance with the Animal Research: Reporting of In Vivo Experiments guidelines (Kilkenny et al., 2010; McGrath and Lilley, 2015). All efforts were made to minimize suffering and harm to animals.
For each experiment, the mice were subjected to stratified randomization according to body weight, and all groups of mice were subjected to behavioral tests carried out during the light phase. The sample sizes were determined according to G. power analysis and our previous studies (Jiang et al., 2019; Wang et al., 2020a, 2020b, 2021; Guan et al., 2021).
Drugs
Vortioxetine, rapamycin, LY294002, and U0126 were bought from Target Mol (Boston, MA, USA). Vortioxetine was dissolved in normal saline and i.p. injected in a volume of 10 mL/kg. Rapamycin, LY294002, and U0126 were dissolved in 1% dimethyl sulfoxide in artificial cerebrospinal fluid and intracerebroventricularly (i.c.v.) infused. The pharmacological doses for vortioxetine (10 mg/kg), rapamycin (0.2 nmol per mouse), LY294002 (10 nmol per mouse), and U0126 (5 μg per mouse) were all determined according to previous reports (Xu et al., 2018; Wang et al., 2020b).
Study Design
The experimental design of the whole study was summarized in Figure 1.
Figure 1.
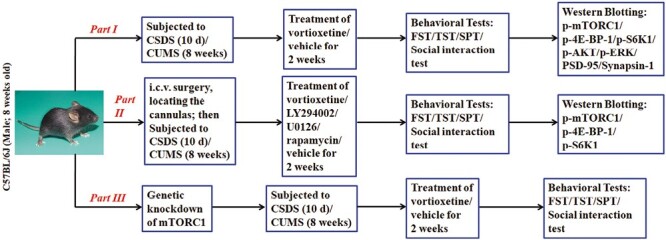
The experimental design of the whole study was provided.
Chronic Unpredictable Mild Stress
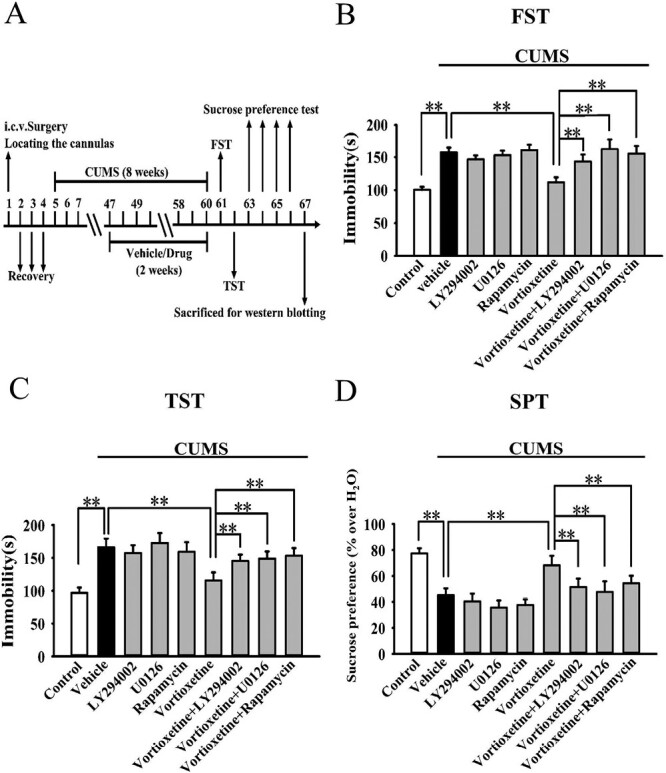
The procedures for CUMS were performed according to our previous studies (Xu et al., 2018; Jiang et al., 2019; Wang et al., 2020b, 2021). Except for the vehicle-treated control group, the mice were individually subjected to various stressors for a period of 8 weeks. These stressors include continuous illumination during the dark cycle, wet bedding for 24 hours, 45° tilting for 12 hours, food or water deprivation for 23 hours, restraint stress for 2 hours, 4°C cold stress for 1 hour, and rotation on a shaker for 1 hour. The stressor order was randomly scheduled during the whole period. Administration of vortioxetine, rapamycin, LY294002, U0126, or vehicle was performed daily during the final 2 weeks. The nonstressed control mice were handled daily without any stress in the housing room. The forced swim test (FST), tail suspension test (TST), and sucrose preference test (SPT) were used together to assay all groups of animals.
Chronic Social Defeat Stress
The paradigm of CSDS was performed as we have frequently reported (Xu et al., 2018; Jiang et al., 2019; Wang et al., 2020a, 2020b, 2021; Guan et al., 2021). Before CSDS, adequate amounts of CD1 aggressors were selected from the retired male CD1 mice according to the experimental design. Except for the vehicle-treated control group, for 10 consecutive days, each C57BL/6J mouse was placed in the home cage of a different CD1 aggressor for up to 10 min/d. After the physical defeat, a perforated divider was used to separate them for 24 hours (allow visual, olfactory, and auditory contact) until the next physical defeat. To minimize harm, when the C57BL/6J mice displayed submissive behavior—including immobility, crouching, trembling, fleeing, and upright posture (usually 5–10 minutes required)—the dividers were immediately set. On the 11th day, all defeated mice were individually housed and received treatment of vortioxetine, rapamycin, LY294002, U0126, or vehicle for another 2 weeks. The nonstressed control mice were handled daily without any stress in the housing room. Afterwards, the FST, TST, SPT, and social interaction test were used together to evaluate all groups of C57BL/6J mice.
Additional materials, methods, and statistics are described in the Supplemental Materials and Methods.
RESULTS
Vortioxetine Reversed Inhibitory Effects of Chronic Stress on mTORC1 Signaling Pathway
Both the CSDS and CUMS models of depression were adopted in the present study. C57BL/6J mice were exposed to 10 days of CSDS and received a daily injection of vehicle (normal saline)/vortioxetine (10 mg/kg) for another 2 weeks, followed by various behavioral tests. As shown in Figure 2A, repeated injection of vortioxetine for 2 weeks not only significantly reversed the CSDS-induced increase in mice immobility in the FST (ANOVA: CSDS, F(1,36) = 22.178, P < .01; vortioxetine, F(1,36) = 18.269, P < .01; interaction, F(1,36) = 15.117, P < .01) and TST (ANOVA: CSDS, F(1,36) = 25.072, P < .01; vortioxetine, F(1,36) = 21.435, P < .01; interaction, F(1,36) = 16.008, P < .01) but also fully reversed the CSDS-induced decrease in sucrose preference (ANOVA: CSDS, F(1,36) = 19.758, P < .01; vortioxetine, F(1,36) = 17.317, P < .01; interaction, F(1,36) = 12.447, P < .01) and social interaction (ANOVA: CSDS, F(1,36) = 35.705, P < .01; vortioxetine, F(1,36) = 28.656, P < .01; interaction, F(1,36) = 23.408, P < .01) in mice (n = 10; P < .01). Similarly, C57BL/6J mice were exposed to 8 weeks of CUMS and received a daily injection of vehicle/vortioxetine during the last 2 weeks, followed by various behavioral tests. Figure 3A indicates that repeated injection of vortioxetine for 2 weeks notably ameliorated not only the promoting effects of CUMS on mice immobility in the FST (ANOVA: CUMS, F(1,36) = 23.305, P < .01; vortioxetine, F(1,36) = 18.154, P < .01; interaction, F(1,36) = 14.112, P < .01) and TST (ANOVA: CUMS, F(1,36) = 26.365, P < .01; vortioxetine, F(1,36) = 20.418, P < .01; interaction, F(1,36) = 15.718, P < .01) but also the decreasing effects of CUMS on mice sucrose preference (ANOVA: CUMS, F(1,36) = 16.882, P < .01; vortioxetine, F(1,36) = 17.342, P < .01; interaction, F(1,36) = 13.005, P < .01) (n = 10; P < .01). Therefore, the efficacy of CSDS, CUMS, and vortioxetine were all confirmed.
Figure 2.
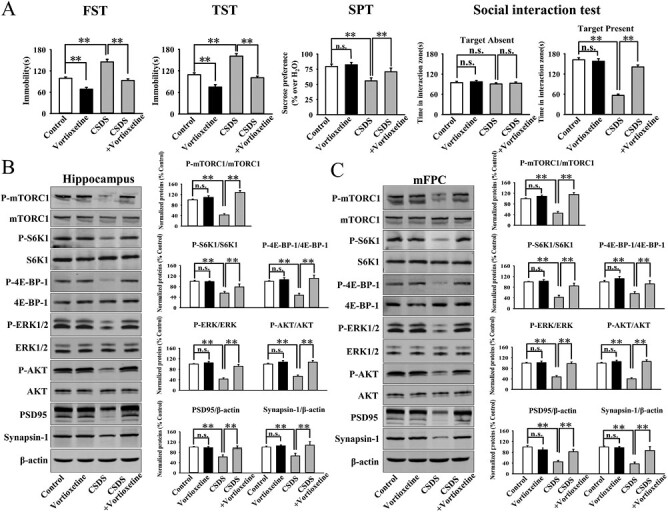
Administration of vortioxetine significantly reversed the chronic social defeat stress (CSDS)-decreased mammalian target of rapamycin complex 1 (mTORC1) signaling cascade in both the hippocampus and mPFC. (A) The antidepressant efficacy of vortioxetine in the CSDS model of depression, which was evaluated by the FST, TST, SPT, and social interaction test (n = 10). (B) The use of vortioxetine notably prevented the CSDS-induced decrease in hippocampal p-mTORC1, p-4E-BP-1, p-S6K1, p-AKT, p-ERK1/2, PSD95, and synapsin-1 expression. (C) The use of vortioxetine also fully prevented the CSDS-induced decrease in the expression of p-mTORC1, p-4E-BP-1, p-S6K1, p-AKT, p-ERK1/2, PSD95, and synapsin-1 in the mPFC (n = 5). The data are expressed as means ± SEM, **P < .01. The comparisons were made by 2-way ANOVA followed by post-hoc Bonferroni test.
Figure 3.
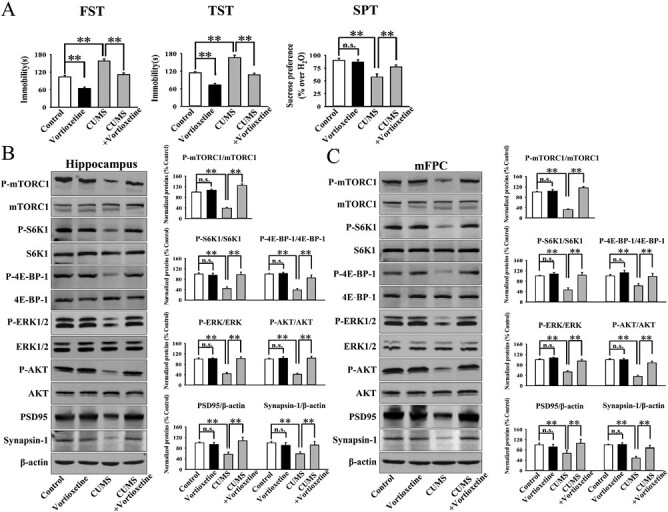
Administration of vortioxetine significantly reversed the chronic unpredictable mild stress (CUMS)-decreased mammalian target of rapamycin complex 1 (mTORC1) signaling cascade in both the hippocampus and mPFC. (A) The antidepressant efficacy of vortioxetine in the CUMS model of depression, which was evaluated by the FST, TST, and SPT (n = 10). (B) The usage of vortioxetine notably prevented the CUMS-induced decrease in the hippocampal p-mTORC1, p-4E-BP-1, p-S6K1, p-AKT, p-ERK1/2, PSD95, and synapsin-1 expression (n = 5). (C) The use of vortioxetine also fully prevented the CUMS-induced decrease in the expression of p-mTORC1, p-4E-BP-1, p-S6K1, p-AKT, p-ERK1/2, PSD95, and synapsin-1 in the mPFC (n = 5). The data are expressed as means ± SEM, **P < .01. The comparisons were made by 2-way ANOVA followed by post-hoc Bonferroni test.
It has been well demonstrated that mTORC1 in both the hippocampus and mPFC regions is closely involved in depression (Abelaira et al., 2014; Xu et al., 2018, 2020; Wang et al., 2020b). Therefore, the levels of the whole mTORC1 signaling cascade in the 2 regions among all groups were examined by western blotting. The hippocampus results in the CSDS model are displayed in Figure 2B. It was found that vortioxetine administration enhanced the protein expression of hippocampal phosphorylated mTORC1 (p-mTORC1) in CSDS-treated mice by 201.9% ± 17.6% (ANOVA: CSDS, F(1,16) = 38.641, P < .01; vortioxetine, F(1,16) = 34.661, P < .01; interaction, F(1,16) = 25.388, P < .01) (n = 5; P < .01). In parallel to p-mTORC1, vortioxetine administration significantly reversed the CSDS-induced downregulation in hippocampal phosphorylated 4E-BP-1 (p-4E-BP-1) (ANOVA: CSDS, F(1,16) = 31.602, P < .01; vortioxetine, F(1,16) = 27.115, P < .01; interaction, F(1,16) = 22.409, P < .01) and phosphorylated S6K1 (p-S6K1) (ANOVA: CSDS, F(1,16) = 25.947, P < .01; vortioxetine, F(1,16) = 17.164, P < .01; interaction, F(1,16) = 12.364, P < .01), 2 downstream molecules of mTORC1 (n = 5; P < .01). Also, vortioxetine administration fully reversed the CSDS-induced downregulation in hippocampal phosphorylated AKT (p-AKT) (ANOVA: CSDS, F(1,16) = 28.991, P < .01; vortioxetine, F(1,16) = 26.557, P < .01; interaction, F(1,16) = 24.222, P < .01) and phosphorylated ERK1/2 (p-ERK1/2) (ANOVA: CSDS, F(1,16) = 27.818, P < .01; vortioxetine, F(1,16) = 24.339, P < .01; interaction, F(1,16) = 21.663, P < .01), 2 upstream molecules of mTORC1 (n = 5; P < .01). The hippocampus results in the CUMS model are displayed in Figure 3B. It was found that vortioxetine administration notably prevented the CUMS-induced decrease in the hippocampal p-mTORC1 (ANOVA: CUMS, F(1,16) = 37.643, P < .01; vortioxetine, F(1,16) = 33.308, P < .01; interaction, F(1,16) = 25.504, P < .01), p-4E-BP-1 (ANOVA: CUMS, F(1,16) = 26.875, P < .01; vortioxetine, F(1,16) = 23.724, P < .01; interaction, F(1,16) = 19.117, P < .01), p-S6K1 (ANOVA: CUMS, F(1,16) = 22.115, P < .01; vortioxetine, F(1,16) = 25.404, P < .01; interaction, F(1,16) = 15.047, P < .01), p-AKT (ANOVA: CUMS, F(1,16) = 32.614, P < .01; vortioxetine, F(1,16) = 27.584, P < .01; interaction, F(1,16) = 22.238, P < .01), and p-ERK1/2 (ANOVA: CUMS, F(1,16) = 30.117, P < .01; vortioxetine, F(1,16) = 24.735, P < .01; interaction, F(1,16) = 17.271, P < .01) expression (n = 5; P < .01).
The mPFC results in the CSDS and CUMS models are displayed in Figures 2C and 3C, respectively. Similarly, the use of vortioxetine evidently ameliorated the inhibitory effects of CSDS on the expression of p-mTORC1 (ANOVA: CSDS, F(1,16) = 35.069, P < .01; vortioxetine, F(1,16) = 28.738, P < .01; interaction, F(1,16) = 21.461, P < .01), p-4E-BP-1 (ANOVA: CSDS, F(1,16) = 20.169, P < .01; vortioxetine, F(1,16) = 23.559, P < .01; interaction, F(1,16) = 15.662, P < .01), p-S6K1 (ANOVA: CSDS, F(1,16) = 22.049, P < .01; vortioxetine, F(1,16) = 18.698, P < .01; interaction, F(1,16) = 11.954, P < .01), p-AKT (ANOVA: CSDS, F(1,16) = 34.607, P < .01; vortioxetine, F(1,16) = 31.772, P < .01; interaction, F(1,16) = 25.308, P < .01), and p-ERK1/2 (ANOVA: CSDS, F(1,16) = 26.665, P < .01; vortioxetine, F(1,16) = 21.432, P < .01; interaction, F(1,16) = 16.337, P < .01) in the mPFC (n = 5; P < .01). Also, the use of vortioxetine significantly attenuated the inhibitory effects of CUMS on the expression of p-mTORC1 (ANOVA: CUMS, F(1,16) = 40.664, P < .01; vortioxetine, F(1,16) = 32.816, P < .01; interaction, F(1,16) = 25.817, P < .01), p-4E-BP-1 (ANOVA: CUMS, F(1,16) = 19.412, P < .01; vortioxetine, F(1,16) = 16.775, P < .01; interaction, F(1,16) = 12.004, P < .01), p-S6K1 (ANOVA: CUMS, F(1,16) = 24.156, P < .01; vortioxetine, F(1,16) = 26.347, P < .01; interaction, F(1,16) = 15.528, P < .01), p-AKT (ANOVA: CUMS, F(1,16) = 33.802, P < .01; vortioxetine, F(1,16) = 27.295, P < .01; interaction, F(1,16) = 23.679, P < .01), and p-ERK1/2 (ANOVA: CUMS, F(1,16) = 25.147, P < .01; vortioxetine, F(1,16) = 20.675, P < .01; interaction, F(1,16) = 14.559, P < .01) in the mPFC (n = 5; P < .01).
Because mTORC1 controls the protein synthesis required for synaptogenesis, we also tested the expression of PSD-95 and synapsin-1. As shown in Figures 2B–C and 3B–C, our results show that injection of vortioxetine fully antagonized the downregulating effects of CSDS (ANOVA of PSD-95 in the hippocampus: CSDS, F(1,16) = 19.185, P < .01; vortioxetine, F(1,16) = 17.024, P < .01; interaction, F(1,16) = 14.133, P < .01. ANOVA of synapsin-1 in the hippocampus: CSDS, F(1,16) = 18.829, P < .01; vortioxetine, F(1,16) = 14.665, P < .01; interaction, F(1,16) = 11.763, P < .01. ANOVA of PSD-95 in the mPFC: CSDS, F(1,16) = 25.059, P < .01; vortioxetine, F(1,16) = 21.617, P < .01; interaction, F(1,16) = 16.325, P < .01. ANOVA of synapsin-1 in the mPFC: CSDS, F(1,16) = 26.703, P < .01; vortioxetine, F(1,16) = 23.044, P < .01; interaction, F(1,16) = 15.817, P < .01), and CUMS (ANOVA of PSD-95 in the hippocampus: CUMS, F(1,16) = 22.449, P < .01; vortioxetine, F(1,16) = 27.071, P < .01; interaction, F(1,16) = 18.016, P < .01. ANOVA of synapsin-1 in the hippocampus: CUMS, F(1,16) = 20.559, P < .01; vortioxetine, F(1,16) = 17.439, P < .01; interaction, F(1,16) = 12.702, P < .01. ANOVA of PSD-95 in the mPFC: CUMS, F(1,16) = 17.304, P < .01; vortioxetine, F(1,16) = 21.556, P < .01; interaction, F(1,16) = 13.414, P < .01. ANOVA of synapsin-1 in the mPFC: CUMS, F(1,16) = 28.066, P < .01; vortioxetine, F(1,16) = 23.505, P < .01; interaction, F(1,16) = 16.338, P < .01) on the 2 proteins in both the hippocampus and mPFC regions (n = 5; P < .01).
In contrast, the levels of total mTORC1, 4E-BP-1, S6K1, AKT, ERK1/2, and β-actin remained constant between all groups (n = 5). Therefore, treatment of vortioxetine notably reversed the inhibitory effects of chronic stress on the mTORC1 signaling pathway in both the hippocampus and mPFC regions.
Blockade of mTORC1 Signaling Pathway Attenuated Antidepressant Effects of Vortioxetine
The selective inhibitors of AKT (LY294002), ERK (U0126), and mTORC1 (rapamycin) were used to test whether the antidepressant effects of vortioxetine in fact require the mTORC1 system. All these inhibitors were i.c.v. infused.
Mice were subjected to CSDS first and then co-treated with vortioxetine and LY294002/U0126/rapamycin for another 2 weeks, followed by behavioral tests and western blotting (Figure 3A). As shown in Figure 4B–C, co-treatment with LY294002, U0126, and rapamycin all significantly blocked the alleviating effects of vortioxetine on the immobility of CSDS-treated mice in the FST (ANOVA: F(8,81) = 17.295, P < .01) and TST (ANOVA: F(8,81) = 21.766, P < .01) (n = 10; P < .01). Figure 4D–E reveal that co-treatment with LY294002, U0126, and rapamycin all fully attenuated the promoting effects of vortioxetine on the sucrose preference (ANOVA: F(8,81) = 14.406, P < .01) and social interaction (ANOVA: F(8,81) = 31.729, P < .01) of CSDS-treated mice (n = 10; P < .01). Moreover, Figure 6A–B show that vortioxetine administration did not reverse the decreased expression of p-mTORC1, p-4E-BP-1, and pS6K1 in the hippocampus (ANOVA for p-mTORC1: F(8,36) = 26.376, P < .01. ANOVA for p-4E-BP-1: F(8,36) = 18.224, P < .01. ANOVA for pS6K1: F(8,36) = 22.601, P < .01) and mPFC (ANOVA for p-mTORC1: F(8,36) = 28.199, P < .01. ANOVA for p-4E-BP-1: F(8,36) = 23.555, P < .01. ANOVA for pS6K1: F(8,36) = 19.301, P < .01) of CSDS-treated mice under the existence of LY294002, U0126, and rapamycin, in line with the behavioral results (n = 5; P < .01). The levels of total mTORC1, 4E-BP-1, S6K1, and β-actin remain unchanged among all groups (n = 5).
Figure 4.
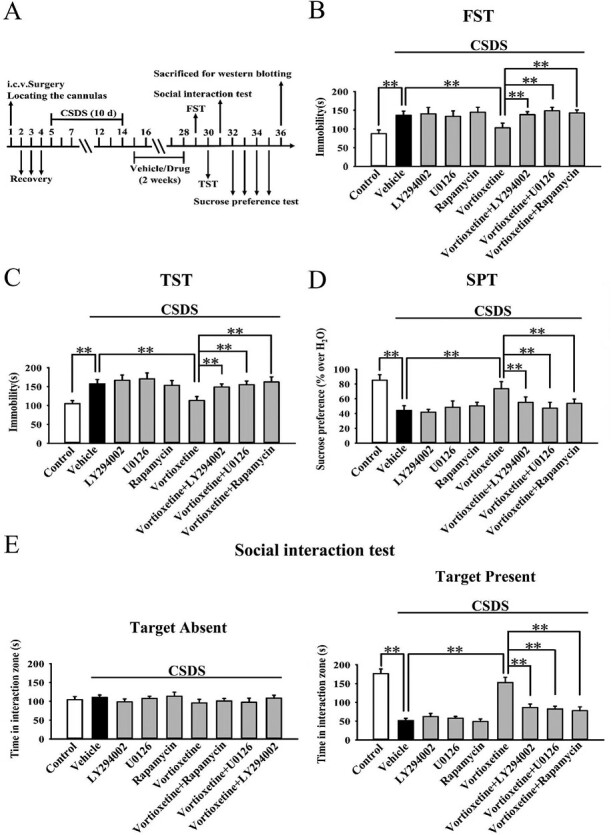
Co-administration with the pharmacological inhibitors of the mammalian target of rapamycin complex 1 (mTORC1) signaling cascade notably attenuated the antidepressant actions of vortioxetine in the chronic social defeat stress (CSDS) model. (A) Detailed schematic timeline of the experimental design is provided. (B) I.c.v. infusion of LY294002, U0126, and rapamycin all significantly blocked the downregulating effects of vortioxetine on the immobility of mice subjected to CSDS in the FST (n = 10). (C) I.c.v. infusion of LY294002, U0126, and rapamycin also blocked the downregulating effects of vortioxetine on the immobility of mice subjected to CSDS in the TST (n = 10). (D) Co-administration with LY294002, U0126, and rapamycin all attenuated the promoting effects of vortioxetine on the sucrose preference of mice subjected to CSDS (n = 10). (E) Co-administration with LY294002, U0126, and rapamycin also attenuated the promoting effects of vortioxetine on the social interaction of mice subjected to CSDS (n = 10). The data are expressed as means ± SEM, **P < .01. The comparisons were made by 1-way ANOVA followed by post-hoc Tukey test.
Figure 6.
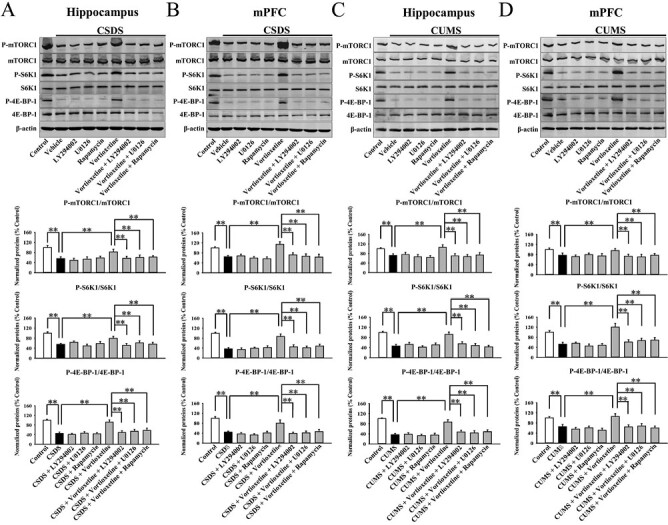
Co-administration with the pharmacological inhibitors of the mammalian target of rapamycin complex 1 (mTORC1) signaling cascade notably attenuated the enhancing effects of vortioxetine on this cascade in mouse models of depression. (A) Mice treated with CSDS + vortioxetine + LY294002/U0126/rapamycin showed significantly less protein expression of p-mTORC1, p-4E-BP-1, and p-S6K1 in the hippocampus than mice treated with CSDS + vortioxetine (n = 5). (B) Mice treated with CSDS + vortioxetine + LY294002/U0126/rapamycin also showed less protein expression of p-mTORC1, p-4E-BP-1, and p-S6K1 in the mPFC than mice treated with CSDS + vortioxetine (n = 5). (C) Mice subjected to CUMS + vortioxetine + LY294002/U0126/rapamycin exhibited evidently lower protein levels of p-mTORC1, p-4E-BP-1, and p-S6K1 in the hippocampus than mice subjected to CUMS + vortioxetine (n = 5). (D) Mice subjected to CUMS + vortioxetine + LY294002/U0126/rapamycin also exhibited lower protein levels of p-mTORC1, p-4E-BP-1, and p-S6K1 in the mPFC than mice subjected to CUMS + vortioxetine (n = 5). The data are expressed as means ± SEM, **P < .01. The comparisons were made by 1-way ANOVA followed by post-hoc Tukey test.
Mice subjected to CUMS were co-treated with vortioxetine and LY294002/U0126/rapamycin during the last 2 weeks, followed by behavioral tests and western blotting (Figure 5A). Figure 5B–C show that the usage of LY294002, U0126, and rapamycin all significantly blocked the alleviating effects of vortioxetine on the immobility of CUMS-treated mice in the FST (ANOVA: F(8,81) = 20.385, P < .01) and TST (ANOVA: F(8,81) = 15.703, P < .01) (n = 10; P < .01). Figure 5D shows that the usage of LY294002, U0126, and rapamycin all fully attenuated the promoting effects of vortioxetine on the sucrose preference (ANOVA: F(8,81) = 13.825, P < .01) of CUMS-treated mice (n = 10; P < .01). Additionally, Figure 6C–D reveal that vortioxetine administration did not reverse the decreased expression of p-mTORC1, p-4E-BP-1, and pS6K1 in the hippocampus (ANOVA for p-mTORC1: F(8,36) = 29.271, P < .01. ANOVA for p-4E-BP-1: F(8,36) = 24.258, P < .01. ANOVA for pS6K1: F(8,36) = 18.317, P < .01) and mPFC (ANOVA for p-mTORC1: F(8,36) = 33.066, P < .01. ANOVA for p-4E-BP-1: F(8,36) = 25.157, P < .01. ANOVA for pS6K1: F(8,36) = 21.221, P < .01) of CUMS-treated mice under the existence of LY294002, U0126, and rapamycin, in line with the behavioral results (n = 5; P < .01). The levels of total mTORC1, 4E-BP-1, S6K1, and β-actin remain unchanged among all groups (n = 5).
Figure 5.
Co-administration with the pharmacological inhibitors of the mammalian target of rapamycin complex 1 (mTORC1) signaling cascade notably attenuated the antidepressant actions of vortioxetine in the chronic unpredictable mild stress (CUMS) model. (A) Detailed schematic timeline of the experimental design is provided. (B) I.c.v. infusion of LY294002, U0126, and rapamycin all significantly blocked the downregulating effects of vortioxetine on the immobility of mice subjected to CUMS in the FST (n = 10). (C) I.c.v. infusion of LY294002, U0126, and rapamycin also blocked the downregulating effects of vortioxetine on the immobility of mice subjected to CUMS in the TST (n = 10). (D) Co-administration with LY294002, U0126, and rapamycin all attenuated the promoting effects of vortioxetine on the sucrose preference of mice subjected to CUMS (n = 10). (E) Co-administration with LY294002, U0126, and rapamycin also attenuated the promoting effects of vortioxetine on the social interaction of mice subjected to CUMS (n = 10). The data are expressed as means ± SEM, **P < .01. The comparisons were made by 1-way ANOVA followed by post-hoc Tukey test.
Taken together, pharmacological blockade of the mTORC1 signaling pathway notably attenuated the antidepressant effects of vortioxetine in mice.
Genetic Blockade of mTORC1 System Prevented Antidepressant Actions of Vortioxetine
Lentiviral-mTORC1-short hairpin RNA-enhanced green fluorescence protein (LV-mTORC1-shRNA-EGFP) was further used to silence the protein expression of mTORC1 in both the hippocampus and mPFC. As shown in Figure 7A–B, the expression of mTORC1-shRNA was stable 2 weeks after its stereotactic injection, and its knockdown efficacy was also confirmed (ANOVA for hippocampus: F(2,12) = 14.789, P < .01. ANOVA for mPFC: F(2,12) = 16.234, P < .01) (n = 5; P < .01).
Figure 7.
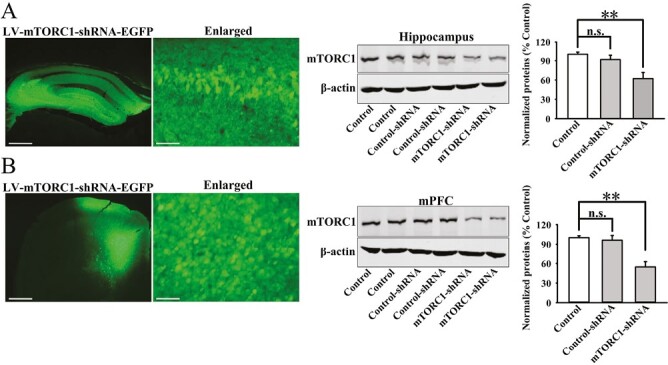
Lentiviral-mammalian target of rapamycin complex 1-short hairpin RNA-enhanced green fluorescence protein (LV-mTORC1-shRNA-EGFP) was stereotactically injected into both the hippocampus and medial prefrontal cortex (mPFC) regions of mice, and its knockdown efficacy was confirmed by western blotting. (A) The fluorescence of a fixed hippocampus slice that expressed mTORC1-shRNA. Corresponding western-blot analysis shows the knockdown effects of mTORC1-shRNA on mTORC1 in the hippocampus (n = 5). (B) The fluorescence of a fixed mPFC slice that expressed mTORC1-shRNA. Corresponding western-blot analysis shows the knockdown effects of mTORC1-shRNA on mTORC1 in the mPFC (n = 5). The scale bar is 200 μm for representative images and 25 μm for enlarged images. The data are expressed as means ± SEM, **P < .01. The comparisons were made by 1-way ANOVA followed by post-hoc Tukey test.
Mice treated with mTORC1-shRNA were subjected to CSDS first and then administration of vortioxetine for another 2 weeks, followed by behavioral tests (Figure 8A). As shown in Figure 8B–E, genetic knockdown of mTORC1 in the hippocampus and mPFC fully abolished the antidepressant actions of vortioxetine in the CSDS model of depression. Detailed data analyses reveal that mice treated with CSDS + vortioxetine + mTORC1-shRNA displayed 28.5% ± 4.73% more immobility in the FST (F(6,63) = 23.052, P < .01), 27.9% ± 5.12% more immobility in the TST (F(6,63) = 19.713, P < .01), 22.3% ± 4.06% less sucrose preference (F(6,63) = 17.118, P < .01), and 51.7% ± 6.35% less social interaction (F(6,63) = 31.804, P < .01) than mice treated with CSDS + vortioxetine (n = 10; P < .01). The use of control-shRNA induced no effects on mouse behavior.
Figure 8.

Co-administration with mammalian target of rapamycin complex 1-short hairpin RNA fully abolished the antidepressant actions of vortioxetine in the chronic social defeat stress (CSDS) model. (A) Detailed schematic timeline of the experimental design is provided. (B) Stereotactic infusion of mTORC1-shRNA notably prevented the protecting effects of vortioxetine against the CSDS-induced behavior of helplessness in mice in the FST (n = 10). (C) Stereotactic infusion of mTORC1-shRNA also prevented the protecting effects of vortioxetine against the CSDS-induced behavior of helplessness in mice in the TST (n = 10). (D) The use of mTORC1-shRNA significantly blocked the protecting effects of vortioxetine against the CSDS-induced behavior of anhedonia in the SPT (n = 10). (E) The use of mTORC1-shRNA also blocked the protecting effects of vortioxetine against the CSDS-induced behavior of social avoidance in the social interaction test (n = 10). The data are expressed as means ± SEM, **P < .01. The comparisons were made by 1-way ANOVA followed by post-hoc Tukey test.
Mice treated with mTORC1-shRNA were subjected to CUMS and vortioxetine administration during the last 2 weeks, followed by behavioral tests (Figure 9A). As shown in Figure 9B–D, genetic knockdown of mTORC1 in the hippocampus and mPFC significantly prevented the antidepressant effects of vortioxetine in the CUMS model of depression. Detailed data analyses reveal that mice treated with CUMS + vortioxetine + mTORC1-shRNA displayed 34.9% ± 6.14% more immobility in the FST (F(6,63) = 15.275, P < .01), 27.9% ± 4.82% more immobility in the TST (F(6,63) = 18.669, P < .01), and 22.2% ± 3.78% less sucrose preference (F(6,63) = 13.351, P < .01) than mice treated with CUMS + vortioxetine (n = 10; P < .01). The use of control-shRNA did not influence mouse behavior.
Figure 9.
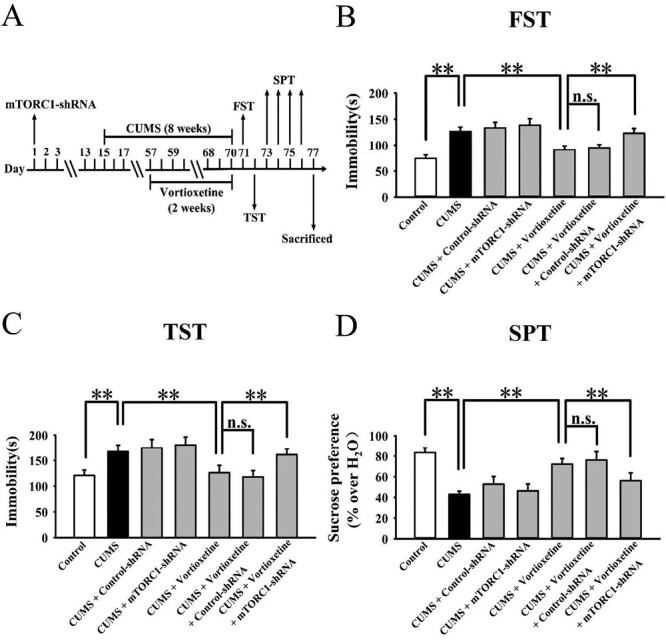
Co-administration with mammalian target of rapamycin complex 1-short hairpin RNA fully abolished the antidepressant actions of vortioxetine in the chronic unpredictable mild stress (CUMS) model. (A) Detailed schematic timeline of the experimental design is provided. (B) Stereotactic infusion of mTORC1-shRNA notably prevented the reversal effects of vortioxetine against the CUMS-induced behavioral changes in mice in the FST (n = 10). (C) Stereotactic infusion of mTORC1-shRNA also prevented the reversal effects of vortioxetine against the CUMS-induced behavioral changes in mice in the TST (n = 10). (D) The use of mTORC1-shRNA significantly blocked the reversal effects of vortioxetine against the CUMS-induced behavioral changes in mice in the SPT (n = 10). The data are expressed as means ± SEM, **P < .01. The comparisons were made by 1-way ANOVA followed by post-hoc Tukey test.
Collectively, genetic blockade of the mTORC1 system fully prevented the antidepressant actions of vortioxetine in mice.
DISCUSSION
As a serine/threonine protein kinase that belongs to the phosphatidylinositol 3-kinase-related kinase family, mTORC1 has been demonstrated to regulate many neuronal actions, such as neurogenesis, neuronal survival, and synaptogenesis (Switon et al., 2017; Liu and Sabatini, 2020). To date, many studies have shown that mTORC1 plays an important role in various neuronal disorders, such as Alzheimer disease, Parkinson disease, and stroke (Wang et al., 2014; Lan et al., 2017; Switon et al., 2017; Querfurth and Lee, 2021; Villa-González et al., 2022). In 2010, Li et al. reported in Science that the mTORC1 signaling cascade in the prefrontal cortex underlay the fast antidepressant actions of ketamine (Li et al., 2010). Since then, mTORC1 has become a popular target in depression research.
Before this study, Liu et al. in 2015 and Xu et al. in 2018 together showed that promotion of hippocampal mTORC1 signaling underlies the antidepressant effects of fluoxetine, a representative serotonin reuptake inhibitor (Liu et al., 2015; Xu et al., 2018). Also, Wang et al. in 2020 showed that activation of the mTORC1 system in both the hippocampus and mPFC participates in the antidepressant mechanism of venlafaxine, a representative serotonin-noradrenaline reuptake inhibitor (Wang et al., 2020b). Like fluoxetine and venlafaxine, vortioxetine was also developed as a monoamine-based antidepressant. Moreover, du Jardin et al. in 2016 preliminarily demonstrated that vortioxetine administration produced enhancing effects on mTORC1 (du Jardin et al., 2016). Therefore, it is possible that mTORC1 is also implicated in the antidepressant effects of vortioxetine, and the present study is the first comprehensive in vivo study, to our knowledge, exploring this assumption.
Two mouse models of depression, CSDS and CUMS, were adopted in this study to obtain a reliable and reasonable conclusion. It is known that CSDS and CUMS are both widely accepted and can mimic several representative depressive-like phenotypes in humans, including helplessness, anhedonia, and social avoidance (Qiao et al., 2016; Antoniuk et al., 2019). As a first step, the antidepressant actions of vortioxetine were successfully reproduced in both CSDS and CUMS, proving the effectiveness of this drug. Detailed analyses showed that vortioxetine treatment for 2 weeks significantly prevented the chronic stress–induced depressive-like behavioral symptoms such as helplessness (as revealed by the FST and TST results), anhedonia (as revealed by the SPT results), and social avoidance (as revealed by the social interaction test results) in mice. Then, it was found that CSDS and CUMS fully decreased the levels of p-AKT, p-ERK1/2, p-mTORC1, p-4E-BP-1, and p-S6K1 in both the hippocampus and mPFC, which is not only consistent with previous reports (Xu et al., 2018; Wang et al., 2020b) but also further supports the reliability of the 2 models. Importantly, repeated administration of vortioxetine notably reversed the downregulated mTORC1 signaling cascade caused by CSDS and CUMS. Moreover, the antidepressant effects of vortioxetine in mouse models of depression were significantly blocked by the use of LY294002, U0126, rapamycin, and mTORC1-shRNA. These results together confirm the speculation of our study from both the positive and negative aspects. However, a shortcoming of our study is using only rodent models, as CSDS and CUMS partially simulate depression behavior in mice. If human tissue samples could be involved, then our conclusion would be considerably strengthened.
Since vortioxetine has promoting effects on mTORC1, this presents an interesting question: Why does vortioxetine not possess fast-onset antidepressant actions as does ketamine? This question may be explained by the phenomenon that vortioxetine administration significantly enhanced the phosphorylation of AKT and ERK1/2, 2 upstream regulators of mTORC1. BDNF, a critical neurotrophic factor in the central nervous system, activates nuclear cyclic adenosine monophosphate (cAMP)-response element binding protein by combining TrkB and then promoting the downstream mitogen-activated protein kinase (MAPK)/ERK and Phosphoinositide 3-kinase (PI3K)/AKT signaling pathways (Sasi et al., 2017). A previous study showed that 5-HT upregulates the expression of BDNF (Morita and Her, 2008). Moreover, vortioxetine has also been demonstrated to enhance the level of BDNF biosynthesis (Yu et al., 2017; Sun et al., 2020). By analyzing this information, we assume that vortioxetine administration first initiates the 5-HT–mediated BDNF biosynthesis through inhibiting the 5-HT reuptake transporter, then promoting the BDNF downstream signaling cascades (MAPK/ERK and PI3K/AKT pathways), and finally inducing the phosphorylation of mTORC1 and antidepressant effects. This is a long-term process and may explain why vortioxetine does not possess fast-onset antidepressant actions, like ketamine. This assumption can be confirmed if the use of 5-HT receptor antagonists, TrkB antagonists, and BDNF-shRNA all prevent the enhancing effects of vortioxetine on mTORC1, and we will perform these experiments in future study. In contrast, ketamine has been shown to activate mTORC1 through α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid (AAMPA) receptors (Zhou et al., 2014; Cavalleri et al., 2018), which seems to be a relatively much shorter pathway.
How does vortioxetine produce antidepressant effects via promotion of mTORC1? The mTORC1 system has been closely linked to protein synthesis required for new spine synapse formation (Li et al., 2010), and the most consistent phenomenon among the findings of altered brain structure and function in depression is reduced volume of the hippocampus and mPFC regions (Krishnan and Nestler, 2008; Dean and Keshavan, 2017; Sheline et al., 2019). The antidepressant effects of ketamine are accompanied with not only mTORC1 activation but also enhanced expression of synaptic proteins such as PSD-95, GluR1, and synapsis (Li et al., 2010). PSD-95 is a critical postsynaptic protein required for synaptic maturation, strengthening, and plasticity (Han and Kim, 2008). Synapses are widely known as markers of presynaptic terminals (Ferreira and Rapoport, 2002). In our results, like ketamine, treatment of vortioxetine notably prevented the decrease induced by chronic stress in the expression of PSD-95 and synapsin-1 in both the hippocampus and mPFC. Thus, vortioxetine may produce antidepressant actions via stimulating mTORC1-related new spine synapse formation, and it can be supported by several reports showing that vortioxetine modulates neuroplasticity (Kugathasan et al., 2017).
It is a difficul task to develop novel antidepressants with strong efficacy and slight adverse effects. Currently, most major pharmaceutical companies have actually abandoned the goal of developing novel antidepressants and instead focused on “me-too” compounds, adding little progress to the treatment of depression because of similar pharmacological targets and efficacy. Failure to develop novel antidepressants has been largely blamed on the neurobiology of depression, which is very complex and elusive. In addition to mTORC1, BDNF, and 5-HT, there are many other molecules reported to play roles in depression, such as vascular endothelial growth factor, PPARα, and so on (Warner-Schmidt and Duman, 2008; Song et al., 2018). These molecules are independent but always produce effects on each other, creating a complex biological network. It is necessary and important to identify the common and primary target for treating depression. For this topic, we are inclined to mTORC1 here. It may be a better way for the pharmaceutical companies to develop novel antidepressants directly targeting at mTORC1 activation (phosphorylation of Ser2448).
Collectively, the present study is new evidence showing the role of mTORC1 in depression and antidepressant responses and extends the understanding of the pharmacological mechanisms for vortioxetine.
Supplementary Materials
Additional materials, methods, and statistics are included.
Acknowledgments
This work was supported by 2 grants from the National Natural Science Foundation of China (no. 82071519 to Bo Jiang and no. 82001606 to Cheng-Niu Wang).
Contributor Information
Wei-Yu Li, Department of Pharmacology, School of Pharmacy, Nantong University, Nantong, Jiangsu, China.
Tian-Shun Shi, Department of Pharmacology, School of Pharmacy, Nantong University, Nantong, Jiangsu, China.
Jie Huang, Department of Pharmacology, School of Pharmacy, Nantong University, Nantong, Jiangsu, China.
Yan-Mei Chen, Department of Pharmacology, School of Pharmacy, Nantong University, Nantong, Jiangsu, China.
Wei Guan, Department of Pharmacology, School of Pharmacy, Nantong University, Nantong, Jiangsu, China.
Bo Jiang, Department of Pharmacology, School of Pharmacy, Nantong University, Nantong, Jiangsu, China.
Cheng-Niu Wang, Institute of Interdisciplinary Integrative Medicine Research, Medical School of Nantong University, Nantong, Jiangsu, China.
Author Contributions
Conceptualization: Bo Jiang and Cheng-Niu Wang. Methodology: Wei-Yu Li, Tian-Shun Shi, Jie Huang, Yan-Mei Chen, and Wei Guan. Investigation: Bo Jiang and Cheng-Niu Wang. Formal analysis: Bo Jiang and Cheng-Niu Wang. Resources: Bo Jiang and Cheng-Niu Wang. Writing—Original Draft: Cheng-Niu Wang. Writing—Review & Editing: Bo Jiang. Visualization: Cheng-Niu Wang. Supervision: Bo Jiang. Project administration: Bo Jiang and Cheng-Niu Wang. Funding acquisition: Bo Jiang and Cheng-Niu Wang.
Interest Statement
The authors declare no conflicts of interest.
Data Availability
The authors declare that all data supporting the findings of this study are available within the paper and its supplementary information files.
References
- Abelaira HM, Réus GZ, Neotti MV, Quevedo J (2014) The role of mTOR in depression and antidepressant responses. Life Sci 101:10–14. [DOI] [PubMed] [Google Scholar]
- Antoniuk S, Bijata M, Ponimaskin E, Wlodarczyk J (2019) Chronic unpredictable mild stress for modeling depression in rodents: meta-analysis of model reliability. Neurosci Biobehav Rev 99:101–116. [DOI] [PubMed] [Google Scholar]
- Asati V, Mahapatra DK, Bharti SK (2016) PI3K/Akt/mTOR and Ras/Raf/MEK/ERK signaling pathways inhibitors as anticancer agents: structural and pharmacological perspectives. Eur J Med Chem 109:314–341. [DOI] [PubMed] [Google Scholar]
- Berton O, Nestler EJ (2006) New approaches to antidepressant drug discovery: beyond monoamines. Nat Rev Neurosci 7:137–151. [DOI] [PubMed] [Google Scholar]
- Cavalleri L, Merlo Pich E, Millan MJ, Chiamulera C, Kunath T, Spano PF, Collo G (2018) Ketamine enhances structural plasticity in mouse mesencephalic and human iPSC-derived dopaminergic neurons via AMPAR-driven BDNF and mTOR signaling. Mol Psychiatry 23:812–823. [DOI] [PubMed] [Google Scholar]
- Chen C, Ma H, Fu Z (2021) Antidepressant-like effect of 3-n-butylphthalide in rats exposed to chronic unpredictable mild stress: modulation of brain-derived neurotrophic factor level and mTOR activation in cortex. Neurochem Res 46:3075–3084. [DOI] [PubMed] [Google Scholar]
- Chen YM, Fan H, Huang J, Shi TS, Li WY, Wang CN, Jiang B, Liu JF (2022) Hippocampal F3/Contactin plays a role in chronic stress-induced depressive-like effects and the antidepressant actions of vortioxetine in mice. Biochem Pharmacol 202:115097. [DOI] [PubMed] [Google Scholar]
- Dean J, Keshavan M (2017) The neurobiology of depression: an integrated view. Asian J Psychiatr 27:101–111. [DOI] [PubMed] [Google Scholar]
- du Jardin KG, Müller HK, Sanchez C, Wegener G, Elfving B (2016) A single dose of vortioxetine, but not ketamine or fluoxetine, increases plasticity-related gene expression in the rat frontal cortex. Eur J Pharmacol 786:29–35. [DOI] [PubMed] [Google Scholar]
- Ferreira A, Rapoport M (2002) The synapsins: beyond the regulation of neurotransmitter release. Cell Mol Life Sci 59:589–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan W, Xu DW, Ji CH, Wang CN, Liu Y, Tang WQ, Gu JH, Chen YM, Huang J, Liu JF, Jiang B (2021) Hippocampal miR-206-3p participates in the pathogenesis of depression via regulating the expression of BDNF. Pharmacol Res 174:105932. [DOI] [PubMed] [Google Scholar]
- Han K, Kim E (2008) Synaptic adhesion molecules and PSD-95. Prog Neurobiol 84:263–283. [DOI] [PubMed] [Google Scholar]
- Ignácio ZM, Réus GZ, Arent CO, Abelaira HM, Pitcher MR, Quevedo J (2016 Nov) New perspectives on the involvement of mTOR in depression as well as in the action of antidepressant drugs. Br J Clin Pharmacol 82:1280–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang B, Wang H, Wang JL, Wang YJ, Zhu Q, Wang CN, Song L, Gao TT, Wang Y, Zhu WZ, Meng GL, Wu F, Huang C, Ling Y, Zhang W, Li JX (2019) Hippocampal salt-inducible kinase 2 plays a role in depression via the CREB-regulated transcription coactivator 1-cAMP response element binding-brain-derived neurotrophic factor pathway. Biol Psychiatry 85:650–666. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG; NC3Rs Reporting Guidelines Working Group (2010) Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol 160:1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V, Nestler EJ (2008) The molecular neurobiology of depression. Nature 455:894–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kugathasan P, Waller J, Westrich L, Abdourahman A, Tamm JA, Pehrson AL, Dale E, Gulinello M, Sanchez C, Li Y (2017) In vivo and in vitro effects of vortioxetine on molecules associated with neuroplasticity. J Psychopharmacol 31:365–376. [DOI] [PubMed] [Google Scholar]
- Lan AP, Chen J, Zhao Y, Chai Z, Hu Y (2017) mTOR signaling in Parkinson’s disease. Neuromolecular Med 19:1–10. [DOI] [PubMed] [Google Scholar]
- Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, Li XY, Aghajanian G, Duman RS (2010) mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 329:959–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu GY, Sabatini DM (2020) mTOR at the nexus of nutrition, growth, ageing and disease. Nat Rev Mol Cell Biol 21:183–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XL, Luo L, Mu RH, Liu BB, Geng D, Liu Q, Yi LT (2015) Fluoxetine regulates mTOR signaling in a region-dependent manner in depression-like mice. Sci Rep 5:16024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Ho CS, McIntyre RS, Wang W, Ho RC (2018) Effects of vortioxetine and fluoxetine on the level of brain derived neurotrophic factors (BDNF) in the hippocampus of chronic unpredictable mild stress-induced depressive rats. Brain Res Bull 142:1–7. [DOI] [PubMed] [Google Scholar]
- McGrath JC, Lilley E (2015) Implementing guidelines on reporting research using animals (ARRIVE etc.): new requirements for publication in BJP. Br J Pharmacol 172:3189–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita K, Her S (2008) Progesterone pretreatment enhances serotonin-stimulated BDNF gene expression in rat c6 glioma cells through production of 5alpha-reduced neurosteroids. J Mol Neurosci 34:193–200. [DOI] [PubMed] [Google Scholar]
- Pehrson AL, Jeyarajah T, Sanchez C (2016) Regional distribution of serotonergic receptors: a systems neuroscience perspective on the downstream effects of the multimodal-acting antidepressant vortioxetine on excitatory and inhibitory neurotransmission. CNS Spectr 21:162–183. [DOI] [PubMed] [Google Scholar]
- Popova NV, Jücker M (2021) The role of mTOR signaling as a therapeutic target in cancer. Int J Mol Sci 22:1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao H, Li MX, Xu C, Chen HB, An SC, Ma XM (2016) Dendritic spines in depression: what we learned from animal models. Neural Plast 2016:8056370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Querfurth H, Lee HK (2021) Mammalian/mechanistic target of rapamycin (mTOR) complexes in neurodegeneration. Mol Neurodegener 16:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasi M, Vignoli B, Canossa M, Blum R (2017) Neurobiology of local and intercellular BDNF signaling. Pflugers Arch 469:593–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Liston C, McEwen BS (2019) Parsing the hippocampus in depression: chronic stress, hippocampal volume, and major depressive disorder. Biol Psychiatry 85:436–438. [DOI] [PubMed] [Google Scholar]
- Shelton RC (2007) The molecular neurobiology of depression. Psychiatr Clin North Am 30:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L, Wang H, Wang YJ, Wang JL, Zhu Q, Wu F, Zhang W, Jiang B (2018) Hippocampal PPARα is a novel therapeutic target for depression and mediates the antidepressant actions of fluoxetine in mice. Br J Pharmacol 175:2968–2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun B, Lv Y, Xu H, Qi C, Li C, Liu P (2020) Effects of vortioxetine on depression model rats and expression of BDNF and TrkB in hippocampus. Exp Ther Med 20:2895–2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Switon K, Kotulska K, Janusz-Kaminska A, Zmorzynska J, Jaworski J (2017) Molecular neurobiology of mTOR. Neuroscience 341:112–153. [DOI] [PubMed] [Google Scholar]
- Villa-González M, Martín-López G, Pérez-Akvarez MJ (2022) Dysregulation of mTOR signaling after brain ischemia. Int J Mol Sci 23:2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Yu JT, Miao D, Wu ZC, Tan MS, Tan L (2014) Targeting the mTOR signaling network for Alzheimer’s disease therapy. Mol Neurobiol 49:120–135. [DOI] [PubMed] [Google Scholar]
- Wang CN, Gong SN, Guan W, Wang JL, Gao TT, Wang Y, Sun F, Jiang B (2020a) Hippocampal overexpression of chordin protects against the chronic social defeat stress-induced depressive-like effects in mice. Brain Res Bull 158:31–39. [DOI] [PubMed] [Google Scholar]
- Wang JL, Wang Y, Gao TT, Liu L, Wang YJ, Guan W, Chen TT, Zhao J, Zhang Y, Jiang B (2020b) Venlafaxine protects against chronic stress-related behaviors in mice by activating the mTORC1 signaling cascade. J Affect Disord 276:525–536. [DOI] [PubMed] [Google Scholar]
- Wang Y, Gu JH, Liu L, Liu Y, Tang WQ, Ji CH, Guan W, Zhao XY, Sun YF, Xu DW, Jiang B (2021) Hippocampal PPARα plays a role in the pharamcological mechanism of vortioxetine, a multimodal-acting antidepressant. Front Pharmacol 12:673221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner-Schmidt JL, Duman RS (2008) VEGF as a potential target for therapeutic intervention in depression. Curr Opin Pharmacol 8:14–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D, Sun Y, Wang C, Wang H, Wang Y, Zhao W, Bao G, Wang F, Cui Z, Jiang B (2018) Hippocampal mTOR signaling is required for the antidepressant effects of paroxetine. Neuropharmacology 128:181–195. [DOI] [PubMed] [Google Scholar]
- Xu D, Wang C, Zhu X, Zhao W, Jiang B, Cui S, Sun Y, Cui Z (2020) The antidepressant-like effects of fluvoxamine in mice involve the mTOR signaling in the hippocampus and prefrontal cortex. Psychiatry Res 285:112708. [DOI] [PubMed] [Google Scholar]
- Yang C, Ren Q, Qu Y, Zhang JC, Ma M, Dong C, Hashimoto K (2018) Mechanistic target of rapamycin-independent antidepressant effects of (R)-ketamine in a social defeat stress model. Biol Psychiatry 83:18–28. [DOI] [PubMed] [Google Scholar]
- Yu H, Chen JJ, Zeng BQ, Zhong QP, Xu JP, Liu YG (2017) [Role of cAMP/CREB/BDNF signaling pathway in anti-depressive effect of vortioxetine in mice]. Nan Fang Yi Ke Da Xue Xue Bao 37:107–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Wang N, Yang C, Li XM, Zhou ZQ, Yang JJ (2014) Ketamine-induced antidepressant effects are associated with AMPA receptors-mediated upregulation of mTOR and BDNF in rat hippocampus and prefrontal cortex. Eur Psychiatry 29:419–423. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that all data supporting the findings of this study are available within the paper and its supplementary information files.


