Abstract
On 10% oleic acid–albumin–dextrose–catalase-enriched 7H11 agar medium, the MIC at which 90% of the isolates are inhibited for 20 strains of Mycobacterium tuberculosis was 0.5 μg of sparfloxacin (SPFX) or moxifloxacin (MXFX) per ml and 1.0 μg of clinafloxacin (CNFX) per ml, indicating that the in vitro activities of SPFX and MXFX were virtually identical and were slightly greater than that of CNFX. However, the in vivo activities of these drugs in a murine tuberculosis model differed considerably. Female Swiss mice were infected intravenously with 6.2 × 106 CFU of the H37Rv strain and treated for 4 weeks, beginning the next day after infection, with isoniazid (INH) serving as the positive control. By the criteria of 30-day survival rate, spleen weight, gross lung lesion, and mean number of CFU in the spleen, treatment with CNFX at up to 100 mg/kg of body weight six times weekly displayed no measurable effect against M. tuberculosis, whereas both SPFX and MXFX were effective; administration six times weekly of either of the latter two drugs demonstrated dosage-dependent bactericidal effects, as measured by enumeration of CFU in the spleens, and MXFX appeared more bactericidal than the same dosage of SPFX. Of the three fluoroquinolones, only MXFX at 100 mg/kg six times weekly appeared as bactericidal as INH at 25 mg/kg six times weekly. Thus, MXFX may be an important component of the newer combined regimens for treatment of tuberculosis.
To treat patients with tuberculosis caused by multidrug-resistant (MDR) strains of Mycobacterium tuberculosis and to improve the efficacy of the standard short-course regimen for the treatment of tuberculosis, new antituberculosis agents acting by mechanisms different from those of the available drugs, especially isoniazid (INH), rifampin, and pyrazinamide, are urgently needed. One possible source of these new agents is the wider-spectrum fluoroquinolones (17).
Compared to the narrow-spectrum fluoroquinolones (e.g., nalidixic acid, norfloxacin, pefloxacin, ciprofloxacin, and ofloxacin [OFLO]), the broad-spectrum compounds (e.g., levofloxacin, clinafloxacin [CNFX], sparfloxacin [SPFX], trovafloxacin, etc.) exhibit greater in vitro activity against several important pathogens as well as improved pharmacokinetic characteristics (21). Our earlier studies revealed that both in vitro and in vivo activities of SPFX and levofloxacin against M. tuberculosis were significantly greater than those of OFLO (12, 14, 16, 17), suggesting that some of the broad-spectrum fluoroquinolones may be more active against M. tuberculosis than are the narrower-spectrum compounds and that the activity of fluoroquinolones against mycobacteria is more closely linked to their general antibacterial activity than to physicochemical characteristics such as lipophilicity (19). SPFX was shown to be far more bactericidal against M. tuberculosis than either levofloxacin or OFLO on a weight-to-weight basis in the treatment of murine tuberculosis (12, 14, 16, 17) and appeared to be the fluoroquinolone with the most bactericidal activity against M. tuberculosis. However, because of the drug’s strong phototoxic potential (9, 24), the manufacturer recommends that, in clinical application, SPFX be administered in a dosage no higher than 200 mg per day, a dosage that appears on the basis of experiments in mice (16) to be suboptimal for the treatment of tuberculosis.
Moxifloxacin (BAY 12-8039; MXFX) is a new member of the broad-spectrum fluoroquinolones. It is highly active against both gram-positive and gram-negative bacteria including anaerobes (1–5, 10, 18, 25), and in vitro, it is generally 10-fold more active than ciprofloxacin and twice as active as SPFX (3). In vivo, MXFX is more active than SPFX against Staphylococcus aureus and as active as SPFX against Streptococcus pneumoniae (3). The MIC of MXFX at which 90% of the isolates are inhibited (MIC90) was found to be 0.25 μg/ml against 25 isolates of M. tuberculosis, 1 dilution (log2) lower than that of SPFX, and 4.0 μg/ml against 28 isolates of Mycobacterium avium-intracellulare complex (MAC), the same as that of SPFX (3a). In healthy male volunteers administered a single 200-mg dose of MXFX, the maximum concentration of drug in serum was 1.16 to 1.17 μg/ml, the half-life at β phase was 14 to 15 h, and the area under the concentration-time curve was 14.3 to 15.4 mg·h/liter (20), indicating that the pharmacokinetic characteristics of this drug are at least as favorable as those of other fluoroquinolones including SPFX (21). Because phototoxicity has not been observed in studies of MXFX either in vitro, in vivo (24), or in humans (6), it may not be a problem during treatment with this drug.
CNFX (CI-960; PD 127391) displayed excellent in vitro activities against a broad spectrum of gram-positive and gram-negative microorganisms and was more active than trovafloxacin and ciprofloxacin (7, 23). In a study which compared almost all available fluoroquinolones except MXFX, the MIC of CNFX against mc2 155, a wild strain of Mycobacterium smegmatis, and the 50% inhibitory concentration against the DNA gyrase purified from this organism were the lowest (19a), suggesting that CNFX may exhibit promising activity against other mycobacteria. To date, however, only limited information is available with respect to its in vitro activity against M. tuberculosis, and to our knowledge, no results of its in vivo activity against M. tuberculosis have been reported. For healthy volunteers administered a single 200-mg dose of CNFX, the time to maximum concentration of drug in serum was 1.5 h, maximum concentration of drug in serum was 1.6 μg/ml, half-life at β phase was 6.3 h, and the area under the concentration-time curve was 11 mg · h/liter (21). Thus, the pharmacokinetic properties of CNFX in humans do not differ significantly from those of other broad-spectrum analogs (21).
To assess the potential role of MXFX and CNFX in the treatment of tuberculosis, the activities of these drugs against M. tuberculosis were compared, both in vitro and in vivo, with those of INH and SPFX. The results of our in vivo experiment demonstrate that MXFX is highly bactericidal against M. tuberculosis.
MATERIALS AND METHODS
Antimicrobial agents.
MXFX was generously provided by Bayer Pharma, Puteaux, France; CNFX was a gift of Parke-Davis, Ann Arbor, Mich.; SPFX was obtained from Rhone D.P.C. Europe, Antony, France; and INH was from Roche, Neuilly, France.
Determination of the MICs against M. tuberculosis.
The MICs of MXFX, CNFX, and SPFX were determined for 20 strains of M. tuberculosis, including 19 clinical isolates and the reference strain H37Rv. Among the 19 clinical isolates, 14 were fully drug susceptible and 5 (strains 4, 6, 10, 14, and 18) were MDR, resistant at least to both INH and rifampin but not to fluoroquinolones. The drugs were initially dissolved in 0.1 N NaOH, subsequently diluted with distilled water, and incorporated into 10% oleic acid–albumin–dextrose–catalase-enriched 7H11 agar medium, with twofold-diluted final concentrations ranging from 2 to 0.03 μg/ml.
The strains were subcultured in Tween 80-containing Dubos broth (Diagnostics Pasteur, Paris, France) at 37°C for 7 days. The turbidity of the resultant suspensions was then adjusted with distilled water to match that of a standard suspension of Mycobacterium bovis BCG (1 mg/ml), the suspensions were further serially 10-fold diluted to a concentration of 10−5 mg/ml, and 0.05-ml portions of the 10−3- and 10−5-mg/ml suspensions were plated on drug-free and drug-containing media, respectively. The MIC was defined as the lowest drug concentration that inhibited more than 99% of the bacterial growth, compared with the growth on drug-free medium, after incubation at 37°C for 28 days.
Comparison of the in vivo activities against M. tuberculosis in mice.
Three hundred ninety 4-week-old, female Swiss mice were infected intravenously with 0.5 ml of a freshly prepared bacterial suspension containing 6.2 × 106 CFU of the H37Rv strain; 30 additional mice were kept as noninfected and untreated controls. The next day after infection (day 1), 30 mice were sacrificed to provide the baseline values of spleen weight, gross lung lesion, and number of CFU per spleen, and the remaining infected mice were randomly allocated to an untreated control group (the negative controls) and 11 treated groups of 30 mice each. The drugs were administered by an esophageal cannula (gavage), beginning on day 1 and continuing for 4 weeks. The treated mice were administered one of the following 11 treatments: INH at 25 mg/kg of body weight six times weekly (the positive controls); SPFX, MXFX, or CNFX in a dosage of 25, 50, or 100 mg/kg six times weekly; and SPFX at 100 mg/kg once weekly, i.e., a total of four doses (the 11th group). The last doses were administered on day 28 at the latest, and all surviving mice were sacrificed on day 30. The severity of infection and the effectiveness of the treatments were assessed by the 30-day survival rate, mean spleen weight, gross lung lesion, and mean number of CFU per spleen. The gross lung lesions were scored from 0 to 2+, with the latter referring to a lung extensively infiltrated with tubercles (22). A regimen was considered bacteriostatic if the mean number of CFU per spleen was significantly lower in treated mice than in untreated controls sacrificed concomitantly but was not lower than the pretreatment value, i.e., the mean number of CFU in the spleens of mice that had been sacrificed on day 1; if the mean number of CFU per spleen in treated mice was significantly lower than the pretreatment value, the regimen was considered to have demonstrated a bactericidal effect.
Enumeration of CFU in the spleen.
At sacrifice, the spleen was removed aseptically and homogenized to a final volume of 3.5 ml. Each suspension was serially diluted in 10-fold steps, and at least three dilutions were plated on Löwenstein-Jensen medium, three to five tubes per dilution, and incubated at 37°C for 6 weeks.
Statistical analysis.
Multiple comparisons among pairs of group means were performed by Bonferroni’s method (8). Because 12 groups were compared, differences were considered significant at the level of 0.00075, i.e., 0.05/[12(12 − 1)/2].
RESULTS
MICs of MXFX, CNFX, and SPFX against M. tuberculosis.
As expected (22), the MICs of each of the three fluoroquinolones demonstrated much the same distributions for the MDR isolates as for the fully drug-susceptible isolates. As shown in Table 1, the MIC50 of SPFX was lower than those of MXFX and CNFX, which were identical. However, the MIC90s of SPFX and MXFX were the same, 0.5 μg/ml, whereas that of CNFX was 1 dilution (log2) higher. Therefore, the in vitro activities of SPFX and MXFX against M. tuberculosis were very similar, and both drugs were slightly more active than was CNFX.
TABLE 1.
MICs of SPFX, MXFX, and CNFX against 19 clinical isolates of M. tuberculosis and the reference strain H37Rv
| Fluroquinolone | MIC range (μg/ml) | MIC50 (μg/ml) | MIC90 (μg/ml) |
|---|---|---|---|
| SPFX | 0.12–0.50 | 0.25 | 0.50 |
| MXFX | 0.12–0.50 | 0.50 | 0.50 |
| CNFX | 0.12–1.0 | 0.50 | 1.0 |
In vivo activities against M. tuberculosis H37Rv strain. (i) Thirty-day survival rate.
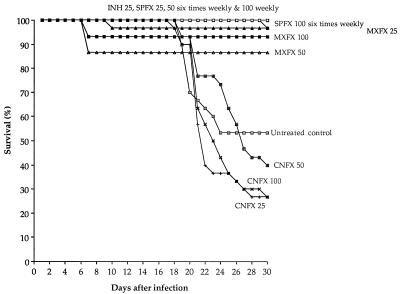
As expected (12, 14, 16, 17), the untreated mice began to die from tuberculosis by day 15 after infection, and about 50% had died by day 30, whereas all of the INH-treated mice survived. As shown in Fig. 1, virtually all of the mice administered SPFX survived, including those treated with SPFX at 100 mg/kg once weekly for four doses. Although not all of the mice treated with MXFX survived, their 30-day survival rates did not differ significantly from 100%. Moreover, all seven of the deaths among the three groups of mice treated with MXFX occurred between day 7 and day 10, earlier than the occurrence of the first deaths among the untreated control mice, suggesting that the deaths among MXFX-treated mice were not a consequence of infection with M. tuberculosis. On the other hand, no dosage of CNFX reduced the mortality from tuberculosis.
FIG. 1.
Survival rate of mice for 30 days after intravenous infection with 6.2 × 106 CFU of M. tuberculosis H37Rv. By the time (day 1) the treatment began, there were 30 mice in each group. Drugs were given by gavage six times weekly, except for one group to which SPFX was given once weekly. The numbers after the abbreviations for the various drugs indicate the milligrams per kilogram per dose.
(ii) Spleen weight.
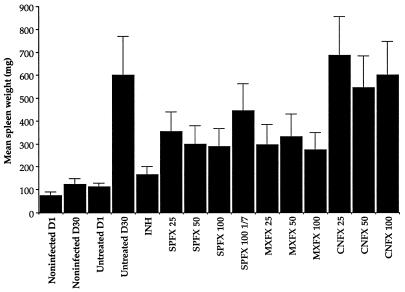
As early as day 1 after infection, the mean spleen weight of infected mice was significantly higher than that among noninfected mice. At day 30, the mean spleen weight of all treated groups was significantly higher than the pretreatment value (Fig. 2). Although none of the treatment regimens was able completely to prevent the development of splenomegaly, the mean spleen weight of INH-treated mice was lower than that of all of the other treated groups. Spleen weights among mice treated six times weekly with either SPFX or MXFX in any dosage were significantly lower than those among the untreated controls, whereas this was not the case among mice treated with CNFX six times weekly in any dosage or with SPFX once weekly.
FIG. 2.
Mean spleen weights for mice surviving at 30 days after intravenous infection with H37Rv. The control D1 bar represents the mean spleen weight for mice sacrificed the next day after infection. Error bars represent standard deviations. The numbers after the abbreviations for the various drugs indicate the milligrams per kilogram per dose.
(iii) Gross lung lesions.
No lung lesions were observed among the infected mice sacrificed on day 1 or among the mice that had been treated with INH, SPFX at 100 mg/kg, or MXFX at 50 or 100 mg/kg six times weekly and sacrificed on day 30. On the other hand, on day 30, severe (2+) lesions were encountered among all surviving untreated control mice and also among mice that had been treated with CNFX six times weekly in any dosage. Lesions of moderate severity (1+) were observed among the remaining groups of mice in various proportions: 29 of 30 (96.7%) mice treated with SPFX at 25 mg/kg, significantly more frequent than those among mice treated with SPFX at 50 mg/kg or MXFX at 25 mg/kg, which were 1 of 30 (3.3%) and 4 of 29 (13.8%), respectively. Among the 30 mice that had been treated with SPFX at 100 mg/kg once weekly, 1+ lesions were detected in 29 mice, and 2+ lesions were detected in one mouse, suggesting a degree of efficacy in preventing the development of gross lung lesions, similar to that of SPFX at 25 mg/kg six times weekly.
(iv) Enumeration of CFU in the spleen.
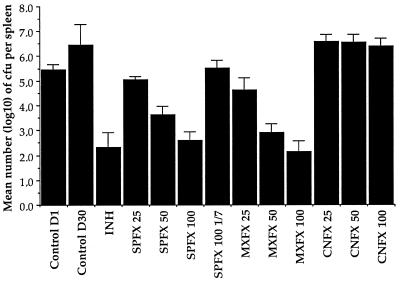
As in all of our earlier experiments (12, 14, 16, 17), treatment with INH for 4 weeks reduced the numbers of M. tuberculosis bacteria by more than 3 log10 from the pretreatment value (Fig. 3). Treatment with SPFX or MXFX six times weekly demonstrated bactericidal activity that was dosage dependent. The mean number of CFU per spleen in mice treated with MXFX was always significantly lower than that among mice treated with the same dosage of SPFX; thus, on a weight-to-weight basis, MXFX was significantly more bactericidal than SPFX. On the other hand, with the exception of MXFX at 100 mg/kg six times weekly, none of the fluoroquinolone regimens was as strongly bactericidal as INH. None of the dosages of CNFX appeared capable of preventing the multiplication of M. tuberculosis in mice. Administration of SPFX at 100 mg once weekly prevented multiplication of M. tuberculosis but did not reduce the bacterial population below the pretreatment value, indicating that, in this dosage, SPFX exerted a purely bacteriostatic effect.
FIG. 3.
Mean numbers of CFU in the spleens of mice surviving at 30 days. Mice were infected intravenously with 6.2 × 106 CFU of M. tuberculosis H37Rv, and treatment was begun the next day after infection. Error bars represent standard deviations. The numbers after abbreviations for the various drugs indicate the milligrams per kilogram per dose.
DISCUSSION
In vitro, MXFX was virtually as active as SPFX against M. tuberculosis, and both were slightly more active than CNFX. In vivo, however, the activities of these drugs differed considerably. Treatment six times weekly with CNFX in any of the dosages examined displayed no measurable effect against M. tuberculosis in mice, suggesting that the prospect for using CNFX for the treatment of tuberculosis is bleak. Nevertheless, treatment six times weekly with either SPFX or MXFX prevented mortality, reduced splenomegaly, and inhibited the development of gross lung lesions among M. tuberculosis-infected mice. However, the results measured by these indicators did not differ significantly among mice treated with various dosages of SPFX or MXFX, suggesting again that these indicators are not sufficiently sensitive to distinguish the efficacy of treatments (17). On the other hand, enumeration of CFU in the spleens clearly revealed that the treatment with SPFX or MXFX displayed a bactericidal effect against M. tuberculosis; more important is that it was able to quantify the efficacy of treatment more precisely, and thus it demonstrated that the bactericidal effects of the two drugs were dosage dependent and that, on a weight-to-weight basis, MXFX was more strongly bactericidal than was SPFX. The discrepancies between the in vitro and in vivo activities of the three fluoroquinolones cannot be immediately explained, although it is likely that they reflect the differences of their pharmacokinetic properties. Unfortunately, only limited pharmacokinetic data for these fluoroquinolones in mice are available at the present time (3, 16), and do not provide insight into the discrepancies.
The finding that MXFX is more bactericidal than is SPFX in mice is encouraging. Moreover, the results from in vitro and in vivo experiments (24) and from human trials (6) all indicate that the phototoxicity may be a negligible issue during treatment with MXFX. Healthy volunteers tolerated well a single dose of 600 mg of MXFX and daily doses of 400 mg (20), suggesting that the clinically tolerated dosage of MXFX may be higher than that of SPFX, which may further enhance the bactericidal effect against M. tuberculosis to a level which would not be achieved by the clinically tolerated dosage of SPFX. Thus, MXFX may represent an important component of newer combined regimens for the treatment of tuberculosis, especially that caused by MDR strains. That MXFX at 100 mg/kg six times weekly appeared to be as bactericidal as was INH in a dosage of 25 mg/kg in mice may also be an important observation. However, we are not yet able to define the human dosage equipotent to MXFX at 100 mg/kg six times weekly in mice.
Up to now, no fluoroquinolone has been shown to be active against MAC (13), the pathogen of the most common disseminated bacterial infection among patients in the later stage of AIDS. In view of the promising bactericidal effect of MXFX against M. tuberculosis, the activity of this drug against MAC should be tested in the beige mouse model. In addition, because OFLO has already become an important component of newer multidrug regimens employed in the treatment of leprosy (15), in order to determine whether MXFX may eventually replace OFLO, the activities of both drugs should be compared in the Mycobacterium leprae-infected mouse footpad system.
One of the current approaches to overcoming the operational difficulties posed by the directly observed treatment strategy for tuberculosis control is to develop once-weekly rifapentine-containing combined regimens, in which all the components are administered once weekly (11). To determine if SPFX may be employed as a component of the once-weekly combined regimen, the activity of SPFX administered once weekly was examined in the present study. Four once-weekly doses of SPFX at 100 mg/kg entirely prevented mortality, inhibited the development of severe (2+) gross lung lesions in the great majority of mice, and demonstrated a bacteriostatic effect against M. tuberculosis. Additional experiments, in which once-weekly SPFX or MXFX is combined with rifapentine and INH, appear to be warranted.
ACKNOWLEDGMENT
The investigation was partially financed by Bayer AG, Wuppertal, Germany.
REFERENCES
- 1.Aldridge K E, Ashcraft D. Comparison of the in vitro activities of BAY 12-8039, a new quinolone, and other antimicrobials against clinically important anaerobes. Antimicrob Agents Chemother. 1997;41:709–711. doi: 10.1128/aac.41.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bauernfeind A. Comparison of the antibacterial activities of the quinolones Bay 12-8039, gatifloxacin (AM1155), trovafloxacin, clinafloxacin, levofloxacin and ciprofloxacin. J Antimicrob Chemother. 1997;40:639–651. doi: 10.1093/jac/40.5.639. [DOI] [PubMed] [Google Scholar]
- 3.Bayer Pharma. Investigator’s drug brochure: Bay 12-8039, version 2. Puteaux, France: Bayer Pharma; 1996. [Google Scholar]
- 3a.Bayer Pharma. Unpublished data.
- 4.Brueggemann A B, Kugler K C, Doern G V. In vitro activity of BAY 12-8039, a novel 8-methoxyquinolone, compared to activities of six fluoroquinolones against Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis. Antimicrob Agents Chemother. 1997;41:1594–1597. doi: 10.1128/aac.41.7.1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dalhoff A, Petersen U, Endermann R. In vitro activity of BAY 12-8039, a new 8-methoxyquinolone. Chemotherapy. 1996;42:410–425. doi: 10.1159/000239474. [DOI] [PubMed] [Google Scholar]
- 6.Ferguson J, Al-Ajmi H, Kubin R, Daggett S, Saggu P S. Program and abstracts of the 8th International Congress on Infectious Diseases. Boston, Mass. 15 to 18 May 1998. 1998. A double-blind, placebo and lomefloxacin controlled human volunteer phototest study to determine the photosensitising potential of oral moxifloxacin (BAY 12-8039), abstr. 59.001. [Google Scholar]
- 7.Fernandes C J, Ackerman V P. Proceedings on posters presented at the 4th International Symposium on New Quinolones, Munich, Germany, 27 to 29 August 1992. 1992. The in vitro activity of CI-960 (clinafloxacin) and CI-990 against routine and multiresistant clinical isolates; pp. 10–11. [Google Scholar]
- 8.Godfrey K. Statistics in practice. Comparing the means of several groups. N Engl J Med. 1985;313:1450–1456. doi: 10.1056/NEJM198512053132305. [DOI] [PubMed] [Google Scholar]
- 9.Goldstein, E. J. C. 1996. Possible role for the new fluoroquinolones (levofloxacin, grepafloxacin, trovafloxacin, clinafloxacin, sparfloxacin, and DU-6859a) in the treatment of anaerobic infections: review of current information on efficacy and safety. Clin. Infect. Dis. 23(Suppl. 1):S25–S30. [DOI] [PubMed]
- 10.Goldstein E J C, Citron D M, Hudspeth M, Gerardo S H, Merriam C V. In vitro activity of Bay 12-8039, a new 8-methoxyquinolone, compared to the activities of 11 other oral antimicrobial agents against 390 aerobic and anaerobic bacteria isolated from human and animal bite wound skin and soft tissue infections in humans. Antimicrob Agents Chemother. 1997;41:1552–1557. doi: 10.1128/aac.41.7.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grosset, J., N. Lounis, C. Truffot-Pernot, R. J. O’Brien, M. C. Raviglione, and B. Ji. Once-weekly rifapentine-containing regimens for treatment of tuberculosis in mice. Am. J. Respir. Crit. Care Med., in press. [DOI] [PubMed]
- 12.Ji B, Truffot-Pernot C, Grosset J. In vitro and in vivo activities of sparfloxacin (AT-4140) against Mycobacterium tuberculosis. Tubercle. 1991;72:181–186. doi: 10.1016/0041-3879(91)90004-c. [DOI] [PubMed] [Google Scholar]
- 13.Ji B, Lounis N, Truffot-Pernot C, Grosset J. Effectiveness of various antimicrobial agents against Mycobacterium avium complex in the beige mouse model. Antimicrob Agents Chemother. 1994;38:2521–2529. doi: 10.1128/aac.38.11.2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ji B, Lounis N, Truffot-Pernot C, Grosset J. In vitro and in vivo activities of levofloxacin against Mycobacterium tuberculosis. Antimicrob Agents Chemother. 1995;39:1341–1344. doi: 10.1128/aac.39.6.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ji B, Levy L, Grosset J H. Chemotherapy of leprosy: progress since the Orlando Congress, and prospects for the future. Int J Lepr. 1996;64:S80–S90. [PubMed] [Google Scholar]
- 16.Lalande V, Truffot-Pernot C, Paccaly-Moulin A, Grosset J, Ji B. Powerful bactericidal activity of sparfloxacin (AT-4140) against Mycobacterium tuberculosis in mice. Antimicrob Agents Chemother. 1993;37:407–413. doi: 10.1128/aac.37.3.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lounis N, Ji B, Truffot-Pernot C, Grosset J. Which aminoglycoside or fluoroquinolone is more active against Mycobacterium tuberculosis in mice? Antimicrob Agents Chemother. 1997;41:607–610. doi: 10.1128/aac.41.3.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petersen U, Bartel S, Bremm K D, Himmler T, Krebs A, Schenke T. The synthesis and biological properties of 6-fluoroquinolone-carboxylic acids. Bull Soc Chim Belg. 1996;105:683–698. [Google Scholar]
- 19.Renau T, Gage J W, Dever J A, Roland G E, Joannides E T, Shapiro M A, Sanchez J P, Gracheck S J, Domagala J M, Jacobs M R, Reynolds R C. Structure-activity relationships of quinolone agents against mycobacteria: effect of structural modifications at the 8 position. Antimicrob Agents Chemother. 1996;40:2363–2368. doi: 10.1128/aac.40.10.2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19a.Sougakoff, W. Personal communication.
- 20.Stass H, Dalhoff A, Kubitza D, Schühly U. Pharmacokinetics, safety, and tolerability of ascending single doses of moxifloxacin, a new 8-methoxy quinolone administered to healthy subjects. Antimicrob Agents Chemother. 1998;42:2060–2065. doi: 10.1128/aac.42.8.2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stein, G. E. 1996. Pharmacokinetics and pharmacodynamics of newer fluoroquinolones. Clin. Infect. Dis. 23(Suppl. 1):S19–S24. [DOI] [PubMed]
- 22.Truffot-Pernot C, Ji B, Grosset J. Activities of pefloxacin and ofloxacin against mycobacteria: in vitro and mouse experiment. Tubercle. 1991;72:57–64. doi: 10.1016/0041-3879(91)90025-n. [DOI] [PubMed] [Google Scholar]
- 23.Visalli M A, Jacobs M R, Appelbaum P C. Activity of CP 99,219 (trovafloxacin) compared with ciprofloxacin, sparfloxacin, clinafloxacin, lomefloxacin and cefuroxime against ten penicillin-susceptible and penicillin-resistant pneumococci by time-kill methodology. J Antimicrob Chemother. 1996;37:77–84. doi: 10.1093/jac/37.1.77. [DOI] [PubMed] [Google Scholar]
- 24.Vohr H W, Wasinska-Kempka G, Ahr H J. Abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. Studies on the phototoxic potential of a new 8-methoxy-quinolone: BAY 12-8039, abstr. F21; p. 103. [Google Scholar]
- 25.Woodcock J M, Andrews J M, Boswell F J, Brenwald N P, Wise R. In vitro activity of BAY 12-8039, a new fluoroquinolone. Antimicrob Agents Chemother. 1997;41:101–106. doi: 10.1128/aac.41.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]