Abstract
Background
Eicosanoids are bioactive lipids that regulate systemic inflammation and exert vasoactive effects. Specific eicosanoid metabolites have previously been associated with pulmonary hypertension (PH), yet their role remains incompletely understood.
Methods
We studied 482 participants with chronic dyspnoea who underwent clinically indicated cardiopulmonary exercise testing (CPET) with invasive haemodynamic monitoring. We performed comprehensive profiling of 888 eicosanoids and eicosanoid-related metabolites using directed non-targeted mass spectrometry, and examined associations with PH (mean pulmonary arterial pressure (mPAP) >20 mmHg), PH subtypes and physiological correlates, including transpulmonary metabolite gradients.
Results
Among 482 participants (mean±sd age 56±16 years, 62% women), 200 had rest PH. We found 48 eicosanoids and eicosanoid-related metabolites that were associated with PH. Specifically, prostaglandin (11β-dhk-PGF2α), linoleic acid (12,13-EpOME) and arachidonic acid derivatives (11,12-DiHETrE) were associated with higher odds of PH (false discovery rate q<0.05 for all). By contrast, epoxide (8(9)-EpETE), α-linolenic acid (13(S)-HOTrE(γ)) and lipokine derivatives (12,13-DiHOME) were associated with lower odds. Among PH-related eicosanoids, 14 showed differential transpulmonary metabolite gradients, with directionality suggesting that metabolites associated with lower odds of PH also displayed pulmonary artery uptake. In individuals with exercise PH, eicosanoid profiles were intermediate between no PH and rest PH, with six metabolites that differed between rest and exercise PH.
Conclusions
Our findings highlight the role of specific eicosanoids, including linoleic acid and epoxide derivatives, as potential regulators of inflammation in PH. Of note, physiological correlates, including transpulmonary metabolite gradients, may prioritise future studies focused on eicosanoid-related pathways as important contributors to PH pathogenesis.
Tweetable abstract
Eicosanoids are bioactive lipids that regulate inflammation. This study found that specific eicosanoid metabolites were associated with pulmonary hypertension, including linoleic acid, prostaglandin, arachidonic acid and epoxide derivatives. https://bit.ly/47Ha6Ni
Introduction
Pulmonary hypertension (PH) affects nearly 1% of the global population and is characterised by vascular remodelling and endothelial cell dysfunction which over time can lead to right-sided heart failure [1]. The aetiology of PH is multifactorial, with the most common contributors being left heart disease and lung disease [1]. Irrespective of subtype, inflammation by various cytokines and macrophages in lung tissue is thought to play a central role in the development of PH, yet specific pro- and anti-inflammatory pathways that lead to pulmonary vascular remodelling remain incompletely understood.
Eicosanoids are bioactive lipids that regulate upstream initiation of inflammation and exert vasoactive properties. Prior studies have demonstrated the important biological contributions of eicosanoids in the development of PH. For example, in both pulmonary arterial hypertension (PAH) and chronic thromboembolic disease samples, arachidonic acid derivatives, including hydroxyeicosatetraenoic acids (HETEs) and leukotrienes, have been associated with PH [2, 3]. Further, prostaglandins are known to be important regulators of vascular tone in PH, with prostaglandin derivatives representing one of the foundational targets for PH therapies [4, 5]. These prior studies have been limited to ascertainment of up to dozens of eicosanoid molecules, and the broader role of eicosanoid and eicosanoid-related metabolites across the spectrum of PH and pulmonary vascular dysfunction has not been well described.
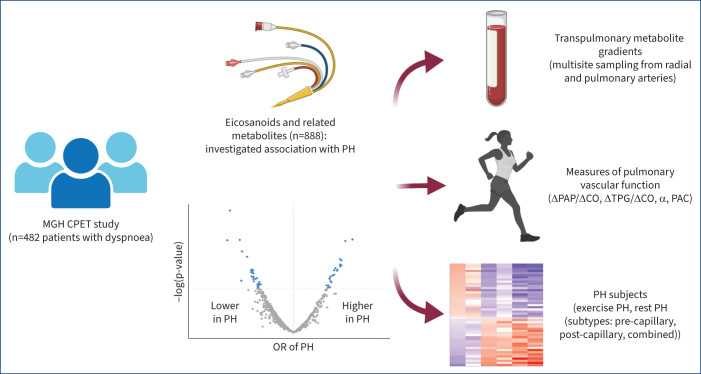
Our group recently demonstrated the use of directed non-targeted liquid chromatography-mass spectrometry (LC-MS) with chemical networking that has allowed for more comprehensive profiling of over 250 eicosanoids and eicosanoid-related molecules [6]. We sought to leverage this molecular profiling platform to study the association of eicosanoids with PH, including PH subtypes, physiological correlates, and pulmonary vascular function at rest and during exercise in a unique sample of individuals who had undergone detailed cardiopulmonary exercise testing (CPET) with invasive haemodynamic monitoring, including multisite blood sampling to ascertain transpulmonary metabolite gradients between the radial and pulmonary artery samples (figure 1). We hypothesise that pro-inflammatory eicosanoids will be associated with a greater odds of PH as well as have higher levels expressed within the pulmonary artery samples.
FIGURE 1.
Study design and approach. We investigated the association of eicosanoid and eicosanoid-related metabolites with pulmonary hypertension (PH). Multivariable models adjusted for age, sex, plate number, statin use, aspirin use, body mass index, diabetes, hypertension, present smoking, prevalent myocardial infarction, heart failure with preserved ejection fraction, obstructive sleep apnoea, COPD and interstitial lung disease. In complementary analyses, we examined PH-associated eicosanoids and their transpulmonary metabolite gradients, association with measures of pulmonary vascular function and PH subtypes. MGH: Massachusetts General Hospital; CPET: cardiopulmonary exercise testing; PAP: pulmonary arterial pressure; CO: cardiac output; TPG: transpulmonary gradient; α: pulmonary vascular distensibility; PAC: pulmonary artery compliance; OR: odds ratio. Partially created with BioRender.com.
Methods
Study sample
The Massachusetts General Hospital (MGH) CPET sample included consecutive patients who underwent clinically indicated CPET for evaluation of chronic dyspnoea between 2009 and 2017. We studied 588 individuals with preserved left ventricular ejection fraction (≥50%) with available blood samples for molecular profiling. From this sample, we excluded participants with the following comorbid conditions: history of cardiac or pulmonary transplant (n=12), complex congenital heart disease (n=14), mitochondrial disorders (n=6), those undergoing lung transplant evaluation (n=10) and those with significant valvular disease (defined as moderate or severe aortic stenosis, aortic regurgitation, mitral stenosis or mitral regurgitation) or lung disease (defined as subjects requiring home oxygen) (n=64), leaving 482 individuals for analysis. The study protocol was approved by the MGH Institutional Review Board and all participants provided informed consent.
Right heart catheterisation and invasive CPET
Participants underwent insertion of a pulmonary artery catheter via the internal jugular vein, as well as radial artery catheterisation. Rest haemodynamic measures were collected in the supine position and measurements recorded at end-expiration. Participants then performed exercise testing using upright cycle ergometry with a ramp protocol that involved an initial period of 3 min of unloaded exercise followed by 5–20 W·min−1 of continuous ramp to maximal volitional effort as previously described [7]. Haemodynamic measures were obtained at rest and during each minute of exercise along with serial gas exchange parameters (MedGraphics, St Paul, MN, USA). These included pulmonary arterial pressure (PAP), pulmonary capillary wedge pressure (PCWP), direct Fick cardiac output (CO), peak oxygen uptake (V′O2) and respiratory exchange ratio (RER).
Definition of PH, subtypes and pulmonary vascular traits
Our primary outcome was rest PH, defined as a mean PAP >20 mmHg as outlined by the most recent European Society of Cardiology/European Respiratory Society recommendations [8, 9]. In secondary analyses, we further classified PH into subtypes as follows: 1) pre-capillary: PCWP ≤15 mmHg and pulmonary vascular resistance (PVR) >2 WU; 2) combined pre- and post-capillary: PCWP >15 mmHg and PVR >2 WU; and 3) post-capillary: PCWP >15 mmHg and PVR ≤2 WU [8, 9]. Exercise PH was defined as the absence of rest PH, with ΔPAP/ΔCO slope >3 mmHg·L−1·min−1 [8, 9], which was calculated using repeated minute-by-minute PAP and CO measures during exercise as previously described [10, 11].
We ascertained measures of pulmonary vascular function, including the ΔTPG/ΔCO slope, calculated using repeated measures of transpulmonary gradient (mean PAP minus PCWP) and CO during exercise. We assessed rest pulmonary artery compliance (PAC), defined as stroke volume divided by pulmonary pulse pressure at end-expiration [12]. Lastly, we estimated pulmonary vascular distensibility (α) using a previous described equation with mPAP, PAWP and pulmonary blood flow (Q; L·min−1) at a minimum of four time-points to calculate the pulmonary vascular pressure–flow relationship [13].
Eicosanoid measurements
Blood samples for eicosanoid measurements were collected after a minimum of 8 h of fasting from the superior vena cava at rest. The samples were processed immediately and stored at −80°C until assayed. To assess transpulmonary metabolite gradients, samples were collected from the radial artery and pulmonary artery at rest. Eicosanoid profiling of the plasma samples was performed at the University of California San Diego (La Jolla, CA, USA). Eicosanoid profiling has been previously described in greater detail [14, 15]. Eicosanoids were assayed using a directed, non-targeted LC-MS approach combined with computational chemical networking of spectral fragmentation patterns. Metabolites were annotated using an in-house library of commercially available standards and by MS/MS fragmentation patterns. Validation of specific metabolites was done using spectral fragmentation pattern networking and manual annotation. For radiolabelled internal standard metabolites, the average coefficient of variation was 10.2% with a range of 8.1–13.1%. Average correlation between metabolites was r=0.07 with a range of −0.62–0.98. Additional information regarding lipid standards, MS characteristics, and naming of eicosanoids and eicosanoid-related metabolites is outlined in LIPID MAPS [16].
Statistical analyses
Baseline clinical and CPET characteristics were summarised by the presence or absence of rest and exercise PH. Pre-processing of metabolite data was performed as follows: missing values indicative of undetectable metabolite levels were imputed as 25% of the minimum value detected for each metabolite. Metabolites with >90% undetectable values in the sample were excluded from the analyses. Among the remaining metabolites, 16% of total values were undetectable. Due to right-skewed distributions, metabolite concentrations were natural-log transformed and then standardised to a mean of 0 and a standard deviation of 1.
We examined the cross-sectional association between single eicosanoid metabolites with rest PH using multivariable logistic regression. Primary analyses were adjusted for age, sex, plate number, statin use and aspirin use, chosen a priori given the known effects of these clinical variables and medications on eicosanoid metabolism [17, 18]. Secondary models were additionally adjusted for body mass index (BMI), diabetes mellitus, hypertension, current smoking status, obstructive sleep apnoea, COPD, interstitial lung disease, prevalent clinical heart failure and prevalent myocardial infarction. To account for multiple hypothesis testing, a false discovery rate (FDR) q<0.05 was deemed statistically significant for primary analyses, with p<0.05 considered as suggestive.
In secondary analyses, we selected PH-associated metabolites identified in primary analyses (n=48) and investigated associations with physiological correlates of PH as well as PH subtypes. We first examined transpulmonary metabolite gradients, defined as radial artery−pulmonary artery metabolite concentrations, to infer potential lung uptake or release of metabolites using paired t-tests [19]. We next tested metabolite associations with pulmonary vascular traits as continuous variables using linear regression, including mean PAP, ΔPAP/ΔCO slope, ΔTPG/ΔCO slope, pulmonary vascular distensibility (α) and PAC. Dependent variables, including mean PAP, ΔPAP/ΔCO slope and ΔTPG/ΔCO slope, were natural-log transformed due to right-skewed distributions. Finally, average PH-associated metabolite concentrations were compared among participants within PH subtypes (pre-capillary PH, post-capillary PH, and combined pre- and post-capillary PH), with between-group differences assessed using ANOVA and subsequent pairwise comparisons as appropriate. Heatmaps were used to summarise directionality and strength of β coefficients and/or average metabolite concentrations in secondary analyses. All analyses were performed using R version 4.2.1 (www.r-project.org).
Results
Among 482 participants (mean±se age 55±16 years, 62% women), 200 had rest PH and 63 had exercise PH. Baseline clinical characteristics and haemodynamic measurements are summarised in table 1. Individuals with rest PH were older (mean age 62 versus 49 years) with greater burden of medical comorbidities, including higher BMI (mean 32 versus 27 kg·m−2), hypertension (69% versus 36%) and diabetes (25% versus 7%) compared with those without PH. Individuals with exercise PH were similar in age to those with rest PH, with comorbidity burden that was intermediate between rest PH and no PH (table 1).
TABLE 1.
Baseline clinical and cardiopulmonary exercise testing (CPET) characteristics by pulmonary hypertension (PH) rest status in the Massachusetts General Hospital CPET sample
| No PH (n=219) | Rest PH (n=200) | Exercise PH (n=63) | |
| Clinical characteristics | |||
| Age, years | 49±16 | 62±13 | 60±14 |
| Women | 148 (68) | 108 (54) | 43 (68) |
| White race | 207 (95) | 189 (95) | 60 (95) |
| BMI, kg·m−2 | 26.8±5.4 | 32.2±6.7 | 27.2±5.0 |
| Hypertension | 78 (36) | 138 (69) | 35 (56) |
| Myocardial infarction | 4 (2) | 9 (5) | 3 (5) |
| Diabetes mellitus | 15 (7) | 49 (25) | 10 (16) |
| Interstitial lung disease | 8 (4) | 13 (7) | 5 (8) |
| COPD | 9 (4) | 26 (13) | 5 (8) |
| Obstructive sleep apnoea | 27 (12) | 68 (34) | 9 (14) |
| Prior HFpEF | 11 (5) | 41 (20) | 10 (16) |
| Connective tissue disease | 24 (11.0) | 14 (7.1) | 8 (12.7) |
| Current smoking | 5 (2) | 8 (4) | 4 (6) |
| Statin use | 51 (23.3) | 96 (48.0) | 24 (38.1) |
| Aspirin use | 58 (26.5) | 93 (46.5) | 21 (33.3) |
| Glucocorticoid use | 15 (6.8) | 19 (9.5) | 7 (11.1) |
| NSAID use | 15 (6.8) | 10 (5.0) | 4 (6.3) |
| PH medication use | 2 (0.9) | 3 (1.5) | 1 (1.6) |
| hsCRP, mg·L−1 | 1.12 (0.52–2.84) | 2.91 (1.30–6.54) | 2.20 (1.00–3.86) |
| LV ejection fraction, % | 65±6 | 65±8 | 66±7 |
| RHC haemodynamics | |||
| RA pressure, mmHg | 4.30±2.41 | 9.09±4.00 | 4.67±2.16 |
| mPAP, mmHg | 14.94±3.24 | 26.09±5.00 | 16.71±2.53 |
| PCWP, mmHg | 9.17±3.02 | 16.08±5.39 | 9.76±3.06 |
| PVR, WU | 1.14±0.66 | 2.18±1.27 | 1.50±0.79 |
| α, % | 1.44±0.53 | 0.99±0.44 | 1.09±0.43 |
| PAC, mL·mmHg−1 | 5.54±2.36 | 3.86±1.80 | 4.78±1.96 |
| Peak RER | 1.18±0.11 | 1.15±0.11 | 1.16±0.12 |
| Peak V′O2, mL·kg−1·min−1 | 19.98±5.78 | 14.64±4.20 | 15.93±3.77 |
| ΔPAP/ΔCO, mmHg·L−1·min−1 | 1.86±0.58 | 3.95±2.52 | 4.75±3.56 |
| ΔTPG/ΔCO, mmHg·L−1·min−1 | 0.59±0.46 | 1.16±1.10 | 1.18±1.39 |
Data are presented as mean±sd, n (%) or median (interquartile range). BMI: body mass index; HFpEF: heart failure with preserved ejection fraction; NSAID: non-steroidal anti-inflammatory drug; hsCRP: high-sensitivity C-reactive protein; LV: left ventricular; RHC: right heart catheterisation; RA: right atrial; mPAP: mean pulmonary arterial pressure; PCWP: pulmonary capillary wedge pressure; PVR: pulmonary vascular resistance; α: pulmonary vascular distensibility; PAC: pulmonary artery compliance; RER: respiratory exchange ratio; V′O2: oxygen uptake; CO: cardiac output; TPG: transpulmonary gradient.
Rest haemodynamics across groups without PH, exercise PH and rest PH showed differences in mean PAP of 15±3, 17±3 and 26±5 mmHg and PVR of 1.1±0.7, 1.5±0.8 and 2.2±1.3 WU, respectively (pANOVA<0.001). Exercise measures showed similar RER indicating maximal effort across groups, with lower overall exercise capacity as measured by peak V′O2 among those with PH (20.0±5.8 mL·kg−1·min−1 (no PH) versus 15.9±3.8 mL·kg−1·min−1 (exercise PH) and 14.6±4.2 mL·kg−1·min−1 (rest PH)). Individuals with rest PH also had worse PAC and pulmonary vascular distensibility (α) compared with those without PH (table 1).
Association of eicosanoids and eicosanoid-related metabolites with rest PH
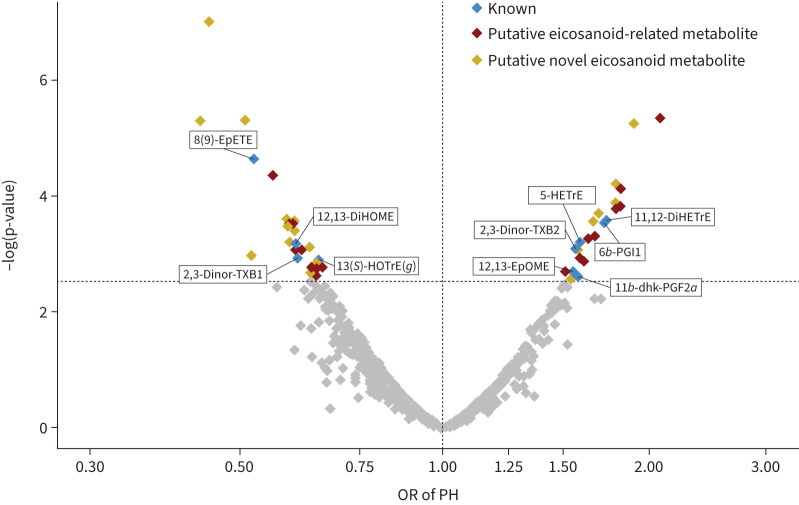
Of 888 eicosanoids and eicosanoid-related metabolites analysed, 48 were associated with rest PH (FDR q<0.05 for all) (figure 2). Of these 48 metabolites, 10 have known molecular identity as shown in table 2 and the remainder are putative eicosanoids (full results in supplementary table S1). Specifically, we found 22 eicosanoids and eicosanoid-related metabolites associated with higher odds of rest PH, including prostaglandins or prostaglandin-like metabolites (11β-dhk-PGF2α and 6β-PGI1) as well as arachidonic acid derivatives (11,12-DiHETrE). For example, 1sd higher 11β-dhk-PGF2α and 6β-PGI1 levels were associated with >1.5-fold higher odds of having rest PH (OR 1.58, 95% CI 1.19–2.18; p=0.003; and OR 1.73, 95% CI 1.30–2.36; p=0.0003, respectively). In addition, linoleic acid (12,13-EpOME) and HETE derivatives (5(S)-HETrE) were associated with higher odds of rest PH. There were 26 eicosanoids and eicosanoid-related metabolites associated with lower odds of PH. These included hydroxyoctadecatrienoic acid derivates, epoxides and lipokines. The largest observed magnitude of effect was for the epoxide 8(9)-EpETE (OR 0.52, 95% CI 0.39–0.70; p=2.25E-05) followed by lipokine 12,13-DiHOME (OR 0.61, 95% CI 0.45–0.80; p=0.0007). In the secondary model after adjusting for additional clinical covariates, including connective tissue disease, the significant eicosanoid results were attenuated. 26 of the 48 had suggestive associations (p<0.05) in the secondary model (supplementary table S2). Of note, when rest PH was defined as mPAP ≥25 mmHg, 37 of the 48 significant eicosanoids were similar (supplementary table S3).
FIGURE 2.
Volcano plot for associations of eicosanoid and eicosanoid-related metabolites with rest pulmonary hypertension (PH). A total of 48 out of 888 eicosanoids and eicosanoid-related metabolites were significantly associated with rest PH as shown in blue, red and yellow (false discovery rate q<0.05), with nonsignificant associations denoted in grey. x-axis displays odds ratio (OR). See table 2 for nomenclature.
TABLE 2.
Association of known eicosanoid and eicosanoid-related metabolites with rest pulmonary hypertension (PH)
| m/z | Retention time, min | Class | Putative metabolite ID | Primary model | Secondary model | ||
| OR# (95% CI) | p-value | OR# (95% CI) | p-value | ||||
| Associated with greater odds of PH | |||||||
| 337.2383 | 3.9405 | Eicosanoid | 11,12-DiHETrE | 1.74 (1.30–2.36) | 0.0003 | 1.50 (1.08–2.10) | 0.02 |
| 353.2336 | 2.2385 | Prostaglandin-like | 6β-PGI1 | 1.73 (1.30–2.36) | 0.0003 | 1.24 (0.93–1.69) | 0.15 |
| 381.2635 | 6.0865 | Eicosanoid | 5-HETrE | 1.59 (1.22–2.09) | 0.0006 | 1.57 (1.16–2.14) | 0.004 |
| 353.2336 | 2.3865 | Eicosanoid | 11β-dhk-PGF2α | 1.58 (1.19–2.18) | 0.0026 | 1.44 (1.04–2.05) | 0.03 |
| 401.2125 | 1.578667 | Fatty acyl-eicosanoids | 2,3-Dinor-TXB2 | 1.57 (1.21–2.06) | 0.0008 | 1.45 (1.09–1.96) | 0.01 |
| 355.2489 | 5.229333 | Eicosanoid | 12,13-EpOME | 1.55 (1.18–2.08) | 0.0021 | 1.58 (1.16–2.19) | 0.005 |
| Associated with lower odds of PH | |||||||
| 377.2305 | 4.840834 | Eicosanoid | 8(9)-EpETE | 0.52 (0.39–0.70) | 0.00002 | 0.70 (0.50–0.98) | 0.04 |
| 313.2388 | 3.447167 | Eicosanoid | 12,13-DiHOME | 0.61 (0.45–0.80) | 0.0007 | 0.85 (0.61–1.19) | 0.35 |
| 403.2295 | 1.406 | Fatty acyl-eicosanoid | 2,3-Dinor-TXB1 | 0.61 (0.45–0.82) | 0.0012 | 0.72 (0.51–1.01) | 0.06 |
| 293.2123 | 4.150167 | Eicosanoid | 13-HOTrE(γ) | 0.65 (0.50–0.84) | 0.0013 | 0.80 (0.59–1.08) | 0.14 |
m/z: mass-to-charge ratio; 11,12-DiHETrE: 11,12-dihydroxy-5Z,8Z,14Z-eicosatrienoic acid; 6β-PGI1: 6β-prostaglandin I1; 5-HETrE: 5-hydroxy-6E,8Z,11Z-eicosatrienoic acid; 11β-dhk-PGF2α: 11β-dihydro-15-keto-prostaglandin F2α; TXB: thromboxane; 12,13-EpOME: 12,13-epoxy-9Z-octadecenoic acid; 8(9)-EpETE: 8(9)-epoxy-5Z,11Z,14Z,17Z-eicosatetraenoic acid; 12,13-DiHOME: 12,13-dihydroxy-9Z-octadecenoic acid; 13-HOTrE: 13S-hydroxy-6Z,9Z,11E-octadecatrienoic acid. Eicosanoid metabolites displayed met the false discovery rate q<0.05 threshold in the primary model. The primary model adjusts for age, sex, plate number, statin use and aspirin use. The secondary model additionally adjusts for body mass index, diabetes, hypertension, present smoking, prevalent myocardial infarction, heart failure with preserved ejection fraction, obstructive sleep apnoea, COPD and interstitial lung disease. #: odds of PH rest status per 1sd higher eicosanoid metabolite concentration.
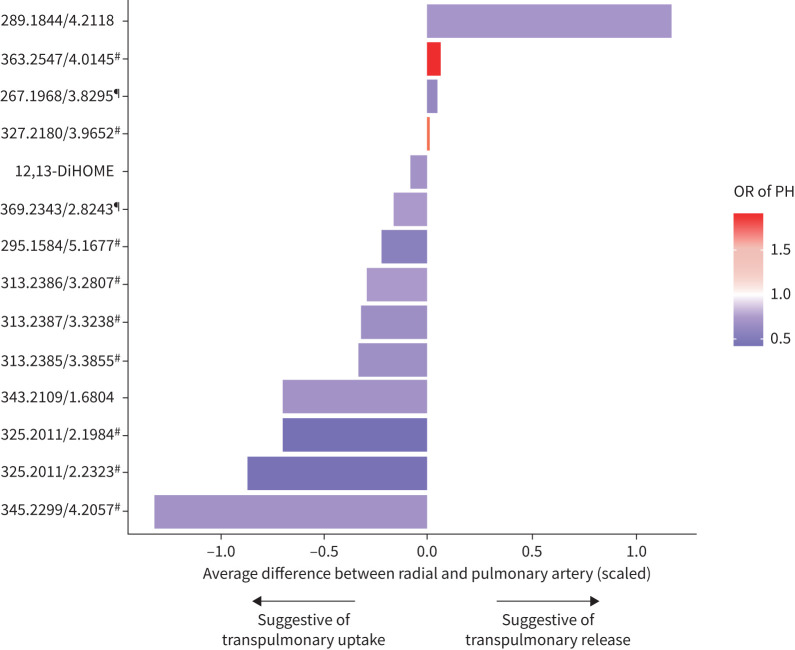
PH-associated eicosanoid metabolites display differential transpulmonary metabolite gradients
Of the 48 metabolites associated with rest PH, we found 14 eicosanoids or eicosanoid-related metabolites with differential transpulmonary metabolite gradients (table 3). When examining directionality, we found that most metabolites associated with a lower odds of PH had a negative gradient across pulmonary to radial artery sampling, suggesting transpulmonary uptake (12,13-DiHOME), whereas metabolites associated with higher odds of PH had positive gradients suggestive of transpulmonary release (figure 3). The greatest metabolite gradients were observed for a putative eicosanoid (mass-to-charge ratio (m/z) 345.2299/retention time 4.2057 min), which was associated with 40% lower odds of PH, with an average difference of 1.3sd between radial and pulmonary artery levels (supplementary table S4). Further, in exploratory analyses examining the association of transpulmonary metabolite gradients with PH, we found that four of the 48 PH-associated resting metabolites showed nominal associations (p≤0.01 for all) (supplementary table S5).
TABLE 3.
Pulmonary hypertension (PH) associated eicosanoids display transpulmonary (TP) metabolite gradients
| m/z | Retention time, min | PUFA | Class/putative ID | Association with rest PH | TP metabolite gradient | ||
| OR (95% CI) | p-value | Radial−PA | p-value | ||||
| Suggestive of TP metabolite release | |||||||
| 289.1844 | 4.211833 | Putative eicosanoid | 0.62 (0.46–0.82) | 0.0009 | 1.17 (1.07–1.27) | <0.00001 | |
| 363.2547 | 4.0145 | Putative eicosanoid | 1.92 (1.46–2.56) | 0.00001 | 0.06 (0.03–0.1) | 0.00033 | |
| 267.1968 | 3.8295 | Putative eicosanoid | 0.56 (0.42–0.73) | 0.0002 | 0.05 (0.02–0.09) | 0.00413 | |
| 327.218 | 3.965167 | Putative eicosanoid | 1.69 (1.29–2.25) | 0.0002 | 0.02 (0.01–0.03) | 0.00266 | |
| Suggestive of TP metabolite uptake | |||||||
| 313.2388 | 3.447167 | 12,13-DiHOME | 0.61 (0.45–0.80) | 0.0007 | −0.07 (−0.1– −0.05) | <0.00001 | |
| 369.2343 | 2.824333 | Unknown | 0.64 (0.47–0.84) | 0.0018 | −0.16 (−0.25– −0.08) | 0.002665 | |
| 295.1584 | 5.167666 | Putative eicosanoid | 0.52 (0.35–0.76) | 0.0012 | −0.22 (−0.27– −0.17) | <0.00001 | |
| 313.2386 | 3.280667 | LA | Putative eicosanoid | 0.64 (0.47–0.85) | 0.0023 | −0.29 (−0.34– −0.23) | <0.00001 |
| 313.2387 | 3.323833 | LA | Putative eicosanoid | 0.59 (0.44–0.78) | 0.0004 | −0.32 (−0.36– −0.28) | <0.00001 |
| 313.2385 | 3.3855 | LA | Putative eicosanoid | 0.59 (0.43–0.79) | 0.0006 | −0.32 (−0.37– −0.28) | <0.00001 |
| 325.2011 | 2.198417 | Putative eicosanoid | 0.44 (0.30–0.61) | 0.00001 | −0.69 (−0.80– −0.59) | <0.00001 | |
| 343.2109 | 1.680417 | AA | Putative eicosanoid | 0.60 (0.44–0.80) | 0.0009 | −0.69 (−0.79– −0.59) | <0.00001 |
| 325.2011 | 2.232333 | Putative eicosanoid | 0.45 (0.33–0.60) | <0.00001 | −0.86 (−0.97– −0.74) | <0.00001 | |
| 345.2299 | 4.205667 | Putative eicosanoid | 0.60 (0.45–0.79) | 0.0004 | −1.30 (−1.39– −1.22) | <0.00001 | |
m/z: mass-to-charge ratio; PUFA: polyunsaturated fatty acid; LA: linoleic acid; AA: arachidonic acid; PA: pulmonary artery; 12,13-DiHOME: 12,13-dihydroxy-9Z-octadecenoic acid. Delta radial–PA represents the average intra-individual difference in standardised metabolites between radial and PA sites. Metabolites are natural-log transformed and standardised to mean of 0 and a standard deviation of 1 from both sites, and then delta was estimated using the paired t-test. Eicosanoid metabolites displayed met the false discovery rate q<0.05 threshold in both the minimally adjusted model for rest PH and radial–PA gradient analysis. Primary model adjusts for age, sex, plate number, statin use and aspirin use.
FIGURE 3.
Pulmonary hypertension (PH)-associated eicosanoids and eicosanoid-related metabolites demonstrate significant transpulmonary metabolite gradients via multisite sampling. Difference between radial and pulmonary artery metabolite levels (standardised) is displayed on the x-axis. A value of 0 indicates no difference between radial and pulmonary artery metabolite levels, with positive values suggestive of transpulmonary release and negative values suggestive of transpulmonary uptake. Red colouring indicates higher metabolite association with higher odds of PH and blue colouring indicates higher metabolite association with lower odds of PH. Metabolites annotated by mass-to-charge (m/z) ratio/retention time (min) unless identity is known (table 2). #: putative eicosanoid metabolite; ¶: putative eicosanoid-related metabolite.
PH-associated eicosanoid metabolites and their associations with pulmonary vascular measures
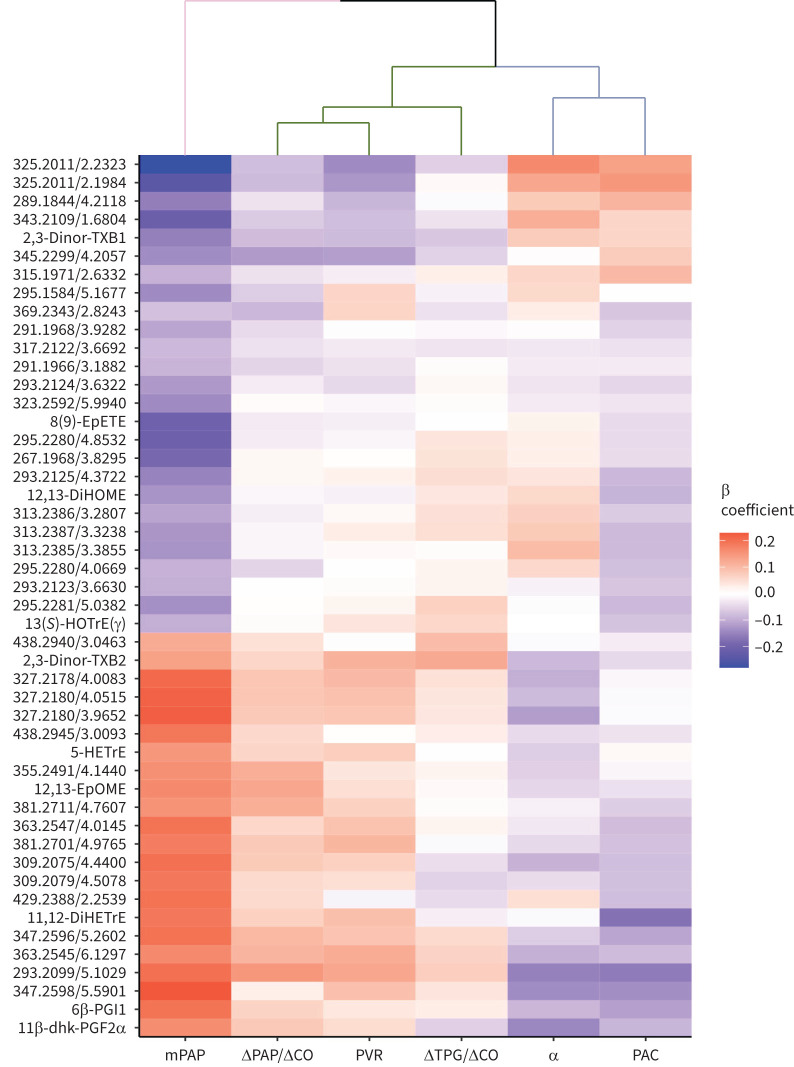
We next examined the association of the 48 PH-related eicosanoids identified in primary analyses with measures of pulmonary vascular function, including mean PAP, ΔPAP/ΔCO slope, rest PVR, ΔTPG/ΔCO slope, pulmonary vascular distensibility (α) and PAC (figure 4). In individual metabolite analyses, we found overlap among the 48 rest PH eicosanoids with pulmonary vascular traits, including seven (15%) that were associated with ΔPAP/ΔCO slope, 11 (23%) with PVR, one (2%) with ΔTPG/ΔCO slope, eight (17%) with α and 11 (23%) with PAC (p<0.05 for all) (supplementary table S6). Using hierarchical clustering we observed that pulmonary vascular traits clustered broadly into two groups, including ΔPAP/ΔCO slope, PVR and ΔTPG/ΔCO slope on one hand, and α and PAC on the other, indicating more closely shared underlying metabolite profile architecture among these groups.
FIGURE 4.
Heatmap of associations of the 48 pulmonary hypertension (PH)-related eicosanoid and eicosanoid-related metabolites with pulmonary vascular function. Colour coding represents the standardised β coefficient in the primary model (xsd change in exercise trait per 1sd change in eicosanoid metabolite). Primary analyses are adjusted for age, sex, plate number, statin use and aspirin use. Clustering is based on pulmonary vascular traits (columns). Colour scale indicates positive associations in red and negative associations in blue. Metabolites annotated by mass-to-charge (m/z) ratio/retention time (min) unless identity is known (table 2). mPAP: mean pulmonary arterial pressure; CO: cardiac output; PVR: pulmonary vascular resistance; TPG: transpulmonary gradient; α: pulmonary vascular distensibility; PAC: pulmonary artery compliance.
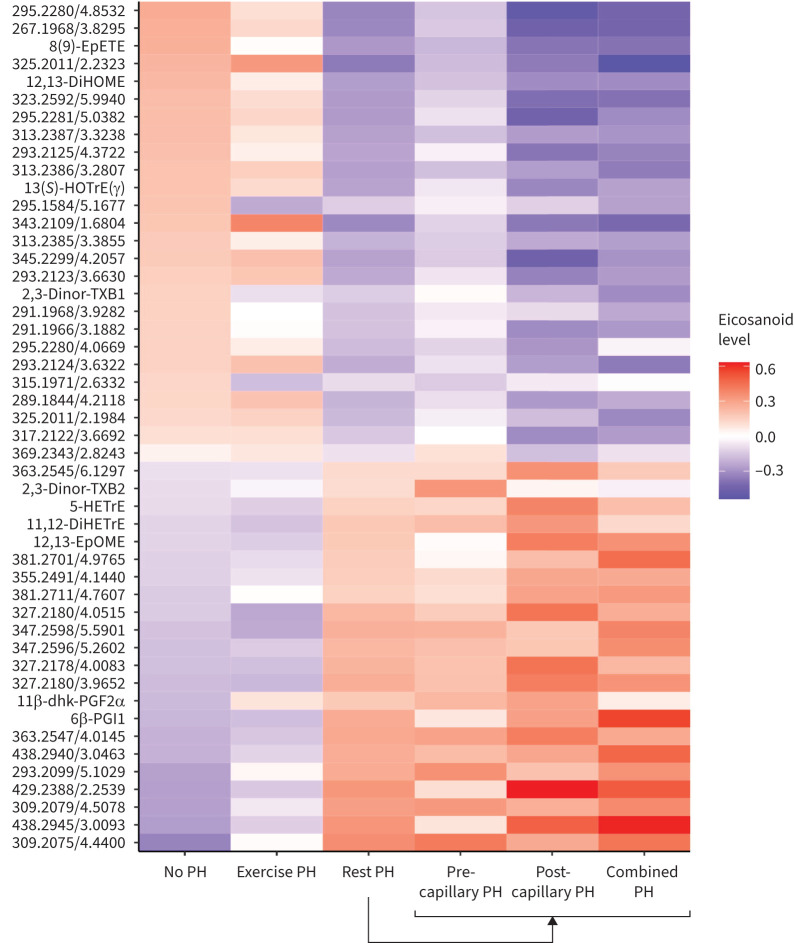
Eicosanoid profiles across PH subtypes
We next focused on PH-associated eicosanoid metabolites and further assessed differences in metabolite concentrations across PH subtypes, including exercise PH, pre-capillary PH, post-capillary PH, and combined pre- and post-capillary PH (figure 5; clinical characteristics in supplementary table S7). In general, eicosanoid profiles among individuals with exercise PH resembled those of individuals with no PH. There were six eicosanoids that differentiated exercise PH from no PH (p<0.05 for all) (supplementary table S8), including the prostaglandin derivative 11β-dhk-PGF2α, which was higher in exercise PH compared with no PH (p=0.018). When comparing individuals with exercise PH versus rest PH, there were 34 eicosanoid or eicosanoid-related metabolites that were significantly different (p<0.05 for all). These included 13(S)-HOTrE(γ), 12,13-DiHOME and 8(9)-EpETE which were higher in exercise PH compared with rest PH (supplementary table S8).
FIGURE 5.
Eicosanoid profiles across pulmonary hypertension (PH) subtypes. Heatmap displays average concentration (expressed in standard deviation units) of PH-associated eicosanoids and eicosanoid-related metabolites among the following groups: 1) no PH, 2) exercise PH and 3) rest PH. The latter is further subdivided into 4) pre-capillary PH, 5) post-capillary PH, and 6) combined pre- and post-capillary PH. Metabolites annotated by mass-to-charge (m/z) ratio/retention time (min) unless identity is known (table 2).
We next examined haemodynamic subtypes of rest PH. A total of 66 participants met the criteria for pre-capillary PH, 50 for post-capillary and 52 for combined PH (figure 5), with 32 participants not falling within a distinct category. In general, there were minor differences across rest PH subtypes. When comparing pre-capillary PH versus post-capillary PH, there were four metabolites that were nominally different, including 12,13-EpOME and other novel eicosanoids (p<0.05 for all). Similarly, when comparing post-capillary PH with combined PH, there were seven metabolites that were different, including 11β-dhk-PGF2α and 12,13-EpOME and other novel eicosanoids (p<0.05 for all). Specifically, individuals with post-capillary PH and combined PH had higher levels of 12,13-EpOME compared with pre-capillary PH (p=0.01 and p=0.04, respectively) (supplementary table S8). There were no significant differences in eicosanoid profiles when comparing post-capillary and combined PH.
Discussion
We performed detailed molecular profiling of eicosanoids and eicosanoid-related metabolites to better understand the role of systemic inflammation in PH in a unique sample of individuals with comprehensive assessment of pulmonary haemodynamics and vascular function with and without exercise. Our findings are four-fold. First, we identified 48 distinct eicosanoid and eicosanoid-related metabolites that were associated with rest PH, including prostaglandins, linoleic acid derivatives and lipokines. Second, we demonstrated that nearly a third of PH-associated eicosanoids display significant transpulmonary metabolite gradients via multisite sampling, including the exercise lipokine 12,13-DiHOME, suggesting possible physiological relevance with respect to pulmonary uptake or release. Third, we demonstrated some overlap of rest PH-associated eicosanoids with specific pulmonary vascular traits, including PAC and pulmonary vascular distensibility, and exercise response, including ΔPAP/ΔCO slope. Finally, when examining PH subtypes, we showed that eicosanoid profiles of individuals with exercise PH more closely resemble those without PH, with a few distinct molecules, including 11β-dhk-PGF2α, that distinguish exercise PH. Taken together, our findings highlight the role of bioactive lipids in PH and suggest a potential physiological role of eicosanoid metabolites representing linoleic acid and arachidonic acid pathways in determining pulmonary vascular function across the spectrum of PH subtypes.
While systemic inflammation is thought to lead to PH through multiple pathways including endothelial activation and pulmonary vascular remodelling, the exact mechanisms remain unclear. Eicosanoids are bioactive lipids that govern the upstream initiation of pro- and anti-inflammatory activity and harbour vasoactive properties [20]. Prior studies have shown that specific eicosanoids including arachidonic acid derivatives such as prostaglandins, leukotrienes and hydroxyeicosanoids are linked to PAH [2, 21]. We now build upon these findings in two important aspects: 1) we leverage a novel directed and non-targeted LC-MS platform to comprehensively profile hundreds of eicosanoids and eicosanoid-related molecules, which was previously not possible; and 2) we studied a unique sample of individuals with and without PH across the spectrum of disease, with in-depth assessment of pulmonary vascular function.
Our study identified 48 eicosanoid and eicosanoid-related metabolites associated with rest PH. Specifically, we show that arachidonic acid derivatives 6β-PGI1, 11β-dhk-PGF2α, thromboxane TXB2 and 11,12-DiHETrE and linoleic acid derivative 12,13-EpOME were associated with higher odds of rest PH, while epoxide 8(9)-EpETE, lipokine 12,13-DiHOME and thromboxane TXB1 were associated with lower odds of rest PH. Prostaglandins and thromboxanes are known to be important contributors to PH: 11β-dhk-PGF2α has been associated with pulmonary vasoconstriction as well as increased airway resistance [22, 23]. Further, elevated levels of 11β-dhk-PGF2α have been associated with elevated PAP and it is thought to act on a nitric oxide synthase inhibitor as well as thromboxane receptors [22]. Lastly, human studies have shown greater urinary excretion of TXB2 metabolites in both primary and secondary PH, concomitant with lower excretion of PGF1α derivatives [24]. Importantly, prostacyclin (PGI) analogues are a mainstay of treatment for PAH, with pre-clinical evidence of benefit with thromboxane receptor antagonists [25, 26]. We now show specific prostaglandin and thromboxane metabolites are associated with PH in a heterogeneous sample of individuals, expanding potential clinical implications across a much broader sample of individuals.
In addition to prostaglandin derivatives, our findings substantiate the role of epoxides in PH. Specifically, we find that 12,13-EpOME, a cytochrome P450-derived linoleic acid metabolite, is associated with higher odds of PH [27]. While no prior studies have described a role in PH, higher levels of EpOMEs have been studied in animal models of cardiac ischaemia and reperfusion injury [27]. In this model, the addition of 12,13-EpOME was associated with decreased post-ischaemic functional recovery, including worse cardiac contractility and relaxation [27].
Importantly, 12,13-EpOME is a direct precursor to 12,13-DiHOME via soluble epoxide hydrolase, which in turn was associated with lower odds of PH in our study [28].
Recent studies have identified 12,13-DiHOME as an important exercise lipokine. In experimental studies, 12,13-DiHOME inhibits the neutrophil respiratory burst, leading to lower levels of reactive oxygen species and inflammation [29], and has been associated with vascular development and repair in animal models [28]. More recently, key metabolic functions have been identified: 12,13-DiHOME levels were positively associated with brown adipose tissue, and negatively correlated with BMI, insulin resistance, triglyceride and leptin levels [28]. When examined in an exercise model, 12,13-DiHOME was associated with greater cardiorespiratory fitness, and greater basal and maximal respiration and fatty acid uptake in skeletal muscle [28, 30]. We now demonstrate a potential role in PH, which is further substantiated by a significant transpulmonary metabolite gradient suggesting net uptake of 12,13-DiHOME across the pulmonary circulation.
Lastly, we show that eicosapentenoic acid (EPA) derivative 8(9)-EpETE, an epoxyeicosatrienoic acid (EET), is also associated with lower risk of PH. Prior studies have shown 8(9)-EpETE to prevent endothelial cell apoptosis in both cardiac and pulmonary cells [31]. In addition, EETs are associated with coronary vasodilation and in animal models associated with decreased PVR and pulmonary artery dilation [32, 33]. While the role of 8(9)-EpETE within the pulmonary vasculature is not fully understood, other related ω-3 fatty acid EPA derivatives are released by mast cells and negatively regulate PH in animal models, with suppression of lung fibroblast activation with treatment with ω-3 epoxides, highlighting the therapeutic potential of the EPA pathway [34].
To refine our understanding of PH-associated eicosanoids, we examined associations with pulmonary vascular traits, including mean PAP, ΔPAP/ΔCO slope, PVR, ΔTPG/ΔCO slope, pulmonary vascular distensibility (α) and PAC. In general, metabolite profiles of pulmonary vascular distensibility, including α and PAC, clustered together while those associated with exercise, including ΔPAP/ΔCO slope, PVR and ΔTPG/ΔCO slope, clustered together. Further, we examined eicosanoid profiles across PH subtypes to compare individuals with no PH, exercise PH and rest PH. We found that in general, eicosanoid profiles of exercise PH more closely resembled individuals without PH, with few specific metabolites that distinguished exercise PH versus no PH, including 11β-dhk-PGF2α which was higher in exercise as well as rest PH. When exercise PH was compared with rest PH, eicosanoids that were lower in exercise PH included 12,12-DiHETrE and 5(S)-HETrE and eicosanoids that were higher in exercise PH included 8(9)-EpETE and 12,13-DiHOME. In addition, α-linolenic acid metabolite 13(S)-HOTrE(γ) was higher in exercise PH versus rest PH. 13(S)-HOTrE(γ) has been associated with anti-inflammatory properties by reducing the activity of lipopolysaccharide-induced inflammation, leading to lower reactive oxygen species, as well as inactivating the NLRP3 inflammasome complex [35]. Further, when subtypes of PH were compared, post-capillary PH and combined pre- and post-capillary PH were found to have elevated levels of 12,13-EpOME compared with pre-capillary PH.
Our study has several limitations worth noting. First, this was a cross-sectional and observational study, and causal inferences around specific eicosanoid metabolites and their transpulmonary gradients with respect to PH cannot be drawn; future studies are needed to expand current mechanistic understanding. Second, we studied individuals who underwent clinically indicated CPET with invasive haemodynamic monitoring and generalisability to other samples may be limited due to potential referral bias. The sample included individuals in whom the primary reason for CPET was dyspnoea. Therefore, individuals with clear diagnoses and/or known aetiology of PH including World Health Organization classification may have been less likely to be included in the study, and our study participants likely had less severe disease. We also acknowledge that rest PH is heterogeneous in our sample and that we had limited power to detect associations with specific PH subtypes including PAH. However, one of the strengths was the broad inclusion of individuals across the spectrum of cardiopulmonary disease and ability to examine pulmonary vascular traits that were carefully assessed with invasive haemodynamic measures. Lastly, in addition to known metabolites, we identified a number of novel eicosanoids of interest for which the exact molecular structure is not known and further studies will need to be performed to determine their exact identity.
In sum, we identified specific linoleic acid, prostaglandin and other arachidonic acid derivatives associated with higher odds of PH, while lipokine and epoxide derivatives were associated with lower odds of PH. Further, many PH-associated eicosanoids displayed gradients suggestive of uptake or release across the pulmonary circulation. Lastly, we provided further granularity of PH-associated eicosanoids with specific pulmonary vascular traits, including PAC and pulmonary vascular distensibility, as well as exercise response. Our findings suggest that bioactive lipids are potentially important markers and potential contributors in the development of PH across the disease spectrum. Further studies are needed to better understand specific mechanisms by which eicosanoid pathways exert pulmonary vascular effects and ensuing potential future therapeutic implications.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-00561-2023.Supplement (157.6KB, xlsx)
Shareable PDF
Footnotes
This article has an editorial commentary: https://doi.org/10.1183/13993003.01547-2023
Conflict of interest: E.S. Lau has previously received modest honoraria from Roche Diagnostics. J.E. Ho has received past research grant support from Bayer AG. M. Jain currently holds equity and a leadership position at Sapient Bioanalytics, LLC, and is engaged in research related to the current study. All other authors have nothing to disclose.
Support statement: E.S. Lau is supported by National Institutes of Health (NIH) grant K23-HL159243 and American Heart Association (AHA) grant 853922. G.D. Lewis is supported by NIH grants 1R01-HL131029 and R01-HL151841, and AHA grant 15GPSGC24800006. J.E. Ho is supported by NIH grants R01-HL134893, R01-HL140224, R01-HL160003 and K24-HL153669. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Hoeper MM, Humbert M, Souza R, et al. A global view of pulmonary hypertension. Lancet Respir Med 2016; 4: 306–322. doi: 10.1016/S2213-2600(15)00543-3 [DOI] [PubMed] [Google Scholar]
- 2.Al-Naamani N, Sagliani KD, Dolnikowski GG, et al. Plasma 12- and 15-hydroxyeicosanoids are predictors of survival in pulmonary arterial hypertension. Pulm Circ 2016; 6: 224–233. doi: 10.1086/686311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alotaibi M, Fernandes T, Tang AB, et al. Sex-related differences in eicosanoid levels in chronic thromboembolic pulmonary hypertension. Am J Respir Cell Mol Biol 2023; 68: 228–231. doi: 10.1165/rcmb.2022-0272LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lang IM, Gaine SP. Recent advances in targeting the prostacyclin pathway in pulmonary arterial hypertension. Eur Respir Rev 2015; 24: 630–641. doi: 10.1183/16000617.0067-2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Safdar Z. Treatment of pulmonary arterial hypertension: the role of prostacyclin and prostaglandin analogs. Respir Med 2011; 105: 818–827. doi: 10.1016/j.rmed.2010.12.018 [DOI] [PubMed] [Google Scholar]
- 6.Palmu J, Watrous JD, Mercader K, et al. Eicosanoid inflammatory mediators are robustly associated with blood pressure in the general population. J Am Heart Assoc 2020; 9: e017598. doi: 10.1161/JAHA.120.017598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eisman AS, Shah RV, Dhakal BP, et al. Pulmonary capillary wedge pressure patterns during exercise predict exercise capacity and incident heart failure. Circ Heart Fail 2018; 11: e004750. doi: 10.1161/CIRCHEARTFAILURE.117.004750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Humbert M, Kovacs G, Hoeper MM, et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J 2022; 43: 3618–3731. doi: 10.1093/eurheartj/ehac237 [DOI] [PubMed] [Google Scholar]
- 9.Humbert M, Kovacs G, Hoeper MM, et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J 2023; 61: 2200879. doi: 10.1183/13993003.00879-2022 [DOI] [PubMed] [Google Scholar]
- 10.Lewis GD, Murphy RM, Shah RV, et al. Pulmonary vascular response patterns during exercise in left ventricular systolic dysfunction predict exercise capacity and outcomes. Circ Heart Fail 2011; 4: 276–285. doi: 10.1161/CIRCHEARTFAILURE.110.959437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lewis GD, Bossone E, Naeije R, et al. Pulmonary vascular hemodynamic response to exercise in cardiopulmonary diseases. Circulation 2013; 128: 1470–1479. doi: 10.1161/CIRCULATIONAHA.112.000667 [DOI] [PubMed] [Google Scholar]
- 12.Ghio S, Schirinzi S, Pica S. Pulmonary arterial compliance: how and why should we measure it? Glob Cardiol Sci Pract 2015; 2015: 58. doi: 10.5339/gcsp.2015.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Linehan JH, Haworth ST, Nelin LD, et al. A simple distensible vessel model for interpreting pulmonary vascular pressure-flow curves. J Appl Physiol 1992; 73: 987–994. doi: 10.1152/jappl.1992.73.3.987 [DOI] [PubMed] [Google Scholar]
- 14.Lagerborg KA, Watrous JD, Cheng S, et al. High-throughput measure of bioactive lipids using non-targeted mass spectrometry. Methods Mol Biol 2019; 1862: 17–35. doi: 10.1007/978-1-4939-8769-6_2 [DOI] [PubMed] [Google Scholar]
- 15.Watrous JD, Niiranen TJ, Lagerborg KA, et al. Directed non-targeted mass spectrometry and chemical networking for discovery of eicosanoids and related oxylipins. Cell Chem Biol 2019; 26: 433–442. doi: 10.1016/j.chembiol.2018.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fahy E, Subramaniam S, Murphy RC, et al. Update of the LIPID MAPS comprehensive classification system for lipids. J Lipid Res 2009; 50: Suppl., S9–S14. doi: 10.1194/jlr.R800095-JLR200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crescente M, Menke L, Chan MV, et al. Eicosanoids in platelets and the effect of their modulation by aspirin in the cardiovascular system (and beyond). Br J Pharmacol 2019; 176: 988–999. doi: 10.1111/bph.14196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gottschall H, Schmocker C, Hartmann D, et al. Aspirin alone and combined with a statin suppresses eicosanoid formation in human colon tissue. J Lipid Res 2018; 59: 864–871. doi: 10.1194/jlr.M078725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilkens H, Bauer M, Forestier N, et al. Influence of inhaled iloprost on transpulmonary gradient of big endothelin in patients with pulmonary hypertension. Circulation 2003; 107: 1509–1513. doi: 10.1161/01.cir.0000056104.49686.4b [DOI] [PubMed] [Google Scholar]
- 20.Lone AM, Tasken K. Proinflammatory and immunoregulatory roles of eicosanoids in T cells. Front Immunol 2013; 4: 130. doi: 10.3389/fimmu.2013.00130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wright L, Tuder RM, Wang J, et al. 5-Lipoxygenase and 5-lipoxygenase activating protein (FLAP) immunoreactivity in lungs from patients with primary pulmonary hypertension. Am J Respir Crit Care Med 1998; 157: 219–229. doi: 10.1164/ajrccm.157.1.9704003 [DOI] [PubMed] [Google Scholar]
- 22.Kang KH, Shim JJ, Banerjee M, et al. PGF2 alpha causes bronchoconstriction and pulmonary vasoconstriction via thromboxane receptors in rat lung. Korean J Intern Med 1996; 11: 74–81. doi: 10.3904/kjim.1996.11.1.74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hussain A, Bennett R, Haqzad Y, et al. The differential effects of systemic vasoconstrictors on human pulmonary artery tension. Eur J Cardiothorac Surg 2017; 51: 880–886. doi: 10.1093/ejcts/ezw410 [DOI] [PubMed] [Google Scholar]
- 24.Christman BW, McPherson CD, Newman JH, et al. An imbalance between the excretion of thromboxane and prostacyclin metabolites in pulmonary hypertension. N Engl J Med 1992; 327: 70–75. doi: 10.1056/NEJM199207093270202 [DOI] [PubMed] [Google Scholar]
- 25.Ruan CH, Dixon RA, Willerson JT, et al. Prostacyclin therapy for pulmonary arterial hypertension. Tex Heart Inst J 2010; 37: 391–399. [PMC free article] [PubMed] [Google Scholar]
- 26.Mulvaney EP, Renzo F, Adao R, et al. The thromboxane receptor antagonist NTP42 promotes beneficial adaptation and preserves cardiac function in experimental models of right heart overload. Front Cardiovasc Med 2022; 9: 1063967. doi: 10.3389/fcvm.2022.1063967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bannehr M, Lohr L, Gelep J, et al. Linoleic acid metabolite DiHOME decreases post-ischemic cardiac recovery in murine hearts. Cardiovasc Toxicol 2019; 19: 365–371. doi: 10.1007/s12012-019-09508-x [DOI] [PubMed] [Google Scholar]
- 28.Hildreth K, Kodani SD, Hammock BD, et al. Cytochrome P450-derived linoleic acid metabolites EpOMEs and DiHOMEs: a review of recent studies. J Nutr Biochem 2020; 86: 108484. doi: 10.1016/j.jnutbio.2020.108484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thompson DA, Hammock BD. Dihydroxyoctadecamonoenoate esters inhibit the neutrophil respiratory burst. J Biosci 2007; 32: 279–291. doi: 10.1007/s12038-007-0028-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stanford KI, Lynes MD, Takahashi H, et al. 12,13-diHOME: an exercise-induced lipokine that increases skeletal muscle fatty acid uptake. Cell Metab 2018; 27: 1357. doi: 10.1016/j.cmet.2018.04.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dhanasekaran A, Al-Saghir R, Lopez B, et al. Protective effects of epoxyeicosatrienoic acids on human endothelial cells from the pulmonary and coronary vasculature. Am J Physiol Heart Circ Physiol 2006; 291: H517–H531. doi: 10.1152/ajpheart.00953.2005 [DOI] [PubMed] [Google Scholar]
- 32.Stephenson AH, Sprague RS, Lonigro AJ. 5,6-Epoxyeicosatrienoic acid reduces increases in pulmonary vascular resistance in the dog. Am J Physiol 1998; 275: H100–H109. doi: 10.1152/ajpheart.1998.275.1.H100 [DOI] [PubMed] [Google Scholar]
- 33.Jacobs ER, Zeldin DC. The lung HETEs (and EETs) up. Am J Physiol Heart Circ Physiol 2001; 280: H1–H10. doi: 10.1152/ajpheart.2001.280.1.H1 [DOI] [PubMed] [Google Scholar]
- 34.Moriyama H, Endo J, Kataoka M, et al. Omega-3 fatty acid epoxides produced by PAF-AH2 in mast cells regulate pulmonary vascular remodeling. Nat Commun 2022; 13: 3013. doi: 10.1038/s41467-022-30621-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumar N, Gupta G, Anilkumar K, et al. 15-Lipoxygenase metabolites of alpha-linolenic acid, [13-(S)-HPOTrE and 13-(S)-HOTrE], mediate anti-inflammatory effects by inactivating NLRP3 inflammasome. Sci Rep 2016; 6: 31649. doi: 10.1038/srep31649 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-00561-2023.Supplement (157.6KB, xlsx)
This one-page PDF can be shared freely online.
Shareable PDF ERJ-00561-2023.Shareable (335.8KB, pdf)