Abstract
The current study, which lasted 45 days, was designed to find a more effective way to use the vast resources of salt-affected land and ground saline water for aquaculture. Biochar made from agrowaste was used as a sediment amendment. The 100 g of biochar was applied to 25 kg of sediment (i.e., 9.0 ton ha–1) in 300L capacity fiber reinforced plastic, and Penaeus vannamei (P. vannamei) (2.74 ± 0.03 g) was stocked at 90 juveniles m–2 in inland ground saline water of salinity 10 ppt fortified with potassium levels that are 50% equivalent to those of seawater. Among different treatments, T1 indicates paddy straw biochar (PSB) application in sediment; T2 indicates sediment amended with KOH-activated PSB; T3 indicates sugar cane bagasse biochar (SBB) application in sediment; and T4 indicates sediment amended with KOH-activated SBB. Compared to the control the potassium (K+), alkalinity, total hardness, calcium/magnesium ratio, and pH of the water increased significantly (P ≤ 0.05) in treatments where biochar was used as an amendment in sediment. The T3 treatment had the best Ca/Mg ratio (1.00:3.12). In water, the magnitude of increase in K+ concentration from high to low followed the order: T2 > T4 > T1 > T3 > control. The concentration of NH4+–N in water was found to be increasing in control, whereas in the rest of the treatments, it decreased significantly from day 1, until the end of the experiment. Compared to control, the bulk density was decreased, and sediment cation exchange capacity and water holding capacity were increased significantly in treatments where biochar was used as an amendment. The soil microbial parameter measured in terms of soil enzyme dehydrogenase was significantly different among treatments at the end of the experiment. Weight gain (%), specific growth rate (SGR), survival (%), and feed conversion ratio of P. vannamei varied significantly in T1, T2, T3, and T4 compared to the control. The SGR (2.38b ± 0.05% day–1) and weight gain (%) in T2, and survival (96.1b ± 2.0%) in T3 treatment were found to be the highest at the end of the experiment. When biochar was mixed with sediment in the inland saline system, an improvement was seen in sediment quality, water quality, and growth characteristics of P. vannamei.
Introduction
The race for boosting aquaculture production has created huge pressure on marine coastal locations and potable water resources. Therefore, alternative strategies that can increase the productivity with minimum harm to the environment using a circular economy approach are the need of the hour. The practice of aquaculture in the inland saline environment, which is deemed to be a degraded system for agricultural purposes, is thought to be a solution to this, keeping in line with the national priority of developing acceptable technology for the use of inland saline groundwater.
Out of the 1000 million ha of salt-affected soil that exists globally, India has nearly 8.62 million ha of salt-affected soils and 1.93 million km2 of ground saline water.1 Salinization of groundwater and land is a major problem in the semiarid agro-climatic zone of India that stretches through the states of Haryana, Rajasthan, Punjab, and Uttar Pradesh. According to several experts, the nonuse of ground saline water has caused water tables to rise, which is responsible for secondary salinization and waterlogging situations in these places. Pumping inland saline groundwater in a landlocked location for aquaculture operations is a sustainable approach since the water might be evapo-transpirated from aquaculture ponds in the long term. The land-based aquaculture exploiting saline groundwater also known as 'inland saline aquaculture' is practiced in many countries including Israel, Australia, the United States of America, and India.
The whiteleg shrimp (Penaeus vannamei) (P. vannamei) is one of the species that is best suited for inland saline farming. P. vannamei has a quicker development rate, a reduced dietary protein demand, tolerance to high stocking density, and tolerance to a broad range of salinities and temperatures.2,3 Being a major candidate species in inland saline aquaculture and considering its export potential, the culture of P. vannamei in inland saline water not only generates income for farmers at a low operating cost but also helps in the utilization of otherwise detrimental environment. However, the inland saline aquaculture ponds are experiencing seepage issues and nutritional deficiencies due to their low potassium, high calcium, and variable magnesium contents when compared to the natural seawater. For the successful inland saline P. vannamei culture, potassium is regarded as the most important limiting element among these minerals. Water modification tactics are thus required for P. vannamei’s survival and development. The ability of shrimp reared in low salinity conditions to grow, live, and osmoregulate appears to be enhanced by modifying the rearing medium with potassium and magnesium fertilizers and nutritional modification methods.4
The gross crop residue potential generated in India is 696.38 million tonnes year–1.5 The cereal crops (rice, maize, wheat, and millet) generate 70% of agricultural waste, with rice alone accounting for 34%. A tonne of rice straw burned is projected to lose 5.5 kg of nitrogen, 2.3 kg of phosphorus, 1.2 kg of sulfur, and 25 kg of potassium in addition to organic carbon. Crop leftovers from various crops typically comprise 80% nitrogen (N), 50% sulfur (S), 25% phosphorus (P), and 20% potassium (K).6 The environmental and socioeconomic impacts caused by the burning of biomass residues (stubble) in open agricultural fields after the harvest of crops are a serious concern. Converting agricultural wastes into biochar is a sustainable technique to mitigate the effects of stubble burning in open agricultural fields as well as exploring biochar’s potential applications, such as a soil supplement.
“Biochar is porous carbonaceous residues produced by the pyrolysis of biomass in an oxygen-limited atmosphere”.7 Biochar is dark and lightweight and contains more carbon. Biochar has the scope to be used for different purposes. The use of biochar in agricultural systems can increase natural carbon sequestration rates in the soil, making it one of the feasible choices for decreasing greenhouse gas emissions; it also decreases farm waste and improves soil quality. Because of this nature of biochar, agricultural experts are interested in producing biochar from bio residues and using it as a soil supplement. Biochar can be made from different raw materials by using a variety of techniques, including pyrolysis, gasification, flash carbonization, and hydrothermal carbonization. The beneficial properties of biochar are determined by the nature of the raw material used to make the biochar. Biochar engineering allows for achieving biochar properties that are optimized for specific applications. Biochar’s large surface area and porosity are advantageous for soil application, particularly for improving soil-water holding capacity (WHC), nutrient retention, housing microorganisms, boosting fertilizer usage efficiency, and so on.8
Inland saline soil may be recovered using biochar, a substance that is climate resilient and is well-accepted as enhancing soil fertility. Recent studies suggest that adding biochar to salt-affected agricultural soils improves their physical, chemical, and biological qualities. The addition of biochar to salt-affected soils enhanced the quantity of nutrients available, especially calcium, magnesium, potassium, nitrogen, and phosphorus.9,10 In this situation, some people think that biochar might offer an advantageous situation for the inland saline soil.11 At the same time, studies on the use of biochar and its beneficial effects in aquaculture and related fields are nonetheless rare.
In this regard, the current study was planned to achieve the following objectives:
Biochar production and characterization.
To investigate the impact of biochar on soil and water quality in an inland saline shrimp farming system.
To evaluate the influence of biochar on the growth parameters of P. vannamei.
Materials and Methods
Production of Biochar and Activated Biochar
The raw biomass of sugar cane bagasse was collected locally from a sugar cane processing plant near the ICAR-CIFE Rohtak center and was air-dried until the moisture content reached 12–15% or below. The paddy straw biochar (PSB) was collected from cattle farmers, and its moisture content was brought down to 12–15% by air drying. Biochar was prepared in an electrical heating kiln of internal biomass holding capacity of 2.0 kg. The dried biomass was tightly packed in the kiln’s biomass holding chamber, the kiln’s mouth was closed with a steel lid, and the gas valve was left open to let volatile gases out. While preparing the sugar cane bagasse biochar (SBB), the kiln was set to 400 °C for 1 h and 30 min, and for the PSB, it was 400 °C for 45 min. After the complete release of volatile gases and the cooling of the kiln to room temperature, the cover lid was opened.
Potassium-activated biochar was produced by mixing biochar with powdered potassium hydroxide (KOH). First, the biochar conversion efficiency of each dried paddy straw and bagasse was calculated. It was 33% for sugar cane bagasse and 13% for paddy straw. The yield of PSB was only 130 g when 1000 g of dried paddy straw residue was used. The potassium hydroxide was mixed with biochar at the rate of 2.0% of the corresponding weight of dried biomass residue required to produce that much amount of biochar. If 20 g of KOH was mixed with 1000 g of dried paddy straw, it would give 130 g of KOH-activated PSB, followed by pyrolysis and activation. The activation was done by heating this mixture at 100 °C inside the kiln for 15–30 min.
Characterization of Biochar
The pH and electrical conductivity (EC) of biochar were tested in triplicate by adding distilled water with biochar samples (1.0 g biochar: 20 mL deionized water) and stirring at low speed with a reciprocating shaker for 1.5 h. The pH of the suspensions was measured using a pH electrode, and the filtrate was used to measure the electrical conductivity using an EC meter after being filtered using filter paper (Whatman No. 42 ).12,13
In a conical flask, 0.5 g of biochar was taken, 10 mL of nitric acid and 2.0 mL of sulfuric acid were added, and the flask was digested at 150 °C until it turned white; this was utilized for total element nutrient analysis. The leftover digested material was diluted with distilled water and filtered by using filter paper. The volumetric flask was filled with 100 mL of filtrate.14 A total of 10 mL of sample was obtained from the content for total phosphorus reading in a spectrophotometer and the remainder for potassium measurement in a flame photometer and calcium estimation by EDTA titrimetry, respectively.
The modified Song and Guo15 technique was used to calculate cation exchange capacity (CEC). Using the gravimetric technique, the biochar’s moisture and ash levels were determined.16 To calculate the ash content, 1.0 g of biochar was dried in a hot air oven for 24 h at 105 °C before being transferred to a muffle furnace and burnt for 6.0 h at 760 °C. electrical conductivity (EC) values were used to calculate total dissolved solids using the equation TDS = EC (dS m–1) × 640.
Functional groups in the biochar were found using Fourier-transform infrared (FTIR) spectroscopy following spectrophotometer investigations in the infrared region (SHIMADZU, FTIR 4100).
Experimental Setup
The trial lasted 45 days (from April 17 to June 2) at the ICAR-CIFE Rohtak center. A completely randomized design was adopted for the experiment, with 15 circular fiber-reinforced plastic tanks of uniform size (300L capacity, 65 cm height, 90 cm diameter). All of the tanks were disinfected with potassium permanganate, cleaned with water, and air-dried. The tanks were labeled to determine the level of water and the sort of treatment used. To achieve a nearly 10 cm deep soil bed in each tank, 25 kg of dry sediment taken from the inland saline pond was put into each tank. After properly mixing the biochar into the sediment, the soil-biochar slurry was created. Biochar (100 g) was used in each tank at an application rate of 9.0 ton ha–1. In the control, no such amendment was done. In treatments T1 and T2, the sediment bed was amended with PSB and activated PSB, respectively. Treatments T3 and T4 consisted of tanks with sediment that were amended using SBB and activated bagasse biochar. After 2 days, all the tanks were filled with inland ground saline water (IGSW) of salinity 10 ppt, fortified with potassium to a level of 50% of the requirement using muriate of potash (MOP) having 50% K. Figure 1 depicts the experimental procedure’s schematic design.
Figure 1.
Schematic diagram of experimental procedure. SBB: sugar cane bagasse biochar; PSB: paddy straw biochar; IGSW: inland ground saline water.
Preparation of 50% Potassium Fortified IGSW
First, the potassium content in MOP was determined and found to be 50%. Raw IGSW was pumped into a few 1000L tanks. The salinity was brought down to 10 from 15 ppt by adding freshwater. The equation relying on Davis et al.17 can be used to modify R-IGSW with MOP (KCl), creating fortified inland ground saline water (F-IGSW), which has potassium levels that are 50% equivalent to those of seawater:
Seed Procurement, Conditioning, and Stocking
The P. vannamei PL10-specific pathogen-free larvae were carried from Sree Sai hatcheries in Yarrayapeta, Andhra Pradesh, India to the experimental location in Rohtak, Haryana, India. The seeds were brought in oxygenated polyethylene bags filled with filtered seawater. To get juveniles (2.74 ± 0.03 g), postlarvae (PL10) were maintained for 45 days in inland saline ponds enriched with potash at potassium levels 100% comparable to seawater at a salinity of 15 ppt. According to the feeding chart, commercial starting feed crumbles from Growel Feeds Pvt Limited in Andhra Pradesh, India were fed to the PLs. The experimental animals were released to each tank at a stocking density of 90 shrimp per square meter. To keep the shrimp from jumping out of the tanks, the tops of the tanks were covered with a thin mesh net. During the midway point of the experiment, water was topped off in each tank with (F-IGSW) with K+ levels 50% equivalent to those of seawater.
Water Quality Parameters
At intervals of 9 days, samples for water quality parameters were taken. The APHA18 standard procedures were used to calculate the pH, salinity, total alkalinity, ammonium-N, potassium, total hardness, calcium, magnesium, dissolved oxygen, and TSS (total suspended solids).
Physicochemical Parameters of Soil
Samples for soil quality parameters were collected at a 9-day interval. Available potassium by Estefan19 CEC by Devis and Freities,20 WHC by ASTM,21 and bulk density by Kadam et al.22
Soil Microbial Activity
Samples for the analysis of soil enzyme dehydrogenase were collected at the end of the experiment. Dehydrogenase activity (DHA) was measured following the method of Casida et al.23
Growth parameters of P. vannamei
The shrimps were weighed, and growth performance was estimated using the formulas provided by Omitoyi24:
Statistical Analysis
Duncan’s multiple range test was performed to statistically analyze the data acquired using the statistical tool SPSS version 26.0. To establish the statistically significant difference between the mean values of different treatments, a 5% threshold of probability (P < 0.05) was used. The data are presented as mean ± S.E. (standard error).
Results
Characterization of Biochar
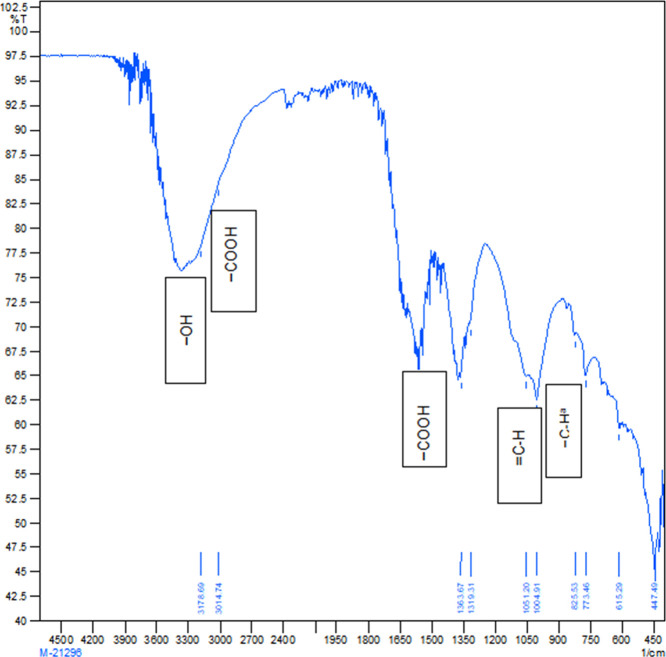
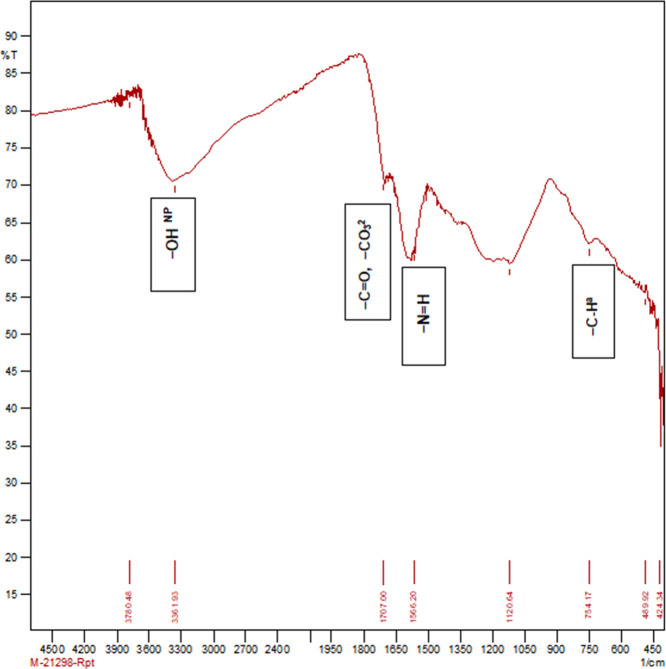
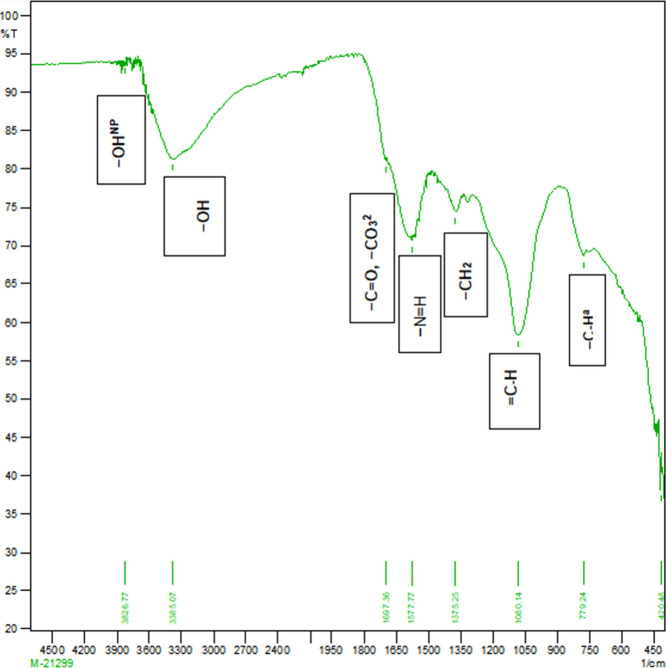
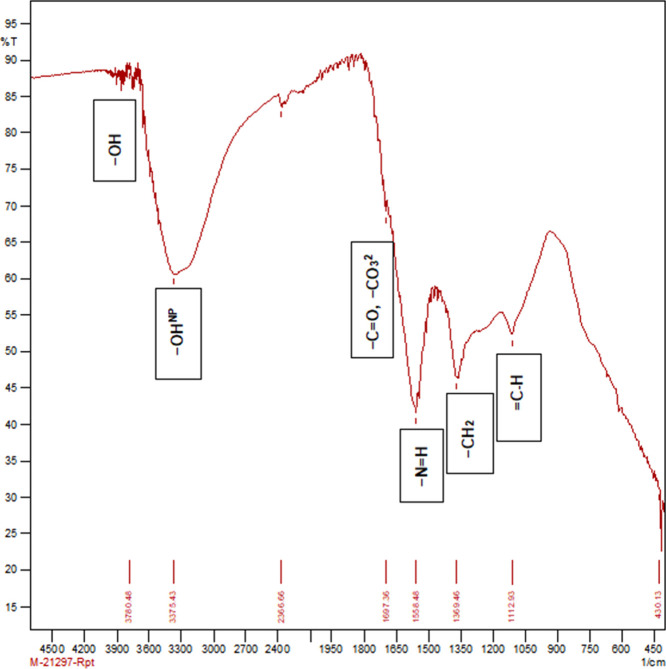
The physicochemical properties and functional groups of PSB and SBB were determined. Table 1 depicts the physical and chemical properties of biochar. Table 2 and Figures 2–6 describe the surface functional groups of biochars. There is a presence of nonpolymeric hydroxyl (−OHNP), methylene (−CH2), secondary amine (−NH+), carbonate (−CO32−), and ketone (−C=O) functional groups. These functional groups play a major role in buffering capacity for pH changes, acid neutralizing capacity for alkalinity changes, and nutrient retention or exchange by contributing to the CEC.
Table 1. Physical and Chemical Characteristics of Biochars Pyrolysed at 400 °Ca.
| characteristics | SBB | SBB-KOH | PSB | PSB-KOH |
|---|---|---|---|---|
| physical properties | ||||
| bulk density (g cm–3) | 0.187 | 0.233 | 0.13 | 0.181 |
| particle density (g cm–3) | 0.68 | 1.12 | 0.84 | 1.34 |
| porosity (%) | 72.5 | 79.1 | 84.5 | 86.4 |
| moisture (%) | 0.462 | 0.54 | 0.41 | 0.687 |
| WHC (%) | 130.31 | 113.55 | 235 | 246.2 |
| Ash (%) | 7.96 | 11.3 | 31 | 36 |
| TDS (mg L–1) | 546 | 582 | 4100 | 4350 |
| chemical properties | ||||
| pH | 7.06–7.17 | 9.1–9.13 | 7.96–8.19 | 10.55–10.65 |
| EC (dS m–1) | 0.84–1.09 | 5.9–6.07 | 5.8–6.31 | 10.34–12.11 |
| CEC (cmol (+) kg–1) | 38.2 | 52.4 | 73.1 | 84.7 |
| total potassium (g kg–1) | 23.94 | 25.12 | 35.8 | 39.84 |
| available potassium (g kg–1) | 19.26 | 23.44 | 31.62 | 36.16 |
| total phosphorus (g kg–1) | 1.6 | 1.8 | 2.1 | 2.3 |
| total calcium (g kg–1) | 6.8 | 4.4 | 9.2 | 8.6 |
SBB: sugar cane bagasse biochar; PSB: paddy straw biochar; SBB-KOH: sugar cane bagasse biochar (KOH activated); PSB-KOH: paddy straw biochar (KOH activated).
Table 2. FTIR Peaks (cm–1) and Functional Groups in Biocharsa.
| wave number range cm–1 | functional groups | SBB biochar | PSB | SBB-KOH | PSB-KOH |
|---|---|---|---|---|---|
| 3220–3600 | –OHNP | 3361.93 (−OHNP) | 3385 (−OHNP) | 3375 (−OHNP) | |
| 3000–4000 | –OH | 3780.48 (−OH) | 3826.77 (−OH) | 3780.48 (−OH) | 3178.69 (−OH) |
| 2500–3300 | –COOH | 3014.74 (−COOH) | |||
| 1670–1820 | –C=O, – CO32− | 1707 (−C=O, – CO32−) | 1697.36 (−C=O, – CO32−) | 1697 (−C=O, – CO32−) | |
| 1550–1650 | –N=H | 1586.2 (−N=H) | 1577 (−N=H) | 1558.48 (−N=H) | |
| 1430–1490 | –CH2 | 1375.25 (−CH2) | 1369.46 (−CH2) | 1363.67 (−CH2) | |
| 1000–1095 | =C–H | 1080.14 (=C–H) | 1112.93 (=C–H) | 1004.91 (=C–H) | |
| 800–700 | –C–Ha | 754.17 (−C–Ha) | 779.24 (−C−Ha) | 825.53 (−C–Ha) | |
| 800–700 | –C–Br | 773.46 (−C–Br) | |||
| 600–500 | –C–I | 615.29 (−C–I) |
A: Aromatic; NP: nonpolymeric; M: metal; SBB: sugar cane bagasse biochar; PSB: paddy straw biochar; SBB-KOH: sugar cane bagasse biochar (KOH activated); PSB-KOH: paddy straw biochar (KOH activated).
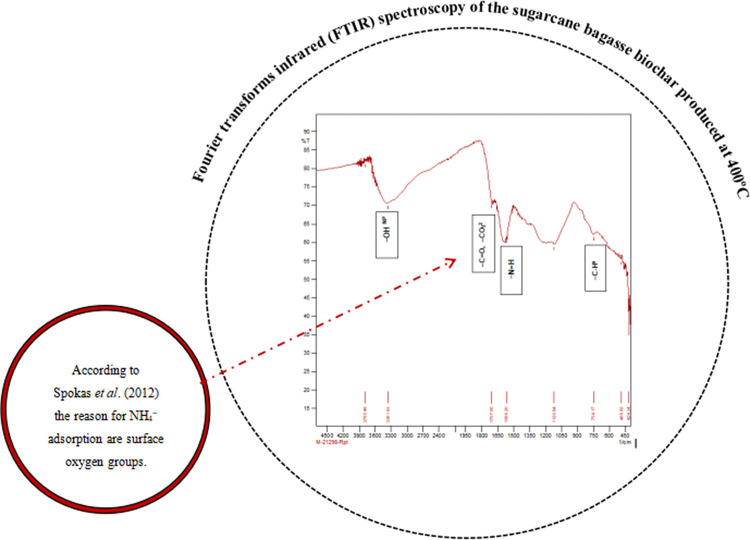
Figure 2.
Fourier transforms infrared (FTIR) spectroscopy confirming the presence of surface oxygen groups in sugar cane bagasse biochar, which is responsible for NH4+ adsorption.
Figure 6.
Molecular absorption in the infrared region with Fourier-transform infrared (FTIR) spectroscopy of the activated paddy straw biochar produced at 400 °C.
Figure 3.
Molecular absorption in the infrared region with Fourier-transform infrared (FTIR) spectroscopy of the sugar cane bagasse biochar produced at 400 °C.
Figure 4.
Molecular absorption in the infrared region with Fourier-transform infrared (FTIR) spectroscopy of the paddy straw biochar produced at 400 °C.
Figure 5.
Molecular absorption in the infrared region with Fourier-transform infrared (FTIR) spectroscopy of the activated sugar cane bagasse biochar produced at 400 °C.
FTIR Analysis of Sediment
Table 3 summarizes the FTIR peaks and functional groups of sediment from control and biochar-amended sediments after 45 days of culture. The sediment contains both (aromatic hydroxyl) −OHA and (nonpolymeric hydroxyl) −OHNP functional groups. The terminal methyl group (−CH2) and nonpolymeric hydroxyl (−OHNP) are unique to biochar and are not found in the final control sediment. However, these functional groups can be found in the biochar-treated sediment. The hydroxyl (−OH) functional group is present in all sediment samples and plays a role in both CEC and buffering of pH.
Table 3. FTIR Peaks (cm–1) and Functional Groups in Final Sediment of Different Treatmentsa,b.
| wave number range cm–1 | functional groups | C | T1 | T2 | T3 | T4 |
|---|---|---|---|---|---|---|
| 3670–3610 | –OHA | 3630.03 (−OHA) | 3630.03 (−OHA) | 3618.46 (−OHA) | ||
| 3220–3600 | –OHNP | 3377.36 (−OHNP) | 3361.93 (−OHNP) | 3392.79 (−OHNP) | ||
| 3000–4000 | –OH | 3874.99 (−OH) | 3776.62 (−OH) | 3743.83 (−OH) | ||
| 2372 | –N=H+ | 2364.73 (−N = H+) | ||||
| 1990–2200 | –C≡C– | 2177.63 (−C≡C−) | ||||
| 1550–1650 | –N=H | 1637.56 (−N=H) | 1647.21 (−N=H) | 1647.21 (−N=H) | 1637.56 (−N=H) | 1651.07 (−N = H) |
| 1430–1490 | –CH2 | 1521.84 (−CH2) | ||||
| 1000–1095 | =C–H | 985.62 (=C–H) | 985.62 (=C–H) | 985.62 (=C–H) | 985.62 (=C–H) | 987.55 (=C–H) |
| 800–700 | –C–Ha | 796.6 (−C–Ha) | 775.38 (−C–Ha) | 773.46 (−C–Ha) | 775.38 (−C–Ha) | |
| 800–700 | –C–Br | 692.44 (−C–Br) | 690.52 (−C–Br) | 688.59 (−C–Br) | 692.44 (−C–Br) | 690.52 (−C–Br) |
| 600–500 | –C–I | 516.92 (−C–I) |
A: aromatic; NP: nonpolymeric; M: metal.
Control: no biochar; T1: paddy straw biochar (PSB) application in sediment; T2: sediment amended with KOH-activated PSB; T3: sugar cane bagasse biochar (SBB) application in sediment; T4: sediment amended with KOH-activated SBB.
Physicochemical Parameters of Sediment
The initial CEC varied from 6.92 ± 0.06 to 7.46 ± 0.09 cmol(+) kg–1, and final values from 6.95 ± 0.05 to 11.60 ± 0.13 cmol(+) kg–1. The CEC of control, T1, T2, T3, and T4 increased by 0.43, 43.30, 59.63, 62.67, and 64.11% from the initial value until the end of the experiment. The available potassium levels between the control and treatments of the final sample varied significantly. The initial value for available potassium varied from 522.36 ± 0.96 to 570.13 ± 1.16 kg ha–1, and final from 664.27 ± 1.27 to 1575.73 ± 1.39 kg ha–1. There was an increase of available potassium in treatments T1, T2, T3, and T4, and in control by 54.14, 176.38, 49.83, 165.90, and 27.17% from the initial value until the end of the experiment, respectively. There was an increase of WHC in treatments T1 by a factor of 2.37, T2 (2.66), T3 (2.18), and T4 (2.38), except in control where the WHC was reduced by a factor of 0.99 from the initial (day 1) value until the end of the experiment. The initial value for BD varied from 1.05 ± 0.012 to 1.09 ± 0.07 g cm–3 and final from 0.79 ± 0.007 to 1.08 ± 0.011 g cm–3. There was a decrease of BD in treatments T1, T2, T3, and T4 by 22.37, 22.56, 23.54, and 35.36% from the initial value until the end of the experiment.
Microbial Activity in Sediment
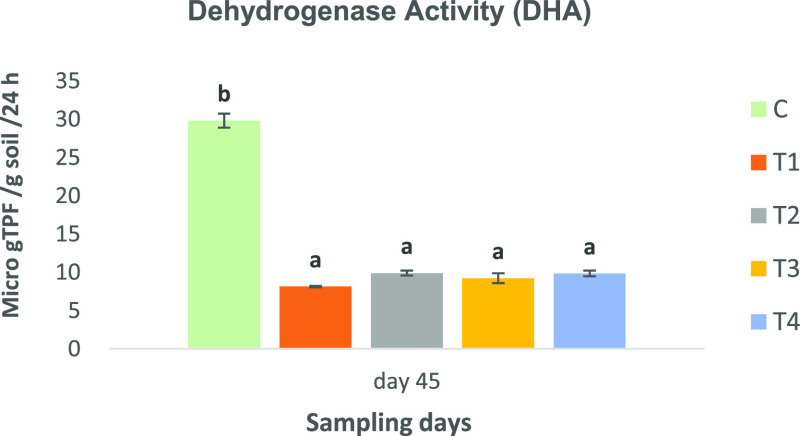
The DHA varied significantly (P < 0.05) in control compared to other treatments in the final sample. The highest DHA was observed in control (29.82 ± 0.92 μgTPF g–1 24 h–1) and lowest in T1 (8.14 ± 0.51 μgTPF g–1 24 h–1) at the end (45th day) of the experiment, respectively (Figure 7).
Figure 7.
Dehydrogenase activity (DHA) μgTPF g–1 24 h–1 in the sediment. Control: no biochar; T1: paddy straw biochar (PSB) application in sediment; T2: sediment amended with KOH-activated PSB; T3: sugar cane bagasse biochar (SBB) application in sediment; T4: sediment amended with KOH-activated SBB.
Physicochemical Parameters of the Water
The concentration of ammonia in water varied significantly in T1, T2, T3, and T4 in comparison to that of the control. The ammonium-N (mg L–1) in treatments T1, T2, T3, and T4 was found to have reduced by 0.72, 0.76, 0.03, and 0.11 units from the starting value (day 1) at the conclusion of the experiment. Ammonia levels in the control were elevated by 0.56 unit over the starting levels.
The pH was significantly increased in treatments T1, T2, T3, and T4. At the end of the 45th day, T4 had the highest pH value (8.47 ± 0.02) and the control had the lowest (8.12 ± 0.02). Total alkalinity increased significantly from the baseline in treatments T1, T2, T3, and T4. Following the completion of the trial, T3 had the greatest total alkalinity (283.33 ± 2.91 ppm), while the control had the lowest (273.33 ± 1.76 ppm).
In contrast to the control, there was a significant increase in potassium levels (mg L–1) in all treatments. For the final water sample, T2 had the highest potassium value (101.10 ± 1.55 mg L–1) and control had the lowest (74.20 ± 0.46 mg L–1). The potassium (mg L–1) concentrations in different treatments T1, T2, T3, and T4, and in the control, increased by 90, 113.70, 82.78, 107.95, and 62.99%, respectively, from the first day of observation to the end of the experiment.
From the beginning to the completion of the experiment, the total hardness in all of the treatments including the control increased significantly. When compared to the other treatments, treatment T3 had the highest total hardness (3713.33 mg L–1). The calcium levels in the treatments changed significantly. The final calcium value varies from 186.67 ± 3.53 to 332 ± 2.31 ppm, with a maximum rise of 34.59% from the initial in control and a decrease of 23.91% from the beginning in treatment T4. There were significant changes in the magnesium levels of the treatments during the experiment. The final value of magnesium ranges from 650.43 ± 4.51 to 767.88 ± 4.86 ppm, with the highest increase of 79.5% from the initial (day 1) value seen in T4, and among all the treatments, lowest increment of magnesium was in control (49.3%) from the initial value. On the last day, there were noticeable changes in the calcium/magnesium ratios of all of the treatments including the control. The end calcium/magnesium ratio ranged from 1.00:1.96 ± 0.03 to 1.00:4.12 ± 0.08, with T4 having the maximum increase of 136.11% from the beginning value and the control having the lowest increment of magnesium (10.91%) from the initial value among all treatments.
The TSS levels in the control varied significantly compared to the rest of the treatments on the final day. The highest value of TSS was recorded in T2 (21.18 ± 0.04 mg L–1), and the lowest value for control (14.13 ± 0.73 mg L–1) on the 45th day.
Growth Parameters of P. vannamei
There was a significantly high weight gain percentage for P. vannamei in treatments T2 and T4 compared to control, T1 and T3. The WG % ranges from 173.23.39 ± 0.73 to 191.75 ± 6.82% with the highest increase of 191.75% from the initial seen in T2 and the lowest increment of 173.23% from the initial in control.
The survival % ranges from 72.5 ± 5.2 to 96.1 ± 2.0% with the highest survival % of 96.1% from the initial seen in T3 and the lowest increment of 72.5% from the initial in control. The FCR ranges from 1.41 ± 0.01 to 1.56 ± 0.01 with the highest FCR of 1.56 seen in control and the lowest of 1.41 in T4. The specific growth rate (SGR) ranges from 2.23 ± 0.01% day–1 to 2.38 ± 0.05% day–1 with the highest SGR of 2.38% day–1 seen in T2 and T4 whereas the lowest of 2.23% day–1 in control.
Discussion
Physicochemical Properties of Biochar
The physical and chemical characteristics of biochar are influenced by the feedstock and pyrolysis conditions.25 Wu et al.26 reported that the pH, ash, CEC, and total phosphorus of rice straw biochar pyrolyzed at 400 °C were 9.96, 288 g kg–1, 61.60 cmol (+) kg–1, and 0.06 g kg–1, respectively. According to Figueredo et al.,27 the pH, ash, CEC, total phosphorus, total potassium, and total calcium of SBB pyrolyzed at 500 °C were 7.16, 7.07%, 52.1 cmol (+) kg–1, 1.6 g kg–1, 39 g kg–1, and 12 g kg–1, respectively. Dejene and Tilahun28 produced rice straw biochar at 300 °C, and the yield was found to be 9.67%. These findings are comparable with the present study, as represented in Table 1. The FTIR spectroscopy revealed that biochar contains secondary amine and hydroxyl functional groups (Table 2), which are fundamental for the reactivity of this material as an excellent natural adsorbent. A similar mechanism is reported in Chitosan-based adsorbents, which are used for the removal of pollutants from aqueous environments.29
Physicochemical Parameters of Water and Soil
During a 45-day experiment, the impact of biochar on physicochemical aspects of inland saline water, including pH, total hardness, total alkalinity, ammonium nitrogen, potassium, calcium, and magnesium, was examined. There was a significant improvement in potassium, pH, total hardness, Ca/Mg ratio, and total alkalinity. The concentration of ammonia nitrogen was decreased initially, and toward the end of the experiment, it increased in all the treatments including the control. However, compared to the control, the ammonia content of water in the tanks with biochar-treated sediment was lower. This is a promising outcome given that just topping was done to make up for the water that was lost through evaporation from the tanks. The highest concentration of ammonium-N was recorded in the control (1.16 mg L–1) treatment and the lowest value for T1 (0.21 mg L–1) in inland saline water (Table 5). According to Lin,30 biochar made from spent mushroom substrate has tremendous potential as a sorbent to adsorb hazardous compounds like ammonia, and therefore the environmental condition of aquaculture ponds may be improved. After the addition of biochar, Atkinsonl et al.31 reported an increase in NH4+ binding. The explanation for NH4+ adsorption, according to Spokas et al.,32 is surface oxygen groups (Figure 2). FTIR examination of biochar, as represented in Table 2, revealed the prevalence of oxidized functional groups (−C=O). The rise in ammonium-N in the control water column may be related to the decomposition of soil organic matter, including uneaten feed and fecal matter, because pond bottom sediment is the primary source of nitrogen fertilizer delivery to the pond water via the breakdown of organic matter.33 Gundale and DeLuca34 found that adding biochar to soil decreased ammonification via adsorption and lowered the possibility for NH3 volatilization.
Table 5. Physicochemical Parameters of the Watera.
| parameters | C | T1 | T2 | T3 | T4 |
|---|---|---|---|---|---|
| ammonium-N (mg L–1) | |||||
| initial | 1.16d ± 0.00 | 0.93a ± 0.03 | 1.00b ± 0.03 | 1.02bc ± 0.01 | 1.09c ± 0.01 |
| final | 1.72c ± 0.01 | 0.21a ± 0.00 | 0.25a ± 0.01 | 0.99b ± 0.01 | 0.98b ± 0.03 |
| total alkalinity (mg L–1) | |||||
| initial | 247.33a ± 1.76 | 254.00b ± 1.15 | 252.00b ± 1.15 | 252.67b ± 0.67 | 260.00c ± 1.15 |
| final | 273.33a ± 1.76 | 276.67a ± 1.76 | 275.33a ± 1.76 | 283.33b ± 2.91 | 278.00ab ± 1.15 |
| pH | |||||
| initial | 7.99a ± 0.00 | 7.93a ± 0.01 | 8.10a ± 0.05 | 7.95a ± 0.02 | 8.13a ± 0.13 |
| final | 8.12a ± 0.02 | 8.31b ± 0.05 | 8.33b ± 0.01 | 8.26b ± 0.05 | 8.47c ± 0.02 |
| potassium (mg L–1) | |||||
| initial | 45.53a ± 0.20 | 46.67a ± 0.16 | 47.31a ± 0.31 | 46.95a ± 0.71 | 46.44a ± 0.94 |
| final | 74.20a ± 0.46 | 88.68c ± 0.55 | 101.10e ± 1.55 | 85.81b ± 0.24 | 96.58d ± 0.60 |
| calcium (mg L–1) | |||||
| initial | 246.67b ± 1.33 | 256.00c ± 2.31 | 228.00a ± 2.31 | 273.33d ± 1.33 | 245.33b ± 1.33 |
| final | 332.00e ± 2.31 | 265.33d ± 3.53 | 254.67c ± 2.67 | 242.00b ± 3.46 | 186.67a ± 3.53 |
| magnesium (mg L–1) | |||||
| initial | 435.78a ± 2.14 | 450.36b ± 2.92 | 444.69b ± 2.43 | 452.79b ± 0.81 | 427.68a ± 3.71 |
| final | 650.43a ± 4.51 | 703.89b ± 7.20 | 726.57c ± 3.71 | 755.32d ± 3.16 | 767.88d ± 4.86 |
| Ca/Mg ratio | |||||
| initial | 1.77b ± 0.01 | 1.76b ± 0.03 | 1.95c ± 0.03 | 1.66a ± 0.01 | 1.74b ± 0.02 |
| final | 1.96a ± 0.03 | 2.65b ± 0.06 | 2.85c ± 0.03 | 3.12d ± 0.04 | 4.12e ± 0.08 |
| TSS (mg L–1) | |||||
| initial | 11.28a ± 0.04 | 11.33ab ± 0.02 | 11.30a ± 0.01 | 11.38b ± 0.01 | 11.32ab ± 0.02 |
| final | 14.13a ± 1.31 | 19.79b ± 0.73 | 21.18b ± 0.04 | 20.02b ± 0.05 | 20.5b ± 0.20 |
Mean values (mean ± S.E.) in each row not sharing a common superscript are significantly different (P < 0.05). One-way ANOVA was used following Duncan’s multiple range test in SPSS-26.0. Control: no biochar; T1: paddy straw biochar (PSB) application in sediment; T2: sediment amended with KOH-activated PSB; T3: sugar cane bagasse biochar (SBB) application in sediment; T4: sediment amended with KOH-activated SBB.
The pH was increased significantly in treatments T1, T2, T3, and T4. According to Najmudeen et al.,35 biochar-embedded feed systems were shown to improve the pH of the system, demonstrating the capacity to reduce water acidity. The existence of sodium carbonate (Na2CO3) (deposits in surface soil owing to excessive evapotranspiration in dry and semiarid climates) in salty soil could be the reason for the pH rise in inland saline water.36,37 When dry saline sediment comes into contact with water, sodium carbonate hydrolyzes, producing highly alkaline sodium hydroxide and unstable weak carbonic acid. The presence of ammonium-N in water increases the concentration of hydroxide ions. The increase in the basic hydroxide ions from sodium hydroxide and ammonia-N raises the pH of water significantly. Because ammonium-N production is stronger in control (Table 5) (which creates more −OH ions), this might explain why pH is increasing in control even in the absence of biochar. Additionally, FTIR analysis (Table 2) revealed that biochar contains alkaline functional groups (hydroxyl, methyl, and secondary amine), which may lead to a greater pH rise in sediment application. The FTIR analysis of biochar showed that the presence of carbonate ion (−CO32−) in biochar (Table 2) causes an increase in total alkalinity in all the treatments and comparatively low alkalinity in control due to the absence of biochar. This supports the significant increase of total alkalinity in treatments T1, T2, T3, and T4 from the initial value.
In all biochar treatments, the potassium content in the water column increased significantly (Table 5). The highest increase of potassium was seen in T2 (113.70%), and the lowest was seen in the control (62.99%) at the end of the trial, respectively. Raul et al.38 observed similar findings; the addition of SBB to sediment increased the critical shortfall nutrient in inland saline water (ISW), potassium. Potassium (K+) is a crucial essential nutrient for primary producers,39 and its availability in soil-water solutions drops dramatically owing to the high salt concentration in inland saline soil.40 Taghavimehr41 reported that biochar application (16 ton ha–1) to saline soil had no effect on exchangeable sodium, calcium, or magnesium concentrations but raised exchangeable potassium concentration by 44%; nevertheless, biochar type and soil parameters may have a role in influencing K+ availability. It has been observed that biochar with a greater ash level has a higher amount of critical plant nutrients, such as phosphorus and potassium.42 The paddy straw and SBB have ash contents of 31 and 7.6%, respectively (Table 1). Since PSB is a richer source of potassium than other mineral components, like sodium, calcium, and magnesium (Table 1), potassium may be more readily accessible in sediment than other minerals. Due to biochar’s CEC, which results in a larger exchange of monovalent cations than divalent cations, the release of potassium is greater than that of calcium and magnesium.43 This is relatable with the current study, as the increase in K+ concentration from high to low occurred in the following order: T2 (sediment amended with KOH-activated PSB) > T4 > T1 > T3 > control. Similarly, for the calcium/magnesium ratio, T4 (sediment amended with KOH-activated SBB) showed the highest increase of 136.11% from the initial value, which was followed by T3 > T2 > T1 > control (Table 5).
TSS levels were found to be increasing in all the treatments including control and were found to be high (21.18 ± 0.04 mg L–1) in treatment T2 and the lowest level (14.13 ± 1.31 mg L–1) was observed in control (Table 5). The reason for the increase in TSS value in all of the treatments might be due to the increased activity of shrimp in sediment as it grows over time, and in biochar-applied treatments, a higher TSS was always expected. According to an experiment done by Kathyayani et al.44 on stress measurement of P. vannamei subjected to varied degrees of turbidity, <30 nephelometric turbidity unit (NTU) water turbidity is a safe threshold for P. vannamei. Since 1 ppm equals 3 NTU, the stress due to turbidity and low potassium level might be the reason for the low survival % in control.
The CEC is an important characteristic of soil, which determines the adsorption/desorption of nutrients and thus their availability in soil-water solution. There was an increase in CEC of final saline sediment in all the treatments (Table 4). The rate of increment in sediment CEC for treatment T1, T2, T3, and T4, and in control were 43.30, 59.63, 62.67, 64.11, and 0.43%, respectively, of the initial CEC. The highest and lowest increase in CEC was in treatment T4 (64.11%) and control (0.43%), respectively. Cheng et al.45 and Atkinson et al.46 reported that the application of paddy straw and wheat straw biochar to saline soil increases CEC, 2.3 and 3.5 times than the initial value. The biochemical basis for the increase in CEC of sediment is due to the presence of oxidized functional groups of biochar, whose presence is indicated by high oxygen and carbon ratios on the surface of charred materials following microbial degradation.47,48 FTIR analysis of biochar (Table 2) also identified that there is a presence of an oxidized functional group (−C=O), which contributes to the effective CEC of sediment.
Table 4. Physicochemical Parameters of Sedimenta.
| parameters | C | T1 | T2 | T3 | T4 |
|---|---|---|---|---|---|
| CEC (cmol(+) kg–1) | |||||
| initial | 6.92a ± 0.06 | 7.46b ± 0.09 | 7.14ab ± 0.12 | 7.27ab ± 0.16 | 7.07a ± 0.09 |
| final | 6.95a ± 0.05 | 10.46b ± 0.36 | 11.27c ± 0.10 | 11.60c ± 0.13 | 11.51c ± 0.06 |
| available K (kg ha–1) | |||||
| initial | 522.36a ± 0.96 | 549.32b ± 1.83 | 570.13c ± 1.16 | 530.30a ± 1.39 | 548.47b ± 1.69 |
| final | 664.27a ± 1.27 | 846.73c ± 1.91 | 1575.73e ± 1.39 | 794.57b ± 1.13 | 1458.37d ± 1.30 |
| water holding capacity (%) | |||||
| initial | 18.68a ± 0.80 | 23.24b ± 1.76 | 22.25ab ± 0.11 | 23.48b ± 1.16 | 20.75ab ± 1.54 |
| final | 18.49a ± 0.42 | 55.05c ± 1.91 | 59.26d ± 0.74 | 51.09b ± 0.99 | 49.45b ± 0.37 |
| bulk density (g cm–3) | |||||
| initial | 1.08ab ± 0.011 | 1.09b ± 0.007 | 1.09ab ± 0.018 | 1.05a ± 0.012 | 1.07ab ± 0.007 |
| final | 1.08d ± 0.011 | 0.89c ± 0.007 | 0.89c ± 0.018 | 0.85b ± 0.012 | 0.79a ± 0.007 |
Mean values (mean ± S.E.) in each row not sharing a common superscript are significantly different (P < 0.05). One-way ANOVA was used following Duncan’s multiple range test in SPSS-26.0. Control: no biochar; T1: paddy straw biochar (PSB) application in sediment; T2: sediment amended with KOH-activated PSB; T3: sugar cane bagasse biochar (SBB) application in sediment; T4: sediment amended with KOH-activated SBB.
There was a decrease in bulk density of the final sediment sample from the initial sample in all biochar treatments, but no changes were observed in the control sediment. The T4 treatment shows the highest decrease (35.36%) and less decrease in T1 (22.37%) (Table 4). Different biochar bulk density varies from 0.08 to 0.43 g cm–3,49 depending on feedstock biomass and process conditions, and is lower than that of mineral soil ranging from 1.16 to 2.00 g cm–3.50 The bulk density of applied bagasse biochar and PSB are 0.187–0.24 and 0.13–0.181 g cm–3 (Table 1), which is very less than the saline sediment 1.08 g cm–3, so a reduction in soil bulk density is anticipated due to low bulk density of biochar and its highly porous structure.51
Soil Microbial Activity
The DHA varied significantly in the control compared to other treatments of the final sample. The highest DHA μgTPF g–1 24 h–1 was observed in control (29.82 ± 0.92 μgTPF g–1 24 h–1) and lowest in T1 (8.14 ± 0.51 μgTPF g–1 24 h–1) at the end of the experiment (Figure 7). According to Serra-Wittling et al.52 Moeskops et al.,53 dehydrogenase is considered correlated with the availability of OM in the soil. Therefore, the reason for increased DHA activity in the control might be due to increased organic matter content available for decomposition. In the context of aquaculture, pond sediment should not contain too much organic matter, especially in shrimp pond sediment, as it creates anoxic conditions in the water. Therefore, the presence of biochar-amended sediment in aquaculture systems may help in attaining a better environmental quality.
Growth Parameters of P. vannamei
When compared to the control, shrimp raised in a culture system with various biochar-amended sediments demonstrated the best growth performance in terms of SGR and weight gain (%). Similar results have been reported for finfish. The addition of 1.0% Eichhornia crassipes biochar to the soil in the culture system increased the SGR of O. mossambicus.31 However, to date, there is no published evidence regarding the effect of biochar on the growth metrics of shrimp.
Prangnell et al.54 found that to maintain a comparable level of shrimp survival, potassium concentrations greater than 76% of those in seawater appear to be required. This is comparable to the present study. The real potassium demand in inland saline water having a salinity of 10 ppt is 97.2 ppm when available potassium in water is 9.2 ppm, according to the equations based on.17 The potassium concentration attained in control at the end of the experiment was 74.20 ppm, when F-IGSW, which has potassium levels that are 50% equivalent to those of seawater, was used initially. Which was only marginally higher than the actual requirement (73.82 ppm K+) of potassium concentration for better survival. This might be the reason for the low survival percentage in the control as compared to all other treatments. Tantulo and Fotedar55 also found that in raw IGSW without supplemented feed, L. vannamei juveniles died completely. It might be attributed to a potassium deficiency in both the water and the feed.
The survival rates of L. vannamei were shown to be reliant on the K+ and Mg2+ availability in low-saline environments, and dietary supplementation with these minerals can greatly increase the survival of shrimps reared in low-saline environments.56,57 The current study observes that the highest survival of L. vannamei can be attained by using a culture system in which biochar-amended sediment was used as a substrate. The relationship between magnesium levels and survival percentage was also observed in this study. The final value of magnesium in the experimental system ranges from (650.43 ± 4.51 mg L–1) to (767.88 ± 4.86 mg L–1) with the highest increase of 79.5 and 66.8% from the initial was seen in T4 and T3, respectively, and among all treatments lowest increment of magnesium was in control (49.3%) from the initial day (Table 5). The survival percentage observed in the control was the lowest among all the treatments (72.5 ± 5.2%). However, in T3 and T4, the survival percentage was 96.1% (Table 6). Reduced magnesium induces a decrease in potassium in the shrimp body, as previously described in L. vannamei.58 Furthermore, magnesium deficiency reduced the survival and growth of postlarval and juvenile shrimps.59 Jahan et al.60 describe similar findings, stating that ion fortification in water still results in greater shrimp survival and growth than feed supplementation.
Table 6. Growth Parameters of Penaeus vannameia.
| parameters | C | T1 | T2 | T3 | T4 |
|---|---|---|---|---|---|
| average body weight initial (ABWI) | 2.74a ± 0.02 | 2.74a ± 0.02 | 2.78a ± 0.02 | 2.73a ± 0.02 | 2.73a ± 0.02 |
| average body weight final (ABWF) | 7.50a ± 0.04 | 7.69a ± 0.02 | 8.11b ± 0.15 | 7.59a ± 0.02 | 7.95b ± 0.04 |
| weight gain percentage | 173.23a ± 0.73 | 180.73ab ± 2.89 | 191.75b ± 6.82 | 178.54a ± 2.17 | 191.70b ± 1.39 |
| survival percentage | 72.5a ± 5.2 | 86.3ab ± 7.1 | 86.3ab ± 2.0 | 96.1b ± 2.0 | 84.3ab ± 2.0 |
| specific growth rate (SGR) % day–1 | 2.23a ± 0.01 | 2.29ab ± 0.02 | 2.38b ± 0.05 | 2.28a ± 0.02 | 2.38b ± 0.01 |
| feed conversion ratio (FCR) | 1.56c ± 0.01 | 1.50bc ± 0.02 | 1.41ab ± 0.05 | 1.51c ± 0.02 | 1.41a ± 0.01 |
Mean values (mean ± S.E.) in each row not sharing a common superscript are significantly different (P < 0.05). One-way ANOVA was used following Duncan’s multiple range test in SPSS-26.0. Control: no biochar; T1: paddy straw biochar (PSB) application in sediment; T2: sediment amended with KOH-activated PSB; T3: sugar cane bagasse biochar (SBB) application in sediment; T4: sediment amended with KOH-activated SBB.
The efficacy of different treatments for the successful culture of P. vannamei in inland saline water are compared and summarized in Table 7. During the 45-day experimental period, it was observed that the magnitude of pH in water varied from high to low in the following order: T4 > T2 > T1 > T3 > control. Except for the control, all treatments met the calcium/magnesium ratio needed for good shrimp production. At the end of the experiment, the treatment T4 had the greatest calcium/magnesium ratio (1.00:4.12), the control treatment had the lowest (1.00:1.96), and the T3 treatment had the best Ca/Mg ratio (1.00:3.12). The potassium concentrations achieved at the end of the trial differed significantly across treatments. The actual potassium requirement for 10 ppt water is 97.2 ppm. Only the T2 treatment was found to be meeting this criterion. The potassium content must be at least 76% higher than that of seawater to have equivalent survival. In this aspect, except for control, all the treatments were found to be effective. A higher concentration of 1.72 ppm ammonia was observed in the control, whereas in all other treatments ammonia concentration was less than 1.00 ppm. In terms of growth metrics, T2 (191.75b ± 6.82%) showed a greater weight increase percentage, which was substantially higher than the weight gain percentage in the control (173.23a ± 0.73%). The highest SGR of 2.38b ± 0.05% day–1 in T2, and the lowest SGR of 2.23a ± 0.01% day–1 in the control, showed a similar tendency. The highest survival percentage was seen in the T3 treatment (96.1b ± 2.0) %, which differed significantly compared to the control (72.5a ± 5.2) %. This might be due to the optimum Ca/Mg ratio in the T3 treatment. When biochar was amended with the sediment, the culture system was found to be maintaining optimum conditions that are conducive to the culture of P. vannamei.
Table 7. Efficacy of Different Treatments: A Comparative Analysis of the Efficacy of Different Treatments for the Successful Penaeus vannamei Culture in Inland Saline Watera.
| parameters | treatments | attained value during the experiment | recommended value for the successful Penaeus vannamei culture | effective treatment |
|---|---|---|---|---|
| pH | C | 8.12a ± 0.02 | 7.8–8.5 | T1, T2, T3, and T4 |
| T1 | 8.31b ± 0.05 | |||
| T2 | 8.33b ± 0.01 | |||
| T3 | 8.26b ± 0.05 | |||
| T4 | 8.47c ± 0.02 | |||
| calcium/magnesium ratio | C | 1.00:1.96 | 1.00:3.00 | T2, T3, and T4 |
| T1 | 1.00:2.65 | |||
| T2 | 1.00:3.85 | |||
| T3 | 1.00:3.12 | |||
| T4 | 1.00:4.12 | |||
| potassium | C | 74.20a ± 0.46 | for comparable survival at least greater than 76% of that in marine water. Actual requirement for 10 ppt water is 97.2 ppm | T1, T2, T3, and T4 highest in T2 |
| T1 | 88.68c ± 0.55 | |||
| T2 | 101.10e ± 1.55 | |||
| T3 | 85.81b ± 0.24 | |||
| T4 | 96.58d ± 0.60 | |||
| ammonium nitrogen | C | 1.72c ± 0.01 | less than 1.00 ppm | T1, T2, T3, and T4 |
| T1 | 0.21a ± 0.00 | |||
| T2 | 0.25a ± 0.01 | |||
| T3 | 0.99b ± 0.01 | |||
| T4 | 0.98b ± 0.03 | |||
| specific growth rate (SGR) % day–1 of Penaeus vannamei | C | 2.23a ± 0.01 | highest in T2 | |
| T1 | 2.29ab ± 0.02 | |||
| T2 | 2.38b ± 0.05 | |||
| T3 | 2.28a ± 0.02 | |||
| T4 | 2.38b ± 0.01 | |||
| weight gain percentage of Penaeus vannamei | C | 173.23a ± 0.73 | highest in T2 | |
| T1 | 180.73ab ± 2.89 | |||
| T2 | 191.75b ± 6.82 | |||
| T3 | 178.54a ± 2.17 | |||
| T4 | 191.70b ± 1.39 | |||
| survival percentage of Penaeus vannamei | C | 72.5a ± 5.2 | highest in T3 | |
| T1 | 86.3ab ± 7.1 | |||
| T2 | 86.3ab ± 2.0 | |||
| T3 | 96.1b ± 2.0 | |||
| T4 | 84.3ab ± 2.0 |
Mean values (mean ± S.E.) in each column not sharing a common superscript are significantly different (P < 0.05). One-way ANOVA was used following Duncan’s multiple range test in SPSS-26.0. Control: no biochar; T1: paddy straw biochar (PSB) application in sediment; T2: sediment amended with KOH-activated PSB; T3: sugar cane bagasse biochar (SBB) application in sediment; T4: sediment amended with KOH-activated SBB.
Conclusions
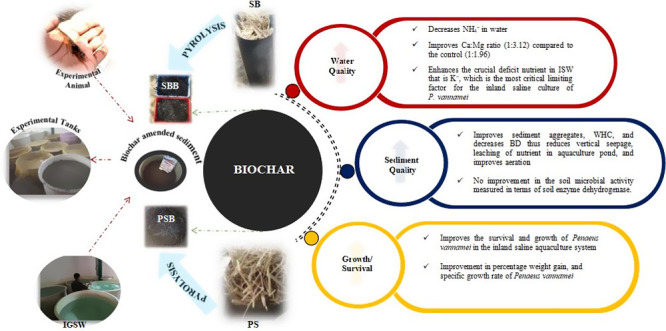
Biochar replaces the role of the mineral mixture and improves the environmental condition of the inland saline shrimp culture system when it is applied as an amendment to the sediment. An improvement was observed in the water quality parameters. Biochar amendment in sediment decreases NH4+ in water compared to the control. Biochar has a key role in controlling the concentration of water quality parameters, such as pH, total alkalinity, available potassium, calcium, magnesium, TSS, and ammonium nitrogen. It improves the important deficient nutrient in IGSW, potassium, which is the most significant limiting factor for P. vannamei inland saline culture. An improvement was seen in survival percentage, percentage weight gain, feed conversion ratio, and SGR of shrimp in all biochar-applied treatments compared to the control at the end of the experiment. This suggests that biochar has the potential to improve the survival and growth of P. vannamei in the inland saline aquaculture system.
Biochar improves sediment aggregates, WHC, and decreases BD thus reducing vertical seepage and leaching of nutrients in aquaculture ponds and improving aeration. Biochar-amended sediment is not favorable for the soil microbial activity measured in terms of soil enzyme dehydrogenase. DHA was more in the control compared to all other treatments. Since DHA is positively correlated with the organic matter in soil. Considering the aquaculture scenario, especially in shrimp farming, high organic matter in the pond bottom is considered undesirable; therefore, this is an encouraging result. Among different treatments, treatments T2 and T4 were found to be most beneficial in improving the water quality and enhancing the growth parameters of P. vannamei.
It can be concluded that converting agrowaste into biochar can be a sustainable option for crop residue management, as well as harnessing the benefits of biochar as a soil amendment for the effective utilization of degraded inland saline environments for the successful culture of P. vannamei in an eco-friendly manner. As it is a kind of experiment, where there is no previously published literature available from India, further field-level experiments are needed to validate these findings.
Recommendations for the Future Research
More study is needed to assess possibly dangerous compounds in biochar and their interactions with cultured animals.
Future studies are needed to investigate the effect of biochar on the growth of shrimp when incorporated through the feed.
Further studies are needed for the technology to produce biochar suiting the local conditions in a farmer-friendly manner.
Further research is needed to evaluate the effect of engineered biochar on soil and water quality in the aquatic system.
Acknowledgments
The present study was a part of regular institutional (ICAR-Central Institute of Fisheries Education, Mumbai, India) activity. No financial support from external agencies was made to conduct this study.
Glossary
Abbreviations
- IGSW
inland ground saline water
- FRP
fiber reinforced plastic
- SBB
sugar cane bagasse biochar
- PSB
paddy straw biochar
- Ca
calcium
- Mg
magnesium
- K+
potassium
- NH4+–N
ammonium nitrogen
- KOH
potassium hydroxide
- MOP
muriate of potash
- FTIR
Fourier-transform infrared (FTIR) spectroscopy
- CEC
cation exchange capacity
- WHC
water holding capacity
- TDS
total dissolved solids
- TSS
total suspended solids
- FCR
feed conversion ratio
- SGR
specific growth rate
- WG
weight gain
Data Availability Statement
All data underlying the results are available as part of the article. Raw data that support the findings of this study are available from the corresponding author, upon reasonable request.
Author Contributions
C.T.A.: execution of work, data collection, analysis, writing—initial draft. V.S.B.: conceptualization, supervision, writing—review and editing. M.C.: supervision, T.K.: data curation, writing—review and editing, S.K.: supervision, A.A.R.: writing—review and editing.
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
The authors declare no competing financial interest.
Notes
The animals used in this study were handled in line with the norms established by the CPCSEA (Committee for the Purpose of Control and Supervision of Experiments on Animals), Ministry of Environment and Forests (Animal Welfare Division), Government of India for the care and use of animals in scientific research.
Notes
All authors read and approved the final manuscript to publish in the ACS Omega journal.
References
- Lakra W. S.; Reddy A. K.; Harikrishna V. Technology for commercial farming of Pacific white shrimp Litopenaeus vannamei in inland saline soils using ground saline water. CIFE Tech. Bull.-1 2014, 1–28. [Google Scholar]
- Menz A.; Blake B. F. Experiments on the growth of Penaeus vannamei Boone. J. Exp. Mar. Biol. Ecol. 1980, 48 (2), 99–111. 10.1016/0022-0981(80)90010-6. [DOI] [Google Scholar]
- Bray W. A.; Lawrence A. L.; Leung-Trujillo J. R. The effect of salinity on growth and survival of Penaeus vannamei, with observations on the interaction of IHHN virus and salinity. Aquaculture 1994, 122 (2–3), 133–146. 10.1016/0044-8486(94)90505-3. [DOI] [Google Scholar]
- Jahan I.; Reddy A. K.; Sudhagar S. A.; Harikrishna V.; Singh S.; Varghese T.; Srivastava P. P. The effect of fortification of potassium and magnesium in the diet and culture water on growth, survival and osmoregulation of Pacific White Shrimp, Litopenaeus vannamei reared in inland ground saline water. Turk. J. Fish. Aquacult. Sci. 2018, 18 (10), 1235–1243. 10.4194/1303-2712-v18_10_10. [DOI] [Google Scholar]
- Venkatramanan V.; Shah S.; Prasad S.; Singh A.; Prasad R. Assessment of bioenergy generation potential of agricultural crop residues in India. Circ. Econ. Sustainability 2021, 1 (4), 1335–1348. 10.1007/s43615-021-00072-7. [DOI] [Google Scholar]
- Kumar S.; Sharma D. K.; Singh D. R.; Biswas H.; Praveen K. V.; Sharma V. Estimating loss of ecosystem services due to paddy straw burning in Northwest India. Int. J. Agric. Sustainability 2019, 17, 146–157. 10.1080/14735903.2019.1581474. [DOI] [Google Scholar]
- Shackley S.; Carter S.; Knowles T.; Middelink E.; Haefele S.; Sohi S.; Cross A.; Haszeldine S. Sustainable gasification–biochar systems. A case study of rice-husk gasification in Cambodia, Part I: Context, chemical properties, environmental and health and safety issues. Energy Policy 2012, 42, 49–58. 10.1016/j.enpol.2011.11.026. [DOI] [Google Scholar]
- Masto R. E.; Kumar S.; Rout T. K.; Sarkar P.; George J.; Ram L. C. Biochar from water hyacinth (Eichhornia crassipes) and its impact on soil biological activity. Catena 2013, 111, 64–71. 10.1016/j.catena.2013.06.025. [DOI] [Google Scholar]
- Lashari M. S.; Liu Y.; Li L.; Pan W.; Fu J.; Pan G.; Zheng J.; Zhang X.; Yu X. Effects of amendment of biochar-manure compost in conjunction with pyroligneous solution on soil quality and wheat yield of a salt-stressed cropland from Central China Great Plain. Field Crops Res. 2013, 144, 113–118. 10.1016/j.fcr.2012.11.015. [DOI] [Google Scholar]
- Jedrum S.; Thanachit S.; Anusontpornperm S.; Wiriyakitnateekul W. Soil amendments effect on yield and quality of jasmine rice grown on typic natraqualfs, Northeast Thailand. Int. J. Soil Sci. 2014, 9 (2), 37. 10.3923/ijss.2014.37.54. [DOI] [Google Scholar]
- Laird D. A. The charcoal vision: a win–win–win scenario for simultaneously producing bioenergy, permanently sequestering carbon, while improving soil and water quality. Agron. J. 2008, 100 (1), 178–181. 10.2134/agrojnl2007.0161. [DOI] [Google Scholar]
- International Biochar Initiative. Standardized product definition and product testing guidelines for biochar that is used in soil. https://biochar-international.org/characterizationstandard/ (accessed September 30, 2022).
- Inyang M.; Gao B.; Yao Y.; Xue Y.; Zimmerman A. R.; Pullammanappallil P.; Cao X. Removal of heavy metals from aqueous solution by biochars derived from anaerobically digested biomass. Bioresour. Technol. 2012, 110, 50–56. 10.1016/j.biortech.2012.01.072. [DOI] [PubMed] [Google Scholar]
- Amaral N. S.; Costa L. D.; De Oliveira C.; Velloso A. X. Heavy metals in some fertilizers and limes. Rev. Bras. Cien. Solo 1992, 16 (2), 271–276. 10.1590/s0100-06831998000200021. [DOI] [Google Scholar]; (in Portugese).
- Song W.; Guo M. Quality variations of poultry litter biochar generated at different pyrolysis temperatures. J. Anal. Appl. Pyrolysis 2012, 94, 138–145. 10.1016/j.jaap.2011.11.018. [DOI] [Google Scholar]
- Novak J. M.; Busscher W. J.; Laird D. L.; Ahmedna M.; Watts D. W.; Niandou M. A. Impact of biochar amendment on fertility of a southeastern coastal plain soil. Soil Sci. 2009, 174 (2), 105–112. 10.1097/SS.0b013e3181981d9a. [DOI] [Google Scholar]
- Davis D. A.; Boyd C. E.; Rouse D. B.; Saoud I. P. Effects of potassium, magnesium and age on growth and survival of Litopenaeus vannamei post-larvae reared in inland low salinity well waters in west Alabama. J. World Aquacult. Soc. 2005, 36 (3), 416–419. 10.1111/j.1749-7345.2005.tb00346.x. [DOI] [Google Scholar]
- APHA . Standard Methods for examination of water and wastewater; Twenty third ed.; American Public Health Association (APHA): Washington D. C., 2017. [Google Scholar]
- Estefan G.; Sommer R.; Ryan J. Methods of soil, plant, and water analysis. A manual for the West Asia and North Africa region 2013, 3, 65–119. [Google Scholar]
- Devis J.; Freitas F. Cation Exchange Capacity, Physical and Chemical Methods of Soil & Water Analysis, Food and Agriculture Organization of the United Nations, Rome, Italy. Soil Bull. 1970, 10, 94–130. [Google Scholar]
- American Society for Testing and Materials . Method D 2216–05. Standard test method for laboratory determination of soil and rock. Annual book of ASTM standards. Construction. Section 4. Soil and rock (I), Vol. 04.08. ASTM: West Conshohocken, PA, 2008. [Google Scholar]
- Kadam J. R.; Shinde P. B.. Practical Manual on Soil Physics -A method manual, Department of Agricultural Chemistry and Soil Science; P.G.I.: Rahuri, 2005; p 24. [Google Scholar]
- Casida L. E. Jr; Klein D. A.; Santoro T. Soil dehydrogenase activity. Soil Sci. 1964, 98 (6), 371–376. 10.1097/00010694-196412000-00004. [DOI] [Google Scholar]
- Omitoyi B. O.Utilization of poultry by-product (feather and officials) in the diet of African catfish clarias gariepinus (burchell1882); PhD thesis; University of Ibadan Nigeria, 1999. [Google Scholar]
- Zhang P.; Sun H.; Yu L.; Sun T. Adsorption and catalytic hydrolysis of carbaryl and atrazine on pig manure-derived biochars: impact of structural properties of biochars. J. Hazard. Mater. 2013, 244, 217–224. 10.1016/j.jhazmat.2012.11.046. [DOI] [PubMed] [Google Scholar]
- Wu W.; Yang M.; Feng Q.; McGrouther K.; Wang H.; Lu H.; Chen Y. Chemical characterization of rice straw-derived biochar for soil amendment. Biomass Bioenergy 2012, 47, 268–276. 10.1016/j.biombioe.2012.09.034. [DOI] [Google Scholar]
- Figueredo N. A.; Costa L. M.; Melo L. C.; Siebeneichlerd E. A.; Tronto J. Characterization of biochars from different sources and evaluation of release of nutrients and contaminants. Rev. Ciên. Agron. 2017, 48 (3), 3–403. 10.5935/1806-6690.20170046. [DOI] [Google Scholar]
- Dereje Dejen; Eyob Tilahun Characterization of Biochar Produced from Different Feed Stocks for Waste Management. Int. J. Environ. Sci. Nat. Res. 2019, 20 (3), 556040 10.19080/IJESNR.2019.20.556040. [DOI] [Google Scholar]
- Ngah W. W.; Teong L. C.; Hanafiah M. M. Adsorption of dyes and heavy metal ions by chitosan composites: A review. Carbohydr. Polym. 2011, 83 (4), 1446–1456. 10.1016/j.carbpol.2010.11.004. [DOI] [Google Scholar]
- Lin J. C.; Cheng A. C.; Shiu Y. L.; Wong Y. C.; Yeh S. P.; Simangunsong T.; Liu C. H. Using the biochar produced from spend mushroom substrate to improve the environmental condition of aquaculture pond. Aquacult. Res. 2021, 52 (8), 3532–3539. 10.1111/are.15194. [DOI] [Google Scholar]
- Atkinsonl C. J.; Fitzgerald J. D.; Hipps N. A. Potential mechanisms for achieving agricultural benefits from biochar application to temperate soils: a review. Plant Soil 2010, 337 (1–2), 1–18. 10.1007/s11104-010-0464-5. [DOI] [Google Scholar]
- Spokas K. A.; Novak J. M.; Venterea R. T. Biochar’s role as an alternative N-fertilizer: ammonia capture. Plant Soil 2012, 350 (1), 35–42. 10.1007/s11104-011-0930-8. [DOI] [Google Scholar]
- Prakash C.Role of nutrient in Aquatic ecosystem. Training manual on recent advances in management of water quality parameters in aquaculture. CAS in fisheries science; C.I.F.E, Versova: Mumbai, 1997; pp 42–48. [Google Scholar]
- Gundale M. J.; DeLuca T. H. Temperature and source material influence ecological attributes of ponderosa pine and Douglas-fir charcoal. For. Ecol. Manage. 2006, 231 (3), 86–93. 10.1016/j.foreco.2006.05.004. [DOI] [Google Scholar]
- Najmudeen T. M.; Arakkal Febna M. A.; Rojith G.; Zacharia P. U. Characterisation of Biochar from Water Hyacinth Eichhornia crassipes and the Effects of Biochar on the Growth of Fish and Paddy in Integrated Culture Systems. J. Coast Res. 2019, 86 (SI), 225–234. 10.2112/SI86-033.1. [DOI] [Google Scholar]
- Handa B. K. Geochemistry and genesis of fluoride containing groundwater in India. Groundwater 1975, 13, 278–281. 10.1111/j.1745-6584.1975.tb03086.x. [DOI] [Google Scholar]
- Chhabra R.Soil Salinity and Water Quality; Routledge: London, 1996. [Google Scholar]
- Raul C.; Bharti V. S.; Dar Jaffer Y.; Lenka S.; Krishna G. Sugarcane bagasse biochar: Suitable amendment for inland aquaculture soils. Aquacult. Res. 2021, 52 (2), 643–654. 10.1111/are.14922. [DOI] [Google Scholar]
- Wakeel A. Potassium–sodium interactions in soil and plant under saline-sodic conditions. J. Plant Nutr. Soil Sci. 2013, 176 (3), 344–354. 10.1002/jpln.201200417. [DOI] [Google Scholar]
- Cakmak I. The role of potassium in alleviating detrimental effects of abiotic stresses in plants. J. Plant Nutr. Soil Sci. 2005, 168 (4), 521–530. 10.1002/jpln.200420485. [DOI] [Google Scholar]
- Taghavimehr J.Effect of Biochar on Soil Microbial Communities, Nutrient Availability, and Greenhouse Gases in Short Rotation Coppice Systems of Central Alberta; Doctoral dissertation; University of Alberta, 2015. [Google Scholar]
- Zhao L.; Cao X.; Masek O.; Zimmerman A. Heterogeneity of biochar properties as a function of feedstock sources and production temperatures. J. Hazard Mater. 2013, 256, 1–9. 10.1016/j.jhazmat.2013.04.015. [DOI] [PubMed] [Google Scholar]
- Dewis J.; Freitas F.. Cation Exchange Capacity. Physical and chemical methods of soil and water analysis. In FAO soils Bulletin, 1970; Vol. 10, pp 94–130.
- Kathyayani S. A.; Muralidhar M.; Kumar T. S.; Alavandi S. V. Stress quantification in Penaeus vannamei exposed to varying levels of turbidity. J. Coast Res. 2019, 86 (SI), 177–183. 10.2112/SI86-027.1. [DOI] [Google Scholar]
- Cheng C. H.; Lehmann J.; Engelhard M. H. Natural oxidation of black carbon in soils: changes in molecular form and surface charge along a climosequence. Geochim. Cosmochim. Acta 2008, 72 (6), 1598–1610. 10.1016/j.gca.2008.01.010. [DOI] [Google Scholar]
- Atkinson C. J.; Fitzgerald J. D.; Hipps N. A. Potential mechanisms for achieving agricultural benefits from biochar application to temperate soils: a review. Plant Soil 2010, 337 (1–2), 1–18. 10.1007/s11104-010-0464-5. [DOI] [Google Scholar]
- Liang B.; Lehmann J.; Solomon D.; Kinyangi J.; Grossman J.; O’neill B.; Skjemstad J. O.; Thies J.; Luizao F. J.; Petersen J.; Neves E. G. Black carbon increases cation exchange capacity in soils. Soil Sci. Soc. Am. J. 2006, 70 (5), 1719–1730. 10.2136/sssaj2005.0383. [DOI] [Google Scholar]
- Preston C. M.; Schmidt M. W. I. Black (pyrogenic) carbon: A synthesis of current knowledge and uncertainties with special consideration of boreal regions’. Biogeosciences 2006, 3, 397–420. 10.5194/bg-3-397-2006. [DOI] [Google Scholar]
- Pastor-Villegas J.; Pastor-Valle J. F.; Rodríguez J. M. M.; García M. G. Study of commercial wood charcoals for the preparation of carbon adsorbents. J. Anal. Appl. Pyrolysis 2006, 76, 103–108. 10.1016/j.jaap.2005.08.002. [DOI] [Google Scholar]
- Chaudhari P. R.; Ahire D. V.; Ahire V. D.; Chkravarty M.; Maity S. Soil bulk density as related to soil texture, organic matter content and available total nutrients of Coimbatore soil. Int. J. Sci. Res. Publ. 2013, 3, 1–8. [Google Scholar]
- Downie A.; Crosky A.; Munroe P.; Lehmann J.; Joseph S.. Biochar for environmental management: science and technology. In Physical Properties of Biochar; Earthscan: London, UK, 2009; pp 13–32. [Google Scholar]
- Serra-Wittling C.; Houot S.; Barriuso E. Soil enzymatic response to addition of municipal solid-waste compost. Biol. Fertil. Soils 1995, 20 (4), 226–236. 10.1007/BF00336082. [DOI] [Google Scholar]
- Moeskops B.; Buchan D.; Sleutel S.; Herawaty L.; Husen E.; Saraswati R.; Setyorini D.; De Neve S. Soil microbial communities and activities under intensive organic and conventional vegetable farming in West Java, Indonesia. Appl. Soil Ecol. 2010, 45 (2), 112–120. 10.1016/j.apsoil.2010.03.005. [DOI] [Google Scholar]
- Prangnell D. I.; Fotedar R. The effect of potassium concentration in inland saline water on the growth and survival of the western king shrimp, (Penaeus latisulcatus Kishinouye, 1896). J. Appl. Aquacult. 2005, 17 (2), 19–34. 10.1300/J028v17n02_02. [DOI] [Google Scholar]
- Tantulo U.; Fotedar R. Osmo and ionic regulation of black tiger prawn (Penaeus monodon Fabricius 1798) juveniles exposed to K+ deficient inland saline water at different salinities. Comp. Biochem. Physiol., Part A: Mol. Integr. Physiol. 2007, 146 (2), 208–214. 10.1016/j.cbpa.2006.10.020. [DOI] [PubMed] [Google Scholar]
- Roy L.Physiological and nutritional requirements for the culture of the Pacific white shrimp, Litopenaeus vannamei, in low salinity waters; Ph. D. Thesis; Auburn university, 2006. [Google Scholar]
- Shiau S. Y.; Hsieh J. F. Dietary potassium requirement of juvenile grass shrimp Penaeus monodon. Fish. Sci. 2001, 67 (4), 592–595. 10.1046/j.1444-2906.2001.00294.x. [DOI] [Google Scholar]
- Davis D. A.; Lawrence A. L.; Gatlin D. M. Mineral requirements of Penaeus vannamei: a preliminary examination of the dietary essentiality for thirteen minerals. J. World Aquacult. Soc. 1992, 23 (1), 8–14. 10.1111/j.1749-7345.1992.tb00745.x. [DOI] [Google Scholar]
- Saoud I. P.; Davis D. A.; Rouse D. B. Suitability studies of inland well waters for Litopenaeus vannamei culture. Aquaculture 2003, 217 (1), 373–383. 10.1016/S0044-8486(02)00418-0. [DOI] [Google Scholar]
- Jahan I.; Reddy A. K.; Sudhagar S. A.; Harikrishna V.; Singh S.; Varghese T.; Srivastava P. P. The effect of fortification of potassium and magnesium in the diet and culture water on growth, survival and osmoregulation of Pacific White Shrimp, Litopenaeus vannamei reared in inland ground saline water. Turk. J. Fish. Aquacult. Sci. 2018, 18 (10), 1235–1243. 10.4194/1303-2712-v18_10_10. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data underlying the results are available as part of the article. Raw data that support the findings of this study are available from the corresponding author, upon reasonable request.