Abstract
Frontotemporal dementia is the second most common form of early onset dementia (<65 years). Despite this, there are few known disease-modifying factors. The anterior cingulate is a focal point of pathology in behavioural variant frontotemporal dementia. Sulcation of the anterior cingulate is denoted by the presence of a paracingulate sulcus, a tertiary sulcus developing, where present during the third gestational trimester and remaining stable throughout life. This study aims to examine the impact of right paracingulate sulcal presence on the expression and prognosis of behavioural variant frontotemporal dementia. This retrospective analysis drew its population from two clinical samples recruited from memory clinics at university hospitals in the USA and The Netherlands. Individuals with sporadic behavioural variant frontotemporal dementia were enrolled between 2000 and 2022 and followed up for an average of 7.71 years. T1-MRI data were evaluated for hemispheric paracingulate sulcal presence in accordance with an established protocol by two blinded raters. Outcome measures included age at onset, survival, cortical thickness and Frontotemporal Lobar Degeneration-modified Clinical Dementia Rating determined clinical disease progression. The study population consisted of 186 individuals with sporadic behavioural variant frontotemporal dementia (113 males and 73 females), mean age 63.28 years (SD 8.32). The mean age at onset was 2.44 years later in individuals possessing a right paracingulate sulcus [60.2 years (8.54)] versus individuals who did not [57.76 (8.05)], 95% confidence interval > 0.41, P = 0.02. Education was not associated with age at onset (β = −0.05, P = 0.75). The presence of a right paracingulate sulcus was associated with an 83% increased risk of death per year after age at onset (hazard ratio 1.83, confidence interval [1.09–3.07], P < 0.02), whilst the mean age at death was similar for individuals with a present and absent right paracingulate sulcus (P = 0.7). Right paracingulate sulcal presence was not associated with baseline cortical thickness. Right paracingulate sulcal presence is associated with disease expression and survival in sporadic behavioural variant frontotemporal dementia. Findings provide evidence of neurodevelopmental brain reserve in behavioural variant frontotemporal dementia that may be important in the design of trials for future therapeutic approaches.
Keywords: brain reserve, cingulate, frontotemporal dementia, paracingulate, sulcation
Harper et al. report an association between right paracingulate sulcal presence and later age at symptom onset (2.44 years) in individuals with behavioural variant frontotemporal dementia. Following onset right paracingulate sulcal presence increased the annual risk of death by 83%. Findings provide evidence for neurodevelopmental brain reserve in frontotemporal dementia.
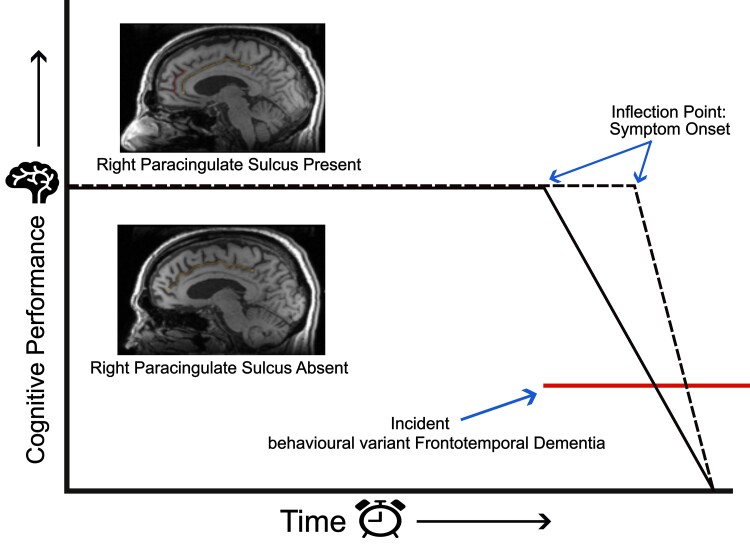
Graphical Abstract
Graphical Abstract.
Introduction
Behavioural variant frontotemporal dementia (bvFTD) is the most common clinicoradiological syndrome within the frontotemporal dementias (FTDs). FTD is highly heritable, ∼30% of suffers have a strong family history with heritability accounted for in 10–20% of FTD by autosomal dominant mutations in the chromosome 9 open reading frame 72 (C9orf72), progranulin (GRN) or microtubule-associated protein tau (MAPT) genes.1,2 The mean age at onset (AAO) is between 45 and 65 years.3 There are however documented cases under the age of 30, whilst up to 30% of patients have a later onset (≥65 years).3 AAO is variably affected by pathological genetic mutations, where present and genetic variation, including the presence of risk allele rs1990622 in the TMEM106B gene.4-6 Despite these few exceptions, there are no known factors affecting AAO in bvFTD. Environmental disease-modifying factors, including educational, occupational attainment and occupational engagement have been shown to provide resilience to the neuropathological burden of FTD, however they have yet to be shown to be associated with AAO.5,7-11 BvFTD atrophy has a predilection for the Anterior Cingulate (AC) and frontoinsula regions.12 Gyrification of the AC may be characterized morphologically by the presence of a paracingulate sulcus (PCS), a tertiary sulcus which, where present develops during the third trimester of gestation denoting the existence of a Paracingulate Gyrus (PCG).13 The presence of a PCS is more frequent in the left hemisphere of healthy individuals.14-17 The PCG is active during performance of cognitively demanding tasks drawing on higher-order executive function where possession of leftward PCS asymmetry (presence of a left but not right hemisphere PCS, as displayed in Fig. 1) has been associated with a performance advantage.18,19 Furthermore, individuals with asymmetric PCS patterns display greater inhibitory control and cognitive efficiency.20-23 Conversely, in schizophrenia, a reduced distribution of leftward PCS asymmetry is observed and interpreted as evidence of a prenatal neurodevelopmental aberration in the pathogenesis of schizophrenia.15,17
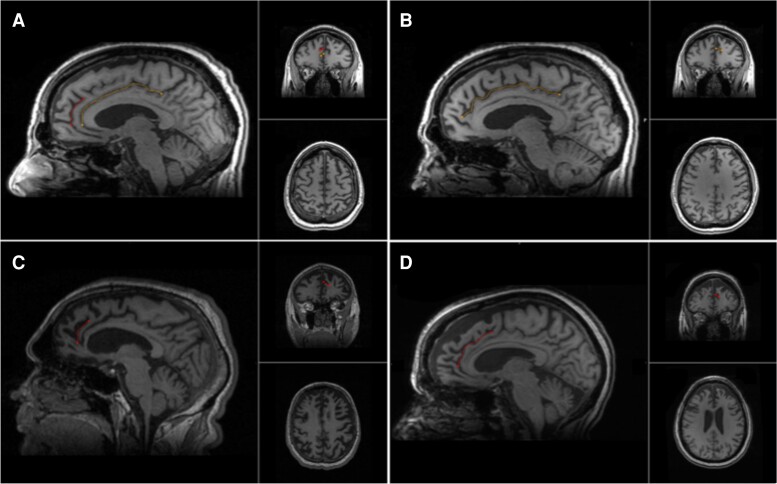
Figure 1.
Cingulate and paracingulate sulci identification and measurement. A 58-year-old male with probable bvFTD displays a leftward pattern of paracingulate asymmetry, A and B. A. The left hemisphere displaying a traced ‘present’ (length ≥ 20 mm), left paracingulate sulcus (red) and a traced cingulate sulcus (yellow). B. The right hemisphere displays a traced cingulate sulcus (yellow) with absence of a right PCS. C. A 62-year-old male with definite bvFTD, images display a traced ‘present’ right paracingulate sulcus, 23 mm in length (red). D. A 60-year-old female with probable bvFTD, images display a traced ‘prominent’ (length ≥ 40 mm) right paracingulate sulcus, 53 mm in length (red).
In previous work, the distribution of hemispheric PCS frequency in sporadic bvFTD was similar to that of healthy individuals.24 Significantly however, right PCS presence was associated with a later AAO in sporadic bvFTD, and is a potential proxy of brain reserve.24 Studied proxies of reserve (including education in AD) provide resilience to disease burden prior to phenoconversion. Following this critical point, compensatory reserve mechanisms become overwhelmed and clinical decline proceeds more rapidly than in those lacking such proxies.25 Despite the possible effect on disease expression, the impact of right PCS presence on disease progression and survival after AAO in bvFTD is not yet known. The present study aims to confirm and expand upon findings from Harper et al.24 in a novel independent cohort with longitudinal data. Our primary hypothesis was an association between right PCS presence and a later AAO in sporadic bvFTD. Secondary hypotheses were that following disease onset, and independent of education, disease progression would occur more rapidly, and survival would be shorter in individuals possessing a right PCS. Our tertiary hypothesis was that whilst at the same clinical stage, bvFTD individuals with a present right PCS would display greater disease burden, demonstrated by cortical atrophy, than those without.
Materials and methods
Participants
This retrospective analysis included individuals with sporadic bvFTD drawn from two clinical samples recruited from memory clinics at university hospitals in the USA (Penn FTDC, enrolment between 2004 and 2018) and The Netherlands (Amsterdam Dementia Cohort,26 enrolment between 2000 and 2022). BvFTD was diagnosed in accordance with revised International bvFTD Consortium criteria27 following multidisciplinary team assessment, clinical examination, standardized symptom assessment, neuropsychological and neurological examination, blood, and cerebrospinal fluid analysis of core Alzheimer’s disease biomarkers and brain MRI. Detailed cohort descriptions are published in the Supplementary material. Individuals with suspected hereditary bvFTD; Woods28 criteria ‘High’ or ‘Medium’ (Penn FTDC) or Goldmans29 criterion ≤ 3.0 (Amsterdam Dementia Cohort) were excluded. C9orf72 mutations were excluded in all and GRN and MAPT mutations were excluded in 92 individuals. Two individuals met subclassification criteria30 for right temporal lobe variant FTD and eight individuals met FTD-ALS criteria31 and were excluded. Neuropathological data were available in 38 individuals. Where neuropathology was consistent with isolated non-FTLD neurodegenerative disease, (n = 4) individuals were excluded whereas individuals with concomitant FTLD and non-FTLD neurodegenerative disease were included (n = 1). All subjects gave informed consent in accordance with the Declaration of Helsinki prior to inclusion in their native studies. Native studies were conducted with approval of respective local ethics committees, as detailed in the Supplementary material.
Magnetic resonance image acquisition and software
High-resolution volumetric whole brain T1-weighted magnetic resonance (MR) images were obtained from all individuals using 1.5 or 3.0 Tesla systems with a minimum spatial resolution of 1.5 × 1.5 × 1.5 mm. Protocols and MRI related details are provided in the Supplementary material.
Prior to analysis, images were pseudo-anonymized and visually inspected. Six individuals were removed due to distortion of their MR data by movement artefact. A further two individuals were removed with postoperative intracranial anatomy obscuring PCS identification. Cortical reconstruction and volumetric segmentation were performed on T1 3D MR images using FreeSurfer Software version 7.3.2 image analysis pipeline (http://surfer.nmr.mgh.harvard.edu/). This procedure is described elsewhere32 and briefly in the Supplementary material. Reconstructed data sets were visually inspected for accuracy by a single rater. Cortical thickness was successfully calculated in 176 individuals. Nineteen scans were excluded following quality control on the basis of poor surface reconstruction.
Paracingulate sulcus measurement and classification criteria
Manual PCS classification was performed radiographically according to a protocol adapted from Garrison’s established protocol for PCS classification,33 which has been used and described in Harper et al.24 and is documented in full in the Supplementary material. The PCS was categorized in a binary fashion; ‘present’ (≥20 mm) or ‘absent’ (<20 mm), as is standard amongst PCS classification protocols.15,17,33-35 Additionally, present PCS were subclassified as ‘prominent’ where their length exceeded 40 mm, as is standard.14,33 Sulcation ratings were performed by two raters, L.H. and A.F.S., who were blinded to individuals’ clinical and demographic data.
Clinical disease expression, progression and survival
AAO was determined by a clinician based on patient and caregiver history as the first date at which typical symptoms, compatible with a diagnosis of bvFTD,27 became apparent. Disease severity was assessed longitudinally in the Penn FTDC cohort at baseline and follow-up according to the Frontotemporal Lobar Degeneration-modified Clinical Dementia Rating (FTLD-CDR)36 The FTLD-CDR was selected over other clinical rating tools due to its superiority in the accurate classification of disease severity in FTD.36,37 Survival data were collected from all individuals.
Statistical analysis
Group differences in categorical variables were tested using chi-squared tests. Continuous measures were compared using two-sample t-tests. Normality was confirmed using the Shapiro–Wilk test. A one-sided t-test was conducted to analyse the association between right PCS presence and AAO. Effect sizes were calculated according to Cohen’s d. Simple and multiple linear regression models were fitted to evaluate covariable and interaction effects on AAO. Correlations between continuous variables were calculated using Pearson’s method whilst correlation between categorical and continuous variables utilized point biserial correlation. Survival analyses were performed from AAO to time of death from any cause (outcome = 1) or censoring date (outcome = 0). The censoring date was recorded as the date of last contact with the individual. Survival analyses were carried out using the Kaplan–Meier method with log rank post hoc testing by means of univariate and multivariate stepwise Cox proportional-hazard regression analysis. Hazard ratios (HRs) are provided with 95% confidence intervals (CIs) and reported as percentage risk of death per year for the right PCS present group compared to the right PCS absent group. A linear mixed effects model with random intercepts and slopes was fitted to analyse clinical disease progression. Cortical thickness calculations were undertaken in FreeSurfer Software version 7.3.2 (http://surfer.nmr.mgh.harvard.edu/). Group differences in cortical thickness according to right PCS presence were analysed by fitting a vertex-based general linear model corrected for the effect of age and sex. Cluster-wise correction for multiple comparisons was performed using Monte Carlo simulation,38 with a threshold of P < 0.05. Statistical analysis was performed using R software (R CoreTeam 2016, https://www.r-project.org/). P < 0.05 was considered statistically significant. Statistical procedures related to primary and secondary hypotheses alongside power calculations were pre-registered and may be accessed at https://aspredicted.org/SKM_6C1. Primary analyses were performed in accordance with the pre-registration, and secondary analyses with relation to PCS presence and local gyrification index are documented in the Supplementary material.
Results
The study population consisted of 186 sporadic bvFTD individuals (113 males and 73 females), with a mean age of 63.28 years (SD 8.32) and AAO of 59.15 (8.4). Demographic and results data are displayed in Table 1 and Supplementary Table 1.
Table 1.
Study population and paracingulate status
| Entire population | Penn FTDC | Amsterdam Dementia Cohort | P-value | |
|---|---|---|---|---|
| Participants | 186 | 94 | 92 | |
| Age, mean (SD), years | 63.28 (8.32) | 63.15 (8.82) | 63.38 (7.98) | 0.87 |
| Age at onset, mean (SD), years | 59.15 (8.4) | 58.16 (8.29) | 60.14 (8.43) | 0.11 |
| Sex | 113M: F73 | 59M: 35F | 54M: 38F | 0.54 |
| Education,a years (SD) | 13.34 (3.79) | 15.88 (2.74) | 10.63 (2.72) | <000.1 |
| Handedness, No. | 0.69 | |||
| Right | 146 | 81 | 65 | |
| Left | 12 | 9 | 3 | |
| Ambidextrous | 5 | 4 | 1 | |
| Unknown | 23 | 0 | 23 | |
| Diagnostic classification,b No | ||||
| Possible | 8 | 8 | 0 | |
| Probable | 140 | 51 | 89 | |
| Definite | 26 | 24 | 2 | |
| Unknown | 11 | 11 | 0 | |
| MMSE, mean (SD) | 24.18 (5.35) | 23.63 (6.07) | 24.8 (4.35) | 0.14 |
| FTLD-CDR sum of boxes, mean (SD) | 7.51 (3.83) | 9.01 (3.74) | 6.71 (3.65) | 0.001 |
| FTLD-CDR global scores, mean (SD) | 1.59 (0.72) | 1.79 (0.59) | 1.45 (0.76) | 0.01 |
| Hemispheric PCS, No. (%) | ||||
| Left present | 145 (78) | 73 (78) | 72 (78) | 1 |
| Left prominent | 59 (32) | 28 (30) | 31 (34) | 0.68 |
| Right present | 106 (57) | 58 (62) | 48 (52) | 0.53 |
| Right prominent | 31 (17) | 18 (19) | 13 (14) | 0.47 |
| Deceased,c No. (%) | 112 (60) | 51 (56) | 61 (66) | 0.13 |
| Age at death, mean (SD), years | 67.19 (8.86) | 67.42 (9.14) | 66.92 (8.62) | 0.79 |
Demographic and results data for entire population and according to cohort, Penn FTDC (The University of Pennsylvania Frontotemporal Degeneration Centre, PA, USA) and Alzheimer Center of the VU University Medical Center, Amsterdam (Amsterdam Dementia Cohort).26 Sex data: male (M); female (F). present = PCS length ≥ 20 mm, prominent = PCS length ≥ 40 mm. Frequency. t-Tests and chi-squared tests were performed to evaluate differences in continuous and nominal data, respectively.
SD, standard deviation; MMSE, Mini Mental State Examination; FTLD-CDR, Frontotemporal Lobar Degeneration-modified Clinical Dementia Rating;36 Sum, sum of boxes; Global, global score; Hemispheric PCS, hemispheric paracingulate sulcus.
aEducation, years data available for 94/94 Penn FTDC and 88/92 Amsterdam Dementia Cohort.
bDiagnostic classification according to revised International bvFTD Consortium criteria.27
cSurvival data were available for 185/186 individuals.
Mean AAO was 2.44 years later (Cohen’s d = 0.29, CI [>0.41], P = 0.02) in individuals with a present [mean AAO 60.20 years (SD 8.54)] versus absent right PCS [57.76 (8.05)]. These data are displayed in Fig. 2. In sensitivity analyses, independently both the Penn FTDC [mean difference (MD) = 2.48 years, P = 0.08] and the Amsterdam Dementia Cohort (MD = 2.8 years, P = 0.06) showed a non-significant, but later AAO in individuals with a present right PCS. Importantly this analysis was powered to identify significance at n = 173. Mean AAO did not differ significantly according to left PCS presence (MD = 1.53, P = 0.16). Right PCS length was not significantly correlated with AAO (r = 0.04, CI [−0.15–0.23], P = 0.66) however mean AAO in individuals with a prominent right PCS was 3.11 years later than in individuals with an absent right PCS [mean AAO = 60.87 years (SD 6.92) and 57.76 (8.05)], respectively (t = 2.02, CI [>0.55], P = 0.02). A one-sided univariate linear regression model identified a significant association between right PCS presence and AAO (β = 2.44, P = 0.03). In univariate linear models, neither education, sex or handedness was independently associated with AAO (P = 0.75, 0.3 and 0.45, respectively) nor was significant associations with AAO identified after including right PCS presence in a multivariate model with these variables (P = 0.64, 0.41 and 0.27, respectively).
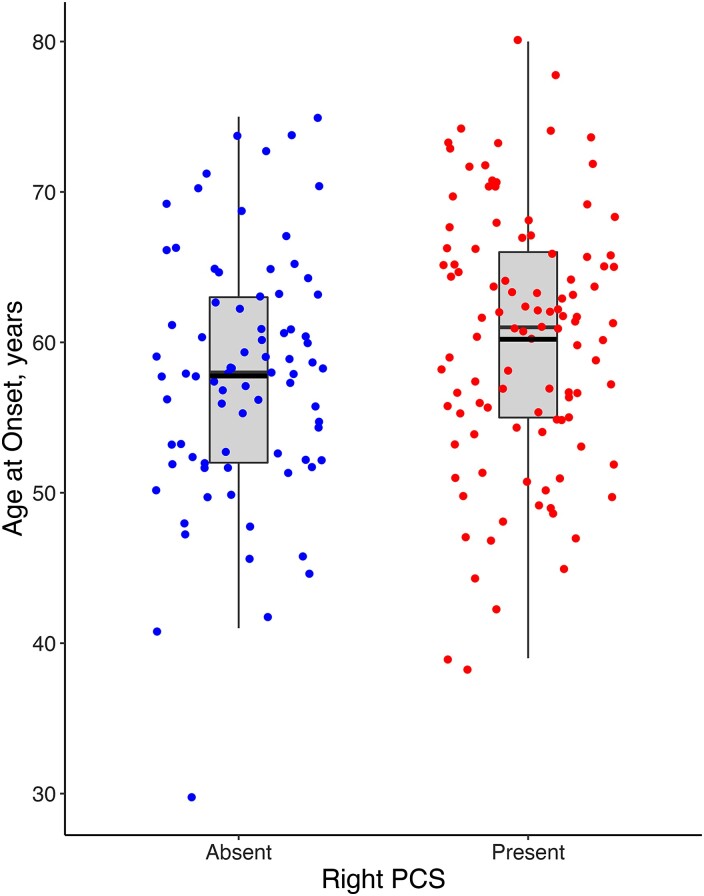
Figure 2.
Age at symptom onset by right hemisphere paracingulate sulcal presence in bvFTD. Red dots represent individuals with a present right paracingulate sulcus (PCS), n = 80. Blue dots represent individuals with an absent right PCS, n = 106. Black lines represent group mean age at symptom onset; 57.76 (SD 8.05) and 60.2 (8.54), respectively, for individuals with an absent and present right PCS, mean difference = 2.44, P = 0.02. Boxes extend from the 25th to 75th percentile, and horizontal black lines within the boxes denote median values.
Education was similar in individuals with a present and absent right PCS (t = 1.12, CI [−0.5–1.82], P = 0.26). A correlation between education and AAO was not observed (r = 0.09, CI [−0.06–0.23], P = 0.25). Furthermore, after correction for education in a one-sided multivariate linear model, the association between right PCS presence and AAO was retained (β = 2.27, P = 0.04). An interaction effect between education (β = 0.05, CI [−0.6–0.71], P = 0.87) and right PCS presence on AAO was not identified. After correcting a one-sided multivariate linear model for sex, the association between right PCS presence and AAO was retained (β = 2.33, P = 0.03). An interaction between sex and right PCS presence was identified such that AAO was greater in males processing a right PCS (β = 5.14, CI [0.17–10.1], P = 0.04), Supplementary Fig. 1.
Individuals were followed for a median of 7.71 years (Interquartile Range 5.00–10.8.7). Mean age at death was similar in individuals with present [66.94 years, (SD 9.66)] and absent [67.62, (7.38)] right PCS, P = 0.7. Survival was significantly affected by right PCS presence (chi-squared 6.6, P = 0.01). The unadjusted risk of death per year after AAO was 65.1% greater in individuals processing a right PCS (HR 1.65, CI [1.13–2.42], P = 0.01). Kaplan–Meier estimates for this result are presented in Fig. 3. Risk of death was enhanced to 83% following correction for baseline FTLD-CDR, AAO, sex and years of education (HR 1.83, CI [1.09–3.07], P < 0.02). Sensitivity analyses after fitting this model identified a significantly increased risk of death following AAO in the Penn FTDC cohort (HR 3.82, CI [1.06–13.82], P = 0.04) and a non-significant increase in the Amsterdam Dementia Cohort (HR 1.64, CI [0.88–3.09], P = 0.12). No interaction effect of sex and right PCS presence on survival was observed (P > 0.1).
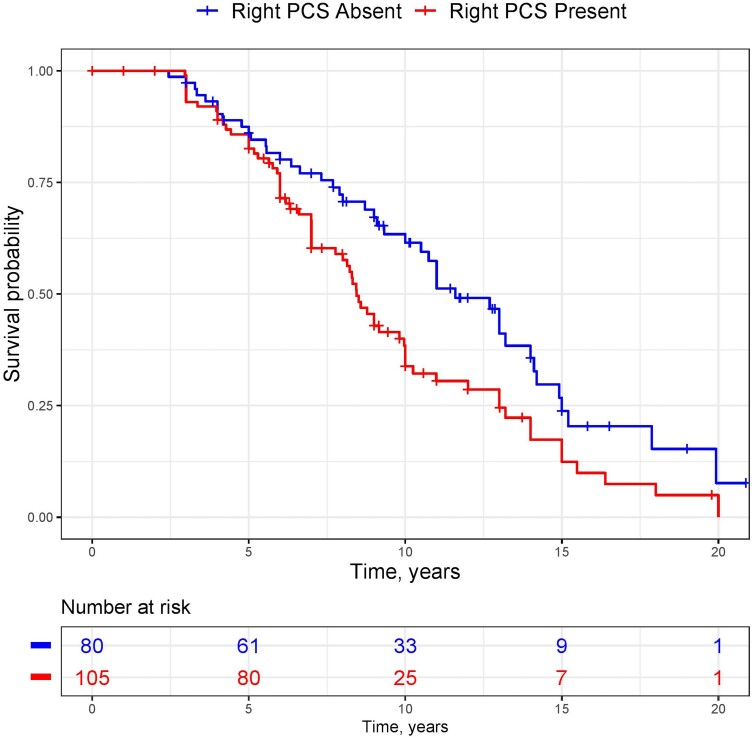
Figure 3.
Kaplan–Meier estimate of survival by right paracingulate sulcal presence. Kaplan–Meier curve for survival in individuals with behavioural variant frontotemporal dementia (n = 185) according to right paracingulate sulcal presence. Red line indicates survival in individuals with a present right paracingulate sulcus (PCS). Blue line represents individuals with an absent right PCS.
Longitudinal FTLD-CDR data were available for 44 individuals with a median follow-up time of 15.83 months. FTLD-CDR sum of boxes and global scores at baseline were similar in individuals with present and absent right PCS after adjusting for age, sex and education (β=−1.02, CI [−2.30–0.27], P = 0.12 and β=−0.24, CI [−0.16–1.89], P = 0.1, respectively). In a linear mixed effects model with fixed effects; age, sex and education, there was a non-significant increase in the rate of clinical disease progression in individuals possessing a right PCS (FTLD-CDR sum of boxes; β=0.26, CI [−0.79–1.32], P = 0.63 and FTLD-CDR global score; β=0.09, CI [−0.11–0.29], P = 0.37). These data are depicted in Supplementary Fig. 2.
Individuals were at a similar clinical disease stage at time of MRI imaging, with no association identified between baseline FTLD-CDR sum of boxes scores and right PCS presence, corrected for time from MRI imaging to CDR-FTLD scoring (β=−0.71, CI [−1.99–0.57], P = 0.27). Twenty-three regions were initially identified with significant differences in cortical thickness according to right PCS presence. All however failed to survive cluster correction for multiple analysis. Cluster details are reported in the Supplementary Tables 2 and 3. In a complementary analysis, cortical thickness differences between groups were investigated based on cortical parcellations according to the Desikan-Killiany atlas,39 traced in regions corresponding of the PCS, displayed in Supplementary Figs 3 and 4. In a multiple linear regression model corrected for age at scan, sex, handedness and years of education, no right hemisphere regions were identified with a significant difference in cortical thickness according to right PCS presence before correction for multiple comparisons. Individuals with a right PCS displayed increased insula cortical thickness (β = 0.1, CI [0.001–0.2], P = 0.048). This result did not however survive correction for multiple analyses.
Discussion
In keeping with Harper et al.,24 this study provides confirmation in a novel, adequately powered cohort of an association between right PCS presence and a later AAO in sporadic bvFTD. Moreover, we demonstrate that this effect is independent of education and that right PCS presence is a prognostic biomarker in sporadic bvFTD, associated with worse survival following AAO. These findings have important consequences; they develop our understanding of the natural history of sporadic bvFTD, give an insight into the implications of neurodevelopmental variability on the expression of a neurodegenerative disease and offer a proxy for brain reserve in bvFTD with potential implications for therapeutic trials.
Reserve theories suggest that individuals possessing adaptable functional brain processes; cognitive reserve, and/or preferential neurobiological capital; and brain reserve possess resilience to the clinical manifestations of a disease despite significant pathological burden.40 Cognitive reserve is a widely accepted concept in AD41-44 where lifetime experiences, including but not limited to educational attainment, are associated with reduced age-specific risk of developing AD.40,45,46 Furthermore, the concept of motor reserve has recently emerged in Parkinson’s disease.47 In FTD, greater occupational attainment,7 degree of occupation8 and active leisure engagement48 have, in some studies, been associated with increased functional and/or structural cerebral impairment despite comparable clinical severity. Furthermore, individuals with greater composite educational and occupational attainment scores process increased brain maintenance, with preservation of frontal anatomical integrity compared to individuals with lower scores.5 Independently, education has also been suggested as a proxy of cognitive reserve in both FTD9-11,48,49 and bvFTD specifically.7 Conversely, others have contested the association in bvFTD50 or restrict this claim to certain disease phenotypes.51 Comparison of studies is difficult due to methodological heterogeneity. Furthermore, there are likely power issues in the published literature. In keeping with Harper et al.24 years of education was not associated with AAO in the present study. As for brain reserve, there is significant support for this model in AD where gross anatomical measures such as head circumference and brain volume are identified proxies.52-54 To the best of our knowledge, a proxy of brain reserve, as defined by Stern et al.,40 has yet to be established in FTD. Results from the present study alongside Harper et al.24 therefore provide evidence for the first proxy of brain reserve in FTD. The rapidity of decline in survival after disease onset in individuals possessing a right PCS is in accordance with reserve theories and the wealth of data in AD, whereby despite initial tolerance of disease burden, following phenoconversion individuals with higher reserve suffer a more rapid rate of clinical decline than individuals with low reserve.25,41,55 This phenomenon has been observed with respect to occupational attainment in bvFTD.56
Longitudinal analysis of clinical disease progression did not reach statistical significance in the present study due to powering, the direction of the observed result however may indicate quicker disease progression in individuals with a right PCS, supporting the rapid decline theory following overload of compensatory mechanisms.
Right PCS presence was not associated with cortical thickness, a surrogate for cortical atrophy in this study. A disconnect between cortical atrophy and clinical disease expression is however described in bvFTD,57,58 suggesting that cortical thickness may not provide an accurate representation of disease burden in all bvFTD sufferers.
The precise neurobiological substrate of reserve remains unknown although greater synaptic density, neuron quantity, brain size, advantageous metabolic properties and increased cerebral blood flow have all been suggested as potential structural and functional underpinnings.5,9,48,49 Functionally, disruption of the salience network, an intrinsic resting state network anchored in the AC, is correlated with clinical severity in bvFTD.50,59,60 PCS presence has been shown to alter the functional architecture of the AC cortex at rest with hemispheres possessing a present PCS displaying enhanced connectivity.61 Furthermore, gyrification is considered to reflect the density of structural neural connectivity, with the degree of cortical folding partially pathway-specific dependent on mechanical tensions.62-66 As such, a salience network topographically and/or structurally altered by the presence of a right PCS may therefore possess resilience to bvFTD.
The right laterality of our findings is relevant for several reasons. The right dorsal ACC (dACC) is active unilaterally, early in decision-making and monitoring of cognitive conflict.67,68 Thus, right but not left dACC could be more closely linked with development of core bvFTD symptoms. Secondly, the salience network is organizationally dominant in the right hemisphere69,70 with multimodal structural and functional imaging studies69-71 identifying stronger and broader intrinsic functional network couplings in the right compared to left dACC. Finally, Von Economo neurons that are selectively targeted in bvFTD are more numerous in the right than left hemisphere.72,73
A non-significant but later AAO in females with sporadic bvFTD74 and all cause bvFTD75 has been reported. Others have demonstrated better than expected executive function in females despite similar levels of atrophy to males.75 In keeping with previous work,24 however, we did not observe a direct association between sex and AAO in sporadic bvFTD. An interaction effect was observed with right PCS presence such that males with a present PCS had a later AAO than other subgroups. As reported by Illan-Gala et al.,75 survival after AAO in the present study was similar in males and females.
This study is subject to limitations, importantly neuropathological diagnostic verification was available in only a minority of individuals. Access to data regarding individual’s presenting symptoms and their phenotypic development was unavailable for this study. It’s recommended that this is addressed in future study preferably aided by neuropsychological testing in relation to PCS presence. Furthermore, the retrospective determination of AAO and assessment of clinical disease severity may be subject to bias. Finally, lifetime exposures with a potential impact on bvFTD onset including but are not limited to occupation, physical exercise and dietary habits were not accounted for in this study but have been considered to impact upon cognitive reserve.40,76
The effect of PCS on disease progression requires further study in a sufficiently powered cohort with neuropathological and longitudinal clinical and radiological data. The impact of PCS presence on disease expression and progression remains unstudied in a genetic bvFTD and is highly indicated given that therapeutic trials of disease-modifying therapies for bvFTD will likely be studied first in genetic cases. The present study identifies that gyrification in a region with a predilection to early and extensive pathological insult by a neurodegenerative disease provides resilience to clinical disease expression. Future study may explore the impact of relevant local gyrification patterns across the spectrum of the neurodegenerative diseases.
Summary
Findings presented in the present study indicate an association between right PCS presence and disease expression and survival in sporadic bvFTD, providing evidence for the first proxy of brain reserve in FTD that may be important in the design of trials for future therapeutic approaches.
Supplementary material
Supplementary material is available at Brain Communications online.
Supplementary Material
Acknowledgements
We express our gratitude towards all participating subjects, their next of kin, all personnel from the four studies contributing with material to this work and the funding bodies.
Contributor Information
Luke Harper, Clinical Memory Research Unit, Department of Clinical Sciences, Lund University, Malmö 20502, Sweden.
Sterre de Boer, Alzheimer Center Amsterdam, Neurology, Vrije Universiteit Amsterdam, Amsterdam UMC location VUmc, Amsterdam 1081 HZ, The Netherlands; Amsterdam Neuroscience, Neurodegeneration, Amsterdam 1105 BA, The Netherlands.
Olof Lindberg, Division of Clinical Geriatrics, Karolinska Institute, Stockholm 17165, Sweden.
Jimmy Lätt, Centre for Medical Imaging and Physiology, Skane University Hospital, Lund 22242, Sweden.
Nicholas Cullen, Clinical Memory Research Unit, Department of Clinical Sciences, Lund University, Malmö 20502, Sweden.
Lyles Clark, Penn Frontotemporal Degeneration Center (FTDC), University of Pennsylvania, Philadelphia, PA 19104, USA; Department of Neurology, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA 19104, USA.
David Irwin, Penn Frontotemporal Degeneration Center (FTDC), University of Pennsylvania, Philadelphia, PA 19104, USA; Department of Neurology, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA 19104, USA.
Lauren Massimo, Penn Frontotemporal Degeneration Center (FTDC), University of Pennsylvania, Philadelphia, PA 19104, USA; Department of Neurology, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA 19104, USA.
Murray Grossman, Penn Frontotemporal Degeneration Center (FTDC), University of Pennsylvania, Philadelphia, PA 19104, USA; Department of Neurology, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA 19104, USA.
Oskar Hansson, Clinical Memory Research Unit, Department of Clinical Sciences, Lund University, Malmö 20502, Sweden; Memory Clinic, Skåne University Hospital, Malmö 22100, Sweden.
Yolande Pijnenburg, Alzheimer Center Amsterdam, Neurology, Vrije Universiteit Amsterdam, Amsterdam UMC location VUmc, Amsterdam 1081 HZ, The Netherlands; Amsterdam Neuroscience, Neurodegeneration, Amsterdam 1105 BA, The Netherlands.
Corey T McMillan, Penn Frontotemporal Degeneration Center (FTDC), University of Pennsylvania, Philadelphia, PA 19104, USA; Department of Neurology, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA 19104, USA.
Alexander F Santillo, Clinical Memory Research Unit, Department of Clinical Sciences, Lund University, Malmö 20502, Sweden.
Funding
Work at the Clinical Memory Research Unit Lund University was supported by the Swedish Research Council (2022-00775), the Knut and Alice Wallenberg foundation (2017-0383), the Strategic Research Area MultiPark (Multidisciplinary Research in Parkinson’s disease) at Lund University, the Swedish Alzheimer Foundation (AF-980907), the Swedish Brain Foundation (FO2021-0293), The Parkinson foundation of Sweden (1412/22), the Cure Alzheimer’s fund, the Konung Gustaf V:s och Drottning Victorias Frimurarestiftelse, the Skåne University Hospital Foundation (2020-O000028), Regionalt Forskningsstöd (2022-1259) and the Swedish federal government under the Avtal om Läkarutbildning och Forskning (ALF) agreement (2022-Projekt0080). L.H., A.F.S. and O.L. are all supported by The Schörling foundation. A.F.S. and L.H. are funded primarily by the Swedish federal government under the Avtal om Läkarutbildning och Forskning (ALF) agreement (ALF YF 2023-2026/0017 and ALF ST 2021-2023/4-43338, respectively).
Work at the University of Pennsylvania was supported by National Institute of Health grants 5P01AG066597-03 6308 (C.T.M.), 5R01NS109260-04 (D.I.) and 1R01AG076832-01A1 (L.M.).
The funding sources had no role in the design and conduct of the study; in the collection, analysis and interpretation of the data; or in the preparation, review or approval of the manuscript.
Competing interests
O.H. has acquired research support (for the institution) from ADx, AVID Radiopharmaceuticals, Biogen, Eli Lilly, Eisai, Fujirebio, GE Healthcare, Pfizer and Roche. In the past 2 years, he has received consultancy/speaker fees from AC Immune, Amylyx, Alzpath, BioArctic, Biogen, Cerveau, Eisai, Fujirebio, Genentech, Novartis, Novo Nordisk, Roche and Siemens.
Data availability
Anonymized data will be shared by request from a qualified academic investigator for the sole purpose of replicating procedures and results presented in the article if data transfer is in agreement with relevant legislation on the general data protection regulation and decisions and by the relevant Ethical Review Boards, which should be regulated in a material transfer agreement.
References
- 1. Greaves CV, Rohrer JD. An update on genetic frontotemporal dementia. J Neurol. 2019;266(8):2075–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gazzina S, Grassi M, Premi E, et al. Education modulates brain maintenance in presymptomatic frontotemporal dementia. J Neurol Neurosurg Psychiatry. 2019;90(10):1124–1130. [DOI] [PubMed] [Google Scholar]
- 3. Onyike CU, Diehl-Schmid J. The epidemiology of frontotemporal dementia. Int Rev Psychiatry. 2013;25(2):130–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gallagher MD, Suh E, Grossman M, et al. TMEM106B is a genetic modifier of frontotemporal lobar degeneration with C9orf72 hexanucleotide repeat expansions. Acta Neuropathol. 2014;127(3):407–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Placek K, Massimo L, Olm C, et al. Cognitive reserve in frontotemporal degeneration: Neuroanatomic and neuropsychological evidence. Neurology. 2016;87(17):1813–1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Heuer HW, Wang P, Rascovsky K, et al. Comparison of sporadic and familial behavioral variant frontotemporal dementia (FTD) in a North American cohort. Alzheimers Dement. 2020;16(1):60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Borroni B, Premi E, Agosti C, et al. Revisiting brain reserve hypothesis in frontotemporal dementia: Evidence from a brain perfusion study. Dement Geriatr Cogn Disord. 2009;28(2):130–135. [DOI] [PubMed] [Google Scholar]
- 8. Dodich A, Carli G, Cerami C, Iannaccone S, Magnani G, Perani D. Social and cognitive control skills in long-life occupation activities modulate the brain reserve in the behavioural variant of frontotemporal dementia. Cortex. 2018;99:311–318. [DOI] [PubMed] [Google Scholar]
- 9. Perneczky R, Diehl-Schmid J, Drzezga A, Kurz A. Brain reserve capacity in frontotemporal dementia: A voxel-based 18F-FDG PET study. Eur J Nucl Med Mol Imaging. 2007;34(7):1082–1087. [DOI] [PubMed] [Google Scholar]
- 10. Premi E, Cristillo V, Gazzina S, et al. Expanding the role of education in frontotemporal dementia: A functional dynamic connectivity (the chronnectome) study. Neurobiol Aging. 2020;93:35–43. [DOI] [PubMed] [Google Scholar]
- 11. Premi E, Garibotto V, Alberici A, et al. Nature versus nurture in frontotemporal lobar degeneration: The interaction of genetic background and education on brain damage. Dement Geriatr Cogn Disord. 2012;33(6):372–378. [DOI] [PubMed] [Google Scholar]
- 12. Seeley WW, Crawford R, Rascovsky K, et al. Frontal paralimbic network atrophy in very mild behavioral variant frontotemporal dementia. Arch Neurol. 2008;65(2):249–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Armstrong E, Schleicher A, Omran H, Curtis M, Zilles K. The ontogeny of human gyrification. Cereb Cortex. 1995;5(1):56–63. [DOI] [PubMed] [Google Scholar]
- 14. Yücel M, Stuart GW, Maruff P, et al. Hemispheric and gender-related differences in the gross morphology of the anterior cingulate/paracingulate cortex in normal volunteers: An MRI morphometric study. Cereb Cortex. 2001;11(1):17–25. [DOI] [PubMed] [Google Scholar]
- 15. Yücel M, Stuart GW, Maruff P, et al. Paracingulate morphologic differences in males with established schizophrenia: A magnetic resonance imaging morphometric study. Biol Psychiatry. 2002;52(1):15–23. [DOI] [PubMed] [Google Scholar]
- 16. Paus T, Tomaiuolo F, Otaky N, et al. Human cingulate and paracingulate sulci: Pattern, variability, asymmetry, and probabilistic map. Cereb Cortex. 1996;6(2):207–214. [DOI] [PubMed] [Google Scholar]
- 17. Le Provost JB, Bartres-Faz D, Paillere-Martinot ML, et al. Paracingulate sulcus morphology in men with early-onset schizophrenia. Br J Psychiatry. 2003;182:228–232. [DOI] [PubMed] [Google Scholar]
- 18. Fornito A, Yücel M, Wood S, et al. Individual differences in anterior cingulate/paracingulate morphology are related to executive functions in healthy males. Cerebral Cortex. 2004;14(4):424–431. [DOI] [PubMed] [Google Scholar]
- 19. Whittle S, Allen NB, Fornito A, et al. Variations in cortical folding patterns are related to individual differences in temperament. Psychiatry Res. 2009;172(1):68–74. [DOI] [PubMed] [Google Scholar]
- 20. Fedeli D, Del Maschio N, Del Mauro G, Defendenti F, Sulpizio S, Abutalebi J. Cingulate cortex morphology impacts on neurofunctional activity and behavioral performance in interference tasks. Sci Rep. 2022;12(1):13684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tissier C, Linzarini A, Allaire-Duquette G, et al. Sulcal polymorphisms of the IFC and ACC contribute to inhibitory control variability in children and adults. eNeuro. 2018;5(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Borst G, Cachia A, Vidal J, et al. Folding of the anterior cingulate cortex partially explains inhibitory control during childhood: A longitudinal study. Dev Cogn Neurosci. 2014;9:126–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Huster RJ, Wolters C, Wollbrink A, et al. Effects of anterior cingulate fissurization on cognitive control during stroop interference. Hum Brain Mapp. 2009;30(4):1279–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Harper L, Lindberg O, Bocchetta M, et al. Prenatal gyrification pattern affects age at onset in frontotemporal dementia. Cerebral Cortex. 2022;32(18):3937–3944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sun J, De Winter F-L, Kumfor F, et al. Neural compensation in manifest neurodegeneration: Systems neuroscience evidence from social cognition in frontotemporal dementia. J Neurol. 2022;270(1):538–547. [DOI] [PubMed] [Google Scholar]
- 26. van der Flier WM, Scheltens P. Amsterdam Dementia Cohort: Performing research to optimize care. J Alzheimers Dis. 2018;62(3):1091–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rascovsky K, Hodges JR, Knopman D, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134(Pt 9):2456–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wood EM, Falcone D, Suh E, et al. Development and validation of pedigree classification criteria for frontotemporal lobar degeneration. JAMA Neurol. 2013;70(11):1411–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Goldman JS, Farmer JM, Wood EM, et al. Comparison of family histories in FTLD subtypes and related tauopathies. Neurology. 2005;65(11):1817–1819. [DOI] [PubMed] [Google Scholar]
- 30. Erkoyun H U, Groot C, Heilbron R, et al. A clinical-radiological framework of the right temporal variant of frontotemporal dementia. Brain. 2020;143(9):2831–2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Strong MJ, Abrahams S, Goldstein LH, et al. Amyotrophic lateral sclerosis—Frontotemporal spectrum disorder (ALS-FTSD): Revised diagnostic criteria. Amyotroph Lateral Scler Frontotemporal Degener. 2017;18(3-4):153–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97(20):11050–11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Garrison JR, Fernyhough C, McCarthy-Jones S, Haggard M, Simons JS. Paracingulate sulcus morphology is associated with hallucinations in the human brain. Nat Commun. 2015;6:8956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ono M, Kubik S, Abernathey CD. Atlas of the cerebral sulci. 1990.
- 35. Del Maschio N, Sulpizio S, Fedeli D, et al. ACC sulcal patterns and their modulation on cognitive control efficiency across lifespan: A neuroanatomical study on bilinguals and monolinguals. Cereb Cortex. 2019;29(7):3091–3101. [DOI] [PubMed] [Google Scholar]
- 36. Knopman DS, Kramer JH, Boeve BF, et al. Development of methodology for conducting clinical trials in frontotemporal lobar degeneration. Brain. 2008;131(11):2957–2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lima-Silva TB, Mioshi E, Bahia VS, et al. Disease progression in frontotemporal dementia and Alzheimer disease: The contribution of staging scales. J Geriatr Psychiatry Neurol. 2021;34(5):397–404. [DOI] [PubMed] [Google Scholar]
- 38. Hagler DJ J, Saygin AP, Sereno MI. Smoothing and cluster thresholding for cortical surface-based group analysis of fMRI data. Neuroimage. 2006;33(4):1093–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Desikan RS, Ségonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–980. [DOI] [PubMed] [Google Scholar]
- 40. Stern Y, Arenaza-Urquijo EM, Bartrés-Faz D, et al. Whitepaper: Defining and investigating cognitive reserve, brain reserve, and brain maintenance. Alzheimers Dement. 2020;16(9):1305–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Stern Y. Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol. 2012;11(11):1006–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lee DH, Lee P, Seo SW, et al. Neural substrates of cognitive reserve in Alzheimer’s disease spectrum and normal aging. Neuroimage. 2019;186:690–702. [DOI] [PubMed] [Google Scholar]
- 43. Kemppainen NM, Aalto S, Karrasch M, et al. Cognitive reserve hypothesis: Pittsburgh Compound B and fluorodeoxyglucose positron emission tomography in relation to education in mild Alzheimer’s disease. Ann Neurol. 2008;63(1):112–118. [DOI] [PubMed] [Google Scholar]
- 44. Bosch B, Bartrés-Faz D, Rami L, et al. Cognitive reserve modulates task-induced activations and deactivations in healthy elders, amnestic mild cognitive impairment and mild Alzheimer’s disease. Cortex. 2010;46(4):451–461. [DOI] [PubMed] [Google Scholar]
- 45. van Loenhoud AC, Wink AM, Groot C, et al. A neuroimaging approach to capture cognitive reserve: Application to Alzheimer’s disease. Hum Brain Mapp. 2017;38(9):4703–4715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Groot C, van Loenhoud AC, Barkhof F, et al. Differential effects of cognitive reserve and brain reserve on cognition in Alzheimer disease. Neurology. 2018;90(2):e149–e156. [DOI] [PubMed] [Google Scholar]
- 47. Chung SJ, Kim HR, Jung JH, Lee PH, Jeong Y, Sohn YH. Identifying the functional brain network of motor reserve in early Parkinson’s disease. Mov Disord. 2020;35(4):577–586. [DOI] [PubMed] [Google Scholar]
- 48. Maiovis P, Ioannidis P, Gerasimou G, Gotzamani-Psarrakou A, Karacostas D. Cognitive reserve hypothesis in frontotemporal dementia: Evidence from a brain SPECT study in a series of Greek frontotemporal dementia patients. Neurodegener Dis. 2018;18(2-3):69–73. [DOI] [PubMed] [Google Scholar]
- 49. Beyer L, Meyer-Wilmes J, Schönecker S, et al. Cognitive reserve hypothesis in frontotemporal dementia: A FDG-PET study. Neuroimage Clin. 2021;29:102535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kim SH, Kim YJ, Lee BH, et al. Behavioral reserve in behavioral variant frontotemporal dementia. Front Aging Neurosci. 2022;14:875589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Premi E, Garibotto V, Gazzina S, et al. Beyond cognitive reserve: Behavioural reserve hypothesis in frontotemporal dementia. Behav Brain Res. 2013;245:58–62. [DOI] [PubMed] [Google Scholar]
- 52. Graves AB, Mortimer JA, Larson EB, Wenzlow A, Bowen JD, McCormick WC. Head circumference as a measure of cognitive reserve. Association with severity of impairment in Alzheimer’s disease. Br J Psychiatry. 1996;169(1):86–92. [DOI] [PubMed] [Google Scholar]
- 53. Perneczky R, Wagenpfeil S, Lunetta KL, et al. Head circumference, atrophy, and cognition: Implications for brain reserve in Alzheimer disease. Neurology. 2010;75(2):137–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Schofield PW, Logroscino G, Andrews HF, Albert S, Stern Y. An association between head circumference and Alzheimer’s disease in a population-based study of aging and dementia. Neurology. 1997;49(1):30–37. [DOI] [PubMed] [Google Scholar]
- 55. Barulli D, Stern Y. Efficiency, capacity, compensation, maintenance, plasticity: Emerging concepts in cognitive reserve. Trends Cogn Sci. 2013;17(10):502–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Massimo L, Xie SX, Rennert L, et al. Occupational attainment influences longitudinal decline in behavioral variant frontotemporal degeneration. Brain Imaging Behav. 2019;13(1):293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Davies RR, Kipps CM, Mitchell J, Kril JJ, Halliday GM, Hodges JR. Progression in frontotemporal dementia: Identifying a benign behavioral variant by magnetic resonance imaging. Arch Neurol. 2006;63(11):1627–1631. [DOI] [PubMed] [Google Scholar]
- 58. Devenney E, Bartley L, Hoon C, et al. Progression in behavioral variant frontotemporal dementia: A longitudinal study. JAMA Neurol. 2015;72(12):1501–1509. [DOI] [PubMed] [Google Scholar]
- 59. Zhou J, Greicius MD, Gennatas ED, et al. Divergent network connectivity changes in behavioural variant frontotemporal dementia and Alzheimer’s disease. Brain. 2010;133(Pt 5):1352–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Day GS, Farb NA, Tang-Wai DF, et al. Salience network resting-state activity: Prediction of frontotemporal dementia progression. JAMA Neurol. 2013;70(10):1249–1253. [DOI] [PubMed] [Google Scholar]
- 61. Fedeli D, Del Maschio N, Caprioglio C, Sulpizio S, Abutalebi J. Sulcal pattern variability and dorsal anterior cingulate cortex functional connectivity across adult age. Brain Connect. 2020;10(6):267–278. [DOI] [PubMed] [Google Scholar]
- 62. Welker W. Why does cerebral cortex fissure and fold? In: Jones EG and Peters A, eds. Cerebral cortex: Comparative structure and evolution of cerebral cortex, part II. Springer US; 1990:3–136. [Google Scholar]
- 63. Van Essen DC. A tension-based theory of morphogenesis and compact wiring in the central nervous system. Nature. 1997;385(6614):313–318. [DOI] [PubMed] [Google Scholar]
- 64. Van Essen DC. A 2020 view of tension-based cortical morphogenesis. Proc Natl Acad Sci U S A. 2020;117(52):32868–32879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hilgetag CC, Barbas H. Role of mechanical factors in the morphology of the primate cerebral cortex. PLoS Comput Biol. 2006;2(3):e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Toro R, Burnod Y. A morphogenetic model for the development of cortical convolutions. Cereb Cortex. 2005;15(12):1900–1913. [DOI] [PubMed] [Google Scholar]
- 67. Lütcke H, Frahm J. Lateralized anterior cingulate function during error processing and conflict monitoring as revealed by high-resolution fMRI. Cereb Cortex. 2008;18(3):508–515. [DOI] [PubMed] [Google Scholar]
- 68. Weiss AR, Gillies MJ, Philiastides MG, et al. Dorsal anterior cingulate cortices differentially lateralize prediction errors and outcome valence in a decision-making task. Front Hum Neurosci. 2018;12:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Zhang Y, Suo X, Ding H, Liang M, Yu C, Qin W. Structural connectivity profile supports laterality of the salience network. Hum Brain Mapp. 2019;40(18):5242–5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Seeley WW, Menon V, Schatzberg AF, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27(9):2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Cauda F, D'Agata F, Sacco K, Duca S, Geminiani G, Vercelli A. Functional connectivity of the insula in the resting brain. Neuroimage. 2011;55(1):8–23. [DOI] [PubMed] [Google Scholar]
- 72. Allman JM, Tetreault NA, Hakeem AY, et al. The von Economo neurons in frontoinsular and anterior cingulate cortex in great apes and humans. Brain Struct Funct. 2010;214(5-6):495–517. [DOI] [PubMed] [Google Scholar]
- 73. González-Acosta CA, Escobar MI, Casanova MF, Pimienta HJ, Buriticá E. Von economo neurons in the human medial frontopolar cortex. Front Neuroanat. 2018;12:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. de Boer SCM, Riedl L, van der Lee SJ, et al. Differences in sex distribution between genetic and sporadic frontotemporal dementia. J Alzheimers Dis. 2021;84(3):1153–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Illan-Gala I, Casaletto KB, Borrego-Ecija S, et al. Sex differences in the behavioral variant of frontotemporal dementia: A new window to executive and behavioral reserve. Alzheimers Dement. 2021;17(8):1329–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Perneczky R, Kempermann G, Korczyn AD, et al. Translational research on reserve against neurodegenerative disease: Consensus report of the International Conference on Cognitive Reserve in the Dementias and the Alzheimer’s Association Reserve, Resilience and Protective Factors Professional Interest Area working groups. BMC Med. 2019;17(1):47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymized data will be shared by request from a qualified academic investigator for the sole purpose of replicating procedures and results presented in the article if data transfer is in agreement with relevant legislation on the general data protection regulation and decisions and by the relevant Ethical Review Boards, which should be regulated in a material transfer agreement.