Abstract
AY 9944 [AY; trans-1,4-bis(chlorobenzylaminomethyl)-cyclohexane dihydrochloride], an inhibitor of sterol synthesis, was found to help restore the normal mitogenic responses and cytokine profiles of peripheral mononuclear cells (PBMCs) from AIDS patients in vitro. Compared to untreated cells, the human immunodeficiency virus type 1 (HIV-1)-infected PBMCs precultured in the presence of AY exhibited a normal rate of either mitogen-induced or recall- and superantigen-induced proliferation. After 2 weeks in the presence of the drug, the percentage of dead CD4+ cells in HIV-1-infected cultures was comparable to that observed in uninfected cultures, while over the same time interval it increased by three- to fivefold in HIV-1-infected cultures maintained in the absence of AY. AY also stimulated by 2- to 12-fold interleukin-12 (IL-12) and (gamma interferon production. For IL-12, this effect appears to be related to an increase in corresponding IL-12 p35 and IL-12 p40 mRNA levels. Moreover, AY restored the expression of the IL-2 receptor, which was severely impaired in HIV-1-infected PBMCs. Although the drug has no direct antiviral effect (it does not significantly inhibit reverse transcriptase activity measured in vitro), it might be considered a potential therapeutic agent for HIV-infected patients, in that it may correct viral infection-related immune system defects by indirectly enhancing the level of resistance to HIV and opportunistic infections.
The consequence of infection with human immunodeficiency virus type 1 (HIV-1) is a remarkable loss of cell-mediated functions. A mechanism that is a determinant of the immune suppression in patients with AIDS is the depletion of circulating CD4+ T cells, which play a central role in the regulation of the immune system. Thus, the progression of the disease in HIV-1-infected patients is characterized at both the early and the late stages by a marked dysregulation of the immune system (14, 17). The virus infects CD4+ cells, inducing the lysis of infected lymphocytes and the subsequent release of virions. However, the direct killing of infected cells cannot by itself account for the progressive immunodeficiency observed in patients with AIDS. HIV-1 infection is also characterized by an impairment of the immunoregulatory network. This dysregulation is in particular illustrated by important decreases in the levels of interleukin-2 (IL-2) and IL-12 production (17).
Various experimental approaches to protecting against CD4+ cell death resulting from virus infection in AIDS patients are under investigation. Anti-HIV compounds that target the viral enzymes responsible for replication are the most widely used at present (19, 26, 39). Another strategy is based on the enhancement of cellular immunity (20, 41), but it remains to be demonstrated whether the immune response against HIV could actually prevent the progression of the disease. Finally, tentative studies that positively modulate the impaired cytokine network with exogenous IL-2 have been conducted (21, 38).
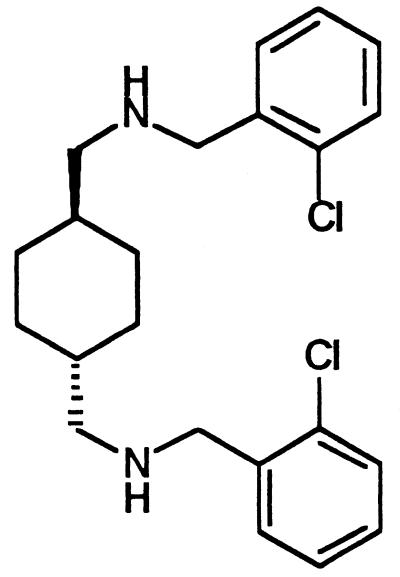
Here we report that AY 9944 (AY; Fig. 1), a cationic amphiphilic compound previously known as a potent inhibitor of cholesterol synthesis (9, 16), partially suppresses HIV replication. More importantly, this drug appears to restore the multiplication of T cells by regenerating CD4+ cells and to enhance the level of expression of the IL-2 α receptor (CD25). AY also markedly stimulates the production of IL-12 in response to activation by increasing the level of expression of IL-12 mRNA (IL-12 p35 and IL-12 p40 chains). Thus, in addition to treatment with anti-HIV compounds, the use of AY-based treatment that restores the cell-mediated functions could offer a complementary approach to AIDS therapy.
FIG. 1.
Chemical structure of the cationic amphiphilic drug AY.
MATERIALS AND METHODS
Chemicals.
The AY molecule (Fig. 1) (molecular weight, 495) was synthesized and provided by Panchim (Evry, France).
Isolation of lymphoid cells and culture conditions.
The study was performed in accordance with local ethical committee standards. Heparinized venous peripheral blood was obtained from consenting HIV-1-seropositive adults with CD4+ T cell counts ranging from 50 to 800/mm3 and from healthy HIV-seronegative controls who signed an informed consent form. Patients were classified according to their absolute CD4+ cell counts (group M, <200/mm3; group S, >500/mm3). Blood samples from healthy HIV-seronegative subjects (group C) were included as controls. Peripheral blood mononuclear cells (PBMCs) were isolated from heparinized venous blood by Ficoll-Hypaque (Histopaque; Sigma, Irvine, United Kingdom) density gradient centrifugation. Cell aliquots were stored in liquid nitrogen in fetal calf serum with 10% dimethyl sulfoxide (1). Frozen cells were cultured in RPMI 1640 medium (Sigma, Irvine, United Kingdom) supplemented with 10% heat-inactivated fetal calf serum (Seromed, Berlin, Germany), 2 mM l-glutamine (Sigma, Irvine, United Kingdom), 1 mM sodium pyruvate, and antibiotics (100 U of penicillin per ml, 100 μg of streptomycin per ml; Sigma, Irvine, United Kingdom). PBMCs were activated with phytohemagglutinin (PHA purified; 3 μg/ml; Sigma) for 3 days. Thereafter, the cells were washed and cultured in the presence or in the absence of 3 × 10−6 M AY in complete medium supplemented with recombinant IL-2 (50 U/ml; Roussel Uclaff, Romainville, France). Cell cultures consisted of 8 × 105 viable cells per ml cultured for 3 days. The cells in the cultures were grown at 37°C in vented upright Costar 3065 flasks containing 5 ml of culture medium. Cells were regularly subcultured in this way over a long period of time. Cell survival, evaluated by the Trypan blue exclusion test, was expressed as the ratio [(final viable cell count − initial viable cell count)/(initial cell count)] × 100.
Lymphocyte proliferation.
PBMCs collected from a patient’s blood were suspended in culture medium (RPMI 1640 medium supplemented with 10% heat-inactivated normal human type AB serum (Institut Jacques Boy, Reims, France) at 2.5 × 106 cells per ml. A total of 100 μl of the cell suspension was added to 100 μl of culture medium containing staphylococcal enterotoxin B (SEB) superantigen (0.1 μg/ml; Sigma, St. Louis, Mo.), purified protein derivative (PPD; 3,000 U/ml), or tetanus toxoid (TT; 1,800 U/ml) (Institut Mérieux, Lyon, France) in the presence or in the absence of the drug. Plates were incubated for 6 days, and during the last 18 h the level of [3H]thymidine (0.5 μCi/well; Amersham Life Science, Buckinghamshire, United Kingdom) incorporation into DNA was measured. All determinations were done in quadriplicate.
Cytokine detection.
Cell-free supernatants were collected and assayed for IL-12 and gamma interferon contents with commercial enzyme-linked immunosorbent assay kits purchased from R&D Systems (Barton Lane Abingdon, United Kingdom).
Cytokine mRNA detection.
Total RNA was extracted from activated peripheral blood cells cultured in the presence or in the absence of AY with an RNA isolation kit (RNAzol; WAK-chemie Medical, Bad Hambourg, Germany) according to the manufacturer’s instructions. The RNA pellet was resuspended in 50 μl of diethylpyrocarbonate-treated distilled water containing 1 mM dithiothreitol (Sigma, St. Louis, Mo.), 5 U of RNasin RNase inhibitor (RNAsin; Promega, Madison, Wis.), and 1 μg of tRNA (Sigma, St. Louis, Mo.). Reverse transcription of cellular RNA into cDNA was performed with Moloney murine leukemia virus reverse transcriptase and subsequent amplification by Taq polymerase treatment of the IL-12 sequences (primers IL-12-p35 [plus strand; 5′-CATGCTTTCAGAATTCGGGC-3′] and IL-12-p35 [minus strand; 5′-GTTAGCTCAGATGCTTTCATG-3′] and primers IL-12-p40 [plus strand; 5′-CCCTGACACCTGGAGTACTC-3′] and IL-12-p40 [minus strand; 5′-GGCTATACCATGAAGCCTAG-3′]). The entire reverse transcription reaction mixture was used in a 100-μl PCR assay mixture. The PCR products were analyzed by electrophoresis on a 1% agarose gel. The amplified PCR fragment was visualized by ethidium bromide staining. All of the cytokine PCR products were normalized according to the amount of the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) detected in the same mRNA sample.
Immunofluorescence staining and flow cytometry.
T-lymphocyte phenotyping was carried out by flow cytometry. For studies of the surface markers, the cells were stained with a phycoerythrin-conjugated anti-CD25 monoclonal antibody (MAb), an anti-CD8 MAb, an anti-CD3 MAb, an fluorescein isothiocyanate-conjugated anti-CD4 MAb, and an anti-CD19 MAb (Becton Dickinson, San Jose, Calif.) for 30 min at 4°C by following the manufacturer’s instructions and were washed twice. The washed cells were analyzed on a flow cytometer (fluorescence-activated cell sorter [FACS]; Becton Dickinson Immunocytometry Systems, San Jose, Calif.).
HIV p24 production.
The production of p24 antigen was measured by an enzyme-linked immunoassay (Abbott Laboratories, North Chicago, Ill.).
Reverse transcriptase assays.
Culture supernatants (1 ml) were clarified by centrifugation through a Millipore membrane (HAWP; pore size, 0.45 μm) and stored at −80°C until they were assayed. The reverse transcriptase activity was measured as described previously (8). Briefly, the virus pellets were resuspended in 0.02 ml of 0.05 M Tris-HCl–0.3 M KCl–0.0014 M dithiothreitol (pH 7.5)–0.015 μg of polyadenylic acid per ml–15 μg of oligothymidylic acid d(pT) 12–18 per ml–3 μCi of [3H]TTP. After 1 h of incubation at 37°C, the reaction was stopped by the addition of 1 ml of 5% trichloroacetic acid containing 0.1 mM sodium pyrophosphate and 0.25 ml of yeast tRNA (0.5 mg/ml), and the mixture was precipitated in 3.5 ml of 20% trichloroacetic acid. The radioactivity incorporated as trichloroacetic acid-precipitable material was measured by scintillation counting with a β counter instrument (Betamatic; Kontron Instruments, Trappes, France). The results were expressed as counts per minute per milliliter of culture medium.
Statistics.
All results are expressed as means ± standard deviations (SDs). Comparisons were done by Student’s t test. A P value of <0.05 was considered significant.
RESULTS
Effect of AY on mitogen-induced proliferative responses of PBMCs.
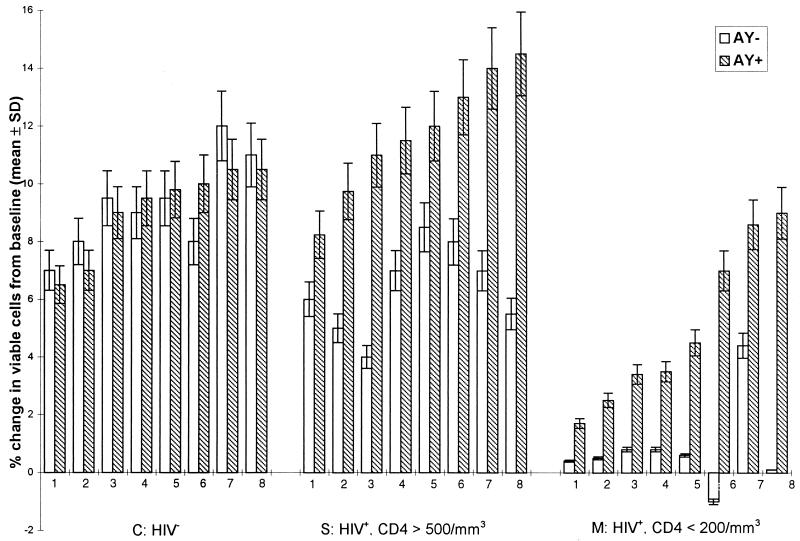
AY was initially developed as a cholesterol synthesis inhibitor. At high concentrations it induces teratogenicity in vivo and cell lethality in vitro (24, 33). Thus, we chose the nontoxic dose of 3 μM to investigate the action of the drug on cultured cells. To assess the effect of AY on the immune response, we first investigated the proliferative responses of PBMCs isolated either from individuals infected with HIV-1 or from healthy seronegative donors after in vitro activation with mitogens such as PHA. The lymphocytes were cultured in complete medium supplemented with recombinant IL-2. The data are presented in Fig. 2. After 15 days of culture in the absence of the drug, it appears that the rate of survival of the PBMCs originating from eight naturally infected donors with clinical symptoms (CD4+ T-cell counts, <200/mm3) was significantly lower than that of the PBMCs obtained from the eight healthy donors used as controls. When the PBMCs were cultured in the presence of the 3 × 10−6 M AY, the proliferative responses of the PBMCs from symptomatic or asymptomatic donors were markedly enhanced compared to the responses of untreated PBMCs (P < 0.001 by Student’s t test). The percent changes (means ± SDs) in the numbers of viable cells in cultures conditioned with AY compared to the numbers in untreated cultures were 887% ± 122% versus 920% ± 160%, 637% ± 153% versus 1,181% ± 220%, and 96% ± 141% versus 502% ± 281% for the control group, the asymptomatic donors (group S) and the patients with AIDS (group M), respectively. By contrast, for PBMCs from noninfected donors, AY had no significant effect on the proliferative responses.
FIG. 2.
Effect of AY on survival (percent change in viable cells from that at the baseline) of PBMCs stimulated with PHA and cultured in complete medium containing recombinant IL-2 in the presence (AY+) or in the absence (AY−) of the drug (3 × 10−6 M). Data represent cell proliferation in 15-day cultures after stimulation of the cells in the absence or in the presence of AY. The cells were derived from three groups of donors: eight healthy HIV-1-seronegative individuals (group C) (HIV−), eight HIV-1-seropositive asymptomatic individuals (group S; CD4+ cell count, >500/mm3), and eight AIDS (symptomatic) patients (group M; CD4+ cell count, <200/mm3). Results are expressed as percent (10−2; means ± SDs of three experimental values).
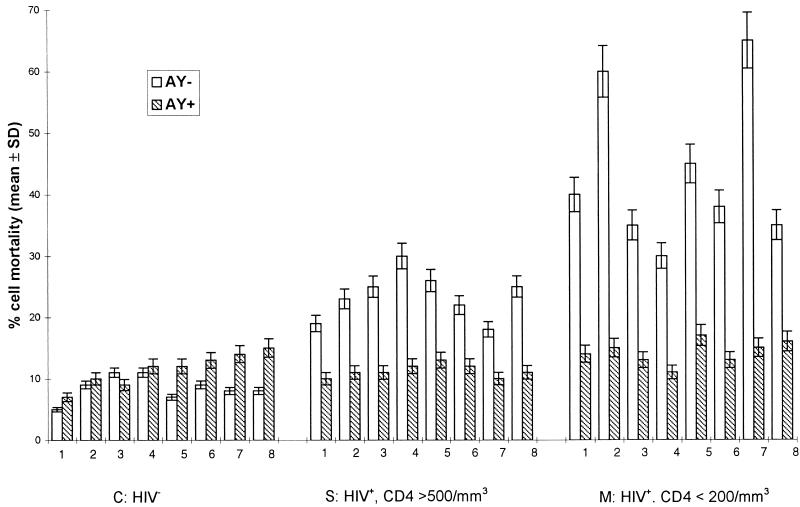
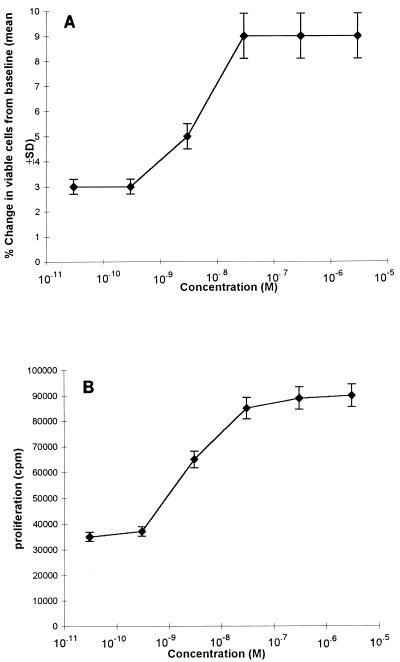
As shown in Fig. 3, under these experimental conditions, the percentage of living cells recovered in cultures derived from seropositive individuals progressively decreased as a consequence of HIV infection: indeed, the percent cell mortality increased from 27.5% ± 7% for PBMCs originating from asymptomatic donors to 47.5% ± 15% for PBMCs from patients with AIDS. In contrast, when these cells were cultured in complete medium supplemented with AY, the lytic effect remained low and the rate of cell mortality was comparable to that for cells derived from seronegative individuals. This effect was maintained over 5 weeks (data not shown). This beneficial effect of AY on cell survival and on the antigen-induced proliferation of PBMCs obtained from HIV-1-infected donors was dose dependent, as indicated in Fig. 4.
FIG. 3.
Effect of AY 9944 on PBMC mortality. The experimental conditions are the same as those described in the legend to Fig. 2. Results are expressed as the percentage of dead cells after 2 weeks of culture, as evaluated by the Trypan blue exclusion test (means ±SDs of three experimental values).
FIG. 4.
Effect of AY on the survival (A) and superantigen-induced proliferation (B) of PBMCs as a function of the drug concentration. PBMCs were obtained from one representative symptomatic AIDS patient and were stimulated with 0.1 μg of SEB per ml, as described in the legend to Fig. 2. The percent change (10−2) in viable cells from that at the baseline was measured after 15 days of culture. Cell proliferation was determined by measuring the level of [3H]thymidine uptake at 6 days after SEB activation. The results are means ± SDs of three experimental values.
Effect of AY on recall antigen- and SEB-induced proliferative responses of PBMCs.
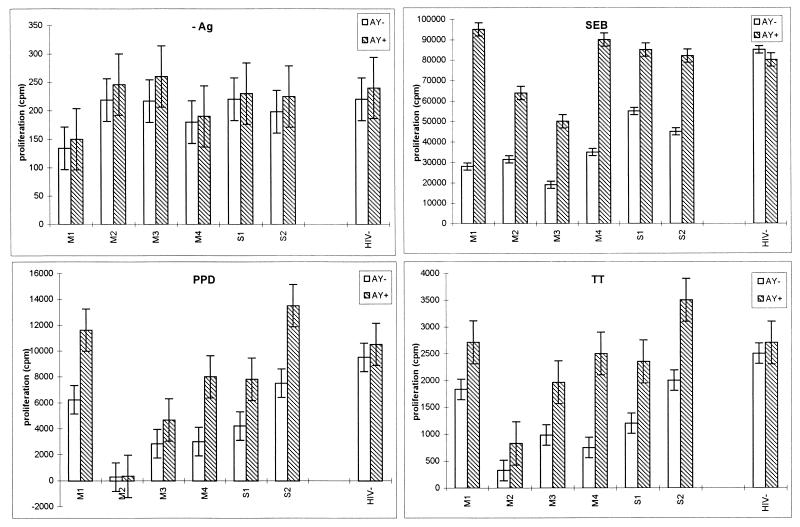
To determine whether AY would also increase T-helper-cell functions with response to recall antigens such as PPD, TT, or superantigen SEB, we stimulated the PBMCs from one healthy HIV-1-seronegative donor, two asymptomatic HIV-1-infected donors (group S), and three patients with AIDS (group M) with these antigens. The data presented in Fig. 5 illustrate that the proliferative responses to SEB of the PBMCs from the five HIV-seropositive individuals were increased two- to fourfold by AY and their TT and PPD responses were increased approximately twofold. By contrast, the intact antigen responses of PBMCs from healthy donors were not significantly affected by AY, which suggests that the drug selectively enhances the deficient responses of the PBMCs from HIV-seropositive individuals. Finally, Fig. 5 also shows that AY does not modify cell proliferation in unstimulated cultures.
FIG. 5.
Human peripheral blood T-helper-cell responses to recall antigens (PPD, TT) and to superantigen (SEB) in the absence (AY−) or in the presence (AY+) of 3 × 10−6 M AY. −Ag, no antigen. The proliferation was studied with PBMCs from six HIV positive subjects (subjects S1 and S2, [CD4+-cell counts, >500/mm3] and subjects M1 to M4 [CD4+-cell counts, <200/mm3) or from one healthy seronegative donor (HIV−). Results are expressed as counts per minute of [3H]thymidine incorporated in cultures at 6 days after stimulation (the results are means ± SDs of three experimental values).
Effect of AY on the PHA-induced IL-12 mRNA and the in vitro production of IL-12 and gamma interferon.
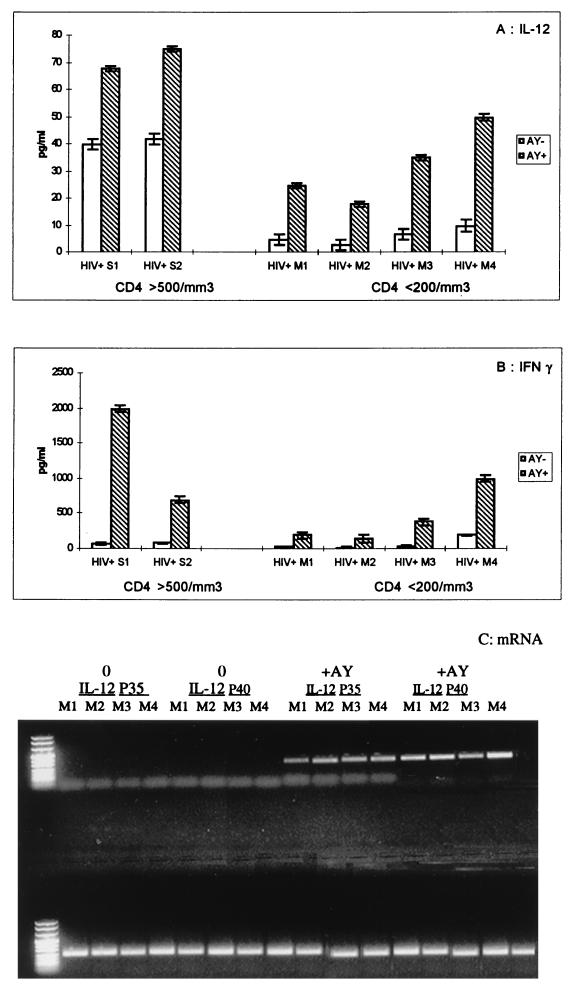
PBMCs from either two HIV-1-seropositive, asymptomatic individuals (group S) or four symptomatic individuals infected with HIV-1 (group M) were stimulated in vitro with PHA and cultured for 2 weeks in the presence of the drug. As shown in Fig. 6, the PBMCs pretreated with AY produced 2- to 12-fold more IL-12 and gamma interferon than the PBMCs from the controls. It is noteworthy that cells obtained from asymptomatic donors produced more IL-12 than cells from patients with AIDS and that their IL-12 production was also stimulated by the drug. In order to specify the mechanisms of this enhancing effect of AY on IL-12 production, we further investigated the effect of the drug on IL-12 mRNA expression in PBMCs originating from the same four AIDS patients (patients M1, M2, M3, and M4; group M). Figure 6 shows that pretreatment with AY of PHA-stimulated PBMCs maintained in a culture medium supplemented with recombinant IL-2 resulted in an induction of IL-12 mRNA (IL-12 p40 and IL-12 p35). For two asymptomatic donors, no significant difference in the levels of expression of IL-12 mRNA between drug-treated and control PBMCs was observed (data not shown).
FIG. 6.
Effect of AY on the in vitro production of IL-12 (A), the synthesis of gamma interferon (B), and the IL-12 mRNA level (C). PBMCs from patients with AIDS were cultured for 15 days in complete medium in the presence (+AY) or in the absence (0) of 3 × 10−6 M AY. Cytokine production (A and B) was measured in cell-free supernatants with an R&D system enzyme-linked immunosorbent assay kit (the results are means ± SDs of three experimental values). The levels of IL-12 and GAPDH mRNA expression (C) were studied in PBMCs from four HIV-1-infected (HIV+) symptomatic individuals (subjects to M1 to M4; CD4+-cells-counts, <200/mm3) as described in Materials and Methods.
Effect of AY on CD4 antigen and IL-2 α receptor (CD25) expression.
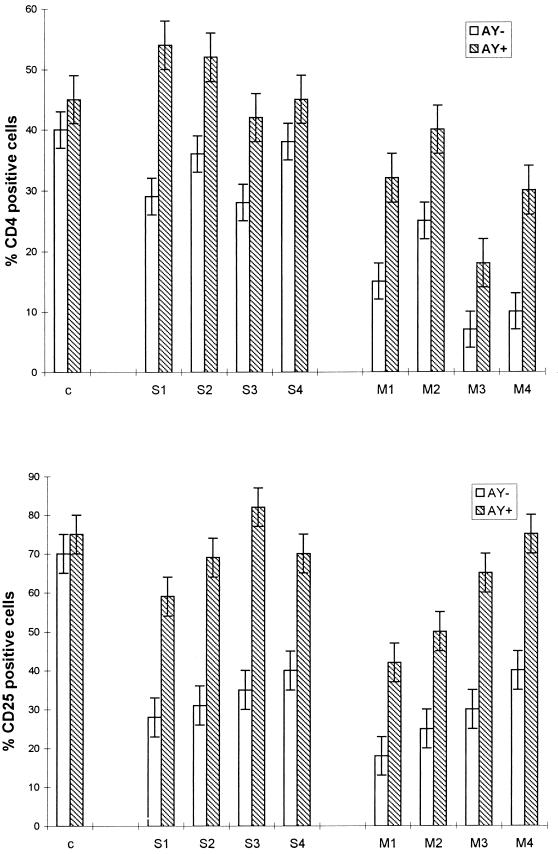
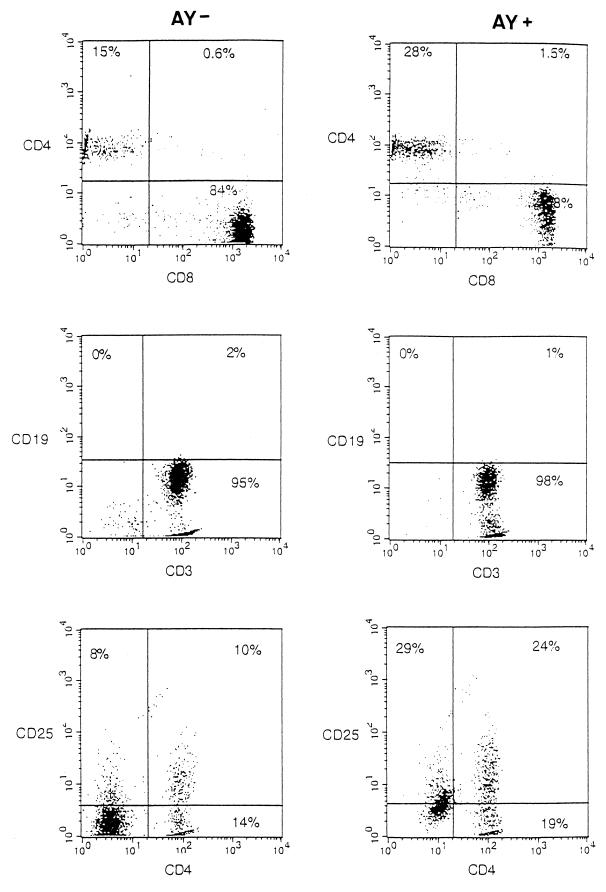
As shown in Fig. 7, analysis by flow cytometry indicated that the percentage of cells expressing the CD4 antigen following in vitro PHA stimulation was significantly higher (30 to 300%; P < 0.01) in cultures treated with AY than in control cultures either for PBMCs originating from asymptomatic subjects (group S) or for PBMCs obtained from patients with AIDS (group M). It should be noted that AY did not affect the percentage of CD4 cells in the PBMCs from a healthy donor. Figure 7 also shows that AY treatment increased by 50 to 100% the level of IL-2 receptor (CD25) expression in PBMCs from asymptomatic or symptomatic subjects, suggesting that the effect of the drug on CD4 proliferation might be related to its ability to restore IL-2-dependent cell activation. Again, AY did not significantly modify the level of CD25 expression in PBMCs from one healthy donor. Figure 8 summarizes the effect of the drug on specific lymphocyte markers of PBMCs obtained from one representative patient (CD4+ count, <200/mm3). As illustrated in Fig. 8, AY acts preferentially on T cells (CD3) but does not have a significant effect on B cells (CD19), positively modulates the IL-2 receptor (CD25), and increases the number of T lymphocytes expressing the CD4 antigen.
FIG. 7.
AY increases the level of PHA-induced CD4 and IL-2 receptor α-chain (CD25) expression. Flow cytometric analysis was performed with T cells from one healthy HIV-negative individual and eight different AIDS patients. The cells were cultured for 4 weeks in complete medium in the presence (AY+) or in the absence (AY−) of the drug. Results are means ± SDs of 10 experimental values. c, control.
FIG. 8.
Two-color FACS analysis of the expression of the T-cell receptor (CD3), the B-cell marker (CD19), the IL-2 α receptor (CD25), and the CD4 antigen (CD4) on cultured cells obtained from one representative AIDS patient+-cell (CD4 count, <200/mm3) at week 4 after the initiation of the cultures in the absence (control) (AY−) or in the presence (AY+) of 3 × 10−6 M. The FACS analyzer was gated for lymphocytes.
Effect of AY on viral replication related to cell mortality and cytokine production.
Having demonstrated the proliferative effect of the drug, we further investigated whether AY could also reduce the level of virus production in naturally infected PBMCs originating from symptomatic subjects (subjects M1 to M8). After in vitro stimulation with PHA, the cells were cultured for more than 5 weeks in IL-2-supplemented medium in the presence or in the absence of AY. As shown in Table 1, for two patients (patients M1 and M2), AY markedly decreased the level of virus production at day 21, as assessed by measuring the viral core p24 protein and virion-associated RNA levels. For two other subjects (subjects M3 and M4), no specific inhibition of HIV-1 production was observed, and for four subjects (subjects M5 to M8), no virus production was observed in untreated or AY-treated cells. It is noteworthy that even when no production of virus was observed, AY-treated cells exhibited lower levels of mortality and higher levels of cytokine production (gamma interferon and IL-12) than their untreated counterparts (Table 2). Moreover, AY had no direct inhibitory effect on enzyme activity, as measured by the reverse transcriptase assay in vitro (data not shown).
TABLE 1.
Effect of AY on HIV-1 production and cell mortality in PBMCs from patients with AIDSa
| Time (days) | Donor | Production of HIV-1 in culture supernatants
|
% Mortality of cultured cells
|
||||
|---|---|---|---|---|---|---|---|
| p24 protein (pg/ml)
|
HIV-1 particles (counts/ml of medium)
|
||||||
| AY− | AY+ | AY− | AY+ | AY− | AY+ | ||
| 10 | M1 | 5 | 4 | 900 | 700 | 23 | 9 |
| 14 | M1 | 10 | 9 | 800 | 770 | 28 | 8 |
| 22 | M1 | 700 | 6 | 5,170 | 822 | 26 | 10 |
| 10 | M2 | −1 | 2 | 650 | 725 | 18 | 10 |
| 14 | M2 | 3 | 5 | 921 | 680 | 55 | 11 |
| 22 | M2 | 2,500 | 25 | 7,500 | 1,000 | 70 | 12 |
| 10 | M3 | 10 | 7 | 1,100 | 652 | 16 | 12 |
| 14 | M3 | 1,800 | 2,200 | 2,541 | 3,161 | 13 | 12 |
| 22 | M3 | 15 | 10 | 650 | 811 | 30 | 13 |
| 10 | M4 | 5 | 4 | 750 | 1,000 | 18 | 7 |
| 14 | M4 | 7 | 10 | 1,000 | 1,400 | 25 | 10 |
| 22 | M4 | 200 | 150 | 1,300 | 1,000 | 28 | 13 |
| 10 | M5 | 5 | 4 | 400 | 500 | 25 | 10 |
| 14 | M5 | 10 | 6 | 700 | 641 | 32 | 11 |
| 22 | M5 | 8 | −2 | 500 | 400 | 35 | 12 |
| 10 | M6 | −1 | 3 | 600 | 400 | 19 | 9 |
| 14 | M6 | 2 | 1 | 500 | 520 | 29 | 8 |
| 22 | M6 | 4 | 3 | 420 | 380 | 40 | 13 |
| 10 | M7 | 4 | 7 | 619 | 542 | 20 | 13 |
| 14 | M7 | 6 | 5 | 531 | 600 | 27 | 12 |
| 22 | M7 | −1 | 2 | 450 | 550 | 31 | 14 |
| 10 | M8 | −1 | 3 | 600 | 575 | 25 | 11 |
| 14 | M8 | 2 | 4 | 500 | 450 | 35 | 14 |
| 22 | M8 | 4 | 3 | 480 | 530 | 42 | 16 |
After activation with PHA, PBMCs were cultured for up to 25 to 30 days in complete medium with recombinant IL-2 in the presence (AY+) or in the absence (AY−) of the molecule. The cells were harvested for cell viability determination, and the culture medium was replaced every 3 days in order to maintain a concentration of 8 × 105 cells/ml. The culture supernatants were used to test both HIV-1 p24 antigen levels by enzyme-linked immunosorbent assay and reverse transcriptase activity. Mortality was assessed by blue Trypan blue coloration. Data are expressed as the means for triplicate cultures. The standard deviation was less than 10% of the mean.
TABLE 2.
Effect of AY on gamma interferon and IL-12 production and cell mortality in PBMCs from patients with AIDSa
| Time (days) | Donor | Cytokine production in culture supernatants
|
% Mortality of cultured cells
|
||||
|---|---|---|---|---|---|---|---|
| Gamma interferon (pg/ml)
|
IL-12 (pg/ml)
|
||||||
| AY− | AY+ | AY− | AY+ | AY− | AY+ | ||
| 10 | M1 | 20 | 200 | 5 | 25 | 23 | 9 |
| 14 | M1 | 15 | 250 | 5 | 20 | 28 | 8 |
| 22 | M1 | 10 | 300 | 7 | 30 | 26 | 10 |
| 10 | M2 | 10 | 40 | 4 | 18 | 18 | 10 |
| 14 | M2 | 20 | 50 | 5 | 20 | 55 | 11 |
| 22 | M2 | 15 | 70 | 7 | 25 | 70 | 12 |
| 10 | M3 | 10 | 350 | 5 | 30 | 16 | 12 |
| 14 | M3 | 10 | 300 | 5 | 25 | 13 | 12 |
| 22 | M3 | 10 | 400 | 5 | 40 | 30 | 13 |
| 10 | M4 | 20 | 750 | 10 | 50 | 18 | 7 |
| 14 | M4 | 15 | 600 | 15 | 60 | 25 | 10 |
| 22 | M4 | 20 | 850 | 20 | 80 | 28 | 13 |
| 10 | M5 | 20 | 600 | 10 | 100 | 25 | 10 |
| 14 | M5 | 15 | 500 | 5 | 90 | 32 | 11 |
| 22 | M5 | 10 | 800 | 5 | 110 | 35 | 12 |
| 10 | M6 | 15 | 200 | 15 | 150 | 19 | 9 |
| 14 | M6 | 20 | 250 | 10 | 100 | 29 | 8 |
| 22 | M6 | 10 | 300 | 5 | 100 | 40 | 13 |
| 10 | M7 | 10 | 150 | 20 | 100 | 20 | 13 |
| 14 | M7 | 15 | 180 | 30 | 150 | 27 | 12 |
| 22 | M7 | 20 | 250 | 25 | 120 | 31 | 14 |
| 10 | M8 | 15 | 350 | 15 | 90 | 25 | 11 |
| 14 | M8 | 20 | 400 | 20 | 80 | 35 | 14 |
| 22 | M8 | 25 | 450 | 25 | 95 | 42 | 16 |
The cells were cultured as described in footnote a of Table 1. The culture supernatants were used to test both gamma interferon and IL-12 levels with an R&D system enzyme-linked immunosorbent assay kit. Mortality was assessed by Trypan blue coloration. Data are expressed as the means for triplicate cultures.
DISCUSSION
The clinical evolution of HIV-1 infection results from immunosuppression characterized by a depletion in the numbers of CD4+ T cells, and CD4+ T-cell counting is currently used to monitor the progress of the disease (14). The depression of antigen-specific T-cell responses is an important feature of HIV infection and involves a qualitative dysfunction of T cells. These alterations of T-cell properties have been attributed in part to the inhibitory effect of proteins encoded by viral genes, e.g., env (11), tat (12, 35), and nef (25). The nef gene has been reported to induce CD4 downregulation (5), which could play an important role in lentivirus-related pathogenesis. Studies with exogenous Tat and Tat-transfected T cells suggested that this regulatory protein plays a role in immune suppression and induces a functional lack of responsiveness of T cells by inhibiting IL-2 mRNA expression and IL-2 secretion (12, 35).
Although the mechanisms by which AY could protect CD4+ cells against death and restore cell proliferation remain to be specified, several hypotheses can be proposed. The drug, initially described as a potent inhibitor of cholesterol biosynthesis acting as an inhibitor of 7-dehydrocholesterol reductase, the last enzyme of the metabolic pathway of cholesterol synthesis (9, 16), has not been used for human therapy due to its teratogenic action demonstrated earlier in rats (33). In a previous report, we have shown that lovastatin, another powerful inhibitor of cholesterol synthesis which reduces the level of hydroxymethyl coenzyme A reductase activity (3), was able to partially inhibit HIV-1 production in an in vitro model (22). Since it has been demonstrated that the virus envelope is enriched in cholesterol compared to the level of cholesterol in cellular membranes (2), which suggests a dependency of virus production on cellular cholesterol synthesis, one can suppose that one of the mechanisms by which AY may inhibit virus production is related to the decrease in the pool of cholesterol available for the formation of viral particles. However, due to its cationic amphiphilic structure, AY is able to act on numerous other cellular targets. As examples, it has been described that this drug alters the activities of membrane-bound enzymes such as ATPase (32), alters in a biphasic manner receptor-mediated low-density lipoprotein processing by cultured human fibroblasts (23), and is a powerful ligand for calmodulin (24). Like many other amphiphilic compounds such as perhexiline maleate, desipramine, or phenothiazines, AY markedly inhibits the lysosomal sphingomyelinase (34, 40), leading to “Niemann-Pick-like” disease in animal models (34). This last property must especially be discussed in relation to preventing the effect of the drug on CD4+ cell mortality described in the present work. Indeed, ceramide generation via sphingomyelinase activation is believed to play an important role in the apoptosis of lymphoid cells (18, 31). Since it has been proposed that the death of CD4+ and even CD8+ T cells observed in patients with AIDS occurs through an apoptotic process (6) in which ceramide might be involved (15, 37), one can suggest that inhibition of sphingomyelinase by AY could be involved in the protective effect of the drug. However, the sphingomyelinase involved in ceramide formation via the sphingomyelin cycle seems not to be the lysosomal enzyme but a membrane-bound enzyme probably located in the inner layer of the plasma membrane (7). There is at present no evidence for an effect of AY on this specific sphingomyelinase, and further experiments are needed to explore this hypothesis.
Beside the hypothesis evoked above, we point in the present work to the newly discovered effects of AY, especially at the level of the cytokine cellular profile. It is noteworthy that these effects are specifically observed in HIV-infected PBMCs either from asymptomatic subjects or from patients with AIDS, while the drug does not significantly affect IL-12 production or IL-2 receptor expression in PBMCs from healthy donors. Several previous findings for HIV-infected patients are compatible with a defect in IL-12 production, in particular, early deficiencies in T-cell responses to antigens (10, 36). IL-12 has been shown to restore antigen-specific lymphocyte proliferation (13). Moreover, it has been reported that CD4+-cell death by apoptosis can be prevented by the addition of IL-12 (29). Thus, the recovery of cell growth and the decrease in CD4+-cell mortality under AY treatment observed in our experiments might be related to the increase in level of IL-12 production induced by the drug. As growth factors, IL-2 and IL-12 regulate vital biological processes such as induction and regulation of immune responses, cellular proliferation, and cellular differentiation (30). The upregulation of IL-12, gamma interferon production, and IL-2 receptor expression by drugs such as AY could be a new way of potentiating defenses toward the immune deficiency related to HIV infection. Clearly, AY has no direct inhibitory action on reverse transcriptase activity and does not confer absolute protection against HIV-1 production.
T-cell lines chronically infected with identified laboratory strains of HIV-1 were usually used in antiviral assays. By this experimental method, a number of compounds have been reported to inhibit HIV-1 production. The aim of our in vitro study was to analyze the effect of AY according to the major cellular mechanisms occurring in vivo. For this purpose, we worked on primary PBMC cultures derived from naturally infected individuals. Our results showed the beneficial effects of the drug due to immunological factors. These new data offer the possibility of establishing the mechanisms of action of AY that may be critical in the development of drugs capable of stimulating protection against HIV-1. However, in our experimental procedure, the potential direct antiviral effect of the drug could not be excluded. Indeed, isolation of HIV-1 from primary cultures of infected PBMCs was not systematically done owing to their poor survival in culture and the presence of HIV-1 suppressor factors. For this reason, it will be necessary to use a single-cell HIV-1 system in which assessment of antiviral activity could be more clearly investigated. Ongoing work with MT-2 and U937 cell lines infected with syncytium-inducing and non-syncytium-inducing viral isolates is aimed at showing whether the effect of the drug is due to immunological factors or antiviral activity, or both.
It can thus be supposed that HIV-1 replication in activated cells may not be the decisive factor regulating the decline in CD4+ cells in patients with AIDS. Indeed, a previous report argued that in vivo HIV-1 kills CD4+ cells by an indirect mechanism (4). Therefore, the protective effect of AY might be related to the restoration of immunoregulatory and antiviral cytokines such as IL-12 and gamma interferon. Such an hypothesis is confirmed by a recent report from Ozmen et al. (27), who demonstrated that in mice infected with encephalomyocarditis virus, IL-12 exerts antiviral activity via the induction of endogenous gamma interferon. To our knowledge, this is the first time that a chemical agent has been demonstrated to enhance CD4+-cell proliferation in PBMCs from HIV-1-infected patients by selectively favoring the production of immunoregulatory and antiviral cytokines.
It was demonstrated that the level of viremia in HIV-1-infected patients is maintained by continuous rounds of viral replication and reinfection of T cells (19, 39). When HIV-1 enters a new host, there is typically a burst of viremia, which is then inhibited by the onset of the immune response. The subsequent level of virus in plasma is a reflection of the equilibrium reached between the virus and the host after the initial battle, and this equilibrium is generally maintained for years. Impressive antiviral effects were reported with triple therapy (26). We can imagine from those reports that additional potent agents like AY could be incorporated into the already powerful regimens of antiretroviral drugs. As reported recently (28), it is crucial to emphasize that immune system-based strategies in combination with antiviral therapy could control HIV-1 infection. Our findings therefore suggest that AY or related molecules may represent important candidates for therapies based on the restoration of CD4+ T-cell functions in HIV-infected individuals.
ACKNOWLEDGMENTS
This work was supported by grants from Néovacs and the Association de Recherche sur le SIDA (ARS), Paris, France. J.-C.M. thanks the Université Picardie-Jules Verne for financial support.
REFERENCES
- 1.Achour A, Bex F, Hermans P, Burny A, Zagury D. Induction of anti-gp160 cytotoxic T cells cross-reacting with various V3 loop p18 peptides in human immunodeficiency virus type 1 envelope-immunized individuals. J Virol. 1996;70:6741–6750. doi: 10.1128/jvi.70.10.6741-6750.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aguilar J J, Anel A, Torres J M, Semmel M, Uriel J. Changes in lipid composition of human peripheral blood lymphocytes infected by HIV. AIDS Res Hum Retroviruses USA. 1991;7:761–765. doi: 10.1089/aid.1991.7.761. [DOI] [PubMed] [Google Scholar]
- 3.Alberts A. HMG-CoA reductase inhibitors—the development. Atherosclerosis Rev. 1988;18:123–132. [Google Scholar]
- 4.Aldrovani G M, Feuer G, Gao L, Jamieson B, Kristeva M, Chen I S Y, Zack J A. The Scid-Hu mouse as a model for HIV-1 infection. Nature (London) 1993;363:732–736. doi: 10.1038/363732a0. [DOI] [PubMed] [Google Scholar]
- 5.Alken C, Konner J, Landau N R, Lenburg M E, Trono D. Nef induces CD4 endocytosis: requirement for a critical dileucine motif in the membrane-proximal CD4 cytoplasmic domain. Cell. 1994;76:853–864. doi: 10.1016/0092-8674(94)90360-3. [DOI] [PubMed] [Google Scholar]
- 6.Ameissen J C, Capron A. Cell dysfunction and depletion in AIDS; the programmed cell death hypothesis. Immunol Today. 1991;12:102–105. doi: 10.1016/0167-5699(91)90092-8. [DOI] [PubMed] [Google Scholar]
- 7.Andrieu N, Salvayre R, Levade T. Comparative study of the metabolic pools of sphingomyelin and phosphatidylcholine sensitive to tumor necrosis factor. Eur J Biochem. 1996;236:738–745. doi: 10.1111/j.1432-1033.1996.00738.x. [DOI] [PubMed] [Google Scholar]
- 8.Barré-Sinoussi F, Chermann J C, Rey F, Nugeyre M T, Gruest J, Dauguet C, Brun-Vezinet F, Rouzioux C, Rozenbaum W, Montagnier L. Isolation of a T-lymphotropic retrovirus from a patient at risk for AIDS. Science. 1983;220:868–871. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- 9.Chappel C, Dubuc J, Dvornik D, Givner M, Humber L, Kraml M, Voith K, Gaudry R. An inhibitor of cholesterol biosynthesis. Nature (London) 1964;201:497–498. doi: 10.1038/201497a0. [DOI] [PubMed] [Google Scholar]
- 10.Chehimi J, Starr S E, Frank I, D’Andrea A, Ma X, MacGregor R B, Sennelier J, Trinchieri G. Impaired interleukin 12 in human immunodeficiency virus-infected patients. J Exp Med. 1994;179:1361–1366. doi: 10.1084/jem.179.4.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chirmule N, Kalyanaraman V S, Oyaizu N, Slade H, Pahwa S. Inhibition of functional properties of tetanus antigen-specific T cell clones by envelope glycoprotein of HIV-1. Blood. 1990;75:152–159. [PubMed] [Google Scholar]
- 12.Chirmule N, Than S, Khan A S, Pahwa S. Human immunodeficiency virus Tat induces functional unresponsiveness in T cells. J Virol. 1995;69:492–498. doi: 10.1128/jvi.69.1.492-498.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clerici M, Lucey D R, Berzofsky J A, Pinto L A, Wynn T A, Blatt S P, Dolan M J, Hendrix G W, Wolf S F, Shearer G M. Restoration of HIV-specific cell-mediated immune responses by interleukin-12 in vitro. Science. 1993;262:1721–1724. doi: 10.1126/science.7903123. [DOI] [PubMed] [Google Scholar]
- 14.Coffin J M. HIV population dynamics in vivo: implications for genetic variation, pathogenesis, and therapy. Science. 1995;267:483–489. doi: 10.1126/science.7824947. [DOI] [PubMed] [Google Scholar]
- 15.De Simone V, Grazia Cifone M, Roncaioli P, Moretti S, Famularo G, Alesse E, Boschini A, Testi R. Ceramide, AIDS and long-term survivors. Immunol Today. 1996;17:48. doi: 10.1016/0167-5699(96)80570-6. [DOI] [PubMed] [Google Scholar]
- 16.Dvornik D, Kraml M, Dubuc J, Givner M, Gaudry R. A novel mode of inhibition of cholesterol biosynthesis. J Am Chem Soc. 1983;85:3309–3310. [Google Scholar]
- 17.Fauci A S. Multifactorial nature of human immunodeficiency virus: implications for therapy. Science. 1993;262:1011–1018. doi: 10.1126/science.8235617. [DOI] [PubMed] [Google Scholar]
- 18.Hannun Y A, Obeid L M. Ceramide: an intracellular signal for apoptosis. Trends Biochem Sci. 1995;20:73–77. doi: 10.1016/s0968-0004(00)88961-6. [DOI] [PubMed] [Google Scholar]
- 19.Ho D, Neumann A U, Perelson A S, Chen W, Leonard J M, Markwitz M. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature (London) 1995;3373:123–126. doi: 10.1038/373123a0. [DOI] [PubMed] [Google Scholar]
- 20.Koup R A, Safrit J T, Cao Y C, Andrews C A, McLeod G, Borkovsky W, Farthing C, Ho D D. Temporal association of cellular immune response with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol. 1995;68:4650–4655. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kovacs J A, Baseler M, Dewar R J, Vogel S, Davey R T, Falloon J, Polis M, Walker R E, Stevens R, Salzman N P, Metcalf J A, Masur H, Lane H C. Increase in CD4+ T lymphocytes with intermittent courses of interleukin-2 in patients with human immunodeficiency virus infection. A preliminary study. N Engl J Med. 1995;332:567–575. doi: 10.1056/NEJM199503023320904. [DOI] [PubMed] [Google Scholar]
- 22.Maziére J C, Landureau J C, Giral P, Auclair M, Fall L, Lachgar A, Achour A, Zagury D. Lovastatin inhibits HIV-1 expression in H9 human T lymphocytes cultured in cholesterol-poor medium. Biomed Pharmacother. 1994;48:63–67. doi: 10.1016/0753-3322(94)90077-9. [DOI] [PubMed] [Google Scholar]
- 23.Maziére J C, Maziére C, Mora L, Gardette J, Wolf C, Rainteau D, Barbu V, Roux C, Polonovski J. Effects of AY 9944 on low density lipoprotein metabolism in cultured human fibroblasts. Biochem Biophys Res Commun. 1984;122:955–959. doi: 10.1016/0006-291x(84)91184-7. [DOI] [PubMed] [Google Scholar]
- 24.Maziére J C, Maziére C, Wolf C, Rainteau D, Sitbon N, Barbu V, Dupuis R, Roux C, Polonovski J. Interaction of AY 9944 with calmodulin. Teratology. 1984;29:29a. [Google Scholar]
- 25.Niederman T M J, Hastings W R, Juria S, Banders J C, Ratner L. HIV-1 nef protein inhibits the recruitment of AP-1 DNA binding activity in human T cells. Virology. 1993;194:338–344. doi: 10.1006/viro.1993.1264. [DOI] [PubMed] [Google Scholar]
- 26.O’Brien W A, Hartigan P M, Martin D, Esinhart J, Hill A, Benoit S, Rubin M, Simberkoff M S, Hamilton J D. Changes in plasma HIV-1 RNA and CD4+ lymphocyte counts and the risk of progression to AIDS. N Engl J Med. 1996;334:426–431. doi: 10.1056/NEJM199602153340703. [DOI] [PubMed] [Google Scholar]
- 27.Ozmen L, Aguet M, Trinchieri G, Garotta G. The in vivo antiviral activity of interleukin-12 is mediated by gamma interferon. J Virol. 1995;69:8147–8150. doi: 10.1128/jvi.69.12.8147-8150.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pantaleo G. How immune-based interventions can change HIV therapy. Nat Med. 1997;5:483–486. doi: 10.1038/nm0597-483. [DOI] [PubMed] [Google Scholar]
- 29.Pasquier J, Idziorek T, Zou W, Emile D, Farber C-M, Bourez J-M, Ameissen J C. T helper type 1/T helper type 2 cytokines and T cell death: preventive effect of interleukin 12 on activation-induced and CD95 (FAS/APO/1)-mediated apoptosis of CD4+ T cells from human immunodeficiency virus-infected persons. J Exp Med. 1995;182:1759–1767. doi: 10.1084/jem.182.6.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paul W E, Seder R A. Lymphocytes responses and cytokines. Cell. 1994;76:241–251. doi: 10.1016/0092-8674(94)90332-8. [DOI] [PubMed] [Google Scholar]
- 31.Perry D K, Obeid L M, Hannun Y A. Ceramide and the regulation of apoptosis. Trends Cardiovasc Med. 1996;6:158–162. doi: 10.1016/1050-1738(96)00044-8. [DOI] [PubMed] [Google Scholar]
- 32.Proverbio F, Rawlins F A. Increment in sodium and potassium-dependent adenosine triphosphatase of brain microsomal fractions from rats treated with the cholesterol biosynthesis inhibitor AY 9944. Mol Pharmacol. 1978;14:911–919. [PubMed] [Google Scholar]
- 33.Roux C, Aubry M M. Action tératogène chez le rat d’un inhibiteur de la synthèse du cholestérol. C R Soc Biol. 1966;160:1353–1357. [PubMed] [Google Scholar]
- 34.Sakuragawa N, Sakuragawa M, Kubawara T, Pentchev P G, Barranger J A, Brady R. Niemann-Pick experimental model: sphingomyelinase reduction by AY 9944. Science. 1977;196:317–319. doi: 10.1126/science.66749. [DOI] [PubMed] [Google Scholar]
- 35.Subramanium M, Gutheil G H, Bachovchin W W, Huber B T. Mechanisms of HIV-1 tat induced inhibition of antigen-specific T cell responsiveness. J Immunol. 1993;150:2544–2553. [PubMed] [Google Scholar]
- 36.Trinchieri G. IL-12 and its role in the generation of TH1 cells. Immunol Today. 1993;14:335–337. doi: 10.1016/0167-5699(93)90230-I. [DOI] [PubMed] [Google Scholar]
- 37.van Veldhoven P P, Matthews T J, Bolognesi D P, Bell R M. Changes in bioactive lipids, alkylacylglycerol and ceramide, occur in HIV-infected cells. Biochem J. 1992;299:597–601. doi: 10.1016/s0006-291x(05)81480-9. [DOI] [PubMed] [Google Scholar]
- 38.Volberding P, Moody D J, Beaedslee D, Bradley E C, Wofsy C B. Therapy of acquired immune deficiency syndrome with recombinant interleukin-2. AIDS Res Hum Retroviruses USA. 1987;3:115–124. doi: 10.1089/aid.1987.3.115. [DOI] [PubMed] [Google Scholar]
- 39.Wei X, Ghosh S J, Taylor M E, Johnson V A, Emini E A, Deutsch P, Lifson J D, Bonheffer S, Nowak M A, Hahn B H, Saag M S, Shaw G M. Viral dynamics in human immunodeficiency virus type 1 infection. Nature (London) 1995;373:117–122. doi: 10.1038/373117a0. [DOI] [PubMed] [Google Scholar]
- 40.Yoshida Y, Arimato K, Sato M, Sakuragawa N, Arima M, Satoyoshi E. Reduction of acid sphingomyelinase activity in human fibroblasts induced by AY 9944 and other cationic amphiphilic drugs. J Biochem. 1985;98:1669–1679. doi: 10.1093/oxfordjournals.jbchem.a135438. [DOI] [PubMed] [Google Scholar]
- 41.Zagury D, Bernard J, Halbreich A, Bizzini B, Carelli C, Achour A, Defer M C, Bertho J M, Lanneval K, Zagury J F, Salaun J J, Lurhuma Z, Mbayo K, Aboud-Pirak E, Lowell G, Lebon P, Burny A, Picard O. One-year follow-up of vaccine therapy in HIV-infected immune-deficient individuals: a new strategy. J Acquired Immune Defic Syndr. 1992;5:676–681. [PubMed] [Google Scholar]