Abstract
Platinum(II) and platinum(IV) compounds were prepared by the stereoselective and regioselective reactions of thiophene-derived cyclohexyl diimine C^N^N-ligands with [Pt2Me4(μ-SMe2)2]. Newly synthesized ligands were characterized by NMR spectroscopy and elemental analysis, and Pt(II)/Pt(IV) compounds were characterized by NMR spectroscopy, elemental analysis, high-resolution mass spectrometry, and single-crystal X-ray diffraction. UV–vis absorbance and photoluminescence measurements were performed on newly synthesized complexes, as well as structurally related Pt(II)/Pt(IV) compounds with benzene-derived cyclohexyl diimine ligands, in dichloromethane solution, as solids, and as 5% by weight PMMA-doped films. DFT and TD-DFT calculations were performed, and the results were compared with the observed spectroscopic properties of the newly synthesized complexes. X-ray total scattering measurements and real space pair distribution function analysis were performed on the synthesized complexes to examine the local- and intermediate-range atomic structures of the emissive solid states.
Introduction
Platinum(II) and platinum(IV) cyclometalated complexes are an important class of organometallic compounds with varied applications in catalysis, inorganic photophysics, and bioinorganic chemistry.1−4 Cyclometalated ligands featuring chelating- or pincer-type binding to the central Pt atom with a strong carbanionic σ-donor are especially well studied in this regard.5,6 In the context of luminescent complexes, these strong field ligands are known to raise the energy of unoccupied metal-centered (MC) states that could otherwise provide nonradiative pathways to the deactivation of the excited state.7 Among cyclometalated Pt compounds, square-planar Pt(II) d8 complexes are the most widely explored for luminescent complexes with long-lived excited states aided by fast intersystem crossing rates.3,8−10 In comparison, octahedral Pt(IV) complexes possess lower energy 5d orbitals that can be expected to have poorer mixing with ligand orbitals, resulting in Pt(IV) compounds being far less studied than Pt(II) compounds as emissive complexes.11 However, the potential of octahedral d6 cyclometalated Pt(IV) complexes as emitters has become more appreciated in recent years.11−13
Cyclometalated complexes of Pt are most often prepared by the well-known C–H and C–X (X = Cl, Br, I) oxidative addition reactions of appropriately designed ligands. The initial coordination of neutral donor atoms in the ligands such as N, O, and P direct the oxidative addition in heteroatom-assisted C–H/C–X activation, leading to the cyclometalated product.4,14−16 In order to realize high-yielding syntheses of cyclometalated Pt complexes, ligands that react with high regioselectivity and stereoselectivity are essential. In general, this requires careful ligand design, as many possible regiochemical and stereochemical products may result from C–H/C–X reactivity of organic ligands. Schiff base ligands with an imine nitrogen donor atom are a commonly used class of ligands for the purposes of cyclometalation due to their ease of synthesis and tendency to metalate to form (most commonly) five- and six-membered cyclometalated rings.5,6,17,18 In one example, Puddephatt and co-workers reported cyclohexyl diimine C^N^N ligands derived from chiral cyclohexyl diamine and benzaldehyde that react to form cyclometalated complexes with very high selectivity for both Pt(II) and Pt(IV) complexes.19,20 Furthermore, cyclometalated C^N^N ligands are known to form luminescent Pt(II) and Pt(IV) complexes.11,21,22
In this work, we explore a new type of cyclohexyl diimine C^N^N ligand featuring a thiophene ring, as is shown in Scheme 1. Conjugated thiophene polymers and oligomers are attractive organic optoelectronic materials in their own right,23−25 and metal complexes featuring metalated or pendant thiophenes with interesting photophysical properties have been reported.26−29 In particular, metalated thiophenes as compared to phenyl groups in structurally analogous Pt(II) complexes have been reported to enhance fluorescence over phosphorescence in recent studies.26,30 Among aromatic rings, thiophenes have been reported to be reactive toward C–H activation and are excellent candidates for preparing cyclometated complexes through C–H activation reactions.14,31 Using this diimine C^N^N ligand framework, we report the synthesis, structural characterization, and photophysical properties of new Pt(II)/Pt(IV) complexes and have compared their properties with the structurally related benzene-derived complexes reported earlier by Puddephatt.19,20 We report their luminescence in both solution and solid media for these four complexes, as a major focus of recent research in luminescent inorganic complexes is the impact of physical state and intermolecular order on photophysical properties.32−35
Scheme 1. Syntheses of New Platinum Compounds, PtIIMTh and PtIVDTh, and the Structures of the Benzene Analogues, PtIIMPh and PtIVDPh.
Results and Discussion
Synthesis and Characterization
The new thiophene-derived diimine ligands were synthesized by condensation reactions, as described in the Experimental Section, and their NMR spectra are included in the Supporting Information (Figures S1–S6). The ligands were characterized by multinuclear NMR spectroscopy, IR spectroscopy, and elemental analysis. The ligands were used to synthesize two platinum compounds by reacting them with the tetramethyl platinum dimer (Scheme 1). The platinum compounds were characterized by multinuclear NMR spectroscopy (Figures S7–S16), IR spectroscopy, elemental analysis, high-resolution mass spectrometry, and single-crystal X-ray diffraction techniques. Ligand A gave a platinum(II) species, PtIIMTh, formed by C–H activation, followed by methane elimination. Ligand B gave a platinum(IV) dimer, PtIVDTh, formed by intramolecular C–Br oxidative addition. These reactions were accompanied by a high degree of stereoselectivity for the isolated products, comparable to analogous species with benzene-derived ligands previously reported.19,20 With our isolated yields of close to 70%, only a single diastereomer is observed. The previously reported platinum compounds could be synthesized by heating and stirring as well as by utilizing a microwave reactor. Similar results were observed in either synthetic case.
The proton spectrum of PtIIMTh contains two imine resonance and one Pt-Me resonance, as expected for a monomeric species with an N^N^C ligand and one methyl ligand in the coordination sphere (Scheme 1). Each of the platinum(IV) species’ 1H and 13C NMR spectra has four methyl resonances and two imine resonances (Figure 1). The J(Pt–H) and J(Pt–C) coupling constants observed for these new complexes are consistent with the literature values for Pt(II) and Pt(IV) complexes.19,20,36−38 The formation of the compounds was indicated in the infrared spectra by a frequency shift of the C=N bond stretching from the free ligand. For PtIVDTh, this band shifted from 1628 to 1582 cm–1, and for PtIIMTh, it shifted from a single band at 1636 to two separate bands at 1621 and 1587 cm–1 due to the existence of one metalated thiophene ring and one dangling thiophene ring (Figures S17–S20). This was corroborated with the DFT-calculated IR spectra (Figures S21 and S22), where visualization of the normal modes shows a higher energy C=N stretch for the imine attached to the dangling thiophene and a lower energy C=N stretch for the imine contained within the cyclometalated ring.
Figure 1.

13C{1H} (top) and 1H (bottom) NMR spectra of imine and methyl resonance regions for PtIVDTh containing 195Pt–13C/1H coupling.
The two new species were also characterized by single-crystal X-ray diffraction techniques (Figures 2 and 3), and the structures confirm those predicted by NMR spectroscopy, as illustrated in Scheme 1. PtIIMTh (Figure 2) has a tridentate pincer ligand with a dangling thiophene ring and one methyl ligand. The Pt–C and Pt–N bond lengths and the bond angles around the platinum center are within acceptable ranges for the platinum(II) species.6PtIVDTh (Figure 3) does not form a monomeric species with the C^N^N ligand but instead forms a dimeric species with one ligand unit bridging two platinum centers with C^N attachments on each metal center. Two bromide ligands, formed by intramolecular oxidative addition, bridge the platinum atoms, and each platinum center also has two methyl ligands. Once again, the bond lengths and angles are within acceptable ranges for platinum(IV) species with C^N chelate and bromide bridging ligands.13,19,20
Figure 2.

ORTEP of Compound PtIIMTh (50% probability thermal ellipsoids). Selected bond lengths (Å) and angles (deg): Pt–C2:1.967(2); Pt–N1:2.045(2); Pt–C1:2.054(3); Pt–N2:2.098(2); C2–Pt–N1:79.85(10); C2–Pt–C1: 96.00(11); N1–Pt–C1:171.12(10); C2–Pt–N2; 160.17(9); N1–Pt–N2:80.32(8); C1–Pt–N2:103.55(9).
Figure 3.

ORTEP of Compound PtIVDTh (50% probability thermal ellipsoids). Selected bond lengths (Å) and angles (deg): Pt1–C3:1.968(4); Pt1–C1:2.054(4); Pt1–C2:2.057(4); Pt1–N1:2.233(4); Pt1–Br2:2.5778(4); Pt1–Br1:2.6104(4); Pt2–C18:1.960(3); Pt2–C20:2.052(3); Pt2–C19:2.060(4); Pt2–N2:2.173(3); Pt2–Br2:2.5777(4); Pt2–Br1:2.6273(4); C3–Pt1–C1:93.81(16); C3–Pt1–C2:91.46(16); C1–Pt1–C2:87.5(2); C3–Pt1–N1:78.05(14); C1–Pt1–N1:171.77(14); C2–Pt1–N1:91.49(17); C3–Pt1–Br2:175.90(11); C1–Pt1–Br2:88.26(12); C2–Pt1–Br2:92.17(12); N1–Pt1–Br2:99.94(9); C3–Pt1–Br1:90.20(11); C1–Pt1–Br1:92.93(13); C2–Pt1–Br1:178.24(13); N1–Pt1–Br1:88.29(9); Br2–Pt1–Br1:86.145(13); C18–Pt2–C20:86.33(14); C18–Pt2–C19:93.98(15); C20–Pt2–C19:89.53(16); C18–Pt2–N2:79.07(12); C20–Pt2–N2:88.35(14); C19–Pt2–N2:172.84(14); C18–Pt2–Br2:174.97(9); C20–Pt2–Br2:94.41(11); C19–Pt2–Br2:91.00(11); N2–Pt2–Br2:95.98(8); C18–Pt2–Br1:93.32(9); C20–Pt2–Br1:178.37(12); C19–Pt2–Br1:92.09(11); N2–Pt2–Br1:90.02(8); Br2–Pt2–Br1:85.797(13); Pt1–Br1–Pt2:89.243(13); Pt2–Br2–Pt1:91.061(14).
The photophysical properties of the compounds were probed to aid in the characterization of the species and compared to those of the benzene-derived compounds. The absorbance spectra (Table S1 and Figure S23) for the platinum(II) species show their lowest energy peaks in the 400–500 nm range, with extinction coefficients of 103 thus tentatively assigned to MLCT transitions (see below for further assignments with the aid of TD-DFT). The platinum(IV) complexes (Table S1 and Figure S23) show their lowest energy band in the UV region, centered at a higher energy at around 350 nm, as expected for the higher oxidation state.11,13 The steady-state emission spectra and lifetime measurements for all four species were conducted for solid compounds, for samples in dichloromethane (DCM) solution, and for 5% by weight doped PMMA films (Table S2 and Figures S24–S29). The emission spectra in DCM solution show wide spectral bands of approximately 200–250 nm with subtly defined multiple and/or shoulder peaks. The samples in solution had low quantum yields of ≤0.1% for all species. The spectra of the solid state samples (Figure 4) also show large spectral width but are slightly red-shifted and are essentially one large, broad band with fewer defined shoulders. The solid state samples also had low quantum yields but greater than those in solution for the platinum(II) species. The quantum yield of PtIIMTh in the solid state was determined to be 1.8%, and for PtIIMPh, it was determined to be 0.8%. Values could not be obtained for the two Pt(IV) solid samples given the limitation of the integration sphere for quantum yields below 0.8%. The steady-state spectra of the 5% doped films of the two Pt(II) species and PtIVDTh were somewhat similar. They consist of broad bands, with the emission maxima centered around 610–650 nm. In contrast, PtIVDPh emits at a lower wavelength than the other three species and has several multiple/shoulder peaks. In contrast to the solid state samples, the quantum yields of the Pt(II) species as PMMA-doped films did not give a measurable quantum yield in the integration sphere due to the sphere’s limitations at low quantum yield and thus presumed to be less than 0.8%; however, measurable results were obtained for the two platinum(IV) PMMA-doped samples. The quantum yield of PtIVDPh was determined to be 2.6% and that of PtIVDTh was determined to be 1.0%. The Pt(IV) species’ quantum yield values were the greatest in the rigid PMMA medium; thus, these samples can be said to exhibit rigidoluminescence.39 Conversely, the Pt(II) species’ values were best in solid state samples, exhibiting an enhancement compared to those of their samples in other states. The lifetimes of emission peaks in both the solid state and in DCM solution (Table S3) are in the hundred nanosecond range and would tentatively indicate an excited state with mixed 3MLCT/3LC character (see below). The lifetime values obtained for the PMMA films were also in the hundreds of nanosecond range. Additionally, all samples also had a second, much shorter lived state in the range 12–23 ns, as the decay curves best fit a double exponential decay (Table S3 and Figure S38). We suggest a possible fluorescence for the faster lifetime.26,29,31
Figure 4.

Solid state emission spectra of platinum compounds excited at 400 nm. Raman spikes have been removed. See Figures S24 and S25 for reference.
TD-DFT and DFT Calculations
Density functional theory (DFT) and time-dependent DFT (TD-DFT) calculations were run on the Pt(II) and Pt(IV) complexes and compared with the observed electronic transitions measured by UV–vis absorbance spectroscopy. As mentioned previously, the large extinction coefficients observed for the UV–vis absorbance features are consistent with MLCT, as has been assigned in structurally similar Pt(II) and Pt(IV) C^N^N, C^N cyclometalated complexes.13,31,40 The DFT-calculated HOMO and LUMO of PtIIMTh and PtIVDTh are shown below. In each case, the HOMO is spread across the π orbitals of the thiophene ligand and Pt d orbitals, while the LUMO is more clearly of π* character across the ligand. The near-frontier orbitals below the HOMO and above the LUMO are also plotted in Supporting Information (Figures S39–S40 and S43–S44) and are similarly distributed across π/π* systems and Pt metal orbitals. The results are analogous in the case of the phenyl diimine ligand Pt(II) and Pt(IV) complexes, except with the π/π* orbitals of the phenyl group, as shown in Figures S41, S42, S45, and S46. The two aromatic rings of the diimine ligand are electronically inequivalent in both Pt(II) and Pt(IV) complexes, which can be clearly seen in the HOMOs of both complexes, as shown in Figure 5.
Figure 5.

DFT-calculated HOMO (left) and LUMO (right) for PtIIMTh (top) and PtIVDTh (bottom). Orbitals are visualized using an 80% isosurface threshold using the IboView program.41
The TD-DFT-simulated absorbance spectrum is shown and compared with the normalized experimental UV–vis spectra in Figure 6. A good qualitative agreement in transition intensities and energies with the experimental data for this level of theory was seen for both Pt(II) and Pt(IV) complexes. The full set of all calculated vertical excitations is shown for all compounds in Figures S36–39. The expected relative blue shift of Pt(IV) versus Pt(II) lowest energy UV–vis absorbance features involving the metal d orbitals is clearly reproduced in these calculations. We conclude that the observed experimental UV–vis absorbance is consistent with the DFT level of theory for the novel compounds, further confirming their identity. Experimental and DFT theory evidence suggest that the UV–vis electronic transitions possess MLCT/LLCT (ligand-to-ligand charge transfer) character, as frontier and near-frontier orbitals are unevenly distributed between the π systems of the two inequivalent aromatic rings in each complex.
Figure 6.

(Top) Observed normalized absorbance spectrum of PtIIMTh and PtIVDTh and TD-DFT-calculated normalized absorbance spectrum. The band shape for the TD-DFT-simulated absorbance spectrum is obtained by broadening the vertical excitations with a 30 nm linewidth (Figures S47 and S48) and then normalizing the oscillator strength.
Real-Space X-ray Pair Distribution Function Analysis
Enhanced emission quantum yields and/or distinct emission profiles in the solid state compared to the solution state for transition-metal complexes are often observed. In many cases, this is believed to be related to the structural coherence as well as the rigidity of the solid state.33 While laboratory powder X-ray diffraction measurements are often performed in these studies and can be used to identify crystalline phases, standard powder diffraction experiments provide limited information regarding amorphous or disordered/nanocrystalline atomic structures.42 Many emissive solid samples of transition-metal complexes obtained from solution synthesis are not crystalline, and the role of structural coherence in aggregation-induced emission for molecular solids has been a focus of several recent reports.32,34,35 A general technique for studying the atomic structure of amorphous, nanocrystalline, and crystalline materials is analysis of the real-space X-ray pair distribution function G(r). The X-ray pair distribution function G(r) is a histogram of the interatomic distances in the sample and can be obtained by the Fourier transform of F(Q), the normalized total scattering structure function obtained from synchrotron X-ray total scattering measurements. The utility of this technique for the structural study of molecular materials has been reviewed recently,43 and several reports highlight the applications for local structural studies of inorganic and organometallic molecular solids.32,44−46
X-ray G(r) spectra were recorded in the solid state for PtIIMTh, PtIIMPh, PtIVDTh, and PtIVDPh, as illustrated in Figure 7, with the reciprocal space F(Q) plotted in Figure S40. The Pt(II) and Pt(IV) complexes for thiophene versus benzene show very similar local- and intermediate-range structures by the inspection of G(r), which are consistent with the similar molecular structures of the benzene versus thiophene analogues. The Pt(II) complexes show their most intense correlation at around 2.05 Å, which is consistent with the overlap of the Pt–C/Pt–N coordination sphere distances listed in the caption of Figure 2 found in our single-crystal X-ray diffraction study. The Pt(IV) complexes on the other hand show the most intense correlation at around 2.60 Å, with a smaller broad first peak around 2.10 Å. In the case of the Pt(IV) complexes, the most intense correlation can be assigned to the Pt–Br distance whose intensity can be explained by (1) the relatively large atomic form factors of the Pt–Br pair and (2) the presence of four Pt–Br distances per molecule in the dimeric structure. The smaller first correlation centered around 2.10 can again be assigned to the overlapped Pt–C/Pt–N distances and is consistent with the distances listed in the caption of Figure 3. Simulation of the PDF using the Debye scattering equation and accompanying partial PDFs for both single molecules and with the local intermolecular structure from the packing of the single-crystal unit cell generated in Mercury47 corroborate the assignments discussed above and are shown in Figures S54 and S55.
Figure 7.

X-ray G(r) data for PtIIMTh (red), PtIIMPh (dashed red), PtIVDTh (blue), and PtIVDPh (dashed blue).
Beyond the local structure in G(r) and extending into the intermolecular distance (r > 5 Å) ranges, the Pt(II) and Pt(IV) complexes with thiophene versus benzene rings show good qualitative agreement with each other. The Pt(II) complexes show extended atomic order past the intramolecular distances, consistent with a high degree of intermolecular order in the solid state. The Pt(IV) complexes also show evidence of intermolecular order in their solid states; however, the absence of sharp peaks past 1 nm is consistent with a more disordered solid state as compared to the Pt(II) complexes. The flat square-planar geometry at Pt(II) may more easily template a close intermolecular packing, as shown in Figure S52 for the crystalline solid state, as compared to the Pt(IV) complexes shown in Figure S53, which are seen cocrystallized with CHCl3 solvent molecules in our single-crystal structure. The observed G(r) for PtIIMTh can be modeled using an established method for treating G(r) of molecular materials that accounts for separate inter- and intramolecular ADPs and a finite crystal coherence length.43,48−51 The fit (rw = 0.32) is shown in Figure 8, while the fit parameters are listed in Table S4 alongside a description of the model. While the virtual crystal model from the single-crystal structure does not fully account for the as-synthesized solid state data, the relatively good agreement in capturing both inter- and intramolecular distance ranges suggests a similar packing of molecules in the bulk synthesized solid sample to the single-crystal solid state.
Figure 8.

G(r) data (blue), fit (red), and difference (green) from r = 1.9 to 40 Å for a virtual crystal model based on the single-crystal intermolecular packing for PtIIMTh.
Concluding Remarks
Thiophene-derived C^N^N diimine ligands were used to synthesize new Pt(II) and Pt(IV) cyclometalated complexes with high reaction selectivity for the isolated products. These products were characterized by multinuclear and 2D NMR spectroscopy, and their atomic structures were determined by single-crystal X-ray diffraction. UV–vis absorbance and photoluminescence measurements of these compounds were performed and compared to those of previously reported compounds synthesized with analogous benzene-derived diimine ligands. The results were compared with the DFT and TD-DFT calculations. The newly synthesized solid sample of PtIIMTh showed the highest recorded quantum yield of the surveyed complexes. The structural order present in the emissive solid state was examined for these complexes by X-ray pair distribution function analysis, with the Pt(II) complexes showing clear evidence of intermolecular ordering. Collectively, these results demonstrate the use of these thiophene-derived C^N^N diimine ligands to selectively prepare photoactive cyclometalated transition-metal complexes with high chemical selectivity.
Experimental Section
General
Solvents and reagents were purchased from Sigma-Aldrich unless otherwise noted. K2PtCl4 was purchased from J. and J. Materials (NJ). NMR spectra were recorded at Bard College using a Varian NMR-400 MHz spectrometer (1H, 400 MHz; 13C, 100.6 MHz). Shifts are given in parts per million and coupling constant J values in Hertz. Abbreviations used: s = singlet; d = doublet; t = triplet; and m = multiplet. Electrospray MS was performed at Vassar College using an Agilent LC/MSD-TOF spectrometer. Samples were run in chloroform in negative mode.
Computational Details
TD-DFT and DFT calculations were performed using Orca ab initio quantum chemistry program version 4.2.1.52,53 All calculations were performed using the B3LYP functional.54,55 The LANL2DZ basis set with an effective core potential used for heavy atoms (Pt, S, and Br) was used during initial geometry optimization and vibrational frequency calculations.56 All final structures were confirmed to be at an energetic minimum by harmonic vibrational analysis. TD-DFT calculations using the Tamm–Dancoff Approximation were performed as single-point calculations, with optimized structures at the ZORA-def2-TZVP level of theory, with an auxiliary basis of SARC/J-ZORA-TZVP for Pt and a CPCM solvation model for the DCM solvent.57 The UV–vis excitations were calculated with the first 30 singlet and triplet excited states and spin–orbit coupling. Theoretical vertical excitations are broadened with a 30 nm linewidth for the purposes of visualization of the band shapes in Figure 6. Initial geometry guesses were generated by using crystallographic data.
Photophysical Measurements
Steady-state emission spectra were recorded by using a PTI QM-40 instrument with a PMT detector, which is sensitive up to 850 nm. In these experiments, the concentration of the platinum complexes ranged from 22 to 41 μM. The fluorimeter emission spectrum was corrected using a method described in the literature which uses four standard fluorophores to calibrate the response of the instrument.58 The slits were set at 2.5 nm bandpass for all solution measurements. Long-pass filters were used to block the excitation light and avoid the detection of its second harmonic. Solid state samples were sandwiched between two pieces of 1 mm thick borosilicate glass slides and mounted at 45° such that the detected luminescence was from the back powder and the specular reflection pointed away from the detector. The slits were set at 1.25 nm bandpass for the solid state samples. The luminescence lifetimes of the complexes were measured by time-correlated single-photon counting (TCSPC) following excitation with a 365, 405, or 450 nm LED. For TCSPC measurements, the slits were adjusted such that <3% of the LED flashes resulted in a detection event ensuring such events are single photons. Solution samples were degassed for 5 min prior to measurement. The 5% by weight doped PMMA films were made by weighing approximately 1 mg of compound and dissolving it in 0.5 mL of HPLC-grade DCM. An appropriate amount of PMMA (poly(methyl methacrylate-co-butyl methacrylate), average Mw ∼ 75 kDa) was weighed to give a weight ratio of 5:95 and dissolved in the DCM solution. After several minutes of stirring, the solution was poured onto a Teflon block and allowed to dry overnight. The films were approximately 1 cm2 in area. The films were mounted at 45°, like the solid state samples, for the luminescence measurements. Quantum yield measurements for solution samples were prepared by serial dilution, all having absorbance values <0.1, and their absorbance and integrated fluorescence signals were compared to aqueous [Ru(bpy)3]Cl2 solutions having similar absorbances. The solid state samples were placed in a Teflon powder holder mounted within a petite integrating sphere (Horiba, K-sphere). During quantum yield measurements, the slit sizes were adjusted, and a neutral density filter with an optical density of 1.0 was used to keep the signal in the linear range of the detector (<1 Mcps). Excitation and emission slits were typically set to ∼1 nm bandpass. BaSO4 was used as the reference compound for the solid state samples, and an undoped PMMA film was used as the reference for the doped PMMA samples. The accuracy of the integrating sphere was checked using Rhodamine 101 in ethanol and also using [Ru(bpy)3]Cl2 in water as standards, with known quantum yields from the literature of 0.92 and 0.042, respectively.59−61 The measured values were found to be 0.93 and 0.041, respectively.
X-ray Diffraction
PtIIMth was crystallized by the slow diffusion of pentane into an acetone solution, while PtIVDTh was crystallized by the slow diffusion of pentane into a chloroform solution. X-ray diffraction data were collected on a Bruker APEX 2 CCD platform diffractometer (Mo Kα (l = 0.71073 Å)) at 125 K, with the crystals mounted on a nylon loop with Paratone-N cryoprotectant oil. The structures were solved using direct methods (SHELXT 2018/2)62 and standard difference map techniques and were refined by full-matrix least-squares procedures on F2 (SHELXL 2017/1).63 In PtIVDTh, a two-fold disorder of the cyclohexyl fragment was modeled and refined (FVAR 0.677(9)) with the help of similarity restraints on bond lengths and angles as well as on displacement parameters. In PtIIMth, a slight (∼10%) two-fold rotational disorder in the S2-containing thiophene did not model sufficiently well and was left unmodeled. All nonhydrogen atoms were refined anisotropically.
X-ray Total Scattering Measurements and Pair Distribution Function Analysis Data Reduction
Total X-ray scattering measurements
were performed at Brookhaven National Laboratory using the 28-ID-2
(XPD) high-energy X-ray powder diffraction beamline at the National
Synchrotron Light Source II (NSLS-II). X-ray scattering data were
collected using a large-area 2D PerkinElmer detector (2048 ×
2048 pixels, 200 × 200 μm2 each) in RA-PDF mode
with a sample-to-detector distance of 224 mm.64 The incident energy of the X-rays was 67.13 keV (λ = 0.1847
Å). Solid samples were loaded into 1.5 mm Kapton polyimide tubes,
and the capillaries were sealed with modeling clay. Beamline calibration
was performed with a Ni powder standard. 2D detector intensity images
were azimuthally integrated using PyFAI to 1D I(Q) curves, where 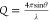 is the magnitude of the elastic scattering
momentum transfer.65 Subtraction of the
sample holder background, polarization correction, normalization to
the reduced total scattering structure function F(Q), and Fourier transformation to obtain the pair
distribution function G(r) were
carried out using PDFgetX3 implemented in xPDFsuite.66,67 The range of scattering vectors used in the Fourier transform to
obtain G(r) was chosen to optimize
the trade-off between real-space resolution and statistical noise
(Qmax = 18 Å–1, Figure S40). PDF simulations of G(r) were performed using custom code written in
Python utilizing PDF calculators in the DiffPy-CMI complex modeling68 framework, and structural refinement was performed
in PDFGui,69 and the refined parameters
and output are listed in Table S4.
is the magnitude of the elastic scattering
momentum transfer.65 Subtraction of the
sample holder background, polarization correction, normalization to
the reduced total scattering structure function F(Q), and Fourier transformation to obtain the pair
distribution function G(r) were
carried out using PDFgetX3 implemented in xPDFsuite.66,67 The range of scattering vectors used in the Fourier transform to
obtain G(r) was chosen to optimize
the trade-off between real-space resolution and statistical noise
(Qmax = 18 Å–1, Figure S40). PDF simulations of G(r) were performed using custom code written in
Python utilizing PDF calculators in the DiffPy-CMI complex modeling68 framework, and structural refinement was performed
in PDFGui,69 and the refined parameters
and output are listed in Table S4.
Preparation of Compounds
See the Supporting Information for additional experimental details, including copies of NMR spectra, UV/vis spectra, and emission spectra. [Pt2Me4(μ-SMe2)2] was prepared according to the literature.70PtIIMPh and PtIVDPh were synthesized according to the literature19,20 and using a microwave reactor. For example, 0.260 g of [Pt2Me4(μ-SMe2)2] and 0.0391g of racemic trans-1,2-(N=CHC4H2SBr)2C6H10 were dissolved in 10 mL of acetone in a CEM microwave reactor vial. The microwave was set at 40 °C and 100 psi for 30 min. The solvent was removed under vacuum, and the resulting solid was triturated with several 1.5 mL portions of diethyl ether before being dried under vacuum. This afforded a gray product (Yield, 0.0303 g, 74.5%).
Ligand A
Racemic trans-1,2-(N=CHC4H3S)2C6H10
To a solution of 3-thiophenecarboxaldehyde (891 mg, 8.0 mmol) in DCM (20 mL), racemic trans-1,2,-diaminocyclohexane (465 mg, 4.07 mmol) was added. Excess magnesium sulfate was added to remove water formed during the reaction. The reaction was stirred at room temperature for 6 h. MgSO4 was removed by gravity filtration. The solvent was removed under vacuum, resulting in a light brown solid. Yield: 62%. 1H NMR (400 MHz, CDCl3): δ = 1.47 [m, 2H, Cy(H)], 1.76 [m, 2H, Cy(H)], 1.84 [m, 4H, Cy(H)], 3.30 [m, 2H, Cy(H)], 7.22 [dd, 2H], 7.40 [dd, 2H], 7.45 [dd, 2H], 8.18 [s, 2H, N=CH]. 13C NMR (100 MHz, CDCl3) δ = 24.6, 33.1, 73.9, 125.9, 126.1, 127.9, 140.7, 155.5. Elemental analysis, % calculated for C16H18N2S2: C, 63.54; H, 6.00; N, 9.26; Found: C, 62.23; H, 5.72; N, 9.00.
Ligand B
Racemic trans-1,2-(N=CHC4H2SBr)2C6H10
To a solution of racemic trans-1,2-diaminocyclohexane (0.184 g) in diethyl ether (10 mL), 2-bromothiophene-3-carbaldehyde (0.366 g) was added. 1 mL of 3 Å molecular sieves was added to remove water formed during the reaction. The mixture was stirred at room temperature for 14 h. The ether was removed by evaporation. 15 mL of DCM was added to the residue with the sieves and brought to a boil. The mixture was filtered while hot by gravity to remove the sieves. The resulting solution was placed in a −20 °C freezer for 24 h. The resulting white solid was collected with a Hirsch funnel and dried under vacuum. Yield: 37% (0.182 g). 1H NMR (400 MHz, CDCl3): δ = 1.48 [m, 2H, Cy(H)], 1.79, 1.85 [m, 6H, Cy(H)], 3.36 [m, 2H, Cy(H)], 7.15 [d, 2H], 7.33 [d, 2H], 8.16 [s, 2H, N=CH]. 13C NMR (100 MHz, CDCl3) δ = 24.6, 33.0, 73.8, 116.7, 126.0, 126.6, 137.8, 154.6. Elemental analysis, % calculated for C16H16Br2N2S2: C, 41.76; H, 3.50; N, 6.09; Found: C, 41.66; H, 3.40; N, 5.94.
PtIIMth
To a solution of ligand A (74 mg) in diethyl ether, [Pt2Me4(μ-SMe2)2] (70 mg) was added. Within minutes, a bright orange precipitate formed. The mixture was stirred for 14 h, and then the solvent was removed under vacuum. The resulting solid was recrystallized from DCM/pentane. The resulting red solid was isolated and washed with 2 mL of ice cold ether and 3 × 2 mL of pentane and then dried under vacuum. Yield: 73% (91 mg). 1H NMR (400 MHz, CDCl3): δ = 0.99 [s, 3H, 2J(PtH) = 83 Hz, Pt-Me], 1.40 [br, m, 2H, Cy(H)], 1.59 [m, 2H, Cy(H)], 2.01 [m, 2H, Cy(H)], 2.41 [d, 1H, Cy(H)], 2.58 [d, 1H, Cy(H)], 3.65 [t, 1H, Cy(H)], 4.21 [t, 1H, Cy(H)], 7.01 [d, 1H, 4J(PtH) = 32], 7.20 [d, 1H], 7.29 [dd, 1H], 7.95 [d, 1H], 8.45 [s, 1H, 3J(PtH) = 52 Hz], 8.48 [d, 1H], 8.86 [s, 1H, 3J(PtH) = 63]. 13C NMR (100 MHz, CDCl3) δ = −20.8 (CH3) 1J(PtC) = 734 Hz, 24.6, 24.8, 30.0 J(PtC) = 12.9 Hz, 30.2, 68.6 J(PtC) = 17.4 Hz, 76.0 J(PtC) = 25.3 Hz, 97.4, 123.3 J(PtC) = 59.7 Hz, 125.6 J(PtC) = 58.2 Hz, 128.5, 133.0, 136.7, 149.3, 155.8 J(PtC) = 20.0 Hz, 156.5, 157.1 J(PtC) = 74.6 Hz. ESI-HR-MS (m/z): Calc. for C17H20N2PtS2Cl: 545.0389, 546.0410, 547.0411 Found: 545.0400, 546.0424, 547.0426. Elemental analysis, % calculated for C17H20N2PtS2: C, 39.91; H, 3.94; N, 5.48; Found: C, 39.58; H, 3.74; N, 5.32.
PtIVDTh
To a solution of ligand B (0.046 g) in toluene (10 mL), complex [Pt2Me4(μ-SMe2)2] (0.055 g) was added, and the mixture was refluxed for 3 h. The solution was filtered through a 2 cm plug of Celite. The filtrate’s solvent was removed under vacuum and redissolved in 2 mL of DCM. A vial-in-vial recrystallization was performed using that solution and pentane. The resulting orangish solid was isolated and washed with 2 mL of ice cold ether and 3 × 2 mL of pentane and then dried under vacuum. Yield: 59 mg; 67%. 1H NMR (400 MHz, CDCl3) δ = 1.07 [s, 3H, 2J(PtH) = 75 Hz], 1.25 [s, 3H, 2J(PtH) = 73 Hz], 1.58 [m, 2H, Cy(H)], 1.88 [s, 3H, 2J(PtH) = 64 Hz], 2.03 [m, 6H, Cy(H)], 2.13 [s, 3H, 2J(PtH) = 68 Hz), 3.99 [m, 1H, Cy(H)], 5.67 [m, 1H, Cy(H)], 7.11 [m, 4H], 8.17 [s, 1H, 3J(PtH)=38 Hz], 8.49 (s, 1H, 3JPt–H = 46 Hz]. 13C NMR (100 MHz, CDCl3) δ = −7.6 (CH3) 1J(PtC) = 583 Hz, −1.0 (CH3) 1J(PtC) = 604 Hz, 1.9 (CH3) 1J(PtC) = 623 Hz, 2.3 (CH3) 1J(PtC) = 616 Hz, 25.4, 25.6, 35.0, 38.5, 63.2 J(PtC) = 11.0 Hz, 78.6, 124.8 J(PtC) = 38.0 Hz, 124.9 J(PtC) = 38.0 Hz, 125.0, 125.3 J(PtC) = 52.0 Hz, 141.7, 142.9, 149.9, 150.6, 163.3 2J(PtC) = 44.0 Hz, 166.1 2J(PtC) = 46.00 Hz. ESI-HR-MS (m/z): Calc. for C20H28Br2N2Pt2S2Cl: m/z 943.9003, 944.9024, 945.9026; found: 943.8952, 944.9014, 945.9074. Elemental analysis, % calculated for C20H28Br2N2Pt2S2: C, 26.38; H, 3.10; N, 3.08; Found: C, 27.22; H, 2.97; N, 2.98.
Acknowledgments
This material is based upon work supported by the U.S. National Science Foundation under CHE-1665435 (C.M.A., P.I.). The authors also acknowledge the Bard Summer Research Institute (BSRI) for support. This research used X-ray powder diffraction (XPD) beamline 28-ID-2 of the National Synchrotron Light Source II, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Brookhaven National Laboratory (BNL) under contract no. DE-SC0012704. Mass spectrometry was run at Vassar College with the help of Karen Wovkulich, and the authors thank her profusely. They thank Sanjit K. Ghose for valuable discussions on X-ray PDF data and assistance during beamline measurements.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.3c05567.
The authors declare no competing financial interest.
Supplementary Material
References
- Yam V. W.-W.; Law A. S.-Y. Luminescent d8 Metal Complexes of Platinum(II) and Gold(III): From Photophysics to Photofunctional Materials and Probes. Coord. Chem. Rev. 2020, 414, 213298 10.1016/j.ccr.2020.213298. [DOI] [Google Scholar]
- Cutillas N.; Yellol G. S.; de Haro C.; Vicente C.; Rodríguez V.; Ruiz J. Anticancer Cyclometalated Complexes of Platinum Group Metals and Gold. Coord. Chem. Rev. 2013, 257 (19–20), 2784–2797. 10.1016/j.ccr.2013.03.024. [DOI] [Google Scholar]
- Ma D.-L.; Ma V. P.-Y.; Chan D. S.-H.; Leung K.-H.; He H.-Z.; Leung C.-H. Recent Advances in Luminescent Heavy Metal Complexes for Sensing. Coord. Chem. Rev. 2012, 256 (23–24), 3087–3113. 10.1016/j.ccr.2012.07.005. [DOI] [Google Scholar]
- Jain V. K. Cyclometalated Group-16 Compounds of Palladium and Platinum: Challenges and Opportunities. Coord. Chem. Rev. 2021, 427, 213546 10.1016/j.ccr.2020.213546. [DOI] [Google Scholar]
- Albrecht M. Cyclometalation Using D-Block Transition Metals: Fundamental Aspects and Recent Trends. Chem. Rev. 2010, 110 (2), 576–623. 10.1021/cr900279a. [DOI] [PubMed] [Google Scholar]
- Newkome G. R.; Puckett W. E.; Gupta V. K.; Kiefer G. E. Cyclometalation of the Platinum Metals with Nitrogen and Alkyl, Alkenyl, and Benzyl Carbon Donors. Chem. Rev. 1986, 86, 451–489. 10.1021/cr00072a006. [DOI] [Google Scholar]
- Amouri H. Luminescent Complexes of Platinum, Iridium, and Coinage Metals Containing N -Heterocyclic Carbene Ligands: Design, Structural Diversity, and Photophysical Properties. Chem. Rev. 2023, 123 (1), 230–270. 10.1021/acs.chemrev.2c00206. [DOI] [PubMed] [Google Scholar]
- Turner E.; Bakken N.; Li J. Cyclometalated Platinum Complexes with Luminescent Quantum Yields Approaching 100%. Inorg. Chem. 2013, 52 (13), 7344–7351. 10.1021/ic302490c. [DOI] [PubMed] [Google Scholar]
- Li G.; Zhao X.; Fleetham T.; Chen Q.; Zhan F.; Zheng J.; Yang Y. F.; Lou W.; Yang Y.; Fang K.; Shao Z.; Zhang Q.; She Y. Tetradentate Platinum(II) Complexes for Highly Efficient Phosphorescent Emitters and Sky Blue OLEDs. Chem. Mater. 2020, 32 (1), 537–548. 10.1021/acs.chemmater.9b04263. [DOI] [Google Scholar]
- Yersin H.; Rausch A. F.; Czerwieniec R.; Hofbeck T.; Fischer T. The Triplet State of Organo-Transition Metal Compounds. Triplet Harvesting and Singlet Harvesting for Efficient OLEDs. Coord. Chem. Rev. 2011, 255 (21–22), 2622–2652. 10.1016/j.ccr.2011.01.042. [DOI] [Google Scholar]
- Dikova Y. M.; Yufit D. S.; Williams J. A. G. Platinum(IV) Complexes with Tridentate, NNC -Coordinating Ligands: Synthesis, Structures, and Luminescence. Inorg. Chem. 2023, 62 (4), 1306–1322. 10.1021/acs.inorgchem.2c04116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliá F.; Bautista D.; Fernández-Hernández J. M.; González-Herrero P. Homoleptic Tris-Cyclometalated Platinum(IV) Complexes: A New Class of Long-Lived, Highly Efficient 3LC Emitters. Chem. Sci. 2014, 5 (5), 1875–1880. 10.1039/C3SC53187B. [DOI] [Google Scholar]
- Vivancos Á.; Jiménez-García A.; Bautista D.; González-Herrero P. Strongly Luminescent Pt(IV) Complexes with a Mesoionic N -Heterocyclic Carbene Ligand: Tuning Their Photophysical Properties. Inorg. Chem. 2021, 60 (11), 7900–7913. 10.1021/acs.inorgchem.1c00410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson C. M.; Brown G.; Greenberg M. W.; Yu D.; Bowen N.; Ahmed R.; Yost-Bido M.; Wray A. Stereoselective C-X and Regioselective C-H Activation to, and Selective C(Sp2)-C(Sp3) Reductive Elimination from, Platinum Compounds with Thiophene-Derived Ligands. Tetrahedron Lett. 2019, 60 (43), 151156 10.1016/j.tetlet.2019.151156. [DOI] [Google Scholar]
- Anderson C. M.; Mastrocinque C.; Greenberg M. W.; McClellan I. C.; Duman L.; Oh N.; Mastrocinque F.; Pizzuto M.; Tran K.; Tanski J. M. Synthesis, Characterization, and Photophysical Properties of Bismetalated Platinum Complexes with Benzothiophene Ligands. J. Organomet. Chem. 2019, 882, 10–17. 10.1016/j.jorganchem.2018.12.010. [DOI] [Google Scholar]
- Rendina L. M.; Puddephatt R. J. Oxidative Addition Reactions of Organoplatinum(II) Complexes with Nitrogen-Donor Ligands. Chem. Rev. 1997, 97 (6), 1735–1754. 10.1021/cr9704671. [DOI] [PubMed] [Google Scholar]
- Crespo M. Diarylplatinum(II) Compounds as Versatile Metallating Agents in the Synthesis of Cyclometallated Platinum Compounds with N-Donor Ligands. Inorganics (Basel) 2014, 2 (1), 115–131. 10.3390/inorganics2010115. [DOI] [Google Scholar]
- Crespo M.; Font-Bardia M.; Granell J.; Martínez M.; Solans X. Cyclometallation on Platinum(Ii) Complexes; the Role of the Solvent and Added Base Donor Capability on the Reaction Mechanisms. J. Chem. Soc., Dalton Trans. 2003, 3 (19), 3763–3769. 10.1039/B306243K. [DOI] [Google Scholar]
- Baar C. R.; Hill G. S.; Vittal J. J.; Puddephatt R. J. Stereoselectivity in Organometallic Reactions: Intramolecular Oxidative Addition of Aryl–Halogen Bonds to Platinum(II). Organometallics 1998, 17 (1), 32–40. 10.1021/om970625e. [DOI] [Google Scholar]
- Baar C. R.; Jenkins H. A.; Vittal J. J.; Yap G. P. A.; Puddephatt R. J. Stereoselectivity in Organometallic Reactions: Oxidative Addition of Alkyl Halides to Platinum(II). Organometallics 1998, 17 (13), 2805–2818. 10.1021/om980065z. [DOI] [Google Scholar]
- Gandioso A.; Valle-Sistac J.; Rodríguez L.; Crespo M.; Font-Bardía M. Platinum(II) Compounds Containing Cyclometalated Tridentate Ligands: Synthesis, Luminescence Studies, and a Selective Fluoro for Methoxy Substitution. Organometallics 2014, 33 (2), 561–570. 10.1021/om401111t. [DOI] [Google Scholar]
- Lázaro A.; Serra O.; Rodríguez L.; Crespo M.; Font-Bardia M. Luminescence Studies of New [C,N,N′] Cyclometallated Platinum(II) and Platinum(IV) Compounds. New J. Chem. 2019, 43 (3), 1247–1256. 10.1039/C8NJ05492D. [DOI] [Google Scholar]
- Amna B.; Siddiqi H. M.; Hassan A.; Ozturk T. Recent Developments in the Synthesis of Regioregular Thiophene-Based Conjugated Polymers for Electronic and Optoelectronic Applications Using Nickel and Palladium-Based Catalytic Systems. RSC Adv. 2020, 10 (8), 4322–4396. 10.1039/C9RA09712K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M. S.; Al-Suti M. K.; Maharaja J.; Haque A.; Al-Balushi R.; Raithby P. R. Conjugated Poly-ynes and Poly(Metalla-ynes) Incorporating Thiophene-Based Spacers for Solar Cell (SC) Applications. J. Organomet. Chem. 2016, 812, 13–33. 10.1016/j.jorganchem.2015.10.003. [DOI] [Google Scholar]
- Kudryashova L. G.; Kazantsev M. S.; Postnikov V. A.; Bruevich V. V.; Luponosov Y. N.; Surin N. M.; Borshchev O. V.; Ponomarenko S. A.; Pshenichnikov M. S.; Paraschuk D. Yu. Highly Luminescent Solution-Grown Thiophene-Phenylene Co-Oligomer Single Crystals. ACS Appl. Mater. Interfaces 2016, 8 (16), 10088–10092. 10.1021/acsami.5b11967. [DOI] [PubMed] [Google Scholar]
- Cao Y.; Wolf M. O.; Patrick B. O. Dual-Emissive Platinum(II) Metallacycles with Thiophene-Containing Bisacetylide Ligands. Inorg. Chem. 2016, 55 (17), 8985–8993. 10.1021/acs.inorgchem.6b01464. [DOI] [PubMed] [Google Scholar]
- Bandini M.; Bianchi M.; Valenti G.; Piccinelli F.; Paolucci F.; Monari M.; Umani-Ronchi A.; Marcaccio M. Electrochemiluminescent Functionalizable Cyclometalated Thiophene-Based Iridium(III) Complexes. Inorg. Chem. 2010, 49 (4), 1439–1448. 10.1021/ic901660m. [DOI] [PubMed] [Google Scholar]
- Anderson C. M.; Coffey B.; Morales L.; Greenberg M. W.; Norman M.; Weinstein M.; Brown G.; Tanski J. M. Platinum Complexes from C-H Activation of Sterically Hindered [C^N] Donor Benzothiophene Imine Ligands: Synthesis and Photophysical Properties. ACS Omega 2020, 5 (41), 26855–26863. 10.1021/acsomega.0c03993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozhevnikov D. N.; Kozhevnikov V. N.; Shafikov M. Z.; Prokhorov A. M.; Bruce D. W.; Gareth Williams J. A. Phosphorescence vs Fluorescence in Cyclometalated Platinum(II) and Iridium(III) Complexes of (Oligo)Thienylpyridines. Inorg. Chem. 2011, 50 (8), 3804–3815. 10.1021/ic200210e. [DOI] [PubMed] [Google Scholar]
- Li K.; Tong G. S. M.; Yuan J.; Ma C.; Du L.; Yang C.; Kwok W.-M.; Phillips D. L.; Che C.-M. Excitation-Wavelength-Dependent and Auxiliary-Ligand-Tuned Intersystem-Crossing Efficiency in Cyclometalated Platinum(II) Complexes: Spectroscopic and Theoretical Studies. Inorg. Chem. 2020, 59 (20), 14654–14665. 10.1021/acs.inorgchem.0c01192. [DOI] [PubMed] [Google Scholar]
- Anderson C. M.; Weinstein M. A.; Morris J.; Kfoury N.; Duman L.; Balema T. A.; Kreider-Mueller A.; Scheetz P.; Ferrara S.; Chierchia M.; Tanski J. M. Biscyclometalated Platinum Complexes with Thiophene Ligands. J. Organomet. Chem. 2013, 723, 188–197. 10.1016/j.jorganchem.2012.10.027. [DOI] [Google Scholar]
- Coffey B.; Clough L.; Bartkus D. D.; McClellan I. C.; Greenberg M. W.; LaFratta C. N.; Tanski J. M.; Anderson C. M. Photophysical Properties of Cyclometalated Platinum(II) Diphosphine Compounds in the Solid State and in PMMA Films. ACS Omega 2021, 6 (42), 28316–28325. 10.1021/acsomega.1c04509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam P.; Climent C.; Alemany P.; Laskar I. R. Aggregation-Induced Emission” of Transition Metal Compounds: Design, Mechanistic Insights, and Applications. J. Photochem. Photobiol. C: Photochem. Rev. 2019, 41, 100317 10.1016/j.jphotochemrev.2019.100317. [DOI] [Google Scholar]
- Zhao J.; Feng Z.; Zhong D.; Yang X.; Wu Y.; Zhou G.; Wu Z. Cyclometalated Platinum Complexes with Aggregation-Induced Phosphorescence Emission Behavior and Highly Efficient Electroluminescent Ability. Chem. Mater. 2018, 30 (3), 929–946. 10.1021/acs.chemmater.7b04708. [DOI] [Google Scholar]
- Pasha S. S.; Alam P.; Dash S.; Kaur G.; Banerjee D.; Chowdhury R.; Rath N.; Roy Choudhury A.; Laskar I. R. Rare Observation of ‘Aggregation Induced Emission’ in Cyclometalated Platinum(II) Complexes and Their Biological Activities. RSC Adv. 2014, 4 (92), 50549–50553. 10.1039/C4RA06623E. [DOI] [Google Scholar]
- Pregosin P. S. Platinum-195 Nuclear Magnetic Resonance. Coord. Chem. Rev. 1982, 44 (2), 247–291. 10.1016/S0010-8545(00)80523-8. [DOI] [Google Scholar]
- Priqueler J. R. L.; Butler I. S.; Rochon F. D. An Overview of 195Pt Nuclear Magnetic Resonance Spectroscopy. Appl. Spectrosc Rev. 2006, 41 (3), 185–226. 10.1080/05704920600620311. [DOI] [Google Scholar]
- Pregosin P. S.Platinum NMR Spectroscopy. In Annual Reports on NMR Spectroscopy; Webb G. A., Ed.. Academic Press: Cambridge, MA, 1986; Vol. 17, pp 285−349. 10.1016/S0066-4103(08)60238-0. [DOI] [Google Scholar]
- McCarthy J. S.; McCormick M. J.; Zimmerman J. H.; Hambrick H. R.; Thomas W. M.; McMillen C. D.; Wagenknecht P. S. Role of the Trifluoropropynyl Ligand in Blue-Shifting Charge-Transfer States in Emissive Pt Diimine Complexes and an Investigation into the PMMA-Imposed Rigidoluminescence and Rigidochromism. Inorg. Chem. 2022, 61 (29), 11366–11376. 10.1021/acs.inorgchem.2c01564. [DOI] [PubMed] [Google Scholar]
- Lu W.; Mi B.-X.; Chan M. C. W.; Hui Z.; Che C.-M.; Zhu N.; Lee S.-T. Light-Emitting Tridentate Cyclometalated Platinum(II) Complexes Containing σ-Alkynyl Auxiliaries: Tuning of Photo- and Electrophosphorescence. J. Am. Chem. Soc. 2004, 126 (15), 4958–4971. 10.1021/ja0317776. [DOI] [PubMed] [Google Scholar]
- Knizia G. Intrinsic Atomic Orbitals: An Unbiased Bridge between Quantum Theory and Chemical Concepts. J. Chem. Theory Comput. 2013, 9 (11), 4834–4843. 10.1021/ct400687b. [DOI] [PubMed] [Google Scholar]
- Billinge S. J. L.; Levin I. The Problem with Determining Atomic Structure at the Nanoscale. Science 2007, 316 (5824), 561–565. 10.1126/science.1135080. [DOI] [PubMed] [Google Scholar]
- Terban M. W.; Billinge S. J. L. Structural Analysis of Molecular Materials Using the Pair Distribution Function. Chem. Rev. 2022, 122, 1208–1272. 10.1021/acs.chemrev.1c00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matassa R.; Carbone M.; Fratoddi I.; Caminiti R. Organometallic Oligomer Resolved by Radial Distribution Function of X-Ray Diffraction Analysis. J. Phys. Chem. B 2010, 114 (7), 2359–2364. 10.1021/jp9099896. [DOI] [PubMed] [Google Scholar]
- Gorelik T. E.; Schmidt M. U.; Kolb U.; Billinge S. J. L. Total-Scattering Pair-Distribution Function of Organic Material from Powder Electron Diffraction Data. Microsc. Microanal. 2015, 21 (2), 459–471. 10.1017/S1431927614014561. [DOI] [PubMed] [Google Scholar]
- Belviso B. D.; Marin F.; Fuertes S.; Sicilia V.; Rizzi R.; Ciriaco F.; Cappuccino C.; Dooryhee E.; Falcicchio A.; Maini L.; Altomare A.; Caliandro R. Structural Insights into the Vapochromic Behavior of Pt- and Pd-Based Compounds. Inorg. Chem. 2021, 60 (9), 6349–6366. 10.1021/acs.inorgchem.1c00081. [DOI] [PubMed] [Google Scholar]
- Macrae C. F.; Sovago I.; Cottrell S. J.; Galek P. T. A.; McCabe P.; Pidcock E.; Platings M.; Shields G. P.; Stevens J. S.; Towler M.; Wood P. A. Mercury 4.0: From Visualization to Analysis, Design and Prediction. J. Appl. Crystallogr. 2020, 53 (1), 226–235. 10.1107/S1600576719014092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Transue W. J.; Nava M.; Terban M. W.; Yang J.; Greenberg M. W.; Wu G.; Foreman E. S.; Mustoe C. L.; Kennepohl P.; Owen J. S.; Billinge S. J. L.; Kulik H. J.; Cummins C. C. Anthracene as a Launchpad for a Phosphinidene Sulfide and for Generation of a Phosphorus-Sulfur Material Having the Composition P 2 S, a Vulcanized Red Phosphorus That Is Yellow. J. Am. Chem. Soc. 2019, 141 (1), 431–440. 10.1021/jacs.8b10775. [DOI] [PubMed] [Google Scholar]
- Ji W.; Kim D. M.; Posson B. M.; Carlson K. J.; Chew A. C.; Chew A. J.; Hossain M.; Mojica A. F.; Ottoes S. M.; Tran D. V.; Greenberg M. W.; Hamachi L. S. COF-300 Synthesis and Colloidal Stabilization with Substituted Benzoic Acids. RSC Adv. 2023, 13 (21), 14484–14493. 10.1039/D3RA02202A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prill D.; Juhás P.; Schmidt M. U.; Billinge S. J. L. Modelling Pair Distribution Functions (PDFs) of Organic Compounds: Describing Both Intra- and Intermolecular Correlation Functions in Calculated PDFs. J. Appl. Crystallogr. 2015, 48 (1), 171–178. 10.1107/S1600576714026454. [DOI] [Google Scholar]
- Rademacher N.; Daemen L. L.; Chronister E. L.; Proffen T. Pair Distribution Function Analysis of Molecular Compounds: Significance and Modeling Approach Discussed Using the Example of p -Terphenyl. J. Appl. Crystallogr. 2012, 45 (3), 482–488. 10.1107/S0021889812016159. [DOI] [Google Scholar]
- Neese F. The ORCA Program System. Wiley Interdiscip. Comput. Mol. Sci. 2012, 2 (1), 73–78. 10.1002/wcms.81. [DOI] [Google Scholar]
- Neese F. Software Update: The ORCA Program System, Version 4.0. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2018, 8 (1), e1327 10.1002/wcms.1327. [DOI] [Google Scholar]
- Becke A. D. Density-functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys. 1993, 98 (7), 5648–5652. 10.1063/1.464913. [DOI] [Google Scholar]
- Lee C.; Yang W.; Parr R. G. Development of the Colle-Salvetti Correlation-Energy Formula into a Functional of the Electron Density. Phys. Rev. B 1988, 37 (2), 785–789. 10.1103/PhysRevB.37.785. [DOI] [PubMed] [Google Scholar]
- Hay P. J.; Wadt W. R. Ab Initio Effective Core Potentials for Molecular Calculations. Potentials for the Transition Metal Atoms Sc to Hg. J. Chem. Phys. 1985, 82 (1), 270–283. 10.1063/1.448799. [DOI] [Google Scholar]
- Pantazis D. A.; Chen X.-Y.; Landis C. R.; Neese F. All-Electron Scalar Relativistic Basis Sets for Third-Row Transition Metal Atoms. J. Chem. Theory Comput. 2008, 4 (6), 908–919. 10.1021/ct800047t. [DOI] [PubMed] [Google Scholar]
- Suzuki K.; Kobayashi A.; Kaneko S.; Takehira K.; Yoshihara T.; Ishida H.; Shiina Y.; Oishi S.; Tobita S. Reevaluation of Absolute Luminescence Quantum Yields of Standard Solutions Using a Spectrometer with an Integrating Sphere and a Back-Thinned CCD Detector. Phys. Chem. Chem. Phys. 2009, 11 (42), 9850. 10.1039/b912178a. [DOI] [PubMed] [Google Scholar]
- Van Houten J.; Watts R. J. Temperature Dependence of the Photophysical and Photochemical Properties of the Tris(2,2’-Bipyridyl)Ruthenium(II) Ion in Aqueous Solution. J. Am. Chem. Soc. 1976, 98 (16), 4853–4858. 10.1021/ja00432a028. [DOI] [Google Scholar]
- Nakamaru K. Synthesis, Luminescence Quantum Yields, and Lifetimes of Trischelated Ruthenium(II) Mixed-Ligand Complexes Including 3,3′-Dimethyl-2,2′-Bipyridyl. Bull. Chem. Soc. Jpn. 1982, 55 (9), 2697–2705. 10.1246/bcsj.55.2697. [DOI] [Google Scholar]
- Lakowicz J. R.Principles of Fluorescence Spectroscopy, 3rd ed.; Lakowicz J. R., Ed.; Springer US: USA, 2006. [Google Scholar]
- Sheldrick G. M. A Short History of SHELX. Acta Crystallogr. A 2008, 64 (1), 112–122. 10.1107/S0108767307043930. [DOI] [PubMed] [Google Scholar]
- Sheldrick G. M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. C Struct Chem. 2015, 71 (1), 3–8. 10.1107/S2053229614024218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chupas P. J.; Qiu X.; Hanson J. C.; Lee P. L.; Grey C. P.; Billinge S. J. L. Rapid-Acquisition Pair Distribution Function (RA-PDF) Analysis. J. Appl. Crystallogr. 2003, 36 (6), 1342–1347. 10.1107/S0021889803017564. [DOI] [Google Scholar]
- Ashiotis G.; Deschildre A.; Nawaz Z.; Wright J. P.; Karkoulis D.; Picca F. E.; Kieffer J. The Fast Azimuthal Integration Python Library: PyFAI. J. Appl. Crystallogr. 2015, 48 (2), 510–519. 10.1107/S1600576715004306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juhás P.; Davis T.; Farrow C. L.; Billinge S. J. L. PDFgetX3: A Rapid and Highly Automatable Program for Processing Powder Diffraction Data into Total Scattering Pair Distribution Functions. J. Appl. Crystallogr. 2013, 46 (2), 560–566. 10.1107/S0021889813005190. [DOI] [Google Scholar]
- Yang X.; Juhas P.; Farrow C. L.; Billinge S. J. L.. XPDFsuite: An End-to-End Software Solution for High Throughput Pair Distribution Function Transformation, Visualization and Analysis. ArXiv (Condensed Matter > Material Science) February 13, 2014, 1402.3163ver. 3. https://arxiv.org/abs/1402.3163.
- Juhás P.; Farrow C. L.; Yang X.; Knox K. R.; Billinge S. J. L. Complex Modeling: A Strategy and Software Program for Combining Multiple Information Sources to Solve Ill Posed Structure and Nanostructure Inverse Problems. Acta Crystallogr. A Found. Adv. 2015, 71 (6), 562–568. 10.1107/S2053273315014473. [DOI] [PubMed] [Google Scholar]
- Farrow C. L.; Juhas P.; Liu J. W.; Bryndin D.; Božin E. S.; Bloch J.; Proffen T.; Billinge S. J. L. PDFfit2 and PDFgui: Computer Programs for Studying Nanostructure in Crystals. J. Phys.: Condens. Matter 2007, 19 (33), 335219 10.1088/0953-8984/19/33/335219. [DOI] [PubMed] [Google Scholar]
- Scott J. D.; Puddephatt R. J. Ligand Dissociation as a Preliminary Step in Methyl-for-Halogen Exchange Reactions of Platinum(II) Complexes. Organometallics 1983, 2 (11), 1643–1648. 10.1021/om50005a028. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




