Abstract

Background Indomethacin as a non-steroidal anti-inflammatory drug (NSAID) is commonly used to treat some ocular inflammatory disorders. Unfortunately, indomethacin is a drug that is poorly soluble in water; therefore, it has low efficacy. An attractive approach is the targeted delivery of indomethacin to the cornea using cationic dextran stearate as a polymeric micelle drug carrier. Methods A dextran stearate-glycidyl trimethylammonium chloride (Dex-St-GTMAC) copolymer was prepared through the reaction of GTMAC, stearoyl chloride, and dextran. Then, Dex-St-GTMAC was characterized by Fourier transform infrared (FT-IR) spectroscopy and 1H NMR spectroscopy. Dex-St-GTMAC forms micelles in the presence of indomethacin. The prepared polymeric micelles were characterized for size, ζ-potential, drug loading, particle morphology, critical micelle concentration, and encapsulation efficiency. To study the irritation potential of the indomethacin-loaded Dex-St-GTMAC, Het-Cam and Draize tests have been performed. Prepared cationic micelles were subjected to the in vitro drug release and ex vivotrans-corneal permeation test. Results The dialysis method was used for the preparation of indomethacin-loaded micelles (10, 20, and 30%). Measurement of the particle size showed a mean diameter of 122.1 and 150.9 nm for the drug-loaded micelles. Scanning electron microscopy (SEM) images showed that the morphology of the particles is spherical. 10% formulation was chosen as the best formulation due to more surface charge and reasonable drug loading. ζ-potential measurement for the 10% drug-containing micelles showed a value of +39.1 mV. Drug loading efficiency and the encapsulation efficiency for 10% drug-containing micelles were 6.36 and 63.61%, respectively. The results of the Het-Cam and Draize tests indicated that the indomethacin-loaded Dex-St-GTMAC formulation had no toxicity to eye tissues. Based on our results, the prepared micelles (indomethacin-loaded Dex-St-GTMAC) exhibited a sustained drug release pattern compared to the control group. Indomethacin penetration from the micelles to the excised bovine cornea was 1.75-fold greater than the control (indomethacin 0.1% in phosphate-buffered saline (PBS)). Conclusions Data from the ζ-potential, SEM, drug loading capacity, and in vitro drug release studies indicated that cationic dextran stearate polymeric micelles are an appropriate carrier for the efficient penetration of indomethacin into cornea tissues.
Introduction
In drug delivery systems, the local use of drugs or target therapy has many advantages, and the most important of them is the reduction of systemic side effects of drugs.1 An important drawback of the target therapy method for ocular drug delivery is the presence of many physiological barriers such as blood–ocular barriers, which prevent the drug from reaching the posterior parts of the eye.2 In general, absorption mechanisms from the eye area can be done in two general ways: (1) corneal absorption and (2) noncorneal absorption.3 In noncorneal absorption, drug absorption occurs through the conjunctiva and sclera of the eye. Due to the large surface area and space, the sclera and conjunctiva of the eye are suitable ways to deliver proteins, peptides, and nucleic acids. However, owing to the presence of many blood vessels, the drug enters the systemic blood flow and is away from the eye area.4 In corneal absorption, the structure of the cornea is a barrier to drug absorption from this area due to the presence of different layers that are different in terms of hydrophilicity and lipophilicity. In addition, eye support mechanisms such as tear production and blinking decrease the drug’s retention time in the cornea. All these factors cause the corneal and noncorneal absorption of the drug to be significantly reduced.4
Today designing a drug delivery system to target a specific eye tissue is the main challenge for researchers in this field.5 A specific solution to ocular drug delivery problems is the use of amphiphilic polymer micelles as excellent nanocarriers. Drug-loaded polymeric micelles are used for ocular drug delivery to improve drug release because they have precious biological advantages such as nontoxicity, biocompatibility, biodegradability, and controlled drug release.6 These polymers can help to solubilize the hydrophobic drugs by using their core–shell structure.7 If the polymer micelle is cationic, the drug delivery to the cornea is better because the cationic property results in a longer residence time for micelles, and they attract the negatively charged cornea and anionic tear film mucins. So, positively charged micelles are a promising vehicle for the topical delivery of hydrophobic drugs for ocular penetration.6,8
In continuation of our studies on the synthesis of polymeric micelles and their use in drug delivery,9,10 in this study, we prepared indomethacin-loaded cationic micelles formed from the reaction of glycidyl trimethylammonium chloride (GTMAC)11 and dextran stearate12 to evaluate the ability of indomethacin penetration.
Results and Discussion
Characterization of the Synthesized Polymer (Dex-St-GTMAC)
FT-IR Study
The Fourier transform infrared (FT-IR) spectra of the dextran and dextran stearate-glycidyl trimethylammonium chloride (Dex-St-GTMAC) polymer are presented in Figure 1. The broadband peak in the 3100–3600 cm–1 range is related to the O–H stretching vibrations. Two peaks at 2919 and 2855 cm–1 show the stretching vibrations of the aliphatic C–H. A weak band at 1735 cm–1 was assigned to the carbonyl group of the ester, which confirmed the binding of the stearate group to the dextran. This peak is seen when the stearate group is attached to polysaccharides.10,13
Figure 1.
FT-IR spectra of dextran and Dex-St-GTMAC.
1H NMR Study
The 1H NMR spectrum confirms the synthesis of Dex-St-GTMAC (Figure 2). The absorption peak at δ = 0.83 ppm was assigned to the terminal CH3 group of the stearate. CH2 groups of the stearate appeared in δ = 1.23 and 1.55 ppm. The region of St peaks in our synthesized polymer is similar to those of previously reported papers. The peaks that appeared in 3.15, 3.43, 3.63, 3.92, 4.89, and 5.09 ppm regions are related to the dextran backbone.12,13 The peaks that appeared in the 2.77 and 2.92 ppm belong to the GTMAC group. The peak that appeared in the region of 4.34 ppm is related to the GTMAC group, which is also reported in Rwei’s paper.14
Figure 2.

1H NMR spectrum of Dex-St-GTMAC in DMSO-d6.
Critical Micelle Concentration (CMC) Assay
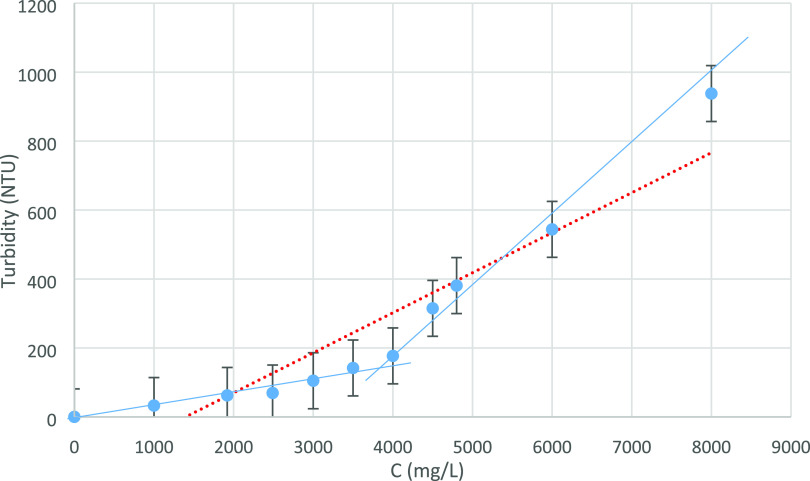
Concentrations of 0 to 8000 mg/L of the synthesized polymer were prepared in deionized water, and its turbidity was measured using the turbidimeter. Figure 3 shows the graph of the results. As seen in the figure, a sudden change in the turbidity of the solution occurs at a concentration of 3700 mg/L, which indicates the formation of micelles at this concentration.
Figure 3.
CMC determination of Dex-St-GTMAC by the turbidity method.
Characterization of Indomethacin-Loaded Polymeric Micelles
Scanning Electron Microscopy (SEM)
The morphology of the obtained micelles is determined by the SEM technique. Scanning electron microscopy (SEM) micrograph of micelles containing indomethacin-loaded Dex-St-GTMAC shows the round homogeneously dispersed particles with an ordered spherical shape (Figure 4).
Figure 4.

SEM micrograph of an indomethacin-loaded polymeric micelle.
Particle Size and ζ-Potential Measurements
Particle size notably affects the drug release, biodistribution, and stability of the formulation.15 Our results indicated that the particle size of the indomethacin-loaded Dex-St-GTMAC nanoparticles ranged from 122.1 to 150.9 nm with PDI values in the 0.379–0.540 range, respectively. It can be depicted that the particle size of the developed nanoparticles is directly proportional to the drug and polymer concentration, which is increased by changing the drug:polymer ratio from 10 to 20 and 30%. NPs with a size range usually <400 nm are appropriate for ophthalmic uses.16
The ζ-potential value of a polymeric micelle affects the stability of NPs as well as the permeability of an encapsulated drug across the corneal barrier.6 ζ-potentials for indomethacin-loaded Dex-St-GTMAC were measured, which showed the surface charge of +39.1 ± 8.83, + 38.5 ± 7.66, and +18.6 ± 6.32 mV for 10, 20, and 30% formulations, respectively. This positive charge is due to the presence of GTMAC moieties in the Dex-St structure, which are present on the surface of the nanoparticles (NPs). Considering that the surface charge is partially positive, Dex-St-GTMAC nanoparticles containing indomethacin have sufficient repulsion force to prevent aggregation during long-term storage. SEM images proved this claim.
In agreement with our results, Li et al. reported positive values for the ζ-potential of poly(ethylene glycol)-poly(ε-caprolactone)-g-polyethylenimine (PEG–PCL-g-PEI) triblock copolymer nanoparticles and stated that the presence of positive charge on the nanoparticle surface increases corneal penetration.6
Encapsulation Efficiency (EE) and Drug Loading Capacity (DL)
The high encapsulation efficiency is because of the high affinity of the drug and the modified polymer in a similar media (aqueous or organic phase).17 Encapsulation efficiency of the indomethacin-loaded Dex-St-GTMAC NPs was found to be 63.61, 106.28, and 1.51% for 10, 20, and 30% formulations, respectively. Also, drug loading of the NPs was found to be 63.36, 21.25, and 0.18% for 10, 20, and 30% formulations, respectively (Table 1).
Table 1. Physical Characteristics of Dex-St-GTMAC Polymeric Micelles.
| formulation | size (nm) | PDI | DL% w/w | EE% w/w | ζ-potential (mv) |
|---|---|---|---|---|---|
| 10% formula | 122.1 | 0.379 | 6.36 ± 0.41 | 63.61% ± 4.13 | +39.1 ± 8.83 |
| 20% formula | 150.9 | 0.540 | 21.25 ± 2.98 | 106.28% ± 14.91 | +38.5 ± 7.66 |
| 30% formula | 128.9 | 0.431 | 0.18 ± 0.0 | 1.51% ± 0.52 | +18.6 ± 6.32 |
After several measurements, the repeatability of the 10% formulation was better than the others in terms of drug loading. Also, its surface charge was almost equivalent to 20% formulation, but compared to 30%, it was higher. The repeatability of the 20% formulation was not as good as that of the 10% formulation. Also, the drug loading in the 30% formulation was very low. Therefore, we chose the 10% formulation as the final formulation because it had a higher ζ-potential and its drug loading was also more reasonable.
In Vitro Drug Release
To determine the drug release behaviors of indomethacin-loaded Dex-St-GTMAC polymeric micelles, release experiments were performed in phosphate-buffered saline (PBS, pH 7.4) by the high-performance liquid chromatography (HPLC) analysis technique. The calibration curve and plot of % cumulative drug release are represented in Figures 5 and 6. As illustrated in Figure 6, 10% indomethacin-loaded micelles showed a small burst release of indomethacin at the first 1.5 h of the experiment, which was followed by sustained release in the next 4.5 h. It should be noted that release efficiency of our formulation after 6 h was 73.5%, which was lower than that of indomethacin 0.1% in PBS (release efficiency = 86.3%). The burst release is related to the high concentrations of drug molecules that are presented on the surfaces of the micelles. The sustained release of indomethacin from the Dex-St-GTMAC micelle is the result of interaction between these hydrophobic indomethacin molecules and the lipophilic core of the micelles that acts as a reservoir of the drug which releases drug molecules gradually. In the literature, similar cationic polymeric nanoparticle-based drug delivery systems indicate similar release behaviors. For example, Mohsen has shown the sustained release of terconazole from terconazole-loaded cationic Eudragit RLPO polymeric nanoparticles.8
Figure 5.
Calibration curve of indomethacin.
Figure 6.
In vitro indomethacin release from the solution (indomethacin 0.1% in PBS) and indomethacin-loaded Dex-St-GTMAC micelles.
Ex Vivotrans-Corneal Permeation
The permeability of the indomethacin solution (indomethacin 0.1% in PBS) and indomethacin-loaded Dex-St-GTMAC micelles (10% formulation) into the corneal tissue was evaluated using isolated corneas from bovine eyes (Figure 7). Our results showed that the indomethacin-loaded Dex-St-GTMAC micelles had a higher permeability coefficient compared to the indomethacin solution (3.44 ± 0.83 × 10–6 vs 1.96 ± 1.25 × 10–6 cm/s). Also, the relatively apparent permeability coefficient can be calculated from the ratio of the permeability of the indomethacin-loaded Dex-St-GTMAC micelles to the control sample (indomethacin solution). This ratio was 1.75.
Figure 7.
In vitro indomethacin permeation from the solution (indomethacin 0.1% in PBS) and indomethacin-loaded Dex-St-GTMAC micelles.
In agreement with the research of Li et al.,6 it seems that the nanoparticle structure of Dex-St-GTMAC micelles with a positive surface charge was able to overcome the corneal barrier and efficiently load and deliver indomethacin.
HET-CAM Assay
As seen in Figure 8, immediately after adding 0.5 M NaOH to the hen’s egg test-chorioallantoic membrane (CAM) surface, blood vessels began to bleed. After 30 s, coagulation was observed, and it became more severe over time. The average cumulative score of NaOH 0.5 M (20.33) indicates the great irritation capacity of NaOH to the ocular tissue. Figure 8 shows that 30 s after the CAM surface was exposed to acetone, minor damage to the capillary walls occurred. After 2 min, vein branches and capillaries began to bleed, and after 5 min, coagulation spots were obviously observed on the CAM surface (score = 11.66). Thirty seconds after the instillation of PG as the positive control, CAM capillaries showed signs of mild hyperemia, which further increased within 2 and 5 min (score = 5.66). After the administration of phosphate buffer (PBS as the negative control), any noticeable vascular injury was not observed. To determine the toxicity effects of developed indomethacin-loaded Dex-St-GTMAC (10% formulation), nanoparticles were applied to the CAM surface and monitored for 5 min. HET-CAM assay results show that the new formulation of indomethacin-loaded Dex-St-GTMAC did not cause any ocular toxicity, including hyperemia, hemorrhage, or clotting/coagulation in egg embryos. Given the high accuracy of the HET-CAM assay, our new formulation is considered safe and nonirritant for ophthalmic administration. Table 2 shows the calculated average cumulative score of stimulations that occurred with each formulation.
Figure 8.

Irritation reactions of the CAM surface after instillation of (A) NaOH (0.5 M), (B) acetone, (C) indomethacin-loaded Dex-St-GTMAC (10% formulation), and (D) propylene glycol.
Table 2. Calculated Mean of the Cumulative Score in the HET-CAM Test.
| cumulative score | ||
|---|---|---|
| formulation | (means ± SD; n = 3) | irritation assessment |
| NaOH 0.5 M (control+) | 20.33 ± 1.15 | severe |
| acetone (control+) | 11.66 ± 0.94 | severe |
| PG (control+) | 5.66 ± 2.35 | moderate |
| PBS (control−) | 0 | nonirritant |
| indomethacin-loaded Dex-St-GTMAC (10% formulation) | 0 | nonirritant |
In Vivo Ocular Irritation Study (Draize Test)
The Draize assay is an excellent ocular toxicity test officially described in the Organization for Economic Cooperation and Development Guidelines for regulating the categorization of irritating chemical substances.8 The Draize trial results showed that all of the tested groups caused no irritation or any irregular changes to the rabbits’ eyes at all times investigated (Figure 9). The irritation index was zero for the tested groups indicating the suitability of the developed cationic polymeric NPs (10% formulation) for ocular delivery of indomethacin. The cornea, iris, and conjunctiva tissues of the rabbits were visually determined for any appearance of irritant reactions after 1–6 h of instillation of control solutions and indomethacin-loaded Dex-St-GTMAC nanoparticles (10% formulation). Based on Table 5, the score of irritation potential was calculated for each test substance, and the results are shown in Table 3. Figure 9 shows that 1 h after the administration of SDS 1%w/v (as positive control), severe ocular irritation including conjunctival discharge, chemosis, and redness was observed (score = 2.33). After the second hour, chemosis and redness were still observed, but there was no sign of conjunctival discharge (score = 1). And after 3 h, redness and chemosis gradually decreased until disappeared during 6 h. These results revealed the high irritancy potential of SDS on the eye tissue of the rabbit. In the group treated with PBS (as a negative control, pH = 7.4), no considerable sign of irritation reactions was detected in the cornea, iris, and conjunctiva tissues (score = 0). One of the groups was treated with Indomethacin 0.1% in PBS and no signs of irritation reactions were observed in the conjunctiva, iris, and cornea (score = 0). After the instillation of indomethacin-loaded Dex-St-GTMAC formulation to the lower cul-de-sac of the conjunctiva, a partial discharge was observed, but the cornea and conjunctival vessels were completely normal (score = 0.33). During the following hours, no sign of irritant responses was observed that confirmed that the prepared formulation is well-tolerated and nonirritant (score = 0).
Figure 9.
Rabbit eye irritation reactions to (A) SDS, (B) indomethacin-loaded Dex-St-GTMAC (10% formulation), (C) indomethacin, and (D) PBS during 6 h.
Table 5. Modified Draize’s Grading Scale for Evaluating the Ocular Irritation Potential of Substances.
| irritation appearance | irritation intensity | score |
|---|---|---|
| conjunctival discharge | normal | 0 |
| slight discharge | 1 | |
| sever discharge covering a small area around the cornea | 2 | |
| sever discharge covering a large around the cornea | 3 | |
| conjunctival chemosis | normal | 0 |
| slight chemosis including the nictitating membrane | 1 | |
| severe chemosis with the eye partially closed | 2 | |
| severe chemosis with the eye closed | 3 | |
| conjunctival redness | blood vessel normal | 0 |
| some blood vessel definitely hyperemic | 1 | |
| diffuse color, individual vessel not easily discernible | 2 | |
| diffuse beefy red | 3 |
Table 3. Irritation Scores of Formulations According to the Modified Draize Test.
| formulation/time (h) | 0 | 1 | 2 | 3 | 4 | 5 | 6 |
| SDS 1% (control +) | 2.33 | 2 | 1 | 0.66 | 0.33 | 0 | 0 |
| PBS (control −) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| indomethacin 0.1% in PBS | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| indomethacin-loaded Dex-St-GTMAC (10% formulation) | 0.33 | 0 | 0 | 0 | 0 | 0 | 0 |
Conclusions
In this study, we synthesized novel cationic dextran stearate (Dex-St-GTMAC) via the reaction of stearoyl chloride, GTMAC, and dextran. FT-IR and H NMR spectra confirmed the formation of Dex-St-GTMAC. CMC analysis by the turbidimetry method shows that this copolymer becomes micelles at a concentration of 3700 mg/L. Scanning electron microscopy images showed that the morphology of the micelles is spherical. Drug loading studies and particle size analysis showed that indomethacin was efficiently loaded onto the modified copolymer, and the micelles were formed successfully. Micelles containing indomethacin were prepared in three formulations (10, 20, and 30%). 10% formulation was the best formulation due to more surface charge and reasonable drug loading. Measuring the size and surface charge of the particles using a nano/zeta sizer showed that the size of the particles is between 122.1 and 150.9 nm and the surface charge of the particles is +39.1 mV. The data of Het-Cam and Draize tests showed that the indomethacin-loaded Dex-St-GTMAC formulation is not an irritant and has no toxicity in eye tissues. Our findings exhibited that this new formulation is able to penetrate corneal tissue efficiently (to a greater extent of 1.75) and is a suitable candidate for the controlled release of lipophilic drugs such as indomethacin.
Materials and Methods
Synthesis of Cationic Dextran (Dex-GTMAC)
In a 100 mL round-bottom flask, 1 g of dextran, 0.4 g of NaOH, and 100 mL of water were poured and mixed under N2 gas at room temperature. After that the temperature of the reaction mixture was raised to 60 °C and 14 mL of GTMAC was added to it. The mixture was stirred for 4 h. Then the reaction mixture was cooled and precipitated with methanol. The resulting precipitate was transferred to a dialysis tube with a cutoff of 12,000 Da and dialyzed in 2 L of distilled water for 24 h. Then, the contents of the dialysis tube were freeze-dried and the product was stored for use in the next step.
Synthesis of Cationic Dextran Stearate (Dex-St-GTMAC) Polymeric Micelles
In a 100 mL round-bottom flask, 1 g of Dex-GTMAC was mixed with 2 g of lithium chloride in 10 mL of dimethylformamide (DMF) at 120 °C. The mixture was stirred while forming a colorless and clear solution. After the temperature was reduced to 80 °C, 180 mg of dimethylaminopyridine and 420 μL of stearoyl chloride were added to the solution and refluxed for 24 h. Then the reaction mixture was cooled and precipitated with methanol. After being cooled to room temperature, the reaction mixture was precipitated with methanol. The obtained precipitate was transferred to a dialysis tube with a 12,000 Da cutoff and dialyzed against 2 L of distilled water for 24 h. Then the contents of the dialysis tube were freeze-dried, and the product was characterized by IR and H NMR.
Critical Micelle Concentration (CMC) Assay
The critical micellar concentration (CMC) of the Dex-St-GTMAC polymer was determined based on light scattering18 using a turbidimeter from Lovibond-TurbDirect apparatus. Various concentrations of the synthesized polymer were prepared in deionized water, and the turbidity of the solution was measured at room temperature.
Loading of Indomethacin onto Polymeric Micelles and Drug Loading Efficiency
The dialysis method was used for the preparation of indomethacin-loaded micelles (10, 20, and 30%). Concisely, 20, 40, and 60 mg of indomethacin were added to 140, 160, and 180 mg of (Dex-St-GTMAC), respectively, and the mixture was dissolved in 20 mL of DMSO and stirred for 1 h at room temperature. To remove unloaded indomethacin and DMSO, the mixture was poured into a dialysis tube (cutoff 2000 Da) and dialyzed against 2 L of distilled water for 1 h, then the dialysis solution was exchanged with an equal amount of fresh water, and the dialysis procedure was continued for 24 h. Subsequently, the lyophilized powder was obtained by freeze-drying. To determine the percentage of indomethacin encapsulated in nanoparticles, the HPLC analysis method was used. In brief, 5 mg of each drug-loaded polymer (10, 20, and 30%) were dissolved in 5 mL of DMSO, and then 1 mL of this solution was mixed with methanol to make 10 mL volume of the final solution. Then, the indomethacin concentration was determined by HPLC with UV detection at 254 nm using a Shimadzu device (Model LC-20AD, Japan). Indomethacin loading efficiency (%) and loading content (%) of the prepared formulations were calculated by the following formula9
Particle Size Distribution Measurements
Particle size, surface charge, and particle size distribution of indomethacin-loaded Dex-St-GTMAC nanoparticles were determined with a nanosizer device (Nano-ZS; Malvern Instruments, U.K.). Five mg of indomethacin-loaded micelle was dispersed in 5 mL of deionized H2O and the micelle size was determined using a Zetasizer Nano ZS device at a wavelength of 633 nm at 25 °C with a detection angle of 90°.
Characterization of Nanoparticle Morphology
SEM assay is performed at the central laboratory of Kermanshah University of Medical Sciences by using a scanning electron microscope device model EM3200 microscope (KYKY Co.).
In Vitro Drug Release Evaluation
To check the release of the drug, 2 groups were investigated. The first group included 3 samples of a 0.1% solution of indomethacin in PBS and the second group included three samples of the drug-containing micelle.
Drug Release from the Solution (as the Control)
One mL of each solution was poured into a dialysis tube with a cutoff of 2000 Da, and then the tube was placed in a beaker containing 100 mL of phosphate buffer with pH 7.4. The beaker was stirred in a shaker incubator at 150 rpm for 360 min at 34 °C. The samples were withdrawn at 30, 60, 90, 120, 180, 240, 300, and 360 min. Then the samples were analyzed by HPLC to measure the amount of released drug.
Drug Release from the Micelles
Evaluation of indomethacin release profiles from the micelle formulations was performed by a dialysis method. In brief, 15.4 mg of indomethacin-loaded micelle powder was dispersed in 1 mL of phosphate buffer (pH 7.4), and then added to the dialysis tubes (cutoff of 2000 Da). The tube was placed in a 100 mL beaker of phosphate buffer (pH 7.4). Then, the beakers were incubated at 150 rpm spinning in a 34 °C shaker incubator. The samples were drawn at 30, 60, 90, 120, 180, 240, 300, and 360 min. Then the concentration of the drug in the samples was determined by the HPLC method.
Permeation
To investigate the penetration of the drug in the cornea of the cow, 2 groups were investigated. The first group included 3 samples of a 0.1% solution of indomethacin in PBS and the second group included three samples of the drug-containing micelle.
Permeability of the Drug from the Solution (as a Control)
One mL of each solution was placed in a Franz diffusion cell containing bovine cornea.19 Then sampling was done at intervals of 30, 60, 90, 120, 180, 240, 300, and 360 min. Then the samples were analyzed with an HPLC device to measure the amount of drug penetrated.
Permeability of the Drug from the Micelle Formulation
Evaluation of the indomethacin penetration profile from the micelle formulation was performed by the Franz diffusion cell method. In brief, 15.4 mg of indomethacin-loaded micelle powder was dispersed in 1 mL of phosphate buffer (pH 7.4), and then added to the Franz cell at 34 °C. The samples were drawn at 30, 60, 90, 120, 180, 240, 300, and 360 min, then the concentration of the drug in the samples was evaluated by the HPLC method.
Toxicity
Het-CAM Test
To evaluate the possible eye irritancy of the prepared formulation using the HET-CAM method,20 eight fresh fertile eggs were obtained from an aviculture farm. Before incubation, the eggs were washed and cleaned with 70% ethanol. The eggs were incubated for 8 days in the germinator under conditions of 66.0 ± 5.0% relative humidity and 37 ± 0.5 °C. During incubation, the eggs were rotated by hand every 12 h in the equatorial axis to avoid the adherence of the embryo to the shell. On the eighth day, the eggs were removed from the incubator and examined using a flashlight to make sure the embryo was produced. The eggshells were cut out deftly to form a window that caused the CAM surface to be exposed. Positive controls were prepared by directly adding 200 μL 0.5 M of NaOH, 200 μL of acetone, and 200 μL of propylene glycol (PG) for coagulation, hemorrhage, and hyperemia, respectively. Phosphate buffer solution (PBS, pH 7.4) was used as a negative control. In total, 200 μL of indomethacin-loaded Dex-St-GTMAC formulation in PBS was added to the clearest vessel of CAM to compare with control solution effects.21 After the application of each substance, the CAM morphology was evaluated in terms of irritation reactions such as blood coagulation, bleeding, and hyperemia after 0, 30, and 2 and 5 min of exposure. According to the presented schedule in Table 4, the irritation potential of each tested substance and indomethacin-loaded Dex-St-GTMAC formulation was scored.
Table 4. Numerical Scores for Each Irritation Reaction in the HET-CAM Test.
| score | cumulative score | irritation assessment | |||
|---|---|---|---|---|---|
| effect/time (min) | 0.5 | 2 | 5 | 0–0.9 | none |
| hyperemia | 5 | 3 | 1 | 1.0–4.9 | slight |
| hemorrhage | 7 | 5 | 3 | 5.0–8.9 | moderate |
| clotting/coagulation | 9 | 7 | 5 | 9.0–21.0 | severe |
Draze Test
The modified Draize irritancy test was accomplished to predict ocular tolerance against sodium dodecyl sulfate (SDS), PBS, indomethacin 0.1% in PBS, and indomethacin-loaded Dex-St-GTMAC nanoparticles. In this test, we used male albino rabbits weighing 3–4 kg. Rabbits were checked in terms of their visual abnormalities and health problems. They were kept in standard laboratory cages at room temperature (22–25 °C) and relative humidity of about 50–70% and also fed at the same time every day until the day of assessment.20 The experiment was accomplished according to the guidelines of the National Institutes of Health for the care and use of Laboratory Animals (NIH publications no 8023, revised 1978) and approved by Hamadan University of Medical Sciences Medical Ethics Review Board (approval number IR. UMSHA.REC. 1398.439). Groups were designed as follows: first, a positive control group that received 100 μL of SDS 1% w/v; second, a negative control group that received 100 μL of PBS; third, blank groups that received 100 μL of indomethacin solution (0.1% indomethacin in phosphate buffer, pH = 7.4) to evaluate the irritancy of indomethacin; and fourth test groups that received 100 μL of indomethacin-loaded Dex-St-GTMAC nanoparticle PBS (0.1% indomethacin in 15.4 mg micelle). Before instillation, each solution was sterilized by UV light. Cornea and conjunctiva tissue were monitored visually for any consequent inflammation or irritation reactions such as discharge, chemosis, and redness after 0, 30, 60, 120, 180, 240, 300, and 360 min. Due to the following table, scores were recorded. In each irritation appearance, a score of >2 was considered clinically significant irritation and consequently irritant for ocular purposes (Table 5).
Acknowledgments
Financial support for this research was provided by the Research and Technology Vice-chancellor of Hamadan University of Medical Sciences with grant No. 9811018466 related to Arabkhni’s thesis.
Glossary
Abbreviations
- Dex
dextran
- DL
drug loading
- DMF
dimethylformamide
- DMSO
dimethyl sulfoxide
- EE
encapsulation efficiency
- GTMAC
glycidyl trimethylammonium chloride
- Het-CAM
Hen’s egg test-chorioallantoic membrane
- In
indomethacin
- NPs
nanoparticles
- PBS
phosphate-buffered saline solution
- PG
propylene glycol
- SDS
sodium dodecyl sulfate
- SEM
scanning electron microscopy
- St
stearate
Author Contributions
F.F. provided the idea of work and took part in drug-release data collection. M.M.M. designed and set up experiments on drug penetration in the cornea. M.M. designed and set up Draize and Het-cam toxicity experiments. Z.A. performed the experiments and data collection. G.C. supervised the research work, interpreted the data and wrote the paper.
The authors declare no competing financial interest.
References
- Tewabe A.; Abate A.; Tamrie M.; Seyfu A.; Siraj E. A. Targeted drug delivery—from magic bullet to nanomedicine: principles, challenges, and future perspectives. J. Multidiscip. Healthcare 2021, 14, 1711–1724. 10.2147/JMDH.S313968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Occhiutto M. L.; Freitas F. R.; Maranhao R. C.; Costa V. P. Breakdown of the blood-ocular barrier as a strategy for the systemic use of nanosystems. Pharmaceutics 2012, 4 (2), 252–275. 10.3390/pharmaceutics4020252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bourlais C.; Acar L.; Zia H.; Sado P. A.; Needham T.; Leverge R. Ophthalmic drug delivery systems-recent advances. Prog. Retinal Eye Res. 1998, 17 (1), 33–58. 10.1016/S1350-9462(97)00002-5. [DOI] [PubMed] [Google Scholar]
- Nayak K.; Choudhari M. V.; Bagul S.; Chavan T. A.; Misra M.. Ocular Drug Delivery Systems. Drug Delivery Devices and Therapeutic Systems, Elsevier: 2021; 515–566. [Google Scholar]
- Lammari N.; Tarhini M.; Miladi K.; Louaer O.; Meniai A.; Sfar S.; Fessi H.; Elaissari A.. Drug Delivery Devices and Therapeutic Systems; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Li J.; Li Z.; Zhou T.; Zhang J.; Xia H.; Li H.; He J.; He S.; Wang L. Positively charged micelles based on a triblock copolymer demonstrate enhanced corneal penetration. Int. J. Nanomed. 2015, 10, 6027–6037. 10.2147/IJN.S90347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y.; Wang X.; Yin Z. Synthesis and evaluation of cationic polymeric micelles as carriers of lumbrokinase for targeted thrombolysis. Asian J. Pharm. Sci. 2019, 14 (2), 144–153. 10.1016/j.ajps.2018.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohsen A. M. Cationic polymeric nanoparticles for improved ocular delivery and antimycotic activity of terconazole. J. Pharm. Sci. 2022, 111 (2), 458–468. 10.1016/j.xphs.2021.09.019. [DOI] [PubMed] [Google Scholar]
- Chehardoli G.; Norouzian P.; Firozian F. Inulin-Grafted Stearate (In-g-St) as the Effective Self-Assembling Polymeric Micelle: Synthesis and Evaluation for the Delivery of Betamethasone. J. Nanomater. 2020, 2020, 1–8. 10.1155/2020/6579538. [DOI] [Google Scholar]
- a Chehardoli G.; Bagheri H.; Firozian F. Synthesis of sodium alginate grafted stearate acid (NaAlg-g-St) and evaluation of the polymer as drug release controlling matrix. J. Polym. Res. 2019, 26, 175 10.1007/s10965-019-1840-3. [DOI] [Google Scholar]; b Firozian F.; Emadi M. A.; Chehardoli G.; Ghafari F. Inulin Stearate Self-assembly Micro-rod Containing Paclitaxel: Synthesis and In Vitro Cytotoxicity MTT Assay in HeLa Cell Line. J. Pharm. Innovation 2021, 17, 1215–1220. 10.1007/s12247-021-09602-0. [DOI] [Google Scholar]
- Kamiński K.; Płonka M.; Ciejka J.; Szczubiałka K.; Nowakowska M.; Lorkowska B.; Korbut R.; Lach R. Cationic derivatives of dextran and hydroxypropylcellulose as novel potential heparin antagonists. J. Med. Chem. 2011, 54 (19), 6586–6596. 10.1021/jm200380w. [DOI] [PubMed] [Google Scholar]
- Varshosaz J.; Hassanzadeh F.; Sadeghi H.; Firozian F.; Mirian M. Effect of molecular weight and molar ratio of dextran on self-assembly of dextran stearate polymeric micelles as nanocarriers for etoposide. J. Nanomater. 2012, 2012, 120. 10.1155/2012/265657. [DOI] [Google Scholar]
- Du Y.-Z.; Weng Q.; Yuan H.; Hu F.-Q. Synthesis and Antitumor Activity of Stearate-g-dextran Micelles for Intracellular Doxorubicin Delivery. ACS Nano 2010, 4 (11), 6894–6902. 10.1021/nn100927t. [DOI] [PubMed] [Google Scholar]
- Rwei S.-P.; Chen Y.-M.; Lin W.-Y.; Chiang W.-Y. Synthesis and rheological characterization of water-soluble glycidyltrimethylammonium-chitosan. Mar. Drugs 2014, 12 (11), 5547–5562. 10.3390/md12115547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caster J. M.; Yu S. K.; Patel A. N.; Newman N. J.; Lee Z. J.; Warner S. B.; Wagner K. T.; Roche K. C.; Tian X.; Min Y.; Wang A. Z. Effect of particle size on the biodistribution, toxicity, and efficacy of drug-loaded polymeric nanoparticles in chemoradiotherapy. Nanomedicine 2017, 13 (5), 1673–1683. 10.1016/j.nano.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorantla S.; Rapalli V. K.; Waghule T.; Singh P. P.; Dubey S. K.; Saha R. N.; Singhvi G. Nanocarriers for ocular drug delivery: current status and translational opportunity. RSC Adv. 2020, 10 (46), 27835–27855. 10.1039/D0RA04971A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou D.; Gui R.; Hu S.; Huang Y.; Feng Z.; Ping Q. Preparation and characterization of novel drug-inserted-montmorillonite chitosan carriers for ocular drug delivery. Adv. Nanopart. 2015, 04 (03), 70–84. 10.4236/anp.2015.43009. [DOI] [Google Scholar]
- Chang C. Y.; Wang S.; Liu I.; Chiu Y. A simple method for determining the critical micellar concentration of a surfactant. J. Chin. Chem. Soc. 1987, 34 (3), 243–246. 10.1002/jccs.198700037. [DOI] [Google Scholar]
- Morrison P. W.; Khutoryanskiy V. V. Enhancement in corneal permeability of riboflavin using calcium sequestering compounds. Int. J. Pharm. 2014, 472 (1–2), 56–64. 10.1016/j.ijpharm.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budai P.; Lehel J.; Tavaszi J.; Kormos É. HET-CAM test for determining the possible eye irritancy of pesticides. Acta Vet. Hung. 2010, 58 (3), 369–377. 10.1556/avet.58.2010.3.9. [DOI] [PubMed] [Google Scholar]
- Talaei S.; Mahboobian M. M.; Mohammadi M. Investigating the ocular toxicity potential and therapeutic efficiency of in situ gel nanoemulsion formulations of brinzolamide. Toxicol. Res. 2020, 9 (4), 578–587. 10.1093/toxres/tfaa066. [DOI] [PMC free article] [PubMed] [Google Scholar]