Abstract
This research focuses on the first demonstration of NO2Lw (2-hydroxy-3-nitronaphthalene-1,4-dione) as a photosensitizer and TiO2, ZnO, and Nb2O5 as photoanode materials for dye-sensitized solar cells (DSSCs). The metal-free organic photosensitizer (i.e., nitro-group-substituted naphthoquinone, NO2Lw) was synthesized for this purpose. As a photoanode material, metal oxides, such as TiO2, ZnO, and Nb2O5, were selected. The synthesized NO2Lw contains an electron-withdrawing group (−NO2) and anchoring groups (−OH) that exhibit absorption in the visible range. The UV–visible absorbance spectrum of NO2Lw demonstrates the absorption ascribed to ultraviolet and visible region charge transfer. The NO2Lw interacts with the TiO2, ZnO, and Nb2O5 photoanode, as shown by bathochromic shifts in wavelengths in the photosensitizer-loaded TiO2, ZnO, and Nb2O5 photoanodes. FT-IR analysis also studied the bonding interaction between NO2Lw and TiO2, ZnO, and Nb2O5 photoanode material. The TiO2, ZnO, and Nb2O5 photoanodes loaded with NO2Lw exhibit a shift in the wavenumber of the functional groups, indicating that these groups were involved in loading the NO2Lw photosensitizer. The amount of photosensitizer loading was calculated, showing that TiO2 has higher loading than ZnO and Nb2O5 photoanodes; this factor may constitute an increased JSC value of the TiO2 photoanode. The device performance is compared using photocurrent–voltage (J–V) curves; electrochemical impedance spectroscopy (EIS) measurement examines the device’s charge transport. The TiO2 photoanode showed higher performance than the ZnO and Nb2O5 photoanodes in terms of photoelectrochemical properties. When compared to ZnO and Nb2O5 photoanodes-based DSSCs, the TiO2 photoanode Bode plot shows a signature frequency peak corresponding to electron recombination rate toward the low-frequency region, showing that TiO2 has a greater electron lifetime than ZnO and Nb2O5 photoanodes based DSSCs.
1. Introduction
Energy becomes sustainable when it is harvested from naturally occurring sources without having a negative impact on the environment.1 Sustainable energy includes all renewable energy sources like geothermal, biomass, wind, tidal, and solar, apart from others.2 It helps in maintaining the natural environment by using eco-friendly materials to serve the ever-increasing needs of the growing world population.3 It is a viable energy source compared to the traditional sources, which are causing a tremendous load on Earth’s ecosystem.4 The entire world is already witnessing global warming and ever-increasing carbon emissions, leading to a pressing need of fulfilling global energy demand by using sustainable sources.5
Most daily activities require tremendous energy usage around the globe, leading to an excessive energy consumption. As the population and industrialization continue to grow, global energy consumption is rising quickly. One of this research’s main aims is to meet the increasing global demand for energy consumption.6,7 As a substitute for fossil fuels, nonfossil energy from clean sources is used. Examples of such sources include wind, water, biomass, geothermal, and solar energy because they are self-sufficient and less harmful to the ecosystem.8−11 Solar energy is one of the most promising nonfossil options for tackling energy challenges with a less detrimental impact on the ecology.12,13 Solar cells are an efficient device to convert solar energy into electrical energy14,15 to meet the rising demand for energy around the globe.
Crystalline silicon performs reliably and efficiently and is considered the main compound in solar cell technology.16 Still, it would be interesting to find cheaper alternatives due to the high cost of these complex technologies and expensive methods. Solar cells made of crystalline silicon are more costly and have a limited application.17,18 Perovskite,19,20 quantum-dot,21 and dye22-sensitized solar cells are examples of third-generation solar cell technology that has met the requirement of a low-cost, straightforward fabrication process.23−26 Over the past few years, dye-sensitized solar cells (DSSCs) have emerged as promising third-generation solar cell technology.27,28 DSSCs make use of photovoltaic energy, making use of nontoxic and environment-friendly materials.29 DSSCs have generated a lot of research attention because of their simple manufacturing process, low cost, ease of scaling up, nontoxicity, lighter weight,30 and potential usage of the flexible substrate with transparent and colorful nature.31
The four main components used in the fabrication of DSSCs are the photoanode material (semiconductor), the dye molecule (a photosensitizer), the redox electrolyte, and the counter electrode.32−37 In 1991, Grätzel and O’Regan published the first study on DSSCs using N3 dye as a photosensitizer.38 Numerous studies in this field since the original work on DSSCs have primarily shown that various dyes (photosensitizers) have been molecularly engineered and used in DSSCs.39−41 The production of novel photosensitizers that can be developed in DSSCs is an essential component affecting device efficiency.42−44
In the DSSCs, dyes based on ruthenium metals are primarily used as photosensitizers. In contrast, metal-free organic dyes have begun to take the position of ruthenium-metal-based dye.45,46 Organic dyes are more affordable and environmentally friendly, with more significant molecular extinction coefficients and easily adjustable photophysical and electrochemical properties than their ruthenium-metal-based dye.47,48 The compatibility of the photosensitizer is determined by understanding the molecular structures of these organic dyes and connecting them to their optical absorption spectral responses.49 The photovoltaic performance of fabricated DSSCs can be reorganized following the photophysical, electrochemical, and structure–property relationships.50 One essential molecular design requirement for DSSCs has been discovered as the donor-bridge-acceptor (D–π–A) system.51 It showed improved solar spectrum light harvesting in visible regions.52 Because it impacts the electrochemical and optical characteristics of the photosensitizer, intermolecular charge transfer (ICT) from donor to acceptor is essential for ensuring that DSSCs function correctly.53 A photosensitizer’s capacity for effective ICT from the HOMO localized donor moiety to the LUMO localized acceptor moiety correlates with enhanced light absorption and a lower HOMO–LUMO gap.54
Henna plant leaves are used to extract henna dyes. Henna leaves contain a derivative of naphthoquinone called 2-hydroxy-1,4-naphthoquinone (Lawsone). Using natural photosensitizers like Lawsone in DSSCs could be an eco-friendly and sustainable alternative to commercially available metal-based photosensitizers, often used to fabricate DSSCs. Semiconducting wide band gap materials such as TiO2, ZnO, Nb2O5, CeO2, and SnO2 are employed as photoanode materials. These materials do not have an environmental impact during their manufacture and disposal process.55 Metal-free organic photosensitizers such as coumarin and triphenylamine-, indoline-, and quinone-based photosensitizers are environmentally friendly compared to metal-containing photosensitizers.56 Organic photosensitizers based on a naphthoquinone derivative has been used as an environmentally safe photosensitizer.57 Naphthoquinone derivatives have an extensive range of molecular structures which could be used as photosensitizers.58Table 1 shows the DSSCs performance of different semiconducting oxides as photoanode materials with several synthesized derivatives of quinone-based photosensitizers from the literature along with NO2Lw photosensitizer.
Table 1. DSSCs Performance of Different Semiconducting Oxides as Photoanode Material with Several Synthesized Derivatives of Quinone-based Photosensitizer from the Literature along with NO2Lw Photosensitizer.
| Photoanode Material | Photosensitizer | λmax (nm) | E0–0 (eV) | Voc (V) | Jsc (mA/cm2) | FF | PCE (%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| TiO2 | 2-chloro-3[(pyridine-2-ylmethyl)amino]naphthalene-1,4-dione (2AMP) | 458 | 2.42 | 0.54 | 0.69 | 61 | 0.22 | (59) |
| TiO2 | 2-chloro-3[(pyridine-3-ylmethyl)amino]naphthalene-1,4-dione (3AMP) | 460 | 2.33 | 0.51 | 0.65 | 83 | 0.27 | (59) |
| TiO2 | 2-chloro-3[(2-pyridine-2-ylethyl)amino)]naphthalene-1,4-dione (2AEP) | 466 | 2.37 | 0.50 | 0.53 | 54 | 0.14 | (59) |
| ZnO | 2-chloro-3[(pyridine-2-ylmethyl)amino]naphthalene-1,4-dione (2AMP) | 458 | 2.42 | 0.53 | 0.68 | 60 | 0.22 | (59) |
| ZnO | 2-chloro-3[(pyridine-3-ylmethyl)amino]naphthalene-1,4-dione (3AMP) | 460 | 2.33 | 0.52 | 0.64 | 69 | 0.23 | (59) |
| ZnO | 2-chloro-3[(2-pyridine-2-ylethyl)amino)]naphthalene-1,4-dione (2AEP) | 466 | 2.37 | 0.30 | 0.57 | 66 | 0.11 | (59) |
| TiO2 | 2-hydroxy-3-[phenyl(phenylamino)methyl] naphthalene-1,4-dione (4a) | 411 | 2.68 | 0.29 | 0.035 | 50 | – | (57) |
| ZnO | 4-(3-chloro-1,4-dioxo-1,4-dihydronaphthalen-2-ylamino) benzoic acid | 482 | 2.75 | 0.392 | 3.196 | 60 | 0.75 | (60) |
| TiO2 | 2-hydroxy-3-[(4-methoxyphenyl](p-tolylamino)methyl] naphthalene-1,4-dione (4-OMe) | 456 | 2.20 | 0.36 | 0.053 | 57 | 0.01 | (58) |
| TiO2 | 2-hydroxy-3-[p-toly (p-tolylamino)methyl] naphthalene-1,4-dione (4-Me) | 444 | 2.20 | 0.37 | 0.045 | 56 | 0.009 | (58) |
| TiO2 | 2-[(4-Bromophenyl) (p-tolylamino)methy]-3-hydroxynaphthalene-1,4-dione (4-Br) | 454 | 2.30 | 0.34 | 0.034 | 58 | 0.007 | (58) |
| TiO2 | 2-[(2-Chlorophenyl) (p-tolylamino)methy]-3-hydroxynaphthalene-1,4-dione (2-Cl) | 433 | 2.30 | 0.31 | 0.026 | 54 | 0.004 | (58) |
| TiO2 | 2-[(2,4-Dichlorophenyl) (p-tolylamino)methy]-3-hydroxynaphthalene-1,4-dione (2,4-diCl) | 455 | 2.30 | 0.26 | 0.022 | 46 | 0.003 | (58) |
| TiO2 NR (300 °C) | 2-bromo-3-(methylamino)naphthalene-1,4-dione (BrA1) | 471 | 2.33 | 0.50 | 0.48 | 44 | 0.10 | (61) |
| TiO2 NR (400 °C) | 2-bromo-3-(methylamino)naphthalene-1,4-dione (BrA1) | 471 | 2.33 | 0.55 | 0.60 | 48 | 0.16 | (61) |
| TiO2 NR (500 °C) | 2-bromo-3-(methylamino)naphthalene-1,4-dione (BrA1) | 471 | 2.33 | 0.52 | 0.63 | 46 | 0.15 | (61) |
| TiO2 NR (600 °C) | 2-bromo-3-(methylamino)naphthalene-1,4-dione (BrA1) | 471 | 2.33 | 0.51 | 0.55 | 40 | 0.11 | (61) |
| ZrO2 | 6-methyl-5H-benzo[α]phenothiazin-5-one | 477 | 1.88 | 0.42 | 3.01 | 20 | 1.64 | (62) |
| ZnO | 2-hydroxy-1,4-naphthoquinone | 462 | – | 0.52 | 1.80 | 62 | 0.56 | (63) |
| TiO2 | 2-hydroxy-1,4-naphthoquinone | 410 | 2.70 | 0.54 | 0.92 | 50 | 0.31 | (64) |
| TiO2 | 3-hydroxy-4-(hydroxyimino) naphthalen -1 (4H)-one (LwOx) | 407 | 2.85 | 0.41 | 0.28 | 37 | 0.04 | (65) |
| TiO2 | 3-hydroxy-4-(hydroxyimino)-2- methylnaphthalen -1(4H)-one (PthOx) | 413 | 2.71 | 0.28 | 0.15 | 32 | 0.01 | (65) |
| TiO2 | 2-chloro-3-hydroxy-4-(hydroxyimino) naphthalen-1(4H)-one (Cl_LwOx) | 402 | 2.87 | 0.47 | 0.78 | 38 | 0.14 | (65) |
| ZnO | 2-propylamine-1,4-naphthoquinone (HA3) | 452 | – | 0.29 | 1.21 | 35 | 0.12 | (66) |
| ZnO | 2-butylamino-1,4-naphthoquinone (HA4) | 452 | – | 0.32 | 0.71 | 38 | 0.09 | (66) |
| ZnO | 2-bromo-3-propylamino-1,4-naphthoquinone (BrA3) | 471 | – | 0.33 | 1.01 | 39 | 0.13 | (66) |
| ZnO | 2-bromo-3-butylamino-1,4-naphthoquinone (BrA4) | 471 | – | 0.31 | 1.66 | 38 | 0.20 | (66) |
| TiO2 | 10-Chloro-6-methyl-5H benzo[α]phenoxazin-5-one (BPO_Cl) | 451 | 2.49 | 0.56 | 0.430 | – | – | (67) |
| TiO2 | 6-methyl-5H-benzo[α]phenoxazin-5-one (BPO) | 440 | 2.52 | 0.53 | 0.440 | – | – | (67) |
| TiO2 | 6-methyl-5H-benzo[α]phenothiazin-5-one (BPT) | 474 | 2.33 | 0.49 | 0.240 | – | – | (67) |
| ZnO | 2-((thiophen-2-yl)methylamino)-3-chloro-naphthalene-1,4-dione (AMT) | 464 | – | 0.21 | 0.17 | 60 | 0.02 | (68) |
| ZnO | 2-((thiophen-2-yl)ethylamino)-3-chloro-naphthalene-1,4-dione (AET) | 469 | – | 0.22 | 0.22 | 59 | 0.03 | (68) |
| TiO2 | 2-((thiophen-2-yl)methylamino)-3-chloro-naphthalene-1,4-dione (AMT) | 464 | – | 0.41 | 1.73 | 33 | 0.23 | (68) |
| TiO2 | 2-((thiophen-2-yl)ethylamino)-3-chloro-naphthalene-1,4-dione (AET) | 469 | – | 0.42 | 1.73 | 44 | 0.32 | (68) |
| TiO2 | 2-hydroxy-1,4-naphthoquinone | – | – | 0.66 | 2.21 | 63 | 0.93 | (69) |
| P-type NiO | KuQCH3 | – | 2.17 | 0.90 | 0.74 | 35 | 0.02 | (70) |
| P-type NiO | KuQ3CO2H | – | 2.21 | 0.92 | 0.74 | 35 | 0.02 | (70) |
| P-type NiO | KuQ8CO2H | – | 2.19 | 0.99 | 0.63 | 36 | 0.02 | (70) |
| TiO2 | 2-hydroxy-3-nitronaphthalene-1,4-dione (NO2Lw) | 391 | 2.87 | 0.44 | 0.82 | 40 | 0.14 | present study |
| ZnO | 2-hydroxy-3-nitronaphthalene-1,4-dione (NO2Lw) | 391 | 2.87 | 0.23 | 0.31 | 32 | 0.02 | present study |
| Nb2O5 | 2-hydroxy-3-nitronaphthalene-1,4-dione (NO2Lw) | 391 | 2.87 | 0.24 | 0.16 | 42 | 0.01 | present study |
In the present investigation, a nitro-substituted naphthoquinone (NO2Lw)-based photosensitizer was synthesized and has the molecular structure depicted in Figure 1a. NO2Lw was synthesized with electron-withdrawing groups (−NO2). On the moiety part, the impact of electron-withdrawing (−NO2) substitutions is investigated concerning the photovoltaic performance of DSSCs. Specific appropriate peripheral (−OH) functional groups for adsorption on the TiO2, ZnO, and Nb2O5 surface are present in the photosensitizer.71,72
Figure 1.
Molecular structure of photosensitizer (a) NO2Lw (2-hydroxy-3-nitronaphthalene-1,4-dione) and schematic of the possible structure interfacial contact between (b) NO2Lw and TiO2, (c) NO2Lw and ZnO, and (d) NO2Lw and Nb2O5.
The lone pair of oxygens (hydroxyl group and carbonyl group) forms the bidentate complex by creating a five-member ring. Thus, the interface between the NO2Lw and TiO2, ZnO, and Nb2O5 is developed. The possible schematic interfacial contact structures of NO2Lw and TiO2, ZnO, and Nb2O5 are shown in Figures 1b, c, and d, respectively. The interaction of the hydroxyl group of NO2Lw molecules with the valence-unfilled TiO2, ZnO, and Nb2O5 surface facilitates the adsorption of the hydroxyl group of NO2Lw molecules on the TiO2, ZnO, and Nb2O5 surface. As a result of our investigation into naphthoquinone-based photosensitizers, the NO2-substituted naphthoquinone-based photosensitizer has been reported by using the approach described in this article. This is the first demonstration of TiO2, ZnO, and Nb2O5 as photoanodes and NO2Lw as a photosensitizer for the DSSC application. Depending upon the energy levels of NO2Lw, the conduction bands (CBs) of TiO2, ZnO, and Nb2O5, and the amount of photosensitizer loading, we have successfully demonstrated the photovoltaic effect.
2. Experimental Section
2.1. Preparation of TiO2, ZnO, and Nb2O5 Pastes
Using commercial TiO2 (P25 Degussa, Nanoshel LLC, USA), ZnO, and Nb2O5 (Merck) nanopowders, TiO2, ZnO, and Nb2O5 pastes, respectively, were prepared by homogenizing each sample with ethylcellulose (SDFCL) and anhydrous α-terpineol (Kemphasol Ltd., India) as follows. First, the respective TiO2, ZnO, and Nb2O5 nanopowders and ethylcellulose were introduced to a mortar and continuously ground for 15 min while adding ethanol (Changshu, China) gradually. Each mixture was then homogenized for 2 h in an ultrasonic bath before adding α-terpineol. Two to three drops of acetylacetone (HPCL, India) were added to the (TiO2, ZnO, and Nb2O5) dispersions, and homogenization was carried out in an ultrasonic bath for 3 h.36,63,67
2.2. Preparation of TiO2, ZnO, and Nb2O5 Photoanodes and Fabrication of DSSCs
Using the chemical bath deposition technique (CBD), the compact TiO2 layer was first deposited before the porous (TiO2 and Nb2O5) layer, as similarly reported.73,74 Before the porous (ZnO) layer was deposited, the compact ZnO layer was prepared using successive ionic layer adsorption and reaction (SILAR) techniques similarly reported.37 The as-deposited compact ZnO and TiO2 film was annealed at 450 °C for 1 h. Compact TiO2-deposited FTO (fluorine-doped tin oxide) deposits a porous layer of (TiO2 and Nb2O5), while compact ZnO-deposited FTO deposits a porous layer of (ZnO). On compact TiO2-deposited FTO, (TiO2 and Nb2O5) paste was coated; in compact ZnO-deposited FTO, (ZnO) paste was coated using the Doctor blade method. All the (TiO2, ZnO, and Nb2O5) photoanodes were dried using an incubator and air-annealed at 450 °C for 1 h.
0.05 M NO2Lw solutions were prepared in ethanol, and the annealed (TiO2, ZnO, and Nb2O5) photoanode were immersed for 72 h. This (TiO2, ZnO, and Nb2O5) photoanode was utilized to fabricate DSSCs after photosensitizer loading.
A 4 μm spacer was added between the platinum-coated FTO counter electrode and the (TiO2, ZnO, and Nb2O5) photoanode to avoid direct contact. The redox electrolyte solution was utilized as a liquid electrolyte. It contained 0.5 M lithium iodide (SRL, India), 0.05 M iodine (Fisher Scientific, USA), and 0.5 M tertiary butylpyridine (ACROS Organics, Belgium) solution in acetonitrile (SDFCL, India, used as received).36
2.3. Characterization
By using X-ray diffraction (Bruker D8, with Cu Kα λ = 0.154 nm) at 20–80°, the crystal structure and crystalline size of the photoanodes were studied. JEM-2010 field emission scanning electron microscopy (FE-SEM) (SUPRA40VP, Germany) was employed to examine the surface morphologies of photoanodes. The shape and size of TiO2 ZnO, and Nb2O5 nanoparticles and selective area electron diffraction (SAED) patterns were studied using transmission electron microscopy (TEM) (TECNAI 12 G2 TEM). X-ray photoelectron spectroscopy analysis of TiO2, ZnO, and Nb2O5 and NO2Lw-loaded TiO2, ZnO, and Nb2O5 was used to characterize the elemental composition with chemical states (XPS: M/s Thermo Fisher Scientific Instrument UK, K Alpha +) with Al Kα monochromator radiation at 12 kV and 6 mA beam current. The UV–visible absorption spectra of the photosensitizer were measured (SHIMADZU UV 1650), and emission spectra were obtained using a spectrofluorometer (JASCO FP-8300). The Fourier-transform infrared spectroscopy (FT-IR) spectra of NO2Lw and the NO2Lw-loaded TiO2 ZnO, and Nb2O5 photoanode were measured (BRUKER VERTEX by SHIMADZUFT 8400 Spectrometer). The Raman spectra are recorded by Renishaw with a He–Ne laser at 785 nm as the excitation source. Using CH equipment with an electrochemical analyzer (CHI 6054E), the photosensitizer cyclic voltammetry (CV) measurement was carried out in ethanol as the solvent. Using a potentiostat (Vertex IVIUM Technologies Netherlands), EIS measurement performs over the frequency range of 106–100 under dark illumination at the voltage that corresponds to open-circuit voltage. Keithley 2400 source meter and solar simulator (ENLITECH model SS-F5–3A) were used to analyze the photocurrent density–voltage (J–V) characteristic curve of the fabricated DSSCs under 100 mW/cm2 illumination.
2.4. Synthesis of NO2Lw (2-Hydroxy-3-nitronaphthalene-1,4-dione)
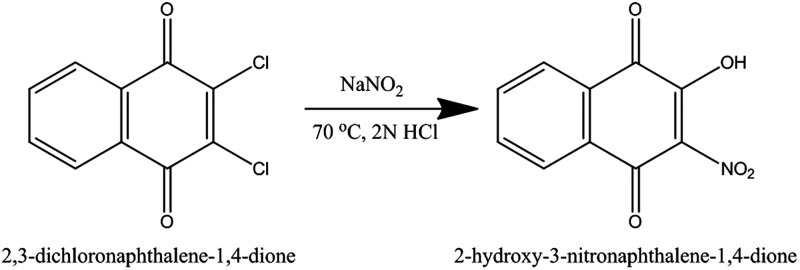
Using Scheme 1, NO2Lw (2-hydroxy-3-nitronaphthalene-1,4-dione) was synthesized. This procedure involved dissolving 0.03 mol (6.81 g) of dichlone (2,3-dichloronaphthalene-1,4-dione) in 38 mL of methanol and 0.10 mol (6.90 g) of sodium nitrite (NaNO2) in 52 mL of water and mixing both solutions in a round-bottom flask. The mixture was warmed at 70 °C continuously for 2.5 h. After heating the solution, the mixture was kept aside for 1 h. A red-colored precipitate was formed, which was then dissolved in warm water. After the reaction mixture was neutralized with 2 N hydrochloric acid, a residue of NO2Lw (2-hydroxy-3-nitronaphthalene-1,4-dione), which was yellow, was produced.75
Scheme 1. Synthesis of NO2Lw (2-Hydroxy-3-nitronaphthalene-1,4-dione).
3. Result and Discussion
3.1. Structural Properties of TiO2, ZnO, and Nb2O5 Photoanode
The TiO2, ZnO, and Nb2O5 photoanode crystal structure and crystalline size were determined using the X-ray diffraction pattern, as shown in Figures 2a/b, c/d, and e/f, respectively.
Figure 2.
X-ray diffraction patterns and crystallite size calculation of (a, b) TiO2, (c, d) ZnO, and (e, f) Nb2O5 photoanodes.
The TiO2 photoanode deposited on FTO is depicted in Figure 2a/b with its X-ray diffraction pattern and the full width at half-maximum (fwhm) of (110) peaks. The obtained XRD pattern was compared to the standard data, and it was found that the crystal planes of the anatase phase were confirmed by comparison with JCPDS card no. 21–1272, while the rutile phase76 was verified by comparison with JCPDS card no. 21–1276. The Scherrer formula77,78 was used to determine the crystallite size of TiO2. The crystallite size of the TiO2 was determined to be ∼37 nm.
The X-ray diffraction patterns and the fwhm of (101) peaks of the ZnO photoanode deposited on FTO are displayed in Figure 2c/d. The obtained XRD pattern was compared to the available data and found to match the JCPDS card no. 36–1451 for ZnO, confirming the formation of the wurtzite structure showing a hexagonal phase for ZnO.79 The crystallite size of the ZnO was found to be ∼52 nm.
The Nb2O5 photoanode deposits on FTO are depicted in Figure 2e/f, which shows the X-ray diffraction patterns and the fwhm of (001) peaks. According to JCPDS card no. 27–1003, the orthorhombic structure of the Nb2O5 photoanode is confirmed by its X-ray diffraction patterns.80,81 The crystallite size of the Nb2O5 was found to be ∼56 nm.
3.2. Morphological Surface Analysis of TiO2, ZnO, and Nb2O5 Photoanodes
The FE-SEM technique was used to examine the surface morphologies of the TiO2, ZnO, and Nb2O5 photoanodes. FE-SEM images of TiO2, ZnO, and Nb2O5 photoanodes are shown in Figure 3a–c. The spherical shape and porous morphology of the high-density TiO2 clusters are depicted in Figure 3a. The TiO2 photoanode porous structure boosts the quantity of photosensitizer loading by offering a high surface area resulting in increased fabricated device efficiency.66,82−84 The ZnO photoanode surface morphology shown in Figure 3b, deposited by using the Doctor blade technique, exhibits uniformly distributed nanograins with porous morphology. Pores of submicrometer size are seen to be evenly dispersed across the entire ZnO photoanode surface. Since light harvesting efficiency depends on the amount of efficient photosensitizer adsorbed on the photoanode surface,68 ZnO photoanode’s porous characteristic is crucial to achieving improved light harvesting.85,86Figure 3c FE-SEM image shows the surface morphology of the Nb2O5 photoanode presented. The Nb2O5 photoanode deposited by the Doctor blade technique reveals irregular spherical particles of well-defined shape.87−90
Figure 3.

Surface morphology of (a) TiO2, (b) ZnO, and (c) Nb2O5 photoanodes.
The porosity observed in the TiO2, ZnO, and Nb2O5 photoanodes facilitates the adsorption of NO2Lw molecules, which allows the photosensitizer molecules to penetrate the porous TiO2, ZnO, and Nb2O5 photoanode so that they get attached to the interfacial surface.91−93
3.3. Transmission Electron Microscopy (TEM)
Figure 4a–i displays TEM micrographs, particle size histograms, and selective area electron diffraction (SAED) patterns of TiO2 nanoparticles (Figure 4a–c), ZnO nanoparticles (Figure 4d–f), and Nb2O5 submicron-particles (Figure 4g–i).
Figure 4.
(a, d, g) TEM micrographs, (b, e, h) particle size histogram, and (c, f, i) selective area electron diffraction pattern of TiO2 nanoparticles, ZnO nanoparticles, and Nb2O5 submicron-particles, respectively.
Figure 4a/d/g shows a TEM micrograph of TiO2, ZnO, and Nb2O5 with spherical TiO2 particles.94 In the instance of ZnO, it was found to have agglomerated nanoparticles,95 whereas Nb2O5 contains irregular submicron-particles.96Figure 4b/e/h depict the particle size distribution histogram of TiO2, ZnO, and Nb2O5 fitted to Gaussian distribution. According to the histogram, the average particle size for TiO2 and ZnO nanoparticles was 45 and 55 nm, respectively, whereas Nb2O5 submicron-particles were 175 nm. TiO2 has a lower average particle size than ZnO and Nb2O5. The smaller the nanoparticles, the greater the surface area and the photosensitizer adsorption capacity of the photoanode material.97 Compared with ZnO and Nb2O5, the TiO2 photoanode exhibits more NO2Lw photosensitizer adsorption. The polycrystalline nature of TiO2, ZnO, and Nb2O5 was deduced from the SAED pattern, which reveals the characteristic ring pattern (Figure 4 c/f/i). Using SAED, the d-spacing values of TiO2, ZnO, and Nb2O5 are computed, and the estimated d-spacing value corresponds with the XRD result.98−100
3.4. X-ray Photoelectron Spectroscopy
X-ray photoelectron spectroscopy (XPS) technique was used to analyze the chemical and electronic states of the elements found in TiO2, ZnO, and Nb2O5 as photoanode materials, as well as NO2Lw-loaded TiO2, ZnO and Nb2O5 photoanodes. The survey spectra displayed in Figure 5a for both TiO2 and TiO2/NO2Lw photoanodes exclusively reveal the existence of Ti, O, and C elements, with TiO2/NO2Lw showing extra N element.
Figure 5.
(a) XPS survey spectra and (b) core level XPS spectra of Ti 2p of TiO2 and TiO2/NO2Lw photoanodes. Core level XPS spectra of (c) C 1s and (d) O 1s of TiO2/NO2Lw photoanode.
The inset of Figure 5a depicts the spectrum of N 1s observed at 402.65 eV, which exhibits nitrogen peaks attributed to the nitro group present.101 The binding energy (BE) values for Ti 2p1/2 and Ti 2p3/2 were 464.6 and 458.9 eV for TiO2 and 464.5 and 458.7 eV for TiO2/NO2Lw, respectively, according to the Ti 2p core level spectra shown in Figure 5b, and these two peaks in TiO2 and TiO2/NO2Lw correspond to the Ti4+ of TiO2.102Figure 5c and d shows the C 1s and O 1s spectra of the TiO2/NO2Lw photoanode. XPSPEAK 41 software was used for fitting and background subtraction. The main peak in the C 1s spectrum at 284.8 eV is attributed to the sp2-hybridized carbon atom fitted by three peaks: the two at 284.70 and 286.35 corresponding to C=C and C–O bonds, and the less intense broader peak near 289.10 eV that is due to the C=O group103 present in the NO2Lw molecule observed for the TiO2/NO2Lw photoanode as shown in Figure 5c. The two peaks at 530.05 and broad peaks around 532.15 in the O 1s spectrum from Figure 5d are ascribed to the TiO2 lattice oxygen and C=O oxygens in the absorbed NO2Lw molecule.104 The XPS study spectra show Ti is present at a +4 oxidation in a state with oxygen vacancies, allowing an acceptable quantity of NO2Lw absorption into the TiO2 photoanode.
The ZnO and ZnO/NO2Lw photoanodes XPS was carried out, and the result is shown in Figure 6. The survey scan spectrum in Figure 6a shows that ZnO and ZnO/NO2Lw photoanodes confirm the presence of Zn, O, and C element, and ZnO/NO2Lw shows the addition of N element. The inside of Figure 6a depicts the spectrum of N 1s observed at 402.4 eV.101 The Zn 2p consists of two peaks, Zn 2p1/2 and Zn 2p3/2 positioned at 1045.7 and 1022.75 eV for the ZnO and 1045.75 and 1022.5 eV105 for the ZnO/NO2Lw, as shown Figure 6b, which were observed for both ZnO and ZnO/NO2Lw. The binding energy differences between Zn 2p1/2 and Zn 2p3/2 for the ZnO and ZnO/NO2Lw was 22.95 and 23.25 eV, the characteristic value of ZnO.106
Figure 6.
(a) XPS survey spectra and (b) core level XPS spectra of Zn 2p of ZnO and ZnO/NO2Lw photoanodes. Core level XPS spectra of (c) C 1s and (d) O 1s of ZnO/NO2Lw photoanode.
Figure 6c shows that the C 1s can be fitted by three peaks at 284.75, 286.45, and 289 eV corresponding to C=C and C–O bonds ZnO/NO2Lw. The less intense broader peak near 289 eV is due to the C=O103 group in the NO2Lw molecule observed for the ZnO/NO2Lw photoanode. Figure 6d shows that the O 1s was fitted using XPSPEAK 41 software by three nearly peaks in the ZnO/NO2Lw photoanode, indicating three different O species in the ZnO/NO2Lw photoanode. The lowest binding energy peaks at 526.8 eV are attributed to oxygen at the lattice site.106 The middle binding energy, 528.85 eV, is attributed to chemically adsorbed oxygen on the surface107 of NO2Lw. The highest component is attributed to interstitial oxygen in ZnO.106
Nb2O5 and Nb2O5/NO2Lw photoanodes were analyzed using XPS. Figure 7a depicts the XPS survey spectra of the Nb2O5 and Nb2O5/NO2Lw photoanodes. Figure 7a inset shows the core level spectrum of N 1s observed at 403.05 eV.101 The core level XPS spectra of Nb 3d of the Nb2O5 and Nb2O5/NO2Lw photoanodes are shown in Figure 7b. The BE values for the Nb 3d3/2 and Nb 3d5/2 were, respectively, 210.35 and 207.65 eV for the Nb2O5 and 209.95 and 207.25 eV for the Nb2O5/NO2Lw; these two peaks in Nb2O5 and Nb2O5/NO2Lw correlate to pentavalent niobium.108 The core level XPS spectra of C 1s are shown in Figure 7c. The sp2-hybridized carbon atom is responsible for the main peak in the C 1s spectrum at 284.8 eV. The C 1s peak is fitted by three peaks at 284.85, 285.55, and 286.45 eV corresponding to C=C, C–O, and C=O bonds.103 These functional groups facilitate the formation of chemical bonds in the Nb2O5 lattice. Figure 7d depicts the core level XPS spectra of O 1s from an Nb2O5/NO2Lw photoanode. The three peaks occurring at 527.35 and 529 eV, and wide peaks at 528.9 correspond to the lattice oxygen in the Nb2O5 and C=O oxygens104 in the absorbed NO2Lw molecules.
Figure 7.
(a) XPS survey spectra and (b) core level XPS spectra of Nb 3d of Nb2O5 and Nb2O5/NO2Lw photoanodes. Core level XPS spectra of (c) C 1s and (d) O 1s of Nb2O5/NO2Lw photoanode.
3.5. Raman Spectra
Raman spectra of TiO2, Nb2O5, and NO2Lw-loaded TiO2 and Nb2O5 photoanodes are shown in Figure 8. The characteristic Raman absorption bands centered at 138, 194, 395, 516, and 636 cm–1 are attributed to TiO2103 phonon modes, as illustrated in Figure 8a, and the characteristic Raman absorption bands centered at 124, 236, 312, 500, 690, 734, and 935 cm–1 are attributed to the phonon modes of Nb2O5,109 as illustrated in Figure 8b. When compared to the Raman spectrum of the TiO2 and Nb2O5, the Raman spectra of NO2Lw-loaded TiO2 and Nb2O5 contain more NO2Lw photosensitizer NO2-group-derived bands (e.g., at 1336 and 1335 cm–1).110 Raman spectra verified the highly ordered TiO2 and Nb2O5 photoanodes, their functionalization via the changes in intensities of the NO2Lw-loaded TiO2 and Nb2O5 photoanodes, and the interaction between the NO2Lw molecule and TiO2 and Nb2O5 photoanodes.111
Figure 8.
Raman spectra of (a) TiO2 and NO2Lw-loaded TiO2 photoanodes and (b) Nb2O5 and NO2Lw-loaded Nb2O5 photoanodes.
3.6. Emission Studies
The photoluminescence (PL) spectra of TiO2, ZnO, and Nb2O5 with deconvoluted PL spectra are shown in Figures 9 a, b, and c, respectively. The emission spectra of TiO2, ZnO, and Nb2O5 photoanodes have been studied to investigate radiative and nonradiative recombination surface characteristics and defect states.112Figure 9a, b, and c shows two emission bands in TiO2, ZnO, and Nb2O5. The second emission band in TiO2, ZnO, and Nb2O5 PL emission spectra represents green emission caused by oxygen vacancies and surface defects. The green emission is quite strong because of radial recombination between photogenerated holes with trap electrons at the oxygen vacancies.113−116
Figure 9.
Photoluminescence (PL) spectra of (a) TiO2, (b) ZnO, and (c) Nb2O5 with deconvoluted PL spectra.
3.7. UV–visible Absorbance of NO2Lw, Photoanodes (TiO2/NO2Lw, ZnO/NO2Lw, Nb2O5/NO2Lw), and Fluorescence Spectra Measurement of NO2Lw
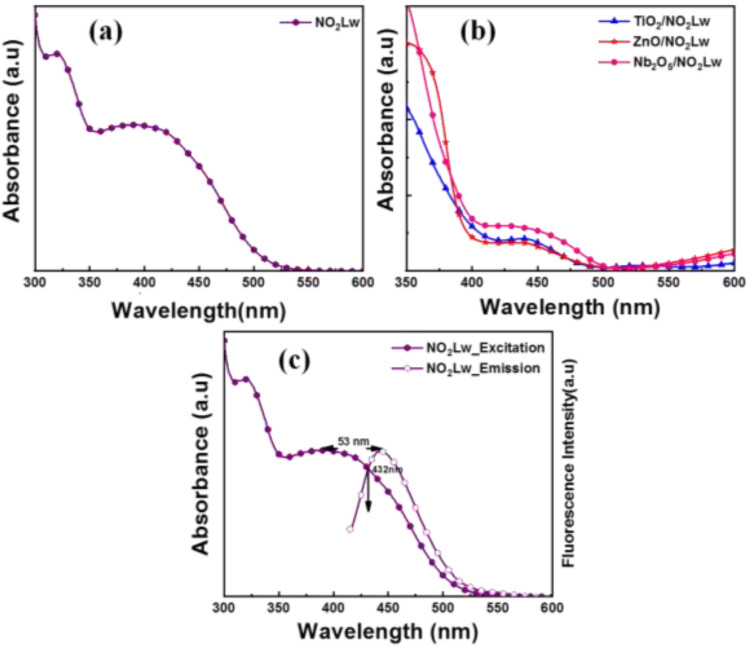
Figure 10a, b depicts the UV–visible absorbance spectra of NO2Lw and NO2Lw-loaded TiO2, ZnO, and Nb2O5 photoanodes.
Figure 10.
UV–visible spectra of (a) NO2Lw and (b) NO2Lw-loaded TiO2, ZnO, and Nb2O5 photoanodes. (c) Combination of UV–visible (excitation) and fluorescence (emission) spectra of NO2Lw.
The UV–visible absorbance for NO2Lw in ethanolic solutions is depicted in Figure 10a as a function of wavelength. NO2Lw was found to have absorptions ranging from 310 to 540 nm. The substituted nitro group electron delocalization effect shows the charge transfer band in NO2Lw. The UV–visible absorbance spectra of NO2Lw demonstrate the absorption ascribed to ultraviolet and visible region charge transfer. NO2Lw has a maximum absorption wavelength (λmax) of ∼391 nm.
Figure 10b shows the optical absorbance spectra of the NO2Lw-loaded TiO2, ZnO, and Nb2O5 photoanodes. The maximum absorption wavelength (λmax) is exhibited at 439, 438, and 427 nm for NO2Lw-loaded TiO2, ZnO, and Nb2O5 photoanodes, respectively. The NO2Lw-loaded TiO2, ZnO, and Nb2O5 photoanodes show the redshift in the visible region, confirming the formation of complexation between NO2Lw and TiO2, ZnO, and Nb2O5 photoanodes, which demonstrates the sufficient amount of photosensitizer adsorbed on the TiO2, ZnO, and Nb2O5 surfaces.117,118
Figure 10c depicts the combined UV–visible (excitation) and fluorescence (emission) spectra of the NO2Lw. The fluorescence (emission) spectra were measured, and the fluorescence emission maxima (λPL) value is displayed in Table 2. It exhibits fluorescence emission maxima at (λPL) ∼444 nm, and the stock shift for NO2Lw is 53 nm. The combination of UV–visible (excitation) and fluorescence (emission) spectra of NO2Lw helps to calculate energy difference,80,81 i.e., the E0–E0 (E0–0) difference of NO2Lw using the formula,
| 1 |
Table 2. Photophysical and Electrochemical Data of NO2Lw.
| Photosensitizer | λmax (nm) | λPL (nm) | Stokes shift (nm) | E0–0 (eV) | EHOMO (eV) | ELOMO (eV) |
|---|---|---|---|---|---|---|
| NO2Lw | 391 | 444 | 53 | 2.87 | –6.73 | –3.86 |
Where E is the energy difference in eV, h represents the Planks constant, λ represents the intersection of excitation and emission spectra in nm, and c is the speed of light. The E0–0 of the NO2Lw is calculated and equals 2.87 eV.119,120
3.8. Electrochemical Characterization Using Cyclic Voltammetry
Figure 11a depicts the NO2Lw cyclic voltammogram in an ethanol solution. The highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) energy levels were calculated using the cyclic voltammeter (CV) approach.
Figure 11.
(a) Cyclic voltammograms of NO2Lw. (b) HOMO–LUMO comparison plot with conduction bands of TiO2, ZnO, and Nb2O5 and redox potential of I–/I3–.
As shown in Figure 11a, the first reduction potential (onset of the first reaction peak) can be used to compute the LUMO.121,122 The NO2Lw has an ELUMO of −3.86 eV. The EHOMO is −6.73 eV and was calculated using the ELUMO and the E0–0 values. Table 2 displays the electrochemical and optoelectrical parameters. Figure 11b depicts the energy level diagram of the NO2Lw and the conduction band edge of the TiO2, ZnO, and Nb2O5 photoanodes. TiO2 and ZnO have conduction bands below the NO2Lw LUMO level, but Nb2O5 has a conduction band above the NO2Lw LUMO level. Iodine/triiodide has a redox potential (−4.8 eV)123 above the HOMO level of NO2Lw. It is possible to regenerate photosensitizers in TiO2, ZnO, and Nb2O5-based DSSCs with a driving force of ∼1.93 eV, which is sufficient.
3.9. FT-IR Analysis
Figure 12a–c displays the FTIR spectra of NO2Lw and NO2Lw-loaded TiO2, ZnO, and Nb2O5 photoanodes. When NO2Lw-loaded TiO2, ZnO, and Nb2O5 photoanodes were compared to NO2Lw, the bonding interaction124,125 was visible in the frequency range of NO2Lw-loaded TiO2, ZnO, and Nb2O5 photoanodes. Table 3 summarizes the characteristics of the frequency band of NO2Lw and photosensitizer-loaded TiO2, ZnO, and Nb2O5 photoanodes. The stretching frequency of the NO2 group is attributed to the bands in the NO2Lw FTIR spectra that are in the range of 1597 cm–1 (antisymmetrical) and 1312 cm–1 (symmetrical).75Figure 12a–c depicts the increase in the NO2 stretching frequency at the photosensitizer-loaded TiO2, ZnO, and Nb2O5 photoanodes from 1312 cm–1 to 1323, 1325, and 1327 cm–1, respectively. Due to the frequency of hydroxyl group (O–H) stretching, the peak occurred at 3563 and 3516 cm–1, as illustrated in Figure 12a–c. One band is visible in photoanodes made of TiO2, ZnO, and Nb2O5 that have been loaded with photosensitizer after coordination126 at 3324, 3334, and 3321 cm–1, respectively
Figure 12.
FT-IR spectra of (a) NO2Lw and NO2Lw-loaded TiO2 photoanodes, (b) NO2Lw and NO2Lw-loaded ZnO photoanodes, and (c) NO2Lw and NO2Lw-loaded Nb2O5 photoanodes.
Table 3. Selected FT-IR Frequencies of the NO2Lw and NO2Lw-loaded TiO2, ZnO, and Nb2O5 Photoanodes.
| Photosensitizer/Photoanode | νO–H (cm–1) | νC=O (cm–1) | νN–O (cm–1) | νC–C (cm–1) | νC–O (cm–1) |
|---|---|---|---|---|---|
| NO2Lw | 3563, 3516 | 1692 | 1597, 1312 | 1271 | 1001 |
| TiO2/NO2Lw | 3324 | 1657 | 1323 | 1273 | 1042 |
| ZnO/NO2Lw | 3334 | 1652 | 1325 | 1273 | 1042 |
| Nb2O5/NO2Lw | 3321 | 1648 | 1327 | 1275 | 1042 |
In photoanodes with photosensitizer-loaded TiO2, ZnO, and Nb2O5, the νC=O frequencies of NO2Lw detected at 1692 cm–1 after coordination are shifted to lower frequencies 1657, 1652, and 1648 cm–1, respectively.120,127 TiO2, ZnO, and Nb2O5 are attached to the NO2Lw anchoring site via νOH and νC=O bonding. The band observed at 1001 cm–1 that was noticed as a result of photosensitizers νC–O stretching frequency of NO2Lw is shifted to 1042 cm–1 in photosensitizer-loaded TiO2, ZnO, and Nb2O5 photoanode after coordination, indicating a significant interaction between the NO2Lw and TiO2, ZnO, and Nb2O5 photoanodes that serves to generate a chain-like structure for the device’s facile electrons transfer.
3.10. Solar Cell Characterization
In comparison to TiO2, ZnO, and Nb2O5, which have conduction bands (CBs) at −4.25, −4.0, and −3.7 eV, respectively, NO2Lw has the LUMO level at −3.86 eV. Thus, based on the energy levels of NO2Lw, TiO2, and ZnO, the electron injection from the LUMO level of NO2Lw into the CB of TiO2 and ZnO is possible. However, the CB position of Nb2O5 (−3.7 eV)36 is above the LUMO level of NO2Lw (−3.86 eV). Some unexpected electron injection has been observed in the present case, and Nb2O5 photoanode-based DSSCs128,129 showed lower performance than ZnO and TiO2.
Figure 13 depicts a schematic of the process flow for NO2Lw-sensitized TiO2, ZnO, and Nb2O5-based DSSCs. The photon incident excites the electron at the NO2Lw, injecting the excited electron into the respective CB of TiO2, ZnO, and Nb2O5. The NO2Lw HOMO level (−6.73 eV) is below the iodine/triiodide electrolyte redox potential (−4.8 eV), facilitating the active regeneration of the oxidized NO2Lw. Due to the removal of an electron in NO2Lw, holes are produced at the HOMO level. The holes absorb the electrons from I–/I3– redox couple, which causes I– to be converted to I3– in the electrolyte. The electrons pass through the counter electrode and are transferred to the external circuit. In an oxidation–reduction reaction, the electrons return to the electrolyte.130−132
Figure 13.

Schematic of the process embedded in DSSCs.
The characteristic curve of NO2Lw-sensitized TiO2, ZnO, and Nb2O5 photoanode-based DSSCs is shown in Figure 14 as photocurrent density (Jsc) versus photovoltage (V). The DSSCs were irradiated with 100 mW cm–2 light intensity during the photovoltaic analyses; a polyiodide solution was employed as an electrolyte. The photovoltaics characteristic for TiO2, ZnO, and Nb2O5 photoanodes, including open circuit voltage (Voc), short-circuit photocurrent density (Jsc), fill factor (FF), and efficiency (η), were computed and summarized in Table 4.
Figure 14.

J–V plot characteristic of TiO2, ZnO, and Nb2O5 photoanode-based devices.
Table 4. Photovoltaic Performance of TiO2, ZnO, and Nb2O5 Photoanode-based Devices.
| Sample/Photosensitizer | Dye Adsorption/10–7 (mol cm–2) | Voc (V) | Jsc (mA/cm2) | FF | Rtr (Ω) | Rrec (Ω) | τe (ms) | η (%) |
|---|---|---|---|---|---|---|---|---|
| TiO2/NO2Lw | 4.96 | 0.44 | 0.82 | 40 | 236 | 102,270 | 63 | 0.14 |
| ZnO/NO2Lw | 3.06 | 0.23 | 0.31 | 32 | 1053 | 47,715 | 52 | 0.02 |
| Nb2O5/NO2Lw | 0.57 | 0.24 | 0.16 | 42 | 275 | 10,111 | 17 | 0.01 |
It was found that TiO2 has a higher Jsc value (Jsc = 0.82 mA/cm2) than ZnO and Nb2O5-based photoanodes. ZnO and Nb2O5 have Jsc values of 0.31 and 0.16 mA/cm2, respectively. On the other hand, the TiO2, ZnO, and Nb2O5 photoanodes had corresponding Voc values of 0.44, 0.23, and 0.24 V, respectively. TiO2, ZnO, and Nb2O5 photoanodes have FF values of 40, 32, and 42, respectively, and their corresponding power conversion efficiency (PEC) η values are 0.14, 0.02, and 0.01%, respectively. Compared to the ZnO and Nb2O5 photoanodes, the TiO2 photoanode-based DSSC performed better in photovoltaic efficiency. This is because the TiO2 photoanode, compared to ZnO and Nb2O5 photoanodes, has a significantly more considerable photosensitizer loading amount.
The photosensitizer loading amounts of NO2Lw are calculated and summarized in Table 4 for TiO2, ZnO, and Nb2O5 photoanodes. In addition, compared to ZnO and Nb2O5 photoanodes, computed Jsc and Voc values for the TiO2 photoelectrode are greater. Finally, a power conversion efficiency (PCE) of 0.14% was shown by the TiO2 photoanode.
3.11. Electrochemical Impedance Spectroscopy (EIS) Analysis
EIS measurement was done at a forward bias of open-circuit voltage under dark illumination conditions in the 1 MHz to 0.1 Hz frequency range to explain the electron transportation resistance and charge recombination resistance at different interfaces and determine the effective electron lifetime of the device.
Figure 15a displays the Bode plot with signature frequency peak corresponding to interfacial electron recombination toward the low-frequency region for the TiO2 in comparison to ZnO and Nb2O5 photoanode-based DSSCs, indicating that the TiO2 has a greater electron lifetime than ZnO and Nb2O5 photoanode-based DSSCs. The average electron lifetime (τeff) of TiO2, ZnO, and Nb2O5 photoanode-based devices can be calculated using the equation133
| 2 |
Figure 15.
(a) Bode plot and (b) Nyquist plot of TiO2, ZnO, and Nb2O5 photoanode-based devices.
Table 4 provides a summary of the values. The average electron lifetime for TiO2 (63 ms) photoanode-based devices was higher in comparison to those for ZnO (52 ms) and Nb2O5 (17 ms) photoanode-based DSSCs. This indicates that as compared to ZnO and Nb2O5 photoanodes, there is reduced electron recombination at the TiO2 photoanode/electrolyte interface-based DSSCs.
Figure 15b displays the Nyquist plot of TiO2, ZnO, and Nb2O5 photoanodes-based DSSCs. The electrochemical parameters of all three devices were calculated using Z View fitted software by fitting the measured EIS entities. Table 4 displays the corresponding values. In Figure 15b, the first semicircle depicts the interface between the counter electrode and an electrolyte, influencing the R2 (Rtr) electron transport resistance. At TiO2/ZnO/Nb2O5/NO2Lw/electrolyte, the charge recombination resistance of electrons was shown by R3 (Rrec).
In the current study, two semicircles in the EIS curves of the TiO2, ZnO, and Nb2O5-based photoanode are merged. This is because the electron recombination resistance at the TiO2/ZnO/Nb2O5/NO2Lw/electrolyte process is significantly greater than the other resistance at the counter electrode and electrolyte interface (electron transport resistance, Rtr). The smaller semicircle associated with the resistance at the interface between the counter electrode and electrolyte may thus be challenging to detect.
The charge recombination resistance (Rrec) is seen to increase in the order Nb2O5 < ZnO < TiO2. Greater Rrec in dark illumination is correlated with a lower recombination rate.134,135 Compared to ZnO and Nb2O5 photoanode-based DSSCs, the Rrec is greater in TiO2 photoanode-based DSSCs, indicating a lower recombination rate at the TiO2 photoanode/electrolyte interface. Thus the optimum values of Rrec led to the highest current density and open circuit voltage for TiO2 compared to ZnO and Nb2O5 photoanode-based DSSCs.
4. Conclusions
In the fabrication and development of DSSCs, the performance of three distinct mesoporous semiconducting oxides, TiO2, ZnO, and Nb2O5, as photoanode materials has been compared. The utilization of NO2Lw as a photosensitizer for DSSCs is shown for the first time in this study. We looked into photovoltaic parameters such as the VOC, JSC, and power conversion efficiency (PEC). Due to their higher photosensitizer loading amount, greater electron lifetime, and reduced electron recombination rate, the fabricated DSSCs function better with the TiO2 photoanode than with ZnO and Nb2O5 photoanodes. The outcomes suggest a role for injecting electrons from higher excited-state energy (LUMO) levels of NO2Lw into the conduction band (CB) of the photoanode material, to which the NO2Lw is chemically bonded.
Acknowledgments
Niyamat Beedri is grateful to Savitribai Phule Pune University, Post-Doctoral Fellowship (SPPU-PDF) program (Grant No. SPPU-PDF/ST/PH/2021/0003).
The authors declare no competing financial interest.
References
- Sonu; Rani G. M.; Pathania D.; Abhimanyu; Umapathi R.; Rustagi S.; Huh Y. S.; Gupta V. K.; Kaushik A.; Chaudhary V. Agro-Waste to Sustainable Energy: A Green Strategy of Converting Agricultural Waste to Nano-Enabled Energy Applications. Science of The Total Environment 2023, 875, 162667. 10.1016/j.scitotenv.2023.162667. [DOI] [PubMed] [Google Scholar]
- Jie H.; Khan I.; Alharthi M.; Zafar M. W.; Saeed A. Sustainable Energy Policy, Socio-Economic Development, and Ecological Footprint: The Economic Significance of Natural Resources, Population Growth, and Industrial Development. Utilities Policy 2023, 81, 101490. 10.1016/j.jup.2023.101490. [DOI] [Google Scholar]
- Fan Q.; Abbas J.; Zhong Y.; Pawar P. S.; Adam N. A.; Alarif G. B. Role of Organizational and Environmental Factors in Firm Green Innovation and Sustainable Development: Moderating Role of Knowledge Absorptive Capacity. Journal of Cleaner Production 2023, 411, 137262. 10.1016/j.jclepro.2023.137262. [DOI] [Google Scholar]
- Ni Z.; Yang J.; Razzaq A. How Do Natural Resources, Digitalization, and Institutional Governance Contribute to Ecological Sustainability through Load Capacity Factors in Highly Resource-Consuming Economies?. Resources Policy 2022, 79, 103068. 10.1016/j.resourpol.2022.103068. [DOI] [Google Scholar]
- Dey S.; Sreenivasulu A.; Veerendra G. T. N.; Rao K. V.; Babu P. S. S. A. Renewable Energy Present Status and Future Potentials in India: An Overview. Innovation and Green Development 2022, 1 (1), 100006. 10.1016/j.igd.2022.100006. [DOI] [Google Scholar]
- Santika W. G.; Anisuzzaman M.; Bahri P. A.; Shafiullah G. M.; Rupf G. V.; Urmee T. From Goals to Joules: A Quantitative Approach of Interlinkages between Energy and the Sustainable Development Goals. Energy Research & Social Science 2019, 50, 201–214. 10.1016/j.erss.2018.11.016. [DOI] [Google Scholar]
- Kabeyi M. J. B.; Olanrewaju O. A. Sustainable Energy Transition for Renewable and Low Carbon Grid Electricity Generation and Supply. Front. Energy Res. 2022, 9, 743114. 10.3389/fenrg.2021.743114. [DOI] [Google Scholar]
- Cai K.; Wu H.; Hua T.; Liao C.; Tang H.; Wang L.; Cao D. Molecular Engineering of the Fused Azacycle Donors in the D-A-π-A Metal-Free Organic Dyes for Efficient Dye-Sensitized Solar Cells. Dyes Pigm. 2022, 197, 109922. 10.1016/j.dyepig.2021.109922. [DOI] [Google Scholar]
- Huang H.; Yan Z. Present Situation and Future Prospect of Hydropower in China. Renewable and Sustainable Energy Reviews 2009, 13 (6–7), 1652–1656. 10.1016/j.rser.2008.08.013. [DOI] [Google Scholar]
- Bejarano M. D.; Sordo-Ward A.; Gabriel-Martin I.; Garrote L. Tradeoff between Economic and Environmental Costs and Benefits of Hydropower Production at Run-of-River-Diversion Schemes under Different Environmental Flows Scenarios. Journal of Hydrology 2019, 572, 790–804. 10.1016/j.jhydrol.2019.03.048. [DOI] [Google Scholar]
- Smakhtin V. U. Low Flow Hydrology: A Review. Journal of Hydrology 2001, 240 (3–4), 147–186. 10.1016/S0022-1694(00)00340-1. [DOI] [Google Scholar]
- Holechek J. L.; Geli H. M. E.; Sawalhah M. N.; Valdez R. A Global Assessment: Can Renewable Energy Replace Fossil Fuels by 2050?. Sustainability 2022, 14 (8), 4792. 10.3390/su14084792. [DOI] [Google Scholar]
- Sen A.; Putra M. H.; Biswas A. K.; Behera A. K.; Groβ A. Insight on the Choice of Sensitizers/Dyes for Dye Sensitized Solar Cells: A Review. Dyes Pigm. 2023, 213, 111087. 10.1016/j.dyepig.2023.111087. [DOI] [Google Scholar]
- Zhao Q.; Lai C.; Zhang H.; Hu Z. A Broad-Spectrum Solar Energy Power System by Hybridizing Stirling-like Thermocapacitive Cycles to Dye-Sensitized Solar Cells. Renewable Energy 2023, 205, 94–104. 10.1016/j.renene.2023.01.069. [DOI] [Google Scholar]
- Sun L.; Chen Y.; Sun M.; Zheng Y. Organic Solar Cells: Physical Principle and Recent Advances. Chemistry An Asian Journal 2023, 18 (5), e202300006. 10.1002/asia.202300006. [DOI] [PubMed] [Google Scholar]
- Vodapally S. N.; Ali M. H. A Comprehensive Review of Solar Photovoltaic (PV) Technologies, Architecture, and Its Applications to Improved Efficiency. Energies 2023, 16 (1), 319. 10.3390/en16010319. [DOI] [Google Scholar]
- Chen L. X. Organic Solar Cells: Recent Progress and Challenges. ACS Energy Lett. 2019, 4 (10), 2537–2539. 10.1021/acsenergylett.9b02071. [DOI] [Google Scholar]
- Sharma K.; Sharma V.; Sharma S. S. Dye-Sensitized Solar Cells: Fundamentals and Current Status. Nanoscale Res. Lett. 2018, 13 (1), 381. 10.1186/s11671-018-2760-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S.; Lee J. Recent Advances and Challenges toward Efficient Perovskite/Organic Integrated Solar Cells. Energies 2023, 16 (1), 266. 10.3390/en16010266. [DOI] [Google Scholar]
- Molina D.; Follana-Berna J.; Sastre-Santos A. Phthalocyanines, Porphyrins and Other Porphyrinoids as Components of Perovskite Solar Cells. J. Mater. Chem. C 2023, 11 (24), 7885. 10.1039/D2TC04441B. [DOI] [Google Scholar]
- Basit M. A.; Aanish Ali M.; Masroor Z.; Tariq Z.; Bang J. H. Quantum Dot-Sensitized Solar Cells: A Review on Interfacial Engineering Strategies for Boosting Efficiency. Journal of Industrial and Engineering Chemistry 2023, 120, 1–26. 10.1016/j.jiec.2022.12.016. [DOI] [Google Scholar]
- Kaur N.; Singh D. P.; Mahajan A. Plasmonic Engineering of TiO2 Photoanodes for Dye-Sensitized Solar Cells: A Review. J. Electron. Mater. 2022, 51 (8), 4188–4206. 10.1007/s11664-022-09707-3. [DOI] [Google Scholar]
- Shao J.-Y.; Li D.; Shi J.; Ma C.; Wang Y.; Liu X.; Jiang X.; Hao M.; Zhang L.; Liu C.; Jiang Y.; Wang Z.; Zhong Y.-W.; Liu S. F.; Mai Y.; Liu Y.; Zhao Y.; Ning Z.; Wang L.; Xu B.; Meng L.; Bian Z.; Ge Z.; Zhan X.; You J.; Li Y.; Meng Q. Recent Progress in Perovskite Solar Cells: Material Science. Sci. China Chem. 2023, 66 (1), 10–64. 10.1007/s11426-022-1445-2. [DOI] [Google Scholar]
- Zhang Q.; Li F.; Xu L. Application of Polyoxometalates in Third-Generation Solar Cells. Polyoxometalates 2023, 2 (1), 9140018. 10.26599/POM.2022.9140018. [DOI] [Google Scholar]
- Xu B.; Wang L.; Li X.; Yang X.; Lü W. A Facile Method to Fabricate Transparent TiO2 Photoanodes for Quantum Dot-Sensitized Solar Cells. Ionics 2022, 28 (6), 3049–3056. 10.1007/s11581-022-04543-1. [DOI] [Google Scholar]
- Pawar S.; Lokhande P. E.; Kaur J.; Dubey R. S.; Pathan H. M. Monochromatic Photochemical Deposition and Characterization of ZnSe Thin Films. ES Energy Environ. 2022, 17, 86–93. 10.30919/esee8c685. [DOI] [Google Scholar]
- Kishore Kumar D.; Kříž J.; Bennett N.; Chen B.; Upadhayaya H.; Reddy K. R.; Sadhu V. Functionalized Metal Oxide Nanoparticles for Efficient Dye-Sensitized Solar Cells (DSSCs): A Review. Materials Science for Energy Technologies 2020, 3, 472–481. 10.1016/j.mset.2020.03.003. [DOI] [Google Scholar]
- Kokkonen M.; Talebi P.; Zhou J.; Asgari S.; Soomro S. A.; Elsehrawy F.; Halme J.; Ahmad S.; Hagfeldt A.; Hashmi S. G. Advanced Research Trends in Dye-Sensitized Solar Cells. J. Mater. Chem. A 2021, 9 (17), 10527–10545. 10.1039/D1TA00690H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maka A. O. M.; Alabid J. M. Solar Energy Technology and Its Roles in Sustainable Development. Clean Energy 2022, 6 (3), 476–483. 10.1093/ce/zkac023. [DOI] [Google Scholar]
- Bandara T. M. W. J.; Hansadi J. M. C.; Bella F. A Review of Textile Dye-Sensitized Solar Cells for Wearable Electronics. Ionics 2022, 28 (6), 2563–2583. 10.1007/s11581-022-04582-8. [DOI] [Google Scholar]
- Mariotti N.; Bonomo M.; Fagiolari L.; Barbero N.; Gerbaldi C.; Bella F.; Barolo C. Recent Advances in Eco-Friendly and Cost-Effective Materials towards Sustainable Dye-Sensitized Solar Cells. Green Chem. 2020, 22 (21), 7168–7218. 10.1039/D0GC01148G. [DOI] [Google Scholar]
- Richhariya G.; Meikap B. C.; Kumar A. Review on Fabrication Methodologies and Its Impacts on Performance of Dye-Sensitized Solar Cells. Environ. Sci. Pollut Res. 2022, 29 (11), 15233–15251. 10.1007/s11356-021-18049-2. [DOI] [PubMed] [Google Scholar]
- Bhand S.; Salunke-Gawali S. Amphiphilic Photosensitizers in Dye Sensitized Solar Cells. Inorg. Chim. Acta 2019, 495, 118955. 10.1016/j.ica.2019.118955. [DOI] [Google Scholar]
- Pawar K. S.; Baviskar P. K.; Inamuddin; Nadaf A. B.; Salunke-Gawali S.; Pathan H. M. Layer-by-Layer Deposition of TiO2-ZrO2 Electrode Sensitized with Pandan Leaves: Natural Dye-Sensitized Solar Cell. Mater. Renew Sustain Energy 2019, 8 (2), 12. 10.1007/s40243-019-0148-x. [DOI] [Google Scholar]
- Dhonde M.; Sahu K.; Das M.; Yadav A.; Ghosh P.; Murty V. V. S. Review—Recent Advancements in Dye-Sensitized Solar Cells; From Photoelectrode to Counter Electrode. J. Electrochem. Soc. 2022, 169 (6), 066507. 10.1149/1945-7111/ac741f. [DOI] [Google Scholar]
- Beedri N. I.; Baviskar P. K.; Bhalekar V. P.; Jagtap C. V.; Asiri A. M.; Jadkar S. R.; Pathan H. M. N3-Sensitized TiO2/Nb2O5: A Novel Bilayer Structure for Dye-Sensitized Solar-Cell Application. Phys. Status Solidi A 2018, 215 (18), 1800236. 10.1002/pssa.201800236. [DOI] [Google Scholar]
- Beedri N. I.; Baviskar P. K.; Mahadik M.; Jadkar S. R.; Jang J. S.; Pathan H. M. Efficiency Enhancement for Cocktail Dye Sensitized Nb2O5 Photoanode Towards Dye Sensitized Solar Cell. Eng. Sci. 2019, 8, 76–82. 10.30919/es8d803. [DOI] [Google Scholar]
- O’Regan B.; Grätzel M. A Low-Cost, High-Efficiency Solar Cell Based on Dye-Sensitized Colloidal TiO2 Films. Nature 1991, 353 (6346), 737–740. 10.1038/353737a0. [DOI] [Google Scholar]
- Güzel E.; Medina D.-P.; Medel M.; Kandaz M.; Torres T.; Rodríguez-Morgade M. S. A Versatile, Divergent Route for the Synthesis of ABAC Tetraazaporphyrins: Molecularly Engineered, Push-Pull Phthalocyanine-Type Dyes. J. Mater. Chem. C 2021, 9 (33), 10802–10810. 10.1039/D1TC00990G. [DOI] [Google Scholar]
- Pashaei B.; Shahroosvand H. Molecularly Engineered Ruthenium Polypyridyl Complexes for Using in Dye-Sensitized Solar Cell. Inorg. Chem. Commun. 2020, 112, 107737. 10.1016/j.inoche.2019.107737. [DOI] [Google Scholar]
- Chandrasekharam M.; Rajkumar G.; Srinivasa Rao C.; Suresh T.; Yella Reddy P.; Yum J.-H.; Khaja Nazeeruddin M.; Graetzel M. A Molecularly Engineered Fluorene-Substituted Ru-Complex for Efficient Mesoscopic Dye-Sensitized Solar Cells. Adv. Nat. Sci: Nanosci. Nanotechnol. 2011, 2 (3), 035016. 10.1088/2043-6262/2/3/035016. [DOI] [Google Scholar]
- Alnakeeb A.; Fadda A. A.; Ismail M. A.; Elmorsy M. R. Efficient Co-Sensitization of Novel Trimethoxybenzene-Based Dyes with N-719 for Highly Efficient Dye-Sensitized Solar Cells. Opt. Mater. 2022, 128, 112344. 10.1016/j.optmat.2022.112344. [DOI] [Google Scholar]
- Sahoo S. S.; Murmu M.; Banerjee P.; Pathan H. M.; Salunke-Gawali S. Tailoring Benzo[α]Phenoxazine Moiety for Efficient Photosensitizers in Dye Sensitized Solar Cells via the DFT/TD-DFT Method. New J. Chem. 2022, 46 (31), 15155–15167. 10.1039/D2NJ02589B. [DOI] [Google Scholar]
- Baptayev B.; Kim S.-M.; Bolatbek B.; Lee S. H.; Balanay M. P. Effect of π-Spacer Length in Novel Xanthene-Linked l -(D-π-A)2 -Type Dianchoring Dyes for Dye-Sensitized Solar Cells. ACS Appl. Energy Mater. 2022, 5 (6), 6764–6771. 10.1021/acsaem.2c00384. [DOI] [Google Scholar]
- Subalakshmi K.; Chung W.; Lee S. Synergistically Improved Photovoltaic Performances of Dye-Sensitized Solar Cells with Metal-Free Organic Cosensitizer and Hybrid RGO-TiO2 Photoanode. Dyes Pigm. 2023, 209, 110892. 10.1016/j.dyepig.2022.110892. [DOI] [Google Scholar]
- Cheng H.-M.; Hsieh W.-F. High-Efficiency Metal-Free Organic-Dye-Sensitized Solar Cells with Hierarchical ZnO Photoelectrode. Energy Environ. Sci. 2010, 3 (4), 442. 10.1039/b915725e. [DOI] [Google Scholar]
- Luo J.; Xu M.; Li R.; Huang K.-W.; Jiang C.; Qi Q.; Zeng W.; Zhang J.; Chi C.; Wang P.; Wu J. N -Annulated Perylene as An Efficient Electron Donor for Porphyrin-Based Dyes: Enhanced Light-Harvesting Ability and High-Efficiency Co(II/III)-Based Dye-Sensitized Solar Cells. J. Am. Chem. Soc. 2014, 136 (1), 265–272. 10.1021/ja409291g. [DOI] [PubMed] [Google Scholar]
- Mathew S.; Yella A.; Gao P.; Humphry-Baker R.; Curchod B. F. E.; Ashari-Astani N.; Tavernelli I.; Rothlisberger U.; Nazeeruddin Md. K.; Grätzel M. Dye-Sensitized Solar Cells with 13% Efficiency Achieved through the Molecular Engineering of Porphyrin Sensitizers. Nature Chem. 2014, 6 (3), 242–247. 10.1038/nchem.1861. [DOI] [PubMed] [Google Scholar]
- Ito S.; Miura H.; Uchida S.; Takata M.; Sumioka K.; Liska P.; Comte P.; Péchy P.; Grätzel M. High-Conversion-Efficiency Organic Dye-Sensitized Solar Cells with a Novel Indoline Dye. Chem. Commun. 2008, (41), 5194. 10.1039/b809093a. [DOI] [PubMed] [Google Scholar]
- Ooyama Y.; Inoue S.; Asada R.; Ito G.; Kushimoto K.; Komaguchi K.; Imae I.; Harima Y. Dye-Sensitized Solar Cells Based on a Novel Fluorescent Dye with a Pyridine Ring and a Pyridinium Dye with the Pyridinium Ring Forming Strong Interactions with Nanocrystalline TiO2 Films. Eur. J. Org. Chem. 2010, 2010 (1), 92. 10.1002/ejoc.200900983. [DOI] [Google Scholar]
- Wu Y.; Zhang X.; Li W.; Wang Z.-S.; Tian H.; Zhu W. Hexylthiophene-Featured D-A-π-A Structural Indoline Chromophores for Coadsorbent-Free and Panchromatic Dye-Sensitized Solar Cells. Adv. Energy Mater. 2012, 2 (1), 149–156. 10.1002/aenm.201100341. [DOI] [Google Scholar]
- Clifford J. N.; Martínez-Ferrero E.; Viterisi A.; Palomares E. Sensitizer Molecular Structure-Device Efficiency Relationship in Dye Sensitized Solar Cells. Chem. Soc. Rev. 2011, 40 (3), 1635–1646. 10.1039/B920664G. [DOI] [PubMed] [Google Scholar]
- Hagfeldt A.; Boschloo G.; Sun L.; Kloo L.; Pettersson H. Dye-Sensitized Solar Cells. Chem. Rev. 2010, 110 (11), 6595–6663. 10.1021/cr900356p. [DOI] [PubMed] [Google Scholar]
- Pfattner R.; Pavlica E.; Jaggi M.; Liu S.-X.; Decurtins S.; Bratina G.; Veciana J.; Mas-Torrent M.; Rovira C. Photo-Induced Intramolecular Charge Transfer in an Ambipolar Field-Effect Transistor Based on a π-Conjugated Donor-Acceptor Dyad. J. Mater. Chem. C 2013, 1 (25), 3985. 10.1039/c3tc30442f. [DOI] [Google Scholar]
- Schoden F.; Schnatmann A. K.; Blachowicz T.; Manz-Schumacher H.; Schwenzfeier-Hellkamp E. Circular Design Principles Applied on Dye-Sensitized Solar Cells. Sustainability 2022, 14 (22), 15280. 10.3390/su142215280. [DOI] [Google Scholar]
- Pu H.; Fang T.; Wu Z.; Sun D.-W. Advancements in Recyclable Photocatalytic Semiconductor Substrates for SERS Detection in Food Safety Applications. Trends in Food Science & Technology 2023, 138, 697–707. 10.1016/j.tifs.2023.07.005. [DOI] [Google Scholar]
- Khanmohammadi Chenab K.; Sohrabi B.; Zamani Meymian M. R.; Mousavi S. V. Naphthoquinone Derivative-Based Dye for Dye-Sensitized Solar Cells: Experimental and Computational Aspects. Mater. Res. Express 2019, 6 (8), 085537. 10.1088/2053-1591/ab2500. [DOI] [Google Scholar]
- Khanmohammadi K.; Sohrabi B.; Zamani Meymian M. R. Effect of Electron-Donating and -Withdrawing Substitutions in Naphthoquinone Sensitizers: The Structure Engineering of Dyes for DSSCs. J. Mol. Struct. 2018, 1167, 274–279. 10.1016/j.molstruc.2018.05.014. [DOI] [Google Scholar]
- Mahadik S. A.; Salunke-Gawali S. 2-Chloro-(n-Alkylamino)Pyridine-1,4-Naphthoquinones as Photosensitizers in TiO2 and ZnO-Based DSSCs. J. Mater. Sci: Mater. Electron 2023, 34 (22), 1609. 10.1007/s10854-023-11020-6. [DOI] [Google Scholar]
- Shinde D.; Tambade P.; Pathan H.; Gadave K. Experimental and Theoretical Study of 1, 4-Naphthoquinone Based Dye in Dye-Sensitized Solar Cells Using ZnO Photoanode. Materials Science-Poland 2017, 35 (4), 746–754. 10.1515/msp-2017-0088. [DOI] [Google Scholar]
- Mahadik S. A.; Pathan H. M.; Salunke-Gawali S.; Butcher R. J. Titania Nanorods Embedded with 2-Bromo-3-(Methylamino)Naphthalene-1,4-Dione for Dye-Sensitized Solar Cells. ACS Omega 2022, 7 (40), 35595–35609. 10.1021/acsomega.2c03208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhand S.; Chadar D.; Pawar K.; Naushad M.; Pathan H.; Salunke-Gawali S. Benzo[α]Phenothiazine Sensitized ZrO2 Based Dye Sensitized Solar Cell. J. Mater. Sci: Mater. Electron 2018, 29 (2), 1034–1041. 10.1007/s10854-017-8003-2. [DOI] [Google Scholar]
- Khadtare S. S.; Ware A. P.; Salunke-Gawali S.; Jadkar S. R.; Pingale S. S.; Pathan H. M. Dye Sensitized Solar Cell with Lawsone Dye Using a ZnO Photoanode: Experimental and TD-DFT Study. RSC Adv. 2015, 5 (23), 17647–17652. 10.1039/C4RA14620D. [DOI] [Google Scholar]
- S. S.; Pesala B. Performance Enhancement of Betanin Solar Cells Co-Sensitized with Indigo and Lawsone: A Comparative Study. ACS Omega 2019, 4 (19), 18023–18034. 10.1021/acsomega.9b01875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beedri N. I.; Mokashi V. B.; Mahadik S. A.; Pathan H. M.; Salunke-Gawali S. Naphthoquinoneoxime-Sensitized Titanium Dioxide Photoanodes: Photoelectrochemical Properties. ACS Omega 2022, 7 (45), 41519–41530. 10.1021/acsomega.2c05334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahadik S. A.; Pathan H. M.; Salunke-Gawali S.; Butcher R. J. Aminonaphthoquinones as Photosensitizers for Mesoporous ZnO Based Dye-Sensitized Solar Cells. J. Alloys Compd. 2020, 845, 156279. 10.1016/j.jallcom.2020.156279. [DOI] [Google Scholar]
- Sahoo S. S.; Chadar D.; Murmu M.; Banerjee P.; Salunke-Gawali S.; Butcher R. J. Evaluation of Physicochemical Properties of Provitamin K3 Derived Benzo[α]Phenoxazine as a Photosensitizer. Eng. Sci. 2021, 14, 94–108. 10.30919/es8d1103. [DOI] [Google Scholar]
- Mahadik S. A.; Patil A.; Pathan H. M.; Salunke-Gawali S.; Butcher R. J. Thionaphthoquinones as Photosensitizers for TiO2 Nanorods and ZnO Nanograin Based Dye-Sensitized Solar Cells: Effect of Nanostructures on Charge Transport and Photovoltaic Performance. Eng. Sci. 2020, 14, 46–58. 10.30919/es8d1160. [DOI] [Google Scholar]
- Lakshmi R.; Krishnakumar G.; Joseph L. K.; Sreelatha K. S.; Jinchu I.. Lawsone Dye Complex: An Efficient Sensitizer for Dye Sensitized Solar Cell. In 2016 International Conference on Electrical, Electronics, and Optimization Techniques (ICEEOT); IEEE: Chennai, India, 2016; pp 4636–4638.
- Bonomo M.; Sabuzi F.; Di Carlo A.; Conte V.; Dini D.; Galloni P. KuQuinones as Sensitizers for NiO Based P-Type Dye-Sensitized Solar Cells. New J. Chem. 2017, 41 (7), 2769–2779. 10.1039/C6NJ03466G. [DOI] [Google Scholar]
- Zhang L.; Cole J. M.; Waddell P. G.; Low K. S.; Liu X. Relating Electron Donor and Carboxylic Acid Anchoring Substitution Effects in Azo Dyes to Dye-Sensitized Solar Cell Performance. ACS Sustainable Chem. Eng. 2013, 1 (11), 1440–1452. 10.1021/sc400183t. [DOI] [Google Scholar]
- Chen Y.-S.; Li C.; Zeng Z.-H.; Wang W.-B.; Wang X.-S.; Zhang B.-W. Efficient Electron Injection Due to a Special Adsorbing Group’s Combination of Carboxyl and Hydroxyl: Dye-Sensitized Solar Cells Based on New Hemicyanine Dyes. J. Mater. Chem. 2005, 15 (16), 1654–1661. 10.1039/B418906J. [DOI] [Google Scholar]
- Rajendra Prasad M. B.; Pathan H. M. Room Temperature Synthesis of Rutile Titania Nanoparticles: A Thermodynamic Perspective. Eur. Phys. J. D 2014, 68 (2), 25. 10.1140/epjd/e2013-40268-1. [DOI] [Google Scholar]
- Prasad M B R.; Pathan H. M. Effect of Photoanode Surface Coverage by a Sensitizer on the Photovoltaic Performance of Titania Based CdS Quantum Dot Sensitized Solar Cells. Nanotechnology 2016, 27 (14), 145402. 10.1088/0957-4484/27/14/145402. [DOI] [PubMed] [Google Scholar]
- Rane S. Y.; Khan E. M.; Khursheed Ah.; Salunke-Gawali S. Ligand Induced Stereoisomers Revealed in Copper(II) Complex of Nitrolawsone Oxime: EPR and Electronic Spectral Studies. Synthesis and Reactivity in Inorganic, Metal-Organic, and Nano-Metal Chemistry 2005, 35 (5), 343–353. 10.1081/SIM-200059192. [DOI] [Google Scholar]
- Zhang Q. Effects of Calcination on the Photocatalytic Properties of Nanosized TiO2 Powders Prepared by TiCl4 Hydrolysis. Applied Catalysis B: Environmental 2000, 26 (3), 207–215. 10.1016/S0926-3373(00)00122-3. [DOI] [Google Scholar]
- A R Sayyed S. A.; Beedri N. I.; Kadam V. S.; Pathan H. M. Rose Bengal-Sensitized Nanocrystalline Ceria Photoanode for Dye-Sensitized Solar Cell Application. Bull. Mater. Sci. 2016, 39 (6), 1381–1387. 10.1007/s12034-016-1279-7. [DOI] [Google Scholar]
- Sayyed S. A. A. R.; Beedri N. I.; Pathan H. M. Spinach Extract and Eosin-Y Co-Sensitized Ceria Photoanode for Dye Sensitized Solar Cell Application: Effect of Dye Adsorption Time. J. Mater. Sci: Mater. Electron 2017, 28 (6), 5075–5081. 10.1007/s10854-016-6145-2. [DOI] [Google Scholar]
- Mani J.; Sakeek H.; Habouti S.; Dietze M.; Es-Souni M. Macro-Meso-Porous TiO2, ZnO and ZnO-TiO2 -Composite Thick Films. Properties and Application to Photocatalysis. Catal. Sci. Technol. 2012, 2 (2), 379–385. 10.1039/C1CY00302J. [DOI] [Google Scholar]
- Lin J.; Yuan Y.; Su Q.; Pan A.; Dinesh S.; Peng C.; Cao G.; Liang S. Facile Synthesis of Nb2O5/Carbon Nanocomposites as Advanced Anode Materials for Lithium-Ion Batteries. Electrochim. Acta 2018, 292, 63–71. 10.1016/j.electacta.2018.09.138. [DOI] [Google Scholar]
- Luo H.; Song W.; Hoertz P. G.; Hanson K.; Ghosh R.; Rangan S.; Brennaman M. K.; Concepcion J. J.; Binstead R. A.; Bartynski R. A.; Lopez R.; Meyer T. J. A Sensitized Nb2O5 Photoanode for Hydrogen Production in a Dye-Sensitized Photoelectrosynthesis Cell. Chem. Mater. 2013, 25 (2), 122–131. 10.1021/cm3027972. [DOI] [Google Scholar]
- Inamdar Y.; Beedri N.; Kodam K.; Shaikh A.; Pathan H. Aggregation of ZnO Nanocrystallites Using Polyol Process for Dye (Reactive Red) Sensitized Solar Cell: Aggregation of ZnO Nanocrystallites Using Polyol. Macromol. Symp. 2015, 347 (1), 52–57. 10.1002/masy.201400047. [DOI] [Google Scholar]
- Kim J. S.; Shin S. S.; Han H. S.; Shin S.; Suk J. H.; Kang K.; Hong K. S.; Cho I. S. Facile Preparation of TiO2 Nanobranch/Nanoparticle Hybrid Architecture with Enhanced Light Harvesting Properties for Dye-Sensitized Solar Cells. J. Nanomater. 2015, 2015, 1–9. 10.1155/2015/139715. [DOI] [Google Scholar]
- Jo M.; Cho J.; Wang X.; Jin E.; Jeong S.; Kang D.-W. Improving of the Photovoltaic Characteristics of Dye-Sensitized Solar Cells Using a Photoelectrode with Electrospun Porous TiO2 Nanofibers. Nanomaterials 2019, 9 (1), 95. 10.3390/nano9010095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inamdar Y. A.; Beedri N. I.; Shaikh A. V.; Kodam K. M.; Pathan H. M. ZnO Photoelectrode for Textile Dye (Reactive Blue 59) Sensitized Solar Cell. Adv. Sci. Lett. 2014, 20 (5), 1155–1158. 10.1166/asl.2014.5443. [DOI] [Google Scholar]
- Sayyed S. A. A. R.; Beedri N. I.; Bhujbal P. K.; Shaikh S. F.; Pathan H. M. Eosin-Y Sensitized Bi-Layered ZnO Nanoflower-CeO2 Photoanode for Dye-Sensitized Solar Cells Application. ES Mater. Manuf. 2020, 10 (2), 45–51. 10.30919/esmm5f939. [DOI] [Google Scholar]
- Beedri N. I.; Sayyed S. A. A. R.; Jadkar S. R.; Pathan H. M.. Rose Bengal Sensitized Niobium Pentaoxide Photoanode for Dye Sensitized Solar Cell Application; In AIP Conference Proceedings, 2017, Vol. 1832 ( (1), ), p 040022.
- Le Viet A.; Jose R.; Reddy M. V.; Chowdari B. V. R.; Ramakrishna S. Nb2O5 Photoelectrodes for Dye-Sensitized Solar Cells: Choice of the Polymorph. J. Phys. Chem. C 2010, 114 (49), 21795–21800. 10.1021/jp106515k. [DOI] [Google Scholar]
- Zhang H.; Wang Y.; Yang D.; Li Y.; Liu H.; Liu P.; Wood B. J.; Zhao H. Directly Hydrothermal Growth of Single Crystal Nb3O7(OH) Nanorod Film for High Performance Dye-Sensitized Solar Cells. Adv. Mater. 2012, 24 (12), 1598–1603. 10.1002/adma.201104650. [DOI] [PubMed] [Google Scholar]
- Lungu J.; Socol G.; Stan G. E.; Ştefan N.; Luculescu C.; Georgescu A.; Popescu-Pelin G.; Prodan G.; Gîrţu M. A.; Mihăilescu I. N. Pulsed Laser Fabrication of TiO2 Buffer Layers for Dye Sensitized Solar Cells. Nanomaterials 2019, 9 (5), 746. 10.3390/nano9050746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lokhande P. E.; Chavan U. S.; Deokar S.; Ingale M.; Bhosale S.; Kale S.; Kamte A. Surfactant Free Chemically Deposited Wheat Spike-like Nanostructure on Cu Foam for Supercapacitor Applications. Materials Today: Proceedings 2019, 18, 979–985. 10.1016/j.matpr.2019.06.537. [DOI] [Google Scholar]
- Lokhande P. E.; Kadam V.; Jagatap C.; Chavan U. S.; R U.; Pathan H. M Hierarchical Ultrathin Nanosheet of Ni(OH)2/RGO Composite Chemically Deposited on Ni Foam for NOx Gas Sensors. ES Mater. Manuf. 2022, 17, 53–56. 10.30919/esmm5e721. [DOI] [Google Scholar]
- Khadtare S. S.; Jadkar S. R.; Salunke-Gawali S.; Pathan H. M. Lawsone Sensitized ZnO Photoelectrodes for Dye Sensitized Solar Cells. JNanoR 2013, 24, 140–145. 10.4028/www.scientific.net/JNanoR.24.140. [DOI] [Google Scholar]
- Usgodaarachchi L.; Thambiliyagodage C.; Wijesekera R.; Vigneswaran S.; Kandanapitiye M. Fabrication of TiO2 Spheres and a Visible Light Active α-Fe2O3 /TiO2 -Rutile/TiO2 -Anatase Heterogeneous Photocatalyst from Natural Ilmenite. ACS Omega 2022, 7 (31), 27617–27637. 10.1021/acsomega.2c03262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essien E. R.; Atasie V. N.; Nwude D. O.; Adekolurejo E.; Owoeye F. T. Characterisation of ZnO Nanoparticles Prepared Using Aqueous Leaf Extracts of Chromolaena Odorata (L.) and Manihot Esculenta (Crantz). S. Afr. J. Sci. 2022, 118 (1/2), 11225. 10.17159/sajs.2022/11225. [DOI] [Google Scholar]
- Ge J.; Wang F.; Xu Z.; Shen X.; Gao C.; Wang D.; Hu G.; Gu J.; Tang T.; Wei J. Influences of Niobium Pentoxide on Roughness, Hydrophilicity, Surface Energy and Protein Absorption, and Cellular Responses to PEEK Based Composites for Orthopedic Applications. J. Mater. Chem. B 2020, 8 (13), 2618–2626. 10.1039/C9TB02456E. [DOI] [PubMed] [Google Scholar]
- Alwin S.; Shajan X. S.; Karuppasamy K.; Warrier K. G. K. Microwave Assisted Synthesis of High Surface Area TiO2 Aerogels: A Competent Photoanode Material for Quasi-Solid Dye-Sensitized Solar Cells. Mater. Chem. Phys. 2017, 196, 37–44. 10.1016/j.matchemphys.2017.04.045. [DOI] [Google Scholar]
- Miao L.; Jin P.; Kaneko K.; Terai A.; Nabatova-Gabain N.; Tanemura S. Preparation and Characterization of Polycrystalline Anatase and Rutile TiO2 Thin Films by Rf Magnetron Sputtering. Appl. Surf. Sci. 2003, 212–213, 255–263. 10.1016/S0169-4332(03)00106-5. [DOI] [Google Scholar]
- Hosseinpour M.; Mirzaee O.; Alamdari S.; Menéndez J. L.; Abdoos H. Novel PWO/ ZnO Heterostructured Nanocomposites: Synthesis, Characterization, and Photocatalytic Performance. Journal of Environmental Management 2023, 345, 118586. 10.1016/j.jenvman.2023.118586. [DOI] [PubMed] [Google Scholar]
- Yang L.; Wei Y.; Song Y.; Peng Y.; Yang Y.; Huang Z. Surface-Enhanced Raman Scattering from Amorphous Nanoflower-Structural Nb2O5 Fabricated by Two-Step Hydrothermal Technology. Materials & Design 2020, 193, 108808. 10.1016/j.matdes.2020.108808. [DOI] [Google Scholar]
- Xu Y.; Li Z.; Zhang F.; Zhuang X.; Zeng Z.; Wei J. New Nitrogen-Rich Azo-Bridged Porphyrin-Conjugated Microporous Networks for High Performance of Gas Capture and Storage. RSC Adv. 2016, 6 (36), 30048–30055. 10.1039/C6RA04077B. [DOI] [Google Scholar]
- Madurai Ramakrishnan V.; Pitchaiya S.; Muthukumarasamy N.; Kvamme K.; Rajesh G.; Agilan S.; Pugazhendhi A.; Velauthapillai D. Performance of TiO2 Nanoparticles Synthesized by Microwave and Solvothermal Methods as Photoanode in Dye-Sensitized Solar Cells (DSSC). Int. J. Hydrogen Energy 2020, 45 (51), 27036–27046. 10.1016/j.ijhydene.2020.07.018. [DOI] [Google Scholar]
- Liu X.; Mao Z.; Liu J.; Meng F.; Shi X.; Xue X.; Zhao B. Probing the Open-Circuit Voltage Improvement of DSSC via Raman Spectroscopy: In Situ Dynamic Tracking Photoanode/Electrolyte Interfaces. ACS Appl. Energy Mater. 2022, 5 (7), 8391–8399. 10.1021/acsaem.2c00924. [DOI] [Google Scholar]
- Lee K. E.; Gomez M. A.; Regier T.; Hu Y.; Demopoulos G. P. Further Understanding of the Electronic Interactions between N719 Sensitizer and Anatase TiO2 Films: A Combined X-Ray Absorption and X-Ray Photoelectron Spectroscopic Study. J. Phys. Chem. C 2011, 115 (13), 5692–5707. 10.1021/jp109869z. [DOI] [Google Scholar]
- Xu L.; Wei B.; Liu W.; Zhang H.; Su C.; Che J. Flower-like ZnO-Ag2O Composites: Precipitation Synthesis and Photocatalytic Activity. Nanoscale Res. Lett. 2013, 8 (1), 536. 10.1186/1556-276X-8-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey S. K.; Pandey S. K.; Mukherjee C.; Mishra P.; Gupta M.; Barman S. R.; D’Souza S. W.; Mukherjee S. Effect of Growth Temperature on Structural, Electrical and Optical Properties of Dual Ion Beam Sputtered ZnO Thin Films. J. Mater. Sci: Mater. Electron 2013, 24 (7), 2541–2547. 10.1007/s10854-013-1130-5. [DOI] [Google Scholar]
- Jing L. Relationships of Surface Oxygen Vacancies with Photoluminescence and Photocatalytic Performance of ZnO Nanoparticles. Sci. China Ser. B 2005, 48 (1), 25. 10.1360/03yb0191. [DOI] [Google Scholar]
- Wang L.; Huang F.; Zhu G.; Dai Z. Nb2O5 Nanocrystals Decorated Graphene Composites as Anode Materials for High-Performance Dual-Ion Batteries. Nano Res. 2023, 1. 10.1007/s12274-023-6013-3. [DOI] [Google Scholar]
- Falk G.; Borlaf M.; López-Muñoz M. J.; Fariñas J. C.; Rodrigues Neto J. B.; Moreno R. Microwave-Assisted Synthesis of Nb2O5 for Photocatalytic Application of Nanopowders and Thin Films. J. Mater. Res. 2017, 32 (17), 3271–3278. 10.1557/jmr.2017.93. [DOI] [Google Scholar]
- Cho F.-H.; Kuo S.-C.; Lai Y.-H. Surface-Plasmon-Induced Azo Coupling Reaction between Nitro Compounds on Dendritic Silver Monitored by Surface-Enhanced Raman Spectroscopy. RSC Adv. 2017, 7 (17), 10259–10265. 10.1039/C7RA00374A. [DOI] [Google Scholar]
- Wang X.; Wang Y.; Sui H.; Zhang X.; Su H.; Cheng W.; Han X. X.; Zhao B. Investigation of Charge Transfer in Ag/N719/TiO2 Interface by Surface-Enhanced Raman Spectroscopy. J. Phys. Chem. C 2016, 120 (24), 13078–13086. 10.1021/acs.jpcc.6b03228. [DOI] [Google Scholar]
- Firtina-Ertis I.; Kerkez-Kuyumcu Ö. Synthesis of NiFe2O4/TiO2-Ag+ S-Scheme Photocatalysts by a Novel Complex-Assisted Vapor Thermal Method for Photocatalytic Hydrogen Production. J. Photochem. Photobiol., A 2022, 432, 114106. 10.1016/j.jphotochem.2022.114106. [DOI] [Google Scholar]
- Bhujbal P. K.; Pathan H. M.; Chaure N. B. Deposition of Amorphous and Crystalline Al Doped ZnO Thin Films by RF Magnetron Sputtering and Their Comparative Properties. ES Energy Environ. 2019, 4, 15–18. 10.30919/esee8c188. [DOI] [Google Scholar]
- Bhujbal P. K.; Pathan H. M.; Chaure N. B. Temperature Dependent Studies on Radio Frequency Sputtered Al Doped ZnO Thin Film. Eng. Sci. 2020, 10, 58–67. 10.30919/es8d1003. [DOI] [Google Scholar]
- Wang M.; Wang H.; Ren Y.; Wang C.; Weng Z.; Yue B.; He H. Construction of G-C3N4-MNb2O5 Composites with Enhanced Visible Light Photocatalytic Activity. Nanomaterials 2018, 8 (6), 427. 10.3390/nano8060427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Q.; Lv K.; Yu J. Pivotal Role of Fluorine in Enhanced Photocatalytic Activity of Anatase TiO2 Nanosheets with Dominant (001) Facets for the Photocatalytic Degradation of Acetone in Air. Applied Catalysis B: Environmental 2010, 96 (3–4), 557–564. 10.1016/j.apcatb.2010.03.020. [DOI] [Google Scholar]
- Vinaayak S. B.; Balasubramani V.; Shkir M.; Manthrammel M. A.; Sreedevi G. Enhancing the Performance of TiO2 Based N-DSSC Using Dye Extracted from Cladophora Columbiana, Ludwigia Repens and Mixed Sensitizer. Opt. Mater. 2022, 133, 112968. 10.1016/j.optmat.2022.112968. [DOI] [Google Scholar]
- Liu B.-Q.; Zhao X.-P.; Luo W. The Synergistic Effect of Two Photosynthetic Pigments in Dye-Sensitized Mesoporous TiO2 Solar Cells. Dyes Pigm. 2008, 76 (2), 327–331. 10.1016/j.dyepig.2006.09.004. [DOI] [Google Scholar]
- Vandewal K.; Benduhn J.; Nikolis V. C. How to Determine Optical Gaps and Voltage Losses in Organic Photovoltaic Materials. Sustainable Energy Fuels 2018, 2 (3), 538–544. 10.1039/C7SE00601B. [DOI] [Google Scholar]
- Sahoo S. S.; Salunke-Gawali S.; Kadam V. S.; Pathan H. M. Canna Lily Red, and Yellow Flower Extracts: A New Power Source to Produce Photovoltage through Dye-Sensitized Solar Cells. Energy Fuels 2020, 34 (8), 9674–9682. 10.1021/acs.energyfuels.0c01482. [DOI] [Google Scholar]
- Ketterer B.; Heiss M.; Livrozet M. J.; Rudolph A.; Reiger E.; Fontcuberta i Morral A. Determination of the Band Gap and the Split-off Band in Wurtzite GaAs Using Raman and Photoluminescence Excitation Spectroscopy. Phys. Rev. B 2011, 83 (12), 125307. 10.1103/PhysRevB.83.125307. [DOI] [Google Scholar]
- Adeniyi A. A.; Ngake T. L.; Conradie J. Cyclic Voltammetric Study of 2-Hydroxybenzophenone (HBP) Derivatives and the Correspondent Change in the Orbital Energy Levels in Different Solvents. Electroanalysis 2020, 32 (12), 2659–2668. 10.1002/elan.202060163. [DOI] [Google Scholar]
- Cardona C. M.; Li W.; Kaifer A. E.; Stockdale D.; Bazan G. C. Electrochemical Considerations for Determining Absolute Frontier Orbital Energy Levels of Conjugated Polymers for Solar Cell Applications. Adv. Mater. 2011, 23 (20), 2367–2371. 10.1002/adma.201004554. [DOI] [PubMed] [Google Scholar]
- Meng S.; Ren J.; Kaxiras E. Natural Dyes Adsorbed on TiO2 Nanowire for Photovoltaic Applications: Enhanced Light Absorption and Ultrafast Electron Injection. Nano Lett. 2008, 8 (10), 3266–3272. 10.1021/nl801644d. [DOI] [PubMed] [Google Scholar]
- Maurya I. C.; Neetu; Gupta A. K.; Srivastava P.; Bahadur L. Callindra Haematocephata and Peltophorum Pterocarpum Flowers as Natural Sensitizers for TiO2 Thin Film Based Dye-Sensitized Solar Cells. Opt. Mater. 2016, 60, 270–276. 10.1016/j.optmat.2016.07.041. [DOI] [Google Scholar]
- Nemade A. M.; Patil K. D.; Kolhe V. C. Synthesis, Compositional and Spectral Studies of Some Transition Metal Complexes with 3-Aminolawsonoxime. Res. J. Chem. Sci. 2017, 7 (1), 25–31. [Google Scholar]
- Sahoo S. S.; Salunke-Gawali S.; Jagtap C. V.; Bhujbal P.; Pathan H. M. Enhanced Photovoltage Production from Canna Dyes with Surface Passivation of ZnO Based Dye Sensitized Solar Cells. Journal of Science: Advanced Materials and Devices 2022, 7 (4), 100513. 10.1016/j.jsamd.2022.100513. [DOI] [Google Scholar]
- Lenzmann F.; Krueger J.; Burnside S.; Brooks K.; Grätzel M.; Gal D.; Rühle S.; Cahen D. Surface Photovoltage Spectroscopy of Dye-Sensitized Solar Cells with TiO2, Nb2O5, and SrTiO3 Nanocrystalline Photoanodes: Indication for Electron Injection from Higher Excited Dye States. J. Phys. Chem. B 2001, 105 (27), 6347–6352. 10.1021/jp010380q. [DOI] [Google Scholar]
- Waghmare M. A.; Naushad Mu.; Pathan H. M.; Ubale A. U. Rose Bengal-Sensitized ZrO2 Photoanode for Dye-Sensitized Solar Cell. J. Solid State Electrochem 2017, 21 (9), 2719–2723. 10.1007/s10008-017-3530-6. [DOI] [Google Scholar]
- Bramhankar T. S.; Pawar S. S.; Shaikh J. S.; Gunge V. C.; Beedri N. I.; Baviskar P. K.; Pathan H. M.; Patil P. S.; Kambale R. C.; Pawar R. S. Effect of Nickel-Zinc Co-Doped TiO2 Blocking Layer on Performance of DSSCs. J. Alloys Compd. 2020, 817, 152810. 10.1016/j.jallcom.2019.152810. [DOI] [Google Scholar]
- Shaikh J. S.; Shaikh N. S.; Mali S. S.; Patil J. V.; Pawar K. K.; Kanjanaboos P.; Hong C. K.; Kim J. H.; Patil P. S. Nanoarchitectures in Dye-Sensitized Solar Cells: Metal Oxides, Oxide Perovskites, and Carbon-Based Materials. Nanoscale 2018, 10 (11), 4987–5034. 10.1039/C7NR08350E. [DOI] [PubMed] [Google Scholar]
- Yeoh M.-E.; Chan K.-Y. Recent Advances in Photo-Anode for Dye-Sensitized Solar Cells: A Review: Recent Advances in Photo-Anode for DSSCs: A Review. Int. J. Energy Res. 2017, 41 (15), 2446–2467. 10.1002/er.3764. [DOI] [Google Scholar]
- Yang W.-G.; Wan F.-R.; Chen Q.-W.; Li J.-J.; Xu D.-S. Controlling Synthesis of Well-Crystallized Mesoporous TiO2Microspheres with Ultrahigh Surface Area for High-Performance Dye-Sensitized Solar Cells. J. Mater. Chem. 2010, 20 (14), 2870. 10.1039/b923105f. [DOI] [Google Scholar]
- Lee K.; Hu C.; Chen H.; Ho K. Incorporating Carbon Nanotube in a Low-Temperature Fabrication Process for Dye-Sensitized TiO2 Solar Cells. Sol. Energy Mater. Sol. Cells 2008, 92 (12), 1628–1633. 10.1016/j.solmat.2008.07.012. [DOI] [Google Scholar]
- Du P.; Song L.; Xiong J.; Yuan Y.; Wang L.; Xi Z.; Jin D.; Chen J. TiO2/Nb2O5 Core-Sheath Nanofibers Film: Co-Electrospinning Fabrication and Its Application in Dye-Sensitized Solar Cells. Electrochem. Commun. 2012, 25, 46–49. 10.1016/j.elecom.2012.09.013. [DOI] [Google Scholar]