Abstract
BACKGROUND: Primary nonadherence (PNA), when a medication is newly prescribed but not filled, has been identified as a major research gap potentially impacting the optimal treatment of patients with overweight and obesity who are newly prescribed antiobesity medications (AOMs).
OBJECTIVES: To assess PNA among patients with newly prescribed AOMs and to examine factors associated with PNA to AOMs.
METHODS: This was a retrospective study that used the Optum Integrated Clinical plus Claims database to identify individuals who had at least 1 prescription order for an AOM the US Food and Drug Administration approved for long-term use. Individuals with prescription orders between January 1, 2012, and February 28, 2019, were identified, and patient demographics, clinical characteristics, medication prescribed, baseline health care utilization, and obesity-related complications were described by PNA status. PNA was defined as no pharmacy claim for the AOM within 60 days of the date of the new prescription order as identified in electronic health record data. A multivariable logistic regression model was used to examine factors associated with PNA.
RESULTS: The study sample included a total of 1,563 patients. The mean body mass index was 38.4 kg/m2; 10.7% were prescribed liraglutide 3.0 mg, 26.0% were prescribed lorcaserin, 36.3% of patients were prescribed naltrexone-bupropion, 5.4% were prescribed orlistat, and 21.6% were prescribed phentermine-topiramate. Most patients (91.1%) exhibited PNA, with only 8.9% filling their newly prescribed AOM within 60 days. Both the adherent and nonadherent groups were predominately female sex, White, and covered by commercial insurance. The mean age was similar between the 2 groups. Most obesity-related complications were less prevalent in the adherent group, although the Charlson comorbidity index score was similar between the 2 groups. After adjustment for patient demographics and clinical characteristics, there was not a statistically significant association between the specific AOM and PNA (P = 0.299). Patients with depression or living in the Midwest or South regions were at significantly increased risk of PNA.
CONCLUSIONS: The rate of PNA to AOMs was very high, suggesting barriers in effective medical management of patients with overweight and obesity. Future research is warranted to understand reasons for PNA to AOMs and how to address these barriers.
DISCLOSURES: Dr Kan, Dr Bae, Dr Dunn, and Dr Ahmad are employees of Eli Lilly and Company. Ms Buysman and Dr Gronroos are employees of Optum. Dr Swindle was an employee of Optum at the time the study was conducted and is currently employed at Evidera. Dr Bengtson is employed at Boehringer Ingelheim Pharmaceuticals, Inc. (Boehringer Ingelheim has no connection to this study), and during the conduct of this study was employed at Optum.
Plain language summary
Patients with overweight and obesity may be prescribed medications to help reduce weight. These patients sometimes do not fill their medication. This study used information from health insurance claims to determine how many patients who were prescribed a new medication did not fill the prescription. We found that most patients do not fill these new prescriptions. Future studies should investigate why this happens.
Implications for managed care pharmacy
Study researchers investigated primary nonadherence among patients newly prescribed antiobesity medications. This study found that 9 in 10 (91.1%) patients exhibited primary nonadherence to new prescriptions. Patients with depression or living in the Midwestern or Southern regions were at higher risk compared with others. Results suggest that there are substantial barriers associated with treatment of overweight and obesity with antiobesity medications.
According to National Health and Nutrition Examination Survey data, the age-adjusted prevalence of obesity in US adults between 2017 and March 2020 was 41.9%.1 Current guidelines recommend the use of antiobesity medications (AOMs) for the treatment of patients with a body mass index (BMI) of at least 30 kg/m2 or at least 27 kg/m2 with at least 1 obesity-related complication (ORC) as adjunct to lifestyle intervention when the latter alone does not sufficiently reduce weight or improve ORCs.2-5 Despite the availability of medications approved by the US Food and Drug Administration (FDA) for long-term use and the high prevalence of overweight and obesity, the use of AOMs remains extremely low. Medications used for the treatment of obesity for at least 12 weeks are referred to in the literature as “FDA approved for long-term use” and “FDA approved for chronic weight management.”6 In this article, AOMs describe the set of medications approved for longterm (chronic) use. MacEwan et al reported that only 0.8% of adults in the United States eligible for AOMs reported use in the past 30 days and only 1.6% had used an AOM in the last 12 months.7
Nonadherence to medication for chronic health conditions has been estimated to cause more than 100,000 deaths and to cost more than $100 billion in preventable health care costs.8 Nonadherence arises either when a patient does not fill their initial prescription (primary nonadherence [PNA])9 or when the patient does not take their medication as prescribed once initiated (secondary nonadherence [SNA]).10 Published data demonstrate SNA to AOMs. In Hemo et al adherence to AOMs was 25% after 4 months, dropping to less than 2% after 12 months of treatment.11 Similarly, Ganguly et al reported adherence after 6 months of treatment was 41.8% for liraglutide 3.0 mg, 15.9% for lorcaserin, 18.1% for naltrexone/bupropion, and 27.3% for phentermine/topiramate.12
Although we are not aware of any previous research investigating PNA to AOMs, studies of PNA to other medications for treatment of chronic conditions, like type 2 diabetes mellitus (T2DM), found rates ranging from 24% to 58%.13,14 In the study by Luo et al, PNA to sodium/glucose cotransporter-2 inhibitors (canagliflozin, empagliflozin) and glucagon-like peptide-1 receptor agonists (dulaglutide, liraglutide, semaglutide) in patients with T2DM was 31.8%,15 with Black race, diabetes-related nephropathy, and hyperlipidemia associated with greater odds of PNA.16 In a 2019 meta-analysis, Cheen et al reported PNA rates of 25% in patients treated for osteoporosis and dyslipidemia.17 Factors such as younger age, higher copayments, and number of concurrent medications were associated with PNA.17
PNA to AOMs has been identified as a research gap that potentially impacts the optimal management of patients with overweight and obesity.18,19 The objective of this study was to estimate the rate of PNA to AOMs among patients who are newly prescribed AOMs and assess patient characteristics associated with PNA to AOMs approved by the FDA for long-term use.
Methods
DATA SOURCE
This was a retrospective study using medical claims, pharmacy claims, and enrollment information with linked electronic health record (EHR) data for commercial and Medicare Advantage with Part D (MAPD) health care plan members in the Optum Integrated Clinical plus Claims database. The study period was from January 1, 2011, to April 30, 2019. Medical claims were identified using International Classification of Diseases, Ninth and Tenth Revisions, Clinical Modification (ICD-9-CM/ICD-10-CM) diagnosis codes and procedure codes (ICD-9-CM/ICD-10-PCS), Current Procedural Terminology codes, Healthcare Common Procedure Coding System codes, place of service codes, provider specialty codes, and revenue codes. Relevant outpatient pharmacy claims were identified using National Drug Code numbers. This study did not require institutional review board approval or waiver of authorization because no identifiable protected health information was accessed.
STUDY SAMPLE
Patients were included in the sample if they had at least 1 record for a prescription order in the EHR data for an AOM from January 1, 2012, through February 28, 2019 (identification period); the index date was the date of the first prescription order for an AOM during the identification period. The AOMs included were those that were FDA approved for long-term use for chronic weight management, specifically, liraglutide 3.0 mg, lorcaserin, orlistat, naltrexone-bupropion, and phentermine-topiramate (Table 1). As the focus of this study was PNA to AOMs approved for long-term (chronic) use among patients engaged in intentional weight reduction, we did not investigate medications FDA approved for short-term (<12 weeks) use (Table 1). We also did not investigate medications prescribed off-label for weight loss but also commonly prescribed for reasons other than obesity. Additional inclusion criteria included age 18 years or older as of the index year, continuous enrollment in the health care plan with medical and pharmacy benefits for at least 12 months prior to the index date (baseline period) and at least 2 months including and following the index date (follow-up period), and evidence of clinical activity in the EHR during the 12-month baseline period. To ensure patients were newly prescribed their index AOM, patients with a prescription ordered in the EHR or a pharmacy claim for an AOM approved for long-term use during the baseline period were excluded. Additionally, patients with a pharmacy claim for an AOM approved for short-term use (namely, benzphetamine, diethylpropion, phendimetrazine, and phentermine) during the baseline period were excluded from the sample. Patients were also excluded if they had any of the following: (1) evidence of pregnancy, labor, or delivery during the baseline or follow-up periods; (2) unknown sex, geographic region, or health care plan type at index; (3) no BMI records in the EHR during the 6 months prior to and including the index date; or (4) prescription orders for multiple AOMs on the index date (excluded to allow for examination of the relationship between the index AOM and PNA).
TABLE 1.
Patient Demographic and Clinical Characteristics in the Overall Sample and Patients With and Without Primary Adherence to AOMs
| Total (N = 1,563) | Nonadherent (n = 1,424) | Adherent (n = 139) | |
|---|---|---|---|
| Age, mean (SD), years | 50.0 (12.2) | 50.0 (12.2) | 49.6 (12.1) |
| Age group, n (%), years | |||
| 18-39 | 320 (20.5) | 286 (20.1) | 34 (24.5) |
| 40-49 | 430 (27.5) | 393 (27.6) | 37 (26.6) |
| 50-59 | 478 (30.6) | 439 (30.8) | 39 (28.1) |
| 60-69 | 248 (15.9) | 226 (15.9) | 22 (15.8) |
| ≥70 | 87 (5.6) | 80 (5.6) | 7 (5.0) |
| Sex, n (%) | |||
| Female | 1,225 (78.4) | 1,119 (78.6) | 106 (76.3) |
| Male | 338 (21.6) | 305 (21.4) | 33 (23.7) |
| Health care plan type, n (%) | |||
| Commercial | 1,357 (86.8) | 1,229 (86.3) | 128 (92.1) |
| Medicare Advantage | 206 (13.2) | 195 (13.7) | 11 (7.9) |
| Geographic region, n (%) | |||
| Northeast | 98 (6.3) | 79 (5.5) | 19 (13.7) |
| Midwest | 607 (38.8) | 544 (38.2) | 63 (45.3) |
| South | 693 (44.3) | 653 (45.9) | 40 (28.8) |
| West | 165 (10.6) | 148 (10.4) | 17 (12.2) |
| CCI score, mean (SD) | 0.5 (1.0) | 0.5 (1.0) | 0.5 (1.0) |
| Race, n (%) | |||
| White | 1,323 (84.6) | 1,200 (84.3) | 123 (88.5) |
| BMI, mean (SD), kg/m2 | 38.4 (8.1) | 38.3 (8.1) | 39.6 (8.3) |
| BMI category, n (%), kg/m2 | |||
| ≥40.0 | 553 (35.4) | 496 (34.8) | 57 (41.0) |
| 30.0-40.0 | 842 (53.9) | 771 (54.1) | 71 (51.1) |
| <30.0a | 168 (10.7) | 157 (11.0) | 11 (7.9) |
| Index year, n (%) | |||
| 2012-2015 | 644 (41.2) | 600 (42.1) | 44 (31.7) |
| 2016-2019 | 919 (58.8) | 824 (57.9) | 95 (68.3) |
| Baseline ED visit, n (%) | 465 (29.8) | 423 (29.7) | 42 (30.2) |
| Baseline hospitalization, n (%) | 70 (4.5) | 68 (4.8) | 2 (1.4) |
aPatients are qualified for AOM prescription with a BMI above 30 or a BMI between 27 and 29.9 with more than 1 obesity-related condition.
AOM = antiobesity medication; BMI = body mass index; CCI = Charlson Comorbidity Index; ED = emergency department.
STUDY VARIABLES
Data obtained from claims included the following: age, sex, health care plan type, geographic region, Charlson Comorbidity Index (CCI) score,20,21 baseline emergency department (ED) visits, and baseline inpatient stays. Index medication, index year, race, and BMI category (latest value 6 months prior to and including index) were obtained from the EHR data. Baseline ORCs were captured based on diagnosis codes in the claims data for an ORC of interest during the baseline period (Figure 1). PNA was defined as no claim for the newly prescribed AOM within 60 days of the index date.22
FIGURE 1.

Baseline Obesity-Related Complications in Patients With and Without Primary Adherence to AOMs
ANALYSIS
Descriptive Statistics. Patient demographic and clinical characteristics were stratified by PNA status (ie, adherent, nonadherent). Frequencies and percentages were provided for categorical variables; means and SDs were provided for continuous variables.
Multivariable Analysis. A logistic regression model was used to examine the association between the specific AOM and PNA. The model was adjusted for age group, sex, geographic region, race, index year, health care plan type, CCI score, baseline BMI, baseline depression, and baseline inpatient stay or ED visit. Covariates were selected for model inclusion based on clinical rationale and statistical significance. An ORC was included in the final model if either of the following conditions were met: (1) statistically related to the outcome of interest (ie, PNA; significance level [α] of (0.05) or (2) controlling for the covariate changed the odds ratio (OR) for one of the index drugs by more than 10%. Multivariable adjusted associations between patient characteristics and PNA were reported. Odds ratios, P values, and CIs were reported.
Results
PATIENT SAMPLE AND PRIMARY ADHERENCE STATUS
The final study sample of patients with new AOM prescriptions included a total of 1,563 patients, of whom 168 (10.7%) were prescribed liraglutide 3.0 mg, 407 (26.0%) were prescribed lorcaserin, 567 (36.3%) were prescribed naltrexone-bupropion, 84 (5.4%) were prescribed orlistat, and 337 (21.6%) were prescribed phentermine-topiramate (Figure 2). There were 1,424 (91.1%) patients who did not have a claim for filling their AOM prescription within 60 days and were classified as nonadherent and 139 (8.9%) patients with a claim for their prescription within 60 days and were classified as adherent.
FIGURE 2.

Patient Identification, Attrition, and Cohort Assignment
BASELINE DEMOGRAPHIC CHARACTERISTICS
Table 2 presents characteristics for patients in the study sample. Both adherent and nonadherent patients were predominately female (76.3% and 78.6%, respectively). Mean age was similar between the adherent and nonadherent groups (49.6 years and 50.0 years, respectively). Most patients in the sample were White (84.6%), although a slightly lower percentage of patients in the adherent cohort were African American (7.9% and 10.4%, respectively) or other/unknown race (3.6% and 5.3%, respectively) than in the nonadherent cohort. Most patients in the sample had commercial health care plan coverage (86.8%), although the percentage of patients with an MAPD plan type was lower in the adherent group than in the nonadherent group (7.9% and 13.7%, respectively). Geography also varied between groups, with the largest percentage of patients in the adherent cohort residing in the Midwestern United States (45.3%) and largest percentage of patients in the nonadherent cohort residing in the Southern United States (45.9%).
TABLE 2.
Medication List
| Medication names |
|---|
| AOMs approved for long-term use |
| Liraglutide 3.0 mg / 0.5 mL Lorcaserin Naltrexone HCl-bupropion Orlistat Phentermine-topiramate |
| AOMs approved for short-term use |
| Benzphetamine Diethylpropion Phendimetrazine Phentermine |
AOM = antiobesity medication.
BASELINE CLINICAL CHARACTERISTICS
The mean BMI across the study sample was 38.4 kg/m2 (SD = 8.1). There were slightly more patients with a BMI of at least 40 kg/m2 in the adherent group than in the nonadherent group (41.0% and 34.8%, respectively). The mean CCI score in both the adherent and nonadherent group was 0.5 (SD = 1.0); however, most ORCs were less prevalent in the adherent group than the nonadherent group, including depression (11.5% and 20.5%), hypertension (39.6% and 46.8%), and T2DM (14.4% and 18.7%). The percentage of patients with at least 1 ED visit during the baseline period was similar between the adherent and nonadherent groups (30.2% and 29.7%, respectively). However, the percentage of patients with at least 1 hospitalization during the baseline period was small in both the adherent and nonadherent groups (1.4% and 4.8%, respectively).
PRIMARY NONADHERENCE
The unadjusted rates of PNA by specific AOM were as follows: 84.5% for liraglutide 3.0 mg, 92.4% for lorcaserin, 90.5% for orlistat, 90.7% for naltrexone-bupropion, and 93.8% for phentermine-topiramate. Before rates were adjusted for patient demographics and clinical characteristics, there was a statistically significant association between the specific AOM and PNA (P = 0.012).
After adjustment for patient demographics and clinical characteristics, there was not a statistically significant association between the specific AOM and PNA (P = 0.299) (Figure 3). However, other attributes were significant, with patients living in the Midwestern United States (OR = 2.3; P = 0.008) or the Southern United States (OR = 3.8; P < 0.001) having greater odds of being nonadherent compared with patients living in the Northeast. Additionally, patients with depression during baseline also had greater odds of PNA (OR = 2.1; P = 0.011).
FIGURE 3.
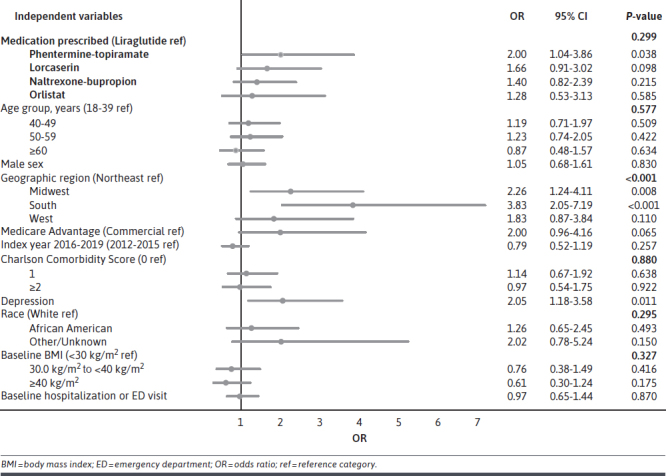
Multivariable Logistic Regression Model of the Association Between Primary Nonadherence and Medication Prescribed
Discussion
This study examines the rate of PNA to AOMs that were FDA approved for long-term, chronic use and describes patient characteristics that were associated with PNA. It was found that 91.1% of patients did not fill their new prescription for an AOM within 60 days, demonstrating a very high rate of PNA to AOMs. After multivariable adjustment, the specific AOM was not associated with PNA. Compared with the Northeast, patients residing in the South and Midwest had higher risk for PNA. This aligns with previous studies investigating primary nonadherence to chronic disease medications that have found lower adherence in the southern region of the United States in adjusted23,24 and unadjusted25 studies. Additionally, patients with baseline depression had an increased risk for PNA. This finding aligns with other work, which reported that depression is associated with SNA in patients with diabetes, and suggests that depression may also play a role in PNA.26
Rates of PNA were higher in this study than in studies of other medications, especially in comparison with medications for other chronic diseases. In a 2012 retrospective cohort study in primary care patients in an outpatient setting, the overall rate of PNA for all newly prescribed medications, excluding only pro re nata medications, was 58%.13 Among studies specifically evaluating adherence to medications for chronic conditions, a meta-analysis by Lemstra et al found a pooled PNA rate of 14.6% among patients prescribed antihypertensive, lipid-lowering, antihyperglycemic, and antidepressant agents.27 Cheen et al reported PNA rates of 25% for patients treated for osteoporosis and dyslipidemia.17 Additionally, Luo et al reported a PNA rate of 31.8% among patients with T2DM prescribed sodium/glucose cotransporter-2 inhibitors and glucagon-like peptide-1 agonists, including liraglutide.15 In the present study, PNA to the weight management dose of liraglutide (liraglutide 3.0 mg) was much higher at 84.5%. Although these comparisons should be treated with caution as studies may have disparate methodologies for measuring PNA, the body of literature suggests that PNA to AOMs found in this study was high compared with other medications.
Patients with obesity may face specific challenges compared with patients with other chronic diseases that may contribute to the very high rate of PNA to AOMs observed in this study.28 There is extensive bias toward patients with obesity, including the misperception that obesity is a personal flaw related to a failure of willpower and not a disease due to pathophysiologic changes. Importantly, weight bias is internalized by people living with overweight or obesity such that they also carry these misperceptions and feelings of self-blame.29,30 Accordingly, Kaplan et al reported that although 45% of people with obesity felt that obesity was a disease, 82% felt “completely” responsible for their weight management.31 Kaplan et al also reported that a barrier to use of AOMs included the belief that lifestyle intervention was the “appropriate” way to lose weight.32 These perceptions likely reduce patient acceptance of medication as necessary to treat overweight or obesity, making patient education a critical component of pharmacologic management of obesity. However, health care professionals are often illequipped to provide the necessary patient education. They tend to share some of the same misperceptions, have inadequate training and experience in obesity management, and report limited awareness of obesity care guidelines, all of which could influence shared decision-making discussions impacting PNA.33 Empowering health care providers to improve patient education could potentially improve PNA.
As previously mentioned, we are not aware of studies investigating PNA to AOMs. However, previous studies have demonstrated that secondary nonadherence to AOMs is also high.11,12 Barriers known to be associated with low secondary adherence such as lack of health care plan coverage for AOMs, resulting in high out-of-pocket costs, and concerns about side effects may also be driving high rates of PNA.11,28,34-36 A study examining Medicare, Medicaid, and Affordable Care Act–established marketplace health care plans in 34 states found that only 11% of marketplace health care plans had coverage for FDA-approved AOMs in 2016, 7 state Medicaid programs had drug coverage, and Medicare policy excluded medications for obesity.37 Current government regulations prohibit inclusion of AOMs on the MAPD formulary.38 Commercial insurers commonly also have formulary restrictions or require prior authorization, limiting access to affordable treatment options.37 In 2017, AOMs were covered by state employee health care plans in only 23 states.31,39 In a survey study published in 2017, only 13% of people with obesity reported that their employer offered health care plan coverage for the medical treatment of obesity.31 Regional variation in health care plan coverage of AOMs may have contributed to the regional variation in PNA observed in this study. Although health care plan type was not statistically significant in the multivariable model in this study, possibly because of small sample size, descriptive results show patients with MAPD coverage in the study sample had a PNA rate of 94.7% and patients with commercial health care plan had a PNA rate of 90.6%.
LIMITATIONS
This study has limitations that are inherent to studies using secondary health care data. The study was conducted in a large US managed care population that may not be representative of the broader population. Because administrative claims are submitted for reimbursement purposes and not for research, claims data lack details such as out-of-pocket costs, social determinants of health, and systemic access issues that would add context to this research. Thus, we were not able to assess practice patterns and other social and cultural factors that may contribute to the geographic difference observed in this study.
There are also limitations related to our study design. We did not investigate baseline levels of PNA to other drugs and were not able to assess how pre-index nonadherence impacted this population. This study only included patients who were prescribed AOMs approved by the FDA for long-term treatment of obesity. It is possible that patients may be taking other medications including individual components of the approved medications for weight loss. These patients were not captured in this study. We did not assess formulary coverage, out-of-pocket costs, or health plan generosity, all of which could influence PNA. Finally, although a multivariable model was used to adjust for covariates that might influence the relationship between the specific AOM and PNA, it is possible that not all relevant covariates were captured.
With respect to generalizability, the inclusion and exclusion criteria were defined in the protocol prior to study execution, and excluded patients were not further reviewed. It is possible that some relevant patients were excluded from the sample. Additionally, because of the specific inclusion and exclusion criteria used, study results may not be generalizable to certain patient populations (eg, patients with pregnancy). Another generalizability limitation is that this study concluded in 2019 and only included AOMs that were FDA approved at the time of the study. Since this study concluded, a new AOM has been approved (semaglutide, 2.4 mg), and additional medications are in the pipeline for FDA approval for the treatment of obesity. Thus, study results may not be generalizable to these newer AOMs.
An additional limitation in interpreting the current findings is that health care providers may prescribe medications off-label for weight management, as these are more often covered by health care plans (eg, liraglutide 1.8 mg vs 3.0 mg weight management dose). Patients with these prescriptions could not have been identified as newly prescribed an AOM in the EHR query. In other instances, commercial health care plans may have required a patient be prescribed phentermine (a medication approved for short-term use) as step therapy before approving the more costly AOMs that were the primary exposures in the present study.37 Additionally, patients prescribed an AOM may have received medications outside of their health care plan, including samples, manufacturer-provided coupons or other discount programs, or over-the-counter AOMs (eg, orlistat 30 mg). In these scenarios, this study may have captured the prescription and not the dispensing, leading to some patients being mis-classified as PNA. Furthermore, this study only examined whether patients filled prescribed medication and did not examine whether alternate AOM was filled, which could lead to some patients being misclassified as PNA.
Conclusions
Less than 10% of patients newly prescribed an AOM filled their prescription within 60 days. The rates of PNA to AOMs reported in this study were much higher than rates reported for medications for other chronic diseases like T2DM. The high rate of PNA observed suggests that antiobesity medication treatment of patients with overweight and obesity has many barriers, which may include lack of understanding of obesity and its treatment and limited health care plan coverage of AOMs. Further research should seek to define these barriers and develop strategies for addressing them to improve pharmacotherapy care of patients with overweight and obesity. Furthering this work would advance the cause of achieving treatment parity for patients with obesity compared with other chronic diseases.
ACKNOWLEDGMENTS
The authors would like to acknowledge Stephanie Gallagher for project management work and Gretchen Hultman for medical writing services.
REFERENCES
- 1.Centers for Disease Control and Prevention. National health and nutrition examination survey 2017–March 2020 Prepandemic data files development of files and prevalence estimates for selected health outcomes. 2021. Accessed November 3, 2022. https://stacks.cdc.gov/view/cdc/106273 [DOI] [PMC free article] [PubMed]
- 2.Apovian CM, Aronne LJ, Bessesen DH, et al. ; Endocrine Society . Pharmacological management of obesity: An endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100(2):342-62. doi:10.1210/jc.2014-3415 [DOI] [PubMed] [Google Scholar]
- 3.Garvey WT, Mechanick JI, Brett EM, et al. ; Reviewers of the AACE/ACE obesity clinical practice guidelines . American Association of Clinical Endocrinologists and American College of Endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity. Endocr Pract. 2016;22(suppl 3):1-203. doi:10.4158/EP161365.GL [DOI] [PubMed] [Google Scholar]
- 4.Jensen MD, Ryan DH, Apovian CM, et al. ; American College of Cardiology/American Heart Association Task Force on practice guidelines; Obesity Society . 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: A report of the American College of Cardiology/American Heart Association task force on practice guidelines and The Obesity Society. J Am Coll Cardiol. 2014;63(25)(25 Pt B):2985-3023. doi:10.1016/j.jacc.2013.11.004 [DOI] [PubMed] [Google Scholar]
- 5.National Heart, Lung, and Blood Institute and North American Association for the Study of Obesity. Practical guide to the identification, evaluation, and treatment of overweight and obesity in adults. Bethesda, MD: National Institutes of Health; 2000. Accessed December 16, 2022. https://www.nhlbi.nih.gov/files/docs/guidelines/prctgd_c.pdf [Google Scholar]
- 6.Calderon G, Gonzalez-Izundegui D, Shan KL, et al. Effectiveness of anti-obesity medications approved for long-term use in a multidisciplinary weight management program: A multi-center clinical experience. Int J Obes. 2022;46(3):555-63. doi:10.1038/s41366-021-01019-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.MacEwan J, Kan H, Chiu K, Poon JL, Shinde S, Ahmad NN. Antiobesity medication use among overweight and obese adults in the United States: 2015-2018. Endocr Pract. 2021;27(11):1139-48. doi:10.1016/j.eprac.2021.07.004 [DOI] [PubMed] [Google Scholar]
- 8.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353(5): 487-97. doi:10.1056/NEJMra050100 [DOI] [PubMed] [Google Scholar]
- 9.Adams AJ, Stolpe SF. Defining and measuring primary medication nonadherence: Development of a quality measure. J Manag Care Spec Pharm. 2016;22(5): 516-23. doi:10.18553/jmcp.2016.22.5.516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hovstadius B, Petersson G. Non-adherence to drug therapy and drug acquisition costs in a national population--A patient-based register study. BMC Health Serv Res. 2011;11(1):326. doi:10.1186/1472-6963-11-326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hemo B, Endevelt R, Porath A, Stampfer MJ, Shai I. Adherence to weight loss medications; post-marketing study from HMO pharmacy data of one million individuals. Diabetes Res Clin Pract. 2011;94(2):269-75. doi:10.1016/j.diabres.2011.08.021 [DOI] [PubMed] [Google Scholar]
- 12.Ganguly R, Tian Y, Kong SX, et al. Persistence of newer anti-obesity medications in a real-world setting. Diabetes Res Clin Pract. 2018;143:348-56. doi:10.1016/j.diabres.2018.07.017 [DOI] [PubMed] [Google Scholar]
- 13.Chang HY, Kan HJ, Shermock KM, Alexander GC, Weiner JP, Kharrazi H. integrating e-prescribing and pharmacy claims data for predictive modeling: Comparing costs and utilization of health plan members who fill their initial medications with those who do not. J Manag Care Spec Pharm. 2020;26(10):1282-90. doi:10.18553/jmcp.2020.26.10.1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fischer MA, Choudhry NK, Brill G, et al. Trouble getting started: Predictors of primary medication nonadherence. Am J Med. 2011;124(11):1081.e9-1.081E22. doi:10.1016/j.amjmed.2011.05.028 [DOI] [PubMed] [Google Scholar]
- 15.Luo J, Feldman R, Rothenberger S, Korytkowski M, Fischer MA, Gellad WF. Incidence and predictors of primary nonadherence to sodium glucose cotransporter 2 inhibitors and glucagon-like peptide 1 agonists in a large integrated healthcare system. J Gen Intern Med. 2022;37(14):3562-9. doi:10.1007/s11606-021-07331-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gellad WF, Grenard JL, Marcum ZA. A systematic review of barriers to medication adherence in the elderly: Looking beyond cost and regimen complexity. Am J Geriatr Pharmacother 2011;9(1):11-23. doi:10.1016/j.amjopharm.2011.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheen MHH, Tan YZ, Oh LF, Wee HL, Thumboo J. Prevalence of and factors associated with primary medication nonadherence in chronic disease: A systematic review and meta-analysis. Int J Clin Pract. 2019;73(6):e13350. doi:10.1111/ijcp.13350 [DOI] [PubMed] [Google Scholar]
- 18.Gellad WF, Grenard J, McGlynn EA.. A review of barriers to medication adherence. A framework for driving policy options. RAND Corporation; 2009. https://www.rand.org/pubs/technical_reports/TR765.html [Google Scholar]
- 19.Adams A, Hubbard T, Stolpe S, Cranston L. The first fill factor: A threat to outcomes, quality, and payment goals. Health Affairs Blog. Accessed [date]. https://www.healthaffairs.org/content/forefront/first-fill-factor-threat-outcomes-quality-and-payment-goals
- 20.Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6): 676-82. doi:10.1093/aje/kwq433 [DOI] [PubMed] [Google Scholar]
- 21.Bayliss EA, Ellis JL, Shoup JA, Zeng C, McQuillan DB, Steiner JF. Association of patient-centered outcomes with patient-reported and ICD-9-based morbidity measures. Ann Fam Med. 2012;10(2):126-33. doi:10.1370/afm.1364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Connor PJ, Schmittdiel JA, Pathak RD, et al. Randomized trial of telephone outreach to improve medication adherence and metabolic control in adults with diabetes. Diabetes Care. 2014;37(12): 3317-24. doi:10.2337/dc14-0596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hernandez I, Divino V, Xie L, et al. A real-world evaluation of primary medication nonadherence in patients with nonvalvular atrial fibrillation prescribed oral anticoagulants in the United States. Am J Cardiovasc Drugs. 2023. doi:10.1007/s40256-023-00588-3 [DOI] [PubMed] [Google Scholar]
- 24.Kirkman MS, Rowan-Martin MT, Levin R, et al. Determinants of adherence to diabetes medications: Findings from a large pharmacy claims database. Diabetes Care. 2015;38(4):604-9. doi:10.2337/dc14-2098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ritchey M, Chang A, Powers C, et al. Vital Signs: Disparities in antihypertensive medication nonadherence among Medicare Part D beneficiaries - United States, 2014. MMWR Morb Mortal Wkly Rep. 2016;65(36):967-76. doi:10.15585/mmwr.mm6536e1 [DOI] [PubMed] [Google Scholar]
- 26.Dirmaier J, Watzke B, Koch U, et al. Diabetes in primary care: prospective associations between depression, nonadherence and glycemic control. Psychother Psychosom. 2010;79(3):172-8. doi:10.1159/000296135 [DOI] [PubMed] [Google Scholar]
- 27.Lemstra M, Nwankwo C, Bird Y, Moraros J. Primary nonadherence to chronic disease medications: A metaanalysis. Patient Prefer Adherence. 2018;12:721-31. doi:10.2147/PPA.S161151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilson ER, Kyle TK, Nadglowski JF Jr, Stanford FC. Obesity coverage gap: Consumers perceive low coverage for obesity treatments even when workplace wellness programs target BMI. Obesity (Silver Spring). 2017;25(2):370-7. doi:10.1002/oby.21746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pearl RL, Puhl RM, Lessard LM, Himmelstein MS, Foster GD. Prevalence and correlates of weight bias internalization in weight management: A multinational study. SSM Popul Health. 2021;13:100755. doi:10.1016/j.ssmph.2021.100755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Verhaak AMS, Ferrand J, Puhl RM, Tishler DS, Papasavas PK, Umashanker D. Experienced weight stigma, internalized weight bias, and clinical attrition in a medical weight loss patient sample. Int J Obes (Lond). 2022;46(6):1241-3. doi:10.1038/s41366-022-01087-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaplan LM, Golden A, Jinnett K, et al. Perceptions of barriers to effective obesity care: Results from the national ACTION study. Obesity (Silver Spring). 2018;26(1):61-9. doi:10.1002/oby.22054 [DOI] [PubMed] [Google Scholar]
- 32.Kaplan L, Kuma R, Kahan S, et al. Perspectives of anti-obesity medication use among persons with obesity and health care providers. Obesity (Silver Spring). 2021;29(S2):8-139 [Google Scholar]
- 33.Turner M, Jannah N, Kahan S, Gallagher C, Dietz W. Current knowledge of obesity treatment guidelines by health care professionals. Obesity (Silver Spring). 2018;26(4):665-71. doi:10.1002/oby.22142 [DOI] [PubMed] [Google Scholar]
- 34.Halpern B, Halpern A. Why are antiobesity drugs stigmatized? Expert Opin Drug Saf. 2015;14(2):185-9. doi:10.1517/14740 338.2015.995088 [DOI] [PubMed] [Google Scholar]
- 35.Horne R, Chapman SC, Parham R, Freemantle N, Forbes A, Cooper V. Understanding patients’ adherence-related beliefs about medicines prescribed for long-term conditions: A meta-analytic review of the Necessity-Concerns Framework. PLoS One. 2013;8(12):e80633. doi:10.1371/journal.pone.0080633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thomas CE, Mauer EA, Shukla AP, Rathi S, Aronne LJ. Low adoption of weight loss medications: A comparison of prescribing patterns of antiobesity pharmacotherapies and SGLT2s. Obesity (Silver Spring). 2016;24(9):1955-61. doi:10.1002/oby.21533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gomez G, Stanford FC. US health policy and prescription drug coverage of FDA-approved medications for the treatment of obesity. Int J Obes. 2018;42(3):495-500. doi:10.1038/ijo.2017.287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Centers for Medicate and Medicaid Services. Medicare prescription drug benefit manual chapter 6 - Part D drugs and formulary requirements. 2016. Accessed June 23, 2023. https://www.cms.gov/medicare/prescription-drug-coverage/prescriptiondrugcovcontra/downloads/part-d-benefits-manual-chapter-6.pdf
- 39.Jannah N, Hild J, Gallagher C, Dietz W. Coverage for obesity prevention and treatment services: Analysis of Medicaid and state employee health insurance programs. Obesity (Silver Spring). 2018;26(12):1834-40. doi:10.1002/oby.22307 [DOI] [PubMed] [Google Scholar]


