Abstract
Background
Congenital or early‐acquired hearing impairment poses a major barrier to the development of spoken language and communication. Early detection and effective (re)habilitative interventions are essential for parents and families who wish their children to achieve age‐appropriate spoken language. Auditory‐verbal therapy (AVT) is a (re)habilitative approach aimed at children with hearing impairments. AVT comprises intensive early intervention therapy sessions with a focus on audition, technological management and involvement of the child's caregivers in therapy sessions; it is typically the only therapy approach used to specifically promote avoidance or exclusion of non‐auditory facial communication. The primary goal of AVT is to achieve age‐appropriate spoken language and for this to be used as the primary or sole method of communication. AVT programmes are expanding throughout the world; however, little evidence can be found on the effectiveness of the intervention.
Objectives
To assess the effectiveness of auditory‐verbal therapy (AVT) in developing receptive and expressive spoken language in children who are hearing impaired.
Search methods
CENTRAL, MEDLINE, EMBASE, PsycINFO, CINAHL, speechBITE and eight other databases were searched in March 2013. We also searched two trials registers and three theses repositories, checked reference lists and contacted study authors to identify additional studies.
Selection criteria
The review considered prospective randomised controlled trials (RCTs) and quasi‐randomised studies of children (birth to 18 years) with a significant (≥ 40 dBHL) permanent (congenital or early‐acquired) hearing impairment, undergoing a programme of auditory‐verbal therapy, administered by a certified auditory‐verbal therapist for a period of at least six months. Comparison groups considered for inclusion were waiting list and treatment as usual controls.
Data collection and analysis
Two review authors independently assessed titles and abstracts identified from the searches and obtained full‐text versions of all potentially relevant articles. Articles were independently assessed by two review authors for design and risk of bias. In addition to outcome data, a range of variables related to participant groups and outcomes were documented.
Main results
Of 2233 titles and abstracts searched, only 13 abstracts appeared to meet inclusion criteria. All 13 full‐text articles were excluded following independent evaluation by two review authors (CGBJ and JW), as they did not meet the inclusion criteria related to the research design. Thus, no studies are included in this review.
Authors' conclusions
This review confirms the lack of well‐controlled studies addressing the use of AVT as an intervention for promoting spoken language development in children with permanent hearing impairments. Whilst lack of evidence does not necessarily imply lack of effect, it is at present not possible for conclusions to be drawn as to the effectiveness of this intervention in treating children with permanent hearing impairments.
Plain language summary
Auditory‐verbal therapy for promoting spoken language development in children with permanent hearing impairments
Permanent hearing impairment greatly restricts a child's speech and language development and hinders his or her behavioural, cognitive and social functioning. Although technological devices, such as hearing aids and cochlear implants, enable the child to hear spoken words, they fail to teach the child how to listen, how to process language or how to talk.
Auditory‐verbal therapy aims to improve the spoken language abilities of a child with hearing impairment to the level of a child with typical hearing by developing his or her listening skills independent of other cues such as speech reading and gestures. It focuses on the context of spoken communication within the family and uses hearing and speech as the primary methods of communication. For this reason, it is thought to be more effective in helping a child reach typical age‐related milestones in speech and language acquisition.
This review was undertaken to assess evidence on the effectiveness of auditory‐verbal therapy in promoting spoken language development in children with permanent hearing impairments. Whilst many studies have examined the effectiveness of AVT, no studies met the criteria for inclusion in this review. Well‐designed studies are urgently needed to examine the effectiveness of AVT in promoting spoken language development in children.
Background
Description of the condition
The most significant effect of hearing impairment in children is its impact on the development of spoken language and communication (Davis 2009). Early detection and effective interventions for children with all types of hearing impairments are essential for the development of age‐appropriate spoken language (Yoshinaga‐Itano 1998). Delays in identification and intervention can have lasting effects on a child's future development, resulting in reduced levels of literacy, educational success and quality of life (Van Eldik 2004; Stacey 2006). The severe implications of later‐identified hearing impairment (at greater than six months of age) for a child's social, behavioural, communicative and cognitive development have prompted the implementation of universal neonatal screening in many countries. As a result, many childhood hearing impairments can be accurately identified soon after birth, allowing (re)habilitative interventions to be sought and implemented far earlier than was previously possible (Davis 1997). The advent of universal neonatal hearing screening should make it possible for many more hearing impaired children to develop spoken language as their primary or sole method of communication, provided the child receives appropriate audiological management and aural (re)habilitation (Flexer 1999).
Estimates of the prevalence of permanent childhood hearing impairment (≥ 40 dBHL (decibels hearing level) in the better ear) in developed countries vary between one and two children per 1000 live births (Martin 1981; Davis 1992; Davis 1994; Fortnum 1997; Fortnum 2001). This figure rises to 3.47 per 1000 children for all types of permanent hearing impairments by the time children reach primary school age (Bamford 2007).
Description of the intervention
Auditory‐verbal therapy (AVT) is a method of working with a hearing impaired child and his or her family to develop spoken communication as the child's primary or sole method of communication, regardless of his or her level of hearing impairment. AVT is normally delivered by someone who is both a qualified teacher of the deaf and a speech and language therapist or audiologist, and is certified as an AV therapist by the Alexander Graham (AG) Bell Academy for Listening and Spoken Language (AG Bell Academy 2013). The primary aim of AVT is for children with hearing impairments to reach the same level of expressive and receptive language abilities as their peers who have typical hearing levels (Eriks‐Brophy 2004).
One of the difficulties in exploring the effectiveness of any speech and language intervention is the fact that interventions are often poorly defined in terms of their underlying principles and the techniques employed (Law 2010). AVT lends itself to this sort of investigation because of its clearly defined set of underlying principles and its very specific regulations for training and registration.
The AVT approach places emphasis on the role of audition in spoken language development and on the importance of the parent as central to the child's (re)habilitation programme (Lim 2005). AVT therefore advocates rigorous audiological management and the use of hearing technology (hearing aids, cochlear implants and assistive listening devices such as personal frequency modulation (FM) systems) to optimise a child's auditory potential. Although AVT shares these features with several other management strategies (e.g. auditory‐oral therapy), it is typically the only therapy approach that specifically promotes the avoidance or exclusion of non‐auditory facial communication, sometimes achieved through use of the 'hand‐cue' as a (re)habilitation technique (McDonald Walker 2001).
A list of the principles of AVT is included in Appendix 1. The following statement is taken from the AG Bell Academy Handbook (AG Bell Academy 2011).
"Auditory‐verbal therapy facilitates optimal acquisition of spoken language through listening by newborns, infants, toddlers and young children who are deaf or hard of hearing. Auditory‐verbal therapy promotes early diagnosis, one‐on‐one therapy and state‐of‐the‐art audiologic management and technology. Parents and caregivers actively participate in therapy. Through guidance, coaching and demonstration, parents become the primary facilitators of their child's spoken language development. Ultimately, parents and caregivers gain confidence that their child will have access to a full range of academic, social and occupational choices. Auditory‐verbal therapy must be conducted in adherence to the 'Principles of LSLSTMAuditory‐Verbal Therapy'."
Although positive outcomes have been reported for children with varying degrees of hearing impairment, the evidence base in relation to AVT is still developing (Eriks‐Brophy 2004).
How the intervention might work
It has been suggested that AVT can result in improved receptive language skills as well as better speech production in children with hearing impairment (Eriks‐Brophy 2004; Dornan 2010). Some studies suggest that AVT has the potential to allow many hearing impaired children to achieve speech and language levels comparable with those of their hearing peers (Hogan 2008).
Whilst numerous approaches are used to promote spoken language development in children with hearing impairments, the mechanism by which AVT might achieve success in facilitating spoken language development is probably best understood by considering possible differences between this and more traditional oral‐aural approaches. The AV method would claim to differ from other oral‐aural approaches in three key areas: emphasis on audition, emphasis on the family and emphasis on following the same developmental processes as a hearing child (Boucher‐Jones 2001).
Emphasis on audition
AVT focuses on developing audition, that is, speech discrimination, through listening alone, rather than through a combination of listening and speech reading (use of visual cues from the mouth and face of the speaker). It is suggested that this focus on audition will facilitate a more normal process of spoken language learning, for example, by allowing situations of joint attention to occur naturally between caregiver and child (Tomasello 2003). This focus on audition also aims to build the child's confidence in his or her hearing and to facilitate more natural speech production, as the child will be focusing on using auditory feedback to imitate auditory rather than visual characteristics of words and phonemes (Richards 2001). Although other auditory‐oral approaches to (re)habilitation also promote rigorous audiological management and the development of auditory speech perception, the AVT approach is more explicit with regard to its emphasis on promoting listening and using a variety of techniques to deliberately exclude visual cues.
Emphasis on the family
Parental and family involvement in the management of children with hearing impairment has been shown to improve language outcomes (Watkin 2007). Again, AVT is not the only approach that stresses the importance of liaison with the child's family and carryover at home, but it is the only method that specifically requires that the parent be both the client and the child's primary therapist (Levasseur 2001). This insistence on the key role of the parent is made possible in part because AVT tends to be delivered privately. Although speech and language therapists and teachers of the deaf who provide standard care for hearing impaired children may strive to achieve high levels of parental involvement in the (re)habilitation process, it is not the case that every parent will be willing or able to engage in this way. In these cases, the teacher or therapist remains responsible for treating the child, and (re)habilitation sessions may take place in the absence of the parent. Once the child starts school, (re)habilitation sessions may often be conducted in the school setting without a parent present for a variety of reasons (Brachmaier 2010). By contrast, an AV therapist never works with a child alone and sees his or her role only as that of training, supporting and working in partnership with the parent(s) (Simser 2001). It is proposed that this emphasis on the parent‐child dyad, rather than the therapist‐child dyad, will result in more natural and effective spoken language learning.
Emphasis on the normal developmental process
The aim of AVT is to facilitate the normal process of spoken language development in the hearing impaired child. More traditional approaches have tended to take a remedial rather than a developmental view, seeing language development of the hearing impaired child as different rather than delayed, resulting in lower expectations in terms of that child's capacity to acquire spoken language and participate in mainstream society (Levasseur 2001; Lim 2005). All AVT sessions are intended to be diagnostic in nature, and the child's language is regularly compared, using standardised assessments, with the language of a hearing child of the same chronological age, with the aim of minimising language delays.
It follows that the proposed effectiveness of AVT is generally considered to be dependent on the following: early identification of hearing impairment, rigorous audiological management to ensure optimal benefit from hearing technology, parents committed to acting as their child's primary therapist and meaningful auditory spoken language input based on the normal process of spoken language learning and appropriate to the child's developmental age. Therefore, the AVT method may not be appropriate for children whose parents are unable or unwilling to participate fully in the programme (Hann 2001).
Why it is important to do this review
Providing effective intervention for children diagnosed with a hearing impairment is vital in promoting language development and improved quality of life (Davis 1997; Yoshinaga‐Itano 1998). Developments in hearing screening and hearing technology have meant that increasing numbers of children with permanent childhood hearing impairments are being diagnosed within the first few weeks of life and are able from an early age to access the sounds of speech through hearing aids or cochlear implants. More than 90% of these children are born to two hearing parents (Mitchell 2004), most of whom would prefer that their child develop spoken communication.
Although AVT is becoming increasingly available in many countries, it is generally delivered by the private sector and often is available only at significant financial cost to families, making AVT less accessible to children from lower socioeconomic backgrounds. Other (re)habilitation methods may be provided by statutory health or education services at little or no cost to families. The stated aims of AVT are to give hearing impaired children access to mainstream education by achieving levels of language development for hearing impaired children that are comparable with their typically hearing peers (Rhoades 2006). Given the global expansion of AVT programmes, it is essential that unbiased information regarding the effectiveness of AVT for hearing impaired children be made available. This will allow parents to make an informed choice as regards the potential benefits and suitability of AVT for their child.
Objectives
To assess the effectiveness of auditory‐verbal therapy (AVT) in developing receptive and expressive spoken language in children who are hearing impaired.
Methods
Criteria for considering studies for this review
Types of studies
Prospective randomised controlled trials (RCTs) and quasi‐randomised controlled trials (e.g. in which participants are allocated by treatment according to date of birth) comparing AVT with a waiting list or treatment as usual control group.
Types of participants
Children from birth to 18 years of age with significant congenital or early‐acquired (before five years of age) bilateral hearing impairment (defined as hearing thresholds ≥ 40 dBHL in the better ear). Children with known significant cognitive or educational impairment will be excluded. AVT participants are to use hearing aids or cochlear implants and receive a formal programme of AVT from an AV therapist certified by the AG Bell Academy for Listening and Spoken Language. Comparison groups should include children with significant congenital or early‐acquired bilateral hearing impairment (≥ 40 dBHL in the better ear) waiting to receive AVT or receiving other standard care (re)habilitative methods.
Types of interventions
We included programmes of AVT administered by a certified AV therapist for a period of at least six months. A minimum time period was required to provide adequate time for any effect of the intervention to be observed. Primarily, we expected to encounter treatment‐as‐usual control groups or different‐dose control groups (where one group receives less intensive therapy). We did not expect to encounter placebo control groups or no treatment control groups. Nor did we anticipate reports of adverse effects resulting from interventions.
Types of outcome measures
Primary outcomes
Level of receptive and expressive language development (e.g. Preschool Language Age Scale, fourth edition (PLS‐4) (Zimmerman 2002); Clinical Evaluation of Language, fourth edition (CELF‐4) (Semel 2003); Reynell Developmental Language Scales (Edwards 2011))*.
Rate of receptive and expressive language development (e.g. PLS‐4 (Zimmerman 2002); CELF‐4 (Semel 2003); Reynell Developmental Language Scales (Edwards 2011))*.
Minimum time periods in which language outcomes should be assessed are within six months of the start of AVT sessions and within six months of completion of the AVT programme. AVT aims to develop expressive (and receptive) language abilities of hearing impaired children to the same level as their peers with typical hearing levels. Therefore, validated assessments, standardised on children with typical hearing, will be used to measure and compare spoken language outcomes (Rhoades 2006).
Change scores (i.e. rate of receptive and expressive language development) can be a more appropriate and meaningful outcome measure in small‐scale studies of language development. These will be analysed separately from end point comparisons (i.e. level of receptive and expressive language development).
Secondary outcomes
Quality of life using validated questionnaires*.
Quality of family life using validated questionnaires.
Behavioural outcomes using standardised measures (e.g. the Child Behaviour Checklist (Achenbach 1991))*.
Educational achievement.
Literacy using standardised measures*.
Socioeconomic status of child and family.
Secondary outcomes (when measured) will be compared with control group(s) within the trial and/or normative values within the standardised measures used for assessment.
*Outcomes intended to be included in a 'Summary of findings' table in the completed review.
Search methods for identification of studies
Electronic searches
The following databases were searched between 25 March 2013 and 20 April 2013 with no restriction on language of publication or publication date. Search strategies for each source are reported in Appendix 2.
The Cochrane Central Register of Controlled Trials (CENTRAL), 2013 Issue 2, part of the Cochrane Library.
Ovid MEDLINE (1946 to Week 2 March 2013).
EMBASE (1980 to Week 12 2013)
PsycINFO (1967 to Week 3 March 2013).
CINAHL Plus (1937 to current).
ERIC (1966 to current).
Science Citation Index (1970 to 22 March 2013).
Social Sciences Citation Index (1970 to 22 March 2013).
Conference Proceedings Citation Index–Science (1990 to 22 March 2013).
Conference Proceedings Citation Index–Social Science & Humanities (1990 to 22 March 2013).
WorldCat (limited to theses) (worldcat.org/).
International Clinical Trials Registry Platform (ICTRP) (apps.who.int/trialsearch/).
ClinicalTrials.gov (clinicaltrials.gov/).
speechBITE (speechbite.com/).
OpenGrey (opengrey.eu/).
Networked Digital Library of Theses and Dissertations (NDLTD) (ndltd.org/).
Trove (National Library of Australia) (trove.nla.gov.au/).
DART–Europe E‐theses portal (DART) (dart‐europe.eu/).
Searching other resources
We searched the reference lists of identified and other relevant original and review articles and contacted several study authors. No additional unpublished RCTs or quasi‐RCTs were identified.
Data collection and analysis
Selection of studies
Two review authors (CGBJ and JW) independently screened titles and abstracts of studies identified in the searches and selected all potentially relevant studies. We obtained copies of relevant articles, which were evaluated independently by the same review authors against the inclusion criteria. Review authors were not blinded to author names or institutions nor to journals of publication of potential studies.
Data extraction and management
No studies met inclusion criteria for this review. For further details of methods, see the protocol (Brennan‐Jones 2012), which has been archived for use in future updates of this review (Appendix 3).
Assessment of risk of bias in included studies
See Appendix 3.
Results
Description of studies
Results of the search
Of 2233 titles and abstracts screened, only 13 appeared to potentially match the inclusion criteria (see Figure 1); full‐text versions were obtained. Most of the other studies were excluded primarily because they did not examine children with hearing impairments, did not specifically examine AVT or were not intervention studies.
1.
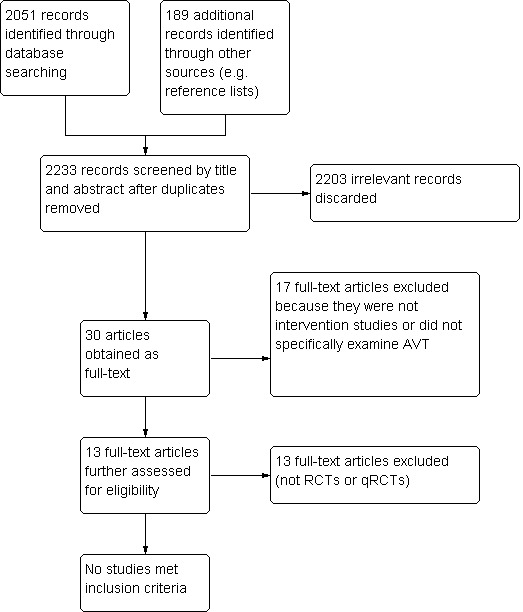
PRISMA study flow diagram.
Included studies
No studies were suitable for inclusion in this review.
Excluded studies
All 13 full‐text studies were excluded following evaluation by review authors JW and CGBJ. All 13 studies were excluded because they were neither randomised nor quasi‐randomised controlled trials (Diller 2001; Doble 2006; Bakhshaee 2007; Dornan 2007; Fairgray 2008; Dornan 2009; Allegro 2010; Dornan 2010; Fairgray 2010 (also excluded for treatment duration less than six months); Hogan 2008; Hogan 2010; Sahli 2011; Fulcher 2012). Randomisation, hearing impaired control groups and blinding of outcome assessors are necessary to limit bias in potential studies of AVT. Randomisation is essential for limiting selection bias by ensuring that participants are not knowingly or unknowingly selected according to their likelihood of success in a particular intervention programme (Odgaard‐Jensen 2011). Ideally, comparison groups should also have been diagnosed with the target condition (hearing impairment in this case), as controls without the target condition are likely to bias results towards a positive effect of the intervention. Hróbjartsson 2012 has noted that significant observer bias towards a more beneficial treatment effect is present in non‐blinded trials using subjective measurement scales, such as the language outcome measures used in AVT (and other (re)habilitative programmes) to monitor language development. Therefore, inclusion of non‐randomised and non‐blinded studies would likely introduce significant observer bias, as the primary outcome measure, language development, is subjectively measured. Blinding of outcome assessors to the allocation of participants, therefore, is required to produce high‐quality, unbiased trials of AVT.
Risk of bias in included studies
As no studies met the inclusion criteria for this review, no risk of bias assessments were undertaken.
Effects of interventions
It was not possible to assess the effects of the interventions, as no studies met the inclusion criteria for this review.
Discussion
Summary of main results
The present review sought to examine the effectiveness of AVT for promoting spoken language development in children with permanent hearing impairments. No studies met the methodological inclusion criteria for this review (i.e. none were RCTs or quasi‐RCTs). Because of the absence of well‐controlled studies, no conclusions can currently be drawn about the effectiveness of AVT for promoting spoken language development. Well‐controlled studies examining AVT are urgently required. Identification of effective interventions for promoting spoken language development will have a significant long‐term positive impact for children with permanent hearing impairments and may make such programmes available to children from a wider range of socioeconomic backgrounds.
Overall completeness and applicability of evidence
As no studies met the inclusion criteria for this review, we are unable to assess the completeness and applicability of the current evidence.
Quality of the evidence
No studies of sufficient quality were identified for inclusion in this review. The overall quality of evidence is low, with no studies using randomisation or blinding of outcome assessors, which are essential for limiting bias when subjective outcome measures are used as the primary outcome measure (see also Excluded studies section).
Potential biases in the review process
A sensitive search strategy was used to identify trials for inclusion in this review, and no restrictions were placed on publication status or language. Two review authors searched all of the electronic databases for relevant trials. Although it is unlikely that we missed any relevant trials identified by the electronic searches, it is possible that some trials may not have been reported. Therefore, we cannot eliminate the possibility that bias may have influenced the results of this review.
Agreements and disagreements with other studies or reviews
To the best of our knowledge, no other systematic review of AVT has been completed. Eriks‐Brophy 2004 completed a narrative review of the topic and also reported a lack of high‐quality evidence investigating the effectiveness of AVT.
Authors' conclusions
Implications for practice.
Given the limited evidence base currently available, it was not possible to reach definitive conclusions regarding the effectiveness of AVT for promoting spoken language development in children with permanent hearing impairments.
Implications for research.
Well‐designed RCTs (reported according to the CONSORT (CONsolidated Standards Of Reporting Trials) guidelines) are urgently needed to examine the effectiveness of AVT. Whilst potential difficulties are encountered in applying RCT designs to speech and hearing (re)habilitation programmes, these studies are essential in building an evidence base from which firm conclusions can be drawn. Morgan 2008 commented on the role of well‐controlled individual case series and other non‐RCT study designs in acting as a catalyst for the completion of larger‐scale RCTs. In the case of AVT, Dornan and colleagues (Dornan 2007;Dornan 2009; Dornan 2010) and Hogan and colleagues (Hogan 2008; Hogan 2010) in particular have produced longitudinal studies of AVT that show a clear intention to improve the evidence base.
The key challenges in conducting an RCT of AVT include the following: (1) acquisition of funding for the intervention, so that children and parents who are willing to participate in the trial can be randomly allocated to another (presumably publicly funded) rehabilitation programme; (2) availability of funding for an independent evaluation of the intervention, including blinded outcome assessors with relevant rehabilitative qualifications who are not involved in the care of children participating in the trial; (3) sample size, depending on the size of the programme, to obtain a sufficient number of participants for a trial it may need to run for several years (this has obvious financial implications); and (4) variability among control groups using a 'best practice' rehabilitation programme. One way to possibly overcome some of these limitations, particularly (3) and (4), would be to implement a standardised RCT protocol for AVT that could be used as the basis for a multi‐site trial. A multi‐site trial between several AVT centres may also be more successful in attracting research funding. A collaborative approach of this kind would significantly advance the current evidence base.
Therefore, it is hoped that this review, although it includes no studies, will serve not only as a call to action (mirroring the words of Eriks‐Brophy 2004 a decade ago) but also as a methodological foundation for future randomised controlled trials of AVT and language development in children with hearing impairments. Many of the organisations providing AVT are not‐for‐profit institutions, in which available funds are often used to offer free places in their intervention programmes to families unable to cover the costs. This leaves little or no funding available to support the additional staffing required to carry out a clinical trial with blinded outcome assessors. We hope that this review, in showing the absence of high‐quality studies examining AVT, will provide justification for initiation of such studies by investigators and for their support by relevant funding bodies.
Acknowledgements
We are grateful to Laura MacDonald, former Managing Editor; Dr Nuala Livingstone, Associate Editor; and Professor Geraldine Macdonald, Co‐ordinating Editor of the CDPLPG, for their comments and advice throughout the review process, and to Margaret Anderson for her assistance with the search strategy. We are also grateful to Sharon Suller, Dr William Whitmer and Dr Michael Akeroyd of the MRC Institute of Hearing Research (Scottish Section) for their assistance during the early stages of protocol development, and to Dr Sophie Brennan‐Jones for her comments on earlier drafts of this review. Christopher Brennan‐Jones was supported by an award from the Vice‐Chancellor's Fund, Queen Margaret University.
Appendices
Appendix 1. Principles of AVT (taken from the AG Bell Academy LSLS Certification Handbook, 2011)
Commitment to these principles is required for certification as an LSLSTM Cert AVT.
Promote early diagnosis of hearing loss in newborns, infants, toddlers and young children, followed by immediate audiological management and auditory‐verbal therapy.
Recommend immediate assessment and use of appropriate, state‐of‐the‐art hearing technology to obtain maximum benefits of auditory stimulation.
Guide and coach parents* to help their child use hearing as the primary sensory modality in developing listening and spoken language.
Guide and coach parents to become the primary facilitators of their child's listening and spoken language development through active consistent participation in individualised auditory‐verbal therapy.
Guide and coach parents to create environments that support listening for the acquisition of spoken language throughout the child's daily activities.
Guide and coach parents to help their child integrate listening and spoken language into all aspects of the child's life.
Guide and coach parents to use natural developmental patterns of audition, speech, language, cognition and communication.
Guide and coach parents to help their child self monitor spoken language through listening.
Administer ongoing formal and informal diagnostic assessments to develop individualised auditory‐verbal treatment plans, to monitor progress and to evaluate the effectiveness of plans for the child and family.
Promote education in regular schools with peers who have typical hearing and with appropriate services from early childhood onwards.
*The term 'parents' also includes grandparents, relatives, guardians and any caregivers who interact with the child.
(Adapted from the Principles originally developed by Doreen Pollack in 1970; adopted by the AG Bell Academy for Listening and Spoken Language on 6 November 2009.)
Appendix 2. Search strategies
Cochrane Central Database of Controlled Trials (CENTRAL; 2013 Issue 2) Searched 26 March 2013 [33 records] #1MeSH descriptor: [Speech Therapy] this term only #2MeSH descriptor: [Language Therapy] this term only #3MeSH descriptor: [Verbal Learning] this term only #4MeSH descriptor: [Auditory Perception] this term only #5MeSH descriptor: [Speech Perception] this term only #6MeSH descriptor: [Communication Methods, Total] explode all trees #7(audition):ti,ab #8(auditory next verbal):ti,ab #9(AVT):ti,ab #10((listening or auditory) near/3 (skill* or activit* or therap* or training)):ti,ab #11#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 #12MeSH descriptor: [Hearing Loss] 1 tree(s) exploded #13MeSH descriptor: [Hearing Impaired Persons] this term only #14(hearing near/3 (loss* or impair*)):ti,ab #15(deaf*):ti,ab #16#12 or #13 or #14 or #15 #17#11 and #16 #18MeSH descriptor: [Correction of Hearing Impairment] this term only #19#17 or #18 156 #20(baby or babies or infant* or toddler* or child* or pre next school* or preschool*) #21 #19 and #20 Ovid MEDLINE (1946 to Week 2 March 2013) Searched 25 March 2013 [441 records] 1 Speech Therapy/ (4900) 2 Language Therapy/ (1148) 3 verbal learning/ (9368) 4 auditory perception/ (20714) 5 speech perception/ (16640) 6 communication methods, total/ (272) 7 audition.tw. (915) 8 auditory verbal.tw. (1097) 9 AVT.tw. (769) 10 ((listening or auditory) adj3 (skill$ or activit$ or therap$ or training)).tw. (2312) 11 or/1‐10 (53690) 12 (hearing adj3 (loss$ or impair$)).tw. (33382) 13 deaf$.tw. (26576) 14 exp Hearing Loss/ (51112) 15 Hearing Impaired Persons/ (1083) 16 or/12‐15 (73085) 17 11 and 16 (6747) 18 "rehabilitation of hearing impaired"/ (1493) 19 or/17‐18 (7906) 20 exp infant/ (892666) 21 exp child/ (1467433) 22 (baby or babies or infant$ or toddler$ or child$ or pre‐school$ or preschool$).tw. (1104719) 23 20 or 21 or 22 (2136523) 24 19 and 23 (2704) 25 randomized controlled trial.pt. (343484) 26 controlled clinical trial.pt. (85451) 27 randomi#ed.ab. (293979) 28 placebo$.ab. (136824) 29 drug therapy.fs. (1589885) 30 randomly.ab. (176590) 31 trial.ab. (253721) 32 groups.ab. (1150140) 33 or/25‐32 (2972733) 34 exp animals/ not humans.sh. (3782732) 35 33 not 34 (2526401) 36 24 and 35 (441) EMBASE (1980 to Week 12 2013) Searched 25 March 2013 [444 records] 1 speech therapy/ (9281) 2 auditory rehabilitation/ (1993) 3 speech perception/ (11025) 4 audition.tw. (1134) 5 auditory verbal.tw. (1696) 6 AVT.tw. (857) 7 ((listening or auditory) adj3 (skill$ or activit$ or therap$ or training)).tw. (2887) 8 or/1‐7 (27751) 9 exp hearing impairment/ (60200) 10 (hearing adj3 (loss$ or impair$)).tw. (41264) 11 deaf$.tw. (30801) 12 or/9‐11 (89791) 13 exp child/ (1652870) 14 (baby or babies or infant$ or toddler$ or child$ or pre‐school$ or preschool$).tw. (1352141) 15 13 or 14 (2128760) 16 8 and 12 and 15 (2147) 17 exp Clinical trial/ (966624) 18 Randomized controlled trial/ (339220) 19 Randomization/ (61054) 20 Single blind procedure/ (17145) 21 Double blind procedure/ (113723) 22 triple blind procedure/ (38) 23 Crossover procedure/ (36496) 24 Placebo/ (215018) 25 Randomi#ed.tw. (439136) 26 RCT.tw. (11133) 27 (random$ adj3 (allocat$ or assign$)).tw. (102358) 28 randomly.ab. (234948) 29 groups.ab. (1513996) 30 trial.ab. (348135) 31 ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw. (151087) 32 Placebo$.tw. (186354) 33 Prospective study/ (228504) 34 (crossover or cross‐over).tw. (63771) 35 prospective.tw. (414735) 36 or/17‐35 (2994007) 37 16 and 36 (444) CINAHL PLus (EBSCO HOST) (1937 to current) Searched 25 March 2013 [178 records] S35 S19 AND S34 178 S34 S20 or S21 or S22 or S23 or S24 or S25 or S26 or S27 or S28 or S29 or S30 or S31 or S32 or S33 382,379 S33 TI (evaluat* study or evaluat* research) or AB (evaluate* study or evaluat* research) or TI (effectiv* study or effectiv* research) or AB (effectiv* study or effectiv* research) OR TI (prospectiv* study or prospectiv* research) or AB(prospectiv* study or prospectiv* research) or TI (follow‐up study or follow‐up research) or AB (follow‐up study or follow‐up research) 89,616 S32 placebo* 27,941 S31 crossover* or "cross over*"12,417 S30 (MH "Crossover Design") S29 (tripl* N3 mask*) or (tripl* N3 blind*) 132 S28 (trebl* N3 mask*) or (trebl* N3 blind*)149,261 S27 (doubl* N3 mask*) or (doubl* N3 blind*) 30,456 S26 (singl* N3 mask*) or (singl* N3 blind*)8,115 S25 (clinic* N3 trial*) or (control* N3 trial*) 164,562 S24 (random* N3 allocat* ) or (random* N3 assign*) 45,322 S23 randomis* or randomiz* 92,674 S22 (MH "Meta Analysis") 15,974 S21 (MH "Clinical Trials+") 157,744 S20 MH random assignment 34,493 S19 S16 OR S18 1,574 S18 S15 AND S17 704 S17 (MH "Rehabilitation of Hearing Impaired") 1,975 S16 S8 AND S12 AND S15 1,073 S15 S13 OR S14 463,734 S14 (baby or babies or infant* or toddler* or child* or pre‐school* or preschool*) 463,734 S13 (MH "Child+") 362,393 S12 (S9 OR S10 OR S11) 23,607 S11 (hearing N3 (loss* or impair*))12,181 S10 deaf* 7,084 S9 (MH "Hearing Disorders+") 19,581 S8 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 13,551 S7 ((listening or auditory) N3 (skill* or activit* or therap* or training))951 S6 AVT 23 S5 (auditory verbal) or (auditory‐ verbal) 357 S4 (MH "Speech and Language Assessment") 1,952 S3 (MH "Speech Perception") OR (MH "Auditory Perception") 6,225 S2 audition 102 S1 (MH "Rehabilitation, Speech and Language+") 4,822 Science Citation Index (SCI; 1970 to 22 March 2013) [searched 26 March 2013; 204 records] Social Science Citation Index (SSCI; 1970 to 22 March 2013) [searched 26 March 2013; 105 records] 10 #9 AND #8 DocType=All document types; Language=All languages; #9 TS=(baby or babies or infant* or toddler* or child* or pre‐school* or preschool*) DocType=All document types; Language=All languages; #8 #7 AND #4 DocType=All document types; Language=All languages; #7 #6 OR #5 DocType=All document types; Language=All languages; #6 TS=(hear* NEAR/5 ( loss* or impair*)) DocType=All document types; Language=All languages; #5 TS= deaf* DocType=All document types; Language=All languages; #4 #3 OR #2 OR #1 DocType=All document types; Language=All languages; #3 TS=(AVT) DocType=All document types; Language=All languages; #2 TS=((listening or auditory) NEAR/3 (activit* or skill* or therap* or train*)) DocType=All document types; Language=All languages; #1 TS=("auditory verbal") DocType=All document types; Language=All languages; Conference Proceedings Citation Index–Science (CPCI‐S; 1990 to 22 March 2013) Conference Proceedings Citation Index–Social Sciences & Humanities (CPCI‐SSH; 1990 to 22 March 2013)
Searched simultaneously 26 March 2013 [36 records] 10 #9 AND #8 DocType=All document types; Language=All languages; #9 TS=(baby or babies or infant* or toddler* or child* or pre‐school* or preschool*) DocType=All document types; Language=All languages; #8 #7 AND #4 DocType=All document types; Language=All languages; #7 #6 OR #5 DocType=All document types; Language=All languages; #6 TS=(hear* NEAR/5 ( loss* or impair*)) DocType=All document types; Language=All languages; #5 TS= deaf* DocType=All document types; Language=All languages; #4 #3 OR #2 OR #1 DocType=All document types; Language=All languages; #3 TS=(AVT) DocType=All document types; Language=All languages; #2 TS=((listening or auditory) NEAR/3 (activit* or skill* or therap* or train*)) DocType=All document types; Language=All languages; #1 TS=("auditory verbal") DocType=All document types; Language=All languages; PsycINFO (1967 to Week 3 March 2013) Searched 25 March 2013 [54 records] 1 speech therapy/ (3433) 2 language therapy/ (253) 3 exp Speech Language Pathology/ (322) 4 auditory verbal.tw. (1418) 5 audition.tw. (1086) 6 AVT.tw. (131) 7 ((listening or auditory) adj3 (skill$ or activit$ or therap$ or train$)).tw. (3128) 8 exp auditory perception/ (29153) 9 or/1‐8 (36839) 10 exp hearing disorders/ (12869) 11 partially hearing impaired/ (2908) 12 deaf$.tw. (11116) 13 (hearing adj3 (loss$ or impair$)).tw. (9247) 14 or/10‐13 (19547) 15 ("100" or "120" or "140" or "160" or "180").ag. (377694) 16 (baby or babies or infant$ or toddler$ or child$ or pre‐school$ or preschool$).tw. (496825) 17 15 or 16 (588730) 18 9 and 14 and 17 (1173) 19 clinical trials/ (6614) 20 (randomis$ or randomiz$).tw. (41412) 21 (random$ adj3 (allocat$ or assign$)).tw. (27017) 22 ((clinic$ or control$) adj trial$).tw. (35255) 23 ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw. (18252) 24 (crossover$ or "cross over$").tw. (6422) 25 random sampling/ (534) 26 Experiment Controls/ (583) 27 Placebo/ (3328) 28 placebo$.tw. (28449) 29 exp program evaluation/ (15200) 30 treatment effectiveness evaluation/ (14229) 31 ((effectiveness or evaluat$) adj3 (stud$ or research$)).tw. (50964) 32 or/19‐31 (163951) 33 18 and 32 (54) ERIC (1966 to current) Searched 26 March 2013 [953 records] (SU.EXACT("Speech Therapy") OR SU.EXACT("Speech Improvement") OR SU.EXACT("Speech Language Pathology") OR TI,AB(audition) OR TI,AB("auditory verbal") OR SU.EXACT ("Hearing Therapy") OR SU.EXACT("Total Communication") OR SU.EXACT("Auditory Training") OR TI,AB((listening or auditory) Near/3 (activit$3 OR skill* OR therap$3 or train$3)) OR TI,AB (AVT)) AND ( SU.EXACT.EXPLODE("Hearing Impairments") OR ti,ab(deaf$4) OR ti,ab(hear$3 Near/3( loss$2 or impair$4)) AND (SU.EXACT ("Longitudinal Studies") OR SU.EXACT("Control Groups") OR SU.EXACT("Program Effectiveness") OR SU.EXACT("Experimental Groups") OR SU.EXACT("Followup Studies") OR SU.EXACT("Comparative Analysis") OR prospective OR "follow up" OR ((evaluat$3 OR compar$5 OR blind$2) NEAR/5 (study OR studies OR research)) OR ((compar$4 OR control$3) NEAR/5 group$1) OR random$7 OR intervention$1 OR experiment$2 OR trial$1) WorldCat (all available years)
Searched 26 March 2013 [six records]
kw:"auditory verbal" and KW:(deaf* or hearing) and limited to theses
International Clinical Trials Registry Platform (ICTRP) (apps.who.int/trialsearch/)
Searched 20 April 2013 [no records]
auditory verbal therapy or auditory verbal
ClinicalTrials.gov (clinicaltrials.gov/)
Searched 20 April 2013 [54 records]
auditory verbal therapy or auditory verbal
speechBITE (speechbite.com/)
Searched 20 April 2013 [14 records]
auditory verbal therapy or auditory verbal
OpenGrey (opengrey.eu/)
Searched 20 April 2013 [no records]
auditory verbal therapy or auditory verbal
Networked Digital Library of Theses and Dissertations (NDLTD) (ndltd.org/)
Searched 20 April 2013 [23 records]
auditory verbal therapy or auditory verbal
Trove (National Library of Australia) (trove.nla.gov.au/)
Searched 20 April 2013 [83 records]
auditory verbal therapy or auditory verbal
DART‐ Europe E‐theses portal (DART) (dart‐europe.eu/)
Searched 20 April 2013 [three records]
auditory verbal therapy or auditory verbal
Appendix 3. Methods for future updates
Assessment of risk of bias in included studies
We will use The Cochrane Collaboration tool for assessing risk of bias (Higgins 2011). Two review authors (CGBJ and JW) will independently assess the risk of bias of included trials based on the six domains listed below. Each study shall be assessed for risk of bias and for the magnitude and direction of any such bias, relative to the effect under consideration. Review authors will independently assess the risk of bias within each included study based on the following six domains with ratings of 'low', 'high' or 'unclear'.
Sequence generation
We will describe in detail the method used to generate the allocation sequence, so as to assess whether it should have produced comparable groups; review authors' judgement: Was the allocation concealment sequence adequately generated?
Allocation concealment
We will describe in sufficient detail the method used to conceal allocation sequence to assess whether intervention schedules could have been foreseen in advance of, or during, recruitment; review authors' judgement: Was allocation adequately concealed?
Blinding
We will describe measures used to blind outcome assessors; review authors' judgement: Was knowledge of the allocated intervention adequately prevented during the study? Blinding of participants and personnel is not always practical in language intervention studies and therefore is not a requirement for inclusion, but it remains a source of potential bias.
Incomplete outcome data
We will extract and report data on attrition and exclusions, as well as the numbers involved (compared with total randomly assigned), reasons for attrition/exclusion (when reported or obtained from investigators) and any reinclusions in analyses performed by review authors; review authors' judgement: Were incomplete outcome data adequately addressed?
Selective outcome reporting
We will attempt to assess the possibility of selective outcome reporting by investigators; review authors' judgement: Are reports of the study free of suggestion of selective outcome reporting? We will also produce a matrix of planned versus collected versus reported outcomes (i.e. outcomes reported in the protocol or conference presentations vs outcomes reported in the publication).
Other sources of bias
We will describe any important concerns about bias not addressed in the other domains in the tool; review authors' judgement: Was the study apparently free of other problems that could put it at high risk of bias? In cluster‐randomised trials, we will examine, in particular, whether recruitment into clusters was different for different intervention groups, which may result from knowledge of which intervention was allocated to the cluster.
Measures of treatment effect
Binary outcomes
Risk ratios (RRs) with 95% confidence intervals (CIs) will be used for binary outcomes. For meta‐analyses of binary outcomes included in the 'Summary of findings' tables, we will express the results as absolute risks, using high and low observed risks among control groups as reference points.
Continuous outcomes
If studies use the same rating scales, we will calculate mean differences (MDs) and 95% CIs.
Multiple outcome measures
When a study provides multiple, interchangeable measures of the same construct at the same point in time (e.g. multiple measures of language development), we will calculate standardised mean differences (SMDs) with 95% CIs across these outcomes, and the average of their estimated variances. This strategy aims to avoid the need to select a single measure and to avoid inflated precision in meta‐analyses (preventing studies that report on more outcome measures receiving more weight in the analysis than comparable studies that report on a single outcome measure). An SMD will also be used for combining continuous data in meta‐analysis when different instruments have been used across studies to measure the same construct (Higgins 2011).
All analyses will include all participants in the treatment groups to which they were allocated if data are available.
Unit of analysis issues
If both individually randomised and cluster‐randomised studies are found, results will be combined when possible using the inverse variance method in Review Manager 2011. The sample size of the cluster‐randomised trial will be adjusted using the intracluster correlation coefficient (ICC), when reported, or an estimate of the ICC from the literature (Donner 2001; Higgins 2011). In the event that no proxy ICCs can be identified, we will undertake sensitivity analyses using a high (0.1), moderate (0.01) and small (0.001) ICC, simply to adjust the effect estimates and their standard errors because of the implausibility that the ICC is, in fact, zero.
We do not anticipate studies with a cross‐over design. This type of study design is considered inappropriate for a treatment such as AVT, which may have a lasting effect (Higgins 2011).
Dealing with missing data
We will assess missing data and dropouts in the included studies. We will investigate and report reasons for, numbers and characteristics of dropouts. When necessary, we will contact the corresponding authors of included studies to request unreported data. We will contact other authors if necessary. If a study reports outcomes only for participants completing the trial or only for participants who followed the protocol, we will contact the study authors and will ask them to provide additional information to permit analyses according to intention‐to‐treat principles. We will describe missing data and dropouts/attrition for each included study in the 'Risk of bias' table, and will discuss the extent to which missing data could alter the results/conclusions of the review. Following Higgins 2008, the sensitivity analysis of unobserved data will separate into two dimensions: (1) the effect of allowing for missing data on effect estimates from individual studies; and (2) the effect of allowing for missing data on standard errors (and hence weights) of these estimates.
We shall select 'informative missingness odds ratios' (IMORs) for the two groups that cover realistic situations, and these results shall be graphed in a L' Abbé plot (experimental group risk vs control group risk) for risks applied to missing participants. When the corners of the plot represent extreme imputation strategies, all points on the plot correspond to IMOR group pairs (IMORE and IMORC).
For a given 'starting point', corresponding to the primary analysis (typically the available case analysis, ACA), we can move in four directions towards the corners of the plot. Moving towards the ICA‐11, imputed case analysis experiencing an event, and ICA‐0 (imputed case analysis not experiencing an event), corners involve assuming the same IMORs in the two treatment groups; moving towards the best‐case and worst‐case corners involves assuming different IMORs in the two treatment groups. These directions are achieved by taking IMORC = IMORE or IMORC = 1/IMORE, respectively. We propose using a weighting scheme that employs standard errors corresponding to the quantity of observed data. Effect estimates will be determined from the imputed case data set, but using standard errors directly from the available case analysis, such that only these estimates are affected by the different IMORs. We shall select from experience combinations of IMORE and IMORC. From this, we shall assess whether results of the ACA are robust to differences in risks between outcomes among missing participants and outcomes of observed participants.
To evaluate the effects of missing participants on weights awarded to the studies, the inflated confidence intervals of Gamble and Hollis will be used (Gamble 2005), expanding the confidence interval by taking the lowest interval level and the highest interval level for a range of estimates, to create an overall uncertainty level.
Assessment of heterogeneity
We will assess clinical heterogeneity across studies by comparing the distribution of important participant factors among trials (e.g. duration and intensity of AVT, age of participants) and methodological heterogeneity (e.g. randomisation concealment, blinding of outcome assessment, losses to follow‐up, treatment type, co‐interventions). We will describe statistical heterogeneity by computing I2 (Higgins 2002), a quantity that describes approximately the proportion of variation in point estimates that is due to heterogeneity rather than to sampling error: I2 values of 0% to 40% might not be important; I2 values of 30% to 60% may represent moderate heterogeneity; I2 values of 50% to 90% may represent substantial heterogeneity; and I2 values of 75% to 100% may indicate considerable heterogeneity. In addition, we will employ a Chi2 test of homogeneity to determine the strength of evidence that heterogeneity is genuine. Chi2 has limited power when studies have a small sample size or are too few in number. Therefore, whilst a statistically significant result may indicate a problem with heterogeneity, a non‐significant result must not be taken as evidence of no heterogeneity. We will therefore use a P value of 0.10 to determine statistical significance (Higgins 2011).
Assessment of reporting biases
We will produce funnel plots (estimated differences in treatment effects against their standard error) if sufficient studies are found. An asymmetrical funnel plot can indicate publication bias but also can be due to a relationship between trial size and effect size. In the event that a relationship is found, clinical diversity of the studies will also be examined (Higgins 2011). As a sensitivity analysis, we will compare the results from published and unpublished trials identified in our search.
Data synthesis
When identified studies are sufficiently homogeneous in terms of participants, interventions and outcomes, we plan to synthesise results in a meta‐analysis using The Cochrane Collaboration statistical software, Review Manager (Review Manager 2011). We will use both a fixed‐effect and a random‐effects model and will compare the results to assess the impact of statistical heterogeneity. Unless the model is contraindicated (e.g. if funnel plot asymmetry is noted), we plan to present the results from the random‐effects model. In the presence of severe funnel plot asymmetry, we will present both fixed‐effect and random‐effects analyses, under the assumption that asymmetry suggests that neither model is appropriate. If both indicate a presence (or absence) of effect, we will be reassured; if they do not agree, we will report this. We will calculate all overall effects using inverse variance methods. If some primary studies report an outcome as a dichotomous measure and others use a continuous measure of the same construct, we will convert results for the former from an odds ratio to an SMD, provided we can assume that the underlying continuous measure has approximately a normal or logistic distribution (otherwise, we will carry out two separate analyses).
Subgroup analysis and investigation of heterogeneity
Subgroup analyses will include the following.
Age at diagnosis: to identify differences in language outcomes for children whose hearing loss is identified early (e.g. before six months of age) compared with those identified later (Yoshinaga‐Itano 1998).
Duration of hearing loss at the start of therapy: to establish whether delays in receiving intervention affect language outcomes.
Socioeconomic status: to establish whether benefit is consistent across a range of socioeconomic groups.
Sensitivity analysis
We will conduct sensitivity analyses to determine whether findings are sensitive to removing studies with a high or unclear risk of bias, thereby restricting analyses to studies judged to be at low risk of bias. We will restrict the analyses to:
only studies with low risk of selection bias (associated with sequence generation or allocation concealment);
only studies with low risk of performance bias (associated with issues of blinding); and
only studies with low risk of attrition bias (associated with completeness of data).
In addition, we will assess the sensitivity of findings to any imputed data.
Characteristics of studies
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Allegro 2010 | Not (quasi‐)RCT |
| Bakhshaee 2007 | Not (quasi‐)RCT |
| Diller 2001 | Not (quasi‐)RCT |
| Doble 2006 | Not (quasi‐)RCT |
| Dornan 2007 | Not (quasi‐)RCT; control group not hearing impaired |
| Dornan 2009 | Not (quasi‐)RCT |
| Dornan 2010 | Not (quasi‐)RCT |
| Fairgray 2008 | Not (quasi‐)RCT |
| Fairgray 2010 | Not (quasi‐)RCT; treatment duration less than six months |
| Fulcher 2012 | Not (quasi‐)RCT |
| Hogan 2008 | Not (quasi‐)RCT |
| Hogan 2010 | Not (quasi‐)RCT |
| Sahli 2011 | Not (quasi‐)RCT |
Contributions of authors
CGBJ and JW conceived of the review, contributed to all drafts of the review and approved the final version. RWR provided statistical expertise, revised drafts of the review and approved the final version. JL provided methodological expertise, revised drafts of the review and approved the final version.
Declarations of interest
Christopher Brennan‐Jones ‐ none known.
Jo White serves as external examiner for the Postgraduate Diploma in Auditory‐Verbal Therapy at Aston University. An annual fee was received and expenses were paid for her to attend an annual exam board meeting.
Robert Rush ‐ none known.
James Law ‐ none known.
New
References
References to studies excluded from this review
Allegro 2010 {published data only}
- Allegro J, Papsin B, Harrison R, Campisi P. Acoustic analysis of voice in cochlear implant recipients with post‐meningitic hearing loss. Cochlear Implants International 2010;11(2):100‐16. [DOI] [PubMed] [Google Scholar]
Bakhshaee 2007 {published data only}
- Bakhshaee M, Ghasemi MM, Shakeri MT, Razmara N, Tayarani H, Tale MR. Speech development in children after cochlear implantation. European Archives of Oto‐Rhino‐Laryngology 2007;264(11):1263‐6. [DOI] [PubMed] [Google Scholar]
Diller 2001 {published data only}
- Diller G, Graser P, Schmalbrock C. Early natural auditory‐verbal education of children with profound hearing impairments in the Federal Republic of Germany: results of a 4 year study. International Journal of Pediatric Otorhinolaryngology 2001;60(3):219‐26. [DOI] [PubMed] [Google Scholar]
Doble 2006 {published data only}
- Doble MG. Development of Oral Communication in Infants with a Profound Hearing Loss: Pre‐ and Post‐cochlear Implantation [PhD thesis]. University of Sydney, 2006. [Google Scholar]
Dornan 2007 {published data only}
- Dornan D, Hickson L, Murdoch B, Houston T. Outcomes of an auditory‐verbal program for children with hearing loss: a comparative study with a matched group of children with normal hearing. The Volta Review 2007;107(1):37‐54. [Google Scholar]
Dornan 2009 {published data only}
- Dornan D, Hickson L, Murdoch B, Houston T. Longitudinal study of speech perception, speech, and language for children with hearing loss in an auditory‐verbal therapy program. The Volta Review 2009;109(2‐3):61‐85. [Google Scholar]
Dornan 2010 {published data only}
- Dornan D, Hickson L, Murdoch B, Houston T, Constantinescu G. Is auditory‐verbal therapy effective for children with hearing loss?. The Volta Review 2010;110(3):361‐87. [Google Scholar]
Fairgray 2008 {published data only}
- Fairgray L, Purdy SC. Benefits of speech and language therapy for hearing impaired children. Poster Presented at Reflecting Connections: A Joint Conference Between New Zealand Speech Language Therapists Association and Speech Pathology Australia; 2008 May 25‐29. 2008.
Fairgray 2010 {published data only}
- Fairgray L, Purdy SC, Smart JL. Effects of auditory‐verbal therapy for school‐aged children with hearing loss: an exploratory study. The Volta Review 2010;110(3):407‐33. [Google Scholar]
Fulcher 2012 {published data only}
- Fulcher A, Purcell AA, Baker E, Munro N. Listen up: children with early identified hearing loss achieve age‐appropriate speech/language outcomes by 3 years‐of‐age. International Journal of Pediatric Otorhinolaryngology 2012;76(12):1785‐94. [DOI] [PubMed] [Google Scholar]
Hogan 2008 {published data only}
- Hogan S, Stokes J, White C, Tyszkiewicz E, Woolgar A. An evaluation of auditory verbal therapy using the rate of early language development as an outcome measure. Deafness & Education International 2008;10(3):143‐67. [Google Scholar]
Hogan 2010 {published data only}
- Hogan S, Stokes J, Weller I. Language outcomes for children of low‐income families enrolled in auditory verbal therapy. Deafness & Education International 2010;12(4):204‐16. [Google Scholar]
Sahli 2011 {published data only}
- Sahli AS, Belgin E. Researching auditory perception performances of children using cochlear Implants and being trained by an auditory verbal therapy. Journal of International Advanced Otology 2011;7(3):385‐90. [Google Scholar]
Additional references
Achenbach 1991
- Achenbach TM. Manual for the Child Behaviour Checklist 4‐18 and 1991 Profile. Burlington, VT: University of Vermont Department of Psychiatry, 1991. [Google Scholar]
AG Bell Academy 2011
- AG Bell Academy. AG Bell Academy. Certification Handbook. Washington DC: AG Bell Academy for Listening and Spoken Language, 2011. [Google Scholar]
AG Bell Academy 2013
- AG Bell Academy. AG Bell Academy for Listening and Spoken Language. www.listeningandspokenlanguage.org/AGBellAcademy/ (accessed 17 October 2013).
Bamford 2007
- Bamford J, Fortnum H, Bristow K, Smith J, Vamvakas G, Davies L, et al. Current practice, accuracy, effectiveness and cost‐effectiveness of the school entry hearing screen. Health Technology Assessment 2007;11(32):1‐168. [DOI] [PubMed] [Google Scholar]
Boucher‐Jones 2001
- Boucher‐Jones M. Does the auditory‐verbal approach differ from the auditory‐oral approach and/or traditional aural habilitation?. In: Estabrooks W editor(s). Fifty FAQs about Auditory‐Verbal Therapy. Toronto, Canada: Learning to Listen Foundation, 2001:17‐9. [Google Scholar]
Brachmaier 2010
- Brachmaier J, Schramm B. Parent observation: an effective assessment method for early speech and language development?. Cochlear Implants International 2010;11(Suppl 1):259‐63. [DOI] [PubMed] [Google Scholar]
Brennan‐Jones 2012
- Brennan‐Jones CG, White J, Rush RW, Law J. Auditory‐verbal therapy for promoting spoken language development in children with permanent hearing impairments. Cochrane Database of Systematic Reviews 2012, Issue 9. [DOI: 10.1002/14651858.CD010100] [DOI] [PMC free article] [PubMed] [Google Scholar]
Davis 1992
- Davis A, Wood S. The epidemiology of childhood hearing impairment: factors relevant to planning of services. British Journal of Audiology 1992;26(2):77‐90. [DOI] [PubMed] [Google Scholar]
Davis 1994
- Davis A, Parving A. Towards appropriate epidemiology data on childhood hearing disability: a comparative European study of birth cohorts 1982‐1988. Journal of Audiological Medicine 1994;3:35‐47. [Google Scholar]
Davis 1997
- Davis A, Bamford J, Wilson I, Ramkalawan T, Forshaw M, Wright S. A critical review of the role of neonatal hearing screening in the detection of congenital hearing impairment. Health Technology Assessment 1997;1(10):1‐176. [PubMed] [Google Scholar]
Davis 2009
- Davis A, Davis K, Mencher G. Epidemiology of permanent childhood hearing impairment. In: Newton VE editor(s). Paediatric Audiological Medicine. 2nd Edition. Chichester, UK: John Wiley & Sons, 2009:1‐26. [Google Scholar]
Donner 2001
- Donner AD, Piaggio G, Villar J. Statistical methods for meta‐analysis of cluster randomization trials. Statistical Methods in Medical Research 2001;10(5):325‐38. [DOI] [PubMed] [Google Scholar]
Edwards 2011
- Edwards S, Letts C, Sinka I. The New Reynell Developmental Language Scales. London: GL‐Assessment, 2011. [Google Scholar]
Eriks‐Brophy 2004
- Eriks‐Brophy A. Outcomes of auditory‐verbal therapy: a review of the evidence and a call for action. The Volta Review 2004;104(1):21‐35. [Google Scholar]
Flexer 1999
- Flexer C. Facilitating Hearing and Listening in Young Children. 2nd Edition. San Diego, CA: Singular Publishing Group, 1999. [Google Scholar]
Fortnum 1997
- Fortnum H, Davis A. Epidemiology of permanent childhood hearing impairment in Trent Region, 1985‐1993. British Journal of Audiology 1997;31(6):409‐46. [DOI] [PubMed] [Google Scholar]
Fortnum 2001
- Fortnum HM, Summerfield AQ, Marshall DH, Davis AC, Bamford JM. Prevalence of permanent childhood hearing impairment in the United Kingdom and implications for universal neonatal hearing screening: questionnaire based ascertainment study. BMJ 2001;323(7312):536‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gamble 2005
- Gamble C, Hollis S. Uncertainty method improved on best‐worst case analysis in a binary meta‐analysis. Journal of Clinical Epidemiology 2005;58(6):579‐88. [DOI] [PubMed] [Google Scholar]
Hann 2001
- Hann FL. Are there situations when auditory‐verbal therapy is not appropriate for the family? How and when is it known that auditory‐verbal therapy is working or not working?. In: Estabrooks W editor(s). Fifty FAQs about Auditory‐Verbal Therapy. Toronto: Learning to Listen Foundation, 2001:113‐6. [Google Scholar]
Higgins 2002
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, White IR, Wood AM. Imputation methods for missing outcome data in meta‐analysis of clinical trials. Clinical Trials 2008;5(3):225‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Hróbjartsson 2012
- Hróbjartsson A, Thomsen AS, Emanuelsson F, Tendal B, Hilden J, Boutron I, et al. Observer bias in randomised clinical trials with binary outcomes: systematic review of trials with both blinded and non‐blinded outcome assessors. BMJ 2012;344:e1119. [DOI] [PubMed] [Google Scholar]
Law 2010
- Law J, Garrett Z, Nye C. Speech and language therapy interventions for children with primary speech and language delay or disorder. Cochrane Database of Systematic Reviews 2010, Issue 5. [DOI: 10.1002/14651858.CD004110] [DOI] [PMC free article] [PubMed] [Google Scholar]
Levasseur 2001
- Levasseur J. What does an auditory‐verbal therapist do that is different from what a speech‐language pathologist does?. In: Estabrooks W editor(s). Fifty FAQs about Auditory‐Verbal Therapy. Toronto: Learning to Listen Foundation, 2001:26‐9. [Google Scholar]
Lim 2005
- Lim SYC, Simser J. Auditory‐verbal therapy for children with hearing impairment. Annals of the Academy of Medicine, Singapore 2005;34(4):307‐12. [PubMed] [Google Scholar]
Martin 1981
- Martin JAM, Bentzen O, Colley JRT, Hennebert D, Holm C, Iurato S, et al. Childhood deafness in the European community. Scandinavian Audiology 1981;10(3):165‐74. [DOI] [PubMed] [Google Scholar]
McDonald Walker 2001
- McDonald Walker W. The therapist covers her mouth and sits across from the child. This is auditory‐verbal therapy, right?. In: Estabrooks W editor(s). Fifty FAQs about Auditory‐Verbal Therapy. Toronto: Learning to Listen Foundation, 2001:30‐2. [Google Scholar]
Mitchell 2004
- Mitchell RE, Karchmer MA. Chasing the mythical ten percent: parental hearing status of deaf and hard of hearing students in the United States. Sign Language Studies 2004;4(2):138‐63. [Google Scholar]
Morgan 2008
- Morgan AT, Vogel AP. Intervention for childhood apraxia of speech. Cochrane Database of Systematic Reviews 2008, Issue 3. [DOI: 10.1002/14651858.CD006278.pub2] [DOI] [PubMed] [Google Scholar]
Odgaard‐Jensen 2011
- Odgaard‐Jensen J, Vist GE, Timmer A, Kunz R, Akl EA, Schünemann H, et al. Randomisation to protect against selection bias in healthcare trials. Cochrane Database of Systematic Reviews 2011, Issue 4. [DOI: 10.1002/14651858.MR000012.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Rhoades 2006
- Rhoades EA. Research outcomes of auditory‐verbal intervention: is the approach justified?. Deafness & Education International 2006;8(3):125‐43. [Google Scholar]
Richards 2001
- Richards L, Simser J. Don't children who are deaf or hard of hearing need lipreading to access and learn spoken language?. In: Estabrooks W editor(s). Fifty FAQs about Auditory‐Verbal Therapy. Toronto: Learning to Listen Foundation, 2001:36‐8. [Google Scholar]
Semel 2003
- Semel E, Wiig EH, Secord WA. Clinical Evaluation of Language (CELF‐4). 4th Edition. Toronto: The Psychological Corporation/A Harcourt Assessment Company, 2003. [Google Scholar]
Simser 2001
- Simser J. Why do parents have to participate in the auditory‐verbal session? Can't they watch through a one‐way mirror?. In: Estabrooks W editor(s). Fifty FAQs about Auditory‐Verbal Therapy. Toronto: Learning to Listen Foundation, 2001:82‐5. [Google Scholar]
Stacey 2006
- Stacey PC, Fortnum HM, Barton GR, Summerfield AQ. Hearing‐impaired children in the United Kingdom I: auditory performance, communication skills, educational achievements, quality of life, and cochlear implantation. Ear and Hearing 2006;27(2):161‐86. [DOI] [PubMed] [Google Scholar]
Tomasello 2003
- Tomasello M. Constructing a Language: A Usage‐Based Theory of Language Acquisition. Cambridge, MA: Harvard University Press, 2003. [Google Scholar]
Van Eldik 2004
- Eldik T, Treffers PD, Veerman JW, Verhulst FC. Mental health problems of deaf Dutch children as indicated by parents' responses to the child behavior checklist. American Annals of the Deaf 2004;148(5):390‐5. [DOI] [PubMed] [Google Scholar]
Watkin 2007
- Watkin P, McCann D, Law C, Mullee M, Petrou S, Stevenson J, et al. Language ability in children with permanent hearing impairment: the influence of early management and family participation. Pediatrics 2007;120(3):e694‐701. [DOI] [PubMed] [Google Scholar]
Yoshinaga‐Itano 1998
- Yoshinaga‐Itano C, Sedey AL, Coulter DK, Mehl AL. Language of early‐ and later‐identified children with hearing loss. Pediatrics 1998;102(5):1161‐71. [DOI] [PubMed] [Google Scholar]
Zimmerman 2002
- Zimmerman IL, Steiner VG, Pond RE. Preschool Language Scale. 4th Edition. San Antonio: The Psychological Corporation, 2002. [Google Scholar]


