Abstract
Background:
Widespread insecticide exposure might be a risk factor for neurodevelopment of our children, but few studies examined the mixture effect of maternal coexposure to organophosphate insecticides (OPPs), pyrethroids (PYRs), and neonicotinoid insecticides (NNIs) during pregnancy on child neurodevelopment, and critical windows of exposure are unknown.
Objectives:
We aimed to evaluate the association of prenatal exposure to multiple insecticides with children’s neurodevelopment and to identify critical windows of the exposure.
Methods:
Pregnant women were recruited into a prospective birth cohort study in Wuhan, China, from 2014–2017. Eight metabolites of OPPs (mOPPs), three metabolites of PYRs (mPYRs), and nine metabolites of NNIs (mNNIs) were measured in 3,123 urine samples collected at their first, second, and third trimesters. Children’s neurodevelopment [mental development index (MDI) and psychomotor development index (PDI)] was assessed using the Bayley Scales of Infant Development at 2 years of age (). Multivariate linear regression models, generalized estimating equation models, and weighted quantile sum (WQS) regression were used to estimate the association between the insecticide metabolites and Bayley scores. Potential sex-specific associations were also examined.
Results:
Single chemical analysis suggested higher urinary concentrations of some insecticide metabolites at the first trimester were significantly associated with lower MDI and PDI scores, and the associations were more prominent among boys. Each 1-unit increase in ln-transformed urinary concentrations of two mOPPs, 3,5,6-trichloro-2-pyridinol and 4-nitrophenol, was associated with a decrease of 3.16 points [95% confidence interval (CI): , ] and 3.06 points (95% CI: , ) respectively in boys’ MDI scores. Each 1-unit increase in that of trans-3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropanecarboxylic acid (trans-DCCA; an mPYR) was significantly associated with a decrease of 2.24 points (95% CI: , ) in boys’ MDI scores and 1.90 points (95% CI: , ) in boys’ PDI scores, respectively. Significantly positive associations of maternal urinary biomarker concentrations [e.g., dimethyl phosphate (a nonspecific mOPP) and desmethyl-clothianidin (a relatively specific mNNI)] with child neurodevelopment were also observed. Using repeated holdout validation, a 1-quartile increase in the WQS index of the insecticide mixture (in the negative direction) at the first trimester was significantly associated with a decrease of 3.02 points (95% CI: , ) in MDI scores among the boys, and trans-DCCA contributed the most to the association (18%).
Conclusions:
Prenatal exposure to higher levels of certain insecticides and their mixture were associated with lower Bayley scores in children, particularly in boys. Early pregnancy may be a sensitive window for such an effect. Future studies are needed to confirm our findings. https://doi.org/10.1289/EHP12097
Introduction
Maternal exposure to environmental chemicals during pregnancy might impair fetal development and increase the future health risks of the children.1–4 Organophosphates (OPPs), pyrethroids (PYRs), and neonicotinoids (NNIs) are three major classes of insecticides widely used in the world.5,6 Neurotoxicity of prenatal exposure to these insecticides has been documented in animal models.7–10 Increasing evidence, mainly from populations with elevated exposure as a result of living near agricultural areas, indicated that prenatal exposure to OPPs,11–14 PYRs,15,16 or NNIs may impair child neurodevelopment,17,18 causing adverse outcomes, such as decreased Bayley scores, or increased risk of autism spectrum disorder (ASD).
Previous studies have investigated neurocognitive development in children associated with gestational exposure to OPPs19–23 or PYRs24,25 or both of them26 in general populations. The available results are inconsistent; some studies have observed harmful effects of OPPs19,20,22 or PYRs,24 including sex-specific effects,21,23 whereas some others found no significant associations25 or positive effects.26 On the other hand, NNIs once accounted for of the global insecticide market27; however, epidemiological studies on the neurodevelopment effects of NNIs in the general population, particularly based on biomonitoring data, are still scarce.
The general population can be exposed to multiple insecticides simultaneously through the intake of contaminated food28,29 and drinking water,30 but few epidemiological studies have explored the mixture effect of maternal exposure to multiple classes of insecticides during pregnancy on child neurodevelopment. In addition, many of those measured exposure biomarkers in urine at only one time point.20,23,26,31 However, for those insecticides that have relatively short half-lives in the human body,32,33 repeated measurements of urinary biomarkers across pregnancy (the three trimesters) are strongly desirable to increase the reliability of exposure assessment and to examine potential sensitive windows for adverse effects of prenatal exposure in relation to neurodevelopment.
In this study, exposure biomarker concentrations of OPPs, PYRs, and NNIs were measured in urine samples repeatedly collected from 1,041 pregnant Chinese women during the first, second, and third trimesters from Wuhan, China, between 2014 and 2017. We explored whether there was a potential sensitive window of prenatal insecticide exposure in association with child neurodevelopment. Because previous studies suggested that prenatal insecticide exposure has stronger adverse effects on neurodevelopment in male offspring,34,35 sex-stratified analyses were performed in this study, and the interaction term between insecticide exposure and child sex was then used to examine the effect modification. Mixture effect of exposure to the selected insecticides on child neurodevelopment was explored and the primary contributors were identified.
Methods
Study Population
Participants in this study were part of a prospective birth cohort that aimed to investigate the association of environmental exposures with the health of pregnant women and their children who were recruited at the first antenatal examination ( of gestation) in the Wuhan Women’s and Children’s Health Care Center, Wuhan, central China. Participants were considered eligible if they were years of age, were Wuhan residents (lived in Wuhan for ) who could understand Chinese well and complete the questionnaire independently without communication problems (i.e., no mental illness), had a singleton pregnancy, planned to deliver at the study hospital, and were willing to provide biospecimens at three trimesters during pregnancy. All participants in this study were urban residents (determined by their permanent address) with no occupational exposure to pesticides. The research protocol was authorized by the ethics committees of Tongji Medical College and the participating health care center, and all the participants provided informed consent.
From January 2014 to June 2017, a total of 5,112 pregnant women were recruited into the cohort and donated at least one urine sample, of which 2,782 mothers had their children complete the neurodevelopment assessment at 2 years of age through May 2020. Of the 2,782 mother–child pairs, 1,041 mothers who donated one spot urine sample in each of the three trimesters during gestation (i.e., who had all three urine samples) were included in this study. The rest, who provided fewer than three urine samples, were not included in this study. No statistically significant differences for the demographic characteristics were observed among the recruited population (), the women whose children completing the evaluation of Bayley scales at 2 years of age (), or the study population () (Table S1).
Data Collection
The data of demographic and socioeconomic characteristics (maternal age, prepregnancy weight, annual household income, and both maternal and paternal education) and lifestyle factors (smoking, passive smoking, alcohol use, and folic acid supplementation during pregnancy) were obtained in face-to-face interviews at enrollment or during prenatal follow-up visits by trained nurses using standardized questionnaires. The data about pregnancy complications (maternal anemia, hypertensive disorders in pregnancy, and gestational diabetes mellitus) and the information of newborns (child sex, gestational age at birth, birth weight, and birth height) were retrieved from the medical records at birth. Breastfeeding status was obtained from questionnaires during the follow-up of children. In addition, self-reported prepregnancy weight and height were applied to calculate the prepregnancy body mass index (PBMI), which was categorized into three groups according to the Guideline of Chinese adults: underweight (), normal weight (), and overweight (). Gestational weight gain (GWG; in kilograms) was divided into three groups: inadequate, recommended, and excessive weight gain during pregnancy (Table S2) according to the standard of recommendation of the Chinese National Health Commission (NHC).36 Sampling seasons were obtained according to the sampling date with May–October and November–April, respectively representing the warm and cold seasons in Wuhan.
Assessment of Child Neurocognitive Development
Neurocognitive development for each child was assessed at 2 years of age (range: 23–26 months) using the locally standardized Chinese revision of the Bayley Scales of Infant Development (BSID-CR).37,38 Cognition, language, and social development was assessed using the mental development index (MDI); fine and gross motor development was assessed using the psychomotor development index (PDI).38
Two well-trained certified psychologists administered the BSID-CR in a quiet room of the study health care center. Videotaped evaluations were used for quality control. Cronbach’s alpha intraclass correlation coefficient39 for the 2-year-old BSID-CR assessments between two psychologists was 0.99 () on the basis of double-scoring among 5% of the randomly selected participants.
Urinary Biomarker Measurements and Quality Control
For each pregnant woman, one spot urine sample was collected at the first (), second (), and third trimesters () during pregnancy, respectively. The samples were stored in polypropylene tubes at for further analysis.
In brief, based on previous studies, we measured six nonspecific metabolites of OPPs [i.e., dialkyl phosphates (DAPs), including dimethyl phosphate (DMP), dimethyl thiophosphate (DMTP), dimethyl dithiophosphate (DMDTP), diethyl phosphate (DEP), diethyl thiophosphate (DETP), diethyl dithiophosphate (DEDTP)], two specific metabolites of OPPs [3,5,6-trichloro-2-pyridinol (TCPy) and 4-nitrophenol (PNP)],40,41 three typical metabolites of PYRs [trans-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylic acid (trans-DCCA), 3-phenoxybenzoic acid (3-PBA), and 4-fluoro-3-phenoxybenzoic acid (4F-3-PBA)],40 and nine typical NNIs/their metabolites [imidacloprid (IMI), acetamiprid (ACE), thiamethoxam (THM), clothianidin (CLO), 5-hydroxy-imidacloprid (5-hydroxy-IMI), imidacloprid-olefin (IMI-olefin), desnitro-imidacloprid (DN-IMI), desmethyl-acetamiprid (DM-ACE), and desmethyl-clothianidin (DM-CLO)].42 The eight metabolites of OPPs (defined as mOPPs), three metabolites of PYRs (defined as mPYRs), and nine NNIs/their metabolites (defined as mNNIs) were measured using isotope-dilution mass spectrometry (MS) methods (Table S3).
Details on specific sample pretreatment steps are as follows. After thawing, of each urine sample was transferred to a centrifuge tube (Corning Inc.), spiked with 850 U of ( of in ammonium acetate with ) and internal standard mixture ( of in acetonitrile: :1), and then incubated (shaken gently) at 37°C overnight. Then, of ethyl acetate was added to each sample for extraction, vortexed in an automatic vortex shaker for 15 min, and centrifuged at for 10 min. The extraction step was repeated. The supernatants were combined and transferred into a glass tube, and then dried under a gentle nitrogen stream at 35°C. Finally, the sample was reconstituted in of acetonitrile/water (3:7) and transferred into a amber liquid chromatography (LC) vial, and stored at for further instrumental analysis.
Because DAPs and DN-IMI cannot be well extracted by the above liquid–liquid extraction method and because PNP had a relatively high background contamination during liquid–liquid extraction, we used the following sample preparation method for those analytes. In brief, of each urine sample was spiked with 85 U of (dissolved in of ammonium acetate buffer, ) and (for , DMTP-d6, , , , , , and ) of each isotope-labeled internal standard, and incubated at 37°C overnight. Then, the sample was diluted five times with of 0.05% formic acid in water, vortexed, transferred into an Amicon Ultra-0.5 Centrifugal Filter Unit,43 and centrifuged at for 30 min. The filtered sample was transferred into an amber LC vial for determination of the target analytes.
Urinary target analyte concentrations were measured by an ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS; ExionLC-QTRAP , AB SCIEX) with an ACQUITY UPLC HSS T3 column (, ; Waters Corporation) maintained at 40°C. Details about the multiple reaction monitoring (MRM) transitions of the target analytes are presented in Table S3. The details on the gradient elution program, mobile phases used, and flow rate are shown in Tables S4 and S5. The separation of the target analytes except for DAPs, PNP, and DN-IMI was achieved with a gradient elution program (Table S4) at a flow rate of . For DAPs, PNP, and DN-IMI, the target analytes were determined separately using another gradient elution program (Table S5) at a flow rate of . The injection volume was . The detection was conducted in MRM mode. mOPPs, IMI-olefin, and DM-CLO were monitored in negative electrospray ionization () mode with the ionization voltage of . The rest target analytes were monitored in with an ionization voltage of . The ion source temperature was 650°C.
Details on the quality assurance/control are as follows: the calibration curves of the analytes were obtained based on 12 levels (0.002, 0.005, 0.01, 0.02, 0.05, 0.10, 0.20, 0.50, 1.00, 2.00, 5.00, and , with of each internal standard), and the regression coefficient () was for each target analyte. Procedural blanks, travel blanks, duplicates (two pooled samples as quality control materials), matrix spikes, and checks of carry-over and instrumental sensitivity drift were included in each batch. The average recoveries of matrix spiked native standards ( of each native standard in the urine sample) ranged from 86.0% to 115%. The relative standard deviations (SDs) of duplicates were . Instrumental limit of quantifications (LOQs) and method detection limits (MDLs) (shown in Table S3) have been defined elsewhere.44 The LOQs of the analytes ranged from 0.005 to (Table S3). The target analytes were not found in any of the blanks or found to be less than the LOQs.
Creatinine adjustment may not be appropriate for metabolite levels in populations undergoing rapid physiologic changes, such as pregnant women, owing to high intra-individual variability in creatinine excretion.45 It has been demonstrated that creatinine can be significantly influenced by age, BMI, and fat-free mass,46 whereas, specific gravity (SG) could be more reliable compared with creatinine.47 Thus, in this study, SG of urine was used to correct urine dilution.44,48 Specifically, the urinary SG was measured by a digital handheld refractometer (PAL-10S, Atago). Concentrations of target pesticides were standardized by urinary SG using the following equation: , where is the SG-adjusted concentration (in nanograms per milliliter), C is the observed concentration (in nanograms per milliliter), and is the median SG at each trimester.
Statistical Analysis
Descriptive statistics on characteristics of the mothers and children were performed, and the chi-square test was conducted to examine the differences in basic characteristics of mother–child pairs among the recruitment population (), the population of their children completing the evaluation of Bayley scales at 2 years of age (), and the study population (). The analysis of variance was used to examine whether child MDI and PDI scores at 2 years of age differed among the participants with different characteristics. Descriptive statistics were also used to characterize the distribution of child Bayley scores at 2 years of age based on the quartiles of average urinary concentrations of insecticide biomarkers throughout pregnancy for the participants.
DMDTP, DEDTP, 4F-3-PBA, and ACE were excluded in the following statistical analysis owing to low detection frequencies (DFs, ). The other mOPPs, mPYRs, and mNNIs had DFs of , and values below the MDLs were imputed as the MDL divided by the square root of 2. To approximate normal distributions, all concentrations were natural logarithm (ln)-transformed for further statistical analysis. We examined Spearman’s rank correlations between target analytes at individual time points and on the basis of the averaged prenatal exposure (the arithmetic mean concentrations of mOPPs, mPYRs, and mNNIs at the three trimesters). Different from many previous studies, we did not calculate the summed concentrations of six nonspecific metabolites of OPPs because DMP and DEP can be originated from not only OPPs but also the organophosphate esters trimethyl phosphate (TMP)49 and triethyl phosphate (TEP),50 which might cause overestimation of the total exposure to OPPs. TMP and TEP can be metabolized into DMP and DEP and excreted through the urine,51 and both TMP and TEP are ubiquitous in the environment.52 In addition, we also compared the concentrations of mOPPs and mPYRs observed in the present study with those in adult females ( of age) from the U.S. National Health and Nutrition Examination Survey (NHANES) in 2013–2014 ()53 and in 2017–2018 ().54
To investigate the effects of insecticide exposure during pregnancy on child neurodevelopment, multivariate linear regression (MLR) models were performed to evaluate the associations between the averaged concentrations of individual analytes across three trimesters and children’s MDI/PDI scores at 2 years of age. Moreover, to uncover the critical windows of exposure susceptibility during pregnancy, generalized estimating equation (GEE) models were used to assess trimester-specific associations of target analyte concentrations with children’s MDI/PDI scores.55 Considering that pesticides may have sex-specific effects, we performed sex-stratified analyses to obtain the effect estimates () and 95% confidence intervals (95% CI), and the corresponding -values. In addition, to examine the effect modification by child sex, we also introduced the interaction term () into to the models (whole population) to derive interaction -values (defined as ). The false discovery rate (FDR) corrections were performed on -values () to account for multiple comparisons.56
Weighted quantile sum (WQS) regression (WQSR) was conducted to identify the major contributors to the association.57 WQSR analysis is an approach that can be used in environmental epidemiology to evaluate associations between potentially highly correlated coexposures and a health outcome, thereby reducing dimensionality and avoiding multicollinearity.58 The WQSR coefficient can be interpreted as the mixture effect of the insecticide mixture exposure on the outcome. The average weight for each biomarker, expressed as the percentage of that sum to one, was estimated from 1,000 bootstraps. Based on the results of MLR and GEE models that suggested the susceptible population and windows of insecticide exposure for adverse effects on neurodevelopment, WQSR analysis was further conducted in the negative direction of the outcomes to identify which biomarker contributed more to the adverse mixture effect. To evaluate the robustness of our estimates, we applied repeated holdout validation in WQSR models.59 Specifically, the full data set was randomly split into 40%/60% training–testing sets and the WQSR analysis was repeated 100 times to examine the distribution of effect estimates and associated weights.59
Covariates were considered as potential confounders if they altered the estimate of the association by 10% or more by a step-wise backward elimination approach,60 including maternal age, PBMI, maternal education level, paternal education level, parity, delivery mode, passive smoking during pregnancy, child sex, and breastfeeding duration (Excel Table S1). In addition, folic acid supplementation during pregnancy61 and season of sample collection42 were also included, considering that these variables have been reported to be significantly associated with child neurodevelopment and insecticide exposure, respectively. Among those covariates above, a multicollinearity between maternal education level and paternal education level was observed in the regression model [], given that VIF values indicate serious multicollinearity. Thus, only maternal education level was included as a covariate in the final models because its adjustment showed a larger impact on the estimates for the associations between some insecticide biomarkers (i.e., DMTP, TCPy, PNP, 3-PBA, trans-DCCA, IMI, IMI-olefin, THM, and DM-CLO) and Bayley scores than adjustment for paternal education level (Excel Table S1). The final models were adjusted for maternal education (categorical: , bachelor’s degree, or ), maternal age (categorical: , 25–29, 30–34, or ), PBMI (categorical: , 18.5–23.9, or ), folic acid supplementation during pregnancy (categorical: yes or no), passive smoking during pregnancy (categorical: yes or no), delivery mode (categorical: vaginal delivery or cesarean delivery), child sex (categorical: boys or girls), parity (categorical: nulliparous or multiparous), breastfeeding duration (categorical: or ), and season of sample collection [categorical: warm season or cold season (except for the average across all trimesters)]. In the present analyses, only breastfeeding duration had missing data (2.9% of values), which were imputed by randomly selecting a value from participants with nonmissing values11 to preserve the size of the study population.
In addition, to investigate whether adverse perinatal factors affected the reliability of the results, we performed three sensitivity analyses based on MLR models to test the robustness of the associations with
Additional adjustment for more covariates—which were related but altered the estimate of the association by , including household income (categorical: , 50,000–100,000, or ), nutritional status during pregnancy [assessed by maternal anemia (categorical: yes or no)], pregnancy weight gain (categorical: inadequate total GWG, adequate total GWG, or excessive total GWG), birth weight (categorical: , 2,500–4,000, or ), and paternal education level (categorical: , bachelor’s degree, or )—to examine the influence of these factors potentially on the associations
Excluding women with gestational diabetes or gestational hypertension
Excluding children with preterm birth (gestation age ) or low birth weight ().
All statistical analyses were conducted using SAS (version 9.4; SAS Institute, Inc.) or the gWQS package of R (version 4.0.4; R Development Core Team). The threshold for statistical significance was set at (two-tailed) or .
Results
Participant Characteristics
As shown in Table 1, the average maternal age at delivery was . Of the 1,041 mothers, the majority were nulliparous (77.4%, ), well-educated (79.4% had a bachelor’s degree or higher, ), had a normal PBMI (; 66.7%, ), had no anemia (96.0%, ), and reported folic acid supplementation (87.6%, ). Approximately 51.3% () of the mothers delivered by cesarean delivery, 42.9% () had excessive total GWG, and 25.1% () reported passive smoking during pregnancy. Among their children, 53.1% () were boys, 56.7% () were breastfed for , and 91.8% () had a normal birth weight (). The average () gestational age at delivery was . Of the 3,123 urine samples, 49.7% () were collected in the warm season (from May to October).
Table 1.
Descriptive characteristics of study participants and child neurodevelopment measures (Bayley scores at 2 years of age) from a birth cohort study in Wuhan, China, 2014–2017 ( mother–child pairs).
| Characteristic | (%) or | MDI scores | PDI scores | ||
|---|---|---|---|---|---|
| -Value | -Value | ||||
| Total | 1,041 | — | — | ||
| Maternal characteristics | |||||
| Age at delivery (y) | — | 0.24 | — | 0.25 | |
| 60 (5.80) | — | — | |||
| 25–29 | 548 (52.6) | — | — | ||
| 30–34 | 330 (31.7) | — | — | ||
| 103 (9.90) | — | — | |||
| PBMI () | — | 0.14 | — | 0.90 | |
| 195 (18.7) | — | — | |||
| 18.5–23.9 | 694 (66.7) | — | — | ||
| 152 (14.6) | — | — | |||
| Maternal education level | — | — | — | 0.36 | |
| 214 (20.6) | — | — | |||
| Bachelor’s degree | 763 (73.3) | — | — | ||
| 64 (6.1) | — | — | |||
| Household income (CNY) | — | — | 0.07 | — | 0.20 |
| 120 (11.5) | — | — | |||
| 50,000–100,000 | 384 (36.9) | — | — | ||
| 537 (51.6) | — | — | |||
| Parity | — | — | 0.007 | — | 0.05 |
| Nulliparous | 806 (77.4) | — | — | ||
| Multiparous | 235 (22.6) | — | — | ||
| Delivery mode | — | — | — | 0.007 | |
| Vaginal delivery | 507 (48.7) | — | — | ||
| Cesarean delivery | 534 (51.3) | — | — | ||
| GWG categories according to NHC | — | — | 0.88 | — | 0.77 |
| Inadequate total GWG | 165 (15.9) | — | — | ||
| Adequate total GWG | 429 (41.2) | — | — | ||
| Excessive total GWG | 447 (42.9) | — | — | ||
| Passive smoking during pregnancy | — | — | 0.37 | — | 0.88 |
| Yes | 261 (25.1) | — | — | ||
| No | 780 (74.9) | — | — | ||
| Folic acid supplementation during pregnancy | — | — | 0.51 | — | 0.97 |
| Yes | 912 (87.6) | — | — | ||
| No | 129 (12.4) | — | — | ||
| Maternal anemia | — | — | 0.71 | — | 0.65 |
| Yes | 42 (4.0) | — | — | ||
| No | 999 (96.0) | — | — | ||
| Hypertension in pregnancy | — | — | 0.83 | — | 0.28 |
| Yes | 24 (2.30) | — | — | ||
| No | 1,017 (97.7) | — | — | ||
| Gestational diabetes | — | — | 0.35 | — | 0.70 |
| Yes | 98 (9.40) | — | — | ||
| No | 943 (90.6) | — | — | ||
| Sampling season | |||||
| First trimester | — | — | 0.32 | — | 0.003 |
| Warm season | 523 (50.2) | — | — | ||
| Cold season | 518 (49.8) | — | — | ||
| Second trimester | — | — | 0.41 | — | 0.69 |
| Warm season | 499 (47.9) | — | — | ||
| Cold season | 542 (52.1) | — | — | ||
| Third trimester | — | — | 0.50 | — | 0.001 |
| Warm season | 530 (50.9) | — | — | ||
| Cold season | 511 (49.1) | — | — | ||
| Paternal education level | — | — | 0.004 | — | 0.49 |
| 209 (20.1) | — | — | |||
| Bachelor’s degree | 783 (75.2) | — | — | ||
| 49 (4.70) | — | — | |||
| Child characteristics | |||||
| Child’s sex | — | — | — | 0.11 | |
| Boy | 553 (53.1) | — | — | ||
| Girl | 488 (46.9) | — | — | ||
| Birth weight (g) | — | 0.23 | — | 0.38 | |
| (low birth weight) | 21 (2.02) | — | — | ||
| 2,500–4,000 | 956 (91.8) | — | — | ||
| 64 (6.15) | — | — | |||
| Gestational age (wk) | — | 0.62 | — | 0.39 | |
| (preterm birth) | 28 (2.70) | — | — | ||
| 1,013 (97.3) | — | — | |||
| Breastfeeding duration (months) | — | — | 0.04 | — | 0.41 |
| 437 (43.2) | — | — | |||
| 574 (56.7) | — | — | |||
| Missing | 30 | — | — | — | — |
Note: Values are or numbers (percentage). —, Not applicable; %, percentage; cold season, November–April; GWG, gestational weight gain; MDI, mental development index; NHC, National Health Commission of the People’s Republic of China; PBMI, prepregnancy body mass index; PDI, psychomotor development index; SD, standard deviation; SD, standard deviation; warm season, May–October. *-Values were derived from the analysis of variance performed to compare MDI and PDI among different characteristic categories. -Values of were considered statistically significant.
The mean BSID-CR MDI and PDI scores () were respectively and for the children (Table 1). Higher MDI scores were observed among children who were breastfed for months () or whose mothers were nulliparous (), had a vaginal delivery (), or had a master’s degree (). Girls’ MDI scores were significantly higher than boys’ MDI scores ( vs. 107, ). Lower PDI scores were observed among children whose mothers’ urine samples of the 1st () collected in the warm season (vs. cold season) and those of the 3rd trimester () collected in the cold season (vs. warm season). Distribution of Bayley scores in this study is shown in Table 2, and the Bayley scores () of participating children according to the averaged urinary quartile concentrations of insecticide biomarkers throughout pregnancy are shown in Table S6.
Table 2.
Distribution of Bayley Scales for Infant Development–Chinese revision (BSID-CR) scores (points) among child participants at 2 years of age from a birth cohort study in Wuhan, China ( participants).
| Development index | 25th | 50th | 75th | 95th | Minimum–maximum | |
|---|---|---|---|---|---|---|
| MDI | 98 | 114 | 126 | 138 | 50–150 | |
| PDI | 99 | 111 | 123 | 140 | 51–150 |
Note: 25th, 50th, 75th, 95th mean the 25th, 50th, 75th, and 95th percentile scores, respectively. MDI, mental development index; PDI, psychomotor development index; SD, standard deviation.
Levels of Maternal Urinary mOPPs, mPYRs, and mNNIs
As shown in Table 3, most insecticide metabolites were detected in of the maternal urine samples, except for IMI (DFs: 72.1%–74.5%) and DN-IMI (DFs: 82.0%–84.2%). Most mOPPs (range of their median SG-adjusted concentrations: ) had higher concentrations than mPYRs () and mNNIs ().
Table 3.
Distribution of maternal urinary concentrations (ng/mL) of mOPPs, mPYRs, and mNNIs in participants from a birth cohort study in Wuhan, China, 2014–2017 ( participants, samples for each trimester).
| Compounds () | MDL () | Average trimester () | First trimester | Second trimester | Third trimester | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GM | Median (25th–75th) | DF [percentage (%)] | GM | Median (25th–75th) | DF [percentage (%)] | GM | Median (25th–75th) | DF [percentage (%)] | GM | Median (25th–75th) | ||
| mOPPs | ||||||||||||
| DEP | ||||||||||||
| Unadjusted | 0.25 | 4.07 | 3.98 (2.53–6.32) | 100 | 3.80 | 3.75 (1.99–6.80) | 100 | 3.13 | 3.23 (1.63–5.63) | 100 | 2.85 | 2.70 (1.48–5.59) |
| SG-adjusted | — | 4.16 | 4.01 (2.63–6.20) | — | 4.08 | 3.88 (2.30–6.54) | — | 3.57 | 3.47 (2.01–6.11) | — | 3.48 | 3.27 (2.04–5.96) |
| DMP | ||||||||||||
| Unadjusted | 0.25 | 4.97 | 4.79 (2.97–7.78) | 100 | 4.83 | 4.74 (2.45–9.55) | 100 | 3.41 | 3.26 (1.72–6.68) | 100 | 3.12 | 2.99 (1.57–5.84) |
| SG-adjusted | — | 5.07 | 4.78 (3.24–7.61) | — | 5.18 | 4.91 (2.87–8.86) | — | 3.89 | 3.57 (2.10–6.93) | — | 3.82 | 3.79 (2.16–6.83) |
| DETP | ||||||||||||
| Unadjusted | 0.05 | 1.90 | 1.88 (1.11–3.29) | 99.9 | 1.79 | 1.72 (0.85–3.68) | 99.9 | 1.27 | 1.23 (0.64–2.60) | 99.8 | 1.20 | 1.18 (0.55–2.54) |
| SG-adjusted | — | 1.98 | 1.90 (1.16–3.14) | — | 2.08 | 2.03 (1.09–3.69) | — | 1.33 | 1.28 (0.66–2.54) | — | 1.23 | 1.21 (0.63–2.41) |
| DMTP | ||||||||||||
| Unadjusted | 0.25 | 0.63 | 0.57 (0.34–1.07) | 95.6 | 0.53 | 0.46 (0.26–1.00) | 93.9 | 0.41 | 0.38 (0.25–0.81) | 94.7 | 0.42 | 0.38 (0.25–0.76) |
| SG-adjusted | — | 0.66 | 0.64 (0.37–1.09) | — | 0.56 | 0.53 (0.28–1.03) | — | 0.46 | 0.42 (0.25–0.96) | — | 0.52 | 0.50 (0.26–0.95) |
| TCPy | ||||||||||||
| Unadjusted | 0.10 | 1.86 | 1.87 (1.29–2.67) | 99.3 | 1.81 | 1.90 (1.04–3.22) | 98.4 | 1.46 | 1.57 (0.89–2.62) | 95.5 | 1.20 | 1.29 (0.73–2.26) |
| SG-adjusted | — | 1.93 | 1.92 (1.31–2.66) | — | 1.94 | 1.89 (1.21–3.14) | — | 1.67 | 1.70 (1.06–2.65) | — | 1.46 | 1.54 (0.95–2.49) |
| PNP | ||||||||||||
| Unadjusted | 0.25 | 2.30 | 2.35 (1.53–3.49) | 99.7 | 2.05 | 2.12 (1.15–3.94) | 99.5 | 1.73 | 1.79 (0.95–3.14) | 100 | 1.79 | 1.71 (0.95–3.30) |
| SG-adjusted | — | 2.36 | 2.34 (1.65–3.40) | — | 2.20 | 2.20 (1.32–3.76) | — | 1.98 | 2.02 (1.17–3.19) | — | 2.19 | 2.18 (1.33–3.66) |
| mPYRs | ||||||||||||
| 3-PBA | ||||||||||||
| Unadjusted | 0.01 | 0.20 | 0.19 (0.11–0.38) | 98.0 | 0.17 | 0.17 (0.08–0.36) | 94.7 | 0.13 | 0.12 (0.05–0.30) | 91.6 | 0.11 | 0.10 (0.05–0.27) |
| SG-adjusted | — | 0.21 | 0.19 (0.12–0.35) | — | 0.19 | 0.17 (0.09–0.34) | — | 0.15 | 0.13 (0.07–0.27) | — | 0.14 | 0.13 (0.06–0.28) |
| trans-DCCA | ||||||||||||
| Unadjusted | 0.02 | 0.25 | 0.24 (0.14–0.43) | 95.6 | 0.21 | 0.21 (0.09–0.45) | 92.9 | 0.14 | 0.15 (0.05–0.34) | 93.0 | 0.14 | 0.12 (0.06–0.31) |
| SG-adjusted | — | 0.25 | 0.23 (0.14–0.42) | — | 0.24 | 0.23 (0.12–0.48) | — | 0.15 | 0.14 (0.07–0.31) | — | 0.14 | 0.13 (0.07–0.28) |
| mNNIs | ||||||||||||
| IMI | ||||||||||||
| Unadjusted | 0.025 | 0.05 | 0.05 (0.03–0.09) | 72.1 | 0.04 | 0.03 () | 74.5 | 0.03 | 0.03 () | 73.8 | 0.03 | 0.03 () |
| SG-adjusted | — | 0.05 | 0.05 (0.03–0.10) | — | 0.04 | 0.04 () | — | 0.04 | 0.03 () | — | 0.04 | 0.03 () |
| IMI-olefin | ||||||||||||
| Unadjusted | 0.025 | 0.57 | 0.53 (0.31–1.01) | 100 | 0.46 | 0.43 (0.21–0.93) | 100 | 0.38 | 0.35 (0.18–0.79) | 100 | 0.37 | 0.33 (0.16–0.83) |
| SG-adjusted | — | 0.60 | 0.55 (0.34–1.01) | — | 0.53 | 0.47 (0.25–1.05) | — | 0.40 | 0.36 (0.19–0.77) | — | 0.38 | 0.33 (0.16–0.79) |
| 5-hydroxy-IMI | ||||||||||||
| Unadjusted | 0.05 | 0.97 | 0.93 (0.48–1.79) | 99.9 | 0.79 | 0.73 (0.35–1.82) | 99.8 | 0.57 | 0.54 (0.25–1.23) | 100 | 0.55 | 0.52 (0.23–1.23) |
| SG-adjusted | — | 1.00 | 0.92 (0.54–1.73) | — | 0.92 | 0.81 (0.43–1.92) | — | 0.60 | 0.56 (0.28–1.23) | — | 0.56 | 0.50 (0.24–1.18) |
| DN-IMI | ||||||||||||
| Unadjusted | 0.025 | 0.13 | 0.11 (0.06–0.22) | 84.2 | 0.11 | 0.09 (0.04–0.21) | 82.0 | 0.08 | 0.06 (0.04–0.13) | 84.0 | 0.09 | 0.07 (0.04–0.17) |
| SG-adjusted | — | 0.14 | 0.12 (0.07–0.22) | — | 0.12 | 0.11 (0.06–0.23) | — | 0.08 | 0.07 (0.04–0.15) | — | 0.09 | 0.08 (0.04–0.16) |
| DM-ACE | ||||||||||||
| Unadjusted | 0.01 | 1.17 | 1.13 (0.70–1.94) | 99.9 | 0.96 | 0.93 (0.47–1.93) | 99.9 | 0.80 | 0.79 (0.39–1.64) | 100 | 0.81 | 0.75 (0.37–1.62) |
| SG-adjusted | — | 1.25 | 1.20 (0.72–2.01) | — | 1.11 | 1.07 (0.54–2.18) | — | 0.84 | 0.83 (0.41–1.69) | — | 0.83 | 0.77 (0.39–1.55) |
| THM | ||||||||||||
| Unadjusted | 0.025 | 0.12 | 0.11 (0.06–0.23) | 95.4 | 0.08 | 0.06 (0.03–0.18) | 96.3 | 0.08 | 0.07 (0.03–0.18) | 95.8 | 0.08 | 0.07 (0.03–0.17) |
| SG-adjusted | — | 0.13 | 0.12 (0.06–0.23) | — | 0.09 | 0.08 (0.03–0.21) | — | 0.08 | 0.07 (0.03–0.17) | — | 0.08 | 0.07 (0.03–0.17) |
| CLO | ||||||||||||
| Unadjusted | 0.05 | 0.16 | 0.15 (0.09–0.27) | 94.0 | 0.12 | 0.11 (0.05–0.23) | 91.8 | 0.10 | 0.09 (0.05–0.20) | 92.9 | 0.11 | 0.10 (0.05–0.23) |
| SG-adjusted | — | 0.17 | 0.15 (0.10–0.28) | — | 0.14 | 0.13 (0.07–0.27) | — | 0.11 | 0.10 (0.05–0.20) | — | 0.11 | 0.10 (0.05–0.21) |
| DM-CLO | ||||||||||||
| Unadjusted | 0.01 | 0.28 | 0.34 (0.17–0.58) | 94.2 | 0.24 | 0.32 (0.14–0.59) | 88.8 | 0.16 | 0.23 (0.07–0.42) | 94.4 | 0.21 | 0.27 (0.12–0.48) |
| SG-adjusted | — | 0.30 | 0.36 (0.18–0.63) | — | 0.28 | 0.38 (0.17–0.67) | — | 0.17 | 0.23 (0.09–0.46) | — | 0.22 | 0.27 (0.11–0.53) |
Note: Other target analytes (including DEDTP, DMDTP, 4F-3-PBA, ACE) were not listed here because they were not found in the samples or the detection frequency was . 25th and 75th means the 25th and 75th percentile concentrations. —, Not applicable; 3-PBA, 3-phenoxybenzoic acid; 4F-3-PBA, 4-fluoro-3-phenoxybenzoic acid; 5-hydroxy-IMI, 5-Hydroxy-imidacloprid; ACE, acetamiprid; CLO, clothianidin; DEDTP, diethyl dithiophosphate; DEP, diethyl phosphate; DETP, diethyl thiophosphate; DF, detection frequency; DM-ACE, desmethyl-acetamiprid; DM-CLO, desmethyl-clothianidin; DMDTP, dimethyl dithiophosphate; DMP, dimethyl phosphate; DMTP, dimethyl thiophosphate; DN-IMI, desnitro-imidacloprid; GM, geometric mean; IMI, imidacloprid; IMI-olefin, imidacloprid-olefin; MDL, method detection limit; mNNIs, metabolites of neonicotinoid insecticides; mOPPs, metabolites of organophosphate insecticides; mPYRs, metabolites of pyrethroids; PNP, para-nitrophenol; SG, urinary specific gravity; TCPy, 3,5,6-trichloro-2-pyridinol; THM, thiamethoxam; trans-DCCA, trans-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylic acid.
Moderate () to strong correlations ( for IMI-olefin and 5-hydroxy-IMI) were observed among the metabolites of the same class insecticides based on the averaged concentrations of three trimesters (Figure S1 and Excel Table S2), and similar Spearman’s correlation coefficients were observed for trimester-specific concentrations (Figures S2–S4 and Excel Tables S3–S5). The correlations between metabolites of different classes are weak ().
Single Chemical Analyses of the Associations between Individual Urinary Biomarkers and Children’s MDI/PDI Scores
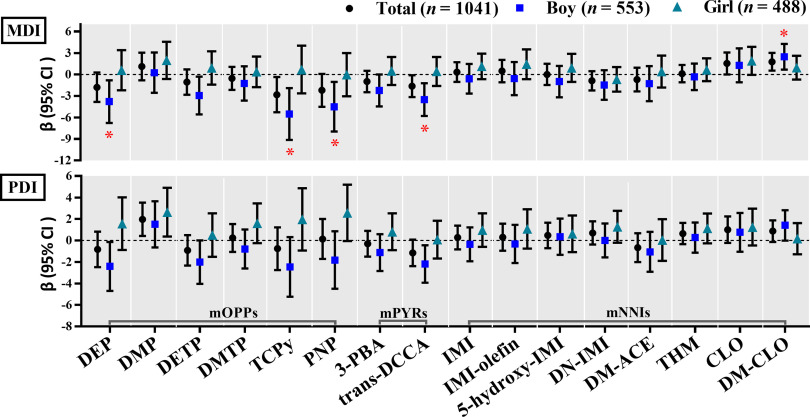
MLR models using the averaged concentrations during pregnancy showed that significant associations with low Bayley scores were observed only among boys (Figure 1; Table S7). Specifically, each 1-unit increase in ln-transformed averaged urinary concentrations of DEP, DETP, TCPy, PNP, and trans-DCCA during pregnancy was significantly associated with a decrease of 3.79 points (95% CI: , ), 2.94 points (95% CI: , ), 5.51 points (95% CI: , ), 4.50 points (95% CI: , ), and 3.50 points (95% CI: , ) respectively in boys’ MDI scores. After multiple testing correction, the associations of DEP (), TCPy (), PNP (), and trans-DCCA () with lower boys’ MDI scores remained significant (Table S7). The interactions between child sex and DEP (), DETP (), TCPy (), PNP (), and trans-DCCA () on Bayley scores were found to be significant (Table S7).
Figure 1.
Adjusted associations between the averaged concentrations (ng/mL, ln-transformed SG-adjusted) of maternal urinary mOPPs, mPYRs, and mNNIs over the three trimesters and children’s MDI and PDI scores using multivariate linear regression (MLR) models for participants from a birth cohort study in Wuhan, China, 2014–2017 ( participants, samples). The models were adjusted for maternal age (categorical), PBMI (categorical), maternal education (categorical), passive smoking (categorical), folic acid supplementation during pregnancy (categorical), parity (categorical), child sex (categorical), breastfeeding duration (categorical), and delivery mode (categorical). Sex-stratified analyses were adjusted for all the abovementioned confounders except child sex. * were considered statistically significant (FDR corrections were performed to adjust for multiple comparisons). The numerical values are listed in Table S7. Note: 3-PBA, 3-phenoxybenzoic acid; 5-hydroxy-IMI, 5-hydroxy-imidacloprid; CLO, clothianidin; DEP, diethyl phthalate; DETP, diethyl thiophosphate; DM-ACE, desmethyl-acetamiprid; DM-CLO, desmethyl-clothianidin; DMP, dimethyl phosphate; DMTP, dimethylthiophosphate; DN-IMI, desnitro-imidacloprid; IMI, imidacloprid; IMI-olefin, imidacloprid-olefin; MDI, mental development index; mNNIs, metabolites of neonicotinoid insecticides; mOPPs, metabolites of organophosphate insecticides; mPYRs, metabolites of pyrethroids; PBMI, prepregnancy body mass index; PDI, psychomotor development index; PNP, 4-nitrophenol; TCPy, 3,5,6-trichloro-2-pyridinol; THM, thiamethoxam; trans-DCCA, trans-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylic acid.
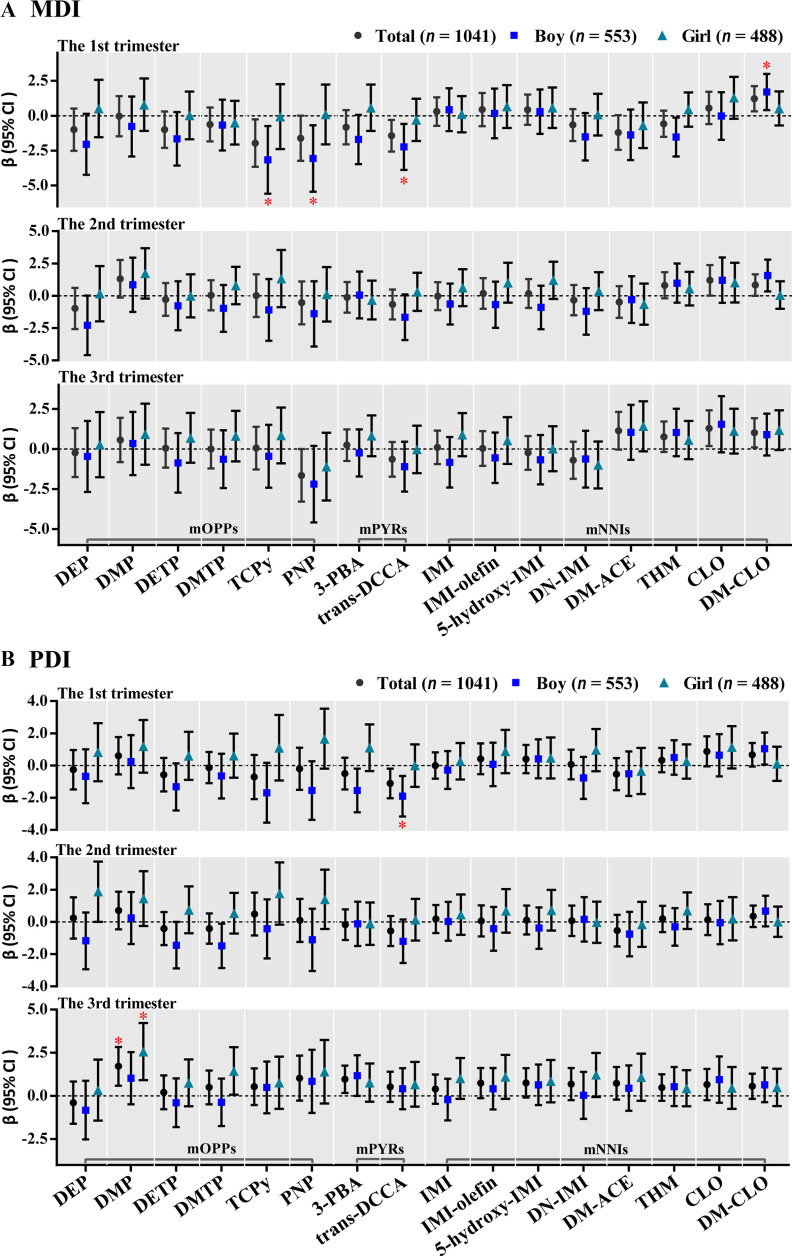
In trimester-specific analyses based on GEE models, only the insecticide metabolites at the first trimester had significantly inverse associations with children’s MDI/PDI scores (Figure 2; Table S8). Among all the children, higher urinary concentrations of TCPy and trans-DCCA of the first trimester were significantly associated with lower MDI or both MDI and PDI. After stratification by child sex, significantly inverse associations were found only in boys (at the first trimester). Specifically, each 1-unit increase in ln-transformed maternal urinary concentrations of two mOPPs (TCPy and PNP), one mPYR (trans-DCCA), and one mNNI (THM) was significantly associated with a decrease of 3.16 points (95% CI: , ), 3.06 points (95% CI: , ), 2.24 points (95% CI: , ), and 1.53 points (95% CI: , ) respectively in boys’ MDI scores; each 1-unit increase in ln-transformed maternal urinary concentrations of two mPYRs (3-PBA and trans-DCCA) was significantly associated with a decrease of 1.55 points (95% CI: , ) and 1.90 points (95% CI: , ) respectively in boys’ PDI scores. After multiple testing correction, the associations of TCPy (), PNP (), and trans-DCCA () at the first trimester with lower MDI scores of boys remained significant (Table S8). At the first trimester, the interactions between child sex and PNP () and trans-DCCA () on Bayley scores were found to be significant (Table S8). No significantly inverse associations were observed at the second or third trimester after multiple testing correction (Table S8).
Figure 2.
Adjusted associations between trimester-specific concentrations (ng/mL, ln-transformed SG-adjusted) of maternal urinary mOPPs, mPYRs, and mNNIs over the three trimesters and children’s (A) MDI and (B) PDI scores using generalized estimating equations (GEE) models for participants from a birth cohort study in Wuhan, China, 2014–2017 ( participants, samples for each specific trimester). The models were adjusted for maternal age (categorical), PBMI (categorical), maternal education (categorical), passive smoking (categorical), folic acid supplementation during pregnancy (categorical), parity (categorical), child sex (categorical), breastfeeding duration (categorical), delivery mode (categorical), and sampling seasons (categorical). Sex-stratified analyses were adjusted for all the abovementioned confounders except child sex. * were considered statistically significant (FDR corrections were performed to adjust for multiple comparisons). Details of chemical abbreviations are provided in Figure 1, and the numerical values are listed in Table S8. Note: FDR, false discovery rate; MDI, mental development index; mNNIs, metabolites of neonicotinoid insecticides; mOPPs, metabolites of organophosphate insecticides; mPYRs, metabolites of pyrethroids; PBMI, prepregnancy body mass index; PDI, psychomotor development index; , -value for false discovery rate; SG, specific gravity.
In the sensitivity analyses that a) additionally adjusted for more covariates, b) excluded women with gestational diabetes or gestational hypertension, and c) excluded children with preterm birth or low birth weight in MLR models, most significant associations between insecticide biomarkers (especially for DEP, TCPy, PNP, trans-DCCA, and DM-CLO) and Bayley scores remained (Excel Tables S6–S8). Although the significant positive associations between CLO and Bayley scores among all the children disappeared in the three sensitivity analyses (Excel Tables S6–S8), some significantly positive associations of maternal urinary biomarker concentrations with Bayley scores were observed (Figures 1–2; Tables S7–S8). Specifically, after multiple testing correction, higher averaged and trimester-specific concentrations of DM-CLO were significantly associated with higher MDI scores among the boys (Tables S7–S8), and higher concentration of DMP in the third trimester was also significantly associated with higher PDI scores among the girls (Table S8).
WQSR Analysis for the Mixture Effect
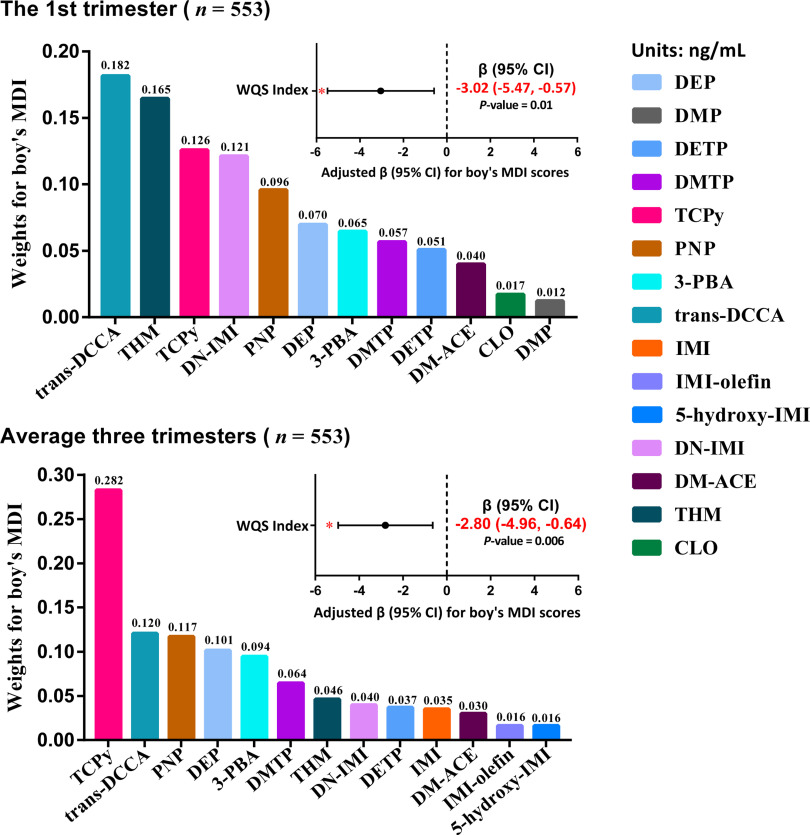
Based on the results of MLR and GEE models, boys might be more susceptible to insecticide exposure than girls, and the first trimester appeared to be a critical window of the susceptibility to insecticide exposures. For such findings with statistical significance, the WQSR analyses were further performed in the negative direction to evaluate the mixture effect of exposure to the selected insecticides on the Bayley scores. At the first trimester, a significant association was observed between a 1-quartile increase in the WQS index and lower MDI scores in boys ( points; 95% CI: , ), and trans-DCCA (18.2%) contributed the most to the mixture effect, followed by THM (16.5%), TCPy (12.6%), DN-IMI (12.1%), PNP (9.6%), DEP (7.0%), and 3-PBA (6.5%) (Figure 3; Table S9). For the averaged concentrations of the three trimesters, the WQS index was also associated with lower boys’ MDI scores ( points; 95% CI: , ), and TCPy made the largest contribution to the association (28.2%), followed by trans-DCCA (12.0%), PNP (11.7%), DEP (10.1%), and 3-PBA (9.4%). We also analyzed the association between the insecticide biomarker mixture and boy’s PDI scores, and found no statistical significance (Table S10).
Figure 3.
Associations between the maternal urinary biomarker mixture and boy’s MDI scores assessed with repeated holdout random subset WQSR in the negative direction, based on the concentrations (ng/mL) in the first trimester ( participants, samples) and the averaged concentrations (ng/mL) over the three trimesters ( participants, samples) for participants from a birth cohort study in Wuhan, China, 2014–2017. The models were adjusted for maternal age (categorical), PBMI (categorical), maternal education (categorical), passive smoking (categorical), folic acid supplementation during pregnancy (categorical), parity (categorical), breastfeeding duration (categorical), delivery mode (categorical), and sampling seasons (except for average concentrations). * were considered statistically significant. Details of chemical abbreviations are provided in Figure 1, and the numerical values are listed in Table S9. Note: CI, confidence interval; MDI, mental development index; PBMI, prepregnancy body mass index; WQSR, weighted quantile sum regression.
Discussion
This study investigated the associations of maternal coexposure to OPPs, PYRs, and NNIs during pregnancy with child neurodevelopment. We found that a) the maternal urinary concentrations of some mOPPs, mPYRs, and mNNIs were significantly associated with lower neurodevelopment scores in children; b) the inverse associations of insecticide concentrations measured in the first trimester appeared to be more pronounced than the associations with second and third trimester measurements; c) the associations in boys were larger than those in girls; and d) trans-DCCA and TCPy were the major biomarkers associated with the mixture effect of the insecticide mixture exposure.
In addition, we compared biomarker concentrations in this study with those in other studies that investigated the associations of prenatal exposure to OPPs and PYRs with child neurodevelopment (Table S11). Maternal urinary biomarker concentrations of OPPs and PYRs observed in our study were generally within the same order of magnitude with those reported previously (Table S11). We also compared them with the data of women ( of age) from NHANES in 2013–201453 or in 2017–201854 and found that pregnant women in this study had higher median concentrations of TCPy (1.29–1.90 vs. ), PNP (1.71–2.12 vs. ), DEP (2.70–3.75 vs. ), DETP (1.18–1.72 vs. ), and DMP (2.99–4.74 vs. ) but lower median concentrations of 3-PBA (0.10–0.17 vs. ) and DMTP ( vs. ) compared with women from the United States (Table S11). So far, few studies have reported the associations between biomarkers of NNI exposure during pregnancy and child neurodevelopment. Nevertheless, a few studies provided data on mNNIs in pregnant women; for example, Anai et al.62 observed that the urinary median concentrations of THM ( creatinine; LOD: ) and CLO ( creatinine; LOD: ) in pregnant women from Japan were higher than those in this study (CLO: ; THM: ).
The results from this study suggest that, compared with the second or third trimester, the first trimester may be a more sensitive window of exposure to the insecticides in association with Bayley scores among boys. The first trimester is the most critical period of fetal brain development,63 and this may explain what we observed in this study. van den Dries et al.64 also found that early pregnancy was the sensitive window of phthalate exposure in association with neurodevelopment in the Generation R study. Nevertheless, very few studies have examined the sensitive window for the relationship between prenatal insecticide exposure and Bayley scores (Table S11). Qi et al.65 found an association of 3-PBA (trans-DCCA was not measured) at the second trimester (vs. the first and third trimesters) with decreased Bayley-III scores of infants at 1 year of age (). However, Jusko et al.22 found an association of elevated DAPs at the third trimester (vs. the first and second trimesters) with lower child intelligence quotient (IQ) at 7 years of age () in the Generation R study. This inconsistency may be attributed to differences in biomarker selection, sample size, exposure level, neurodevelopment outcome assessed, child age of neurodevelopment assessment, and exposure profiles of insecticides. Our findings of trimester-specific analysis may provide clues for future studies and health risk interventions related to pesticide exposure.
The Bayley PDI or MDI scores of the boys were slightly lower than the girls at the same age in this study (Table 1), which was generally consistent with a very recent study in Shanghai, China.66 We further observed stronger associations between the higher concentrations of the insecticide metabolites and lower MDI scores for the boys. Such associations were not significant in the girls, possibly owing to the (well-documented) resilience of the female brain to neurotoxic injury caused by contaminants.67 Estrogen itself is neuroprotective,67 and estrogen-mediated receptors can activate multiple molecular neuroprotective and neuro-reparative responses.67,68 Interference with development of sex-dimorphic neuroendocrine pathways (e.g., sex-specific expression level of estrogen receptors in the brain),68,69 neuroepigenetics,70 thyroid hormone homeostasis,71,72 neuropeptide and neurotransmitter signaling,73,74 and neuroinflammation34 have been suggested to be responsible for such sex-specific neurotoxic effects of environmental chemicals like OPPs. Several previous epidemiological studies have also found similar sex-specific associations of prenatal exposure to OPPs (based on biomonitoring data of urinary DAPs13,21,23,75 or chlorpyrifos in cord blood76) with adverse neurodevelopment in boys. Such sex-specific findings for prenatal exposure to PYRs and NNIs have not been reported in human beings by others yet, and further confirmation is needed. Nevertheless, the sex-specific effects of PYRs and NNIs on neurotoxicity have been characterized in animal models. For instance, prenatal exposure of mice to deltamethrin () induced behavioral abnormalities only in male offspring,77 and higher striatal dopamine transporter protein levels and D1 dopamine receptor levels in male offspring may be responsible for the differed neurodevelopment changes.77 In another animal study, conducted by Kubo et al.,78 administration of CLO at the chronic no observed adverse effect level (NOAEL) dose () reduced locomotor activities and elevated anxiety-like behaviors more in male mice than in females. They suggested that male-dominant changes in neuroactivities in several brain regions (including the paraventricular thalamic nucleus and the dentate gyrus) could explain the sex-related differences in neurotoxicity induced by CLO.78
Single chemical analyses in this study showed the significant associations of higher maternal urinary concentrations of certain mOPPs (i.e., DEP, TCPy, and PNP) and mPYRs (i.e., trans-DCCA) with lower MDI scores. For each 1-unit increase in ln-transformed concentrations of these metabolites, the Bayley scores decreased by 2–4 points. In previous studies that explored the associations between pesticide exposure during pregnancy and Bayley scores in children (Table S11), some studies also found that prenatal exposure to OPPs (e.g., assessed using the averaged urinary concentrations of TCPy and DAPs at the first and second trimesters,11 urinary DAPs at the third trimester,20,79 or chlorpyrifos in cord blood19,80) or PYRs (assessed using urinary mPYRs prior to delivery,81 at the first and second trimesters,65 or at the third trimester82) were associated with poorer Bayley scores of children (2–36 months of age). In these studies, for each 1-unit,65,82 an interquartile range,79 or 10-fold increase11,20,81 in urinary insecticide biomarkers during pregnancy, the children’s Bayley scores decreased by 0.03–4.42 points. A couple of studies categorized the exposure levels into high and low, and found higher chlorpyrifos exposure () produced an -point decrease in Bayley scores compared with those with lower exposure ()19,80 (Table S11). However, Donauer et al.83 (using maternal urinary DAPs at gestational weeks 16 and 26) and Watkins et al.25 (using maternal urinary 3-PBA at the third trimester) did not find such associations.
In addition to Bayley scores, some studies (Table S11) have shown that prenatal exposure to OPPs or PYRs or both of them were associated with other neurodevelopment outcomes of children [e.g., developmental quotients,13,21,76 IQ scores,22,23,84,85 neurobehavioral development of neonates,86 traits related to ASD,87,88 traits related to attention deficit hyperactivity disorder,31,89 decreased brain activity90]. However, other studies did not find such associations of childhood outcomes with prenatal exposure to OPPs,91–93 PYRs,24,94,95 or both.26 Differences in sample size, time point of sample collection, biomarkers selected, child age at examination, exposure levels, exposure characteristics, and individual susceptibility might partly account for the inconsistency across these studies.
We also examined the association of maternal urinary concentrations of PNP and mNNIs during pregnancy with child neurodevelopment. Urinary PNP has been recommended as a biomarker of exposure to parathion and methyl parathion.96 Consistent with that reported by Li and Kannan,40 PNP was detected with high DF (99.5%–100%) in this study. However, parathion and methyl parathion have been banned in China since 2007 owing to their high toxicity.97 Meanwhile, PNP can be a metabolite of itself98,99 and nitrobenzene.100,101 Therefore, the concentrations of PNP do not necessarily represent OPP exposure, and exposure sources of PNP parent compounds for the general population merit further studies. In addition, PNP,99 nitrobenzene,100 parathion,102 and methyl parathion103 have all been demonstrated to have neurotoxicity, and their neurodevelopmental toxicities need further studies.
In the present study, we found some significantly positive associations of DM-CLO or DMP with Bayley scores, which might be confounded by some unmeasured protective factors such as those from the consumption of fruits and vegetables.22,104 Inconsistent with our study, three previous studies from California have identified potential relationships between prenatal residential proximity to agricultural use of neurotoxic pesticides (including IMI) and an increased risk of neural tube defects105 or lower IQ scores in 7-y-old children.17,18 In China, the general population is exposed to both traditional insecticides (e.g., OPPs and PYRs) and new emerging insecticides (e.g., NNIs) mainly through the diet, but residue concentrations differ among various foodstuffs. Specifically, CLO (the parent compound of DM-CLO) is mainly ingested through fruits and vegetables in China,29 in contrast to the traditional insecticide chlorpyrifos106 (the parent compound of TCPy, DETP, and DEP).107,108 Such comparison data were not available on dichlorvos (a major parent insecticide of DMP),108 malathion (a major parent insecticide of DMP and DMTP),108 and cypermethrin/permethrin (the major parent insecticides of trans-DCCA),82 so we had to resort to data on their registration amounts in China. According to registration information of pesticides109 in China, dichlorvos (62.8%) and malathion (57.1%) are mainly registered for use on fruits and vegetables,109 whereas cypermethrin/permethrin are mainly registered as a sanitary insecticide (e.g., for mosquito control) and for use on cotton (55.3%) rather than on fruits and vegetables (31.6%).109 Thus, the nutritional benefits of fruits and vegetables may counteract or outweigh the adverse effects of CLO/DM-CLO and dichlorvos/malathion/DMP on child neurodevelopment. On the other hand, we cannot rule out the influence of some other unmeasured confounding factors of socioeconomic status and dietary habits that may mask the adverse effects of such insecticides.26 The reason why higher DMP levels in the third trimester were associated with better PDI scores only in girls is unclear; more studies are warranted to understand whether the underlying mechanisms are related to estrogen and its receptors.
Based on the WQSR model, which can help reduce the likelihood of collinearity, model instability, and the reversal paradox,57 we found that higher insecticide mixture exposure levels were significantly related to lower MDI scores among the boys, with trans-DCCA and TCPy identified as the major biomarkers of concern. Similarly, in a mixture analysis (i.e., WQSR) of prenatal exposure to 26 endocrine-disrupting chemicals (EDCs, including TCPy and 3-PBA but not trans-DCCA) measured in the first trimester urine or blood, Tanner et al.58 observed an inverse association of EDC mixture with IQ scores of 7-y-old boys, and they identified TCPy and 3-PBA as the major biomarkers of concern. Another study observed that prenatal exposure (using averaged concentrations at , , and of gestation) to selected nonpersistent chemical mixtures [assessed by measurements of phthalate metabolites, bisphenol A, and five nonspecific mOPPs (DEP, DETP, DMP, DMTP, and DMDTP) in urine] was associated with lower nonverbal IQ in 6-y-old children,110 although they found the association was mainly driven by a phthalate mixture.
Although trans-DCCA and TCPy were detected with lower concentrations compared with other biomarkers (e.g., DMP and DEP), they were identified as the major biomarkers of concern affecting neurodevelopment. First, this finding may be attributed to the much higher neurotoxicity of their major parent insecticides (cypermethrin and chlorpyrifos, respectively) than the major parent insecticides of DMP108 (dichlorvos and malathion)111 applied in China,109,112 although available toxicological data did not contain sufficient information on the effects on the cognitive domain (learning and memory).113 For example, concerning functional alteration of the motor division of rodent nervous system, the NOAEL values of cypermethrin and chlorpyrifos [both are per body weight (BW) per day]111 were times lower than that of malathion [( per BW per day),111 which is a major parent compound of DMP and DMTP108 applied in China109]. Second, DAPs are nonspecific metabolites of OPPs,108 and the related NOAEL values differed largely among various OPPs [for example, the NOAEL value is 17 and per BW per day for malathion and dimethoate, respectively, concerning the effect of acetylcholinesterase inhibition,111 and malathion is frequently used in China but dimethoate is not,109 despite both being parent insecticides of DMP and DMTP108], thus, it would be hard to examine/interpret their effects only on the basis of the concentrations of the nonspecific DAPs (without knowing the exposure levels of the specific parent OPPs). Third, some OPPs would be transformed rapidly into DAPs after agricultural application [e.g., dichlorvos has a very short half-life (4.6–6.8 h) in beans, potato, and tomato, being transformed into DMP, methyl phosphate, and others114]; thus DAP residues (which are not considered toxic108) in fruits and vegetables may confound exposure biomonitoring and risk assessment.115 Last, DMP and DEP could also be metabolized from TMP and TEP,51 which are not insecticides but are instead organophosphate esters (another class of widespread environment contaminants),52 and TMP116 and TEP117 are neurotoxic only at high doses. This may also make it hard to interpret the effects related to DMP and DEP.
The well-known toxicity of OPPs occurs via inhibition of cholinesterase, but in recent years the detrimental effects of exposure to low-dose OPPs have been attributed to some noncholinergic mechanisms,118 such as exacerbated oxidative stress119 and disruption of thyroid hormone homeostasis.120 The neurotoxic mechanisms of cypermethrin were hypothesized to include perturbation of the dopamine system74 and increase of neuroinflammation.121 It is also noteworthy that chlorpyrifos has been gradually banned in many countries owing to its reported risks to nontarget organisms, particularly developmental neurotoxicity in children,122,123 but is still widely used in China. Similarly, some isomers of cypermethrin (including alpha-cypermethrin, beta-cypermethrin, and theta-cypermethrin) are not approved for use in the European Union anymore,124 owing to their developmental neurotoxicity in rats [range of acceptable daily intake values for those isomers: BW per day].125 Permethrin is not approved for use in the European Union either.124 It is the right time for China to further regulate them and other parent insecticides of TCPy and tran-DCCA, as well as THM.
The strength of this study was analyzing the mixture effect of multiple insecticides on child neurodevelopment with a relatively large sample size and repeated measurements across trimesters. However, some limitations should be noted. First of all, we did not collect information on dietary patterns of the pregnant women, which may cause some potential bias of our findings. Further, the confounding effects of other pollutants were not considered in this work. Finally, we did not assess the associations between Bayley scores and children’s postnatal exposure to these insecticides, which might also have some impact. Future prospective studies are warranted to confirm our findings by considering the abovementioned limitations.
Conclusions
Our findings suggest that maternal insecticide exposure in the first trimester may compromise child neurodevelopment, and the associations are particularly pronounced in boys. In addition, we found that trans-DCCA and TCPy contributed the most to the mixture effect. In addition to those factors that are commonly recognized to affect child neurodevelopment (e.g., nutrition in pregnancy, socioeconomic status, genetics), our findings highlight the necessity for public health and policy measures to reduce pesticide exposures among pregnant women and warrant additional investigations.
Supplementary Material
Acknowledgments
Y.W. is grateful to Prof. Kurunthachalam Kannan for mentoring. This study was funded by the National Natural Science Foundation of China [grants U21A20397 (to S.X.), 42277428 (to W.X.), 21407117 (to Y.W.), and 81402649 (to W.X.)], the Hubei Province Health and Family Planning Scientific Research Project [grant WJ2019H307 (to W.X.)], the Wuhan Preventive Medicine Research Project [grant WY19A03 (to W.X.)], and the Program for HUST Academic Frontier Youth Team [grant 2018QYTD12 (to W.X.)]. The manuscript contents are solely the responsibility of the authors and do not necessarily represent the official views of the department.
References
- 1.Ferguson KK, McElrath TF, Meeker JD. 2014. Environmental phthalate exposure and preterm birth. JAMA Pediatr 168(1):61–67, PMID: , 10.1001/jamapediatrics.2013.3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hu J, Xia W, Pan X, Zheng T, Zhang B, Zhou A, et al. 2017. Association of adverse birth outcomes with prenatal exposure to vanadium: a population-based cohort study. Lancet Planet Health 1(6):e230–e241, PMID: , 10.1016/S2542-5196(17)30094-3. [DOI] [PubMed] [Google Scholar]
- 3.Hu J, Peng Y, Zheng T, Zhang B, Liu W, Wu C, et al. 2018. Effects of trimester-specific exposure to vanadium on ultrasound measures of fetal growth and birth size: a longitudinal prospective prenatal cohort study. Lancet Planet Health 2(10):e427–e437, PMID: , 10.1016/S2542-5196(18)30210-9. [DOI] [PubMed] [Google Scholar]
- 4.Ouidir M, Buck Louis GM, Kanner J, Grantz KL, Zhang C, Sundaram R, et al. 2020. Association of maternal exposure to persistent organic pollutants in early pregnancy with fetal growth. JAMA Pediatr 174(2):149–161, PMID: , 10.1001/jamapediatrics.2019.5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abreu-Villaça Y, Levin ED. 2017. Developmental neurotoxicity of succeeding generations of insecticides. Environ Int 99:55–77, PMID: , 10.1016/j.envint.2016.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang D, Lu S. 2022. Human exposure to neonicotinoids and the associated health risks: a review. Environ Int 163:107201, PMID: , 10.1016/j.envint.2022.107201. [DOI] [PubMed] [Google Scholar]
- 7.Abou-Donia MB, Goldstein LB, Bullman S, Tu T, Khan WA, Dechkovskaia AM, et al. 2008. Imidacloprid induces neurobehavioral deficits and increases expression of glial fibrillary acidic protein in the motor cortex and hippocampus in offspring rats following in utero exposure. J Toxicol Environ Health A 71(2):119–130, PMID: , 10.1080/15287390701613140. [DOI] [PubMed] [Google Scholar]
- 8.Imanishi S, Okura M, Zaha H, Yamamoto T, Akanuma H, Nagano R, et al. 2013. Prenatal exposure to permethrin influences vascular development of fetal brain and adult behavior in mice offspring. Environ Toxicol 28(11):617–629, PMID: , 10.1002/tox.20758. [DOI] [PubMed] [Google Scholar]
- 9.De Felice A, Greco A, Calamandrei G, Minghetti L. 2016. Prenatal exposure to the organophosphate insecticide chlorpyrifos enhances brain oxidative stress and prostaglandin E2 synthesis in a mouse model of idiopathic autism. J Neuroinflammation 13(1):149, PMID: , 10.1186/s12974-016-0617-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laporte B, Gay-Quéheillard J, Bach V, Villégier AS. 2018. Developmental neurotoxicity in the progeny after maternal gavage with chlorpyrifos. Food Chem Toxicol 113:66–72, PMID: , 10.1016/j.fct.2018.01.026. [DOI] [PubMed] [Google Scholar]
- 11.Eskenazi B, Marks AR, Bradman A, Harley K, Barr DB, Johnson C, et al. 2007. Organophosphate pesticide exposure and neurodevelopment in young Mexican-American children. Environ Health Perspect 115(5):792–798, PMID: , 10.1289/ehp.9828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bouchard MF, Chevrier J, Harley KG, Kogut K, Vedar M, Calderon N, et al. 2011. Prenatal exposure to organophosphate pesticides and IQ in 7-year-old children. Environ Health Perspect 119(8):1189–1195, PMID: , 10.1289/ehp.1003185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu P, Wu C, Chang X, Qi X, Zheng M, Zhou Z. 2016. Adverse associations of both prenatal and postnatal exposure to organophosphorous pesticides with infant neurodevelopment in an agricultural area of Jiangsu Province, China. Environ Health Perspect 124(10):1637–1643, PMID: , 10.1289/EHP196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sagiv SK, Kogut K, Harley K, Bradman A, Morga N, Eskenazi B. 2021. Gestational exposure to organophosphate pesticides and longitudinally assessed behaviors related to attention-deficit/hyperactivity disorder and executive function. Am J Epidemiol 190(11):2420–2431, PMID: , 10.1093/aje/kwab173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shelton JF, Geraghty EM, Tancredi DJ, Delwiche LD, Schmidt RJ, Ritz B, et al. 2014. Neurodevelopmental disorders and prenatal residential proximity to agricultural pesticides: the CHARGE study. Environ Health Perspect 122(10):1103–1109, PMID: , 10.1289/ehp.1307044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.von Ehrenstein OS, Ling C, Cui X, Cockburn M, Park AS, Yu F, et al. 2019. Prenatal and infant exposure to ambient pesticides and autism spectrum disorder in children: population based case-control study. BMJ 364:l962, PMID: , 10.1136/bmj.l962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gunier RB, Bradman A, Harley KG, Kogut K, Eskenazi B. 2017. Prenatal residential proximity to agricultural pesticide use and IQ in 7-year-old children. Environ Health Perspect 125(5):057002, PMID: , 10.1289/EHP504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coker E, Gunier R, Bradman A, Harley K, Kogut K, Molitor J, et al. 2017. Association between pesticide profiles used on agricultural fields near maternal residences during pregnancy and IQ at age 7 years. Int J Environ Res Public Health 14(5):506, PMID: , 10.3390/ijerph14050506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rauh VA, Garfinkel R, Perera FP, Andrews HF, Hoepner L, Barr DB, et al. 2006. Impact of prenatal chlorpyrifos exposure on neurodevelopment in the first 3 years of life among inner-city children. Pediatrics 118(6):e1845–e1859, PMID: , 10.1542/peds.2006-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Engel SM, Wetmur J, Chen J, Zhu C, Barr DB, Canfield RL, et al. 2011. Prenatal exposure to organophosphates, paraoxonase 1, and cognitive development in childhood. Environ Health Perspect 119(8):1182–1188, PMID: , 10.1289/ehp.1003183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y, Zhang Y, Ji L, Hu Y, Zhang J, Wang C, et al. 2017. Prenatal and postnatal exposure to organophosphate pesticides and childhood neurodevelopment in Shandong, China. Environ Int 108:119–126, PMID: , 10.1016/j.envint.2017.08.010. [DOI] [PubMed] [Google Scholar]
- 22.Jusko TA, van den Dries MA, Pronk A, Shaw PA, Guxens M, Spaan S, et al. 2019. Organophosphate pesticide metabolite concentrations in urine during pregnancy and offspring nonverbal IQ at age 6 years. Environ Health Perspect 127(1):17007, PMID: , 10.1289/EHP3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ntantu Nkinsa P, Muckle G, Ayotte P, Lanphear BP, Arbuckle TE, Fraser WD, et al. 2020. Organophosphate pesticides exposure during fetal development and IQ scores in 3 and 4-year old Canadian children. Environ Res 190:110023, PMID: , 10.1016/j.envres.2020.110023. [DOI] [PubMed] [Google Scholar]
- 24.Viel JF, Warembourg C, Le Maner-Idrissi G, Lacroix A, Limon G, Rouget F, et al. 2015. Pyrethroid insecticide exposure and cognitive developmental disabilities in children: the PELAGIE mother–child cohort. Environ Int 82:69–75, PMID: , 10.1016/j.envint.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 25.Watkins DJ, Fortenberry GZ, Sánchez BN, Barr DB, Panuwet P, Schnaas L, et al. 2016. Urinary 3-phenoxybenzoic acid (3-PBA) levels among pregnant women in Mexico City: distribution and relationships with child neurodevelopment. Environ Res 147:307–313, PMID: , 10.1016/j.envres.2016.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andersen HR, Dalsager L, Jensen IK, Timmermann CAG, Olesen TS, Trecca F, et al. 2021. Prenatal exposure to pyrethroid and organophosphate insecticides and language development at age 20–36 months among children in the Odense Child Cohort. Int J Hyg Environ Health 235:113755, PMID: , 10.1016/j.ijheh.2021.113755. [DOI] [PubMed] [Google Scholar]
- 27.Bass C, Denholm I, Williamson MS, Nauen R. 2015. The global status of insect resistance to neonicotinoid insecticides. Pestic Biochem Physiol 121:78–87, PMID: , 10.1016/j.pestbp.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 28.Xu X, Li L, Huang X, Lin H, Liu G, Xu D, et al. 2018. Survey of four groups of cumulative pesticide residues in 12 vegetables in 15 provinces in China. J Food Prot 81(3):377–385, PMID: , 10.4315/0362-028X.JFP-17-197. [DOI] [PubMed] [Google Scholar]
- 29.Chen D, Zhang Y, Lv B, Liu Z, Han J, Li J, et al. 2020. Dietary exposure to neonicotinoid insecticides and health risks in the Chinese general population through two consecutive total diet studies. Environ Int 135:105399, PMID: , 10.1016/j.envint.2019.105399. [DOI] [PubMed] [Google Scholar]
- 30.Wan Y, Tran TM, Nguyen VT, Wang A, Wang J, Kannan K. 2021. Neonicotinoids, fipronil, chlorpyrifos, carbendazim, chlorotriazines, chlorophenoxy herbicides, bentazon, and selected pesticide transformation products in surface water and drinking water from northern Vietnam. Sci Total Environ 750:141507, PMID: , 10.1016/j.scitotenv.2020.141507. [DOI] [PubMed] [Google Scholar]
- 31.Dalsager L, Fage-Larsen B, Bilenberg N, Jensen TK, Nielsen F, Kyhl HB, et al. 2019. Maternal urinary concentrations of pyrethroid and chlorpyrifos metabolites and attention deficit hyperactivity disorder (ADHD) symptoms in 2-4-year-old children from the Odense Child Cohort. Environ Res 176:108533, PMID: , 10.1016/j.envres.2019.108533. [DOI] [PubMed] [Google Scholar]
- 32.Egeghy PP, Cohen Hubal EA, Tulve NS, Melnyk LJ, Morgan MK, Fortmann RC, et al. 2011. Review of pesticide urinary biomarker measurements from selected US EPA children’s observational exposure studies. Int J Environ Res Public Health 8(5):1727–1754, PMID: , 10.3390/ijerph8051727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klimowska A, Amenda K, Rodzaj W, Wileńska M, Jurewicz J, Wielgomas B. 2020. Evaluation of 1-year urinary excretion of eight metabolites of synthetic pyrethroids, chlorpyrifos, and neonicotinoids. Environ Int 145:106119, PMID: , 10.1016/j.envint.2020.106119. [DOI] [PubMed] [Google Scholar]
- 34.Comfort N, Re DB. 2017. Sex-Specific neurotoxic effects of organophosphate pesticides across the life course. Curr Environ Health Rep 4(4):392–404, PMID: , 10.1007/s40572-017-0171-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vester AI, Chen M, Marsit CJ, Caudle WM. 2019. A neurodevelopmental model of combined pyrethroid and chronic stress exposure. Toxics 7(2):24, PMID: , 10.3390/toxics7020024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.NHC (National Health Commission). 2022. National Health Commission of the People’s Republic of China, Standard of recommendation for weight gain during pregnancy period. http://www.nhc.gov.cn/wjw/fyjk/202208/864ddc16511148819168305d3e576de9/files/4c0e42b584dd4c25b1a4004dd260d561.pdf [accessed 17 May 2023].
- 37.Bayley N. 1969. Manual for the Bayley Scales of Infant Development. San Antonio, TX: Psychological Corporation. [Google Scholar]
- 38.Yi SH, Liu XH, Yang ZW, Wan GB. 1993. The revising of the Bayley Scales of Infant Development (BSID) in China [in Chinese]. Chin J Clin Psychol 1(2):71–75. [Google Scholar]
- 39.Bravo G, Potvin L. 1991. Estimating the reliability of continuous measures with Cronbach’s alpha or the intraclass correlation coefficient: toward the integration of two traditions. J Clin Epidemiol 44(4–5):381–390, PMID: , 10.1016/0895-4356(91)90076-l. [DOI] [PubMed] [Google Scholar]
- 40.Li AJ, Kannan K. 2018. Urinary concentrations and profiles of organophosphate and pyrethroid pesticide metabolites and phenoxyacid herbicides in populations in eight countries. Environ Int 121(pt 2):1148–1154, PMID: , 10.1016/j.envint.2018.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li AJ, Kannan K. 2020. Profiles of urinary neonicotinoids and dialkylphosphates in populations in nine countries. Environ Int 145:106120, PMID: , 10.1016/j.envint.2020.106120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mahai G, Wan Y, Xia W, Wang A, Qian X, Li Y, et al. 2022. Exposure assessment of neonicotinoid insecticides and their metabolites in Chinese women during pregnancy: a longitudinal study. Sci Total Environ 818:151806, PMID: , 10.1016/j.scitotenv.2021.151806. [DOI] [PubMed] [Google Scholar]
- 43.Qian X, Wan Y, Wang A, Xia W, Yang Z, He Z, et al. 2021. Urinary metabolites of multiple volatile organic compounds among general population in Wuhan, central China: inter-day reproducibility, seasonal difference, and their associations with oxidative stress biomarkers. Environ Pollut 289:117913, PMID: , 10.1016/j.envpol.2021.117913. [DOI] [PubMed] [Google Scholar]
- 44.Wang A, Mahai G, Wan Y, Yang Z, He Z, Xu S, et al. 2020. Assessment of imidacloprid related exposure using imidacloprid-olefin and desnitro-imidacloprid: neonicotinoid insecticides in human urine in Wuhan, China. Environ Int 141:105785, PMID: , 10.1016/j.envint.2020.105785. [DOI] [PubMed] [Google Scholar]
- 45.Boeniger MF, Lowry LK, Rosenberg J. 1993. Interpretation of urine results used to assess chemical exposure with emphasis on creatinine adjustments: a review. Am Ind Hyg Assoc J 54(10):615–627, PMID: , 10.1080/15298669391355134. [DOI] [PubMed] [Google Scholar]
- 46.Barr DB, Wilder LC, Caudill SP, Gonzalez AJ, Needham LL, Pirkle JL. 2005. Urinary creatinine concentrations in the U.S. population: implications for urinary biologic monitoring measurements. Environ Health Perspect 113(2):192–200, PMID: , 10.1289/ehp.7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Middleton DRS, Watts MJ, Polya DA. 2019. A comparative assessment of dilution correction methods for spot urinary analyte concentrations in a UK population exposed to arsenic in drinking water. Environ Int 130:104721, PMID: , 10.1016/j.envint.2019.03.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shin HM, Oh J, Kim K, Busgang SA, Barr DB, Panuwet P, et al. 2021. Variability of urinary concentrations of phenols, parabens, and triclocarban during pregnancy in first morning voids and pooled samples. Environ Sci Technol 55(23):16001–16010, PMID: , 10.1021/acs.est.1c04140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.PubChem. 2022. Metabolism/metabolites. Trimethyl phosphate. https://pubchem.ncbi.nlm.nih.gov/compound/Trimethyl-phosphate#section=Metabolism-Metabolites [accessed 25 June 2023].
- 50.Wang Y, Li W, Martínez-Moral MP, Sun H, Kannan K. 2019. Metabolites of organophosphate esters in urine from the United States: concentrations, temporal variability, and exposure assessment. Environ Int 122:213–221, PMID: , 10.1016/j.envint.2018.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jones AR. 1970. The metabolism of tri-alkyl phosphates. Experientia 26(5):492–493, PMID: , 10.1007/BF01898464. [DOI] [PubMed] [Google Scholar]
- 52.Li S, Wan Y, Wang Y, He Z, Xu S, Xia W. 2022. Occurrence, spatial variation, seasonal difference, and ecological risk assessment of organophosphate esters in the Yangtze River, China: from the upper to lower reaches. Sci Total Environ 851(pt 1):158021, PMID: , 10.1016/j.scitotenv.2022.158021. [DOI] [PubMed] [Google Scholar]
- 53.NHANES (National Health and Nutrition Examination Survey). 2020. National Health and Nutrition Examination Survey, 2013–2014 Data Documentation, Codebook, and Frequencies. Pyrethroids, Herbicides, & Organophosphorus Metabolites. Data File: UPHOPM_H.xpt. https://wwwn.cdc.gov/Nchs/Nhanes/2013-2014/UPHOPM_H.htm [accessed 29 May 2023].
- 54.NHANES. 2022. National Health and Nutrition Examination Survey, 2017–2018 Data Documentation, Codebook, and Frequencies. Organophosphate Insecticides – Dialkyl Phosphate Metabolites – Urine (OPE-J). Data File: OPD_J.xpt. Last revised April 2022. https://wwwn.cdc.gov/Nchs/Nhanes/2017-2018/OPD_J.htm [accessed 29 May 2023].
- 55.Sánchez BN, Hu H, Litman HJ, Téllez-Rojo MM. 2011. Statistical methods to study timing of vulnerability with sparsely sampled data on environmental toxicants. Environ Health Perspect 119(3):409–415, PMID: , 10.1289/ehp.1002453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Benjamini Y, Yekutieli D. 2001. The control of the false discovery rate in multiple testing under dependency. Ann Statist 29(4):1165–1188, 10.1214/aos/1013699998. [DOI] [Google Scholar]
- 57.Carrico C, Gennings C, Wheeler DC, Factor-Litvak P. 2015. Characterization of weighted quantile sum regression for highly correlated data in a risk analysis setting. J Agric Biol Environ Stat 20(1):100–120, PMID: , 10.1007/s13253-014-0180-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tanner EM, Hallerbäck MU, Wikström S, Lindh C, Kiviranta H, Gennings C, et al. 2020. Early prenatal exposure to suspected endocrine disruptor mixtures is associated with lower IQ at age seven. Environ Int 134:105185, PMID: , 10.1016/j.envint.2019.105185. [DOI] [PubMed] [Google Scholar]
- 59.Tanner EM, Bornehag CG, Gennings C. 2019. Repeated holdout validation for weighted quantile sum regression. MethodsX 6:2855–2860, PMID: , 10.1016/j.mex.2019.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Atashili J, Ta M. 2007. A SAS® macro for automating the ‘change-in-estimate’ strategy for assessing confounding. Paper 032-2007. https://support.sas.com/resources/papers/proceedings/proceedings/forum2007/032-2007.pdf [accessed 22 May 2023].
- 61.McNulty H, Rollins M, Cassidy T, Caffrey A, Marshall B, Dornan J, et al. 2019. Effect of continued folic acid supplementation beyond the first trimester of pregnancy on cognitive performance in the child: a follow-up study from a randomized controlled trial (FASSTT Offspring Trial). BMC Med 17(1):196, PMID: , 10.1186/s12916-019-1432-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Anai A, Hisada A, Yunohara T, Iwasaki M, Arizono K, Katoh T. 2021. Urinary neonicotinoids level among pregnant women in Japan. Int J Hyg Environ Health 236:113797, PMID: , 10.1016/j.ijheh.2021.113797. [DOI] [PubMed] [Google Scholar]
- 63.Rice D, Barone S Jr.. 2000. Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect 108(suppl 3):511–533, PMID: , 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.van den Dries MA, Guxens M, Spaan S, Ferguson KK, Philips E, Santos S, et al. 2020. Phthalate and bisphenol exposure during pregnancy and offspring nonverbal IQ. Environ Health Perspect 128(7):077009, PMID: , 10.1289/EHP6047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Qi Z, Song X, Xiao X, Loo KK, Wang MC, Xu Q, et al. 2022. Effects of prenatal exposure to pyrethroid pesticides on neurodevelopment of 1-year-old children: a birth cohort study in China. Ecotoxicol Environ Saf 234:113384, PMID: , 10.1016/j.ecoenv.2022.113384. [DOI] [PubMed] [Google Scholar]
- 66.Zhang T, Luo ZC, Ji Y, Chen Y, Ma R, Fan P, et al. 2023. The impact of maternal depression, anxiety, and stress on early neurodevelopment in boys and girls. J Affect Disord 321:74–82, PMID: , 10.1016/j.jad.2022.10.030. [DOI] [PubMed] [Google Scholar]
- 67.Kern JK, Geier DA, Homme KG, King PG, Bjørklund G, Chirumbolo S, et al. 2017. Developmental neurotoxicants and the vulnerable male brain: a systematic review of suspected neurotoxicants that disproportionally affect males. Acta Neurobiol Exp (Wars) 77(4):269–296, PMID: , 10.21307/ane-2017-061. [DOI] [PubMed] [Google Scholar]
- 68.Azcoitia I, Barreto GE, Garcia-Segura LM. 2019. Molecular mechanisms and cellular events involved in the neuroprotective actions of estradiol. Analysis of sex differences. Front Neuroendocrinol 55:100787, PMID: , 10.1016/j.yfrne.2019.100787. [DOI] [PubMed] [Google Scholar]
- 69.Venerosi A, Tait S, Stecca L, Chiarotti F, De Felice A, Cometa MF, et al. 2015. Effects of maternal chlorpyrifos diet on social investigation and brain neuroendocrine markers in the offspring - a mouse study. Environ Health 14:32, PMID: , 10.1186/s12940-015-0019-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Walker DM, Gore AC. 2017. Epigenetic impacts of endocrine disruptors in the brain. Front Neuroendocrinol 44:1–26, PMID: , 10.1016/j.yfrne.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Leemans M, Couderq S, Demeneix B, Fini JB. 2019. Pesticides with potential thyroid hormone-disrupting effects: a review of recent data. Front Endocrinol (Lausanne) 10:743, PMID: , 10.3389/fendo.2019.00743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fan P, Chen Y, Luo ZC, Shen L, Wang W, Liu Z, et al. 2021. Cord blood thyroid hormones and neurodevelopment in 2-year-old boys and girls. Front Nutr 8:773965, PMID: , 10.3389/fnut.2021.773965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Aldridge JE, Meyer A, Seidler FJ, Slotkin TA. 2005. Alterations in central nervous system serotonergic and dopaminergic synaptic activity in adulthood after prenatal or neonatal chlorpyrifos exposure. Environ Health Perspect 113(8):1027–1031, PMID: , 10.1289/ehp.7968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Elwan MA, Richardson JR, Guillot TS, Caudle WM, Miller GW. 2006. Pyrethroid pesticide-induced alterations in dopamine transporter function. Toxicol Appl Pharmacol 211(3):188–197, PMID: , 10.1016/j.taap.2005.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Marks AR, Harley K, Bradman A, Kogut K, Barr DB, Johnson C, et al. 2010. Organophosphate pesticide exposure and attention in young Mexican-American children: the CHAMACOS study. Environ Health Perspect 118(12):1768–1774, PMID: , 10.1289/ehp.1002056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chiu KC, Sisca F, Ying JH, Tsai WJ, Hsieh WS, Chen PC, et al. 2021. Prenatal chlorpyrifos exposure in association with PPARγ H3K4me3 and DNA methylation levels and child development. Environ Pollut 274:116511, PMID: , 10.1016/j.envpol.2021.116511. [DOI] [PubMed] [Google Scholar]
- 77.Richardson JR, Taylor MM, Shalat SL, Guillot TS III, Caudle WM, Hossain MM, et al. 2015. Developmental pesticide exposure reproduces features of attention deficit hyperactivity disorder. FASEB J 29(5):1960–1972, PMID: , 10.1096/fj.14-260901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kubo S, Hirano T, Miyata Y, Ohno S, Onaru K, Ikenaka Y, et al. 2022. Sex-specific behavioral effects of acute exposure to the neonicotinoid clothianidin in mice. Toxicol Appl Pharmacol 456:116283, PMID: , 10.1016/j.taap.2022.116283. [DOI] [PubMed] [Google Scholar]
- 79.Kongtip P, Techasaensiri B, Nankongnab N, Adams J, Phamonphon A, Surach A, et al. 2017. The impact of prenatal organophosphate pesticide exposures on Thai infant neurodevelopment. Int J Environ Res Public Health 14(6):570, PMID: , 10.3390/ijerph14060570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lovasi GS, Quinn JW, Rauh VA, Perera FP, Andrews HF, Garfinkel R, et al. 2011. Chlorpyrifos exposure and urban residential environment characteristics as determinants of early childhood neurodevelopment. Am J Public Health 101(1):63–70, PMID: , 10.2105/AJPH.2009.168419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Eskenazi B, An S, Rauch SA, Coker ES, Maphula A, Obida M, et al. 2018. Prenatal exposure to DDT and pyrethroids for malaria control and child neurodevelopment: the VHEMBE cohort, South Africa. Environ Health Perspect 126(4):047004, PMID: , 10.1289/EHP2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fluegge KR, Nishioka M, Wilkins JR III.. 2016. Effects of simultaneous prenatal exposures to organophosphate and synthetic pyrethroid insecticides on infant neurodevelopment at three months of age. J Environ Toxicol Public Health 1:60–73, PMID: , 10.5281/zenodo.218417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Donauer S, Altaye M, Xu Y, Sucharew H, Succop P, Calafat AM, et al. 2016. An observational study to evaluate associations between low-level gestational exposure to organophosphate pesticides and cognition during early childhood. Am J Epidemiol 184(5):410–418, PMID: , 10.1093/aje/kwv447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rauh V, Arunajadai S, Horton M, Perera F, Hoepner L, Barr DB, et al. 2011. Seven-year neurodevelopmental scores and prenatal exposure to chlorpyrifos, a common agricultural pesticide. Environ Health Perspect 119(8):1196–1201, PMID: , 10.1289/ehp.1003160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Furlong MA, Herring A, Buckley JP, Goldman BD, Daniels JL, Engel LS, et al. 2017. Prenatal exposure to organophosphorus pesticides and childhood neurodevelopmental phenotypes. Environ Res 158:737–747, PMID: , 10.1016/j.envres.2017.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang Y, Han S, Liang D, Shi X, Wang F, Liu W, et al. 2014. Prenatal exposure to organophosphate pesticides and neurobehavioral development of neonates: a birth cohort study in Shenyang, China. PLoS One 9(2):e88491, PMID: , 10.1371/journal.pone.0088491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Furlong MA, Engel SM, Barr DB, Wolff MS. 2014. Prenatal exposure to organophosphate pesticides and reciprocal social behavior in childhood. Environ Int 70:125–131, PMID: , 10.1016/j.envint.2014.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lizé M, Monfort C, Rouget F, Limon G, Durand G, Tillaut H, et al. 2022. Prenatal exposure to organophosphate pesticides and autism spectrum disorders in 11-year-old children in the French PELAGIE cohort. Environ Res 212(pt C):113348, PMID: , 10.1016/j.envres.2022.113348. [DOI] [PubMed] [Google Scholar]
- 89.Lee KS, Lim YH, Lee YA, Shin CH, Kim BN, Hong YC, et al. 2022. The association of prenatal and childhood pyrethroid pesticide exposure with school-age ADHD traits. Environ Int 161:107124, PMID: , 10.1016/j.envint.2022.107124. [DOI] [PubMed] [Google Scholar]
- 90.Binter AC, Bannier E, Saint-Amour D, Simon G, Barillot C, Monfort C, et al. 2020. Exposure of pregnant women to organophosphate insecticides and child motor inhibition at the age of 10–12 years evaluated by fMRI. Environ Res 188:109859, PMID: , 10.1016/j.envres.2020.109859. [DOI] [PubMed] [Google Scholar]
- 91.Fortenberry GZ, Meeker JD, Sánchez BN, Barr DB, Panuwet P, Bellinger D, et al. 2014. Urinary 3,5,6-trichloro-2-pyridinol (TCPY) in pregnant women from Mexico City: distribution, temporal variability, and relationship with child attention and hyperactivity. Int J Hyg Environ Health 217(2–3):405–412, PMID: , 10.1016/j.ijheh.2013.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cartier C, Warembourg C, Le Maner-Idrissi G, Lacroix A, Rouget F, Monfort C, et al. 2016. Organophosphate insecticide metabolites in prenatal and childhood urine samples and intelligence scores at 6 years of age: results from the mother-child PELAGIE cohort (France). Environ Health Perspect 124(5):674–680, PMID: , 10.1289/ehp.1409472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Guo J, Zhang J, Wu C, Lv S, Lu D, Qi X, et al. 2019. Associations of prenatal and childhood chlorpyrifos exposure with neurodevelopment of 3-year-old children. Environ Pollut 251:538–546, PMID: , 10.1016/j.envpol.2019.05.040. [DOI] [PubMed] [Google Scholar]
- 94.Viel JF, Rouget F, Warembourg C, Monfort C, Limon G, Cordier S, et al. 2017. Behavioural disorders in 6-year-old children and pyrethroid insecticide exposure: the PELAGIE mother–child cohort. Occup Environ Med 74(4):275–281, PMID: , 10.1136/oemed-2016-104035. [DOI] [PubMed] [Google Scholar]
- 95.Barkoski JM, Philippat C, Tancredi D, Schmidt RJ, Ozonoff S, Barr DB, et al. 2021. In utero pyrethroid pesticide exposure in relation to autism spectrum disorder (ASD) and other neurodevelopmental outcomes at 3 years in the MARBLES longitudinal cohort. Environ Res 194:110495, PMID: , 10.1016/j.envres.2020.110495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.CDC (U.S. Centers for Disease Control and Prevention). 2013–2014. Specific Organophosphorous Pesticides, Synthetic Pyrethroids, and Select Herbicides (Universal Pesticides). Method No. 6103.05. https://wwwn.cdc.gov/nchs/data/nhanes/2013-2014/labmethods/UPHOPM-H-MET-508.pdf [accessed 29 July 2022].
- 97.MOA (Ministry of Agriculture). 2004. Announcement No. 322 of the Ministry of Agriculture of the People’s Republic of China. https://www.moa.gov.cn/nybgb/2004/deq/201806/t20180623_6152926.html [accessed 15 June 2023].
- 98.Machida M, Morita Y, Hayashi M, Awazu S. 1982. Pharmacokinetic evidence for the occurrence of extrahepatic conjugative metabolism of p-nitrophenol in rats. Biochem Pharmacol 31(5):787–791, PMID: , 10.1016/0006-2952(82)90464-6. [DOI] [PubMed] [Google Scholar]
- 99.ATSDR (Agency for Toxic Substances and Disease Registry). 2022. Toxicological Profile for Nitrophenols. CAS#: 2-nitrophenol 88-75-5; 3-nitrophenol 554-84-7; 4-nitrophenol 100-02-7. https://wwwn.cdc.gov/TSP/ToxProfiles/ToxProfiles.aspx?id=880&tid=172 [accessed 15 June 2023]. [PubMed]
- 100.ATSDR. 2022. Toxicological Profile for Nitrobenzene. CAS#: 2-nitrophenol 88-75-5; 3-nitrophenol 540-84-7; 4-nitrophenol 100-02-7. https://wwwn.cdc.gov/TSP/ToxProfiles/ToxProfiles.aspx?id=532&tid=95 [accessed 15 June 2023].
- 101.IARC (International Agency for Research on Cancer). 1996. Summaries & Evaluations. Nitrobenzene (group 2B). https://inchem.org/documents/iarc/vol65/nitrobenzene.html [accessed 15 June 2023].
- 102.ATSDR. 2017. Toxicological Profile for Parathion. CAS#: 56-38-2. https://wwwn.cdc.gov/TSP/ToxProfiles/ToxProfiles.aspx?id=1425&tid=246 [accessed 15 June 2023].
- 103.ATSDR. 2014. Toxicological Profile for Methyl Parathion. CAS#: 298-00-0. https://wwwn.cdc.gov/TSP/ToxProfiles/ToxProfiles.aspx?id=636&tid=117 [accessed 15 June 2023].
- 104.Guxens M, Aguilera I, Ballester F, Estarlich M, Fernández-Somoano A, Lertxundi A, et al. 2012. Prenatal exposure to residential air pollution and infant mental development: modulation by antioxidants and detoxification factors. Environ Health Perspect 120(1):144–149, PMID: , 10.1289/ehp.1103469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yang W, Carmichael SL, Roberts EM, Kegley SE, Padula AM, English PB, et al. 2014. Residential agricultural pesticide exposures and risk of neural tube defects and orofacial clefts among offspring in the San Joaquin Valley of California. Am J Epidemiol 179(6):740–748, PMID: , 10.1093/aje/kwt324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sang C, Sørensen PB, An W, Andersen JH, Yang M. 2020. Chronic health risk comparison between China and Denmark on dietary exposure to chlorpyrifos. Environ Pollut 257:113590, PMID: , 10.1016/j.envpol.2019.113590. [DOI] [PubMed] [Google Scholar]
- 107.Timchalk C, Busby A, Campbell JA, Needham LL, Barr DB. 2007. Comparative pharmacokinetics of the organophosphorus insecticide chlorpyrifos and its major metabolites diethylphosphate, diethylthiophosphate and 3,5,6-trichloro-2-pyridinol in the rat. Toxicology 237(1–3):145–157, PMID: , 10.1016/j.tox.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 108.CDC. 2017. Biomonitoring Summary. Organophosphorus Insecticides: Dialkyl Phosphate Metabolites. https://www.cdc.gov/biomonitoring/OP-DPM_BiomonitoringSummary.html [accessed 15 June 2023].
- 109.CPIN (China Pesticide Information Network). 2023. Pesticide registration data. www.chinapesticide.org.cn/zwb/dataCenter [accessed 29 May 2023].
- 110.van den Dries MA, Ferguson KK, Keil AP, Pronk A, Spaan S, Ghassabian A, et al. 2021. Prenatal exposure to nonpersistent chemical mixtures and offspring IQ and emotional and behavioral problems. Environ Sci Technol 55(24):16502–16514, PMID: , 10.1021/acs.est.1c04455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.EFSA (European Food Safety Authority), Crivellente F, Hart A, Hernandez-Jerez AF, Hougaard Bennekou S, Pedersen R, Terron A, et al. 2019. Establishment of cumulative assessment groups of pesticides for their effects on the nervous system. EFSA J 17(9):e05800, PMID: , 10.2903/j.efsa.2019.5800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Shu F, Li Y, Wei Q. 2019. Pesticide use in plantation industry in 2018 and demand analysis in 2019 China plant protection guide [in Chinese]. China Plant Prot 39(04):73–76. https://kns.cnki.net/kcms/detail/detail.aspx?FileName=ZBJS201904016&DbName=CJFQ2019. [Google Scholar]
- 113.EFSA Panel on Plant Protection Products their Residues. 2013. Scientific opinion on the identification of pesticides to be included in cumulative assessment groups on the basis of their toxicological profile. EFSA J 11(7):3293, 10.2903/j.efsa.2013.3293. [DOI] [Google Scholar]
- 114.Food and Agriculture Organization. Dichlorvos (025); pp 63–70. https://www.fao.org/fileadmin/templates/agphome/documents/Pests_Pesticides/JMPR/Evaluation12/Dichlorvos.pdf [accessed 8 September 2023].
- 115.Zhang X, Driver JH, Li Y, Ross JH, Krieger RI. 2008. Dialkylphosphates (DAPs) in fruits and vegetables may confound biomonitoring in organophosphorus insecticide exposure and risk assessment. J Agric Food Chem 56(22):10638–10645, PMID: , 10.1021/jf8018084. [DOI] [PubMed] [Google Scholar]
- 116.Bomhard EM, Krinke GJ, Rossberg WM, Skripsky T. 1997. Trimethylphosphate: a 30-month chronic toxicity/carcinogenicity study in Wistar rats with administration in drinking water. Fundam Appl Toxicol 40(1):75–89, PMID: , 10.1006/faat.1997.2374. [DOI] [PubMed] [Google Scholar]
- 117.PubChem. 2022. Triethyl phosphate 11.1.8 Toxicity Data. https://pubchem.ncbi.nlm.nih.gov/compound/Triethyl-phosphate#section=Toxicity-Data [accessed 15 June 2023].
- 118.Hertz-Picciotto I, Sass JB, Engel S, Bennett DH, Bradman A, Eskenazi B, et al. 2018. Organophosphate exposures during pregnancy and child neurodevelopment: recommendations for essential policy reforms. PLoS Med 15(10):e1002671, PMID: , 10.1371/journal.pmed.1002671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Slotkin TA, Seidler FJ. 2009. Oxidative and excitatory mechanisms of developmental neurotoxicity: transcriptional profiles for chlorpyrifos, diazinon, dieldrin, and divalent nickel in PC12 cells. Environ Health Perspect 117(4):587–596, PMID: , 10.1289/ehp.0800251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ghassabian A, Trasande L. 2018. Disruption in thyroid signaling pathway: a mechanism for the effect of endocrine-disrupting chemicals on child neurodevelopment. Front Endocrinol (Lausanne) 9:204, PMID: , 10.3389/fendo.2018.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Maurya SK, Mishra J, Abbas S, Bandyopadhyay S. 2016. Cypermethrin stimulates GSK3β-Dependent Aβ and p-tau proteins and cognitive loss in young rats: reduced HB-EGF signaling and downstream neuroinflammation as critical regulators. Mol Neurobiol 53(2):968–982, PMID: , 10.1007/s12035-014-9061-6. [DOI] [PubMed] [Google Scholar]
- 122.EFSA. 2019. Chlorpyrifos: assessment identifies human health effects. https://www.efsa.europa.eu/en/press/news/chlorpyrifos-assessment-identifies-human-health-effects [accessed 8 December 2022].
- 123.U.S. EPA (U.S. Environmental Protection Agency). 2021. EPA takes action to address risk from chlorpyrifos and protect children’s health. EPA measures will stop the use of the pesticide chlorpyrifos on food [Press release]. https://www.epa.gov/newsreleases/epa-takes-action-address-risk-chlorpyrifos-and-protect-childrens-health [accessed 8 December 2022].
- 124.PPDB (Pesticide Properties DataBase). http://sitem.herts.ac.uk/aeru/ppdb/en/atoz_insect.htm [accessed 8 October 2023].
- 125.EFSA (European Food Safety Authority). 2023. Review of the residue definitions for risk assessment of pyrethroids forming common metabolites. EFSA J 21(5):e08022, PMID: , 10.2903/j.efsa.2023.8022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.