Abstract
A commercially available Lipase B from Candida antarctica immobilized onto a macroporous support (Novozym 435) has been employed in the presence of H2O2 as a benign oxidant for the epoxidation of various biorenewable terpenes. This epoxidation protocol was explored under both heterogeneous batch and continuous flow conditions. The catalyst recyclability was also investigated demonstrating good activity throughout 10 cycles under batch conditions, while the same catalyst system could also be productively used under continuous flow operation for more than 30 h. This practical and relatively safe sustainable flow epoxidation of di- and trisubstituted alkenes by H2O2 allows for the production of gram quantities of a range of terpene epoxides. As a proof of principle, the same protocol can also be applied to the epoxidation of biobased polymers as a means to post-functionalize these macromolecules and equip them with cross-linkable epoxy groups.
Keywords: continuous flow, enzymatic catalysis, epoxidation, polymers, terpenes
Short abstract
The batch and flow-based biocatalytic epoxidation of terpenes using H2O2 offers a sustainable alternative to conventional oxidation methods.
Introduction
The development of simple and efficient processes capable to convert biorenewable feedstocks into fine chemicals, drugs, and polymers that are currently obtained from petroleum-based precursors is one of the key challenges of the 21st century. An increasing amount of attention is focused on the use of biopolymers (e.g., lignocellulose, chitin, polysaccharides, etc.) as potential biorenewable feedstocks for sustainable chemical production. In addition, the use of biobased feedstocks as starting materials for the synthesis of monomers en route to biosourced polymers has greatly expanded.1,2 In this context, non-edible food components of plants as well as industrial food waste offer a rich source of low-value, small molecules that can be converted into high-value building blocks and monomers through selective transformations but may also be directly applied in upstream chemical manufacturing.3−9
Terpenes and terpenoids are widespread and are produced in large amounts as secondary metabolites from plants and microbes. They are widely used as biorenewable feedstocks for the production of chemicals, flavors, fragrances, drugs, and polymers.10−15 Typically, they are forged through reactions of C5-based isoprene-containing precursors and afford low-oxygenated, unsaturated acyclic and cyclic C10 frameworks containing one or more alkene functionalities.16 Several unsaturated terpenes are currently available in large volumes (estimated amount of more than 330 000 tonnes per year) as waste products from forestry (e.g., pinenes from paper milling) and agricultural industries (e.g., d-limonene from citrus juice production).4 Therefore, they represent attractive starting materials that can be used as (pre)monomers for the preparation of biobased polymers.17−19
The development of new efficient catalytic protocols to transform abundant terpene feedstock into synthetically versatile intermediates represents a great opportunity since it would broaden the range of biorenewable products available from terpene biorefineries, thus helping to improve their economic feasibility.20,21 In this realm, epoxides are one of the most synthetically useful functional groups,22,23 which have been frequently used for the preparation of cyclic carbonates or monomers in ring-opening (ROP) and ring-opening copolymerization (ROCOP) reactions leading to polyethers, polyesters, and polycarbonates. The availability of sustainable catalytic protocols that can be used to epoxidize the double bonds of terpene precursors is of high importance to replace environmentally less attractive oxidation methods that rely on, inter alia, meta-chloroperbenzoic acid (mCPBA) as a reagent.
In the recent past, our group used terpene oxides as structurally versatile precursors for the preparation of new types of biobased polymers.17,24 The main challenge we encountered in the preparation of terpene epoxides is the formation of side-products such as diols through epoxide hydrolysis. This, combined with the regular use of organic peroxides instead of employing greener oxidants such as H2O2 or O2, prompted us to consider a more benign oxidation approach that could help to increase the atom economy of the process while maximizing the process chemoselectivity. We decided to explore the possibility of using a commercially available catalyst to conduct epoxidation reactions under both heterogeneous batch and continuous flow conditions. We considered Candida antarcticaLipase B (CALB) immobilized onto poly(methyl methacrylate) cross-linked with a divinyl-benzene macroporous support (known as Novozym 435) as a promising candidate. Commercial CALB is an attractive epoxidation catalyst in the presence of an externally added or in situ generated carboxylic acid and allows to prepare various types of terpene oxides as originally and individually reported by Björkling, Skouridou, Moreira/Nascimento, and Sieber.25−37
To further increase the value of CALB-promoted epoxidation, the development of a continuous flow process could increase catalyst recyclability/lifetime and facilitate product/substrate separation within a sustainable oxidation context. Continuous flow operations have emerged as a powerful tool in synthetic chemistry,38,39 and compared to batch processes, they offer significant advantages such as improved reagent mixing, better mass transfer, increased thermal control, minimization of the risk of handling hazardous substances (cf. H2O2), and increasing productivity.40 Recent studies have demonstrated that enzymes are feasible catalyst systems for continuous flow operation in, for instance, the preparation of biobased oligomers.41−45 However, as far as we know, the use of enzymes to facilitate continuous flow production of terpene oxides remains unknown despite the importance and relevance of these targets to advance biopolymer development.
Here, we report heterogeneous batch and continuous flow epoxidation of biobased terpenes using H2O2 as a benign oxidant in the presence of Novozym 435. Our protocol offers advantages in terms of product scalability to gram quantities, low toxicity of the byproducts, higher productivity under flow operation, and easy processing. The biocatalytic oxidation protocol is also demonstrated to be suitable for the functionalization of biobased macromolecules to equip these with synthetically versatile epoxy groups.
Results and Discussion
Epoxidation of Terpenes under Batch Conditions
Based on previously reported work using Novozym 435,46 we decided to start our investigation using the trisubstituted alkene (+)-3-carene as the substrate. Ethyl acetate (EtOAc) was applied as the medium and 2.4 equiv of H2O2 (30% aq solution) as oxidant was added to the terpene precursor at r.t.47 This resulted in complete substrate consumption after 30 min, affording carene oxide (CO) as a single diastereoisomer (see Figure S1). The isolation of the crude reaction product was achieved by simple filtration of the reaction mixture, and the catalyst was collected and used for the study of its recyclability under similar reaction conditions. The product (CO) was purified through column chromatography to afford carene oxide (97%) in a higher yield compared to a previously reported procedure that utilized mCPBA (85%).48 We decided to continue our studies with (diluted) H2O2 for its attractive characteristics, including being easily handled, cheap, mild, and environmentally benign.
In view of the relatively fast epoxidation rate of (+)-3-carene, we decided to explore the possibility of recycling the catalyst (Novozym 435) and monitoring its activity in 10 consecutive epoxidation cycles (Figure 1) under similar reaction conditions (r.t., 30 min, 2.4 equiv of H2O2; note that the use of 1.2 equiv of oxidant also produces CO though a longer reaction time of 1 h was needed). After the first two cycles, the activity of the supported catalyst started slowly to decrease; however, a 75% yield of carene oxide was still achieved in the 10th cycle. This slow decrease in catalytic activity is attributed to oxidative damage of the enzyme by H2O2, possible enzyme leakage from the matrix, and inactivation by the peracid intermediate (the peracid intermediate is formed according to the mechanism reported in Scheme 1) and/or the epoxide product as previously noted.25 Additionally, mechanical instability of this type of supported biocatalyst when repeatedly used under batch conditions has also been previously observed, while an increased stability was observed using a continuous flow approach.25 In any case, a total amount of 8.95 mmol of CO (1.22 g) was produced during these recycling studies, with an average batch productivity of 1.79 mmolproduct h–1 gcat–1.
Figure 1.
Recycling experiments for the preparation of carene oxide (CO) from (+)-3-carene.
Scheme 1. Mechanism of Chemoenzymatic Epoxidation of Alkenes in the Presence of Novozym 435.
The same heterogeneous batch protocol was then applied to other terpene substrates containing multiple C=C bonds (Scheme 2). Recently, our group reported the epoxidation of triolefin β-elemene in the presence of mCPBA, giving access to both mono- and diepoxy products, while the preparation of its trisepoxy derivative was only possible in low yield (33%) due to side-reactions.46 Unlike the preparation of CO promoted by Novozym 435, the selective synthesis of β-elemene monoxide (BEM, ≤50% yield) is difficult as β-elemene dioxide (BED) is always observed as a byproduct as both disubstituted alkene groups have rather similar reactivity. Therefore, it remains crucial to develop a practical epoxidation of (terpene) substrates bearing multiple double bonds that can provide both high chemoselectivity and scalability.
Scheme 2. Epoxidation and Bis-Epoxidation of Common Terpenes Using Novozym 435 under Heterogeneous Batch Conditions.
Reaction conditions: terpene precursor (1.0 mmol, 1.0 equiv), H2O2 (30% in H2O, 1.2 equiv), EtOAc (5 mL), r.t., 1 h. [a] H2O2 (30% in H2O, 2.4 equiv), EtOAc (5 mL), 4 h, 50 °C, using the corresponding monoxide as the starting material. [b] Mixture of four stereoisomers.
Even though various terpene oxides are commercially available, most reported laboratory-scale procedures for their preparation suffer from low yield and poor selectivity for the desired, partially or fully epoxidized products. Remarkably, the epoxidation of a cis/trans mixture of d-limonene by H2O2 promoted by Novozym 435 proceeds at r.t. leading to full substrate conversion, yielding a 79:21 mixture of limonene oxide (LO) and limonene dioxide (LDO) and providing a 70% yield of LO after column separation. The synthesis of LDO (78% yield) was achieved from LO at 50 °C using 2.4 equiv of H2O2. The requirement for a slightly higher reaction temperature can be rationalized by the less-activated nature of the terminal olefin. Notably, the epoxidation reaction using mCPBA to afford LDO gave a low yield (32%).34 Lastly, the nonconjugated trisubstituted alkene bond of acyclic myrcene could be epoxidized by Novozym 435/H2O2 to give (rac)-myrcene oxide (MO) with full conversion after 1 h along with the preferential formation of MO with only a low amount of myrcene dioxide formed (MO/MDO = 93:7). This is synthetically useful as MO retains a reactive 1,3-diene fragment for follow-up chemistry, and the isolated yield of MO (90%) far outranges the one previously obtained using mCPBA as the oxidant (38%).49 The preparation of the bisepoxy derivative MDO was carried out using MO as the starting material, giving full conversion of MO in 2 h at 50 °C, producing two possible regioisomers of MDO (rr = 1:4) in a combined 95% yield. When departing directly from myrcene, the addition of 2.4 equiv of H2O2 leads to a mixture of both MO and MDO.
In all of these aforementioned epoxidation reactions using Novozym 435/H2O2, the formation of 1,2-diols (cf. epoxide hydrolysis) was not detected. The epoxidation reactions of d-limonene and (+)-3-carene could also be easily scaled up to 10 mmol (>1.3 g obtained after a 1 h reaction time) with comparable results compared to the reactions performed on a smaller scale (1 mmol); see the Supporting Information for details.
To extend the application of this enzyme-based epoxidation process, we wondered whether it could be applied to more complex macromolecules such as a biobased polycarbonate, polyester, and polydiene (Scheme 3) featuring pendant olefin groups. The first example of an enzyme-catalyzed modification of the backbone of a synthetic polymer was reported by St Pourçain and co-workers.50 They showed that a selective epoxidation of polybutadiene (Mn ∼ 1300 g mol–1) in organic solvents in the presence of hydrogen peroxide is feasible using catalytic quantities of acetic acid and Novozym 435 as the catalyst.50 In this reference work, only the internal cis- and trans-configured double bonds (55% of the total of C=C bonds) along the polymer backbone could be epoxidized with an overall cis/trans C=C conversion of 60%, leaving the pendent vinyl groups (45% of the total of C=C bonds) unaffected. Later, the same protocol was employed for the enzyme-promoted polycondensation of fatty acids and for the subsequent epoxidation of the unsaturated fatty acid moiety in the side chain of the resulting polymer.51
Scheme 3. Biobased Polymer Post-Modification through Enzymatic Epoxidation under Heterogeneous Batch Conditions.
Reaction conditions: polymer (1 mmol, 1 equiv), H2O2 (30% in H2O, 3 equiv), EtOAc (0.2 M), 0.100 g of catalyst. [a] Double-bond conversion was determined by 1H NMR (CDCl3). Mn’s and Đ values were determined by GPC in tetrahydrofuran (THF) calibrated with PS standards. Tg values were determined by DSC analysis at a 10 °C min–1 heating/cooling rate; data are from the second heating cycle. The Td values were measured by thermogravimetric analysis; data refer to the values at 5 wt % loss. [b] The solvent was toluene/EtOAc, 4:1 v/v. [c] The solvent was CH2Cl2/EtOAc, 4:1 v/v.
With our ongoing interest in using biobased epoxies in the production of new types of coatings and adhesives,52,53 we decided to first explore the possibility to derivatize the pendant alkene moiety present in poly(limonene carbonate), here denoted as PLC. The virtual complete epoxidation of the PLC to poly(limonene carbonate oxide), PLCO, was previously carried out using mCPBA in CH2Cl2 at 0 °C for 12 h and, importantly, the epoxidation did not affect the polycarbonate backbone.54 We thus applied our enzyme-based epoxidation protocol to a sample of PLC (Mn = 5.8 kg mol–1, Đ = 1.37; see Scheme 3) and found that 94% conversion of the pendant C=C bonds had occurred after 4 h at 50 °C. The same procedure was also employed for the epoxidation of poly(β-elemene monoxide-alt-phthalic anhydride), abbreviated as poly(BEM-alt-PA), with an Mn of 2.4 kg mol–1 and Đ of 1.15. The resultant oxidized poly(BED-alt-PA) had a C=C functionalization degree of 70% after 4 h (Scheme 3), with a strong regio-preference for the disubstituted terminal olefin bond as readily identified by 1H NMR. Interestingly, in our previous work,55 both C=C bonds could be addressed sequentially depending on the amount of mCPBA added to the reaction mixture (1.2 or 2.5 equiv), giving high yields of the epoxidized polymer with >90% double-bond functionalization in each of these cases. Although a lower degree of epoxidation of poly(BEM-alt-PA) is noted when using Novozym 435/H2O2, the results do suggest that a high amount of epoxy groups can still be produced with this enzymatic procedure. It is remarkable that this enzyme-mediated epoxidation affords an orthogonally disubstituted polymer whose functional moieties (epoxide, double bond) can be used for further site-selective functionalization.
As a third example, we successfully probed the enzymatic epoxidation with H2O2 of polymyrcene (PM: Mn = 70.1 kg mol–1, Đ = 1.19) that features two distinct trisubstituted C=C bonds (Scheme 3). The target product (PMO) was obtained with a 89% functionalization degree, and the procedure allowed to selectively convert the sterically more accessible alkene group without noticeable changes for the alkene fragments present in the backbone of the parent polymer. Also, for this resultant polymer, post-synthetic site-selective transformation of the epoxy or alkene groups may be feasible.
Continuous Flow Epoxidation
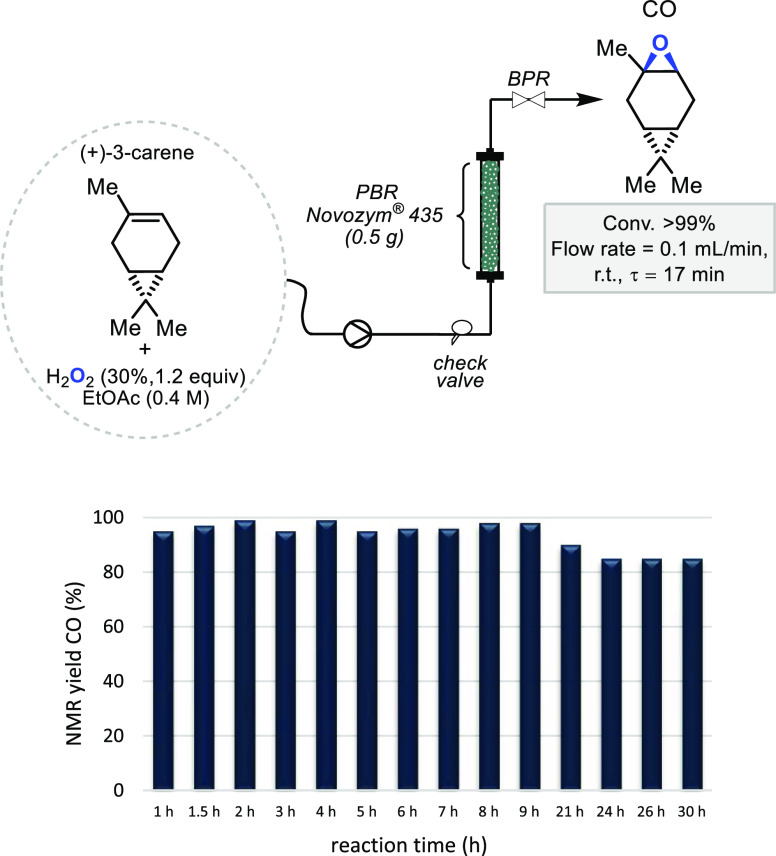
The results obtained for the heterogeneous batch epoxidation of several terpenes and terpene-based polymers prompted us to investigate and develop a continuous flow process with the aim to increase the lifetime of the catalyst, thus improving the lipase performance and productivity. The optimized heterogeneous batch epoxidation conditions developed for the epoxidation of (+)-3-carene were chosen as a starting point, and a packed bed reactor (PBR) was prepared with 0.5 g of Novozym 435. A solution of the selected terpene in EtOAc (0.4 M) in the presence of H2O2 (30% in H2O, 1.2 equiv) was pumped inside this system at r.t. After reaching a steady state, a flow rate of 0.1 mL min–1 and a residence time (τ)56 of 17 min resulted in a 99% NMR yield of CO (Figure 2). Considering this excellent outcome, no further optimization experiments were conducted while the study of the stability of the catalytic system under the previously reported batch conditions was taken into account providing, altogether, a starting point for a long-run experiment. To our delight, spectroscopic analyses (Figure 2) showed that this flow setup was stable during a time frame of 30 h, furnishing almost 8.7 g (57 mmol) of the product. Samples were collected every 1 h during the first 9 h and further regularly checked after 21 h.
Figure 2.
NMR yield of CO (%) with time in a long-run (30 h) continuous flow catalysis experiment using Novozym 435 under optimized conditions. For details, see the Supporting Information.
The overall experimental outcome corresponds to a catalyst productivity of 4.33 mmolproduct h–1 gcat–1, considering an average substrate conversion of 90% over the entire time span. This value is more than 2-fold higher than the average batch productivity (1.79 mmol h–1 gcat–1), thus demonstrating the beneficial role of the flow regime. Compared to the reaction conducted in batch mode, under continuous flow conditions, it is possible to minimize catalyst exposure to high peroxide concentration and therefore increase the lifetime of lipase. Moreover, the continuous flow approach also helps to increase the stability of the system, as under these conditions, the time during which the enzyme is in contact with the alcohol (ethanol) is minimized by continuously refreshing the reaction feed inside the system.
We further examined whether the addition (instead of in situ generation) of a carboxylic acid influenced the performance of the enzyme catalyst. A slight decrease in enzyme reactivity was observed (see Section S17) being ascribed to the presence of oxidative inhibition along with the formation of short-chain alcohols (EtOH) that proved to be harmful to the enzyme (see Scheme 1).44,57−59 To avoid the generation of EtOH, the possibility of directly using acetic acid and conducting the catalysis in 2-methyl-tetrahydrofuran (Me-THF) was evaluated. A long-run experiment for the epoxidation of (+)-3-carene by Novozym 435/H2O2 was conducted (CH3COOH: 5.0 equiv, H2O2: 1.2 equiv; see Figures S4 and S5). The process outcome from the two flow processes (with and without the addition of CH3COOH) showed comparable results, and therefore, we proceeded with our studies with in situ formed acetic acid.
Other terpenes were then investigated (Scheme 4) under comparable continuous flow conditions. First, d-limonene was epoxidized affording an 83:17 mixture of limonene oxide (LO) and limonene dioxide (LDO) at 92% substrate conversion with a yield for LO of 76%. Further attempts to achieve full substrate conversion and minimize the formation of LDO were not successful. Similar continuous flow conditions though using 1 g of enzyme catalyst in the PBR while flowing through a liquid stream containing 2.4 equiv of H2O2 to limonene proved to be productive toward the preparation of LDO (65% isolated yield) starting from LO. Notably, an increase in the reaction temperature and/or further raising the amount of catalyst along with the reaction time did not help to improve the yield of LDO. Additionally, attempts to prepare LDO directly from limonene did not provide encouraging results.
Scheme 4. Epoxidation and Bis-Epoxidation of Various Terpenes Using Novozym 435/H2O2 under Continuous Flow Conditions.
Reaction conditions: [a] alkene (1 mmol, 1 equiv), H2O2 (30% in H2O, 1.2 equiv), EtOAc (0.4 M), 0.5 g of catalyst. NMR yield after reaching a steady-state regime. [b] Productivity of the catalyst system expressed in mg (product) h–1. [c] A 1.0 g catalyst was used. [d] H2O2 (30% in H2O, 2.4 equiv) using the corresponding monoepoxide as the starting material. τ stands for residence time and P stands for productivity.
The developed flow epoxidation protocol was then applied to myrcene, and full conversion of this substrate was easily achieved providing a 93:7 mixture of MO (yield: 93%) and MDO (Scheme 4). The bisepoxy derivative MDO (mixture of two regioisomers) could be obtained by conducting the flow experiment at 50 °C in the presence of an excess of H2O2 (2.4 equiv) and using a packed bed column with 1.0 g of the catalyst while using MO as the substrate. Under these conditions, the highest conversion (60%) to MDO was detected (54% isolated yield) with a regioisomeric ratio (rr) of 1:4, similar to what was observed under batch conditions for this transformation.
Previous batch process experiments involving the use of mCPBA as the oxidant showed that β-elemene can be epoxidized to both its monoepoxide (BEM, 50% yield) and bisepoxy derivative (BED, 97% yield) using 1.0 and 2.2 equiv of oxidant, respectively.55 Under continuous flow (Scheme 4, bottom part), BEM can be obtained in >99% yield using 1.0 g of enzyme catalyst at 50 °C without any observable formation of the bisepoxy derivative BED, which is a significant improvement of the batch process outcome. Lower reaction temperatures and lower amounts of the catalyst only resulted in the partial conversion of the substrate. Similar reaction conditions were applied for the synthesis of BED, and to obtain a high yield of product (90%), it was necessary to use BEM as the starting point. The latter could be simply recovered from this flow process and used for subsequent oxidations enabling a high atom economy. The second oxidation step (BEM → BED) required the presence of higher amounts (2.4 equiv) of H2O2 as this alkene fragment is less reactive due to a higher degree of steric hindrance.55
According to the results illustrated in Scheme 4, the benefits arising from the use of a continuous flow setup are (1) shorter reaction times (the residence times under continuous flow are shorter than the reaction times required for the batch conversions), (2) the possibility to operate the catalytic system for a longer period under flow without losing significant activity (cf. the catalyst lifetime) as supported by the calculated catalyst productivities in the epoxidation of (+)-3-carene in batch and continuous flow mode, and as a consequence, (3) easier scale-up of the process.
Finally, as a proof of concept, the protocol was extended to the same biobased polymers explored under a batch regime (Scheme 3). Under continuous flow, poly(myrcene oxide) (PMO) was prepared with a functionalization degree of 87% (Scheme 5) similar to the one (89%) obtained under heterogeneous batch conditions while utilizing 1.0 g of the catalyst, a flow rate of 0.05 mL min–1, and a residence time (τ) of 70 min. However, substantially lower degrees of alkene epoxidation were achieved using PLC and poly(BEM-alt-PA). In the case of PLC as the substrate, only 34% of the pendant alkene groups were converted into oxiranes (94% under batch operation), and for poly(BEM-alt-PA), 26% of monosubstituted double bonds were epoxidized as opposed to 70% in the batch experiment. Although these functionalization degrees attained under continuous flow are lower with respect to the ones obtained under batch conditions, for polymer cross-linking, a partial functionalization is often preferred with the objective to minimize steric hindrance issues that could subsequently lead to a lower reactivity in the presence of curing agents.60,61 A residual presence of reactive groups after a curing procedure is also not desired as it could cause undesired colorization of a material and/or other side-reactions within the polymer matrix lowering the material’s functionality and stability. Since both batch and continuous flow approaches deliver distinct functionalization degrees of PLC and poly(BEM-alt-PA), different polymer formulations may be prepared, thereby controlling the amount of cross-linking in the matrix upon curing.
Scheme 5. Epoxidation of Terpene-Based Polymers using Novozym 435 under Continuous Flow Conditions.
Reaction conditions: polymer (1.0 mmol, 1.0 equiv), H2O2 (30% in H2O, 2.4 equiv), EtOAc (0.4 M), 1.0 g of catalyst. Mn and Đ values were determined by GPC in THF calibrated with PS standards. Tg values were determined by DSC analysis at a 10 °C min–1 heating/cooling rate; data are from the second heating cycle. Td values were obtained by thermogravimetric analysis; data refer to the values at 5 wt % loss. P stands for productivity expressed in mmol (product) h–1 g(cat)−1 of the polymer obtained with the achieved functionalization degree. [a] Functionalization degrees were determined by 1H NMR (CDCl3). [b] The solvent was CH2Cl2/EtOAc, 4:1 v/v. [c] The solvent was toluene/EtOAc, 4:1 v/v.
Conclusions
In summary, we have applied a heterogeneous epoxidation protocol for the preparation of terpene oxides using a recyclable supported Lipase B obtained from Candida antarctica (Novozym 435) in the presence of H2O2 and EtOAc. This protocol represents a general tool for terpene epoxidation and biobased polymer epoxidation avoiding the use of toxic reagents and minimizing the formation of byproducts. The process has been explored both under heterogeneous batch and continuous flow conditions. The use of enzymes as catalysts in chemoenzymatic epoxidation shows some advantages over traditional methodologies, e.g., mCPBA epoxidation, such as mild reaction conditions and lower environmental impact. The use of a continuous flow strategy makes the process operationally simple to perform and simplifies catalyst recovery and recycling to improve the overall sustainability of the process. Compared to batch operation, continuous flow epoxidation reported here allows typically for faster epoxidation kinetics (see comparative Table S6) at similar product yields. Additionally, the protocol is more attractive when polymers are designed for biomedical applications since no metal catalysts are used and the downstream purification of the products is easily achieved. This enables epoxide compounds to be safely prepared in short reaction times, with the flow protocol used to produce multigram quantities. Overall, this green, sustainable, and scalable catalytic epoxidation protocol provides a valuable synthetic tool for transforming cheap biorenewable feedstock into valuable chemical building blocks within a biorefinery context.
Acknowledgments
The authors thank the CERCA program/Generalitat de Catalunya, ICREA, MICINN (PID2020-112684GB-100, PID2019-109236RB-I00, the Severo Ochoa Excellence Accreditation 2020–2023 CEX2019-000925-S) and the ERDF (European Regional Development Fund) through the POCTEFA Interreg program (EFA308/19, TRIPyr) for support. D.H.L. acknowledges MICINN for a postdoctoral fellowship (PDC2021-120952-I00, MacroLemon). The authors further thank Isobionics for a kind donation of β-elemene and Novozyme for the generous donation of Novozym 435.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acssuschemeng.3c00370.
Experimental and analytical procedures and relevant spectra of compounds including NMR, GPC, DSC, and TGA analyses (PDF)
Author Present Address
∥ Department of Environment and Prevention Sciences, University of Ferrara, Via L. Borsari, 46, I-44121 Ferrara, Italy
The authors declare no competing financial interest.
Supplementary Material
References
- Cywar R. M.; Rorrer N. A.; Hoyt C. B.; Beckham G. T.; Chen E. Y. -X. Bio-based polymers with performance-advantaged properties. Nat. Rev. Mater. 2022, 7, 83–103. 10.1038/s41578-021-00363-3. [DOI] [Google Scholar]
- Zhu Y.; Romain C.; Williams C. Sustainable polymers from renewable resources. Nature 2016, 540, 354–362. 10.1038/nature21001. [DOI] [PubMed] [Google Scholar]
- Sheldon R. A. Green and Sustainable Manufacture of Chemicals from Biomass: State of the Art. Green Chem. 2014, 16, 950–963. 10.1039/C3GC41935E. [DOI] [Google Scholar]
- Sheldon R. A.; Arends I.; Hanefield U.. Green Chemistry and Catalysis; Wiley-VCH, 2007. [Google Scholar]
- Shanks B. H.; Keeling P. L. Bioprivileged molecules: creating value from biomass. Green Chem. 2017, 19, 3177–3185. 10.1039/C7GC00296C. [DOI] [Google Scholar]
- Zhang X.; Fevre M.; Jones G. O.; Waymouth R. M. Catalysis as an Enabling Science for Sustainable Polymers. Chem. Rev. 2018, 118, 839–885. 10.1021/acs.chemrev.7b00329. [DOI] [PubMed] [Google Scholar]
- Lee S. Y.; Kim H. U.; Chae T. U.; Cho J. S.; Kim J. W.; Shin J. H.; Kim D. I.; Ko Y.-K.; Jang W. D.; Jang Y.-S. A comprehensive metabolic map for production of bio-based chemicals. Nat. Catal. 2019, 2, 18–33. 10.1038/s41929-018-0212-4. [DOI] [Google Scholar]
- Wheeldon I.; Christopher P.; Blanch H. Integration of heterogeneous and biochemical catalysis for production of fuels and chemicals from biomass. Curr. Opin. Biotechnol. 2017, 45, 127–135. 10.1016/j.copbio.2017.02.019. [DOI] [PubMed] [Google Scholar]
- Huo J.; Shanks B. H. Bioprivileged Molecules: Integrating Biological and Chemical Catalysis for Biomass Conversion. Annu. Rev. Chem. Biomol. Eng. 2020, 11, 63–85. 10.1146/annurev-chembioeng-101519-121127. [DOI] [PubMed] [Google Scholar]
- Tsolakis N.; Barn W.; Srai J. S.; Kumar M. Renewable chemical feedstock supply network design: The case of terpenes. J. Cleaner Prod. 2019, 222, 802–822. 10.1016/j.jclepro.2019.02.108. [DOI] [Google Scholar]
- Schwab W.; Fuchs C.; Huang F. C. Transformation of terpenes into fine chemicals. Eur. J. Lipid Sci. Technol. 2013, 115, 3–8. 10.1002/ejlt.201200157. [DOI] [Google Scholar]
- Golets M.; Ajaikumar S.; Mikkola J. P. Catalytic Upgrading of Extractives to Chemicals: Monoterpenes to “EXICALS”. Chem. Rev. 2015, 115, 3141–3169. 10.1021/cr500407m. [DOI] [PubMed] [Google Scholar]
- Lamparelli D. H.; Paradiso V.; Della Monica F.; Proto A.; Guerra S.; Giannini L.; Capacchione C. Toward more sustainable elastomers: Stereoselective copolymerization of linear terpenes with butadiene. Macromolecules 2020, 53, 1665–1673. 10.1021/acs.macromol.9b02646. [DOI] [Google Scholar]
- Lamparelli D. H.; Paradiso V.; Capacchione C. New elastomeric materials from biomass: stereoselective polymerization of linear terpenes and their copolymerization with butadiene by using a cobalt complex with phosphane ligands. Rubber Chem. Technol. 2020, 93, 605–614. 10.5254/rct.20.79972. [DOI] [Google Scholar]
- Lamparelli D. H.; Winnacker M.; Capacchione C. Stereoregular Polymerization of Acyclic Terpenes. ChemPlusChem 2022, 87, e20210036 10.1002/cplu.202100366. [DOI] [PubMed] [Google Scholar]
- Silvestre A. J. D.; Gandini A.. Terpenes: Major Sources, Properties, and Applications in Monomers, Polymers and Composites from Renewable Resources; Belgacem M. N.; Gandini A., Eds.; Elsevier: Amsterdam, 2008; Chapter 2, p 17. [Google Scholar]
- Della Monica F.; Kleij A. W. From terpenes to sustainable and functional polymers. Polym. Chem. 2020, 11, 5109–5127. 10.1039/D0PY00817F. [DOI] [Google Scholar]
- Wilbon P. A.; Chu F.; Tang C. Progress in Renewable Polymers from Natural Terpenes, Terpenoids, and Rosin. Macromol. Rapid Commun. 2013, 34, 8–37. 10.1002/marc.201200513. [DOI] [PubMed] [Google Scholar]
- Mosquera M. E. G.; Jiménez G.; Tabernero V.; Vinueza-Vaca J.; García-Estrada C.; Kosalková K.; Sola-Landa A.; Monje B.; Acosta C.; Alonso R.; Valera M. A. Terpenes and Terpenoids: Building Blocks to Produce Biopolymers. Sustainable Chem. 2021, 2, 467–492. 10.3390/suschem2030026. [DOI] [Google Scholar]
- Tibbetts J. D.; Cunningham W. B.; Vezzoli M.; Plucinskic P.; Bull S. D. Sustainable catalytic epoxidation of biorenewable terpene feedstocks using H2O2 as an oxidant in flow microreactors. Green Chem. 2021, 23, 5449–5455. 10.1039/D1GC01734A. [DOI] [Google Scholar]
- Tibbetts J. D.; Bull S. D. p-Menthadienes as Biorenewable Feedstock for a Monoterpene-Based Biorefinery. Adv. Sustainable Syst. 2021, 5, 2000292 10.1002/adsu.202000292. [DOI] [Google Scholar]
- Sienel G.; Rieth R.; Rowbottom K. T.. Epoxides, in Ullmann’s Encyclopedia of Industrial Chemistry. 2012; 139.
- Reeves R.; Lawrence M.. Epoxides, Synthesis, Reactions and Uses Nova Science, Lancaster (UK) 2017.
- Brandolese A.; Kleij A. W. Catalyst Engineering Empowers the Creation of Biomass-Derived Polyesters and Polycarbonates. Acc. Chem. Res. 2022, 55, 1634–1645. 10.1021/acs.accounts.2c00204. [DOI] [PubMed] [Google Scholar]
- Ortiz C.; Ferreira M. L.; Barbosa O.; dos Santos J. C. S.; Rodrigues R. C.; Berenguer-Murcia Á.; Briand L. E.; Fernandez-Lafuente R. Novozym 435: the “perfect” lipase immobilized biocatalyst?. Catal. Sci. Technol. 2019, 9, 2380–2420. 10.1039/C9CY00415G. [DOI] [Google Scholar]
- Björkling F.; Godtfredsen S. E.; Kirk O. J. Lipase-mediated formation of peroxycarboxylic acids used in catalytic epoxidation of alkenes. J. Chem. Soc., Chem. Commun. 1990, 19, 1301–1303. 10.1039/C39900001301. [DOI] [Google Scholar]
- Björkling F.; Frykman H.; Godtfredsen S. E.; Kirk O. Lipase catalyzed synthesis of peroxycarboxylic acids and lipase mediated oxidations. Tetrahedron 1992, 48, 4587–4592. 10.1016/S0040-4020(01)81232-1. [DOI] [Google Scholar]
- Skouridou V.; Stamatis H.; Kolisis F. N. Lipase-mediated epoxidation of α-pinene. J. Mol. Catal. B: Enzym. 2003, 21, 67–69. 10.1016/S1381-1177(02)00141-8. [DOI] [Google Scholar]
- Su W.; Li Q.; Liu Y.; Qin Y.; Liu H.; Tang A. Improved efficiency of lipase-mediated epoxidation of α-pinene using H2O2 in single-phase systems. Mol. Catal. 2021, 508, 111585–111592. 10.1016/j.mcat.2021.111585. [DOI] [Google Scholar]
- Skouridou V.; Stamatis H.; Kolisis F. N. A Study on the Process of Lipase-catalyzed Synthesis of α-pinene Oxide in Organic Solvents. Biocatal. Biotransform. 2003, 21, 285–290. 10.1080/10242420310001597801. [DOI] [Google Scholar]
- Salvi H. M.; Yadav G. D. Chemoenzymatic Epoxidation of Limonene Using a Novel Surface-Functionalized Silica Catalyst Derived from Agricultural Waste. ACS Omega 2020, 5, 22940–22950. 10.1021/acsomega.0c02462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiemann L. O.; Faltl C.; Sieber V. Lipase-mediated Epoxidation of the Cyclic Monoterpene Limonene to Limonene Oxide and Limonene Dioxide. Z. Naturforsch., B 2012, 67, 1056–1060. 10.5560/znb.2012-0167. [DOI] [Google Scholar]
- Melchiors M. S.; Vieira T. Y.; Pereira L. P. S.; Carciofi B. A. M.; de Araujo P. H. H.; de Oliveira D.; Sayer C. Epoxidation of (R)-(+)-Limonene to 1,2-Limonene Oxide Mediated by Low-Cost Immobilized Candida antarctica Lipase Fraction B. Ind. Eng. Chem. Res. 2019, 58, 13918–13925. 10.1021/acs.iecr.9b02168. [DOI] [Google Scholar]
- Tzialla A. A.; Kalogeris E.; Enotiadis A.; Taha A. A.; Gournis D.; Stamatis H. Effective immobilization of Candida antarctica lipase B in organic-modified clays: Application for the epoxidation of terpenes. Mater. Sci. Eng. B 2009, 165, 173–177. 10.1016/j.mseb.2009.09.003. [DOI] [Google Scholar]
- Salvi H. M.; Yadav G. D. Chemoenzymatic Epoxidation of Limonene Using a Novel Surface-Functionalized Silica Catalyst Derived from Agricultural Waste. ACS Omega 2020, 5, 22940–22950. 10.1021/acsomega.0c02462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira M. A.; Nascimento M. G. Chemo-enzymatic epoxidation of (+)-3-carene. Catal. Commun. 2007, 8, 2043–2047. 10.1016/j.catcom.2007.02.032. [DOI] [Google Scholar]
- Ranganathan S.; Sieber V. Development of semi-continuous chemo-enzymatic terpene epoxidation: combination of anthraquinone autooxidation and the lipase-mediated epoxidation process. React. Chem. Eng. 2017, 2, 885–895. 10.1039/C7RE00112F. [DOI] [Google Scholar]
- McQuade D. T.; Seeberger P. H. Applying Flow Chemistry: Methods, Materials, and Multistep Synthesis. J. Org. Chem. 2013, 78, 6384–6389. 10.1021/jo400583m. [DOI] [PubMed] [Google Scholar]
- Britton J.; Majumdar S.; Weiss G. A. Continuous flow biocatalysis. Chem. Soc. Rev. 2018, 47, 5891–5918. 10.1039/C7CS00906B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plutschack M. B.; Pieber B.; Gilmore K.; Seeberger P. H. The Hitchhiker’s Guide to Flow Chemistry. Chem. Rev. 2017, 117, 11796–11893. 10.1021/acs.chemrev.7b00183. [DOI] [PubMed] [Google Scholar]
- De Santis P.; Meyer L.-E.; Kara S. The rise of continuous flow biocatalysis – fundamentals, very recent developments and future perspectives. React. Chem. Eng. 2020, 5, 2155–2184. 10.1039/D0RE00335B. [DOI] [Google Scholar]
- Britton J.; Majumdar S.; Weiss G. A. Continuous Flow Biocatalysis,. Chem. Soc. Rev. 2018, 47, 5891–5918. 10.1039/C7CS00906B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santi M.; Sancineto L.; Nascimento V.; Azeredo J. B.; Orozco; Erika V. M.; Andrade L. H.; Gröger H.; Santi C. A Challenging Alternative for the Synthesis of APIs and Natural Compounds. Int. J. Mol. Sci. 2021, 22, 990–1022. 10.3390/ijms22030990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiles C.; Hammond M. J.; Watts P. The development and evaluation of a continuous flow process for the lipase-mediated oxidation of alkenes. Beilstein J. Org. Chem. 2009, 5, 27 10.3762/bjoc.5.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baraldi S.; Fantin G.; Di Carmine G.; Ragno D.; Brandolese A.; Massi A.; Bortolini O.; Marchetti N.; Giovannini P. P. Enzymatic synthesis of biobased aliphatic–aromatic oligoesters using 5,5′-bis(hydroxymethyl)furoin as a building block. RSC Adv. 2019, 9, 29044–29050. 10.1039/C9RA06621G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maquilón C.; Brandolese A.; Alter C.; Hoevelmann C.; Della Monica F.; Kleij A. W. Renewable Beta-Elemene Based Cyclic Carbonates for the Preparation of Oligo(hydroxyurethane)s. ChemSusChem 2022, 15, e202201123 10.1002/cssc.202201123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The addition of 2.4 equiv of H2O2 to the catalytic mixture resulted into an emulsion, whereas addition of higher amounts of the oxidant results into a biphasic system. Please note that we did not check for residual (volatile) H2O2 at the end of the reaction. Caution: this aspect may be of significance when larger-scale operations are conducted as H2O2 is potentially explosive, and proper safety measures need to be considered.
- Paquette L. A.; Ross R. J.; Shi Y.-J. Regioselective routes to nucleophilic optically active 2- and 3-carene systems. J. Org. Chem. 1990, 55, 1589–1598. 10.1021/jo00292a039. [DOI] [Google Scholar]
- Fiorani G.; Stuck M.; Martín C.; Martinez Belmonte M.; Martín E.; Escudero-Adán E. C.; Kleij A. W. Catalytic Coupling of Carbon Dioxide with Terpene Scaffolds: Access to Challenging Bio-Based Organic Carbonates. ChemSusChem 2016, 9, 1304–1311. 10.1002/cssc.201600238. [DOI] [PubMed] [Google Scholar]
- Jarvie A. W. P.; Overton N.; St Pourçain C. B. Enzyme catalysed modification of synthetic polymers. J. Chem. Soc., Perkin Trans. 1999, 1, 2171–2176. 10.1039/a902165e. [DOI] [Google Scholar]
- Uyama H.; Kuwabara M.; Tsujimoto T.; Kobayashi S. Enzymatic Synthesis and Curing of Biodegradable Epoxide-Containing Polyesters from Renewable Resources. Biomacromolecules 2003, 4, 211–215. 10.1021/bm0256092. [DOI] [PubMed] [Google Scholar]
- Moreira V. B.; Alemán C.; Rintjema J.; Bravo F.; Kleij A. W.; Armelin E. A Biosourced Epoxy Resin for Adhesive Thermoset Applications. ChemSusChem 2022, 15, e202102624 10.1002/cssc.202102624. [DOI] [PubMed] [Google Scholar]
- Bonamigo Moreira V.; Rintjema J.; Bravo F.; Kleij; A W.; Franco L.; Puiggalí J.; Alemán; Armelin E. Novel Biobased Epoxy Thermosets and Coatings from Poly(limonene carbonate) Oxide and Synthetic Hardeners. ACS Sustainable Chem. Eng. 2022, 10, 2708–2719. 10.1021/acssuschemeng.1c07665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindermann N.; Cristofol À.; Kleij A. W. Access to Biorenewable Polycarbonates with Unusual Glass-Transition Temperature (Tg) Modulation. ACS Catal. 2017, 7, 3860–3863. 10.1021/acscatal.7b00770. [DOI] [Google Scholar]
- Della Monica F.; Kleij A. W. Synthesis and Characterization of Biobased Polyesters with Tunable Tg by ROCOP of Beta-Elemene Oxides and Phthalic Anhydride. ACS Sustainable Chem. Eng. 2021, 9, 2619–2625. 10.1021/acssuschemeng.0c09189. [DOI] [Google Scholar]
- The residence time (τ) for flow mode experiments were determined using a methyl red solution: flow rate = 0.1 mL/min; 1.0 g of catalyst, residence time = 35 min.
- José C.; Bonetto R. D.; Gambaro L. A.; del Pilar Guauque Torres M.; Foresti M. L.; Ferreira M. L.; Brian L. E. Investigation of the causes of deactivation–degradation of the commercial biocatalyst Novozym 435® in ethanol and ethanol–aqueous media. J. Mol. Catal. B: Enzym. 2011, 71, 95–107. 10.1016/j.molcatb.2011.04.004. [DOI] [Google Scholar]
- Mangiagalli M.; Ami D.; de Divitiis M.; Brocca S.; Catelani T.; Natalello A.; Lotti M. Short-chain alcohols inactivate an immobilized industrial lipase through two different mechanisms. Biotechnol. J. 2022, 17, 2100712 10.1002/biot.202100712. [DOI] [PubMed] [Google Scholar]
- Zhou P.; Wang X.; Yang B.; Hollmannc F.; Wang Y. Chemoenzymatic epoxidation of alkenes with Candida antarctica lipase B and hydrogen peroxide in deep eutectic solvent. RSC Adv. 2017, 7, 12518–12523. 10.1039/C7RA00805H. [DOI] [Google Scholar]
- Functional Polymers by Post-Polymerization Modification: Concepts; Theato P.; Klok H.-A., Eds.; Wiley: Weinheim (Germany), 2012. [Google Scholar]
- Gauthier M. A.; Gibson M. I.; Klok H.-A. Synthesis of Functional Polymers by Post-Polymerization Modification. Angew. Chem., Int. Ed. 2009, 48, 48–58. 10.1002/anie.200801951. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.