Abstract
The blood–brain barrier (BBB) plays a critical role in maintaining the equilibrium between amyloid beta (Aβ) levels in blood and the brain by regulating Aβ transport. Our previous publications demonstrated that BBB trafficking of Aβ42 and Aβ40 is distinct and is disrupted under various pathophysiological conditions. However, the intracellular mechanisms that allow BBB endothelium to differentially handle Aβ40 and Aβ42 have not been clearly elucidated. In this study, we identified mechanisms of Aβ endocytosis in polarized human cerebral microvascular endothelial cell monolayers. Our studies demonstrated that Aβ peptides with fluorescent label (F-Aβ) were internalized by BBB endothelial cells via energy, dynamin, and actin-dependent endocytosis. Interestingly, endocytosis of F-Aβ40 but not F-Aβ42 was substantially reduced by clathrin inhibition, whereas F-Aβ42 but not F-Aβ40 endocytosis was reduced by half after inhibiting the caveolae-mediated pathway. Following endocytosis, both isoforms were sorted by the endo-lysosomal system. Although Aβ42 was shown to accumulate more in the lysosomes, which could lead to its higher degradation and/or aggregation at lower lysosomal pH, Aβ40 demonstrated robust accumulation in recycling endosomes, which may facilitate its exocytosis by the endothelial cells. These results provide a mechanistic insight into the selective ability of BBB endothelium to transport Aβ40 versus Aβ42. This knowledge contributes to the understanding of molecular pathways underlying Aβ accumulation in the BBB endothelium and associated BBB dysfunction. Moreover, it allows us to establish mechanistic rationale for altered Aβ40:Aβ42 ratios and anomalous amyloid deposition in the cerebral vasculature as well as brain parenchyma during Alzheimer’s disease progression.
SIGNIFICANCE STATEMENT
Differential interaction of Aβ40 and Aβ42 isoforms with the blood–brain barrier (BBB) endothelium may contribute to perturbation in Aβ42:Aβ40 ratio, which is associated with Alzheimer’s disease (AD) progression and severity. The current study identified distinct molecular pathways by which Aβ40 and Aβ42 are trafficked at the BBB, which regulates equilibrium between blood and brain Aβ levels. These findings provide molecular insights into mechanisms that engender BBB dysfunction and promote Aβ accumulation in AD brain.
Introduction
Mounting evidence suggests that reduction in the ratio of amyloid beta peptide 42 (Aβ42) and Aβ40, two major Aβ isoforms that accumulate in Alzheimer’s disease (AD) patient plasma and the cerebrospinal fluid is associated with the severity of AD pathology and is emerging as a reliable biomarker for AD diagnosis (Lewczuk et al., 2004; Doecke et al., 2020). Changes in Aβ42:Aβ40 ratio during AD progression may not be solely driven by the differential amyloidogenicity of Aβ42 and Aβ40; differential handling of Aβ0 and Aβ42 by the blood–brain barrier (BBB) and other disposition (distribution, metabolism, and elimination) pathways may also play a substantial role.
BBB is a physiologic interface between blood and the brain that maintains the brain homeostasis by delivering essential nutrients to the brain and removing toxic metabolites out of the brain (Ballabh et al., 2004; Abbott et al., 2010). The carrier and barrier functions of the BBB are coordinated by selective internalization and intricate intracellular trafficking apparatus (Villaseñor et al., 2019). The luminal-to-abluminal transport of Aβ was reported to occur via the receptor for advanced glycation end products (Deane et al., 2003), whereas the low-density lipoprotein receptor related protein-1 (Shibata et al., 2000; Storck et al., 2016) and P-glycoprotein (Candela et al., 2010) were claimed to mediate brain-to-blood efflux. However, the molecular machinery by which these receptors and/or transporters mediate cell entry and subsequent intracellular transit of Aβ in the BBB endothelial cells is only partially characterized.
Impairment of Aβ transport at the BBB is believed to promote the formation of cerebrovascular amyloid deposits and amyloid plaques found in the brains of AD patients. Disrupted Aβ trafficking at the BBB is also presumed to increase anomalous accumulation of Aβ peptides in the BBB endothelium that compromise BBB integrity (Wan et al., 2015) and function (Hooijmans et al., 2007) and trigger inflammatory changes (Miao et al., 2005). Therefore, elucidating molecular mechanisms underlying Aβ trafficking disruption is critical for identifying novel therapeutic strategies.
Our recent study demonstrated that these two isoforms exhibit distinct trafficking and accumulation kinetics at the BBB endothelium (Wang et al., 2021), which could be attributed to distinct trafficking apparatus employed by Aβ40 and Aβ42, although they are thought to be transported by the same receptors/transporters. Elucidation of the underlying mechanisms by which Aβ isoforms are differentially internalized and sorted in the BBB endothelial cells may lead to the identification of molecular mediators that drive pathologic shifts in Aβ42:Aβ40 ratios in plasma and the brain during AD progression.
Extensive research describing the mechanisms underlying intraneuronal accumulation of Aβ peptides has been reported. Previous work conducted by us and others has demonstrated that Aβ40 and Aβ42 are internalized by distinct mechanisms (Kandimalla et al., 2009; Wesén et al., 2017) and follow different itineraries in neuronal cells (Omtri et al., 2012). However, similar studies have not been conducted in BBB endothelial cells. Based on these reports, we hypothesize that Aβ40 and Aβ42 employ distinct molecular mechanisms to enter and traffic within the BBB endothelium. To test this hypothesis, we systematically dissected the cellular uptake and intracellular itineraries of fluorescently labeled soluble Aβ40 and Aβ42 (F-Aβ40 or F-Aβ42) in BBB cell culture models in vitro. We quantified the intracellular accumulation of F-Aβ using flow cytometry and confocal microscopy following pharmacological inhibition and small interfering RNA (siRNA) knockdowns to investigate the contributions of known endocytic mechanisms. Further, we assessed the intracellular distributions of F-Aβ isoforms by labeling various endo-lysosomal organelles followed by live cell imaging. Our studies revealed that F-Aβ40 and F-Aβ42 are endocytosed by BBB endothelial cells via distinct molecular pathways and are differentially sorted by the endo-lysosomal system, which could lead to their distinct trafficking and accumulation kinetics in the BBB endothelium.
Materials and Methods
Cell Culture
The immortalized human cerebral microvascular endothelial (hCMEC/D3) cell line was a gift from P-O Couraud (Institut Cochin, France). Cells were grown according to the culture conditions reported previously (Weksler et al., 2013). Polarized hCMEC/D3 cell monolayers were cultured on collagen-coated (Corning, MA) coverslip bottomed dishes (Mattek, MA), on 6-well plates, or on Transwell filters (Corning Costar, MA) at 5% CO2 and 37°C. All the monolayers were moved to low-serum (1% serum) medium, one night before the experiment. Bovine brain microvascular endothelial (BBME) cells were obtained from Cell Applications Inc. (San Diego, CA, Cat# B840-05). The BBME cellular monolayers were grown as reported previously (Agyare et al., 2013). Formation of monolayers on Transwell filters with good tight junctional integrity was confirmed by measuring the trans-endothelial electrical resistance across the monolayers. Cell cultures were routinely inspected for possible mycoplasma contamination.
Microscopy and Cellular Imaging
The hCMEC/D3 or BBME monolayers were treated with 1 µM of F-Aβ40, F-Aβ42, sulforhodamine 101 (SR101)-labeled Aβ40, or SR101-Aβ42 for 60 minutes followed by 20 µg/ml of Alexa Fluor-labeled transferrin (AF633-TRF) treatment for 30 minutes. In case of inhibitor experiments, the cells were pretreated with inhibitor for the designated time and then incubated with F-Aβ. Then the cells were washed with PBS and the nuclei were stained by incubating with Hoechst dye (0.5 µg/ml in PBS) for 5 minutes. At the end, the dishes were fixed in 4% para-formaldehyde (PFA) at 4°C for 60 minutes, mounted with ProLong gold antifade reagent (Life Technologies, OR), and dried overnight before imaging by laser confocal microscopy as described in our previous publication (Kandimalla et al., 2009).
Clathrin/Caveolin-1 Knockdowns and K44-Negative Dominant Dynamin Transfections
Clathrin and caveolin-1 knockdown in polarized hCMEC/D3 cell monolayers was performed using siRNA kit containing RNAi mix (Invitrogen, CA) and reduced serum medium, Opti-MEM (Gibco, NY). The cells were allowed to recover for 48 hours in hCMEC/D3 medium (5% FBS) before the uptake experiment was performed. GFP and non-GFP K44-negative–dominant dynamin transfections of hCMEC/D3 cell monolayers were performed using Lipofectamine reagent (Invitrogen, CA) per manufacturer’s protocol. The cells were allowed to recover for 24 hours in hCMEC/D3 medium (1% FBS), and the experiment was performed the next day.
Rab Protein Transfections
m-Cherry fluorescent protein fused to Rab5 (m-CFP/Rab5), green fluorescent protein fused to Rab7 (GFP/Rab7), and green fluorescent protein fused to Rab11 (GFP/Rab11) plasmids were obtained as described in our previous publications (Omtri et al., 2018). Transfections of hCMEC/D3 cell monolayers were performed using Lipofectamine reagent as per manufacturer’s protocol. The cells were allowed to recover for 24 hours in hCMEC/D3 medium (1% FBS), and the experiment was performed the following day.
Flow Cytometry
Following the treatment with 1 µM F-Aβ for 60 minutes, hCMEC/D3 cells were washed thoroughly with PBS and gently trypsinized with trypsin-EDTA for 2 minutes and neutralized with FBS. In case of inhibitor experiments, the cells were pretreated with inhibitor for the designated time and then incubated with F-Aβ. The dislodged cells were washed twice using ice cold PBS, fixed with 4% PFA solution, and analyzed for intracellular fluorescence using BD FACSCalibur flow cytometer. The F-Aβ40 and F-Aβ42 intracellular fluorescence intensities were measured using a 488 nm laser fitted with a 530/30 filter. Data were acquired with BD CellQuest Pro and analyzed using FlowJo software. The F-Aβ uptake was represented as histograms of intracellular fluorescence intensities. All sample analyses were performed within 1 hour from the completion of the experiment.
Western Blotting
Following the treatment with 0.25 µM Aβ40 or Aβ42 in Dulbecco’s modified Eagle’s medium (DMEM) for 15 minutes, the cells were washed three times with PBS and lysed with a radioimmunoprecipitation assay buffer containing protease and phosphatase inhibitors (Sigma-Aldrich, St. Louis. MO). Total protein concentration in the lysates was determined by bicinchoninic acid assay (Pierce, Waltham, MA). Lysates (25 µg protein per lane) were loaded onto 4%–12% Criterion XT precast gels, and proteins were separated by SDS-PAGE under reducing conditions (Bio-Rad Laboratories, Hercules, CA). The proteins were then electroblotted onto a 0.45 µm nitrocellulose membrane. Membranes were blocked with 5% nonfat dry milk protein (Bio-Rad Laboratories), followed by overnight incubation at 4°C with primary antibodies (1:1000) against phospho-caveolin-1 (Y14) (#3251), caveolin-1 (#3267), and vinculin (#13901) (Cell Signaling Technology, Danvers, MA). Afterward, the membrane was incubated with IR-dye conjugated secondary antibody (1:2000) (LI-COR Inc., Lincoln, NE; LIC-926-32211) for 1 hour at room temperature. Immunoreactive bands were then imaged (Odyssey CLx; LI-COR Inc.), and the band intensities were quantified by densitometry (Image Studio Lite Software, LI-COR Inc.).
ELISA
Following the 48-hour recovery of vehicle/siRNA transfections, hCMEC/D3 cells were incubated with 1 µM Aβ40/42 for 30 minutes. Afterward, cells were washed thoroughly with ice-cold PBS twice and harvested in 0.25% trypsin-EDTA. The resulting cell pellets were lysed in 50 µl radioimmunoprecipitation assay buffer containing protease inhibitor. The cell lysates were centrifuged at 10,000 rpm for 10 minutes to remove cell debris and used as samples for ELISA. Aβ levels were determined with Aβ40- and Aβ42-specific ELISA kits (KHB3481 and KHB3544, ThermoFisher) according to the manufacturer’s instructions. For data analysis, Aβ concentration was normalized by the total protein concentration and fold change compared with untreated control was calculated. The same cells lysates were also subjected to western blotting as described above to confirm the successful knockdown of caveolin-1.
Live Cell Imaging
Following the incubation with various fluorophores, the cells were washed twice, maintained in an atmosphere humidified with 5% CO2 in air, and imaged live using a TE-2000-S inverted microscope (Chiyoda-ku, Tokyo, Japan) equipped with Nikon FITC HQ and m-Cherry-A-zero filters. The images were captured using Nikon’s NIS elements AR 3.0 software and processed using ZEN Imaging software.
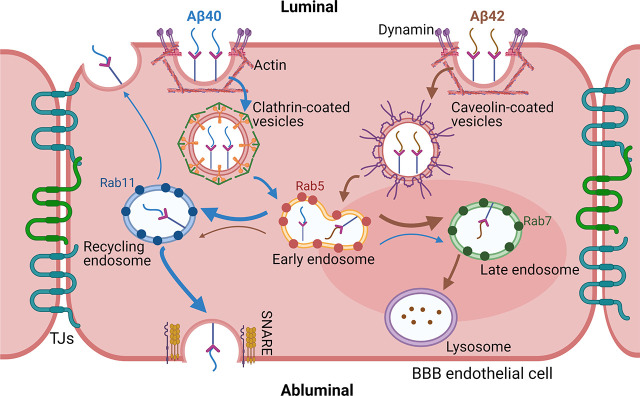
Mechanisms of F-Aβ40 and F-Aβ42 Endocytosis at the BBB Endothelium
Energy Dependence of F-Aβ Uptake
The polarized hCMEC/D3 cell monolayers grown on Transwell filters, and 6-well culture plates were preincubated with glucose-free DMEM supplemented with 50 mM 2-deoxy glucose and 0.1% sodium azide (ATP depletion medium) for 30 minutes before start of the experiment. The control cells were incubated with regular DMEM medium containing glucose. Then, 1 µM of either F-Aβ40 or F-Aβ42 was added to the luminal side of the Transwell filter and incubated for 30 minutes. The resultant intracellular fluorescence was assessed by flow cytometry.
Dynamin, Actin, Clathrin, and Lipid Raft-Mediated F-Aβ Endocytosis
Small molecule inhibitors were used to inhibit the dynamin-dependent vesicle pinch-off, actin polymerization, clathrin-dependent endocytosis, or lipid raft-mediated uptake to assess the contribution of these processes to the internalization of FAβ40 and F-Aβ42 by the BBB endothelium. In these experiments, polarized hCMEC/D3 monolayers were preincubated with medium containing 1% serum for at least 1 hour before the addition of inhibitors. The hCMEC/D3 cells grown on 6-well culture plates or on Transwell filters were preincubated with inhibitors for 30 minutes, then 1 µM of F-Aβ40 or F-Aβ42 was added and the monolayers were incubated for 60 minutes. The uptake of F-Aβ peptides was determined using flow cytometry. The following inhibitors and concentrations were used: 80 µM Dynasore (dynamin inhibitor) (Tocris Bioscience, MO), 10 µM cytochalasin A (actin inhibitor) (Cayman Chemical, MI), 200 µM monodansylcadaverine (MDC, specific clathrin inhibitor), 10 mM chlorpromazine (CPZ, nonspecific clathrin inhibitor) (MP Biomaterials, OH), 2.5 µM nystatin (specific lipid raft inhibitor), and 5 mM methyl-β-cyclodextrin (mβCD, nonspecific cholesterol chelator) (Acros Organics, NJ). All the control experiments were performed in a similar fashion without the addition of inhibitors, and the intracellular fluorescence was assessed by flow cytometry. For knockdown studies, control or knock-down cells were treated with F-Aβ peptides and AF633-Trf for 60 minutes. The confocal images were obtained after washing the cells with PBS and fixing with 4% PFA for 1 hour at 4°C. In a separate experiment, polarized BBME cell monolayers were also preincubated with 10 mM mβCD for 30 minutes, followed by incubation with 1 µM of F-Aβ40 or F-Aβ42, and 20 μg/ml AF633-TRF for 60 minutes. The z-series confocal micrographs were obtained after washing the filters with PBS and fixing with 4% PFA for 1 hour at 4°C. Then Transwells were mounted with Vectashield Antifade mounting medium with 4′,6-diamidino-2-phenylindole (Vector Laboratories, CA).
Intracellular Itineraries of F-Aβ40 and F-Aβ42 at the BBB Endothelium
Accumulation in Early Endosomes
hCMEC/D3 cells expressing m-CFP/Rab5 were incubated with 1 µM F-Aβ40 for 15 minutes. Live cell imaging was conducted up to 60 minutes after washing the cells with DMEM.
Accumulation in Secondary Endosomes
The cells were treated with 1 µM F-Aβ40 and Dil–low-density lipoprotein (LDL) (15 μg/ml) for 60 minutes. Then live cell imaging was conducted after washing the cells with DMEM.
Accumulation in Late Endosomes
hCMEC/D3 cells expressing GFP-labeled Rab7 were incubated with 1 µM SR101-Aβ40 or SR101-Aβ42 for 15 minutes. Then the cells were washed with DMEM and imaged by live cell imaging up to 60 minutes.
Accumulation in Recycling Endosomes
hCMEC/D3 cells expressing GFP/Rab11 were incubated with 1 µM SR101-Aβ40 or SR101-Aβ42 for 15 minutes. Then the cells were washed with DMEM and imaged by live cell imaging up to 60 minutes.
Accumulation in Lysosomes
The cells grown on coverslip-bottom dishes were treated with 1 µM F-Aβ40 or F-Aβ42 and 75 nM LysoTracker Red (Invitrogen-Molecular Probes, Carlsbad, CA) for 30 minutes at 37°C. Thereafter, the cells were washed with ice-cold PBS three times and imaged by confocal microscopy. The fluorescein and LysoTracker Red signals in the image were superimposed, and the extent of colocalization was estimated by Pearson’s correlation coefficients.
Statistical Analysis
Unless otherwise indicated in the figure legends, all the sample sizes (N) refer to the number of independent replicates used for statistical testing. For studies where technical replicates were used as the sample size to conduct statistical tests, the results were confirmed by at least two independent experiments. Error bars in all the bar charts represent the standard deviation. Statistical significance of differences between two groups was ascertained by Student’s t test. For differences among more than two groups, one-way ANOVA followed by Bonferroni’s multiple comparison test was used. All statistical tests were performed using Prism GraphPad software version 9 (La Jolla, CA). The studies described above were not designed to test a prespecified null hypothesis and hence are deemed exploratory.
Results
Energy-Dependent Uptake of F-Aβ40 and F-Aβ42 in BBB Endothelial Cells
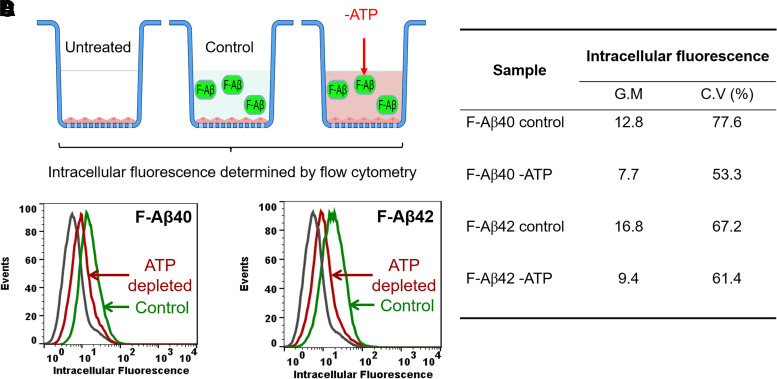
Receptor-mediated endocytosis requires energy produced by ATP hydrolysis. To determine if the uptake of F-Aβ40 or F-Aβ42 in human cerebral microvascular endothelial cell (hCMEC/D3) monolayers is mediated by energy-dependent endocytosis, the extent of F-Aβ internalization with or without ATP depletion was determined by flow cytometry (Fig. 1A). As shown in the histograms, the intra-endothelial F-Aβ40 (Fig. 1B) and F-Aβ42 (Fig. 1C) accumulation was substantially reduced in ATP-depleted cells compared with control cells. The corresponding geometric mean of fluorescence intensity and coefficient of variance were displayed in Fig. 1D.
Fig. 1.
Energy-dependent uptake of F-Aβ peptides in polarized hCMEC/D3 monolayers. (A) Experimental design. Cells grown on Transwell filters were preincubated with glucose-free DMEM supplemented with 50 mM 2-deoxy glucose and 0.1% sodium azide for 30 minutes. Then 1 µM F-Aβ was added to the luminal compartment and incubated for 30 minutes. Intracellular fluorescence was assessed by flow cytometry. (B and C) Histograms of hCMEC/D3 cells incubated with (B) F-Aβ40 or (C) F-Aβ42 in control and ATP-depleted group. (D) Comparison of geometric means of F-Aβ40 and F-Aβ42 fluorescence in control and ATP-depleted cells.
Dynamin-Dependent Uptake of F-Aβ40 and F-Aβ42 in BBB Endothelial Cells
To determine if F-Aβ endocytosis is dependent on dynamin, a protein involved in pinching off the endocytotic vesicles (Ferguson and De Camilli, 2012; Antonny et al., 2016), the effect of Dynasore, a potent dynamin inhibitor, on F-Aβ uptake was assessed by flow cytometry (Fig. 2A). It was shown that uptake of F-Aβ40 was inhibited in Dynasore pretreated cells compared with the control cells and the difference was statistically significant (Fig. 2B). Uptake of F-Aβ42 also appeared to decrease in dynamin-treated cells compared with the control cells; however, the difference was not statistically significant (Fig. 2C). Effect of dynamin on F-Aβ internalization was further verified in hCMEC/D3 cells overexpressing dominant-negative mutant K44-dynamin (Fig. 2D). Substantial decrease in green puncta of F-Aβ40 and red puncta of AF633-Trf were observed in cells expressing K44-dynamin mutant (Fig. 2F) compared with control cells (Fig. 2E). Similarly, uptake of F-Aβ42 was also inhibited in K44-dynamin transfected cells (Fig. 2H) compared with control cells (Fig. 2G). Moreover, cells that were transfected with GFP-K44 dynamin (green puncta, Fig. 2J) clearly showed lower uptake of both SR101-Aβ40 (orange puncta, Fig. 2K) and AF633-Trf (red puncta, Fig. 2L). However, in the same image, nontransfected cells without GFP expression demonstrated the uptake of both SR101-Aβ40 and AF633-Trf.
Fig. 2.
Dynamin-dependent endocytosis of F-Aβ40 and F-Aβ42 in hCMEC/D3 cells. (A) Experiment design of flow cytometry study. Cells grown on Transwell filters were preincubated with 80 µM Dynasore for 30 minutes. Then 1 µM F-Aβ was added to the luminal compartment and incubated for 60 minutes. Intracellular fluorescence was assessed by flow cytometry. (B) Fold change of intracellular median fluorescence intensity (N, number of independent replicates; SD, standard deviation). *P < 0.05, Student’s t test, Dynasore-treated group vs. control. (C and H) Experiment design of confocal microscopy. Cells grown on coverslip bottom dishes were transfected with GFP or non-GFP K44-negative–dominant dynamin. Transfected cells were incubated with 1 µM F-Aβ or SR101-labeled Aβ (SR-Aβ) for 60 minutes followed by 20 µg/ml of AF633-TRF treatment of 30 minutes. Following fixation and nuclear staining, the cells were imaged by laser confocal microscopy. (D–G) Confocal micrographs depicting internalization of F-Aβ (green) and AF633-Trf (red) (D and F) control cells and in cells (E and G) expressing negative-dominant K44-dynamin mutant [K44 Dyn knock out (KO)]. (I–K) Intracellular uptake of AF633-Trf (red) and SR-Aβ40 (orange) in cells expressing GFP-labeled negative-dominant K44-dynamin (GFP-K44 Dyn KO). (I) GFP-K44 Dyn KO cells marked by dashed white line, (J) SR-Aβ40, and (K) AF633-Trf.
Actin-Dependent Uptake of F-Aβ40 and F-Aβ42 in BBB Endothelial Cells
To investigate the impact of actin on F-Aβ uptake, the intracellular accumulation of F-Aβ40 and F-Aβ42 in hCMEC/D3 cells was determined by flow cytometry with or without actin inhibition (Fig. 3A). The histograms demonstrated that uptake of F-Aβ40 (Fig. 3B) and F-Aβ42 (Fig. 3C) was substantially reduced in cells pretreated with cytochalasin A, which disrupts actin dynamics. Further, the reductions were found to be statistically significant for both F-Aβ40 (Fig. 3D) and F-Aβ42 (Fig. 3E) from three independent experiments.
Fig. 3.
Actin-dependent endocytosis of F-Aβ peptides in polarized hCMEC/D3 monolayers. (A) Experimental design. Cells were preincubated with cytochalasin A (CytoA, 110 µM) for 30 minutes. Then 1 µM F-Aβ was added and incubated for 1 hour. Intracellular fluorescence was assessed by flow cytometry. (B and C) Histograms of hCMEC/D3 cells incubated with (B) F-Aβ40 or (C) F-Aβ42 in control and CytoA-treated group. (D) Fold change of intracellular median fluorescence intensity (N, number of technical replicates). The results were confirmed by two independent experiments. ***P < 0.001, Student’s t test, CytoA-treated vs. control.
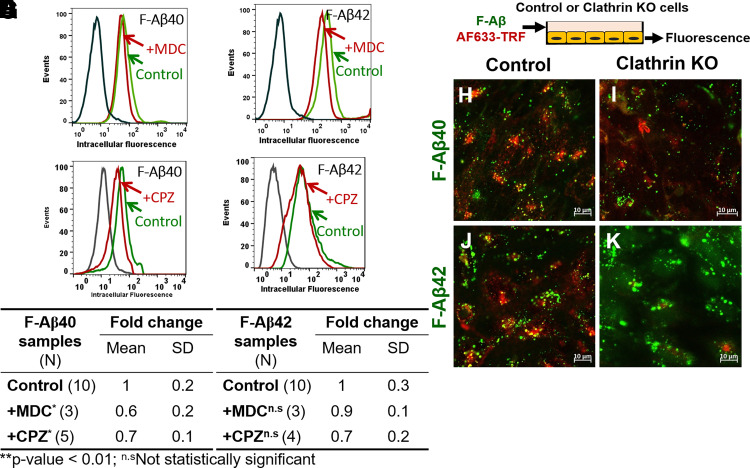
F-Aβ40 Uptake by BBB Endothelial Cells via Clathrin-Mediated Endocytosis
Flow cytometry analysis showed lower accumulation of F-Aβ40 in cells treated with MDC (Fig. 4A) and CPZ (Fig. 4C), and the difference was found to be statistically significant (Fig. 4E). Although, F-Aβ42 uptake was reduced in MDC (Fig. 4B) and CPZ (Fig. 4D) pretreated hCMEC/D3 cells, the difference was not significant (Fig. 4F). To further confirm the role of clathrin-mediated endocytosis on F-Aβ uptake, knockdown of clathrin in hCMEC/D3 cells was performed using siRNA. Then the cells were incubated with AF633-Trf and F-Aβ (Fig. 4G). The AF633-Trf (red puncta, Fig. 4, H–K) was used as a marker of clathrin-mediated endocytosis and its uptake was inhibited in the cells transfected with clathrin siRNA compared with the control cells that underwent mock transfection. In these cells, intracellular uptake of F-Aβ40 was also substantially reduced compared with the control cells (green puncta, Fig. 4, H and I). However, no substantial change was observed in F-Aβ42 uptake (green puncta, Fig. 4, J and K).
Fig. 4.
Role of clathrin-mediated endocytosis in the internalization of F-Aβ40 and F-Aβ42 in polarized hCMEC/D3 monolayers. (A–D) Histograms of hCMEC/D3 cells incubated with (A) F-Aβ40 and (B) F-Aβ42 in control and MDC (a specific clathrin inhibitor)-pretreated group, (C) F-Aβ40 and (D) F-Aβ42 in control and CPZ (a nonspecific clathrin inhibitor)-pretreated group. (E and F) Fold change of median cellular fluorescence intensity (N, number of independent replicates). *P < 0.05 compared with F-Aβ alone group, one-way ANOVA with Bonferroni post-tests. (G) Experiment design of confocal microscopy study. Control or clathrin knocked-down cells were treated with 1 µM F-Aβ peptides and 20 µg/ml AF633-Trf and then subjected to confocal microscopy. (H–K) Confocal micrographs depicting internalization of F-Aβ (green) and AF633-Trf (red) in control cells (H and J) and clathrin knocked-down cells (I and K).
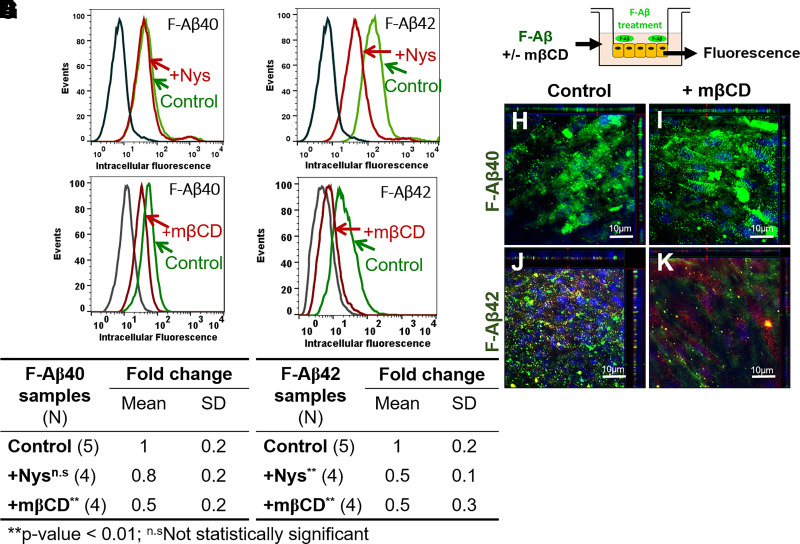
F-Aβ42 Uptake by BBB Endothelial Cells via Caveolae-Mediated Endocytosis
Flow cytometry studies conducted on hCMEC/D3 cells demonstrated that nystatin (Fig. 5B) or mβCD (Fig. 5D) treatment reduced the F-Aβ42 uptake, and the difference was statistically significant (Fig. 5F). However, no impact of nystatin on F-Aβ40 uptake was observed as indicated by the histogram (Fig. 5A) and statistical analysis (Fig. 5E), although the fluorescence intensity of F-Aβ40 was still reduced after mβCD treatment (Fig. 5, C and E). In a separate study, primary BBME cells grown on Transwell inserts were treated with mβCD and F-Aβ, followed by confocal microscopy imaging (Fig. 5G). The z-stack images demonstrated reduced intracellular uptake of F-Aβ42 (green puncta) in mβCD-treated monolayers (Fig. 5K) in comparison with control cells (Fig. 5J). In contrast, the uptake of F-Aβ40 was unaffected by mβCD pretreatment (Fig. 5, H and I).
Fig. 5.
Role of lipid rafts on the internalization of F-Aβ peptides in hCMEC/D3 and BBME cells. (A–D) Histograms of hCMEC/D3 cells incubated with (A) F-Aβ40 and (B) F-Aβ42 in control and Nystatin (a specific inhibitor of lipid raft-caveolae endocytosis pathway)-pretreated group, and (C) F-Aβ40 and (D) F-Aβ42 in control and mβCD (a nonspecific cholesterol chelator)-pretreated group. (E and F) Fold change of median cellular fluorescence intensity (N, number of independent replicates). *P < 0.05, **P < 0.01 compared with F-Aβ alone group, one-way ANOVA with Bonferroni post-tests. (G) Experiment design of confocal microscopy study. Polarized BBME cell monolayers cultured on Transwell filters were preincubated with or without mβCD in DMEM and then incubated with 1 µM F-Aβ peptides and/or 20 µg/ml AF633-Trf. (H–K) The z-series confocal micrographs demonstrating F-Aβ (green) fluorescence in control cells (H and J) and mβCD-treated cells (I and K). Images were shown in x-y (large square), x-z (top horizontal panel), and y-z (right vertical panel) plane.
Tyrosine Phosphorylation of Caveolin-1 Is Induced by Aβ42 but Not by Aβ40 and Mediates Aβ42 Uptake
Caveolin-1 is a major structural component of caveolae. To further confirm the impact of caveolin-1 on cellular uptake of Aβ, knockdown of caveolin-1 in hCMEC/D3 cells was performed using siRNA, and intracellular Aβ was measured by ELISA. Successful knockdown of caveolin-1 was demonstrated by western blotting as shown in Fig. 6A. Moreover, caveolin-1 knockdown was shown to reduce Aβ42 cellular uptake and the difference was statistically significant, whereas no difference was observed for Aβ40 (Fig. 6B). It has been reported that phosphorylation of caveolin-1 is associated with caveolae-dependent endocytosis (Orlichenko et al., 2006; Zimnicka et al., 2016). To study the effect of Aβ40 and Aβ42 on the phosphorylation of caveolin-1, we exposed hCMEC/D3 cells to soluble Aβ peptides predominantly containing monomers and determined the phospho-caveolin-1 expression by western blotting. Incubation with Aβ42 resulted in a statistically significant increase in the phosphorylation of caveolin-1 on tyrosine 14 and pretreatment of Src kinase inhibitor PP1 was shown to block the phosphorylation (Fig. 6C). By contrast, Aβ40 has no impact on caveolin-1 phosphorylation (Fig. 6D).
Fig. 6.
Tyrosine phosphorylation of caveolin-1 is induced by Aβ42 but not by Aβ40 and mediates Aβ42 uptake. (A) Representative immunoblots confirming caveolin-1 knockdown using siRNA in hCMEC/D3 cells. (B) Intracellular Aβ accumulation in hCMEC/D3 cells transfected with caveolin-1 siRNA. N, number of independent replicates. *P < 0.05, Student’s t test. (C and D) Representative immunoblots and quantification of phosphorylated caveolin-1 (Y14) and total caveolin-1 with (C) Aβ42 and (D) Aβ40 stimulation. N, number of technical replicates from one representative experiment, the results were confirmed by two independent experiments. **P < 0.01, ***P < 0.005, one-way ANOVA with Bonferroni post-tests.
Intracellular Itineraries of Aβ40 versus Aβ42 in BBB Endothelial Cells
Accumulation of Aβ40 and Aβ42 in Early and Late Endosomes
hCMEC/D3 cells were transfected with m-Cherry Rab5 protein (marker for early endosome) and then subjected to incubation with F-Aβ40. Confocal microscopy studies demonstrated that Aβ40 accumulated in the early endosomes as shown by substantial colocalization of F-Aβ40 (Fig. 7B) and m-Cherry Rab5 (Fig. 7A). Further, F-Aβ40 was found to accumulate in the late endosomes, as indicated by colocalization of F-Aβ40 and Dil-LDL (Fig. 7, D–F). To confirm the accumulation of F-Aβ in the late endosomes, hCMEC/D3 cells transfected with GFP-labeled Rab7 protein (marker for late endosome) were incubated with SR-Aβ40 or SR-Aβ42 and then imaged live for up to 60 minutes. Only minor accumulation of SR-Aβ40 in the late endosomes was observed at early time points (Fig. 8, A and B). Contrarily, substantial colocalization between SR-Aβ42 and GFP-labeled Rab7 was found after 14 minutes of incubation (Fig. 8, D and E). Both peptides were shown to accumulate in the late endosome at later time points (Fig. 8, C and F).
Fig. 7.
Accumulation of F-Aβ40 peptides in early and secondary endosomes in hCMEC/D3 cells. (A–C) Colocalization of F-Aβ40 with m-Cherry fluorescent Rab5. hCMEC/D3 cells were transfected with m-Cherry fluorescent Rab5 and then incubated with F-Aβ40 for 15 minutes. Then live cell imaging was conducted after washing the cells with DMEM. (A) m-Cherry fluorescent Rab5, (B) F-Aβ40, and (C) merge image of (A) and (B). (D–F) Colocalization of F-Aβ40 with Dil-LDL. hCMEC/D3 cells were treated with 1 µM F-Aβ40 and Dil-LDL (15 μg/ml) for 60 minutes. Then live cell imaging was conducted after washing the cells with DMEM. (D) Dil-LDL, (E) F-Aβ40, and (F) merge image of (D) and (E).
Fig. 8.
Time-dependent accumulation of sulforhodamine-labeled Aβ (SR-Aβ) peptides in late endosomes. hCMEC/D3 cells expressing GFP-Rab7 were incubated with SR-Aβ for 15 minutes. Then the cells were washed with DMEM and imaged by live cell imaging up to 60 minutes. (A–C) Accumulation of SR-Aβ40 following incubations at (A) 17 minutes, (B) 37 minutes, and (C) 62 minutes. (D–F) Accumulation of SR-Aβ42 following incubations at (D) 14 minutes, (E) 36 minutes, and (F) 51 minutes.
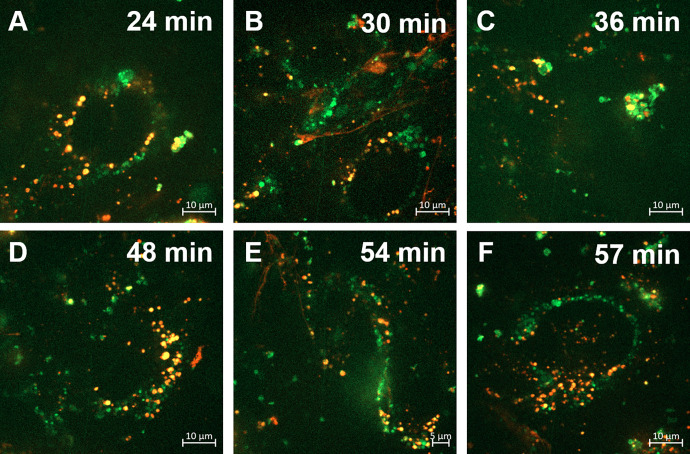
Accumulation of Aβ40 in Recycling Endosomes
Accumulation of Aβ40 in recycling endosomes was examined in hCMEC/D3 cells transfected with GFP-labeled Rab11, which is a marker for recycling endosomes. Considerable colocalization of SR-Aβ40 and GFP-labeled Rab11 was observed throughout the incubation period (Fig. 9), confirming Aβ40 accumulation in recycling endosomes.
Fig. 9.
Time-dependent accumulation of sulforhodamine-labeled Aβ40 peptides in recycling endosomes. hCMEC/D3 cells expressing GFP-Rab11 were incubated with SR-Aβ40 for 15 minutes. Then the cells were washed with DMEM and imaged by live cell imaging up to 60 minutes. Accumulation of SR-Aβ40 following incubations at (A) 24 minutes, (B) 30 minutes, (C) 36 minutes, (D) 48 minutes, (E) 54 minutes, and (F) 57 minutes.
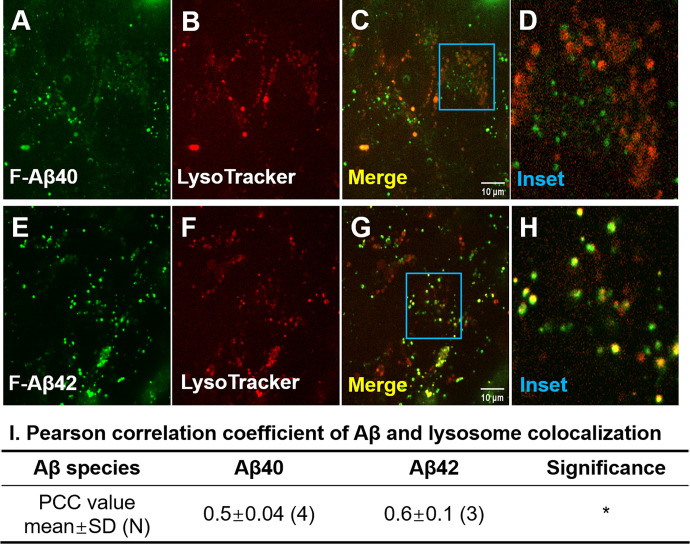
Accumulation of Aβ40 and Aβ42 in Lysosomes
hCMEC/D3 cells were coincubated with F-Aβ and LysoTracker (predominantly a marker for lysosomes) to examine lysosomal accumulation of F-Aβ. The F-Aβ40 displayed only a partial localization in the lysosomes (Fig. 10, A–D), whereas F-Aβ42 exhibited considerable lysosomal accumulation (Fig. 10, E–H). Pearson’s correlation coefficient of F-Aβ42, which describes its extent of colocalization with lysosomes was found to be higher than that of F-Aβ40, and the difference was statistically significant (Fig. 10I).
Fig. 10.
Accumulation of F-Aβ40 and F-Aβ42 peptides in lysosomes in hCMEC/D3 cells. Representative images showing colocalization of F-Aβ (green) and LysoTracker (red) in hCMEC/D3 cells. (A–C) F-Aβ40; (E–G) F-Aβ42; and (D and H) inset showing the magnified images of (C) and (G). (I) Pearson correlation coefficient of F-Aβ and LysoTracker. N, number of independent replicates. *P < 0.05, Student’s t test.
Discussion
Reduction in soluble Aβ42/Aβ40 ratio has been observed in both plasma and cerebrospinal fluid of AD patients. Although several investigators attributed this phenomenon to the propensity of Aβ42 to form insoluble fibril and amyloid plaques, contribution of Aβ42 and Aβ40 trafficking disruptions at the BBB endothelium that could affect the levels of both isoforms in cerebrospinal fluid and plasma cannot be ruled out. Therefore, we hypothesize that the BBB endothelium discriminates Aβ isoforms and traffics them independently via distinct transcytosis mechanisms. Impaired transcytosis of Aβ peptides at the BBB could promote their accumulation in the BBB endothelium and induce BBB dysfunction, which is associated with AD progression. However, the molecular mechanisms regulating Aβ trafficking at the BBB endothelial cells are not well understood. Therefore, in this study, we investigated cellular mechanisms involved in Aβ internalization and intracellular transit at the BBB endothelium.
Our previous studies have shown that the uptake of Aβ by brain microvascular endothelial cells is temperature-dependent (Kandimalla et al., 2009) and saturable (Sharda et al., 2021). The current study confirms that the uptake of both Aβ40 and Aβ42 in hCMEC/D3 monolayers require energy and is inhibited on ATP depletion (Fig. 1). Taken together, these results indicate that both Aβ40 and Aβ42 are internalized by BBB endothelial cells via receptor-mediated endocytosis. Next, using small molecule inhibitors of dynamin, such as Dynasore, or by transfecting hCMEC/D3 monolayers with inactive mutant form of dynamin, we demonstrate that dynamin is critical for Aβ endocytosis at the BBB (Fig. 2). Previous studies have shown that the uptake of Aβ42 but not Aβ40 is reduced in Dynasore-treated PC-12 cells (Omtri et al., 2012) and SH-SY5Y cells (Wesén et al., 2017). The discrepancy between BBB endothelial cells and neuronal cells could be due to differential expression of dynamin isoforms. Although dynamin 1 and 3 are highly expressed in neurons, endothelial cells mostly express dynamin 2 (Raimondi et al., 2011). Finally, we found that both Aβ40 and Aβ42 use actin-dependent endocytosis in BBB endothelial cells (Fig. 3). Similar results were reported in neurons (Wesén et al., 2017) and astrocytes (Lee et al., 2015), where disruption of actin polymerization by cytochalasin D was shown to significantly reduce Aβ40 and Aβ42 endocytosis. These studies have indicated that both Aβ40 and Aβ42 are internalized by BBB endothelial cells via energy, dynamin, and most likely, actin-dependent endocytosis.
We further investigated the role of clathrin versus caveolae-mediated endocytosis in Aβ uptake at the BBB endothelium. These are two well-studied mechanisms that were shown to facilitate the receptor-mediated transcytosis of various macromolecules at the BBB: endocytosis of critical endogenous proteins such as transferrin (Roberts et al., 1993) and LDL (Anderson et al., 1977) is mediated by clathrin. In contrast, caveolae-mediated transcytosis mediates the blood-to-brain entry of viruses and extracellular proteins such as albumin (Razzak, 2014). Using chemical inhibitors and siRNA knockdown of clathrin, we have shown that the endocytosis of Aβ40 but not Aβ42 is clathrin-mediated in hCMEC/D3 monolayers (Fig. 4). It has been reported that clathrin-mediated endocytosis pathway is disrupted in AD (Ando et al., 2013), which may lead to impaired Aβ40 clearance via the BBB. On the other hand, inhibitors of caveolae-mediated endocytosis and siRNA knockdown of caveolin-1 were shown to substantially decrease the uptake of Aβ42 but not Aβ40 (Figs. 5 and 6B). Moreover, western blot results demonstrated that only Aβ42, not Aβ40, increased the tyrosine-14 phosphorylation of caveolin-1 (Fig. 6), which engenders conformational change in caveolin-1 leading to the formation, maturation, and internalization of caveolae into the cytoplasm (Nomura and Fujimoto, 1999; Orlichenko et al., 2006; Botos et al., 2007). Together, these studies indicate that endocytosis of Aβ40 at the BBB endothelium is primarily clathrin-dependent whereas the endocytosis of Aβ42 is caveolae-mediated.
Understanding the mechanisms that govern the transport of Aβ40 and Aβ42 across the BBB may help to shed light on how their disposition is affected by various AD risk factors, such as insulin resistance, aging, and apolipoprotein E4 (APOE4). Our earlier studies have shown that insulin exposure on the luminal side of BBB decreased the influx of Aβ42 into the brain but increased that of Aβ40. Additionally, insulin treatment enhanced clathrin but reduced caveolae-mediated endocytosis in hCMEC/D3 monolayers (Swaminathan et al., 2018). These findings suggest that the different effects of insulin on Aβ trafficking may be due to the distinct endocytic pathways employed by these two peptides, with insulin activating clathrin-mediated endocytosis for Aβ40 and inhibiting caveolae-mediated endocytosis for Aβ42. In another study, we demonstrated that blood-to-brain influx of Aβ40 decreased but Aβ42 increased with aging in wild-type mice (Zhou et al., 2022). Age-related shift from constitutive clathrin-mediated endocytosis to nonspecific caveolar transcytosis at the BBB has also been reported previously (Yang et al., 2020). Based on the current finding that Aβ42 endocytosis at the BBB endothelium is mediated by caveolae, it is likely that the preferential influx of Aβ42 during aging is due to changes in transcytosis involving caveolae-coated vesicles compared with clathrin. Literature evidence has shown that AD genetic risk factor APOE ε4 allele is associated with decreased Aβ clearance in the brain (Verghese et al., 2013). Although previous studies have investigated the APOE effect on Aβ42 trafficking in neurons (Li et al., 2012), its impact on the BBB endothelium remains unknown and warrants further investigation.
In the second part of this study, we investigated the intracellular itineraries of Aβ40 and Aβ42 following receptor-mediated endocytosis in polarized hCMEC/D3 cell monolayers. We discovered that most of the internalized Aβ40 was trafficked through the early and late endosomes (Fig. 7). Compared with Aβ40, Aβ42 accumulated more in the late endosomes (Fig. 8) and subsequent lysosomes (Fig. 10). These results were consistent with our previous findings in neuronal cells (Omtri et al., 2012) and imply that Aβ42 is more susceptible to lysosomal degradation compared with F-Aβ40. On the other hand, F-Aβ40 accumulated in GFP-Rab11–labeled recycling endosomes for up to 1 hour of incubation (Fig. 9). The Rab11 has been reported to mediate the transcytosis of polymeric immunoglobulin A across the polarized epithelial cell (Fan et al., 2021). Therefore, accumulation of F-Aβ40 in Rab11-positive recycling endosomes may facilitate its exocytosis on the abluminal side. Indeed, our previous study demonstrated that the exocytosis rate of intracellular Aβ40 to the abluminal side was 3-fold higher than that of Aβ42 (Swaminathan et al., 2018). These results indicate that internalized Aβ40 and Aβ42 follow distinct trafficking paths in the BBB endothelium, which may determine their fate of transcytosis potential versus intracellular degradation.
Published studies indicated that caveolae-mediated vesicle trafficking directs monocarboxylic acid transporter 1 (Mct1) into late endosome/lysosome compartments in BBB endothelial cells, whereas clathrin-mediated endocytosis directs Mct1 to Rab11-positive recycling endosomes (Smith et al., 2012). Therefore, caveolae-mediated endocytosis could sort Aβ42 for lysosomal degradation while clathrin-mediated endocytosis moves Aβ40 into recycling endosomes. Another potential reason for the preferential accumulation in lysosomes versus recycling endosomes could be related to their binding affinity to the receptors. Preclinical studies have shown that the transcytosis efficiency of the transferrin antibody across the BBB decreased with its affinity to the transferrin receptor (Johnsen et al., 2018). Both in vivo pharmacokinetic modeling (Wang et al., 2021) and cellular transcytosis (Sharda et al., 2021) studies in our previous publications demonstrated that Aβ42 has higher binding affinity to the BBB endothelium. As observed with transferrin receptor antibody, it is likely that such high binding affinity reduces Aβ42 transcytosis and increases its likelihood of lysosomal entrapment.
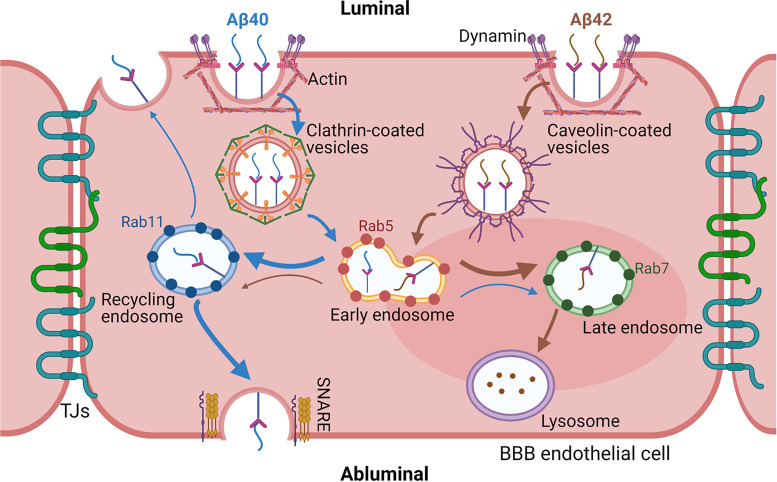
Taken together, our study demonstrates that Aβ40 and Aβ42 are internalized by BBB endothelial cells via dynamin and actin-dependent processes but employ different endocytotic pathways. Endocytosis of Aβ40 is clathrin-mediated, whereas Aβ42 endocytosis is caveolae-mediated. Following the endocytosis, both Aβ40 and Aβ42 are sorted and processed within the endo-lysosomal system but their accumulation patterns differ substantially. The Aβ42 was found to accumulate more in lysosomes than Aβ40 that could lead to higher degradation and/or aggregation of Aβ42 (Fig. 11). We acknowledge the limitations of using hCMEC/D3 cell line as the in vitro BBB model. Due to its modest tight junctional integrity, it is not appropriate for studying paracellular permeability of substances, including that of Aβ(Biemans et al., 2017). However, the current study focuses on Aβ transcytosis, which consist of endocytosis, intracellular sorting, and exocytosis. Previous proteomics studies demonstrated that the hCMEC/D3 cell line expresses most of the receptors and/or transporters that are involved in Aβ transcytosis (Ohtsuki et al., 2013). The limitations posed by hCMEC/D3 monolayers as a BBB model will apply to both Aβ40 and Aβ42. Because the major goal of the current study is to identify relative differences in the endocytosis mechanisms of Aβ40 and Aβ42, the limitations posed by hCMEC/D3 monolayers are expected to not affect the conclusions. Our results provided molecular insights into mechanisms that regulate the transport of Aβ40 versus Aβ42 in the BBB endothelium. Further research is needed to understand the mechanisms behind these differences in the sorting and degradation of Aβ40 and Aβ42 within the endo-lysosomal system and their potential role in the development and progression of BBB dysfunction in AD.
Fig. 11.
Summary of Aβ40 and Aβ42 endocytosis and intracellular trafficking pathway in the BBB endothelial cells. Both peptides were internalized via energy, dynamin, and actin-dependent endocytosis. Endocytosis of Aβ40 was found to be clathrin-mediated whereas Aβ42 was majorly dependent on caveolae. Following endocytosis, the peptides were sorted by the endo-lysosomal system of endothelial cells. Aβ42 was found to accumulate more in the late endosomes and lysosomes (brown thick arrow) but Aβ40 was more likely to get transcytosed to the abluminal side (blue thick arrow) via recycling endosome and soluble N-ethylmaleimide–sensitive fusion protein attachment protein receptors protein-facilitated exocytosis.
Data Availability
The authors declare that all the data supporting the findings of this study are contained within the paper.
Abbreviations
- Aβ
amyloid beta
- AD
Alzheimer’s disease
- AF633-Trf
AlexaFluor 633-transferrin
- APOE4
apolipoprotein E4
- BBB
blood–brain barrier
- BBME
bovine brain microvascular endothelial
- CPZ
chlorpromazine
- CytoA
cytochalasin A
- DMEM
Dulbecco’s modified Eagle’s medium
- F-Aβ
fluorescent label amyloid beta
- hCMEC/D3
human cerebral microvasculature endothelial cells
- LDL
low-density lipoprotein
- mβCD
methyl-β-cyclodextrin
- MDC
monodansylcadaverine
- PFA
para-formaldehyde
- siRNA
small interfering RNA
- SR101
sulforhodamine 101
Authorship Contributions
Participated in research design: Wang, Sharda, Omtri, Kandimalla.
Conducted experiments: Wang, Sharda, Omtri.
Performed data analysis: Wang, Sharda.
Wrote or contributed to the writing of the manuscript: Wang, Sharda, Omtri, Li, Kandimalla.
Footnotes
This study was supported by the National Institutes of Health National Institute on Aging [Grant AG058081] and National Institute of Neurologic Disorders and Stroke [Grant R01NS125437].
1Current affiliation: Clinical Pharmacology and Pharmacometrics, Bristol-Myers Squibb, Princeton, New Jersey.
2Z.W. and N.S. contributed equally to this work.
Rajesh S. Omtri is deceased.
No author has an actual or perceived conflict of interest with the contents of this article.
A preprint of this article was deposited in bioRxiv [https://www.biorxiv.org/content/10.1101/2022.11.17.516996v1].
References
- Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ (2010) Structure and function of the blood-brain barrier. Neurobiol Dis 37:13–25. [DOI] [PubMed] [Google Scholar]
- Agyare EK, Leonard SR, Curran GL, Yu CC, Lowe VJ, Paravastu AK, Poduslo JF, Kandimalla KK (2013) Traffic jam at the blood-brain barrier promotes greater accumulation of Alzheimer’s disease amyloid-β proteins in the cerebral vasculature. Mol Pharm 10:1557–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RG, Brown MS, Goldstein JL (1977) Role of the coated endocytic vesicle in the uptake of receptor-bound low density lipoprotein in human fibroblasts. Cell 10:351–364. [DOI] [PubMed] [Google Scholar]
- Ando K, Brion JP, Stygelbout V, Suain V, Authelet M, Dedecker R, Chanut A, Lacor P, Lavaur J, Sazdovitch V, et al. (2013) Clathrin adaptor CALM/PICALM is associated with neurofibrillary tangles and is cleaved in Alzheimer’s brains. Acta Neuropathol 125:861–878. [DOI] [PubMed] [Google Scholar]
- Antonny B, Burd C, De Camilli P, Chen E, Daumke O, Faelber K, Ford M, Frolov VA, Frost A, Hinshaw JE, et al. (2016) Membrane fission by dynamin: what we know and what we need to know. EMBO J 35:2270–2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballabh P, Braun A, Nedergaard M (2004) The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis 16:1–13. [DOI] [PubMed] [Google Scholar]
- Biemans EALM, Jäkel L, de Waal RMW, Kuiperij HB, Verbeek MM (2017) Limitations of the hCMEC/D3 cell line as a model for Aβ clearance by the human blood-brain barrier. J Neurosci Res 95:1513–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botos E, Turi A, Müllner N, Kovalszky I, Tátrai P, Kiss AL (2007) Regulatory role of kinases and phosphatases on the internalisation of caveolae in HepG2 cells. Micron 38:313–320. [DOI] [PubMed] [Google Scholar]
- Candela P, Gosselet F, Saint-Pol J, Sevin E, Boucau MC, Boulanger E, Cecchelli R, Fenart L (2010) Apical-to-basolateral transport of amyloid-β peptides through blood-brain barrier cells is mediated by the receptor for advanced glycation end-products and is restricted by P-glycoprotein. J Alzheimers Dis 22:849–859. [DOI] [PubMed] [Google Scholar]
- Deane R, Du Yan S, Submamaryan RK, LaRue B, Jovanovic S, Hogg E, Welch D, Manness L, Lin C, Yu J, et al. (2003) RAGE mediates amyloid-beta peptide transport across the blood-brain barrier and accumulation in brain. Nat Med 9:907–913. [DOI] [PubMed] [Google Scholar]
- Doecke JD, Pérez-Grijalba V, Fandos N, Fowler C, Villemagne VL, Masters CL, Pesini P, Sarasa M; AIBL Research Group (2020) Total Aβ42/Aβ40 ratio in plasma predicts amyloid-PET status, independent of clinical AD diagnosis. Neurology 94:e1580–e1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X, Zhou D, Zhao B, Sha H, Li M, Li X, Yang J, Yan H (2021) Rab11-FIP1 and Rab11-FIP5 regulate pIgR/pIgA transcytosis through TRIM21-mediated polyubiquitination. Int J Mol Sci 22:10466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SM, De Camilli P (2012) Dynamin, a membrane-remodelling GTPase. Nat Rev Mol Cell Biol 13:75–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooijmans CR, Graven C, Dederen PJ, Tanila H, van Groen T, Kiliaan AJ (2007) Amyloid beta deposition is related to decreased glucose transporter-1 levels and hippocampal atrophy in brains of aged APP/PS1 mice. Brain Res 1181:93–103. [DOI] [PubMed] [Google Scholar]
- Johnsen KB, Bak M, Kempen PJ, Melander F, Burkhart A, Thomsen MS, Nielsen MS, Moos T, Andresen TL (2018) Antibody affinity and valency impact brain uptake of transferrin receptor-targeted gold nanoparticles. Theranostics 8:3416–3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandimalla KK, Scott OG, Fulzele S, Davidson MW, Poduslo JF (2009) Mechanism of neuronal versus endothelial cell uptake of Alzheimer’s disease amyloid beta protein. PLoS One 4:e4627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SJ, Seo BR, Koh JY (2015) Metallothionein-3 modulates the amyloid β endocytosis of astrocytes through its effects on actin polymerization. Mol Brain 8:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewczuk P, Esselmann H, Otto M, Maler JM, Henkel AW, Henkel MK, Eikenberg O, Antz C, Krause WR, Reulbach U, et al. (2004) Neurochemical diagnosis of Alzheimer’s dementia by CSF Abeta42, Abeta42/Abeta40 ratio and total tau. Neurobiol Aging 25:273–281. [DOI] [PubMed] [Google Scholar]
- Li J, Kanekiyo T, Shinohara M, Zhang Y, LaDu MJ, Xu H, Bu G (2012) Differential regulation of amyloid-β endocytic trafficking and lysosomal degradation by apolipoprotein E isoforms. J Biol Chem 287:44593–44601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao J, Xu F, Davis J, Otte-Höller I, Verbeek MM, Van Nostrand WE (2005) Cerebral microvascular amyloid beta protein deposition induces vascular degeneration and neuroinflammation in transgenic mice expressing human vasculotropic mutant amyloid beta precursor protein. Am J Pathol 167:505–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura R, Fujimoto T (1999) Tyrosine-phosphorylated caveolin-1: immunolocalization and molecular characterization. Mol Biol Cell 10:975–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsuki S, Ikeda C, Uchida Y, Sakamoto Y, Miller F, Glacial F, Decleves X, Scherrmann JM, Couraud PO, Kubo Y, et al. (2013) Quantitative targeted absolute proteomic analysis of transporters, receptors and junction proteins for validation of human cerebral microvascular endothelial cell line hCMEC/D3 as a human blood-brain barrier model. Mol Pharm 10:289–296. [DOI] [PubMed] [Google Scholar]
- Omtri RS, Davidson MW, Arumugam B, Poduslo JF, Kandimalla KK (2012) Differences in the cellular uptake and intracellular itineraries of amyloid beta proteins 40 and 42: ramifications for the Alzheimer’s drug discovery. Mol Pharm 9:1887–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omtri RS, Thompson KJ, Tang X, Gali CC, Panzenboeck U, Davidson MW, Kalari KR, Kandimalla KK (2018) Differential effects of Alzheimer’s disease Aβ40 and 42 on endocytosis and intraneuronal trafficking. Neuroscience 373:159–168. [DOI] [PubMed] [Google Scholar]
- Orlichenko L, Huang B, Krueger E, McNiven MA (2006) Epithelial growth factor-induced phosphorylation of caveolin 1 at tyrosine 14 stimulates caveolae formation in epithelial cells. J Biol Chem 281:4570–4579. [DOI] [PubMed] [Google Scholar]
- Raimondi A, Ferguson SM, Lou X, Armbruster M, Paradise S, Giovedi S, Messa M, Kono N, Takasaki J, Cappello V, et al. (2011) Overlapping role of dynamin isoforms in synaptic vesicle endocytosis. Neuron 70:1100–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razzak M (2014) Glomerular disease: albumin endocytosis is caveolin-mediated. Nat Rev Nephrol 10:242. [DOI] [PubMed] [Google Scholar]
- Roberts RL, Fine RE, Sandra A (1993) Receptor-mediated endocytosis of transferrin at the blood-brain barrier. J Cell Sci 104:521–532. [DOI] [PubMed] [Google Scholar]
- Sharda N, Ahlschwede KM, Curran GL, Lowe VJ, Kandimalla KK (2021) Distinct uptake kinetics of Alzheimer disease amyloid-β 40 and 42 at the blood-brain barrier endothelium. J Pharmacol Exp Ther 376:482–490. [DOI] [PubMed] [Google Scholar]
- Shibata M, Yamada S, Kumar SR, Calero M, Bading J, Frangione B, Holtzman DM, Miller CA, Strickland DK, Ghiso J, et al. (2000) Clearance of Alzheimer’s amyloid-ss(1-40) peptide from brain by LDL receptor-related protein-1 at the blood-brain barrier. J Clin Invest 106:1489–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JP, Uhernik AL, Li L, Liu Z, Drewes LR (2012) Regulation of Mct1 by cAMP-dependent internalization in rat brain endothelial cells. Brain Res 1480:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storck SE, Meister S, Nahrath J, Meißner JN, Schubert N, Di Spiezio A, Baches S, Vandenbroucke RE, Bouter Y, Prikulis I, et al. (2016) Endothelial LRP1 transports amyloid-β(1-42) across the blood-brain barrier. J Clin Invest 126:123–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaminathan SK, Ahlschwede KM, Sarma V, Curran GL, Omtri RS, Decklever T, Lowe VJ, Poduslo JF, Kandimalla KK (2018) Insulin differentially affects the distribution kinetics of amyloid beta 40 and 42 in plasma and brain. J Cereb Blood Flow Metab 38:904–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verghese PB, Castellano JM, Garai K, Wang Y, Jiang H, Shah A, Bu G, Frieden C, Holtzman DM (2013) ApoE influences amyloid-β (Aβ) clearance despite minimal apoE/Aβ association in physiological conditions. Proc Natl Acad Sci USA 110:E1807–E1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villaseñor R, Lampe J, Schwaninger M, Collin L (2019) Intracellular transport and regulation of transcytosis across the blood-brain barrier. Cell Mol Life Sci 76:1081–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan W, Cao L, Liu L, Zhang C, Kalionis B, Tai X, Li Y, Xia S (2015) Aβ(1-42) oligomer-induced leakage in an in vitro blood-brain barrier model is associated with up-regulation of RAGE and metalloproteinases, and down-regulation of tight junction scaffold proteins. J Neurochem 134:382–393. [DOI] [PubMed] [Google Scholar]
- Wang Z, Sharda N, Curran GL, Li L, Lowe VJ, Kandimalla KK (2021) Semimechanistic population pharmacokinetic modeling to investigate amyloid beta trafficking and accumulation at the BBB endothelium. Mol Pharm 18:4148–4161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weksler B, Romero IA, Couraud PO (2013) The hCMEC/D3 cell line as a model of the human blood brain barrier. Fluids Barriers CNS 10:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesén E, Jeffries GDM, Matson Dzebo M, Esbjörner EK (2017) Endocytic uptake of monomeric amyloid-β peptides is clathrin- and dynamin-independent and results in selective accumulation of Aβ(1-42) compared to Aβ(1-40). Sci Rep 7:2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang AC, Stevens MY, Chen MB, Lee DP, Stähli D, Gate D, Contrepois K, Chen W, Iram T, Zhang L, et al. (2020) Physiological blood-brain transport is impaired with age by a shift in transcytosis. Nature 583:425–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou AL, Sharda N, Sarma VV, Ahlschwede KM, Curran GL, Tang X, Poduslo JF, Kalari KR, Lowe VJ, Kandimalla KK (2022) Age-dependent changes in the plasma and brain pharmacokinetics of amyloid-β peptides and insulin. J Alzheimers Dis 85:1031–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimnicka AM, Husain YS, Shajahan AN, Sverdlov M, Chaga O, Chen Z, Toth PT, Klomp J, Karginov AV, Tiruppathi C, et al. (2016) Src-dependent phosphorylation of caveolin-1 Tyr-14 promotes swelling and release of caveolae. Mol Biol Cell 27:2090–2106. [DOI] [PMC free article] [PubMed] [Google Scholar]