Abstract
There is a need to characterize the potential susceptibility of older adults to toxicity from environmental chemical exposures. Liver xenobiotic metabolizing enzymes (XMEs) play important roles in detoxifying and eliminating xenobiotics. We examined global gene expression in the livers of young (21–45 years) and old (69+ years) men and women. Differentially expressed genes (DEG) were identified using two-way ANOVA (p≤0.05). We identified 1437 and 1670 DEGs between young and old groups in men and women, respectively. Only a minor number of the total number of genes overlapped (146 genes). Aging increased or decreased pathways involved in inflammation and intermediary metabolism, respectively. Aging led to numerous changes in the expression of XME genes or genes known to control their expression (~90 genes). Out of 10 cytochrome P450s activities examined, there were increased activities of CYP1A2 and CYP2C9 enzymes in the old groups. We also identified sex-dependent genes that were more numerous in the young group (1065) than in the old group (202) and included changes in XMEs. These studies indicate that the livers from aging humans when compared to younger adults exhibit changes in XMEs that may lead to differences in the metabolism of xenobiotics.
Keywords: Human liver, transcript profiling, age, sex, P450 enzyme activities, xenobiotic metabolizing genes
Introduction
The demographics of the United States are undergoing rapid change. By 2030, the number of individuals older than 65 are expected to more than double to 71.5 million [1], resulting in one of every five Americans being older than 65 [2]. There is an increasing need to understand the impact that environmental conditions have on the aging population. The 2016 Frank Lautenberg Chemical Safety for the 21st Century Act (Lautenberg TSCA) amended the 1976 Toxic Substances Control Act (TSCA) to explicitly mandate protection of highly exposed and susceptible populations. The Act directs EPA to identify and protect “potentially exposed or susceptible sub-populations,” defined as “a group of individuals within the general population identified by the [US EPA] Administrator who, due to either greater susceptibility or greater exposure, may be at greater risk than the general population of adverse health effects from exposure to a chemical substance or mixture, such as infants, children, pregnant women, workers, or the elderly” (15 USC §2602 [3]).
The rapid growth in the number of older Americans has a number of implications for public health [4], including the need for increased efforts to better understand the risks posed to older adults by environmental exposures. A large body of work has shown that biological capacity declines with normal aging, and the decline may be exacerbated in individuals with pre-existing health conditions [5, 6]. This decline can result in compromised pharmacokinetic and pharmacodynamic responses to environmental exposures encountered in daily activities [7]. Many age-related differences in drug and toxicant responsiveness are based on altered absorption, distribution, metabolism, and elimination (ADME) that determines the dose of an environmental agent reaching a target organ (summarized in [4]). Changes in these processes could mean that the same external dose results in a very different internal dose or distribution to different target organs in older versus younger adults.
Much of the research focus to define the changes in older populations has been based on animal models in which tissues are readily available. Many studies have examined changes in the liver, the primary tissue involved in drug and chemical metabolism. Previous work showed that between 3 and 7% of all gene changes in the livers of aging rats were those encoding ADME-related xenobiotic metabolism enzymes (XMEs) [8]. In the mouse liver, XMEs were identified that were commonly altered with age across multiple studies. However, the altered expression of many of the XMEs with aging were those that also exhibited sex-specific gene expression patterns and exhibited a feminized gene expression pattern in the male livers [9]. The feminization occurs due to decreases in the male-specific pulsatile growth hormone (GH) expression pattern, a mechanism that occurs in rodents but not humans [10]. Thus, while the effects of aging on XMEs have been characterized in rodents, many of these changes are likely not relevant to humans. An examination of a targeted set of ~100 XMEs found changes due to age in the human liver including decreases in cytochrome P450 reductase [11] and increases in the transporter gene MRP3 [12]. A comprehensive analysis of the impact of aging on human liver XMEs has not been conducted.
In this study, we examined global gene expression differences between the livers from young adult (21–45 years) and old (69+ years) humans with the goal of characterizing differences in expression of genes that are known to be involved in xenobiotic metabolism. We found that aging leads to extensive changes in the human liver transcriptome, most of which are unique to each sex. The genes differentially regulated between old and young groups include many that are involved in xenobiotic metabolism or transport. Our examination of 10 CYP enzyme activities found increased activities of CYP1A2 and CYP2C9 in the old group. While not the main focus of the study, we also identified gene expression differences between males and females that were found to diminish with age. Many XMEs were differentially expressed between sexes. These studies support the hypothesis that aging leads to differences in xenobiotic metabolism, and these changes impact not only susceptibility to xenobiotic chemicals but adds support for evaluation of life-stage dependent differences to refine risk assessment approaches.
Materials and Methods
Human livers
Human livers, male and female, young (21–45 years old) and old (69+ years old), were obtained frozen from CellzDirect (Durham, NC). All information about the individuals were anonymized. The characteristics of the individuals are summarized in Table 1. Ten samples were excluded from the gene expression analysis because of partially degraded RNA (RNA Integrity Number < 8). Liver samples were obtained from individuals who died from accidents. Livers were obtained within 6 hrs of death. The study was reviewed and approved by the EPA and CCTE human subjects institutional review board.
Table 1.
Human liver demographics
| Donor# | Age | Sex | Race | Height (inches) | Weight (lbs) | BMI | BMI category | Diabetes | Microaray | CYP activity |
|---|---|---|---|---|---|---|---|---|---|---|
| Hu0131 | 42 | m | Caucasian | √ | X | |||||
| Hu0133 | 43 | m | Caucasian | √ | X | |||||
| Hu0162 | 77 | f | X | |||||||
| Hu0177 | 37 | f | Afr. American | 66 | 170 | 27.4 | Overweight | √ | X | |
| Hu0243 | 45 | f | X | |||||||
| Hu0244 | 38 | m | X | |||||||
| Hu0264 | 45 | f | X | |||||||
| Hu0286 | 39 | f | X | |||||||
| Hu0300 | 71 | f | Afr. American | 66 | 178 | 28.7 | Overweight | Yes | √ | X |
| Hu0340 | 21 | m | Hispanic | 72 | 213 | 28.9 | Overweight | No | √ | X |
| Hu0342 | 39 | m | Caucasian | 74 | 217 | 27.9 | Overweight | No | √ | X |
| Hu0369 | 69 | M | Hispanic | 65 | 156 | 26 | Overweight | No | √ | |
| Hu0405 | 44 | m | Caucasian | 70 | 220 | 31.6 | Obese | No | √ | X |
| Hu0416 | 24 | m | Caucasian | 68 | 141 | 21.4 | Ideal weight | No | √ | X |
| Hu0582 | 36 | f | Caucasian | 63 | 143 | 25.3 | Overweight | No | √ | X |
| Hu0634 | 84 | f | X | |||||||
| Hu0637 | 86 | f | Afr. American | 63 | 151 | 26.7 | Overweight | No | √ | X |
| Hu0698 | 84 | f | Caucasian | 64 | 170 | 29.2 | Overweight | √ | X | |
| Hu0703 | 84 | m | Caucasian | 61 | 192 | 36.3 | Obese | √ | X | |
| Hu0710 | 70 | m | Caucasian | 69 | 167 | 24.7 | Ideal weight | √ | X | |
| Hu0711 | 78 | m | X | |||||||
| Hu0712 | 76 | m | Afr. American | 66 | 161 | 26 | Overweight | √ | X | |
| Hu0717 | 72 | f | Caucasian | 62 | 179 | 32.7 | Obese | √ | X | |
| Hu0722 | 78 | m | X | |||||||
| Hu0742 | 74 | f | X | |||||||
| Hu0746 | 73 | m | Caucasian | 71 | 199 | 27.8 | Overweight | √ | X | |
| Hu0752 | 80 | f | Caucasian | 63 | 134 | 23.7 | Ideal weight | √ | X | |
| Hu0784 | 79 | m | Caucasian | 76.5 | 159 | 19.1 | Ideal weight | √ | X | |
| Hu8002 | 34 | m | X | |||||||
| Hu8007 | 33 | f | Caucasian | √ | X | |||||
| Hu8032 | 24 | f | Caucasian | 67 | 154 | 24.1 | Ideal weight | No | √ | X |
| Hu8046 | 34 | f | Hispanic | 33 | Obese | √ | X | |||
| Hu8050 | 83 | m | Caucasian | 66 | 147 | 23.7 | Ideal weight | √ | X |
RNA Isolation
Total RNA was isolated from human livers according to the TriReagent procedure (Molecular Research Center, Cincinnati, OH) and purified using the Qiagen RNeasy mini RNA cleanup protocol (Qiagen, Valencia, CA). The integrity of each RNA sample was determined using an Agilent 2100 Bioanalyzer (Agilent, Foster City, CA), and RNA quantity was determined using a Nanodrop® ND-100.
Microarray hybridizations
Liver gene expression analysis was performed according to the Affymetrix recommended protocol using Affymetrix Human Genome U133 Plus 2.0 GeneChips® containing probes for over 47,000 genes. Total RNA (5 μg per sample) was labeled using the Affymetrix® One-Cycle cDNA Synthesis protocol and hybridized to Affymetrix® Human U133 2.0 arrays as described by the manufacturer (Affymetrix®, Santa Clara, CA). The cRNA hybridization cocktail was incubated overnight at 45°C while rotating in a hybridization oven. After 16 hours of hybridization, the cocktail was removed and the arrays were washed and stained in an Affymetrix GeneChip® fluidics station 450 according to the Affymetrix-recommended protocol. Arrays were scanned on an Affymetrix GeneChip® scanner. The raw data files can be found at Gene Expression Omnibus (GSE133815).
Analyses of Microarray data
The Affymetrix.cel files were imported into Partek Flow (Partek Inc, St. Louis MO) and standard procedures recommended by the manufacturer were followed. Probe level intensity of these arrays were normalized using RMA and probe level alignment is performed using Bowtie. This was followed by Partek E/M annotation model to quantify the intensity data at probe set level. Principle Component Analysis (PCA) was used to visualize the gene expression profiles of 23 samples with regard to age and sex (old female (n=5), old male (n=7), young female (n=5), young male (n=6). Differentially expressed genes were identified using 2-way ANOVA considering age and sex (p < 0.05 and absolute fold-change ≥ 1.5). All gene changes are found in Table S1.
Two-dimensional clustering
The Gene Cluster version 3.0 (https://cluster2.software.informer.com/3.0/) was used to generate hierarchical complete linkage of DEGs, and the generated.cdt file was then uploaded into TreeView (https://treeview.software.informer.com/1.6/) to generate the heatmap for visualization.
BaseSpace Correlation Engine analysis
BaseSpace Correlation Engine (BSCE, Illumina, CA) is an ‘omic database that includes DEG lists generated from RNA-Seq and microarray experiments. The database contains over 23,000 scientific studies to enable identification of linkages between genes, experiments, chemicals and phenotypes. DEGs generated as described above were uploaded into BSCE and compared with all biosets in the database using the Running Fisher test [13]. (A bioset is a list of significantly altered genes.) This method provides an assessment of the statistical significance of the correlation of the overlapping genes between two gene lists resulting in a summary p-value [13]. The results were exported, and each p-value was converted to a -log(p-value). Biosets with negative correlation were converted to a negative number. Biosets with absolute value (-log(p-value)) ≥ 4 were considered significant [14–16] [17]. Biosets that were positively correlated with the DEGs were predicted to produce similar effects, either directly or indirectly; biosets that were negatively correlated were predicted to have the opposite biological responses.
Identification of genes commonly altered by aging in human and mouse livers.
Genes that exhibited similar directional changes in expression in statistically significant gene lists were first compiled using the meta-analysis function in BSCE. The gene lists were derived from the three aging comparisons from this study and from three lists of genes from old versus young comparisons from mice (GSE63943, GSE75192). The overlapping genes were exported and filtered for consistent directional changes in 5 or 6 out of 6 of the comparisons resulting in 52 genes.
Ingenuity Pathway Analysis
The filtered genes were analyzed using the canonical pathway and upstream analysis functions of Ingenuity Pathway Analysis (IPA, Qiagen Bioinformatics, Redwood City, California). IPA uses a right-tailed Fisher’s exact test to calculate a significant overlap between the genes and the IPA pathway gene lists. The upstream analysis uses the number of differentially expressed genes to predict upstream regulators of the biomarker genes. The p-values for the canonical pathway analysis and upstream regulator were converted to −log(p-value)s. The results for the 6 gene lists described above are found in Tables S2, S3. The results for the genes that are commonly altered by aging in human and mouse livers are found in Tables S4, S5.
Human liver microsome preparation
Each of the liver samples was processed into cytosol and microsomes at CellzDirect. Frozen liver tissues were thawed on ice in homogenization buffer (50 mM Tris at pH 7.4, 150 mM KCl, 2 mM EDTA), homogenized with a Potter-Elvehjem type tissue grinder, and centrifuged at low speed (∼13,000gmax) for 20 min at 4°C to remove plasma membranes, mitochondria, nuclei, and lysosomes. The resulting supernatant was centrifuged at high speed (∼100,000gmax) for 60 min at 4°C to yield a cytosolic fraction and microsomal pellet. The pellet was resuspended in homogenization buffer, recentrifuged, and resuspended in 0.25 M sucrose with a Potter-Elvehjem tissue grinder fitted with a Teflon pestle. A small aliquot of the microsomal preparation was retained for protein determination, and the remainder was stored at −70°C. The concentration of microsomal protein was determined with a bicinchoninic acid kit. The concentration of microsomal protein per gram of liver tissue was determined (Pierce Chemical, Dallas, TX). Microsomes were diluted to an equivalent concentration before evaluation.
P450 enzyme activity measurements
The activities of CYP1A2, CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, CYP2E1, and CYP3A4 were measured in human liver microsomal preparations at CellzDirect. The substrates used were acetaminophen (APAP) for CYP1A2, 7-hydroxycoumarin (7OHCMN) for CYP2A6, hydroxybupropion (OHBUP) for CYP2B6, 6-hydroxypaclitaxel (6OHTAX) for CYP2C8, hydroxytolbutamide (HTB) for CYP2C9, 4′-hydroxymephenytoin (4HMPN) for CYP2C19, dextromethorphan/dextrorphan metabolic ratio (DRR) for CYP2D6, and 6-hydroxychlorzoxazone (6HCLZ) for CYP2E1. Two substrates were used for the CYP3A4 activity measurement: 6beta-testosterone (6BT) and 10-hydroxymidazolam (10HMDZ), with the corresponding enzyme activity designated as CYP3A4T and CYP3A4M, respectively. Microsomal incubations were performed in triplicate in a final incubation volume of 150 μL. The substrate, in 0.1 M phosphate buffer (pH 7.4), was prewarmed to 37°C with a total organic solvent content of ≤ 1%. Microsomal protein was added to a final concentration of ∼0.1 mg/mL (±0.05 mg/mL). Reactions were initiated by addition of NADPH (1 mM final concentration) and incubated for the indicated times with gentle agitation. The rate of substrate turnover was linear under these reaction conditions during method validations. Control incubations included pooled human liver microsomes (CellzDirect; pooled from 15 male and female individuals) as positive control samples as well as samples without NADPH. The reactions were terminated by the addition of organic solvent, and internal standards were added, mixed, and centrifuged at ∼3000 rpm for 5 min. The organic layer was transferred to a clean tube and evaporated to dryness at ∼40°C under nitrogen. The sample was reconstituted and analyzed by LC-MS/MS.
Metabolite standard curves with eight concentrations were included with each analytical run. In addition, bioanalytical metabolite quality control samples (QC) at three concentrations with four replicates spaced throughout the analytical run were measured. All analytical runs were evaluated based on predefined acceptance criteria as follows: having at least two-thirds of the quality control samples, with 50% at each level, and within ±20% of the nominal values.
Mass spectrometry data were acquired, integrated, regressed, and quantified with MassLynx software, version 3.4 (Micromass). Data were graphed and calculated using Microsoft Excel 97. Reaction velocities (v) were calculated using the following equation: v(nmol/min/mg) = calculated ng/ml x 0.5 mL/molecular weight in ng/nmol/min/mg protein.
Statistical Analysis
The Bowtie aligned reads were quantified in the annotation model (Partek E/M) to generate gene counts, followed by ANOVA with differential expression analysis setting p < 0.05. For human P450 activities, the data presented were mean ± SEM. Comparisons between young and old were made via Student’s t-test, the significance was set at p < 0.05.
Results
Identification of genes altered by aging in the human liver.
Gene expression changes due to aging and sex were examined in a set of 23 human livers (5–6/group) derived from individuals with the demographics detailed in Table 1. Principle components analysis (PCA) of all genes showed that the primary factor determining separation of the samples was age (Figure 1A). While there was one old individual that grouped with the young group, there were three young individuals that were found in the old group. We attempted to find explanations for why one of the old males and three of the young individuals (2 males, 1 female) separated into the opposite groups. Factors including race, body mass index or diabetes could not explain the behavior of these outliers. Sex as a factor did not result in separation of the samples.
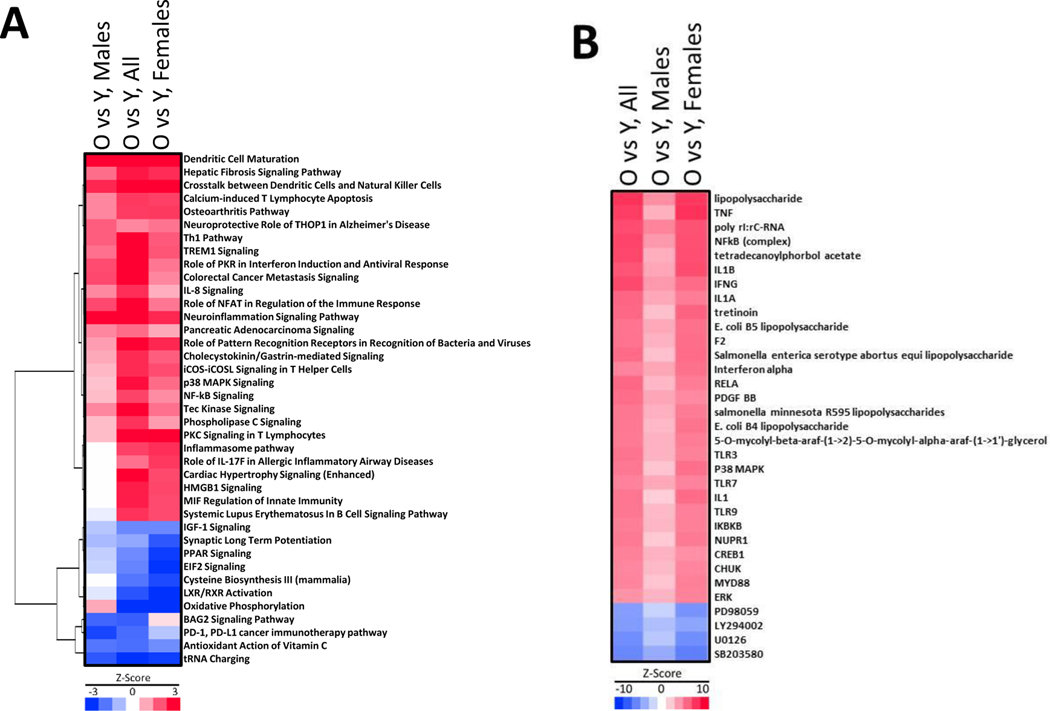
Figure 1. Identification of gene expression changes in the livers of aging humans.
A. Principal Component Analysis of the distribution of 23 samples subjected to microarray analysis. Females in blue, males in red, old (69+ yr) in spheres, and young in cubes.
B. Number of differentially expressed genes among the indicated comparisons.
C. Two dimensional clustering of genes significantly altered by age. Specific clusters of genes altered by aging are shown.
Genes that were differentially expressed between old vs young and between males vs females were generated as detailed in the Methods (p-value < 0.05; ≥ absolute 1.5-fold-change). The numbers of genes altered by age are shown in Figure 1B. The greatest number of altered genes by age were found when all old individuals were compared to all young individuals regardless of sex (up 1044, down 973), likely due to increased statistical power with a greater number of samples to detect altered genes. When the analysis was repeated by sex, there were 1437 genes altered between old males versus young males (up 780, down 657) and 1670 genes altered between old females and young females (up 662, down 1008). Figure 1C shows the two-dimensional clustering analysis of the DEGs. There were 635 genes (303 up in cluster 1 and 332 down in cluster 4) unique to aged males, and 804 genes (298 up in cluster 4 and 506 down in cluster 6) unique to aged females. There were only 146 genes (70 up in cluster 2 and 76 down in cluster 5) that were common to all groups. These results indicate that in large part males and females exhibit sex-specific aging patterns in gene expression. The complete dataset can be found in Table S1.
Aging in the liver affects pathways involved in inflammation and intermediary metabolism.
The genes differentially expressed by age in males, females or all individuals were examined by Ingenuity Pathways Analysis (IPA). The canonical pathways that were altered are shown in Figure 2A. Many of the pathways that had a positive Z-score across all comparisons were linked to inflammatory processes including “Role of Pattern Recognition Receptors in Recognition of Bacteria and Viruses”, “NF-kB Signaling”, and “p38 MAPK Signaling”. Three other pathways were activated of note including “Hepatic Fibrosis Signaling Pathway”, “Colorectal Cancer Metastasis Signaling”, and “Pancreatic Adenocarcinoma Signaling”, diseases with an inflammatory component that occur with increased frequency in aged individuals. There were 6 pathways with significant positive Z-scores that were altered in females but not males (“Inflammasome pathway”, “Role of IL-17F in Allergic Inflammatory Airway Diseases”, “Cardiac Hypertrophy Signaling (Enhanced)”, “HMGB1 Signaling”, “MIF Regulation of Innate Immunity”, “Systemic Lupus Erythematosus In B Cell Signaling Pathway”). These findings indicate that the aging process may activate some pathways including those involved in inflammation unique to females. Although aged males express a unique set of genes compared to aged females, there were no pathways that were uniquely activated in males. The entire dataset is found in Table S2.
Figure 2. Effects of aging on inflammation and intermediary metabolism pathways.
A. IPA canonical pathway analysis. Pathways were clustered by one dimensional clustering. Only upstream regulators that had a combined Z-score across the three comparisons of ≥ 4 or ≤ −4 are shown.
B. IPA upstream activator analysis. Upstream effectors common to all groups were ranked by the sum of the Z-score across all comparisons. Only upstream regulators that had a combined Z-score across the three comparisons of ≥ 12 or ≤ −12 are shown.
A smaller subset of pathways were down-regulated with aging. There were two pathways down-regulated in both males and females (“Antioxidant Action of Vitamin C”, “tRNA Charging”). Multiple pathways down-regulated predominantly in females are involved in intermediary metabolism (“Cysteine Biosynthesis III (mammalia)”, “LXR/RXR Activation”, “Oxidative Phosphorylation”, “PPAR Signaling”). Additional pathways predominantly affected in females included “EIF2 Signaling”, “IGF-1 Signaling”, and “Synaptic Long Term Potentiation”. There were two pathways that were down-regulated in males but not females (“BAG2 Signaling Pathway”, “PD-1, PD-L1 cancer immunotherapy pathway”). This analysis indicates that there were sex differences in the canonical pathways that were altered with aging.
The genes differentially expressed by age in males, females or all individuals were also examined by IPA upstream analysis which identifies upstream effectors of the same set of genes. In general, there was consistency in the effectors between the sexes although the Z-scores were almost always lower in males than females (Figure 2B). The analysis identified large molecule effectors that would be expected to activate genes involved in the inflammatory response (lipopolysaccharide, tumor necrosis factor (TNF), E. coli B4 lipopolysaccharide, E. coli B5 lipopolysaccharide, interleukin 1 (IL1), IL1A, IL1B, Salmonella enterica serotype abortus equi lipopolysaccharide, Salmonella minnesota R595 lipopolysaccharides). The effectors also included proteins in the NF-kB signaling pathway that are activated under inflammatory conditions (Inhibitor of Nuclear Factor Kappa B Kinase Subunit Beta (IKBKB), nuclear factor kappa-light-chain-enhancer of activated B cells (NFkB) (complex), RELA, toll-like receptor 3 (TLR3), TLR7, TLR9). The MYD88 is involved in signaling within immune cells acting as an adapter, connecting proteins that receive signals from outside the cell. In addition, conserved helix-loop-helix ubiquitous kinase (CHUK) is an inhibitor of nuclear factor kappa-B kinase subunit alpha (IKK-α). Nuclear Protein 1, Transcriptional Regulator (NUPR1) is a stress-inducible transcription factor that has been linked to inflammation. The effectors also included those associated with viral infections (interferon gamma (IFNG), interferon alpha, poly rI:rC-RNA). The entire dataset is found in Table S3.
Multiple pathways involved in cell growth may also be activated including p38 MAPK and ERK. Activation of a growth pathways is supported by negative Z-scores for inhibitors of mitogen activated kinases (PD98059, U0126, SB203580) or phosphatidylinositol 3 (PI3) kinase (LY294002). Additionally, platelet-derived growth factor B subunit (PDGF-BB), is a potent mitogen for cells of mesenchymal origin, including fibroblasts, smooth muscle cells and glial cells. Tetradecanoylphorbol Acetate (TPA) through induction of protein kinase C (PKC) activity induces maturation and differentiation of hematopoietic cell lines, including leukemic cells.
In our past studies, we have built and characterized multiple gene expression biomarkers that are predictive of transcription factor activity in human tissues. Each of the three gene lists was compared to the gene expression biomarkers for heat shock factor 1 [18], oxidant-induced Nrf2 [19], metal induced factor 1 [20], NF-kB [21], and a biomarker predictive of DNA damage (TGx-DDI) [22] using the Running Fisher test in BSCE [13]. There was significant activation (-log(p-value) ≥ 4) of MTF1 and NF-kB in the female old versus young but not the other groups. Additionally, there was suppression of Nrf2 in all the old versus young group but not the others. The biomarker analysis indicates that the older females have increases in two transcription factors associated with inflammation.
Comparison of genes altered in aged humans to those in mice and rats.
The three sets of genes altered in aging human livers were compared to lists of genes that were significantly altered by aging in humans and rodents. In the database, there was only one study that examined the effects of aging in the human liver (our study; GSE133815). Because BSCE derived their gene lists using a method different than ours, we compared the gene lists using our method to those in BSCE. Figure 3A shows the -Log(p-value)s of the correlations in the 9 pair-wise comparisons. All comparisons gave -Log(p-value)s > 17 indicating very significant correlation between the two methods.
Figure 3. Comparison of genes altered by aging in humans to those in aging mice.
The three old vs. young comparisons were uploaded to BaseSpace Correlation Engine and compared to gene lists in the database using the Running Fisher test. The -Log(p-value)s of the correlation between the gene lists are shown.
A. Different analysis methods result in similar sets of genes between old and young humans. The three old vs. young comparisons generated in the present study were compared to the three parallel gene lists generated in BSCE. The results show highly significant correlations between the comparisons.
B. Comparisons of human aging profiles to those from the livers of aged mice. The three old vs. young human comparisons were each compared to the indicated 5 gene lists generated from the livers of old vs. young mice. The results show that three studies have significant (-log(p-value) ≥ 4) overlap with those in humans.
C. Common genes altered by aging in humans and mice. The genes altered in the human or mouse liver with aging were compared to find genes with consistent directional changes across at least 5 out of the 6 groups examined.
The three gene sets were compared to old versus young comparisons derived from the livers of mice in 5 separate studies (Figure 3B). There was no apparent consistency in the correlations based on the -Log(p-value)s across the studies, even though these gene sets were all derived from the livers of old mice (20–30 months old) vs. young mice (2–4 months old). This inconsistency has been noted before in a meta-analysis study of some of these same individual studies [9]. The highest correlations were from GSE63943 [23] in which 20-mo old mice were compared to 3-mo young mice and GSE75912 [24] in which 24- and 30-mo old mice were compared to 2-mo young mice. The lowest correlations were from GSE62762 [25] in which 22-mo old mice were compared to 3-mo young mice and GSE3150 [26] in which 22-mo old mice were compared to 4-mo old mice. All comparisons were from wild-type male mice and the basis for lack of correlation for two of the studies is not apparent.
Given that there was an overlap in genes between some of the mouse studies and the human groups, we sought to characterize the common changes between the two species. The gene lists derived from our study were compared to those from two mouse studies with the three lists that had the highest correlations (GSE63943, GSE75192). The overlapping genes were filtered for consistent directional changes in 5 or 6 out of 6 of the comparisons resulting in the identification of 52 genes including 44 up-regulated and 8 down-regulated with aging (Figure 3C). An IPA analysis of the genes showed that the top 10 significant canonical pathways included those involved in inflammation (Th1 and Th2 Activation Pathway, Th2 Pathway, Th1 Pathway, Antigen Presentation Pathway, Crosstalk between Dendritic Cells and Natural Killer Cells, B Cell Development, Dendritic Cell Maturation, Neuroinflammation Signaling Pathway, IL-10 Signaling, Toll-like Receptor Signaling). Upstream analysis identified transcription factors involved in inflammation (NFAT5, FOXP3, SPI1, STAT3, NFKBIA, ID3, ECSIT, NR3C1, IRF8, CIITA). The entire dataset is found in Table S4, S5.
Identification of genes that exhibit sex differences in the human liver.
There are substantial gene expression differences in the rodent liver between sexes [10]. We determined if sex-dependent genes could be identified in the individuals in our analysis and whether there were differences in sex-dependent genes between young and old groups. We identified genes that were differentially expressed between males and females in all individuals, in young only, or in old only. Figure 4A shows the number of genes differentially expressed between males and females in each of the groups. There were fewer genes altered by sex than by aging (see PCA in Figure 1A) which may explain why the samples did not separate by sex in the PCA. The greatest numbers of genes altered by sex were from comparisons between young males vs young females. Only ~20% of the genes in the young group were found in the old males vs old females, indicating that aging leads to decreases in sexual dimorphism. Figure 4B shows 2-dimentional clustering of the differentially expressed genes across the three groups. Only a small set of genes were commonly regulated in all three comparisons.
Figure 4. Identification of genes with altered expression between males and females in the human liver.
A. Number of differentially expressed genes in the three comparisons.
B. Two-dimensional clustering of genes significantly different between sexes. Only the clades for genes are shown.
C. Expression differences between males and females of genes encoded by the Y and X chromosomes. The genes were organized by sex chromosome with Y-encoded genes on the left panel and X-encoded genes on the right panel.
D. Comparison to genes significantly different between sexes in organs from humans. The male vs. female comparisons were compared to the male vs female comparisons from the GTEx study analyzed in BSCE. The -log(p-value) of the Running Fisher correlation test are shown for that tissue.
E. Comparison to genes significantly different between sexes in mouse and rat livers. The male vs. female comparisons were compared to the male vs female comparisons from adult mice and rats. The Gene Expression Omnibus number for the study is shown. The -log(p-value) of the Running Fisher correlation test for the pair-wise comparison is shown.
F. The expression changes of genes encoding transporters (left) and miscellaneous XMEs and transcriptional regulators (right).
We hypothesized that a subset of the genes which exhibited differential expression between the sexes would be encoded by the sex chromosomes. Approximately 4% of all genes were encoded on the sex chromosomes in the young male vs female group and that number increased to ~11% in either the all male vs female group or old male vs old female group (Figure 4C). Eight of the genes encoded on the Y chromosome (RPS4Y2, RPS4Y1, DDX3Y, EIF1AY, USP9Y, TXLNG2P, KDM5D, ZFY) and 7 of the genes encoded on the X chromosome (TSIX, XIST, HDHD1, ARSD-AS1, KDM6A, ARSD, RPS4X) exhibited a common pattern of expression across the three comparisons. Those genes encoded on the Y chromosome exhibited higher expression levels in males than females, and the genes encoded on the X chromosome had lower expression levels in males than females, as expected.
Similar to the analysis for the aging genes, we compared the three male vs. female comparisons to gene lists in BSCE. Most notably, the Genotype-Tissue Expression (GTEx) Project is a large study in which 5–13 samples from 39 human tissues were examined for gene expression differences between males and females. Figure 4D shows the consistently high correlation -log(p-value)s (8.5 – 13.5) between the comparisons in the GTEX study and our gene lists. Surprisingly, the tissues with the highest correlations were derived from the brain (anterior cingulate cortices, frontal cortices, cerebellar hemispheres), while the livers from the GTEx study were in the bottom third of the correlation –log(p-value)s. This analysis indicates that there is a common set of tissue-independent genes that exhibit sex differences.
We also compared the male vs female gene lists to those derived from the livers of mice and rats (Figure 4E). There was little consistency between the human male vs female gene lists and those derived from the livers of mice or rats. However, the significance of the correlations was higher between the mouse and rat comparisons and those in old males vs old females group indicating sex-dependent gene expression converges between species upon aging in humans. These results are consistent with the fact that sex-dependent differences in gene expression in rodents but not humans are driven mainly by the growth hormone pulsatile pattern from the pituitary [27]. Any overlap in the genes altered by sex would be independent of the growth hormone-dependent pathways.
XMEs differentially expressed between sexes were identified and are shown in Figure 4F. XME genes including those involved in absorption, distribution, metabolism, and excretion in humans came from two sources [28, 29]. There were 14 transporter genes that were differentially expressed between one or more groups, most of which were down-regulated in males compared to females. Most of the changes were in the young comparison. There were a minor number of changes in CYP genes (CYP1B1, CYP26A1, CYP2C18, CYP2J2, CYP8B1, CYP4X1) or transcriptional regulators (FOXA1, ADH5, CEBPA, NR1H4, NR5A1) between sexes that did not exhibit consistent directional changes between sexes.
Impact of aging on expression of cytochrome P450 genes/enzyme activities
We determined whether there were differences in the cytochrome P450 activities between old and young groups. While the genomic analysis used 23 human livers, there were 32 samples (8 per group) used for the P450 enzyme activity analysis. PCA of the 10 P450 enzyme activities across the 32 samples is shown in Figure 5A. Overall, there was no obvious separation of samples either by age or sex. The P450 enzyme activities were plotted vs age of the individual and compared between males and females (Figure 5B). There were trends towards either increased activity (CYP1A2, CYP2C9) or decreased activity (CYP2A6, CYP2B6, CYP2D6, CYP2E1, CYP3A4) in the older vs. younger individuals. Figure 5C shows the mean of enzyme activity in each of the groups. There were statistically significant increases in CYP1A2 and CYP2C9 activities in old compared to young. There were no significant decreases in any of the activities with age. There were no changes in the gene expression of CYP1A2 and CYP2C9 between the groups analyzed.
Figure 5. Alteration of xenobiotic metabolizing enzyme expression and activity by age and sex.
A. PCA of 32 individuals plotted based on the P450 activities of 10 enzyme activities. Samples were colored by age. Females are depicted by spheres and males by cubes.
B. The cytochrome P450 activities among all 32 individuals. P450 activities were plotted by age of the individual. Males are depicted by circles and females by triangles. The lines are the result of a regression analysis.
C. Cytochrome P450 activity levels in old and young individuals. CYP3A4 (6BT) levels were reduced 10-fold to put the values in scale with the rest of the activities. *Indicates a significant difference between old and young (p-value < 0.05).
D. The expression changes of genes encoding transporters (left) and miscellaneous XMEs and transcriptional regulators (right).
We determined whether any of the genes altered by aging were those involved in xenobiotic metabolism. Figure 5D (left) shows that a number of transporters exhibited altered expression in the aged groups including SLC family members. A number of genes that either regulate the XMEs (FOS, HNF4G, MYC, NR0B2, NR1I3, NR3C1, NR4A1, NR4A2, NR4A3, NR5A2, STAT4) or are XMEs themselves (ADH1A, ADH1C, ADH4, ADH6, AKR7A3, AOC3, EPHX2, FMO1, FMO3, FMO4, FMO5, GUSB, HSD17B2) were found at higher expression levels in the aged individuals. Fewer genes were down-regulated including potential transcriptional regulators (ARNTL, NR1I2, NR2F6, RXRA) and XMEs themselves (AKR1C2, AKR1C3, CYP4F11, NOSIP, POR). Thus, genes involved in regulating XMEs or the XMEs themselves are altered by age in human livers.
Discussion
The aged represent a potential sensitive subpopulation due to reduced resilience and greater sensitivity to the adverse effects of drugs and xenobiotic chemical exposure [4]. The impact of aging on basal gene expression in human tissues is not completely known. Here, we characterized the global changes in the expression of XMEs in 23 human liver samples. We found that aging has a significant impact on the expression of the human genome with 1437 and 1670 genes being identified as significantly altered between old versus young groups in males or females, respectively. Through a canonical pathway analysis, the changes implied that aging led to increased inflammation and suppression of intermediary metabolism. The relatively minor overlap in genes altered in aging (147) between the sexes paralleled sex-specific alterations in many canonical pathways. Common genes altered by aging in the livers of humans and mice were identified and many were involved in inflammatory responses. The set of samples analyzed allowed us to characterize differences in gene expression between males and females. Many genes were identified in the young male versus young female comparisons; only ~20% of the total number of gene changes in the young comparison persisted in the old male versus old female comparison. Of the sex-dependent genes, only a small number of the genes were encoded on the X or Y chromosomes, indicating other factors are involved in determining sex-specific gene expression patterns. Many genes involved in xenobiotic metabolism were identified that were differentially regulated by age or sex and included CYP enzymes, phase-II metabolism genes, transporter genes, and transcription factors known to control these genes. An analysis of 10 P450 enzyme activities showed that the aged group exhibited higher activities of CYP1A2 and CYP2C9 enzymes compared to the young group. These studies demonstrate that in the human liver, age and sex have a profound impact on basal gene expression including those involved in xenobiotic metabolism. These findings lead to the hypothesis that differences in the expression and activity of the xenobiotic metabolism machinery result in differences in the metabolism and phenotypic effects of xenobiotics in older adults.
Inflammation is a hallmark of aging [7]. There are increases in the circulating levels of proinflammatory cytokines such as IL-6, TNF-α, and IL-1 with age in mice and humans [30, 31]. “Inflamm-aging” has been associated with most of the major age-related diseases, including atherosclerosis, diabetes, Alzheimer’s disease, rheumatoid arthritis, cancer, and aging itself [32]. Our analysis indicated that the aged livers exhibited increased expression of genes associated with inflammation. We found that canonical pathways annotated in IPA involved in inflammatory processes were predicted to be increased in activity and that the increases were either unique to or more prominent in females than males. The IPA upstream activator analysis supported the activation of NF-kB, a central mediator of inflammatory responses in the aged livers. Furthermore, using a gene expression biomarker that accurately predicts NF-kB activation in human tissues [21], we confirmed that NF-kB was activated but only in the female old versus young group. Many of the gene expression changes linked to inflammation are likely associated with tertiary lymphoid neogenesis (TLN). TLN is the phenomenon in which ectopic lymphoid structures are formed in chronically inflamed tissues (Hjelmstrom, 2001) [33]. These structures have been observed in a transgenic model of chronic hepatic inflammation and Helicobacter infection in mouse liver and in humans with liver inflammation due to infectious agents such as hepatitis C [34–36]. In a comparison between the aged human liver samples and livers from aged mice, we identified a common set of genes that were altered in the same direction in both species; many of these genes were linked to inflammatory processes. These genes did not include those identified earlier in mice that were associated with the presence of activated B-lymphocytes including a number of immunoglobulin genes regulated by ITF-2 and TCF4 [9]. Given that most of the genes that overlap between humans and mice have functions in immune cells or in inflammatory responses, alteration of these genes is a consequence of either increased levels of immune cells in the tissues and/or increased levels of cytokines, as demonstrated previously in other tissues of aged mice [37, 38]. Our observations suggest that normal aging in the liver is associated with a transcriptional pattern indicative of heightened immune and inflammatory responses that appear to be more prominent in women. These findings support the idea that a set of transcriptional biomarkers of aging could be used to estimate the efficacy of general and tissue-specific aging interventions.
While not the primary focus of the study, we determined the differences in basal gene expression between males and females. There are substantial gene expression differences in the rodent liver between sexes [10], but the differences in humans have not been well defined. When comparing young males versus young females, we identified 1065 genes. In the old groups, this number was greatly reduced (202). Remarkably, only a small percentage of the genes were encoded on the sex chromosomes. These findings indicate that sexual dimorphism in gene expression is determined by sex chromosome-independent changes and are most likely due to differences in the circulating levels of sex hormones. Additionally, by comparing our gene lists to those in the Genotype-Tissue Expression (GTEx) database Project [39, 40], we found consistently high correlations between the sexually-dimorphic genes in our study and the 39 tissues examined in the GTEx study indicating that there are common sets of tissue-independent genes that exhibit sex differences across tissues, including the liver. The definition of these gene sets will be an interesting focus in future studies to determine if any of the genes are linked to differences in disease and longevity between the sexes. For example, women suffer fewer obesity-related disorders than men [41]. Sex-specific gene expression could contribute to longer life span in women [42]. However, women tend to have longer periods of frailty and disability, the so-called “male-female health-survival paradox” [43]. The role of sexually dimorphic genes in these sex differences in health and disease may be a fruitful area of investigation.
An important outcome of our study was the identification of XMEs that exhibit age- and sex-dependent gene expression. Hepatic metabolism genes play important roles in xenobiotic and endogenous molecule metabolism contributing to individual susceptibility to drugs and toxicants. Studies show that levels of liver enzyme activity drop with aging [44], and this decreased activity could result in slowed detoxification of some compounds and reduced excretion rates, which can result in higher effective circulating levels and longer half-lives. Our analysis of the liver xenobiotic metabolism genes (Phase I, Phase II, and Phase III) along with 10 P450 activities indicates that there were no consistent decreases in XME gene expression or enzyme activity in the older groups. In fact, the only differences in enzyme activity between old and young were small but significant increases in the activity of CYP1A2 and CYP2C9. While there were decreases in the expression of some of the XMEs, there were more increases in expression than XMEs with decreased expression. The effects of these changes in XMEs on xenobiotic metabolism might be predicted by examining linkages between the enzyme encoded by the gene and effects of that enzyme on metabolism of specific chemicals in the Comparative Toxicogenomics Database which has annotations gathered from the literature linking specific enzyme activities to individual chemical metabolism [45]. While we identified many differences in gene expression and enzyme activity in the aged group, the impact on xenobiotic metabolism remains to be determined. Additionally, we examined a relatively small set of humans and findings should be confirmed in another cohort. It should be acknowledged that differences in xenobiotic metabolism may be minor compared to changes in liver volume, blood flow, and glomerular filtration rate. Studies have found that most age-related changes in hepatic activity are likely due to declines in liver volume and blood flow with age [46, 47].
In summary, we profiled human liver gene expression and identified sets of genes that are differentially expressed with age and sex. Aging affected pathways involved in inflammation and intermediary metabolism. The young group exhibited more sex-related gene expression alterations than the old group. Many XMEs were identified that exhibited changes with age or sex. The cytochrome P450 activity towards 10 substrates showed that the old group had higher CYP1A2 and CYP2C9 activities than the young. These studies provide information on the effects of aging and sex on hepatic metabolic gene expression that could be informative for the evaluation of life-stage dependent differences and toxicity in human health risk assessments.
Supplementary Material
Table S1: Differentially expressed genes identified in the study.
Table S2: Canonical pathway analysis of the differentially expressed genes.
Table S3: Upstream activator analysis of the differentially expressed genes.
Table S4: Canonical pathway analysis of genes commonly altered by aging in human and mouse livers.
Table S5: Upstream activator analysis of genes commonly altered by aging in human and mouse livers
Acknowledgements
We thank Drs. Elaina Kenyon and James Samet for critical review of this manuscript. Funding for this study came from the U.S. EPA Office of Research and Development.
Abbreviations:
- XME
xenobiotic metabolizing enzyme
- CYP
cytochrome P450 enzyme
- IPA
Ingenuity Pathway Analysis
- BSCE
BaseSpace Correlation Engine
References:
- 1.AOA, A., Profile of Older Americans: 2009. Washington, DC: US Administration on Aging. Available, 2004. [Google Scholar]
- 2.Census UBOT, Population projections of the United States by age, sex, race, and Hispanic origin: 1995 to 2050. 1996, US Government Printing Office; Washington DC. p. 25–1130. [Google Scholar]
- 3.Landrigan PJ, et al. , Environmental pollutants and disease in American children: estimates of morbidity, mortality, and costs for lead poisoning, asthma, cancer, and developmental disabilities. Environmental health perspectives, 2002. 110(7): p. 721–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Geller AM and Zenick H, Aging and the environment: a research framework. Environ Health Perspect, 2005. 113(9): p. 1257–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tan JL, et al. , Age-Related Changes in Hepatic Function: An Update on Implications for Drug Therapy. Drugs Aging, 2015. 32(12): p. 999–1008. [DOI] [PubMed] [Google Scholar]
- 6.Mitzner TL, Sanford JA, and Rogers WA, Closing the Capacity-Ability Gap: Using Technology to Support Aging With Disability. Innov Aging, 2018. 2(1): p. igy008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hunt NJ, et al. , Hallmarks of Aging in the Liver. Comput Struct Biotechnol J, 2019. 17: p. 1151–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee JS, et al. , Coordinated changes in xenobiotic metabolizing enzyme gene expression in aging male rats. Toxicol Sci, 2008. 106(1): p. 263–83. [DOI] [PubMed] [Google Scholar]
- 9.Lee JS, et al. , Meta-analysis of gene expression in the mouse liver reveals biomarkers associated with inflammation increased early during aging. Mech Ageing Dev, 2012. 133(7): p. 467–78. [DOI] [PubMed] [Google Scholar]
- 10.Waxman DJ and Holloway MG, Sex differences in the expression of hepatic drug metabolizing enzymes. Mol Pharmacol, 2009. 76(2): p. 215–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu J, et al. , Expression of cytochrome P450 isozyme transcripts and activities in human livers. Xenobiotica, 2021. 51(3): p. 279–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu J, et al. , Sex-, Age-, and Race/Ethnicity-Dependent Variations in Drug-Processing and NRF2-Regulated Genes in Human Livers. Drug Metab Dispos, 2021. 49(1): p. 111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kupershmidt I, et al. , Ontology-based meta-analysis of global collections of high-throughput public data. PLoS One, 2010. 5(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oshida K, et al. , Identification of chemical modulators of the constitutive activated receptor (CAR) in a gene expression compendium. Nucl Recept Signal, 2015. 13: p. e002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oshida K, et al. , Screening a mouse liver gene expression compendium identifies modulators of the aryl hydrocarbon receptor (AhR). Toxicology, 2015. 336: p. 99–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oshida K, et al. , Identification of modulators of the nuclear receptor peroxisome proliferator-activated receptor alpha (PPARalpha) in a mouse liver gene expression compendium. PLoS One, 2015. 10(2): p. e0112655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corton JC, Kleinstreuer NC, and Judson RS, Identification of potential endocrine disrupting chemicals using gene expression biomarkers. Toxicol Appl Pharmacol, 2019. 380: p. 114683. [DOI] [PubMed] [Google Scholar]
- 18.Cervantes PW and Corton JC, A Gene Expression Biomarker Predicts Heat Shock Factor 1 Activation in a Gene Expression Compendium. Chem Res Toxicol, 2021. 34(7): p. 1721–1737. [DOI] [PubMed] [Google Scholar]
- 19.Rooney J, Chorley B, Corton JC, Mining a human transcriptome database for chemical modulators of Nrf2. Submitted, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jackson AC, et al. , Identification of novel activators of the metal responsive transcription factor (MTF-1) using a gene expression biomarker in a microarray compendium. Metallomics, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Korunes KL, et al. , A gene expression biomarker for predictive toxicology to identify chemical modulators of NF-κB. PLoS One, 2022. 17(2): p. e0261854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Corton JC, Williams A, and Yauk CL, Using a gene expression biomarker to identify DNA damage-inducing agents in microarray profiles. Environ Mol Mutagen, 2018. 59(9): p. 772–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levin M, et al. , The mid-developmental transition and the evolution of animal body plans. Nature, 2016. 531(7596): p. 637–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aramillo Irizar P, et al. , Transcriptomic alterations during ageing reflect the shift from cancer to degenerative diseases in the elderly. Nat Commun, 2018. 9(1): p. 327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baruch K, et al. , Aging. Aging-induced type I interferon response at the choroid plexus negatively affects brain function. Science, 2014. 346(6205): p. 89–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boylston WH, DeFord JH, and Papaconstantinou J, Identification of longevity-associated genes in long-lived Snell and Ames dwarf mice. Age (Dordr), 2006. 28(2): p. 125–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Waxman DJ and O’Connor C, Growth hormone regulation of sex-dependent liver gene expression. Mol Endocrinol, 2006. 20(11): p. 2613–29. [DOI] [PubMed] [Google Scholar]
- 28.Slatter JG, et al. , Compendium of gene expression profiles comprising a baseline model of the human liver drug metabolism transcriptome. Xenobiotica, 2006. 36(10–11): p. 938–62. [DOI] [PubMed] [Google Scholar]
- 29.Bleasby K, et al. , Expression profiles of 50 xenobiotic transporter genes in humans and pre-clinical species: a resource for investigations into drug disposition. Xenobiotica, 2006. 36(10–11): p. 963–88. [DOI] [PubMed] [Google Scholar]
- 30.Ershler W, et al. , Interleukin-6 and aging: blood levels and mononuclear cell production increase with advancing age and in vitro production is modifiable by dietary restriction. Lymphokine and cytokine research, 1993. 12(4): p. 225–230. [PubMed] [Google Scholar]
- 31.Ershler WB, Interleukin-6: A Cytokine for Gerontolgists. Journal of the American Geriatrics Society, 1993. 41(2): p. 176–181. [DOI] [PubMed] [Google Scholar]
- 32.Rea IM, et al. , Age and Age-Related Diseases: Role of Inflammation Triggers and Cytokines. Front Immunol, 2018. 9: p. 586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luo S, et al. , Chronic Inflammation: A Common Promoter in Tertiary Lymphoid Organ Neogenesis. Front Immunol, 2019. 10: p. 2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heikenwalder M, et al. , Chronic lymphocytic inflammation specifies the organ tropism of prions. Science, 2005. 307(5712): p. 1107–1110. [DOI] [PubMed] [Google Scholar]
- 35.Freni MA, et al. , Focal lymphocytic aggregates in chronic hepatitis C: occurrence, immunohistochemical characterization, and relation to markers of autoimmunity. Hepatology, 1995. 22(2): p. 389–394. [PubMed] [Google Scholar]
- 36.Shomer NH, et al. , Helicobacter-induced chronic active lymphoid aggregates have characteristics of tertiary lymphoid tissue. Infection and immunity, 2003. 71(6): p. 3572–3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu D, et al. , Aging up-regulates expression of inflammatory mediators in mouse adipose tissue. The Journal of Immunology, 2007. 179(7): p. 4829–4839. [DOI] [PubMed] [Google Scholar]
- 38.Ye S-M and Johnson RW, Increased interleukin-6 expression by microglia from brain of aged mice. Journal of neuroimmunology, 1999. 93(1–2): p. 139–148. [DOI] [PubMed] [Google Scholar]
- 39.Carithers LJ and Moore HM, The Genotype-Tissue Expression (GTEx) Project. Biopreserv Biobank, 2015. 13(5): p. 307–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Consortium TG, The Genotype-Tissue Expression (GTEx) project. Nat Genet, 2013. 45(6): p. 580–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heindel JJ, et al. , Metabolism disrupting chemicals and metabolic disorders. Reprod Toxicol, 2017. 68: p. 3–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tower J, Sex-Specific Gene Expression and Life Span Regulation. Trends Endocrinol Metab, 2017. 28(10): p. 735–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Le Couteur DG, Anderson RM, and de Cabo R, Sex and Aging. J Gerontol A Biol Sci Med Sci, 2018. 73(2): p. 139–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Youssef J and Badr M, Biology of senescent liver peroxisomes: role in hepatocellular aging and disease. Environmental Health Perspectives, 1999. 107(10): p. 791–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Davis AP, et al. , Comparative Toxicogenomics Database (CTD): update 2021. Nucleic Acids Res, 2021. 49(D1): p. D1138–d1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schmucker DL, Liver function and phase I drug metabolism in the elderly. Drugs & aging, 2001. 18(11): p. 837–851. [DOI] [PubMed] [Google Scholar]
- 47.Vestal RE, Aging and determinants of hepatic drug clearance. Hepatology, 1989. 9(2): p. 331–334. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Differentially expressed genes identified in the study.
Table S2: Canonical pathway analysis of the differentially expressed genes.
Table S3: Upstream activator analysis of the differentially expressed genes.
Table S4: Canonical pathway analysis of genes commonly altered by aging in human and mouse livers.
Table S5: Upstream activator analysis of genes commonly altered by aging in human and mouse livers