Abstract
Aims:
(1) To determine the dose-response relationship of therapeutic ultrasound for TMD-related pain in the masseter muscle among four doses comprised of two intensities (0.4 W/cm2 and 0.8 W/cm2) and two duty cycles (50% and 100%); and (2) to determine if therapeutic ultrasound applied to the masseter muscle would elicit a segmental effect on the ipsilateral temporalis muscle.
Methods:
A total of 28 adult women with bilateral myalgia were randomly allocated to one of the four intervention doses. Therapeutic ultrasound was applied on each side of the masseter sequentially for 5 minutes. The following outcomes were measured before and immediately after each intervention: self-reported pain score, pressure pain thresholds for the masseter and temporalis muscles, and intraoral temperature adjacent to the treated masseter.
Results:
Self-reported pain scores showed neither significant main effects nor significant interaction among the intensity or duty cycle doses (all P > .05). The change in the pressure pain threshold of the masseter showed a significant interaction (P = .02) attributed to the 0.4 W/cm2 and 100% duty cycle dose. Intraoral temperature was significantly increased and associated with the duty cycle (P = .01). A significant segmental effect of the pressure pain threshold of the temporalis was found for intensity (P = .01).
Conclusion:
There was an increase in the pressure pain threshold of the painful masticatory muscles and an increase in intraoral temperature adjacent to the treated area immediately after the use of ultrasound at 0.4 W/cm2 with a 100% duty cycle. J Oral Facial Pain Headache 2022;36:263–271.doi: 10.11607/ofph.2836
Keywords: dose response, masseter myalgia, pressure pain threshold, randomized clinical trial, temporomandibular disorders, ultrasound therapy
Definition and Pathophysiology of TMD
Temporomandibular disorders (TMD) are a group of musculoskeletal and neuromuscular conditions that involve the temporomandibular joints (TMJs), the masticatory muscles, and all associated tissues.1 These disorders are characterized by regional pain and limitation of mandibular range of motion2 and affect approximately 5% to 12% of the population, with an 83.8% female prevalence.3 A dual-axis diagnostic instrument (Diagnostic Criteria for TMD [DC/TMD]) has been developed for categorizing TMD conditions and provides simple, reliable, and valid operational definitions for diagnosis using evidence-based criteria.4
One of the commonly diagnosed pain disorders associated with masticatory muscles is “myalgia,” defined as “pain of muscle origin that is affected by jaw movements, function, or parafunction, and replication of this pain occurs with provocation testing of the masticatory muscles.”4 While the pathophysiology of this pain is poorly understood, multiple risk factors have been identified.3,5 A risk factor model describing the contribution of environmental, as well as some known genetic predisposing factors, to a state of high psychologic distress was identified.6 Because of the multifactorial nature of these disorders, a multimodal conservative treatment approach is recommended that includes patient education, behavioral and psychologic management, physical therapy, pharmacotherapy, and occlusal splints.7
Reviews of Ultrasound for Muscle Pain
Ultrasound has been reported since 19538 and has been applied to the jaw.9 There have been many reviews of ultrasound, and all agree its use is widespread.10–13 However, these reviews report a lack of evidence supporting ultrasound as a therapeutic modality,11,14–19 and unfortunately these reviews rarely report on the effect immediately after treatment or what measure of pain was used. In contrast, one recent systematic review and meta-analysis20 reported the immediate effects of ultrasound via the use of a visual analog scale to measure pain and pressure pain threshold (PPT). This review concluded that ultrasound reduced pain and increased PPT at the end of treatment. In addition, an indirect antinociceptive effect has been demonstrated clinically on painful trigger points present on segmental muscles that are neurologically linked but anatomically distinct.21 Thus, there is a rationale for the present research.
Animal experiments suggest that ultrasound appears to reduce nitric oxide synthase–like cells in the dorsal horn after injury,22 increase antinociception after trigeminal nerve constriction,23 and lower the thresholds for head withdrawal reflex after inferior alveolar nerve transection.24 Moreover, genetic manipulation suggests that wild-type reversal behavior is preserved without thermosensation, but is absent without mechanosensation.25 Together, these studies suggest that the mechanisms for ultrasound may in part extend beyond heating the tissue. Application of these ideas to human subjects will require future reports.
The first purpose of this study was to investigate the dose-response relationship between duty cycles of 100% and 50% and between intensities of 0.4 and 0.8 W/cm2 on painful masticatory muscles by measuring the following variables for the masseter muscle: self-reported verbal pain scale and PPT to assess the effect on pain, and intraoral temperature to assess the effect of any heating of the tissue. The second purpose was to determine if therapeutic ultrasound applied to the masseter muscle is capable of eliciting segmental effects on the ipsilateral temporalis muscle by measuring PPTs of the temporalis.21
Materials and Methods
Study Design and Subjects
This study was approved by the University at Buffalo Institutional Review Board (UBIRB 030-633338), was registered at clinicaltrials.gov (NCT02295644), and took place at the University at Buffalo School of Dental Medicine. The sample size was estimated using an alpha of .05, power of 0.8, and data from Srbely and Dickey,26 which led to a size of six; thus, seven subjects were recruited per group. The design was a parallel-group randomized clinical trial.
A total of 28 women were recruited from the Orofacial Pain and TMD Clinic and were identified during their initial visit. Subjects who participated in previous research conducted in the school and who had agreed to be contacted for future research studies were also invited to participate after confirming their eligibility. Written informed consent was obtained from each subject before participation in this single-blinded, randomized clinical trial.
All participants had a diagnosis of myalgia according to the DC/TMD,4 specifically bilaterally on masseter muscles, as modified for this protocol. At the time of recruitment, subjects had a pain intensity of 4 or more on a scale from 0 to 10, where 0 represented no pain and 10 the worst pain ever. Subjects who had a history of or who were diagnosed with a systemic musculoskeletal disorder or rheumatologic diseases (eg, fibromyalgia, muscular atrophy) were excluded from this trial. Those who had conditions such as neoplasms, fractures, or any neuropathies and/or degenerative neurologic disorders were also excluded. In addition, those who had undergone any form of physical therapy for the jaw area targeting muscle or TMJ symptoms within the last 60 days were not eligible to participate. Participants who were taking muscle relaxants or analgesics that were prescribed or over-the-counter were asked to stop any intake for at least 48 hours prior to the trial.
Randomization, Blinding, and Study Intervention Device
Computer-generated randomization was used. A random two-digit number was generated by a computer spreadsheet. The first digit was used to assign the treatment group. The second digit was used to assign the starting side of treatment. The participants were randomly assigned to one of four ultrasound intervention groups that included two settings for intensity (0.4 and 0.8 W/cm2) and two settings for duty cycle (50% and 100%). The treatments were assigned in blocks of four. Numbered, sealed envelopes were generated for the total number of subjects and opened after the subject was seated. The subject was unaware of the ultrasound dose settings.
Once the research visit was scheduled and participants had arrived, the investigators explained the study and obtained informed consent. A custom template of a clear vinyl sheet was made for each subject for each side of the face. The template was indexed to the ear and the ala of the nose and then attached by tape, remaining in place throughout the procedure. The first purpose of this template was to demarcate the anatomical borders of the masseter muscle to confine delivery of therapeutic ultrasound to the anatomical palpable limits of the masseter. The second purpose was to ensure reproducibility of the predetermined points on the masseter and temporalis muscles at which the PPTs were obtained. Baseline parameters on each side were taken in a particular order: first, self-reported pain scale, followed by PPT, then intraoral temperature. A single operator (Y.F.) performed the interventions at a consistent light pressure to ensure reliable application. The transducer head of the ultrasound unit was moved within the confines of the template cutout. A coupling agent gel was applied to ensure proper wave transmission and transfer of energy. The gel was warmed by a gel warmer to a level of normal body temperature in order to reduce error in postapplication muscle temperature measurement. Figure 1 shows an overview of the protocol.
Fig 1.

Flowchart of experimental protocol.
Following intervention, the same parameters were assessed in the following chronologic order: intraoral temperature, self-reported pain, and PPT. Therapeutic intervention was then delivered to the contralateral masseter through the attached template. Parameters were measured again in the same order.
At the end of the trial, subjects were given a gift card as compensation for their time, travel, and effort. Subjects were then dismissed with copies of their consent forms and contact information.
The ultrasound settings were selected based on the anatomical location and previously conducted studies.11,17 The therapeutic ultrasound intervention device (Sonicator 740, Mettler Electronics) has a transducer head (applicator) of 5 cm2, an effective radiating area (ERA) of 5 cm2 ± 20%, and a beam nonuniformity ratio (BNR) of 6:1. The frequency was set to 1 MHz. There is uncertainty in the literature about the efficacy of 1 vs 3 MHz, but no differences were found in human brachial artery dilation27 or in pig cadaver legs,28 and the present authors were not aware of any evidence in humans. Moveover, a fully cross-over design was desired, and so using both frequencies would have complicated the statistics and doubled the number of subjects. Since 1 MHz is reported to penetrate more deeply,29,30 1 MHz was used.
The assigned intervention was delivered to the assigned starting side. Therapeutic ultrasound was delivered for 5 minutes per muscle. The 5-minute duration of ultrasound was determined according to Grey.31 Planned average local exposure time was 2.5 minutes, the tissue area of the masseter was 10 cm2, and the ERA was 5 cm2. The total treatment time was equal to 5 minutes per side.
The ultrasound dose settings were: intensity of 0.4 or 0.8 W/cm2, and duty cycle of 50% or 100%.
Outcome Measures and Assessment
The primary outcomes were self-reported pain scale and PPT. The secondary outcome was intraoral temperature at the approximate site of the masseter muscle. The measurements were taken as follows:
Self-reported pain scale: This was obtained on a verbal scale from 0 to 10, where 0 represented no pain and 10 the worst pain ever. Self-reported pain was obtained verbally for each side of the face separately.
PPT: The PPT was measured at four sites: one on the masseter on each side, and one on the temporalis on each side. PPT sites on the masseter (PPT-M) and temporalis (PPT-T) muscles on each side were determined by palpating the bulkiest prominent sites of the body of the muscles while asking the subjects to repeatedly tap their teeth together. At each site, measurements were repeated until two readings less than 20 units apart were obtained. There was a 30-second time interval between recordings. A digital algometer (Somedic) was used under a constant rate of 0.67 kg/cm2 per second. The device was equipped with a pressure-terminating button that was handed to the subject; subjects were instructed to push the button as soon as they felt the sensation change from pressure to pain.
Intraoral temperature: The intraoral temperature was recorded in degrees Celsius using a digital thermometer. The recording metal end was 2 cm long. The temperature was recorded as two digits with one decimal. The metal end of the thermometer was placed against the buccal mucosa as an approximation of the location of the body of the masseter, and the temperature was measured as a surrogate of the internal masseter muscle temperature at the buccal mucosa. Intraoral thermal temperature was measured immediately after ultrasound delivery to minimize the temperature fade.
Statistical Methods
Changes in the outcome measurements before and after therapeutic intervention for all parameters were averaged for both sides (right and left) for each subject. The average changes were then statistically analyzed (Fig 2). Shapiro-Wilk test27 as well as visual inspection of the histograms, normal quantile-quantile (Q-Q) plots, and box plots of all measured parameters were performed. Fligner-Killeen test was performed to verify the equality of variance among groups.28 No tests showed violation of these assumptions. Data were analyzed using the “Anova” function in the “car” package in R,32 with the alpha set at .05.
Fig 2.
Timing of data collection and analysis. Intervention of the first side is shown on the left, and intervention on the second side on the right. Pretreatment scores (T1 for first side, T2 for second side) and posttreatment scores (T2 for first side, T3 for second side) were averaged; then, the posttreatment minus pretreatment scores were computed. T1 = before any intervention; T2 = after first-side intervention; T3 = after second side intervention
Two-way analysis of variance (ANOVA) of ultrasound dose settings (intensity [0.4 and 0.8 W/cm2] and duty cycle [50% and 100%]) were conducted to test the dose-response relationship for self-reported pain, PPT, and temperature, with ultrasound intensity and duty cycle as the between-subject factors.
Results of the segmental effects were obtained by conducting two-way ANOVA of ultrasound intensity and duty cycle on the PPTs of the temporalis muscle.
Results
Subject Characteristics
A total of 28 adult women with a mean ± SD age of 37 ± 11.41 years, ranging from 23 to 65 years, were recruited in the study in 2014 and 2015. Recruiting ended when all 28 were enrolled. Three subjects were lost for not meeting the inclusion requirements: 1 in the 0.8 W/cm2 + 50% duty cycle group, and 2 in the 0.8 W/cm2 + 100% duty cycle group. In addition to bilateral masseter myalgia, 16 subjects had a diagnosis of bilateral arthralgia, 4 had unilateral arthralgia, and 4 had no arthralgia. A total of 21 subjects also had a diagnosis of headache attributed to TMD, 19 of which had a bilateral condition (Table 1).
Table 1.
Baseline Characteristics of the Intervention Groups
| Intervention group | Subjects, n | Mean age, y | Range baseline pain, 0–10 VAS |
Mean baseline pain at time of recruitment, 0–10 VAS |
Baseline diagnosis, n/total subjects |
||
|---|---|---|---|---|---|---|---|
| Intensity (W/cm2) |
Duty cycle (%) |
Headache attributed to TMD |
Arthralgia | ||||
| 0.4 | 50 | 7 | 39.1 | 4 to 6 | 5.0 | Bilateral: 4/7 Unilateral: 2/7 None: 1/7 |
Bilateral: 3/7 Unilateral: 1/7 None: 3/7 |
| 0.4 | 100 | 7 | 34.7 | 4 to 6 | 4.9 | Bilateral: 6/7 None: 1/7 |
Bilateral: 5/7 Unilateral: 1/7 NA: 1/7 |
| 0.8 | 50 | 6 | 38.1 | 4 to 7 | 5.5 | Bilateral: 5/6 None: 1/6 |
Bilateral: 5/6 Unilateral: 1/6 |
| 0.8 | 100 | 5 | 29.8 | 5 to 7 | 5.6 | Bilateral: 4/5 None: 1/5 |
Bilateral: 3/5 Unilateral: 1/5 None: 1/5 |
Dose-Response Relationships
Self-reported pain scale.
Table 2 shows the change in scores, posttreatment minus pretreatment, for the self-reported pain score (SRPS). The scores averaged over both sides showed negative values, which indicates some reduction in pain and therefore an improvement of the pain condition. The two groups receiving 0.4 W/cm2 had 95% CI that did not include 0. Two-way ANOVA (Table 3) failed to find significant main effects or interactions (F < 2, P > .2 for all). Mean changes are shown in Fig 3.
Table 2.
Mean (± SD) and 95% CI of Changes for Self-Reported Pain Scale (Pain), PPT-M, PPT-T, and Intraoral Temperature (Temp)
| Duty cycle | Intensity 0.4 W/cm2 | Pain | Intensity 0.8 W/cm2 | |||||
|---|---|---|---|---|---|---|---|---|
| Pain | PPT-M | Temp | PPT-T | PPT-M | Temp | PPT-T | ||
| 50% | –0.93 (± 1.41), –2.48 to –0.116 |
–8.8 (± 30), –31 to 14 |
–0.007 (± 0.10), –0.08 to 0.07 |
15.4 (± 23), –1.9 to 33 |
–1.33 (± 1.2), –2.3 to –0.36 |
–2.0 (± 11), –7.2 to 11 |
0.083 (± 0.13), –0.02 to 0.19 |
–2.8 (± 18) , –17 to 12 |
| 100% | –2.0 (± 0.87), –2.64 to –0.36 |
15.21 (± 5.8), 10.9 to 19.5 |
0.15 (± 0.21), –0.003, 0.30 |
16.04 (± 15.5), 4.6 to 28 |
–1.3 (± 1.35), –1.94 to 0.084 |
–10.25 (± 12.04), –20.8 to 0.304 |
0.42 (± 0.40), 0.07 to 0.77 |
–9.2 (± 16), –23 to 4.6 |
Table 3.
Two-Way ANOVA for Self-Reported Pain Scale (Pain), PPT-M, PPT-T, and Intraoral Temperature (Temp)
| Variable | Factor | df | Sum of squares | F | Sig. |
|---|---|---|---|---|---|
| Pain | Duty cycle | 1 | 1.65 | 1.14 | .30 |
| Intensity | 1 | 0.134 | 0.092 | .76 | |
| Interaction | 1 | 1.87 | 1.29 | .27 | |
| Error | 21 | 1.42 | |||
| PPT-M | Duty cycle | 1 | 210 | 0.64 | .43 |
| Intensity | 1 | 332 | 1.00 | .33 | |
| Interaction | 1 | 2,010 | 6.09 | .022 | |
| Error | 21 | 38.97 | |||
| Temp | Duty cycle | 1 | 0.37 | 7.63 | .01 |
| Intensity | 1 | 0.20 | 4.07 | .06 | |
| Interaction | 1 | 0.05 | 1.01 | .33 | |
| Error | 24 | 0.045 | |||
| PPT-T | Duty cycle | 1 | 52 | 0.15 | .69 |
| Intensity | 1 | 2,907 | 8.3 | .009 | |
| Interaction | 1 | 76 | 0.22 | .65 | |
| Error | 21 | 387.46 |
Fig 3.

Mean changes in self-reported pain scores. Scores are calculated as posttreatment values minus pretreatment values, so negative values represent less reported pain.
Pressure pain threshold of the masseter muscle.
The largest increase in PPT-M was shown in the group with an intervention of 0.4 W/cm2 + 100% duty cycle, resulting in a mean of 15.21 ± 5.8 (Table 2). The 95% CI for that group did not include 0. An increase in PPT is interpreted as an improvement or reduction in pain. A decrease in PPT-M was shown in the other three groups, which indicates some increase in pain or no improvement. Mean changes are presented in Fig 4. Two-way ANOVA showed a significant interaction (P = .022) between the intensity and duty cycle (Table 3). This significant interaction of the PPT response suggests that the 0.4 W/cm2 + 100% duty cycle was the most effective dose.
Fig 4.
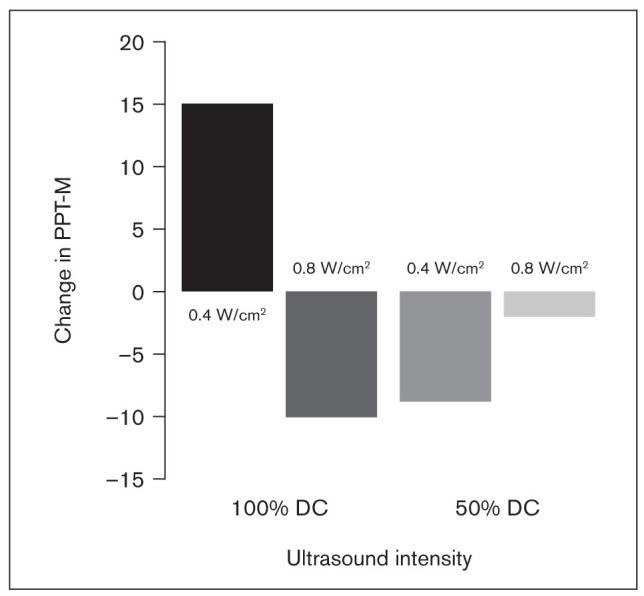
Mean changes in PPT-M. Changes are calculated as posttreatment minus pretreatment values, so positive numbers represent an increase in the pain threshold.
Intraoral temperature.
Intraoral temperature changes from pretreatment to posttreatment were positive for the 100% duty cycle groups (Table 2), with means of 0.15ºC and 0.42ºC (Fig 5). Two-way ANOVA showed no significant interaction, but duty cycle showed a significant main effect on intraoral temperature increase (P = .01, Table 3). These data suggest that ultrasound heats the masseter muscle in a dose-dependent relation.
Fig 5.

Mean changes in intraoral temperature. Changes are calculated as posttreatment values minus pretreatment values, so positive numbers suggest an increase in temperature.
Segmental effects: Pressure pain threshold of the temporalis muscle.
PPT-T increased in the two groups utilizing 0.4 W/cm2 (Table 2), which indicates improvement, whereas the two groups with an intervention of 0.8 W/cm2 showed a decrease in pain threshold, which may indicate an exacerbation of the pain condition (Fig 6). Only the 0.4 W/cm2 + 100% duty cycle had a 95% CI that did not include 0. Two-way ANOVA showed no significant dose interaction (P = .65); however, a main effect of intensity setting was significant (P = .009; Table 3). This significant main effect for intensity was interpreted as the ultrasound treatment applied to the masseter providing a segmental effect to the untreated temporalis muscle.
Fig 6.

Mean PPT-T changes. Changes are calculated as posttreatment minus pretreatment values, so positive numbers represent an increase in the pain threshold.
Discussion
One main finding in this dose-response, single-blinded study was the increase in PPT-M utilizing therapeutic ultrasound with settings of 0.4 W/cm2 and 100% duty cycle. This finding was also observable in the interaction plot. The changes in the SRPS were consistent with the PPT results but not significant.
A second main finding was that two of the doses led to an increase in PPT in the untreated ipsilateral temporalis muscle after the masseter muscle was treated with ultrasound. Srbely et al21,26 observed similar results in back muscles. In the present study, there was an average increase in scores for the intervention groups using 0.4 W/cm2, while the groups utilizing the setting of 0.8 W/cm2 showed a decrease in PPT scores. Significant main effects were attributed to the intensity setting. The use of 0.4 W/cm2 was more capable of providing an indirect antinociceptive effect on the temporalis muscle. These findings are consistent with the findings of Srrbely et al and raise the possibility of involvement of a central modulatory mechanism. Thus, experimental and clinical evidence suggest that peripheral application of ultrasound waves may have central modulatory effects.
In animal models, cellular changes have occurred in the dorsal horn of the spinal cord, and the number of neurons with nitric oxide synthase-like enzyme (nNOS-L1) involved in the pain-modulating pathway was reduced.22,33 These findings suggest that ultrasound may act on central mechanisms of sensitization, but data from human trials have not been established.
A third finding in this study was an increase in intraoral temperature for the 100% duty cycle doses. Therapeutic ultrasound is delivered through the reverse piezoelectric phenomenon. Thermal effects are thought to be achieved upon the transfer of acoustic energy to mechanical vibration, which generates frictional heat. The present data provide indirect evidence of heating of the masseter. The increase in intraoral temperature is an approximation of the muscle temperature.
Limitations
Limitations of this study include that the study was short term, from before to immediately after ultrasound therapy. In light of the dearth of dose-response studies, the present authors believe that this variable should be optimized to increase the chance of success. Future studies will be needed to determine if the successful dose found here provides relief for longer times.
Although the sample size was adequate to suggest an optimal dose among the four tested groups using PPTs and to suggest a segmental effect, these results should be considered as exploratory due to the modest sample size.
This study also did not include a placebo group to test whether the results were more efficacious than a placebo effect. However, Hussain et al34 reported evidence that the most successful dose used in the present study was better than placebo.
In general, there are a dearth of dose-response studies in therapeutic ultrasound; for example, the frequency of 1 MHz is thought to penetrate more deeply than 3 MHz, but neither textbooks29,30 nor experiments27,28 provide definitive guidance. Frequency and other factors affecting dose response await future experiments.
Strengths
This study was the first, to the present authors’ knowledge, to investigate a dose-response relationship for the management of TMD-related pain using therapeutic ultrasound. It was also the first to examine indirect effects of ultrasound on segmentally linked masticatory muscles. In addition, it was also the first ultrasound paper attempting to measure the intraoral temperature approximating the masseter muscle temperature to suggest that the ultrasound therapy did heat the masseter muscle. Finally, the diagnostic criteria for subject selection was based on the DC/TMD, so the selection was reliable and valid.
Conclusions
The use of ultrasound as a method for immediate management of painful masticatory muscles was shown to be successful.
Treatment directed to painful masseter muscles with an intensity of 0.4 W/cm2 with a 100% duty cycle increased the PPT of the masseter itself as well as the untreated temporalis muscle.
Changes in PPT of the masseter muscle, as well as the temporalis, were dependent on the intensity and duty cycle settings. Intraoral temperature rise was associated with the duty cycle.
Highlights
A dose of 0.4 W/cm2 + 100% duty cycle appears to be better than other doses tested.
This study was the first, to the authors’ knowledge, to investigate a dose-response relationship in the management of TMD-related pain using ultrasound.
This study was the first, to the authors’ knowledge, to report the indirect effects of ultrasound on segmentally linked masticatory muscles, as well as the first ultrasound paper attempting to measure intraoral temperature approximating the masseter muscle to suggest that ultrasound did heat the masseter muscle.
The diagnostic criteria for subject characterization were based on the DC/TMD and were thus reliable and valid.
Acknowledgments
The authors would like to acknowledge the American Academy of Orofacial Pain Research Committee for their support. Data acquisition was mostly by Y.F. and W.M. All authors contributed to the design, interpretation, writing, and revision of the manuscript.
The authors report no conflicts of interest. This work was presented at the American Academy or Orofacial Pain Annual Meeting, May 7–10, 2015, Orlando, Florida.
References
- Greene CS. Diagnosis and treatment of temporomandibular disorders: Emergence of a new “standard of care”. Quintessence Int. 2010;41:623–624. [PubMed] [Google Scholar]
- Drangsholt M, LeResche L. Temporomandibular disorder pain. In: Crombie IK, Croft PR, Linton SJ, Leresche L, Von Korff M, editors. Epidemiology of Pain: A Report of the Task Force on Epidemiology of the International Association for the Study of Pain. Seattle: IASP; 1999. pp. 203–233. [Google Scholar]
- Slade GD, Bair E, By K, et al. Study methods, recruitment, sociodemographic findings, and demographic representativeness in the OPPERA study. J Pain. 2011;12(suppl 11):T12–T26. doi: 10.1016/j.jpain.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffman E, Ohrbach R, Truelove E, et al. Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) for Clinical and Research Applications: Recommendations of the International RDC/TMD Consortium Network and Orofacial Pain Special Interest Group. J Oral Facial Pain Headache. 2014;28:6–27. doi: 10.11607/jop.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SB, Maixner DW, Greenspan JD, et al. Potential genetic risk factors for chronic TMD: Genetic associations from the OPPERA case control study. J Pain. 2011;12(suppl 11):T92–T101. doi: 10.1016/j.jpain.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maixner W, Diatchenko L, Dubner R, et al. Orofacial pain prospective evaluation and risk assessment study–the OPPERA study. J Pain. 2011;12(suppl 11):T4–11. e1–2. doi: 10.1016/j.jpain.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- List T, Axelsson S. Management of TMD: Evidence from systematic reviews and meta-analyses. J Oral Rehabil. 2010;37:430–451. doi: 10.1111/j.1365-2842.2010.02089.x. [DOI] [PubMed] [Google Scholar]
- Lehmann JF. The biophysical basis of biologic ultrasonic reactions with special reference to ultrasonic therapy. Arch Phys Med Rehabil. 1953;34:139–151. [PubMed] [Google Scholar]
- Erickson RI. Ultrasound-a useful adjunct in temporomandibular joint therapy. Oral Surg Oral Med Oral Pathol. 1964;18:176–179. doi: 10.1016/0030-4220(64)90422-0. [DOI] [PubMed] [Google Scholar]
- Armijo-Olivo S, Fuentes J, Muir I, Gross DP. Usage patterns and beliefs about therapeutic ultrasound by Canadian physical therapists: An exploratory population-based cross-sectional survey. Physiother Can. 2013;65:289–299. doi: 10.3138/ptc.2012-30BC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gam AN, Johannsen F. Ultrasound therapy in musculoskeletal disorders: A meta-analysis. Pain. 1995;63:85–91. doi: 10.1016/0304-3959(95)00018-N. [DOI] [PubMed] [Google Scholar]
- Warden SJ, McMeeken JM. Ultrasound usage and dosage in sports physiotherapy. Ultrasound Med Biol. 2002;28:1075–1080. doi: 10.1016/s0301-5629(02)00552-5. [DOI] [PubMed] [Google Scholar]
- Wong RA, Schumann B, Townsend R, Phelps CA. A survey of therapeutic ultrasound use by physical therapists who are orthopaedic certified specialists. Phys Ther. 2007;87:986–994. doi: 10.2522/ptj.20050392. [DOI] [PubMed] [Google Scholar]
- Allen RJ. Physical agents used in the management of chronic pain by physical therapists. Phys Med Rehabil Clin N Am. 2006;17:315–345. doi: 10.1016/j.pmr.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Baker KG, Robertson VJ, Duck FA. A review of therapeutic ultrasound: Biophysical effects. Phys Ther. 2001;81:1351–1358. [PubMed] [Google Scholar]
- Loyola-Sánchez A, Richardson J, MacIntyre NJ. Efficacy of ultrasound therapy for the management of knee osteoarthritis: A systematic review with meta-analysis. Osteoarthritis Cartilage. 2010;18:1117–1126. doi: 10.1016/j.joca.2010.06.010. [DOI] [PubMed] [Google Scholar]
- Robertson VJ. Dosage and treatment response in randomized clinical trials of therapeutic ultrasound. Phys Ther Sport. 2002;3:124–133. [Google Scholar]
- Robertson VJ, Baker KG. A review of therapeutic ultrasound: Effectiveness studies. Phys Ther. 2001;81:1339–1350. [PubMed] [Google Scholar]
- van der Windt DAWM, van der Heijden GJMG, van den Berg SGM, Ter Riet G, de Winter AF, Bouter LM. Ultrasound therapy for musculoskeletal disorders: A systematic review. Pain. 1999;81:257–271. doi: 10.1016/S0304-3959(99)00016-0. [DOI] [PubMed] [Google Scholar]
- Xia P, Wang X, Lin Q, Cheng K, Li X. Effectiveness of ultrasound therapy for myofascial pain syndrome: A systematic review and meta-analysis. J Pain Res. 2017;10:545–555. doi: 10.2147/JPR.S131482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srbely JZ, Dickey JP, Lowerison M, Edwards MA, Nolet PS, Wong LL. Stimulation of myofascial trigger points with ultrasound induces segmental antinociceptive effects: A randomized controlled study. Pain. 2008;139:260–266. doi: 10.1016/j.pain.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Hsieh YL. Reduction in induced pain by ultrasound may be caused by altered expression of spinal neuronal nitric oxide synthase-producing neurons. Arch Phys Med Rehabil. 2005;86:1311–1317. doi: 10.1016/j.apmr.2004.12.035. [DOI] [PubMed] [Google Scholar]
- Savernini Á, Savernini N, de Amaral FA, Romero TR, Duarte ID, de Castro MS. Assay of therapeutic ultrasound induced-antinociception in experimental trigeminal neuropathic pain. J Neurosci Res. 2012;90:1639–1645. doi: 10.1002/jnr.23056. [DOI] [PubMed] [Google Scholar]
- Sato M, Motoyoshi M, Shinoda M, Iwata K, Shimizu N. Low-intensity pulsed ultrasound accelerates nerve regeneration following inferior alveolar nerve transection in rats. Eur J Oral Sci. 2016;124:246–250. doi: 10.1111/eos.12271. [DOI] [PubMed] [Google Scholar]
- Kubanek J, Shukla P, Das A, Baccus SA, Goodman MB. Ultrasound elicits behavioral responses through mechanical effects on neurons and ion channels in a simple nervous system. J Neurosci. 2018;38:3081–3091. doi: 10.1523/JNEUROSCI.1458-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srbely JZ, Dickey JP. Randomized controlled study of the antinociceptive effect of ultrasound on trigger point sensitivity: Novel applications in myofascial therapy? Clin Rehabil. 2007;21:411–417. doi: 10.1177/0269215507073342. [DOI] [PubMed] [Google Scholar]
- Hauck M, Noronha Martins C, Borges Moraes M, et al. Comparison of the effects of 1MHz and 3MHz therapeutic ultrasound on endothelium-dependent vasodilation of humans: A randomised clinical trial. Physiotherapy. 2019;105:120–125. doi: 10.1016/j.physio.2017.08.010. [DOI] [PubMed] [Google Scholar]
- Demmink JH, Helders PJ, Hobaek H, Enwemeka C. The variation of heating depth with therapeutic ultrasound frequency in physiotherapy. Ultrasound Med Biol. 2003;29:113–118. doi: 10.1016/s0301-5629(02)00691-9. [DOI] [PubMed] [Google Scholar]
- Michlovitz SL, Bellew JW, Nolan T, editors. Modalities for Therapeutic Intervention. ed 5. Philadelphia: F. A. Davis; 2012. [Google Scholar]
- Bellew JW, Michlovitz SL, Nolan TP. Modalities for Therapeutic Intervention. ed 6. Philadelphia: F.A. Davis; 2016. [Google Scholar]
- Grey K. Distribution of treatment time in physiotherapeutic application of ultrasound. Physiotherapy. 2003;89:696–707. [Google Scholar]
- R Core Team. Vienna, Austria: Foundation for Statistical Computing; 2013. R: A language and environment for statistical computing. [Google Scholar]
- Hsieh YL. Effects of ultrasound and diclofenac phonophoresis on inflammatory pain relief: Suppression of inducible nitric oxide synthase in arthritic rats. Phys Ther. 2006;86:39–49. doi: 10.1093/ptj/86.1.39. [DOI] [PubMed] [Google Scholar]
- Hussain H, Crow H, Gonzalez Y, McCall WD. Immediate effect of continuous ultrasound vs sham ultrasound for bilateral masseter myalgia: A double-blinded trial. J Oral Facial Pain Headache. 2018;32:304–308. doi: 10.11607/ofph.2000. [DOI] [PubMed] [Google Scholar]



