Abstract
Aims:
To systematically review the scientific literature for evidence concerning the clinical use of botulinum toxin (BTX) for the management of various temporomandibular disorders (TMDs).
Methods:
A comprehensive literature search was conducted in the Medline, Web of Science, and Cochrane Library databases to find randomized clinical trials (RCT) published between 2000 and the end of April 2021 investigating the use of BTX to treat TMDs. The selected articles were reviewed and tabulated according to the PICO (patients/problem/population, intervention, comparison, outcome) format.
Results:
A total of 24 RCTs were selected. Nine articles used BTX injections to treat myofascial pain, 4 to treat temporomandibular joint (TMJ) articular TMDs, 8 for the management of bruxism, and 3 to treat masseter hypertrophy. A total of 411 patients were treated by injection of BTX. Wide variability was found in the methods of injection and in the doses injected. Many trials concluded superiority of BTX injections over placebo for reducing TMD pain levels and improving maximum mouth opening; however, this was not universal.
Conclusion:
There is good scientific evidence to support the use of BTX injections for treatment of masseter hypertrophy and equivocal evidence for myogenous TMDs, but very little for TMJ articular disorders. Studies with improved methodologic design are needed to gain better insight into the utility and effectiveness of BTX injections for treating both myogenous and TMJ articular TMDs and to establish suitable protocols for treating different TMDs. J Oral Facial Pain Headache 2022;36:6–20. doi: 10.11607/3023.
Keywords: botulinum toxin, bruxism, myofascial, pain, temporomandibular disorders, temporomandibular joint
Temporomandibular disorders (TMDs) are a heterogenous group of conditions causing pain and/or dysfunction of the masticatory muscles and/or temporomandibular joints (TMJ). Common signs and symptoms of TMDs include pain in and around the jaw aggravated by jaw movement and function, restricted or limited jaw movements, and TMJ noises, such as clicking or crepitus.1 TMDs are a leading cause of orofacial pain and can be associated with significant morbidity and negative impact on a patient’s quality of life.2 It is well recognized that TMDs have a multifactorial etiology arising from an interplay of predisposing, precipitating, and perpetuating factors.2 Internationally accepted dual-axis, evidence-based diagnostic criteria have been developed to enable clinicians to diagnose various TMDs with validated physical examination methods and psychosocial instruments.3 Imaging studies may be required for additional diagnostic information, such as to assess the status of the TMJ articular discs or the osseous structures of the TMJ.
The initial management of TMDs usually involves conservative and reversible measures such as patient education, reassurance, soft diet, home exercises, application of moist heat, simple analgesics/nonsteroidal anti-inflammatory medications, and occlusal splints.2 Surgical interventions are indicated in specific circumstances, such as when nonsurgical treatments have failed and severe TMJ pathology has been established as the cause of pain.4,5
Botulinum toxin (BTX) is a 150-kDa exotoxin produced by the anaerobic bacterium Clostridium botulinum. It consists of a heavy chain (100 kDa) and a light chain (50 kDa) linked by a disulfide bridge.6 Seven distinct serotypes (A through G) have been identified, with further subtypes.7 Four types of serotype A (BTX-A) are commercially available for use in humans.7 BTX acts by presynaptically blocking the release of the neurotransmitter acetylcholine into the neuromuscular junction.6–8 The heavy chain of the BTX molecule binds to the neuronal membrane presynaptically, and the light chain enters the motor neuron cell via receptor-mediated endocytosis. Once in the cell, the light chain binds to SNAP-25 (synaptosomal-associated protein of 25 kDa) docking proteins and cleaves the synaptic SNARE proteins (SNAP receptors) that form the synaptic fusion complex.6–8 This inhibits the fusion of acetylcholine–containing vesicles to the cell membrane at the synapse and thus the release of acetylcholine into the synaptic cleft at the neuromuscular junction. Reducing acetyl choline in the synapse results in reduced postsynaptic muscle contraction.8 The effects are temporary, lasting about 3 months. In addition to the well-known action on cholinergic nerve endings, such as the neuromuscular junction and salivary and sweat glands, BTX-A has also been found to act on other nerve endings and to reduce pain by both peripheral and central actions.9,10 In rat models, peripheral injection of BTX has been shown to inhibit the secretion of substance P, glutamate, and calcitonin gene-related peptide (CGRP) from sensory neurons.10–12 It also reduces local inflammation11 around nerve endings, inhibits sodium channels that are essential for pain transmission,13 is retrogradely transported to the spinal cord, and inhibits release of substance P and c-Fos expression.14 In humans, there is evidence that BTX therapy is effective for a number of neuropathic pain conditions, including postherpetic neuralgia, trigeminal neuralgia, and posttraumatic neuralgia.15
BTX is currently used to treat a number of head and neck pain and dysfunction disorders, especially when the problem is assumed to be primarily of muscular origin.16–18 It is the approved treatment of choice from the Food and Drug Administration for certain movement disorders, such as blepharospasm, strabismus, and torticollis.17 Despite a paucity of studies supporting high-grade evidence of its effectiveness, BTX has been increasingly used to treat muscle spasms and myofascial pain in patients with TMDs.16 This is based on the assumption that most TMDs are myogenous and associated with increased masticatory muscle tension and myofascial pain secondary to excessive masticatory muscle activity.16 Thus, a reduction of TMD pain and associated symptoms might be expected after reducing muscle hyperactivity.19
To date, the use of BTX to treat various TMDs remains controversial. Previous systematic reviews of studies on the use of BTX in TMDs have discussed outcome measures in terms of pain intensity and range of motion for myofascial pain patients, but generally have not discriminated, nor separately assessed, the outcomes for other types of TMDs.16–19 Based on this premise, the aim of the present review was to review the current scientific evidence concerning the effectiveness of BTX injections for treating various TMDs, with suggestions for future clinical research.
Methods
Search Strategy and Criteria for Selecting Articles
A thorough review of the relevant literature addressing the use of BTX to treat different TMD diagnostic categories was performed. The literature search was carried out using the Medline, Web of Science, and Cochrane Library databases. Additional studies identified within the references of the reviewed articles were also included if they met the inclusion criteria.
The search queries used for PubMed were a combination of the keywords “botulinum toxin,” “toxin,” and “Botox,” with relevance to the following terms: temporomandibular disorder, temporomandibular joint disorders, TMJ clicking, bruxism, masseter hypertrophy, TMJ degenerative joint disease, TMJ osteoarthritis, TMJ arthrosis/arthritis, myalgia, myofascial pain, myospasm, TMJ arthrocentesis, TMJ disc displacement, TMJ ankylosis, and TMJ pain. The full list of keyword combinations used can be seen in Appendix 1.
Inclusion Criteria
Randomized controlled clinical trials (RCTs) that evaluated the use of BTX to treat TMDs and other musculoskeletal pain in the head and neck were included. Studies had to be written in English.
Exclusion Criteria
The following publication types were excluded: nonrandomized controlled trials, systematic reviews, meta-analyses, nonsystematic reviews, studies not reporting data on the use of BTX in TMDs, studies reporting redundant data from previous publications, opinion papers, letters to the editor, and articles published before 2000.
Selection of Participants
Adults of either gender with a diagnosis of TMD, bruxism, myofascial pain, TMJ articular disc displacements, and/or any painful disorders involving the head and neck who underwent any treatment with BTX (of any type) were included.
In relation to the effects of BTX in patients diagnosed with bruxism, this work aims to evaluate the efficacy of BTX in relation to symptoms connected to bruxism, not to the reduction in electromyography (EMG) values or the EMG-documented frequency of bruxist events.
Methods
The review process first involved screening of titles and abstracts and then considered the full texts of relevant papers for inclusion. Three different reviewers (M.V., R.D., D.M.) independently performed the screening process, and discordant evaluations were discussed. The selected articles were obtained in full text. In each study, the data extracted included the following items: author(s), year of publication, study design, sample size, gender and age of participants, follow-up period, TMD diagnosis, outcome variables, and results. A PICO-like20 structured reading (ie, P = patients/problem/population, I = intervention, C = comparison, and O = outcome) was adopted based on the following question: In patients with various TMDs, including those with bruxism and masseter hypertrophy (P), do BTX injections (I) as compared to other treatments (C) lead to reduced jaw pain levels, jaw stiffness, and muscle tenderness, and improve jaw range of motion (O)? Afterwards, a descriptive analysis was conducted to evaluate the selected studies. It was not possible to structure the review strictly according to PICOT (where T = time) due to the extremely wide variation of the timelines used to assess the outcomes of the selected studies.
Statistical Analysis
Although a meta-analysis was intended to be performed for this systematic review, this proved impossible due to the marked heterogeneity of the studies.
Quality of Studies
The quality of the selected studies was assessed by grading the level of evidence according to Sackett et al21 and is summarized in Table 1. The Jadad score22 (0–5) was used to assess the quality of the randomization, double-blinding, and flow of subjects for the selected studies (Table 2).
Table 1.
Levels of Evidence
| Level | Type of study |
|---|---|
| I |
|
| II |
|
| III |
|
| IV |
|
| V |
|
Table 2.
Jadad Scores
| Item | Maximum point |
Description |
|---|---|---|
| Randomization | 2 |
|
| Blinding | 2 |
|
| An account of all patients | 1 |
|
Outcome
The primary outcome measures were as follows:
Pain levels assessed by any validated scale, such as a visual analog scale (VAS)
Maximum mouth opening in millimeters
Health-related quality of life assessed by any validated tool (if available)
Adverse events; eg, serious disability and/or incapacity, hospitalization, infection, dysphagia, anaphylaxis, allergic reactions, urticaria, soft tissue edema, etc
The secondary outcome measures were as follows:
Jaw function assessed by a validated questionnaire
Use of pain medication and dosage used per day
Results
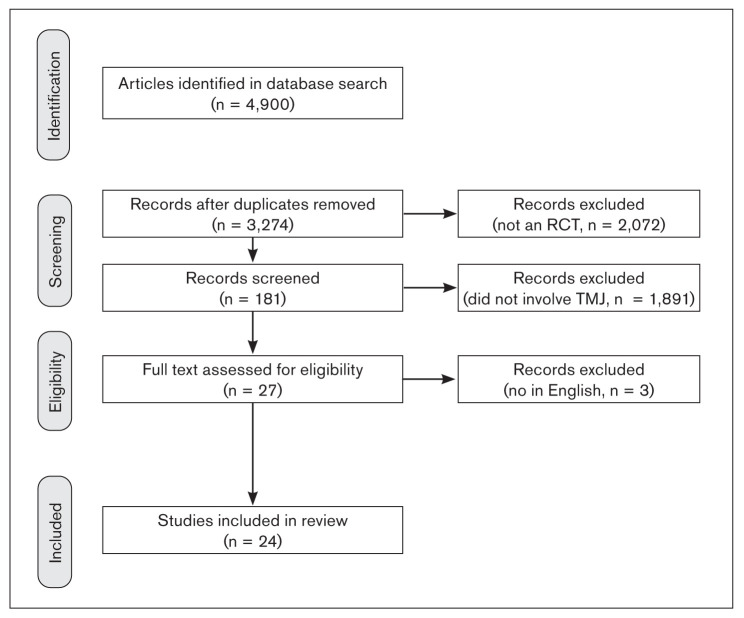
A total of 4,900 papers were found. From the analysis of the literature, 24 randomized clinical trials were selected on the basis of the keywords searched. A flowchart illustrating article selection is shown in Fig 1. Due to the considerable variations in study methods and evaluation of results and the very low number of papers, particularly in relation to TMJ articular disorders, a meta-analysis could not be performed.
Fig 1.
Flowchart of article selection.
Study Characteristics
Table 3 shows the relevant characteristics of the included studies (published between 2000 and 2021).23–46 The studies included a total of 698 patients, 411 of whom were treated by injection of BTX. Women markedly outnumbered men, reflecting the epidemiology of the condition(s).
Table 3.
Characteristics of the Included Studies
| Study, y | Diagnosis | Sample size (test/control), n | Test (T)/control (C) interventions | Primary outcome(s) | Primary outcome results | Secondary outcome(s) | Secondary outcome results |
|---|---|---|---|---|---|---|---|
| Lee et al,23 2013 | Masseter hypertrophy | 15 (1 dropout) | Single injections of BTX-A or BTX-B at a dose ratio of 1:50 or 1:70 in a split-face study | Masseter volume reduction | Both BTX-A and BTX-B produced significant improvements in masseter hypertrophy. The maximum volume reduction, as determined by CT scanning, at 12 wk was comparable between BTX-A and BTX-B at a dose ratio of 1:70 (15.6% and 14.2%, respectively). At 24 wk, only masseters treated with BTX-A maintained a significant volume reduction. | N/A | N/A |
| Nixdorf et al,24 2002 | TMD (RDC/TMD groups Ia and IIb and myofascial pain without and with limited mouth opening) | 15/15 | T = BTX C = saline |
VAS pain (0–100 mm), no raw data present | Baseline to 8 wk: mean difference in VAS Pain intensity: C = 1-mm reduction, T = 19-mm reduction Pain unpleasantness: C = 5-mm reduction, T = 13-mm reduction |
MMO (with and without pain) | MMO with/without pain: increase from baseline to 8 wk (mm): C = 5/10, T = 3/0 |
| Ernberg et al,25 2011 | TMD (myofascial pain) | 21/21 | T = BTX C = isotonic saline |
VAS pain (0–100 mm) | Mean results, baseline: C = 54, T = 58 1 mo: C = 11%, T = 30% (to baseline) 3 mo: C = 4%, T = 23% (to baseline) |
MMO | Increase from baseline to 1/3 mo: C = 0.9 mm/0.1 mm, T = 1.6 mm/1.6 mm |
| Guarda-Nardini et al,26 2008 | TMD (RDC/TMD groups Ia and IIb) and bruxism | 10/10 | T = BTX C = saline |
VAS pain (0–10) | Baseline: C = 4.1, T = 6.2 1 wk: C = 3.8, T = 5.2 1 mo: C = 3.7, T = 3.6 6 mo: C = 4.7, T = 3.6 |
MMO (nonassisted and assisted) | Increase from baseline to 6 mo (mm): C = 2.1 and 1.8, T = 0.3 and 1.0 |
| Guarda-Nardini et al,27 2012 | TMD (myofascial pain) | 15/15 | T = BTX C = fascial manipulation technique |
VAS pain (0–10) | Baseline: C = 6.0, T = 7.3 Immediately postoperative: C = 2.1, T = 5.2 3 mo: C = 2.5, T = 4.8 |
MMO | Increase from baseline to 3 mo: C = 0.44 mm, T = 2.7 mm |
| von Lindern et al,28 2003 | TMD (myofascial pain) and bruxism | 60/30 | T = BTX C = saline |
VAS pain (0–10), no raw data present | Baseline to 4 wk: C = 0.4 improvement, T = 3.2 improvement |
N/A | |
| Kurtoglu et al,29 2008 | TMD (myofascial pain) | 12/12 | T = BTX C = saline |
RDC/TMD Axis II biobehavioral questionnaire, questions 7–9 (related to pain) | Baseline: C = 58.9, T = 56.1 14 d: C = 51.1, T = 45.8 28 d: C = 51.4, T = 43.9 |
EMG readings at rest and maximal clenching of the anterior temporalis and masseter muscles bilaterally |
Calculated mean EMG (for temporalis and masseter muscles bilaterally) when rest/clenching (mV): Baseline: C = 200.0/529.3, T = 206.3/296.0 14 d: C = 252.3/498.8, T = 165.0/199.0 28 d: C = 212.8/540.8, T = 221.0/256.0 |
| Patel et al,30 2017 | TMD | 10/10 (1 dropout) | T = BTX C = saline |
Pain scale (1–10) | Baseline: C = 5.43, T = 5.4 1 mo: C = 4.5 reduction, T = 1.7 reduction |
N/A | N/A |
| Altaweel et al,31 2019 | TMD | 7/7 | T = BTX, extraoral injection C = BTX, intraoral injection |
Mouth-opening assessment, lateral pterygoid muscle tenderness, TMJ clicking, and tenderness | Significant improvement in mouth opening from 8 wk postinjection was reported in both approaches. There was a significant improvement in TMJ clicking from 1 wk postinjection, with no group difference. The EMG assessment documented LPM hyperactivity preinjection followed by significantly decreased muscle activity at 8 and 16 wk postinjection, without a statistical difference. | Disc position | MRI showed no change in disc position after injection. |
| Kütük et al,32 2019 | TMD (myofascial pain) | 20/20 | T = BTX C = dry needling |
VAS pain, crepitation, functional limitation | Pain at rest was relieved more effectively in Group C at the end of 6 wk. Improvement in jaw protrusion angles on the right (P = .009) and left (P = .002) sides was more evident in Group C after 6 wk. |
MMO, jaw strength | In Group C, recovery of TMJ function was more obvious at 6 wk following dry needling (P = .002). |
| Cahlin et al,33 2019 | Bruxism | 6/6 | T = BTX C = saline |
Bite force, chewing efficiency, VAS pain (0–100) | No significant changes between the two groups | N/A | N/A |
| Jadhao et al,34 2017 | Myofascial pain and bruxism | 8/8/8 | T = BTX C1 = saline C2 = no treatment |
VAS pain (0–5) | Baseline: T = 3.8, C1 = 4, C2 = 4 7 d: T = 3.55, C1 = 3.85, C2 = 3.80 3 mo: T = 3.2, C1 = 4.1, C2 = 4 6 mo: T = 3, C1 = 3.8, C2 = 3.9 |
Maximum occlusal force | Baseline: 7 d: T = –32.43, C1 = –5.81, C2 = 0.63 3 mo: T = –37.64, C1 = –11.32, C2 = –5.77 6 mo: T = –30.12, C1 = –24.34, C2 = –3.56 |
| De Carli et al,35 2016 | Myofascial pain | 7/8 (3 drop outs) | T = BTX C = low-level laser therapy |
VAS pain (0–10) | Baseline: C = 7 (approx), T = 7 (approx) 30 d: C = 3.5 (approx), T = just under 3.5 (approx) |
MMO | Baseline: C = 42, T = 38 30 d: C = 42, T = 36 |
| De la Torre Canales et al,36 2020 | TMD (myofascial pain) | 4/4/4/4/4 | T = oral appliance C1 = saline solution C2, C3, C4 = three BTX groups with different doses |
VAS pain | BTX reduced pain intensity (P < .0001) and increased PPT (P < .0001) for up to 24 wk compared to placebo. | Muscle contraction, masticatory performance, muscle thickness, and mandibular bone | A transient decline in masticatory performance (P < .05) and muscle contraction (P < .0001). A decrease in muscle thickness (P < .05) and coronoid and condylar process bone volume (P < .05) were found as dose-related adverse effects of BTX. |
| Pihut et al, 37 2017 | TMD | 5/5 | T = BTX C = occlusal splint |
Assessment of the loads of four disc zones of the TMJs based on the results of clinical studies | Average load values for all evaluated zones of the right and left articular discs differed in a statistically significant way in favor of the BTX group, with the exception of the external mid part of the discs. | Numeric model tests | In the anterior right disc, the load was lower in patients belonging to group I than in those in the BTX group. |
| Shandilya et al,38 2020 | TMD (after surgical approach to TMJ) | 10/10 | T = BTX C = saline |
Pain and ease of active physiotherapy at the 1-wk and 1-, 3-, and 6-mo follow-up visits using a questionnaire | Mouth opening achieved with BTX was significantly greater than that achieved for the C group. None of the patients in the T group reported pain during the jaw movements over follow-up. | EMG recordings were performed of the individual masticatory muscles in each patient before injection and at 1 and 3 mo after injection. | Transient decrease in the microvolt value in EMG analysis of the masticatory muscles on injection of BTX. |
| Ondo et al,39 2018 | Bruxism | 13/9 (1 dropout) | T = BTX C = saline |
CGI | CGI improvement (P < .05) favored the BTX group. | VAS of change in bruxism and pain at 4 to 8 wk after injection | VAS of change (P < .05) favored the BTX group. |
| Lee et al,40 2010 | Bruxism | 6/6 | T = BTX (Dysport) C = saline |
No. of bruxism events during sleep | No. of EMG bruxism events/h during sleep (using the 20% MVC criterion) at baseline/4/8/12 wk: C = (2.48 ± 1.26)/(2.24 ± 1.06)/(2.50 ± 1.37)/(2.66 ± 1.44) T = (2.77 ± 1.86)/(0.15 ± 0.29)/(0.26 ± 0.35)/(0.26 ± 0.24) |
N/A | N/A |
| Shim et al,41 2014 | Bruxism | 11/11 (2 drop outs) | T = BTX injected bilaterally into the masseter muscle C = BTX injected bilaterally into both the masseter and temporalis muscles |
No. of bruxism events during sleep | 9 (4 from T and 5 from C) subjects with a self-reported decrease of tooth grinding had a significantly higher RMMA index (3.83 ± 1.50/h) compared to the other 11 subjects, who self-reported no change (2.38 ± 0.87/h; P = .015). The reduction in the sensation of morning jaw stiffness after BTX injection was 47.50% ± 15.86% in group T and 57.50% ± 30.30% in group C | N/A | N/A |
| Shim et al,42 2020 | Bruxism | 13/10 (8 dropouts) | T = BTX C = saline |
No. of bruxism events during sleep | RMMA episode variables did not change significantly during the 12 wk in either group except for the number of RMMA episodes per hour of sleep (RMMA episodes/h) in the placebo group (P = .036). For RMMA episodes/h, significant differences were found between baseline and 4 wk (P = .011) and between 4 wk and 12 wk (P = .020) in the placebo group. MVC MA and RMMA MA significantly decreased only in the T group for 12 wk. For these variables, a significant difference was found only between baseline and 4 wk (paired t test: P = .001 for MVC MA, P < .0001 for RMMA MA) |
N/A | N/A |
| Zhang et al,43 2016 | TMD and bruxism or daytime clenching for > 2/12 h | 10/10/10 (two control groups) | T = BTX C1 = saline C2 = no treatment |
Occlusal force | Changes in mean (SEM) maximum bite force (kg) from baseline to 1/3/6 mo: C1: 7.97/13.33/22.52 C2: 0.94/8.63/3.77 T: 41.97/48.17/39.79 |
N/A | N/A |
| Kaya and Ataoglu,44 2021 | TMD and bruxism | 20/20 | T = BTX C = occlusal splint |
Maximum bite force | 0–2 wk: T = 57.7 0–6 wk: T = 66.6 0–3 mo: T = 13.7 0–6 mo: T = 40.15 |
Pain VAS (0–10) | 0–2 wk: T = 2.9, C = 2.0 0–6 wk: T = 3.3, C = 1.8 0–3 mo: T = 2.2, C = 2.4 0–6 mo: T = 2.1, C = 2.5 |
| Lee et al,45 2014 | Masseter hypertrophy | 56 | Split-face study, onabotulinum vs incobotulinum | Masseter volume reduction | The efficacy and safety of incobotulinum were not inferior to those of onabotulinum in treating periocular rhytides and masseter hypertrophy up to 16 wk after injection. There were no noteworthy differences in the onset time of effect between two botulinum toxins for periocular wrinkles and masseter hypertrophy. | N/A | N/A |
| Wei et al,46 2015 | Masseter hypertrophy | 49/49 | BTX into the masseter muscles: T = instructed to strengthen C = no instruction |
Masseter volume reduction | The duration of the masseter muscle hypertrophy was significantly prolonged in the T group (stretching) patients. The thickness and the volume of the other masticatory muscles were significantly increased in the T group, but were either slightly decreased or insignificantly different in the C group. | N/A | N/A |
CGI = Clinical Global Impression; CT = computed tomography; EMG = electromyographic; MA = muscle activity; MMO = maximum mouth opening; MRI = magnetic resonance imaging; = MVC = maximal voluntary clenching; N/A = not applicable; PPT = pressure pain threshold; RDC/TMD = Research Diagnostic Criteria for Temporomandibular Disorders; RMMA = rhythmic masticatory muscle; SEM = standard error of the mean; VAS = visual analog scale.
Quality of Selected Articles
Table 4 depicts the levels of evidence and Jadad scores of the 24 selected articles.
Table 4.
Level of Evidence and Jadad Score of Selected Studies
| Study | Level of evidence | Jadad score |
|---|---|---|
| Lee et al23 | II | 3 |
| Nixdorf et al24 | II | 4 |
| Ernberg et al25 | II | 4 |
| Guarda-Nardini et al26 | II | 2 |
| Guarda-Nardini et al27 | II | 2 |
| von Lindern et al28 | II | 2 |
| Kurtoglu et al29 | II | 4 |
| Patel et al30 | II | 4 |
| Altaweel et al31 | II | 1 |
| Kütük et al32 | II | 1 |
| Cahlin et al33 | II | 5 |
| Jadhao et al34 | II | 2 |
| De Carli et al35 | II | 2 |
| De la Torre Canales et al36 | II | 5 |
| Pihut et al37 | II | 2 |
| Shandilya et al38 | II | 1 |
| Ondo et al39 | II | 5 |
| Lee et al40 | II | 3 |
| Shim et al41 | II | 2 |
| Shim et al42 | II | 2 |
| Zhang et al43 | II | 2 |
| Kaya and Ataoglu44 | II | 1 |
| Lee et al45 | II | 3 |
| Wei et al46 | II | 1 |
Types, Number, Sites, and Doses of BTX
The majority of the selected studies evaluated the effectiveness of BTX type A. One study compared BTX type A and type B for the management of masseter hypertrophy.23 Only 2 studies24,25 utilized a crossover design. In most studies, both the masseter and temporalis muscles were injected,24,26–29 with only 3 papers injecting either the masseter or lateral pterygoid muscles alone.30–32 Most papers16,20,23,25–32,35–38 used palpation to establish injection sites. EMG confirmation of needle location within the target muscle was utilized in six studies.24,25,29–31,33 Kütük et al32 and Jadhao et al34 used ultrasound guidance to perform injections. Guarda-Nardini et al26 used ultrasound guidance in 2008, but only palpation in 2012.27 The total BTX units injected and the dose per individual muscle varied enormously, even where the same (myogenous) pathology was being treated, and therefore cannot be properly compared.
BTX in Myofascial Pain/Myospasm
From the analysis of the literature, 9 RCTs (Table 5) were selected that assessed the management of myofascial pain in the head and neck, with a total of 315 patients analyzed.24,25,27–29,32,34–36 No RCTs were found that specifically treated masticatory myospasm. The BTX-A serotype was injected in all studies in doses ranging from 25 U to 300 U. Most studies compared BTX-A injections to saline injections,24,25,28,29,34,36 while 4 studies compared BTX-A to low-level laser therapy,35 physiotherapy,27 dry needling,32 and oral appliances.36 The number, location(s), and frequency of injections differed between studies. Although individual studies used a standardized injection approach, no identifiable standardized protocol was common between studies. Patient follow-up time also varied from 28 days29 to 7 months25 across studies. Furthermore, with the exception of the VAS scale and millimeter measurement of mouth opening used as outcome variables in all papers, no other common outcome variables could be identified to evaluate the success of the treatment. De Carli et al35 utilized EMG values to evaluate the effects of BTX on occlusal forces, while De la Torres Canales et al36 used EMG, CBCT (to evaluate TMJ osseous changes), and ultrasound (to evaluate changes in size of the affected muscles). No significant adverse events were reported in any studies. Three double-blinded RCTs24–26 failed to find significant differences between patients treated with BTX and saline injections. Nixdorf et al24 failed to find statistically significant differences in regard to pain intensity (P = .10), unpleasantness (P = .40), and muscle palpation tenderness (P = .91), but found statistical significance in maximum opening without pain (P = .02) and with pain (P = .005). Interestingly, the BTX group demonstrated a relative decreased mouth opening. Ernberg et al25 found minimal pain reduction at 3 months after BTX injections compared to saline, concluding that BTX has low clinical efficacy for treating persistent myofascial TMD pain. However, a number of studies27,29,32,35,36 showed statistically significant improvements in pain levels and jaw movements in the group of patients treated with BTX. Interestingly, other studies have shown that low-level laser therapy35 and dry needling32 resulted in faster reduction of pain than BTX injections.
Table 5.
Characteristics of Studies on BTX for Treatment of Myofascial Pain/Spasm
| Study | Total dose (U) | Muscles injected | Sites of injection |
|---|---|---|---|
| Nixdorf et al24 | 150 | Both groups: masseter and temporalis | Both groups: 50-U masseter, 25-U temporalis (single session, bilaterally); 0.6 mL for each dose divided over 3 injection sites in each muscle |
| Ernberg et al25 | 50 or 100 | Both groups: masseter | Both groups: 3 standard points on each masseter, deep portion 0.1 mL (10 U) ×1, superficial portion 0.2 mL (20 U) ×2 (single session, bilaterally) |
| Guarda-Nardini et al27 | 300 | Group 1, BTX: masseter and temporalis Group 2, facial manipulation: not applicable |
Group 1: single session, dosing not specified Group 2: weekly (facial manipulation) |
| von Lindern et al28 | Average: 70 | Both groups: masseter, temporalis, or medial pterygoid, depending on areas of maximum tenderness and pain | Both groups: dependent on areas with pain (single session, bilaterally) |
| Kurtoglu et al29 | 100 | Both groups: masseter and temporalis | Both groups: three injections within the masseter (30 U) and two injections within the temporalis (20 U; single session, bilaterally) |
| Kütük et al32 | 25–150 | Group 1, BTX: masseter, temporalis, and lateral pterygoid Group 2, dry needling: masseter, temporalis, and lateral pterygoid |
Group 1 BTX: not declared Group 2 dry needling: The needle was inserted into the muscle until the trigger point in the muscle band with the tip was found. The same point was needled rapidly 8 to 10 times with the tip of the needle mounted to the empty syringe. |
| Jadhao et al34 | 100 | Group 1, BTX: masseter and temporalis Group 2, saline: masseter and temporalis Group 3: no injection |
Group 1: intramuscular injections for each side (30 U) within the masseter muscles and three injections (20 U) within the anterior temporalis muscles |
| De Carli et al35 | 500 | Group 1, BTX: masseter and temporalis Group 2, laser: masseter and temporalis |
Group 1: 30 U were applied per point in two points of the superficial bundle of the masseter muscle (in the upper portion and the lower portion) and in one point of the temporal muscle (central portion); 15 U 15 d later Group 2: GaAlAs active medium, 100 mW of power, at a continuous emission mode, wavelength of 830 nm, and dose of 80 J/cm2 per application point |
| De la Torre Canales et al36 | 40–100 | Group 1, oral appliance: N/A Group 2 saline solution: masseter, temporalis Groups 3, 4, and 5, different doses of BTX = masseter, temporalis |
Group 2, saline: Temporal 0.4 mL, masseter 1 mL Group 3, BTX: Temporal 10 U, masseter 30 U Group 4, BTX: Temporal 20 U, masseter 50 U Group 5, BTX: Temporal 25 U, masseter 75 U |
GaAlAs = gallium arsenide and aluminum.
BTX in TMJ Disorders
Only 4 articles were included for BTX in TMJ intra-articular disorders (Table 6), with a total of 64 patients.30,31,37,38 Only 3 studies specified the parameters for the diagnosis of TMD in the selected patients. BTX-A was used in all studies, but the studies could not be compared due to the diversity of TMJ diagnoses, doses of BTX used, and the sites and methods used for the injections. Patel et al30 reported a statistically significant reduction of pain levels after BTX injections when compared to saline in a crossover setting, but the diagnosis and patient selection are unclear. Although not strictly meeting the criteria, the authors noted a prospective, uncontrolled, and unblinded study31 comparing BTX injections for treatment of TMJ clicking via intra- and extraoral approaches to the lateral pterygoid muscle in patients with MRI-confirmed TMJ articular disc displacement with reduction. Both approaches led to significantly reduced TMJ clicking noise, but the MRI-determined position of the articular disc did not change. Pihut et al37 demonstrated that BTX injections into masticatory muscles significantly reduced average loading forces on the TMJ articular disc. Shandilya et al38 assessed the effectiveness of presurgical BTX injections in patients who were surgically treated for TMJ ankylosis and demonstrated a significant reduction in pain levels and improved mouth-opening range.
Table 6.
Characteristics of Studies on BTX for Treatment of TMDJ Articular Disorders
| Study | Total dose | Muscles injected | Sites of injection |
|---|---|---|---|
| Patel et al30 | 170 U | Both groups: masseter, temporalis, and external pterygoid, crossover setting after 1 mo | 50 units to each masseter, 25 units to each temporalis, 10 units to each external pterygoid |
| Altaweel et al31 | 20 U | Group 1: Injection of LPM using extraoral approach Group 2: Injection of LPM using intraoral approach |
Both groups: 20 U per side |
| Pihut et al37 | N/A | Both groups: not described | 21 U for each muscle in 3 points of 7 U each |
| Shandilya et al38 | 48 U | Both groups: masseter and temporalis | Two injections (2 x 4 U) were given 1 cm above the inferior border of mandible; two (2 x 4 U) were given 1 cm below the inferior border of the zygomatic arch; and one (1 x 4 U) was given at the center of the masseter muscle. The injections into each temporalis muscle were given at the junction of the scalp and non–hair-bearing skin in the temporal region. Three points of injections of 4 U each (3 x 4 U) were given horizontally at a distance of 1 cm below the level of the temporalis muscle origin. |
BTX in Bruxism
Eight articles reporting on the use of BTX-A for the treatment of bruxism were extrapolated26,33,39–44 (Table 7). A total 36 patients were studied. All articles used the BTX-A serotype in the management of sleep bruxism. Total doses of BTX-A ranged from 24 U44 to 100 U.40 Time of follow-up ranged from 12 weeks40–42 to 6 months.44 Subjective bruxism symptoms were significantly decreased in the BTX groups.26,39–41,43,44 Ondo et al39 compared BTX-A and saline solution injections into the masseter and temporalis muscles of bruxers. Reduced VAS pain levels, improved sleep time, and reduced number and duration of bruxist episodes were found in the BTX group. Shim et al41,42 evaluated the efficacy of different BTX injection protocols and found that BTX injections reduced the peak amplitude of EMG bursts in the masseter and temporalis, but not the number of rhythmic masticatory muscle activity (RMMA) episodes per hour during sleep. Investigating bruxism in patients with cerebral palsy, Cahlin et al33 failed to find any significant effect of BTX-A compared to placebo. No dysphagia or masticatory adverse events were reported.
Table 7.
Characteristics of Studies on BTX for Treatment of Bruxism
| Study | Total dose | Muscles injected | Sites of injection |
|---|---|---|---|
| Guarda-Nardini et al26 | 100 U | Both groups: masseter and temporalis | Both groups: four intramuscular injections within the masseter (30 U) and three intramuscular injections within the anterior temporalis (20 U; single session, bilaterally) |
| Cahlin et al33 | 100 U | Both groups: masseter and temporalis | Group 1: 30 U in masseter muscles and 20 U in the temporalis muscles on each side |
| Ondo et al39 | 200 U | Both groups: bilaterally into masseter and temporalis | Group 1: Total 60 U injected into each masseter muscle (2 sites) Group 2: Total 40 U injected into each temporalis muscle (3 sites) |
| Lee et al40 | 80 U | Both groups: masseter | Both groups: masseter (3 points) |
| Shim et al41 | 50–100 U | Group 1 = BTX bilaterally into the masseter muscle Group 2 = BTX bilaterally into both the masseter and temporalis muscles |
25 U of BTX-A was injected into each muscle. |
| Shim et al42 | 100 U | Both groups: masseter | Group 1: 25 U of BTX-A injected into each masseter muscle (2 points) |
| Zhang et al43 | 100 U | Both groups: masseter | Both groups: masseter (3 points) |
| Kaya and Ataoglu44 | 24 U | Group 1, BTX: masseter Group 2, occlusal splint: N/A |
Group 1, BTX: 24 units of BTX-A were applied on one side of the masseter muscle |
BTX in Masseter Hypertrophy
Three RCTs on BTX for masseter hypertrophy were highlighted, accounting for a total of 169 patients23,45,46 (Table 8). All three studies performed a single administration of BTX, but different serotypes were evaluated, making it difficult to compare doses. The follow-up time varied from 16 weeks45 to 12 months.46 The efficacy and safety of incobotulinum were not inferior to those of onabotulinum in treating masseter hypertrophy up to 16 weeks after injection.45 There were no noteworthy differences in the onset time of effect between two BTX serotypes for masseter hypertrophy.45 Lee et al23 highlighted that, at week 24, only the BTX-A injected group maintained a significant volume reduction; however, if both patient satisfaction scores and objective computed tomography (CT) measurements were assessed, both BTX-A and BTX-B were considered effective for the treatment of masseter hypertrophy. It appears that BTX-B at a dose ratio of 1:70 has a comparable efficacy but a shorter duration of action than BTX-A.23 No adverse side effects were reported in any of the studies.
Table 8.
Characteristics of Studies on BTX for Treatment of Masseter Hypertrophy
| Study | Total dose | Muscles injected | Sites of injection |
|---|---|---|---|
| Lee et al23 | 30 U BTX-A, 1.5 U or 2.1 BTX-B |
Both groups: masseter | Fixed dose (30 U) of BTX-A injected into the masseter muscle on one side and either 1.5 U or 2.1 U of BTX-B injected symmetrically into the muscle on the other side |
| Lee et al45 | 50 U (incobotulinum or onabotulinum) | Both groups: masseter | Both groups: injection of 25 U (incobotulinum or onabotulinum) into the lower posterior portion of the masseter muscle on each side |
| Wei et al46 | 70 U BTX-A | Group 1 = BTX bilaterally into the masseter muscle + stretching Group 2 = bilateral BTX into both the masseter muscles |
Both groups: intramasseter muscle injections of 35 U, once per side |
Discussion
The aim of this systematic review was to summarize the current scientific knowledge on the effectiveness of BTX injections for the management of various TMDs. Unlike previous reviews that limited their scope to myogenous TMDs, the purpose of the present article was to investigate the specific use of BTX injection for treating other TMD diagnoses and to suggest strategies for future research.
Four main categories of TMDs were identified: (1) myofascial pain (and/or myospasm); (2) TMJ articular disorders; (3) bruxism; and (4) masseter hypertrophy. Extensive searches using multiple search terms and words were carried out in order to include as many relevant studies as possible. All studies had small sample sizes, with only 4 of the 24 studies being conducted in more than 30 participants.28,44–46 The high cost of BTX, the short-term nature of its effects, and the absence of approved indications for BTX injections into the masticatory muscles could explain the small number of participants in these studies. Such low numbers resulted in obvious consequences regarding the external validity of the findings.
Furthermore, the absence of an established injection protocol has led to a wide range of BTX treatment methods. Most studies evaluated the results of a single BTX injection into the masseter muscle without considering other masticatory muscles injected, and there was no consistency in the number, method, or site of injections. Other masticatory muscles were rarely mentioned, and the effect of BTX injections into multiple masticatory muscles during one visit requires further study. Only one study41 compared a single BTX injection into the masseter muscles with additional injection into the temporalis muscles. Most studies used palpation to locate the sites,25,29,32,34–41,44–46 while others used ultrasound or EMG guidance.26,32,34 It is evident that more controlled trials evaluating the outcomes of different doses, numbers, and sites of injections are needed to establish the most effective and safe protocol for treating a particular TMD. Most of the studies failed to provide information on needle size, without any studies mentioning the depth of injection into the muscles. The relevance of injection depth also requires further study.
Consistent with previously published systematic reviews,16–19 most data supporting the efficacy of BTX injection for treating TMDs came from studies on myofascial pain or muscle spasms. This was a logical expectation based on the well-known primary mechanism of action of BTX. Similar difficulties were encountered when comparing studies due to the differing study designs, poorly defined TMD groups, and different outcome assessment criteria. Despite benefits being found in many studies, consensus on the utility of BTX injections to treat myofascial TMDs is still lacking.4 The reported superiority of BTX injections over placebo27,29,30,32,35,36,38,39 for reducing pain levels was not universally replicated for increasing mouth opening and mandibular range of motion values. Interestingly, there are some suggestions that other treatments (eg, low-level laser therapy35 and dry needling23) may achieve faster pain relief than BTX, possibly supporting the hypothesis that multiple visits with the caregiver may enhance the results of a therapeutic regimen. Clearly, further RCTs with larger samples of well-defined diagnostic groups according to the Diagnostic Criteria for TMD (DC/TMD) and longer follow-up periods are required.
Concerning TMJ articular disorders, only 4 studies were eligible for the review,30,31,37,38 all utilizing different methods, diagnosis, and outcome variables, making comparison of these studies and the drawing of conclusions impossible. In particular, despite a tendency to report BTX superiority over placebo, comparison of the effect of BTX on different articular TMJ disorders was impossible. No blinded RCTs were found regarding injection of BTX into the lateral pterygoid muscle to reduce TMJ clicking and improve articular disc displacement. Similarly, no high-quality evidence regarding the use of BTX injections to specifically treat TMJ arthralgia, nor other effects of directly injecting BTX into the TMJs, was found. If effectiveness is proven, such injections could be a potential alternative for treatment of TMJ osteoarthritis. Recent rheumatologic literature has demonstrated improvement in arthritis pain and joint function after BTX injections directly into a number of other joints, although the results are variable. To date, human studies have been small and with inconsistent results.47 Clinical RCTs with accurate phenotyping and defined patient criteria are required to determine the characteristics of, and selection criteria for, the OA population that would most likely benefit.48 The utility of intra-articular injections of BTX directly into the TMJ to treat TMJ articular disorders such as osteoarthritis and internal derangement—both directly via its analgesic effects and indirectly via muscle relaxation effects—needs to be investigated.
The literature on bruxism is difficult to analyze because of the difficulty discriminating between BTX effects on the reduction of bruxism (ie, a decrease in the number of sleep bruxism events) and on the control of bruxism-related symptoms (ie, a decrease in pain levels in populations of individuals diagnosed with bruxism).49–51 The available research is inconclusive and does not show enough evidence that bruxism can be treated with BTX injections. Although promising results have been shown in individual studies, further clinical RCTs are needed. Evolving works concerning bruxism definition paradigms must be taken into account for designing future trials on the topic, with a focus on the possible etiology and proper assessment of bruxism, which is much more than just sleep-time tooth grinding in relation to arousals.47 Studies also need to define the treatment indications for bruxism and use standardized protocols regarding dose, number, and location of injection sites, etc.
Regarding esthetic indications, the existing literature supports the usefulness of BTX injections for masseter hypertrophy, demonstrating superiority over placebo.23,45,46 As demonstrated elsewhere, concerns were found here regarding the quality of currently available material, given the poor description of procedures that have been used to evaluate muscle thickness and hypertrophy.
These findings confirm that a better refinement and increased homogeneity of research protocols are needed to achieve tailored indications for the use of BTX to treat different TMDs. In particular, two possible critical concerns must be considered for designing future trials. First, given further evidence of the pain modulation properties of BTX and its potential effectiveness for managing symptoms of neuropathic pain, it is fundamental that differential diagnosis using validated diagnostic criteria, such as the DC/TMD,3 is established before enrolling patients in clinical trials. This is important when considering the overlap of symptoms that is often seen in the clinical setting, which is seldom considered in the research setting. Second, there is an almost universal absence of information concerning Axis II (ie, psychosocial) findings as prognostic predictors of the effectiveness of various treatments. Again, given the well-known impact of those factors in the clinical setting, consideration must be given to the biopsychosocial model of pain when gathering evidence-based information.
Conclusions
Orofacial musculoskeletal conditions are very common disorders that are usually well managed with a number of reversible and conservative approaches. It appears that BTX is gaining popularity due to its effects in reducing muscle contractions and modulation of peripheral and central pain. However, with particular respect to both myogenous and arthrogenous TMDs, high-quality, evidence-based data utilizing the DC/TMD with consideration of Axis II factors is lacking. The present evaluation of the literature was performed to summarize the current knowledge on this topic. Only 24 RCTs were identified, all with limitations, therefore not allowing validated protocols for managing different TMD conditions to be identified. Despite the limitations, the data to date justify further research to better identify the patients most likely to benefit and to establish the best protocol (eg, site, number, and dose of injections) for treating different TMDs (eg, myofascial pain, TMJ osteoarthritis, and internal derangement). Such research is required before routine clinical use of BTX to treat TMDs can be recommended.
Key Findings
BTX treatment is gaining popularity; however, the results of studies to date are somewhat equivocal, and BTX is not necessarily superior to currently available less invasive and less expensive conservative treatments.
There is good scientific evidence that BTX treatment is effective for treating masseter hypertrophy and equivocal evidence for its use in treating masticatory myofascial pain. However, there is a paucity of evidence concerning the effectiveness of treating TMJ articular disorders.
Further controlled studies of improved methodologic design are required to identify the types of TMD patients most likely to benefit from BTX injection treatment and to establish standardized protocols.
Acknowledgments
All authors participated in the analysis of the literature and preparation of the manuscript. R.D.: conceived, wrote, and edited the manuscript; M.V.: conducted literature searches, tabulated the results, and wrote sections of the text; L.G.N.: assisted the literature search and reviewed the manuscript; D.M.: helped design, coordinate, write, and edit the manuscript.
The authors report no conflicts of interest.
Appendix 1.
Supplementary List of Search Keywords
| Generic | ((((“botulinum toxins”[MeSH Terms] OR (“botulinum”[All Fields] AND “toxins”[All Fields])) OR “botulinum toxins”[All Fields]) OR (“botulinum”[All Fields] AND “toxin”[All Fields])) OR “botulinum toxin”[All Fields]) AND ((“head neck”[Journal] OR (“head”[All Fields] AND “neck”[All Fields])) OR “head neck”[All Fields]);B |
|---|---|
| BTX: TMJ | (“botulinum toxins”[MeSH Terms] OR (“botulinum”[All Fields] AND “toxins”[All Fields]) OR “botulinum toxins”[All Fields] OR (“botulinum”[All Fields] AND “toxin”[All Fields]) OR “botulinum toxin”[All Fields]) AND (“temporomandibular joint”[MeSH Terms] OR (“temporomandibular”[All Fields] AND “joint”[All Fields]) OR “temporomandibular joint”[All Fields] OR “tmj”[All Fields]) |
| BTX: TMD | (“botulinum toxins”[MeSH Terms] OR (“botulinum”[All Fields] AND “toxins”[All Fields]) OR “botulinum toxins”[All Fields] OR (“botulinum”[All Fields] AND “toxin”[All Fields]) OR “botulinum toxin”[All Fields]) AND “TMD”[All Fields] |
| BTX: Bruxism | ((((“botulinum toxins”[MeSH Terms] OR (“botulinum”[All Fields] AND “toxins”[All Fields])) OR “botulinum toxins”[All Fields]) OR (“botulinum”[All Fields] AND “toxin”[All Fields])) OR “botulinum toxin”[All Fields]) AND (“bruxism”[MeSH Terms] OR “bruxism”[All Fields]) |
| BOTULINUM TOXIN BRUXER | (“botulinum toxins”[MeSH Terms] OR (“botulinum”[All Fields] AND “toxins”[All Fields]) OR “botulinum toxins”[All Fields] OR (“botulinum”[All Fields] AND “toxin”[All Fields]) OR “botulinum toxin”[All Fields]) AND (“bruxer”[All Fields] OR “bruxers”[All Fields]) |
| BTX: Masseter hypertrophy | (“botulinum toxins”[MeSH Terms] OR (“botulinum”[All Fields] AND “toxins”[All Fields]) OR “botulinum toxins”[All Fields] OR (“botulinum”[All Fields] AND “toxin”[All Fields]) OR “botulinum toxin”[All Fields]) AND (“masseter muscle”[MeSH Terms] OR (“masseter”[All Fields] AND “muscle”[All Fields]) OR “masseter muscle”[All Fields] OR “masseter”[All Fields] OR “masseters”[All Fields]) AND (“hypertrophy”[MeSH Terms] OR “hypertrophy”[All Fields] OR “hypertrophied”[All Fields] OR “hypertrophies”[All Fields] OR “hypertrophying”[All Fields]) |
| BTX: Ankylosis | (“botulinum toxins”[MeSH Terms] OR (“botulinum”[All Fields] AND “toxins”[All Fields]) OR “botulinum toxins”[All Fields] OR (“botulinum”[All Fields] AND “toxin”[All Fields]) OR “botulinum toxin”[All Fields]) AND (“ankylose”[All Fields] OR “ankylosed”[All Fields] OR “ankylosis”[MeSH Terms] OR “ankylosis”[All Fields] OR “ankyloses”[All Fields]) |
| BTX: Arthrosis | (“botulinum toxins”[MeSH Terms] OR (“botulinum”[All Fields] AND “toxins”[All Fields]) OR “botulinum toxins”[All Fields] OR (“botulinum”[All Fields] AND “toxin”[All Fields]) OR “botulinum toxin”[All Fields]) AND (“joint diseases”[MeSH Terms] OR (“joint”[All Fields] AND “diseases”[All Fields]) OR “joint diseases”[All Fields] OR “arthrosis”[All Fields]) |
| BTX: Arthritis | (“botulinum toxins”[MeSH Terms] OR (“botulinum”[All Fields] AND “toxins”[All Fields]) OR “botulinum toxins”[All Fields] OR (“botulinum”[All Fields] AND “toxin”[All Fields]) OR “botulinum toxin”[All Fields]) AND (“arthritis”[MeSH Terms] OR “arthritis”[All Fields] OR “arthritides”[All Fields] OR “polyarthritides”[All Fields]) |
| BTX: Osteoarthritis | (“botulinum toxins”[MeSH Terms] OR (“botulinum”[All Fields] AND “toxins”[All Fields]) OR “botulinum toxins”[All Fields] OR (“botulinum”[All Fields] AND “toxin”[All Fields]) OR “botulinum toxin”[All Fields]) AND (“osteoarthritis”[MeSH Terms] OR “osteoarthritis”[All Fields] OR “osteoarthritides”[All Fields]) |
| BTX: Myofascial pain | ((((“botulinum toxins”[MeSH Terms] OR (“botulinum”[All Fields] AND “toxins”[All Fields])) OR “botulinum toxins”[All Fields]) OR (“botulinum”[All Fields] AND “toxin”[All Fields])) OR “botulinum toxin”[All Fields]) AND “MYOFASCIAL”[All Fields] AND (“pain”[MeSH Terms] OR “pain”[All Fields]) “MYOFASCIAL”[All Fields] AND (“spasm”[MeSH Terms] OR “spasm”[All Fields] OR “spasms”[All Fields]) AND (“botulinum toxins”[MeSH Terms] OR (“botulinum”[All Fields] AND “toxins”[All Fields]) OR “botulinum toxins”[All Fields] OR (“botulinum”[All Fields] AND “toxin”[All Fields]) OR “botulinum toxin”[All Fields]) |
| BTX: Myalgia | (“botulinum toxins”[MeSH Terms] OR (“botulinum”[All Fields] AND “toxins”[All Fields]) OR “botulinum toxins”[All Fields] OR (“botulinum”[All Fields] AND “toxin”[All Fields]) OR “botulinum toxin”[All Fields]) AND (“myalgia”[MeSH Terms] OR “myalgia”[All Fields] OR “myalgias”[All Fields]) |
| BTX: TMJ | ((((“botulinum toxins”[MeSH Terms] OR (“botulinum”[All Fields] AND “toxins”[All Fields])) OR “botulinum toxins”[All Fields]) OR (“botulinum”[All Fields] AND “toxin”[All Fields])) OR “botulinum toxin”[All Fields]) AND ((“temporomandibular joint”[MeSH Terms] OR (“temporomandibular”[All Fields] AND “joint”[All Fields])) OR “temporomandibular joint”[All Fields]); “botulinum toxin and TMJ dislocation” and ““botulinum toxin and TMJ subluxation”; |
| BTX: TMDs | ((((“botulinum toxins”[MeSH Terms] OR (“botulinum”[All Fields] AND “toxins”[All Fields])) OR “botulinum toxins”[All Fields]) OR (“botulinum”[All Fields] AND “toxin”[All Fields])) OR “botulinum toxin”[All Fields]) AND ((((“temporomandibular joint disorders”[MeSH Terms] OR ((“temporomandibular”[All Fields] AND “joint”[All Fields]) AND “disorders”[All Fields])) OR “temporomandibular joint disorders”[All Fields]) OR (“temporomandibular”[All Fields] AND “disorder”[All Fields])) OR “temporomandibular disorder”[All Fields]) |
| BTX: TMJ surgery | (“botulinum toxins”[MeSH Terms] OR (“botulinum”[All Fields] AND “toxins”[All Fields]) OR “botulinum toxins”[All Fields] OR (“botulinum”[All Fields] AND “toxin”[All Fields]) OR “botulinum toxin”[All Fields]) AND (“temporomandibular joint”[MeSH Terms] OR (“temporomandibular”[All Fields] AND “joint”[All Fields]) OR “temporomandibular joint”[All Fields]) AND (“surgery”[MeSH Subheading] OR “surgery”[All Fields] OR “surgical procedures, operative”[MeSH Terms] OR (“surgical”[All Fields] AND “procedures”[All Fields] AND “operative”[All Fields]) OR “operative surgical procedures”[All Fields] OR “general surgery”[MeSH Terms] OR (“general”[All Fields] AND “surgery”[All Fields]) OR “general surgery”[All Fields] OR “surgery s”[All Fields] OR “surgerys”[All Fields] OR “surgeries”[All Fields]) |
| BTX: Meniscectomy | (“botulinum toxins”[MeSH Terms] OR (“botulinum”[All Fields] AND “toxins”[All Fields]) OR “botulinum toxins”[All Fields] OR (“botulinum”[All Fields] AND “toxin”[All Fields]) OR “botulinum toxin”[All Fields]) AND (“meniscectomy”[MeSH Terms] OR “meniscectomy”[All Fields] OR “meniscectomies”[All Fields]) |
| BTX: Arthroplasty | (“botulinum toxins”[MeSH Terms] OR (“botulinum”[All Fields] AND “toxins”[All Fields]) OR “botulinum toxins”[All Fields] OR (“botulinum”[All Fields] AND “toxin”[All Fields]) OR “botulinum toxin”[All Fields]) AND (“arthroplasty”[MeSH Terms] OR “arthroplasty”[All Fields] OR “arthroplasties”[All Fields]) |
| BTX: Pain | (“botulinum toxins”[MeSH Terms] OR (“botulinum”[All Fields] AND “toxins”[All Fields]) OR “botulinum toxins”[All Fields] OR (“botulinum”[All Fields] AND “toxin”[All Fields]) OR “botulinum toxin”[All Fields]) AND (“pain”[MeSH Terms] OR “pain”[All Fields]) |
| BTX: Disc displacement | (“botulinum toxins”[MeSH Terms] OR (“botulinum”[All Fields] AND “toxins”[All Fields]) OR “botulinum toxins”[All Fields] OR (“botulinum”[All Fields] AND “toxin”[All Fields]) OR “botulinum toxin”[All Fields]) AND “DISC”[All Fields] AND (“displace”[All Fields] OR “displaced”[All Fields] OR “displacement, psychological”[MeSH Terms] OR (“displacement”[All Fields] AND “psychological”[All Fields]) OR “psychological displacement”[All Fields] OR “displacement”[All Fields] OR “displacements”[All Fields] OR “displaces”[All Fields] OR “displacing”[All Fields]) |
| BTX: Clicking | ((((“botulinum toxins”[MeSH Terms] OR (“botulinum”[All Fields] AND “toxins”[All Fields])) OR “botulinum toxins”[All Fields]) OR (“botulinum”[All Fields] AND “toxin”[All Fields])) OR “botulinum toxin”[All Fields]) AND “TEMPOROMANDIBULAR”[All Fields] AND ((((“click”[All Fields] OR “clicked”[All Fields]) OR “clicking”[All Fields]) OR “clickings”[All Fields]) OR “clicks”[All Fields]) |
| BTX: TMJ internal derangement | (“temporomandibular joint”[MeSH Terms] OR (“temporomandibular”[All Fields] AND “joint”[All Fields]) OR “temporomandibular joint”[All Fields] OR “tmj”[All Fields]) AND (“internal”[All Fields] OR “internally”[All Fields] OR “internals”[All Fields]) AND (“derange”[All Fields] OR “deranged”[All Fields] OR “derangement”[All Fields] OR “derangements”[All Fields] OR “deranges”[All Fields] OR “deranging”[All Fields]) AND (“botulinum toxins”[MeSH Terms] OR (“botulinum”[All Fields] AND “toxins”[All Fields]) OR “botulinum toxins”[All Fields] OR (“botulinum”[All Fields] AND “toxin”[All Fields]) OR “botulinum toxin”[All Fields]) |
| BTX: TMJ arthrocentesis | (“arthrocentesis”[MeSH Terms] OR “arthrocentesis”[All Fields] OR (“tmj”[All Fields] AND “arthrocentesis”[All Fields]) OR “tmj arthrocentesis”[All Fields]) AND (“botulinum toxins”[MeSH Terms] OR (“botulinum”[All Fields] AND “toxins”[All Fields]) OR “botulinum toxins”[All Fields] OR (“botulinum”[All Fields] AND “toxin”[All Fields]) OR “botulinum toxin”[All Fields]) |
| BTX: Degenerative joint disease | (“osteoarthritis”[MeSH Terms] OR “osteoarthritis”[All Fields] OR (“degenerative”[All Fields] AND “joint”[All Fields] AND “disease”[All Fields]) OR “degenerative joint disease”[All Fields]) AND (“botulinum toxins”[MeSH Terms] OR (“botulinum”[All Fields] AND “toxins”[All Fields]) OR “botulinum toxins”[All Fields] OR (“botulinum”[All Fields] AND “toxin”[All Fields]) OR “botulinum toxin”[All Fields]) |
References
- Ohrbach R, Bair E, Fillingim RB, et al. Clinical orofacial characteristics associated with risk of first-onset TMD: The OPPERA prospective cohort study. J Pain. 2013;14(12 suppl):T33–T50. doi: 10.1016/j.jpain.2013.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The National Academies of Sciences, Engineering, and Medicine. Temporomandibular Disorders: Priorities for Research and Care. Washington, DC: The National Academies Press; 2020. [PubMed] [Google Scholar]
- Schiffman E, Ohrbach R, Truelove E, et al. Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) for clinical and research applications: Recommendations of the International RDC/TMD Consortium Network and Orofacial Pain Special Interest Group. J Oral Facial Pain. 2014;28:6–27. doi: 10.11607/jop.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouloux G, Koslin MG, Ness G, Shafer D. Temporomandibular joint surgery. J Oral Maxillofac Surg. 2017;75(8S):e195–e223. doi: 10.1016/j.joms.2017.04.027. [DOI] [PubMed] [Google Scholar]
- Guarda-Nardini L, Manfredini D, Berrone S, Frerronato G. Total temporomandibular joint prosthesis as a surgical option for severe mouth opening restriction, A case report of a bilateral intervention. Reumatismo. 2007;59:322–327. doi: 10.4081/reumatismo.2007.322. [DOI] [PubMed] [Google Scholar]
- Foster KA, Bigalke H, Aoki KR. Botulinum neurotoxin - from laboratory to bedside. Neurotox Res. 2006;9:133–140. doi: 10.1007/BF03033931. [DOI] [PubMed] [Google Scholar]
- Dressler D. Therapeutically relevant features of botulinum toxin drugs. Toxicon. 2020;175:64–68. doi: 10.1016/j.toxicon.2019.12.005. [DOI] [PubMed] [Google Scholar]
- Dressler D, Benecke R. Pharmacology of therapeutic botulinum toxin preparations. Disabil Rehabil. 2007;29:1761–1768. doi: 10.1080/09638280701568296. [DOI] [PubMed] [Google Scholar]
- Aoki KR. Review of a proposed mechanism for the antinociceptive action of botulinum toxin type A. Neurotox. 2005;26:785–793. doi: 10.1016/j.neuro.2005.01.017. [DOI] [PubMed] [Google Scholar]
- Durham PL, Cady R, Cady R. Regulation of calcitonin gene-related peptide secretion from trigeminal nerve cells by botulinum toxin type A: Implications for migraine therapy. Headache. 2004;44:35–42. doi: 10.1111/j.1526-4610.2004.04007.x. discussion 42–33. [DOI] [PubMed] [Google Scholar]
- Lucioni A, Bales GT, Lotan TL, McGehee DS, Cook SP, Rapp DE. Botulinum toxin type A inhibits sensory neuropeptide release in rat bladder models of acute injury and chronic inflammation. BJU Int. 2008;101:366–370. doi: 10.1111/j.1464-410X.2007.07312.x. [DOI] [PubMed] [Google Scholar]
- Cui M, Khanijou S, Rubino J, Aoki KR. Subcutaneous administration of botulinun toxin A reduces formain-induced pain. Pain. 2004;107:125–133. doi: 10.1016/j.pain.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Kharatmal SB, Singh JN, Sharma SS. Voltage-gated sodium channels as therapeutic targets for treatment of painful diabetic neuropathy. Min Rev Med Chem. 2015;15:1134–1147. doi: 10.2174/1389557515666150722112621. [DOI] [PubMed] [Google Scholar]
- Matak I, Riederer P, Lacković Z. Botulinum toxin’s axonal transport from periphery to the spinal cord. Neurochem Int. 2012;61:236–239. doi: 10.1016/j.neuint.2012.05.001. [DOI] [PubMed] [Google Scholar]
- Safarpour Y, Jabbari B. Botulinum toxin treatment of pain syndromes -an evidence based review. Toxicon. 2018;147:120–128. doi: 10.1016/j.toxicon.2018.01.017. [DOI] [PubMed] [Google Scholar]
- Thambar S, Kulkarni S, Armstrong S, Nikolorakos D. Botulinum toxin in the management of temporomandibular disorders: A systematic review. Br J Oral Maxillofac Surg. 2020;58:508–519. doi: 10.1016/j.bjoms.2020.02.007. [DOI] [PubMed] [Google Scholar]
- Chen YW, Chiu YW, Chen CY, Chuang SK. Botulinum toxin therapy for temporomandibular joint disorders: A systematic review of randomized controlled trials. Int J Oral Maxillofac Surg. 2015;44:1018–1026. doi: 10.1016/j.ijom.2015.04.003. [DOI] [PubMed] [Google Scholar]
- Patel J, Cardoso JA, Mehta S. A systematic review of botulinum toxin in the management of patients with temporomandibular disorders and bruxism. Br Dent J. 2019;226:667–672. doi: 10.1038/s41415-019-0257-z. [DOI] [PubMed] [Google Scholar]
- Machado D, Martimbianco ALC, Bussadori SK, Pacheco RL, Riera R, Santos EM. Botulinum toxin type A for painful temporomandibular disorders: Systematic review and meta-analysis. J Pain. 2020;21:281–293. doi: 10.1016/j.jpain.2019.08.011. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. J Clin Epidemiol. 2009;62:1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Sackett DL, Strauss SE, Richardson WS, Rosenberg W, Haynes RB. Evidence-Based Medicine: How to Practice and Teach EBM. ed 2. Edinburgh: Churchill Livingstone; 2000. [Google Scholar]
- Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- Lee DH, Jin SP, Cho S, et al. RimabotulinumtoxinB versus OnabotulinumtoxinA in the treatment of masseter hypertrophy: A 24-week double-blind randomized split-face study. Dermatology. 2013;226:227–232. doi: 10.1159/000349984. [DOI] [PubMed] [Google Scholar]
- Nixdorf DR, Heo G, Major PW. Randomized controlled trial of botulinum toxin A for chronic myogenous orofacial pain. Pain. 2002;99:465–473. doi: 10.1016/S0304-3959(02)00240-3. [DOI] [PubMed] [Google Scholar]
- Ernberg M, Hedenberg-Magnusson B, List T, Svensson P. Efficacy of botulinum toxin type A for treatment of persistent myofascial TMD pain: A randomized, controlled, double-blind multicenter study. Pain. 2011;152:1988–1996. doi: 10.1016/j.pain.2011.03.036. [DOI] [PubMed] [Google Scholar]
- Guarda-Nardini L, Manfredini D, Salamone M, Salmaso L, Tonello S, Ferronato G. Efficacy of botulinum toxin in treating myofascial pain in bruxers: A controlled placebo pilot study. Cranio. 2008;26:126–135. doi: 10.1179/crn.2008.017. [DOI] [PubMed] [Google Scholar]
- Guarda-Nardini L, Stecco A, Stecco C, Masiero S, Manfredini D. Myofascial pain of the jaw muscles: Comparison of short-term effectiveness of botulinum toxin injections and fascial manipulation technique. Cranio. 2012;30:95–102. doi: 10.1179/crn.2012.014. [DOI] [PubMed] [Google Scholar]
- von Lindern JJ, Niederhagen B, Bergé S, Appel T. Type A botulinum toxin in the treatment of chronic facial pain associated with masticatory hyperactivity. J Oral Maxillofac Surg. 2003;61:774–778. doi: 10.1016/s0278-2391(03)00153-8. [DOI] [PubMed] [Google Scholar]
- Kurtoglu C, Gur OH, Kurkcu M, Sertdemir Y, Guler-Uysal F, Uysal H. Effect of botulinum toxin-A in myofascial pain patients with or without functional disc displacement. J Oral Maxillofac Surg. 2008;66:1644–1651. doi: 10.1016/j.joms.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Patel AA, Lerner MZ, Blitzer A. IncobotulinumtoxinA injection for temporomandibular joint disorder. Ann Otol Rhinol Laryngol. 2017;126:328–333. doi: 10.1177/0003489417693013. [DOI] [PubMed] [Google Scholar]
- Altaweel AA, Elsayed SA, Baiomy AABA, Abdelsadek SE, Hyder AA. Extraoral versus intraoral botulinum toxin type A injection for management of temporomandibular joint disc displacement with reduction. J Craniofac Surg. 2019;30:2149–2153. doi: 10.1097/SCS.0000000000005658. [DOI] [PubMed] [Google Scholar]
- Kütük SG, Özkan Y, Kütük M, Özdaş T. Comparison of the efficacies of dry needling and Botox methods in the treatment of myofascial pain syndrome affecting the temporomandibular joint. J Craniofac Surg. 2019;30:1556–1559. doi: 10.1097/SCS.0000000000005473. [DOI] [PubMed] [Google Scholar]
- Cahlin BJ, Lindberg C, Dahlström L. Cerebral palsy and bruxism: Effects of botulinum toxin injections-A randomized controlled trial. Clin Exp Dent Res. 2019;5:460–468. doi: 10.1002/cre2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadhao VA, Lokhande N, Habbu SG, Sewane S, Dongare S, Goyal N. Efficacy of botulinum toxin in treating myofascial pain and occlusal force characteristics of masticatory muscles in bruxism. Indian J Dent Res. 2017;28:493–497. doi: 10.4103/ijdr.IJDR_125_17. [DOI] [PubMed] [Google Scholar]
- De Carli BM, Magro AK, Souza-Silva BN, et al. The effect of laser and botulinum toxin in the treatment of myofascial pain and mouth opening: A randomized clinical trial. J Photochem Photobiol. 2016;159:120–123. doi: 10.1016/j.jphotobiol.2016.03.038. [DOI] [PubMed] [Google Scholar]
- De la Torre Canales G, Alvarez-Pinzon N, Muñoz-Lora VRM, et al. Efficacy and safety of botulinum toxin type A on persistent myofascial pain: A randomized clinical trial. Toxins (Basel) 2020;12:395. doi: 10.3390/toxins12060395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pihut ME, Margielewicz J, Kijak E, Wiśniewska G. Evaluation of articular disc loading in the temporomandibular joints after prosthetic and pharmacological treatment in model studies. Adv Clin Exp Med. 2017;26:455–460. doi: 10.17219/acem/62216. [DOI] [PubMed] [Google Scholar]
- Shandilya S, Mohanty S, Sharma P, Chaudhary Z, Kohli S, Kumar RD. Effect of preoperative intramuscular injection of botulinum toxin A on pain and mouth opening after surgical intervention in temporomandibular joint ankylosis cases: A controlled clinical trial. J Oral Maxillofac Surg. 2020;78:916–926. doi: 10.1016/j.joms.2020.02.011. [DOI] [PubMed] [Google Scholar]
- Ondo WG, Simmons JH, Shahid MH, Hashem V, Hunter C, Jankovic J. Onabotulinum toxin-A injections for sleep bruxism: A double-blind, placebo-controlled study. Neurology. 2018;90:e559–e564. doi: 10.1212/WNL.0000000000004951. [DOI] [PubMed] [Google Scholar]
- Lee SJ, McCall WD, Kim YK, Chung SC, Chung JW. Effect of botulinum toxin injection on nocturnal bruxism: A randomized controlled trial. Am J Phys Med Rehabil. 2010;89:16–23. doi: 10.1097/PHM.0b013e3181bc0c78. [DOI] [PubMed] [Google Scholar]
- Shim YJ, Lee MK, Kato T, Park HU, Heo K, Kim ST. Effects of botulinum toxin on jaw motor events during sleep in sleep bruxism patients: A polysomnographic evaluation. J Clin Sleep Med. 2014;10:291–298. doi: 10.5664/jcsm.3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim YJ, Lee HJ, Park KJ, Kim HT, Hong IH, Kim ST. Botulinum toxin therapy for managing sleep bruxism: A randomized and placebo- controlled trial. Toxins. 2020;12:168. doi: 10.3390/toxins12030168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang LD, Liu Q, Zou DR, Yu LF. Occlusal force characteristics of masseteric muscles after intramuscular injection of botulinum toxin A(BTX - A)for treatment of temporomandibular disorder. Br J Oral Maxillofac Surg. 2016;54:736–740. doi: 10.1016/j.bjoms.2016.04.008. [DOI] [PubMed] [Google Scholar]
- Kaya DI, Ataoglu H. Botulinum toxin treatment of temporomandibular joint pain in patients with bruxism: A prospective and randomized clinical study. Niger J Clin Pract. 2021;24:412–417. doi: 10.4103/njcp.njcp_251_20. [DOI] [PubMed] [Google Scholar]
- Lee JH, Park JH, Lee SK, et al. Efficacy and safety of incobotulinum toxin A in periocular rhytides and masseter hypertrophy: Side-by-side comparison with onabotulinum toxin A. J Dermatol Treat. 2014;25:326–330. doi: 10.3109/09546634.2013.769041. [DOI] [PubMed] [Google Scholar]
- Wei J, Xu H, Dong J, Li Q, Dai C. Prolonging the duration of masseter muscle reduction by adjusting the masticatory movements after the treatment of masseter muscle hypertrophy with botulinum toxin type A injection. Dermatol Surg. 2015;41(suppl 1):s101–s109. doi: 10.1097/DSS.0000000000000162. [DOI] [PubMed] [Google Scholar]
- Khenioui H, Houvenagel E, Catanzariti JF, Guyot MA, Agnani O, Donze C. Usefulness of intraarticular botulinum injections: A systematic review. Joint Bone Spine. 2016;83:149–154. doi: 10.1016/j.jbspin.2015.10.001. [DOI] [PubMed] [Google Scholar]
- Arendt-Nielsen L, Jiang GL, DeGryse R, Turkel CC. Intra-articular onabotulinumtoxinA [sic] in osteoarthritis knee pain: Effect on human mechanistic pain biomarkers and clinical pain. Scand J Rheumatol. 2017;46:303–316. doi: 10.1080/03009742.2016.1203988. [DOI] [PubMed] [Google Scholar]
- Lobbezoo F, Ahlberg J, Raphael KG, et al. International consensus on the assessment of bruxism: Report of a work in progress. J Oral Rehabil. 2018;45:837–844. doi: 10.1111/joor.12663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manfredini D, Winocur E, Guarda-Nardini L, Paesani D, Lobbezoo F. Epidemiology of bruxism in adults: A systematic review of the literature. J Orofac Pain. 2013;27:99–110. doi: 10.11607/jop.921. [DOI] [PubMed] [Google Scholar]
- Manfredini D, Serra-Negra J, Carboncini F, Lobbezoo F. Current concepts of bruxism. Int J Prosthodont. 2017;30:437–438. doi: 10.11607/ijp.5210. [DOI] [PubMed] [Google Scholar]