Abstract
Aims:
To evaluate the efficacy and safety of melatonin for migraine prophylaxis in adults.
Methods:
After a comprehensive literature search in the MEDLINE, Cochrane Database, and International Clinical Trial Registry Platform databases, reviewers extracted data from three relevant articles. PRISMA guidelines were followed in the selection, analysis, and reporting of the findings. Quality assessment was performed using the Cochrane risk of bias assessment tool. A random-effects model was used to estimate the effect size, and meta-regression was performed for variables with a likely influence on effect size. Subgroup analysis was performed based on the comparison used in the included studies.
Results:
Melatonin therapy in migraine was associated with a significantly higher responder rate when compared to both placebo and standard therapy (OR = 1.84; 95% CI: 1.08 to 3.14; P = .03). The results of the meta-analyses indicated that melatonin can achieve a significant reduction in frequency of migraine attacks (MD = 1.00; 95% CI: 0.02 to 1.98; P = .04), migraine attack duration (MD = 5.02; 95% CI: 0. 91 to 9.13; P = .02), use of analgesics (MD = 1.43; 95% CI: 0.38 to 2.48; P = .008), and migraine severity (MD = 1.93; 95% CI: 1.23 to 2.63; P < .0001) over placebo, but had no significant effects in comparison to amitriptyline or valproate. There was no significant difference in the occurrence of common adverse drug reactions, such as drowsiness and fatigue, between the melatonin group and the comparison groups.
Conclusions:
Melatonin showed a beneficial prophylactic role in migraine, with a better responder rate in comparison to placebo in reducing migraine severity, mean attack duration, mean attack frequency, and analgesic use, but did not show significant effects in comparison to amitriptyline or valproate. J Oral Facial Pain Headache 2022;36:207–219. doi: 10.11607/ofph.3211
Keywords: frequency of migraine attacks, melatonin, meta-analysis, migraine, migraine attack duration, migraine severity, prophylaxis, responder rate
Migraine is a chronic multifactorial brain disorder with an estimated global prevalence of 12% to 20%.1–3 It is one of the most disabling neurologic illnesses, with the resultant low quality of life leading to a considerable social and economic burden.2,4 Epidemiologic studies have shown that prophylactic therapy is used by 13% of patients out of the 38% who need it.5 Preventive therapy helps reduce the severity, frequency, and duration of migraine attacks, thereby leading to increased responsiveness to acute treatment and incidentally improving the patient’s quality of life.4 Although there are various migraine prophylaxis guidelines in place, only a very small proportion of patients receive adequate prophylactic medications.6 A real-world study by Piccinni et al concluded that the current use of prophylactic therapies is inadequate, with negligible benefits, and that there is a lack of effective preventive strategies.7
The present prophylactic therapy for migraine includes anti-epileptics, beta blockers, antidepressants, calcium channel blockers, and triptans, with adequate supporting evidence.5,8 Recently, researchers have shown an increased interest in the various neurotransmitters involved in migraine pathophysiology.1 One among the many neurotransmitters being studied is melatonin. Many published scholarly articles have elucidated the antimigraine effects of melatonin, which incidentally prevents toxic molecular damage to the brain.9–15 Its inherent analgesic and antioxidant properties, along with its potential to normalize circadian rhythm, empower melatonin as a prophylactic agent for reducing migraine attacks.10,16 The antinociceptive activity of melatonin is made up of a milieu of mechanisms, such as potentiation of gamma-aminobutyric acid (GABA) and opioid activity, promotion of neurovascular regulation due to calcium entry, inhibition of nitric oxide synthase activity and dopamine release, modulation of 5-hydroxytryptamine (5-HT2) receptors on cerebral vasculature, and a structure similar to indomethacin.13,17–19
Clinical trials conducted over the last decade have generated mixed evidence on the effect of melatonin as prophylactic therapy for migraine. Among the various clinical trials that compared melatonin to standard prophylactic medications, Gonçalves et al2 and Ebrahimi-Monfared et al20 found beneficial effects of melatonin for migraine. However, Alstadhaug et al did not find any significant effect of melatonin over placebo for migraine prophylaxis.21 Long et al performed a systematic review on the therapeutic role of melatonin in migraine prophylaxis and cited heterogeneity as their reason for not performing further meta-analysis; yet, they combined pediatric and adult age groups in their forest plots.22 The conflicting results in these clinical trials and the dubious statements in the systematic review by Long et al signal an immediate need to generate conclusive evidence regarding the efficacy of melatonin in migraine prophylaxis. Hence, this meta-analysis was carried out to evaluate the efficacy and safety of melatonin for prophylaxis of migraine in adults when compared to placebo and standard therapy.
Materials and Methods
Development and Registration of Protocol
A standard meta-analysis protocol was developed following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis Protocol (PRISMA-P) 2015 guidelines.23 The protocol was registered in the International Prospective Register of Ongoing Systematic Reviews (PROSPERO registration number: CRD42020183241). This meta-analysis was conducted and reported in accordance with the PRISMA statement.24 The Cochrane Handbook for Systematic Reviews of Interventions was used as a methodologic reference.25
Literature Search
A systematic literature search was performed using the MEDLINE, Cochrane Database, and World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) databases for prospective clinical trials on the efficacy of melatonin for migraine prophylaxis in adults up to January 2022. Embase and the Allied and Complementary Medicine Database (AMED) could not be used due to financial constraints. The search strategy was not restricted by date of publication, ethnicity of population, or clinical variants of migraine. Search terms were constructed following the PICO (population, intervention, comparison, outcome) method using MeSH terms. The key elements used in the search were: P: (migraine/migraine disorders/hemicrania/headache/status migrainosus); I: (melatonin/melatonin extended-release); C: (placebo/amitriptyline/valproic acid/propranolol/flunarizine/any other drug; O: (efficacy/responder rate/intensity/attack duration/rescue medications/safety/adverse drug reactions).
Study Selection Criteria
Types of studies.
Randomized controlled clinical trials evaluating the efficacy of melatonin based on the response rate and the frequency, severity, and/or duration of migraine headache were included in this meta-analysis. Observational studies, preclinical/in vitro studies, review articles, letters to the editor, comments, case series, case reports, and studies where it was impossible to retrieve or calculate data of interest were excluded.
Types of participants.
Adult male or female patients ≥ 18 years of age with a history of disabling migraine with or without aura for ≥ 1 year and fulfilling the International Headache Society (IHS) diagnostic criteria, second and third editions, were included in this meta-analysis.26,27 The age of onset of migraine was before 50 years of age, and the patients had a total Migraine Disability Assessment (MIDAS) score of ≥ 11 and a history of 3 to 8 migraine attacks per month (< 15 headache days per month). The exclusion criteria included a history of psychiatric disorder(s), use of preventive medications (eg, tricyclic antidepressants, calcium channel blockers, beta blockers), patients on any combination of analgesics, patients with basic medical conditions and surgeries requiring medical interventions, history of drug allergy, and/or recent intake of drugs in any other research.
Types of interventions.
For available clinical trials, melatonin (2 to 3 mg) used as prophylaxis for migraine was considered to be the intervention.
For the control intervention, placebo or use of any prophylactic medication was considered as a comparator for these meta-analyses. However, studies were available only for valproate and amitriptyline.
Types of outcome measures.
The primary outcome considered was responder rate, defined as > 50% reduction in migraine headache days from baseline, as recommended by the guidelines of the IHS for controlled trials of preventive treatment of chronic migraine in adults.28
Secondary outcomes included change in the frequency of migraine attacks from baseline, change in mean migraine attack duration, mean decrease in analgesic medications, reduction in migraine severity (assessed by scoring on a visual analog scale [VAS]), and common adverse drug reactions (ADRs).
Study Selection and Data Collection
Selection of studies.
The selection of relevant studies was made in a stepwise manner. First, three review authors (H.M.P., R.M., and M.J.) independently checked the titles, abstracts, and keywords of all references retrieved. Then, the full texts of all selected studies were retrieved and assessed by the same three authors, and the studies that met the inclusion criteria were included in the meta-analysis.
Data extraction and management.
Three review authors (H.M.P., B.R.M., and A.M.) independently collected the data and assessed study quality using guidelines published by the Cochrane Collaboration.25 Any disagreement among the three review authors was resolved by consensus. Extracted data included study design, participants, intervention, comparators, and outcome measures.
Data analysis.
Meta-analysis was conducted using the Cochrane Program Review Manager software (version 5.3), and meta-regression was performed using the “Metapackage” function in R programming language (version 3.4).29,30
Assessment of risk of bias in included studies.
Three review authors (R.M., A.M., B.R.M.) independently assessed the internal validity of the eligible studies according to the Cochrane Collaboration risk of bias tool (version 1.0)25 and resolved any disagreement via discussion until consensus was obtained.
Unit of analysis issue.
This meta-analysis considered study as the unit of design. In the included studies, placebo or a different standard therapy was used as a comparator to assess efficacy and safety; for the purposes of the present meta-analysis, the different comparators were considered as separate units of analysis.
Measures of treatment effect.
The outcome measures of responder rate and occurrence of ADRs are presented as categorical data, for which the odds ratio (OR) was calculated and presented with a 95% CI to estimate the effect size. Outcome measures such as change in the frequency of migraine attacks from baseline, change in migraine attack duration, reduction in analgesic medications, and change in migraine severity are presented as continuous data, for which mean differences (MDs) were calculated and presented with a 95% CI to estimate the effect size of the outcome measures between the experiment (melatonin) and control (placebo/standard therapy) groups. In case of missing the SD of the MD for any continuous variable, the pooled variance and SD were calculated. A random-effects model was used for overall between-group analyses irrespective of the heterogeneity between individual studies.
Assessment of heterogeneity.
Keeping in mind that statistical heterogeneity is inevitable due to clinical and methodologic diversity in clinical studies, it is still important to quantify it across studies included in a meta-analysis. Chi-square test was used to assess whether the observed differences in the results were likely due to chance alone; a low P value (or a large chi-square statistic relative to degrees of freedom) provides evidence of significant heterogeneity of the intervention effects (ie, a variation in effect estimates beyond chance). The I2 statistic, which describes the percentage of the variability in effects due to heterogeneity, was calculated for quantifying inconsistency. A L’Abbé Plot was also made for showing variations in the observed results by plotting the event rate (ie, responder rate) in the treatment group on the vertical axis and the rate in the control group on the horizontal axis.
Subgroup analysis.
Subgroup analysis was performed for all outcome parameters depending on the comparator used (ie, placebo or standard therapy).
Meta-regression.
As different study characteristics, such as the drug dose of melatonin and duration of therapy, could potentially modify the effect size of the intervention, meta-regression across the included studies was performed to estimate how the outcome variable (the intervention effect) changes with a unit change in the explanatory variable (the potential effect modifier), which can be described as the regression coefficient. The statistical significance of the regression coefficient is a test of whether there is a linear relationship between the intervention effect and the explanatory variable.
Assessment of publication bias.
Publication bias across studies was also assessed quantitatively using the Begg and Mazumdar rank correlation test.
Assessment of certainty of the evidence.
Standard Cochrane methodology and Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) Working Group guidance were followed to create a summary of findings table, and five considerations (risk of bias, consistency, imprecision, indirectness, and publication bias) regarding the methods and results of the included studies were considered to draw conclusions about the certainty of the evidence for each outcome.31
Data availability.
As the present study is secondary research, the meta-analysis herein involves an analysis of already published data. All analyzed data have been incorporated into the manuscript.
Results
Description of Included Studies
The database searches identified 126 references for title and abstract screening for eligibility, and 118 of these studies were excluded. The reasons for exclusion were: review article; preclinical/in vitro study, observational study, hypothesis, editorial, expert comment, letter to the editor, non-English language, etc. The study selection and screening process is shown in Fig 1. After screening, 8 records were retrieved for full-text assessment. During the final screening, 5 full-text records were excluded, and so 3 studies were included for meta-analysis (Table 1).2,20,21 Two studies by Fallah et al were excluded, as they were conducted in a pediatric population.32,33 Another two studies were excluded due to insufficient data or because it was impossible to retrieve or calculate data of interest.34,35 Bougea et al could not be included, as it was a single-arm study without any comparator group.36Assessment of risk of bias is summarized in Table 2.
Fig 1.
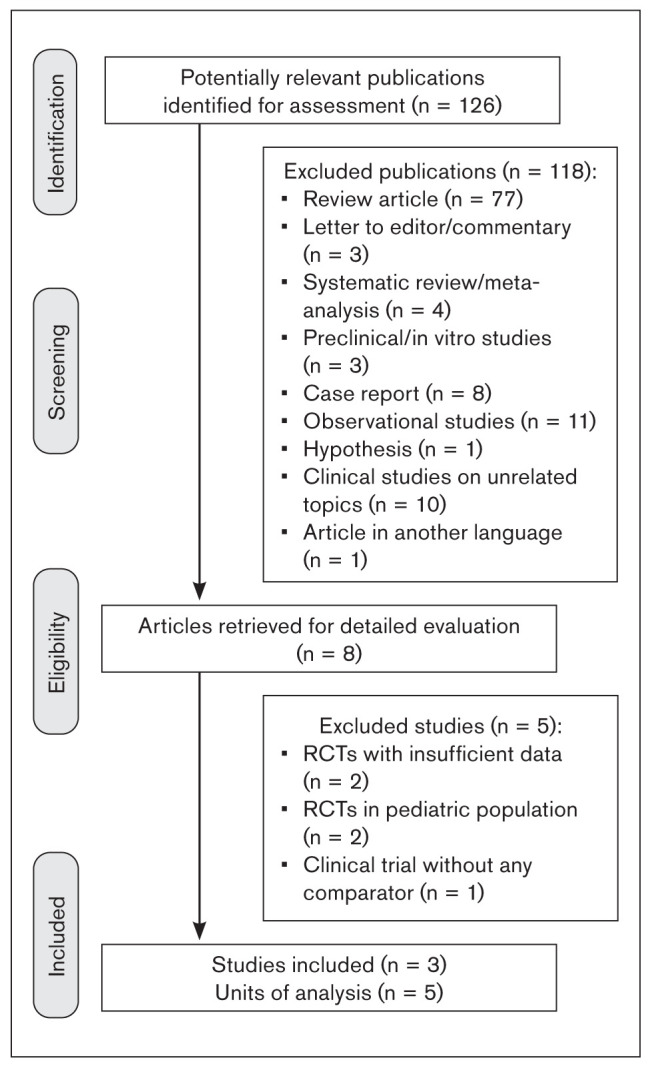
PRISMA flowchart showing the study selection process.
Table 1.
Characteristics of Studies Included in the Meta-Analyses
| Study (year), country | Methods | Participants | Interventions | No. of participants | Duration | Outcomes | Notes/remarks |
|---|---|---|---|---|---|---|---|
| Alstadhaug et al21 (2010), Norway | Randomized, double blinded, single center | Migraine with and without aura | Placebo Melatonin: 2 mg/8 wk |
Placebo Melatonin: 2 mg |
22 wk | Responder rate, frequency of migraine attacks, migraine attack duration, analgesic medications, ADRs | Prolonged-release melatonin did not have any significant effect on migraine. |
| Gonçalves et al2 (2016), Brazil | Randomized, double blinded, multicenter | Migraine with and without aura | Placebo Amitriptyline: 25 mg/12 wk Melatonin: 3 mg/12 wk |
Placebo: 65 Amitriptyline: 66 Melatonin: 65 |
12 wk | Responder rate, frequency of migraine attacks, migraine attack duration, analgesic medications, migraine severity, ADRs | Melatonin was effective and proved better than placebo and amitriptyline in responder rate. |
| Ebrahimi-Monfared et al20 (2017), Iran | Randomized, double blinded, multicenter | Migraine with and without aura | Placebo Sodium valproate: 200 mg/8 wk Melatonin: 3 mg/8 wk |
Placebo: 35 Amitriptyline: 35 Melatonin: 35 |
8 wk | Responder rate, frequency of migraine attacks, migraine attack duration, analgesic medications, migraine severity, ADRs | Melatonin was effective and proved better than placebo but comparable to sodium valproate in responder rate. |
Table 2.
Risk of Bias Assessment
| Included studies | Risk of bias | ||||||
|---|---|---|---|---|---|---|---|
| B1 | B2 | B3 | B4 | B5 | B6 | B7 | |
| Alstadhaug et al,21 2010 | Low | Unclear | Low | Unclear | Low | Low | Unclear |
| Gonçalves et al,2 2016 | Low | Low | Low | Low | High | Low | Unclear |
| Ebrahimi-Monfared et al,20 2017 | Low | Low | Low | Low | High | Low | Unclear |
B1 = random sequence generation (selection bias); B2 = allocation concealment (selection bias); B3 = blinding of participants and personnel (performance bias); B4 = blinding of outcome assessment (detection bias); B5 = incomplete outcome data (attrition bias); B6 = selective reporting (reporting bias); B7 = other type of bias.
Effects of Intervention
To evaluate the effect of melatonin, the responder rate and change in duration of migraine attacks, migraine severity, use of analgesic medications, and frequency of migraine attacks were assessed. The occurrence of ADRs in patients following the intervention was also estimated. The effect sizes of included studies were entered into Cochrane Review Manager software (version 5.3)29 using a random-effects model.
Responder rate.
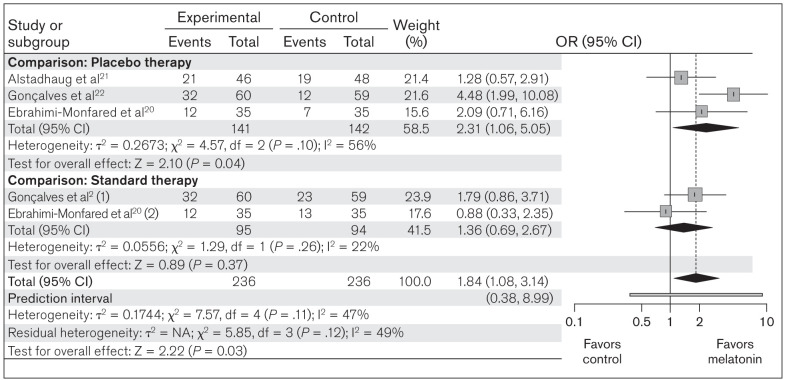
All three studies included in this meta-analysis compared response to melatonin in terms of headache frequency in a melatonin group (n = 236) vs placebo/standard therapy groups (n = 236). The test for heterogeneity among the included studies was not significant (c2 = 7.57; P = .11; I2 = 47%). In the forest plot, the CIs for the results of individual studies (depicted graphically using horizontal lines) were found to have good overlap, indicating nonsignificant heterogeneity. Random-effects model analysis revealed an OR of 1.84 (95% CI: 1.08 to 3.14; prediction interval [PI]: 0.38 to 8.99; z = 2.22; P = .03) indicating that the responder rate was significantly greater with melatonin vs the comparators (Fig 2).
Fig 2.
Forest plot of included studies assessing responder rate between melatonin and placebo/standard therapy. Mantel-Haenszel, random-effects model. (1) = melatonin vs amitriptyline; (2) melatonin vs valproic acid.
Subgroup analysis was performed based on the comparator group (placebo or standard therapy). Heterogeneity was not significant in either subgroup. The OR of 2.31 (95% CI: 1.06 to 5.05; z = 2.10; P = .04) in the placebo group and the OR of 1.36 (95% CI: 0.69 to 2.67; z = 0.89; P = .37) in the standard therapy group suggest that melatonin is beneficial in comparison to placebo, but showed no significant difference compared to standard therapy (Fig 2). A L’Abbé plot was also generated (Appendix 1) to depict the apparent effectiveness of melatonin over placebo in terms of response rate. The cumulative distribution calculator predicted a chance of 9.8% that future studies will show a result opposite to that of the present meta-analysis (ie, favoring placebo/standard therapy).
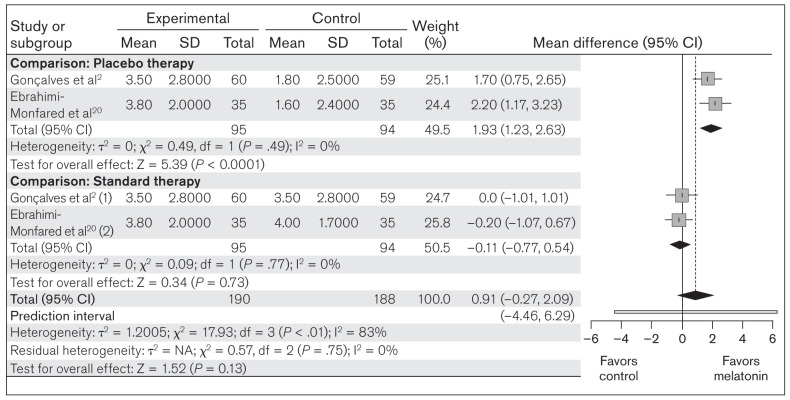
Migraine severity.
This meta-analysis also compared the change in migraine severity between melatonin (n = 190) and placebo/standard treatment (n = 188), as measured in all included studies. Random-effects model analysis revealed an MD of 0.91 (95% CI: –0.27 to 2.09; PI: –4.46 to 6.29; z = 1.52; P = .13), indicating that melatonin showed no significant overall effect on the change in migraine severity (Fig 3). In the subgroup analysis, no significant heterogeneity was found in either of the groups. The placebo group showed an MD of 1.93 (95% CI: 1.23 to 2.63; z = 5.39; P < .0001), and the standard therapy group showed an MD of –0.11 (95% CI: –0.77 to 0.54; z = 0.34; P = .73), which suggests that melatonin can reduce migraine severity significantly compared to placebo but not to standard therapy (Fig 3).
Fig 3.
Forest plot of included studies assessing change in mean migraine severity. Inverse variance, random-effects model. (1) = melatonin vs amitriptyline; (2) melatonin vs valproic acid.
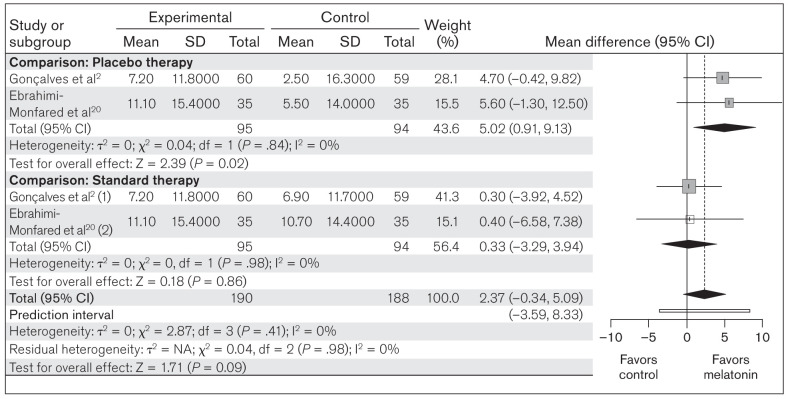
Migraine attack duration.
This meta-analysis compared the change in mean migraine attack duration between melatonin (n = 190) and placebo/standard therapy (n = 188) in the included studies. The heterogeneity among the included studies was not significant (χ2 = 2.87; P = .41; I2 = 0%). Random-effects model analysis for melatonin revealed an OR of 2.37 (95% CI: –0.34 to 5.09; PI: –3.59 to 8.33; z = 1.71; P = .09), suggesting a nonsignificant effect of melatonin on change in mean migraine attack duration (Fig 4). Subgroup analysis with placebo as comparator showed an OR of 5.02 (95% CI: 0.91 to 9.13; z = 2.39; P = .02), but against the standard therapy, the OR was 0.33 (95% CI: –3.29 to 3.94; z = 0.18; P = .86) (Fig 4). These results suggest that melatonin has a significant beneficial effect over placebo in reducing mean migraine attack duration, but not when compared to standard therapy.
Fig 4.
Forest plot of included studies assessing change in mean migraine attack duration. Inverse variance, random-effects model. (1) = melatonin vs amitriptyline; (2) melatonin vs valproic acid.
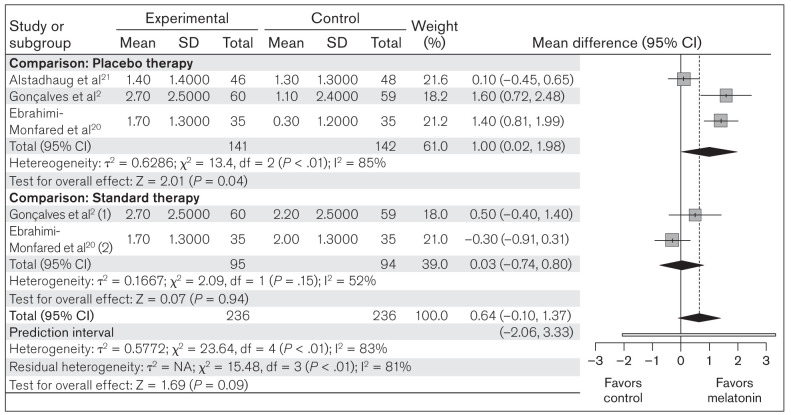
Frequency of migraine attacks.
All three studies included in this meta-analysis compared the change in frequency of migraine attacks from baseline between melatonin (n = 236) and placebo/standard therapy (n = 236). Random-effects model analysis revealed an MD of 0.64 (95% CI: –0.10 to 1.37; PI: –2.06 to 3.33; z = 1.69; P = .09), showing a nonsignificant result of the overall effect of melatonin on change in frequency of migraine attacks (Fig 5). Subgroup analysis comparing melatonin to placebo revealed an MD of 1.00 (95% CI: 0.02 to 1.98; z = 2.01; P = .04), which was significant, and an MD of 0.03 (95% CI: –0.74 to 0.80; z = 0.07; P = .94) in comparison to standard treatment (Fig 5). These results suggest that melatonin has a significant beneficial effect over placebo in reducing the frequency of migraine attacks, but not compared to standard therapy.
Fig 5.
Forest plot of included studies assessing change in mean migraine attack frequency. Inverse variance, random-effects model. (1) = melatonin vs amitriptyline; (2) melatonin vs valproic acid.
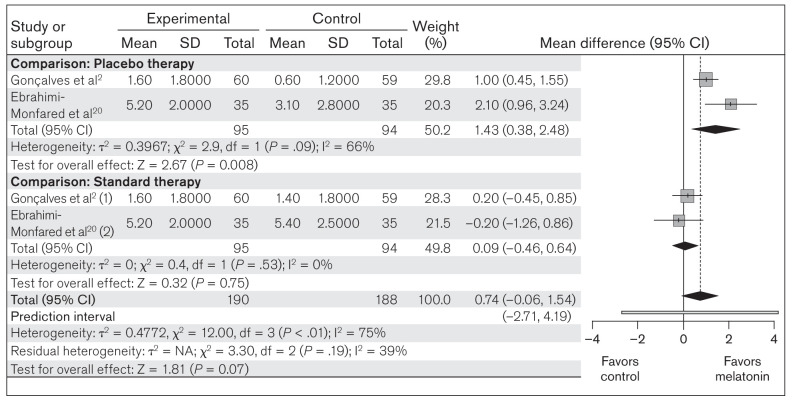
Use of analgesic medications.
This meta-analysis compared the use of analgesic medications between melatonin (n = 190) and placebo/standard therapy (n = 188) in all included studies. Random-effects model analysis revealed an MD of 0.74 (95% CI: –0.06 to 1.54; PI: –2.71 to 4.19; z = 1.81; P = .07), indicating that the overall effect of melatonin on the mean decrease in the use of analgesic medications was nonsignificant (Fig 6). In subgroup analysis, no significant heterogeneity was found in either of the groups. The MD of 1.43 (95% CI: 0.38 to 2.48; z = 2.67; P = .008) in the placebo group and the MD of 0.09 (95% CI: –0.46 to 0.64; z = 0.32; P = .75) in the standard treatment group suggest that melatonin can significantly reduce the use of analgesic medications over placebo, but not compared to standard therapy (Fig 6).
Fig 6.
Forest plot of included studies assessing mean decrease in analgesic medications. Inverse variance, random-effects model. (1) = melatonin vs amitriptyline; (2) melatonin vs valproic acid.
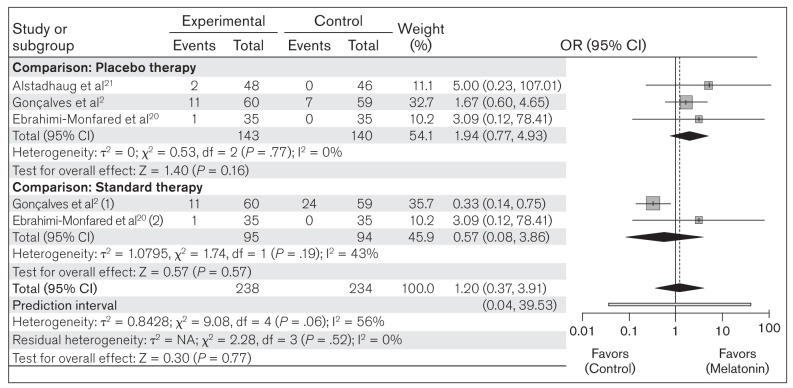
Occurrence of ADRs.
The included studies reported different ADRs in response to melatonin. Among the reported ADRs, drowsiness and fatigue were found to be very common in all included studies. The other commonly reported ADRs included dry mouth, weight gain, epigastralgia, night sweats, and abnormally high dream activity. The present meta-analysis evaluated the occurrence of the two most common ADRs (drowsiness and fatigue) between melatonin (n = 238) and placebo/standard therapy (n = 234) in all included studies. The heterogeneity among the included studies was not significant (χ2 = 9.08; P = .06; I2 = 56%). Random-effects model analysis revealed an OR of 1.20 (95% CI: 0.37 to 3.91; PI: 0.04 to 39.53; z = 0.30; P = .77), indicating that the occurrence of an ADR was not significantly greater in the melatonin group compared to placebo/standard therapy (Fig 7). In the subgroup analysis, no significant heterogeneity was found in either of the groups. The OR of 1.94 (95% CI: 0.77 to 4.93; z = 1.40; P = .16) in the placebo group and the OR of 0.57 (95% CI: 0.08 to 3.86; z = 0.57; P = .57) in the standard therapy group suggest that there is no significant difference in the occurrence of drowsiness and fatigue in the melatonin group when compared to either comparison group separately (Fig 7).
Fig 7.
Forest plot of included studies for assessing ADRs (drowsiness and fatigue). Mantel-Haenszel, random-effects model. (1) = melatonin vs amitriptyline; (2) melatonin vs valproic acid.
Meta-Regression
Meta-regression showed no statistically significant association between effect sizes for response rate and the dose or duration of therapy (Appendix 2). The result showed the slope coefficient to be 0.18 (P = .06) for duration of therapy and 0.48 (P = .31) for the dose of melatonin, where the slope coefficient quantifies the change in response rate due to a unit change in the predictor variables.
Publication Bias in Included Studies
The assessment of publication bias using the Begg and Mazumdar rank correlation test showed a Kendall’s tau value of –0.20 (with continuity correction) with a two-tailed P value of .624, which was not significant.
Certainty of Evidence
Details of the effect estimates and GRADE ratings are summarized in Table 3. Compared to placebo, the certainty of the evidence was found to be high for responder rate, suggesting it is very likely that the true effect lies close to the estimate of the effect. For migraine severity, mean attack duration, and a mean decrease in analgesic use and ADRs, the certainty of the evidence was found to be moderate, suggesting that the true effect is likely to be close to the estimate of the effect, but with the possibility remaining that it is substantially different.
Table 3.
Summary of Findings
| Outcome | No. of participants | Relative effect | Anticipated absolute risk | Certainty of evidence | |
|---|---|---|---|---|---|
| Risk with control | Risk difference with melatonin | ||||
| Responder rate | |||||
| Overall | 472 (3 RCTs) |
OR 1.84 (1.08 to 3.14) |
314 per 1,000 | 143 more per 1,000 (17 to 276 more) |
⊕⊕⊕○ MODERATE |
| Comparison: Placebo therapy | 283 (3 RCTs) |
OR 2.31 (1.06 to 5.05) |
268 per 1,000 | 190 more per 1,000 (12 to 381 more) |
⊕⊕⊕⊕ HIGH |
| Comparison: Standard therapy | 189 (2 RCTs) |
OR 1.36 (0.69 to 2.67) |
383 per 1,000 | 75 more per 1,000 (83 fewer to 241 more) |
⊕⊕○○ LOW |
| Migraine severity | |||||
| Overall | 378 (2 RCTs) |
– | – | MD 0.91 higher (0.27 lower to 2.09 higher) |
⊕⊕○○ LOW |
| Comparison: Placebo therapy | 189 (2 RCTs) |
– | – | MD 1.93 higher (1.23 to 2.63 higher) |
⊕⊕⊕○ MODERATE |
| Comparison: Standard therapy | 189 (2 RCTs) |
– | – | MD 0.11 lower (0.77 lower to 0.54 higher) |
⊕⊕⊕○ MODERATE |
| Mean attack duration | |||||
| Overall | 378 (2 RCTs) |
– | – | MD 2.37 higher (0.34 lower to 5.09 higher) |
⊕⊕⊕○ MODERATE |
| Comparison: Placebo therapy | 189 (2 RCTs) |
– | – | MD 5.02 higher (0.91 to 9.13 higher) |
⊕⊕⊕○ MODERATE |
| Comparison: Standard therapy | 189 (2 RCTs) |
– | – | MD 0.33 higher (3.29 lower to 3.94 higher) |
⊕⊕⊕○ MODERATE |
| Mean attack frequency | |||||
| Overall | 472 (3 RCTs) |
– | – | MD 0.64 higher (0.1 lower to 1.37 higher) |
⊕⊕○○ LOW |
| Comparison: Placebo therapy | 283 (3 RCTs) |
– | – | MD 1 higher (0.02 to 1.98 higher) |
⊕⊕○○ LOW |
| Comparison: Standard therapy | 189 (2 RCTs) |
– | – | MD 0.03 higher (0.74 lower to 0.8 higher) |
⊕⊕○○ LOW |
| Mean decrease in use of analgesics | |||||
| Overall | 378 (2 RCTs) |
– | – | MD 0.74 higher (0.06 lower to 1.54 higher) |
⊕⊕○○ LOW |
| Comparison: Placebo therapy | 189 (2 RCTs) |
– | – | MD 1.43 higher (0.38 to 2.48 higher) |
⊕⊕⊕○ MODERATE |
| Comparison: Standard therapy | 189 (2 RCTs) |
– | – | MD 0.09 higher (0.46 lower to 0.64 higher) |
⊕⊕⊕○ MODERATE |
| ADRs | |||||
| Overall | 472 (3 RCTs) |
OR 1.20 (0.37 to 3.91) |
132 per 1,000 | 22 more per 1,000 (79 fewer to 241 more) |
⊕○○○ VERY LOW |
| Comparison: Placebo therapy | 283 (3 RCTs) |
OR 1.94 (0.77 to 4.93) |
50 per 1,000 | 43 more per 1,000 (11 fewer to 156 more) |
⊕⊕⊕○ MODERATE |
| Comparison: Standard therapy | 189 (2 RCTs) |
OR 0.57 (0.08 to 3.86) |
255 per 1,000 | 92 fewer per 1,000 (229 fewer to 314 more) |
⊕○○○ VERY LOW |
Discussion
The main hurdle in generating conclusive evidence is the paucity of clinical studies performed in this domain. The main purpose of the present meta-analysis was to evaluate the efficacy and safety of melatonin as a prophylactic medication in adult migraineurs; therefore, responder rate and change in migraine severity, mean attack duration, mean attack frequency, and mean decrease in analgesic use were compared between melatonin and placebo or standard therapy. Subgroup analysis was performed depending on the comparator used (placebo/standard therapy). Melatonin showed a significant overall greater responder rate than both comparators. In subgroup analyses, the responder rate for melatonin was significantly greater than placebo, but there was no significant difference observed when compared to standard therapy. Similarly, melatonin therapy showed a significantly beneficial role in terms of reduction of migraine severity, mean attack duration, and decrease in analgesic use in comparison to placebo. However, the effect of melatonin was not significant in comparison to standard prophylactic therapy with amitriptyline or valproic acid. The certainty of the evidence for the standard therapy subgroup is moderate to very low, which suggests that the true effect may be substantially different from the estimate of the effect, whereas the certainty of the evidence for the placebo subgroup is moderate to high. Thus, there is a need for a greater number of RCTs for more conclusive evidence on the efficacy of melatonin, especially in comparison to standard prophylactic therapy.
ADRs in response to melatonin were reported in all included studies, and the occurrence of the two most common ADRs (drowsiness and fatigue) was evaluated. Over the course of the treatment, no serious adverse events were observed. The majority of ADRs were either mild or moderate in intensity and occurred more commonly in the standard therapy group than in the melatonin group. The study by Gonçalves et al2 reported some common ADRs apart from drowsiness and fatigue, such as daytime sleepiness, dry mouth, and constipation. Similarly, Ebrahimi-Monfared et al20 and Alstadhaug et al21 reported drowsiness, fatigue, nervousness, and nightmares. The two most common ADRs in these studies were evaluated, and the results showed that the occurrence of ADRs with melatonin was not higher than the comparators, suggesting that melatonin is safe.
The differences within the three included studies with respect to dose and duration of treatment were addressed by performing meta-regression, which showed no statistically significant influence of dose or duration of treatment on effect size. As the results of the present meta-analysis are based on only three studies, the most glaring limitation of this study is its generalizability to the population, reinforcing the need for more studies to establish the effect of melatonin for migraine prophylaxis. A previous meta-analysis by Long et al combined adult and pediatric populations, and thus the true effect of melatonin in adults could not be obtained.22 However, the present meta-analysis combined studies including adult populations and calculated the PI and probability. Further studies are warranted for robust results as per prediction statistics and certainty of evidence.
The beneficial role of melatonin in comparison to placebo for migraine prophylaxis may be due to multiple mechanisms, as explained in previous literature. Through the free radical scavenging property and inhibition of inflammatory factors, melatonin can protect the brain from direct toxic damages and maintain its structural and functional integrity.37,38 Melatonin is also known to benefit migraine treatment by reducing nitric oxide synthase activity, inhibiting dopamine release, antagonizing glutamate-induced excitotoxicity, and suppressing calcitonin gene-related peptide (CGRP).9,11,12,14 Additionally, melatonin exerts its analgesic property by increasing β-endorphin release, activating melatonin receptors, and augmenting GABAergic action.10,39 The pleiotropic effects of melatonin strongly support its probable role in migraine; however, the dearth of clinical data is the only impediment to translating these findings into clinical practice. The major limitation of the present meta-analysis is the small number of RCTs available for analysis. Second, in the absence of the SD of the MD, the pooled variance was calculated, which gave only an approximate estimate of the SD.
Conclusions
Melatonin showed a beneficial role as a prophylactic medication for migraine in terms of better responder rate and reduction in migraine severity, mean attack duration, mean attack frequency, and analgesic use in comparison to placebo. Future clinical trials on melatonin in comparison to standard prophylactic therapy in migraine are warranted to generate conclusive evidence and a preventive strategy for better clinical outcomes.
Highlights
The role of melatonin for migraine prophylaxis assessed in previous clinical trials is nonconclusive.
This meta-analysis evaluated the efficacy and safety of melatonin for prophylaxis in migraine in adults.
Melatonin therapy in migraine is associated with a significantly higher responder rate and reduced migraine attack frequency, duration, severity, and use of analgesics over placebo.
Melatonin may have a beneficial prophylactic role in migraine in comparison to placebo.
Acknowledgments
PROSPERO registration number: CRD42020183241.
The authors report no conflicts of interest.
Author contributions: H.M.P.: study concept and design; literature search; study selection and data collection; data extraction, analysis, management, and interpretation; critical revision and final approval of manuscript; R.M.: literature search; study selection and data collection; data extraction and management; critical revision and final approval of manuscript; A.M.: literature search; data extraction and management; drafting and final approval of manuscript; M.J.: literature search, study selection and data collection; drafting and final approval of manuscript; B.R.M.: study concept and design; data extraction, analysis, and interpretation; critical revision and final approval of manuscript.
Appendix 1.
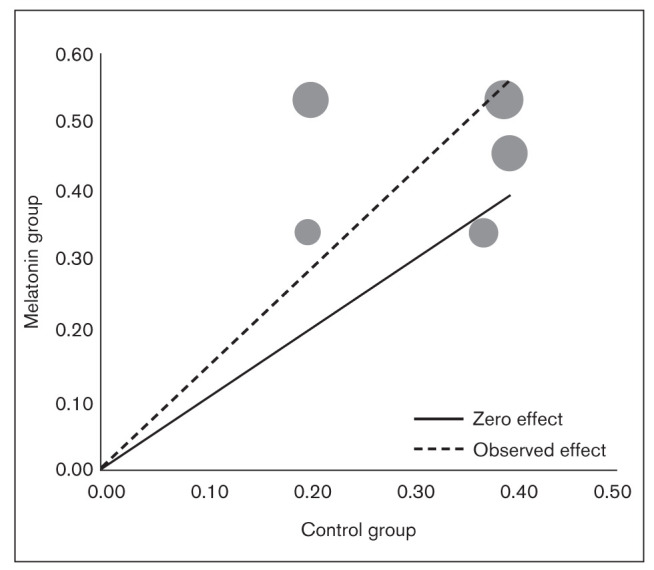
L’Abbé plot depicting the effectiveness of melatonin over control treatments (placebo/standard therapy) on responder rate in adult migraineurs. The solid line represents the line of no effect. The dotted line represents the combined effect of all the studies as OR. The circle represents individual studies and size variations as a function of weight.
Appendix 2.
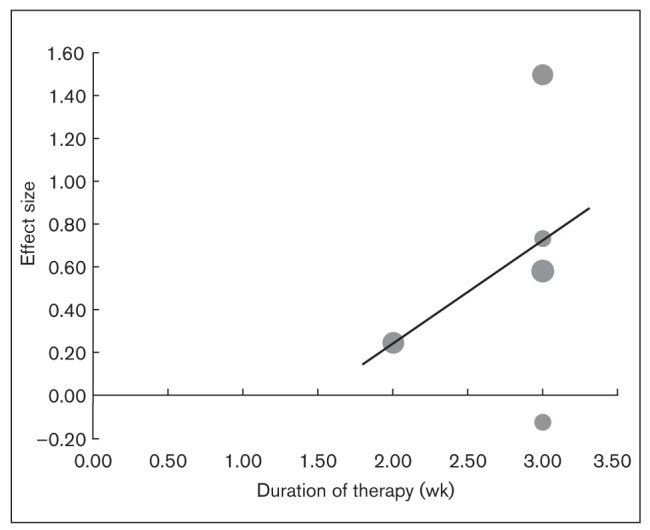
Bubble plot showing the effect of duration of therapy on the OR for responder rate across studies. Individual studies are depicted by circles along the line of meta-regression.
Appendix Table 1.
Search Strategy
| Search: (migraine) AND (melatonin) Sort by: Most Recent | ("migrain"[All Fields] OR "migraine disorders"[MeSH Terms] OR ("migraine"[All Fields] AND "disorders"[All Fields]) OR "migraine disorders"[All Fields] OR "migraine"[All Fields] OR "migraines"[All Fields] OR "migraine s"[All Fields] OR "migraineous"[All Fields] OR "migrainers"[All Fields] OR "migrainous"[All Fields]) AND ("melatonin"[MeSH Terms] OR "melatonin"[All Fields] OR "melatonin s"[All Fields] OR "melatonine"[All Fields] OR "melatonins"[All Fields]) |
|---|---|
| Translations | migraine: "migrain"[All Fields] OR "migraine disorders"[MeSH Terms] OR ("migraine"[All Fields] AND "disorders"[All Fields]) OR "migraine disorders"[All Fields] OR "migraine"[All Fields] OR "migraines"[All Fields] OR "migraine’s"[All Fields] OR "migraineous"[All Fields] OR "migrainers"[All Fields] OR "migrainous"[All Fields] |
| melatonin: "melatonin"[MeSH Terms] OR "melatonin"[All Fields] OR "melatonin’s"[All Fields] OR "melatonine"[All Fields] OR "melatonins"[All Fields] |
References
- Puledda F, Messina R, Goadsby PJ. An update on migraine: Current understanding and future directions. J Neurol. 2017;264:2031–2039. doi: 10.1007/s00415-017-8434-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonçalves AL, Martini Ferreira A, Ribeiro RT, Zukerman E, Cipolla-Neto J, Peres MF. Randomised clinical trial comparing melatonin 3 mg, amitriptyline 25 mg and placebo for migraine prevention. J Neurol Neurosurg Psychiatry. 2016;87:1127–1132. doi: 10.1136/jnnp-2016-313458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buse DC, Manack A, Serrano D, Turkel C, Lipton RB. Sociodemographic and comorbidity profiles of chronic migraine and episodic migraine sufferers. J Neurol Neurosurg Psychiatry. 2010;81:428–432. doi: 10.1136/jnnp.2009.192492. [DOI] [PubMed] [Google Scholar]
- Lipton RB, Bigal ME, Diamond M, et al. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68:343–349. doi: 10.1212/01.wnl.0000252808.97649.21. [DOI] [PubMed] [Google Scholar]
- Silberstein SD, Holland S, Freitag F, et al. Evidence-based guideline update: Pharmacologic treatment for episodic migraine prevention in adults: Report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1337–1345. doi: 10.1212/WNL.0b013e3182535d20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart WF, Shechter A, Lipton RB. Migraine heterogeneity. Disability, pain intensity, and attack frequency and duration. Neurology. 1994;44(6 Suppl 4):S24–S39. [PubMed] [Google Scholar]
- Piccinni C, Cevoli S, Ronconi G, et al. A real-world study on unmet medical needs in triptan-treated migraine: Prevalence, preventive therapies and triptan use modification from a large Italian population along two years. J Headache Pain. 2019;20:74. doi: 10.1186/s10194-019-1027-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Kadian R. Migraine Prophylaxis. Treasure Island: StatPearls; 2020. [PubMed] [Google Scholar]
- Bettahi I, Pozo D, Osuna C, Reiter RJ, Acuña-Castroviejo D, Guerrero JM. Melatonin reduces nitric oxide synthase activity in rat hypothalamus. J Pineal Res. 1996;20:205–210. doi: 10.1111/j.1600-079x.1996.tb00260.x. [DOI] [PubMed] [Google Scholar]
- Danilov A, Kurganova J. Melatonin in chronic pain syndromes. Pain Ther. 2016;5:1–17. doi: 10.1007/s40122-016-0049-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zisapel N. Melatonin-dopamine interactions: From basic neurochemistry to a clinical setting. Cell Mol Neurobiol. 2001;21:605–616. doi: 10.1023/A:1015187601628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadan NM. The link between glutamate and migraine. CNS Spectr. 2003;8:446–449. doi: 10.1017/s1092852900018757. [DOI] [PubMed] [Google Scholar]
- Peres MF, Masruha MR, Zukerman E, Moreira-Filho CA, Cavalheiro EA. Potential therapeutic use of melatonin in migraine and other headache disorders. Expert Opin Investig Drugs. 2006;15:367–375. doi: 10.1517/13543784.15.4.367. [DOI] [PubMed] [Google Scholar]
- Vogler B, Rapoport AM, Tepper SJ, Sheftell F, Bigal ME. Role of melatonin in the pathophysiology of migraine: Implications for treatment. CNS Drugs. 2006;20:343–350. doi: 10.2165/00023210-200620050-00001. [DOI] [PubMed] [Google Scholar]
- Gagnier JJ. The therapeutic potential of melatonin in migraines and other headache types. Altern Med Rev. 2001;6:383–389. [PubMed] [Google Scholar]
- Leite Pacheco R, de Oliveira Cruz Latorraca C, Adriano Leal Freitas da Costa A, Luiza Cabrera Martimbianco A, Vianna Pachito D, Riera R. Melatonin for preventing primary headache: A systematic review. Int J Clin Pract. 2018;72:e13203. doi: 10.1111/ijcp.13203. [DOI] [PubMed] [Google Scholar]
- Chen IJ, Yang CP, Lin SH, Lai CM, Wong CS. The circadian hormone melatonin inhibits morphine-induced tolerance and inflammation via the activation of antioxidative enzymes. Antioxidants (Basel) 2020;9:780. doi: 10.3390/antiox9090780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakhri S, Ahmadpour Y, Rezaei H, et al. The antinociceptive mechanisms of melatonin: Role of L-arginine/nitric oxide/cyclic GMP/KATP channel signaling pathway. Behav Pharmacol. 2020;31:728–737. doi: 10.1097/FBP.0000000000000579. [DOI] [PubMed] [Google Scholar]
- Zhu S, McGeeney B. When indomethacin fails: Additional treatment options for “indomethacin responsive headaches”. Curr Pain Headache Rep. 2015;19:7. doi: 10.1007/s11916-015-0475-2. [DOI] [PubMed] [Google Scholar]
- Ebrahimi-Monfared M, Sharafkhah M, Abdolrazaghnejad A, Mohammadbeigi A, Faraji F. Use of melatonin versus valproic acid in prophylaxis of migraine patients: A double-blind randomized clinical trial. Restor Neurol Neurosci. 2017;35:385–393. doi: 10.3233/RNN-160704. [DOI] [PubMed] [Google Scholar]
- Alstadhaug KB, Odeh F, Salvesen R, Bekkelund SI. Prophylaxis of migraine with melatonin: A randomized controlled trial. Neurology. 2010;75:1527–1532. doi: 10.1212/WNL.0b013e3181f9618c. [DOI] [PubMed] [Google Scholar]
- Long R, Zhu Y, Zhou S. Therapeutic role of melatonin in migraine prophylaxis: A systematic review. Medicine (Baltimore) 2019;98:e14099. doi: 10.1097/MD.0000000000014099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumpston M, Li T, Page MJ, et al. Updated guidance for trusted systematic reviews: A new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019;10:ED000142. doi: 10.1002/14651858.ED000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Headache Classification Subcommittee of the International Headache Society. The International Classification of Headache Disorders: 2nd edition. Cephalalgia. 2004;(24 Suppl 1):9–160. doi: 10.1111/j.1468-2982.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- Headache Classification Committee of the International Headache Society. The International Classification of Headache Disorders, 3rd edition (beta version) Cephalalgia. 2013;33:629–808. doi: 10.1177/0333102413485658. [DOI] [PubMed] [Google Scholar]
- Tassorelli C, Diener HC, Dodick DW, et al. Guidelines of the International Headache Society for controlled trials of preventive treatment of chronic migraine in adults. Cephalalgia. 2018;38:815–832. doi: 10.1177/0333102418758283. [DOI] [PubMed] [Google Scholar]
- Review Manager. Version 3.5. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration; 2014. [Google Scholar]
- Vienna: R Foundation for Statistical Computing; 2013. R: A Language and Environment for Statistical Computing. Version 3.4. [Google Scholar]
- Hamilton, Ontario, Canada: McMaster University, Evidence Prime; 2015. GRADEpro Guideline Development Tool. [Google Scholar]
- Fallah R, Fazelishoroki F, Sekhavat L. A randomized clinical trial comparing the efficacy of melatonin and amitriptyline in migraine prophylaxis of children. Iran J Child Neurol. 2018;12:47–54. [PMC free article] [PubMed] [Google Scholar]
- Fallah R, Shoroki FF, Ferdosian F. Safety and efficacy of melatonin in pediatric migraine prophylaxis. Curr Drug Saf. 2015;10:132–135. doi: 10.2174/1574886309666140605114614. [DOI] [PubMed] [Google Scholar]
- Gelfand AA, Qubty W, Patniyot I, et al. Home-based trials in adolescent migraine: A randomized clinical trial. JAMA Neurol. 2017;74:744–745. doi: 10.1001/jamaneurol.2017.0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peres MF, Zukerman E, da Cunha Tanuri F, Moreira MF, Cipolla-Nieto J. Melatonin, 3 mg, is effective for migraine prevention. Neurology. 2004;63:757. doi: 10.1212/01.wnl.0000134653.35587.24. [DOI] [PubMed] [Google Scholar]
- Bougea A, Spantideas N, Lyras V, Avramidis T, Thomaidis T. Melatonin 4 mg as prophylactic therapy for primary headaches: A pilot study. Funct Neurol. 2016;31:33–37. doi: 10.11138/FNeur/2016.31.1.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter RJ, Calvo JR, Karbownik M, Qi W, Tan DX. Melatonin and its relation to the immune system and inflammation. Ann N Y Acad Sci. 2000;917:376–386. doi: 10.1111/j.1749-6632.2000.tb05402.x. [DOI] [PubMed] [Google Scholar]
- Gurunathan S, Kang MH, Kim JH. Role and therapeutic potential of melatonin in the central nervous system and cancers. Cancers (Basel) 2020;12:1567. doi: 10.3390/cancers12061567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M, Jadhav HR. Melatonin: Functions and ligands. Drug Discov Today. 2014;19:1410–1418. doi: 10.1016/j.drudis.2014.04.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
As the present study is secondary research, the meta-analysis herein involves an analysis of already published data. All analyzed data have been incorporated into the manuscript.