Abstract
Background:
Hypertension (HTN) is associated with renal proinflammatory immune cell infiltration and increased sodium retention. We reported previously that renal lymphatic vessels, which are responsible for trafficking immune cells from the interstitial space to draining lymph nodes, increase in density under hypertensive conditions. We also demonstrated that augmenting renal lymphatic density can prevent HTN in mice. Whether renal lymphangiogenesis can treat HTN in mice is unknown. We hypothesized that genetically inducing renal lymphangiogenesis after the establishment of HTN would attenuate HTN in male and female mice from three different HTN models.
Methods:
Mice with inducible kidney-specific overexpression of VEGF-D (KidVD) experience renal lymphangiogenesis upon doxycycline administration. HTN was induced in KidVD+ and KidVD- mice by subcutaneous release of angiotensin II, administration of the nitric oxide synthase inhibitor L-NAME, or consumption of a 4% salt diet following a L-NAME priming and washout period. After a week of HTN stimuli treatment, doxycycline was introduced. Systolic blood pressure (SBP) readings were taken weekly. Kidney function was determined from urine and serum measures. Kidneys were processed for RT-qPCR, flow cytometry, and imaging.
Results:
Mice that underwent renal-specific lymphangiogenesis had significantly decreased SBP and renal proinflammatory immune cells. Additionally, renal lymphangiogenesis was associated with a decrease in sodium transporter expression and increased fractional excretion of sodium, indicating improved sodium handling efficiency.
Conclusions:
These findings demonstrate that augmenting renal lymphangiogenesis can treat HTN in male and female mice by improving renal immune cell trafficking and sodium handling.
Introduction
Hypertension (HTN), defined by the American Heart Association as having a blood pressure of 130/80 mmHg or above, afflicts nearly half of adults [1]. The condition is a major risk factor for cardiovascular disease, which is the leading cause of death in the U.S.A. and worldwide [2]. Despite the availability of therapeutic options and lifestyle modifications to treat HTN, many patients with HTN struggle to regulate their blood pressure. Of the 116 million U.S. adults with HTN, only 23.9 million (roughly one in four) have their blood pressure under control [3]. In the context of the current COVID-19 pandemic, it has become even more critical to treat HTN, as patients with HTN have a nearly 2.5-fold increased risk of developing severe or fatal COVID-19 and are at risk for developing the poorly understood long-term effects associated with SARS-CoV-2 infection [4]. For adults with HTN, particularly those with uncontrolled HTN due to medication noncompliance or intolerance and those with resistant HTN who are on three or more medications but still have uncontrolled HTN, there is a need for new treatment options.
The kidney’s involvement in blood pressure regulation is unquestionable and has been acknowledged for many years. Several cross-transplantation studies have verified the essential role that kidneys play in the establishment and maintenance of HTN [5–7]. Unique mechanisms, such as the renin-angiotensin system and regulation of sodium excretion, enable the kidney to manipulate blood pressure by modulating fluid volumes and arterial pressure [8,9]. Renal immune cell infiltration and inflammation are associated with HTN and can contribute to end organ damage. In particular, infiltrating T lymphocytes and macrophages have been implicated in the progression and maintenance of various types of HTN, including angiotensin II-induced HTN (A2HTN), salt-sensitive HTN (SSHTN), and nitric oxide synthase inhibitor L-NAME-induced HTN (LHTN) [10–14]. Numerous studies have reported that limiting the immune response in rodents with HTN lowers blood pressure and limits kidney damage, and Guzik et al. demonstrated that returning lymphocytes to mice lacking T and B cells restored A2HTN [14–17]. These infiltrating proinflammatory immune cells contribute to renal inflammation and increased sodium transport. In turn, increased sodium accumulates in the interstitium and propagates renal inflammation, creating a cycle that results in increased blood pressure and renal fibrosis.
The lymphatic system is involved in many disease pathologies including arthritis, diabetes, and HTN due to its relationship with the immune system under chronic inflammatory conditions [18,19]. Renal lymphatic vessels uptake immune cells, antigens, and cellular waste from the interstitial space and transport them to draining lymph nodes. These vessels serve as a highway for immune cells and facilitate transport to areas in need. In HTN, the kidney experiences a compensatory increase in lymphatic vessel density [20,21]. This can be interpreted as the body’s attempt to increase routes for fluid and immune cell clearance; however, by this point in the disease pathology, endogenous renal lymphangiogenesis is insufficient to fully resolve inflammation in the kidneys. We have demonstrated previously that augmenting the renal lymphatic network prior to the introduction of HTN stimuli can prevent the development of three forms of HTN in male and female mice [21,22]. Although these studies were impactful, the practicality of inducing renal lymphangiogenesis as a preventive measure in the billions of at-risk adults worldwide is questionable. Therefore, it is of interest whether this type of renal lymphangiogenic therapy could treat HTN when initiated after HTN has been established.
With our goal being to effectively combat the effects of HTN by way of renal lymphangiogenesis, we utilized mice with inducible kidney-specific overexpression of vascular endothelial growth factor D (VEGF-D) (KidVD), which have been previously reported to overexpress the prolymphangiogenic VEGF-D specifically in the kidney upon doxycycline administration [23]. KidVD+ and KidVD- male and female littermates were made hypertensive by one of three methods, intended to represent hypertensive patients with elevated angiotensin II levels, salt-sensitive HTN, or elevated levels of the endogenous nitric oxide synthase inhibitor asymmetric dimethylarginine. One week following initiation of HTN stimuli, the establishment of HTN was confirmed and renal lymphangiogenesis was then induced in KidVD+ mice. We hypothesized that the increase in renal lymphatic density in KidVD+ mice would attenuate the HTN, and that there would be a corresponding decrease in renal proinflammatory immune cells and improved sodium handling.
Methods
Mice
Inducible kidney-specific overexpression of VEGF-D (KidVD) mice backcrossed over at least seven generations to C57BL6/J mice have been previously described [23]. TRE-VEGF-D mice were crossed with mice carrying a kidney promoter-driven rtTA (Cdh16; KSP) to generate transgenic KidVD mice that overexpress VEGF-D specifically in the kidney upon doxycycline administration. The KSP-rtTA mouse was made available by the University of Texas-Southwestern George M. O’Brien Kidney Research Core Center (P30DK079328). KidVD+ and KidVD- littermates were made to have either A2HTN, Nω-nitro-l-arginine methyl ester hydrochloride (L-NAME)-induced HTN (LHTN), or salt-sensitive HTN (SSHTN), as described in the following paragraph. In addition to receiving hypertensive stimuli specific to their assigned hypertensive model, mice received doxycycline (0.2 mg/mL; doxycycline hyclate, Sigma, St. Louis, MO) in their drinking water beginning 1 week, following the induction of HTN: 1 week following pump implantation for A2HTN, 1 week following initial L-NAME administration for LHTN, and 1 week after salt diet became available to SSHTN mice. KidVD+ and KidVD- littermates assigned to the LHTN group received doxycycline in their drinking water in addition to L-NAME.
A2HTN
Male and female KidVD+ and KidVD- littermates (n=13–20) were assigned to the A2HTN group. At 10–14 weeks of age, these mice were surgically implanted with osmotic minipumps (Alzet, model 1004, Cupertino, CA) loaded with angiotensin II (490 ng/kg/min; BACHEM, Torrance, CA) under inhaled isoflurane (1.5–5%) anesthesia (A2HTN) and treated with topical lidocaine. These pumps diffused angiotensin II into the mice for 4 weeks.
LHTN
Male and female KidVD+ and KidVD- littermates 10–14 weeks of age (n=12–18) were given L-NAME (0.5 mg/mL; Sigma, St. Louis, MO) in their drinking water for 4 weeks.
SSHTN
Male and female KidVD+ and KidVD- littermates 10–14 weeks of age (n=11–16) were given L-NAME (0.5 mg/mL) in their drinking water for 2 weeks. Following a 2-week washout period, standard chow was replaced with 4% high salt diet for 4 weeks.
Water and diets were provided ad libitum. All mice were euthanized 4 weeks following induction of HTN by exsanguination under deep isoflurane anesthesia, with death confirmed by cervical dislocation prior to tissue collection. All procedures performed in mice occurred at the Texas A&M Health Science Center and were approved by the Texas A&M University IACUC in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
Blood pressure
Systolic blood pressure (SBP) readings were taken weekly via tail cuff to confirm the presence/absence of HTN. The IITC Life Science noninvasive blood pressure acquisition system for mice (IITC Inc., Woodland Hills, CA) was used for all measurements. Mice were acclimatized for 30 min to a designated quiet area before being transferred to prewarmed restrainers and placed into a warming chamber heated to 34°C. Mice acclimatized to the restrainers and tail cuffs for an additional 5–10 min. SBP measurements were derived from pressure tracings by two independent, blinded investigators.
Immunofluorescence
After euthanization, kidneys were collected and cut sagittally into halves. Kidney halves were fixed in 10% buffered formalin solution (Sigma, St. Louis, MO) for 48 h. Following fixation, kidney halves were washed in 100% ethanol and embedded in paraffin. The paraffinized tissues were cut into 5–7 μm sections and adhered to slides. Sections were deparaffinized, rehydrated, and permeabilized with 0.1% Triton solution (BioRad, Hercules, CA). Sections were then blocked with 10% AquaBlock solution (EastCoastBio, North Berwick, ME) prior to being immunolabeled with lymphatic endothelial cell markers podoplanin (PDPN; goat polyclonal; R&D Systems, Minneapolis, MN) and Lyve1 (goat polyclonal; R&D Systems), in addition to α smooth muscle actin (αSMA; rabbit polyclonal; Invitrogen, Carlsbad, CA) by overnight incubation at 4°C. Samples were incubated with Alexafluor 594 (donkey antigoat; Life Technologies, Carlsbad, CA) and Alexafluor 488 (donkey antirabbit; Life Technologies) for an hour at room temperature to achieve lymphatic vessel visualization. Negative controls were incubated with only a secondary antibody. All labeled slides were mounted with ProLong Gold antifade reagent with DAPI (Invitrogen, Carlsbad, CA). Sections were imaged using an Olympus Q5 camera with an Olympus BX51 fluorescence microscopy system. Images were captured at 20× magnification using Olympus CellSens imaging software (Olympus, Shinjuju, Tokyo, Japan). Representative images were acquired at 20× magnification on an Olympus Fluoview FV3000 confocal laser scanning microscope system using Fluoview FV31S-SW imaging software v2.6.1.243 (Olympus).
Renal immunofluorescence quantification
From each kidney section, 5–8 images were collected at 20× from predetermined areas, generally avoiding tissue defects, minor calyx, and large numbers of glomeruli. ImageJ was used to collect area values measuring the total number of PDPN+ pixels after the threshold was set for positive endothelium.
qRT-PCR
Kidneys were decapsulated, cut into quarters, and flash frozen in liquid nitrogen prior to storage at −80°C. Kidney quarters were dissociated, and total RNA was isolated using the Quick-RNA Miniprep Plus Kit (Zymo Research, Irvine, CA), using the manufacturer’s instructions. Samples underwent reverse transcription and cDNA synthesis of 0.5 μg total RNA using the RT2 First Strand Kit (Qiagen, Hilden, Germany) and following the manufacturer’s instructions. Kidney cDNA was combined with PowerUp SYBR Green Master Mix (Applied Biosystems, Waltham, MA), nuclease-free water (Invitrogen, Carlsbad, CA), and primers (10 μM; IDT, Coralville, IA; Sigma, St. Louis, MO) for qPCR reactions. Reactions of 10 μL volumes were plated into a 384-well plate and ran in duplicate in a 7900HT Fast Real-Time PCR Thermal Cycler (Applied Biosystems). Gene expression fold-changes were calculated using the comparative Ct (ΔΔCT) method, using ribosomal protein S18 (RPS18) as the endogenous control. Forward and reverse primer sequences were generated using NCBI Gene DataBase and PrimerBank. These sequences are listed in Online Table IV.
Serum and urine collection and measures
Mice acclimated overnight to single capacity metabolic collection cages (Hatteras, Cary, NC). The following morning, urine collection tubes were replaced, and urine was collected over a 24-h period immediately prior to euthanization. Blood was collected via the left ventricle and serum was isolated and stored with the urine samples at −80°C until analysis. Serum and urine were analyzed by capillary electrophoresis for sodium, potassium, and chlorine concentrations using a DxC 700 AU Chemistry Analyzer (Beckman Coulter, Brea, CA) and by direct potentiometry for creatinine using a P/ACE MDQ Plus Capillary Electrophoresis System (Sciex, Redwood City, CA). These measures were used to calculate urinary excretion, fractional excretion of sodium, and creatinine clearance.
Flow cytometry
After euthanization, kidneys were decapsulated and minced thoroughly before being placed in digestion buffer containing Collagenase D (2.5 mg/mL; Roche Sigma, St. Louis, MO) and Dispase II (1 mg/mL; Sigma). Spleens were placed in digestion buffer lacking enzymes. Kidneys incubated at 37°C for 35 min with constant disruption provided by a gentleMACS™ Octo Dissociator with heaters (Miltenyi Biotec, Bergisch Gladbach, Germany), while spleens incubated at room temperature for 1 min with constant disruption. All digested samples were filtered and rinsed through 100 and 40 μm strainers. Flow-through was centrifuged to isolate cellular components, and red blood cells were lysed in Ammonium-Chloride-Potassium (ACK) Lysing Buffer (Life Technologies). Renal cells were resuspended in 0.1% FBS solution and incubated with an antimouse CD16/CD32 antibody (BD Pharmingen, San Jose, CA) for 10 min on ice to prevent nonspecific Fc binding. Cell solutions from each sample were split into thirds by volume and assigned to one of three antibody panels. Depending on what antibody panel cells were assigned to, they were stained with an appropriate viability stain: Ghost Dye Violet 510, Ghost Dye Red 710 (Tonbo Biosciences, San Diego, CA), or Zombie Red Fixable Viability Kit (BioLegend, Inc., San Diego, CA). All cells were incubated with their assigned viability dye for 30 min on ice. Cells were then incubated with fluorescently conjugated antibodies against CD45, Cd11b, CD11c, Gr1 (Ly6G+Ly6G), F4/80, CD64, CD38, and CD206; CD3e, CD8, gdTCR, NK1, and CD19; or CD45, CD4, CD25, CD44, and CD62L, depending on their panel assignment (see Online Table V for the descriptive panel). Cells assigned to the panel containing CD4 underwent intracellular staining using a Foxp3/Transcription Factor Staining Buffer Set (eBioscience, Inc., San Diego, CA). These cells were incubated with antibodies against Ifng, IL4, Foxp3, TNFa, and IL17 for 30 min on ice. Upon completion of staining, cells were washed, resuspended in 0.1% FBS solution, and filtered through sterile 35 μm strainers. A BD LSR Fortessa X-20 flow cytometer with FACS DIVA software (BD Biosciences, San Jose, CA) was used for acquisition. Populations of up to 500000 cells were analyzed using FlowJo v10.8 (FlowJo, LLC, Ashland, OR). Gating strategies for each panel are outlined in Supplementary Figures 9–11. Results are expressed as a percentage of total CD45+ cells per kidney and spleen. Antibody compensation controls were created using UltraComp eBeads Compensation Beads and viability dye compensation controls were created using an ArC Amine Reactive Compensation Bead Kit (Invitrogen).
Statistics
Results are presented as dot plots with means or bar graphs showing mean ± SEM. Differences between two groups were assessed by a two-tailed unpaired Student’s t-test. The significance level was set at 0.05 for all comparisons. All statistical analyses were performed using SigmaPlot 10.0 software (Systat, San Jose, CA).
Study approval
All procedures performed in mice were approved by the Texas A&M University IACUC in accordance with the NIH Guide for the Care and Use and Care of Laboratory Animals.
Results
Renal-specific lymphangiogenesis was successfully induced in KidVD+ A2HTN, SSHTN, and LHTN mice
To observe the effects of renal lymphangiogenesis in different types of HTN, three mouse models of HTN were utilized: A2HTN, SSHTN, and LHTN. To establish A2HTN, mice were subcutaneously implanted with an osmotic pump that released angiotensin II over a 4-week period. SSHTN mice were given L-NAME in their drinking water for 2 weeks, followed by a 2-week washout period. Then, SSHTN was induced by administration of a 4% salt diet over a 4-week period. LHTN was established by administering L-NAME in the drinking water for 4 weeks. All KidVD- and KidVD+ mice were given doxycycline 1 week following the induction of HTN, but this only induces renal-specific VEGF-D overexpression in KidVD+ mice. Doxycycline was continued until euthanization to keep this signaling constant. There were no significant differences in body weight between groups as a whole; however, KidVD+ mice had significantly increased spleen weight-to-body weight ratios and decreased left and right kidney weight-to-body weight ratios compared with KidVD- mice (Online Tables I, II, and II). Immunofluorescent labeling for the lymphatic marker PDPN demonstrated that across all three models of HTN, KidVD+ kidneys had significantly more lymphatic vessel density (Figure 1A). ImageJ quantification of PDPN+ pixels confirmed these visual observations (Figure 1B). Serial kidney sections stained with lymphatic vessel endothelial hyaluronan receptor 1 (LYVE1) are accessible in the data supplement (Supplementary Figure 1). Across all models of HTN, KidVD+ mice displayed significantly increased renal expression of the lymphatic markers Lyve1, Prox1, and Pdpn; the lymphangiogenic factors Vegfd and Vegfr3; and the immune cell-lymphatic homing genes Ccl21 and Ccr7 (Figure 1C). All RT-qPCR primer sequences are described in the data supplement (Online Table IV).
Figure 1. Renal lymphangiogenesis was successfully induced in KidVD+ mice with A2HTN, SSHTN, and LHTN.
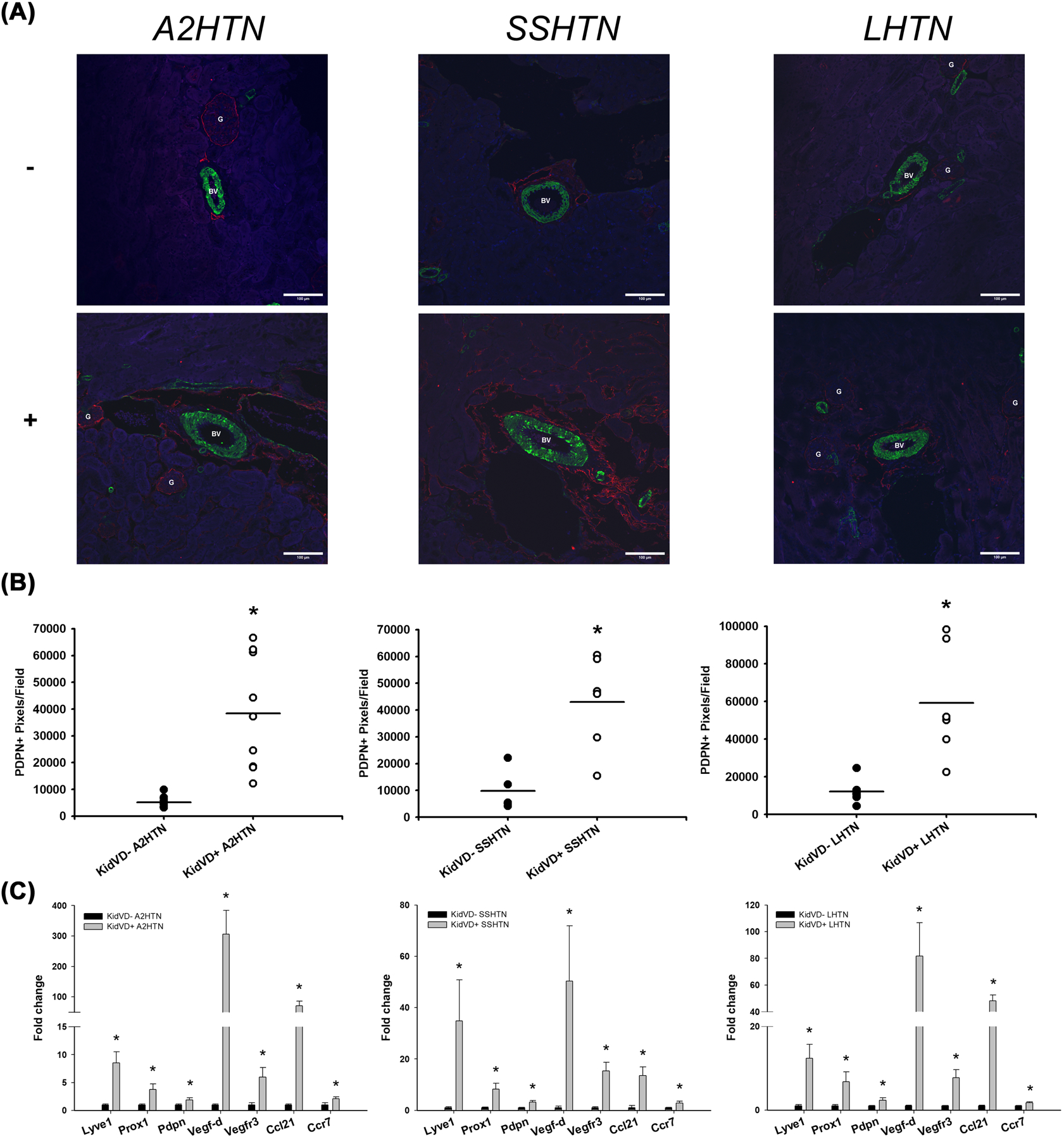
(A) PDPN immunofluorescence in the kidneys of male and female A2HTN, SSHTN, and LHTN mice, including those who underwent renal-specific lymphangiogenesis (KidVD+) and their littermates who did not experience renal lymphangiogenesis (KidVD-). (B) Quantification of PDPN+ pixels in kidneys from KidVD- and KidVD+ mice. (C) mRNA expression of lymphatic gene markers in kidneys from KidVD- and KidVD+ hypertensive mice. Images were taken at 20×. PDPN is labeled red, aSMA is labeled green, and DAPI is labeled blue. Scale bars = 100 μm. Results are expressed as dot plots with mean or bar graphs with mean + SEM (n=6–13 per group) and statistical analyses were performed with Student’s t-test. *P<0.05 vs KidVD- mice.
Renal-specific lymphangiogenesis attenuated SBP in KidVD+ A2HTN, SSHTN, and LHTN mice
All three mouse models developed HTN following 1 week of receiving their assigned HTN stimulus (Figure 2). KidVD+ mice in each HTN model began to experience a significant decrease in SBP over the weeks following VEGF-D overexpression. By week 4 of HTN treatment, KidVD+ mice had significantly decreased SBP when compared with their KidVD- counterparts (Figure 2).
Figure 2. Renal lymphangiogenesis decreased SBP in KidVD+ mice with A2HTN, SSHTN, and LHTN.

SBP measures in male and female KidVD- and KidVD+ mice with A2HTN, SSHTN, and LHTN, presented as (A) group averages and (B) individual data points. Blood pressures were taken weekly via tail cuff. Results are expressed as mean ± SEM (n=6–13 per group) and statistical analyses were performed with Student’s t-test. *P<0.05 vs KidVD- mice.
Attenuation of A2HTN, SSHTN, and LHTN by renal lymphangiogenesis in KidVD+ mice was associated with a decrease in proinflammatory renal immune cells
To assess how renal immune cell populations were impacted by renal-specific lymphangiogenesis, immune cells were isolated from decapsulated kidneys, stained for either CD4-lineage T cells, CD8-lineage T cells, or innate immune cells, and analyzed by flow cytometry. Although KidVD+ mice from all hypertensive models exhibited an increase in total renal CD45+ immune cells, they experienced a decrease in traditionally proinflammatory renal immune cells, such as F4/80-CD11c+CD38+ activated dendritic cells (DCs) and CD11b+ myeloid cells (Figure 3). Additionally, KidVD+ A2HTN and KidVD+ SSHTN mice experienced a decrease in CD4+CD62L-CD44+ effector memory T (TEM) cells (Figure 3). CD4+ helper T cells were increased in A2HTN KidVD+ and SSHTN KidVD+ mice but decreased in LHTN KidVD+ mice (Figure 3).
Figure 3. Renal lymphangiogenesis increases overall renal immune cells but decreases proinflammatory renal immune populations.

Flow cytometric data examining renal immune cell populations in KidVD- and KidVD+ mice with A2HTN, SSHTN, and LHTN. CD45+ renal immune cell populations are shown as percentages of total live single cells, while all other populations are shown as percentages of CD45+ cells. Results are expressed as dot plots and mean ± SEM (n=3–9 per group) and statistical analyses were performed with Student’s t-test between KidVD- and KidVD+. *P<0.05 vs KidVD- mice.
Despite the decrease in proinflammatory immune cells, A2HTN and SSHTN KidVD+ mice did experience an increase in CD11b+F4/80+CD206-CD11c+ M1 macrophages and CD8+ cytotoxic T cells, but these groups also exhibited a significant decrease in renal gamma-delta CD8+ T cells (Supplementary Figures 2 and 3). Additional changes in renal immune cell populations are displayed in the data supplement (Supplementary Figures 2 and 3). Lastly, there were some significant changes in splenic populations for both innate immune cells (Supplementary Figure 4) and T cells (Supplementary Figure 5) in KidVD+ mice compared with KidVD- mice. Antibody panels are described in the data supplement (Online Table V), along with their associated gating strategies (Supplementary Figures 6–8).
Attenuation of A2HTN, SSHTN, and LHTN by renal lymphangiogenesis in KidVD+ mice was associated with various cytokine and chemokine changes
To assess the degree of renal inflammation in mice with and without genetically induced renal lymphatic expansion, qPCR was performed for cytokine and chemokine gene markers. KidVD+ mice in all hypertensive models experienced an increase in Tgfb1 and Tgfb3 expression (Figure 4). Aside from these general results, there were a few isolated changes: the KidVD+ SSHTN mice had higher expression of Mcp1 compared with their KidVD- counterparts, and KidVD+ LHTN mice expressed Il1b at a higher level than their KidVD- counterparts (Figure 4).
Figure 4. Renal lymphangiogenesis increases TGFβ1 and TGFβ3 mRNA expression.

mRNA expression changes in cytokine and chemokine marker genes in kidneys from KidVD- and KidVD+ mice with A2HTN, SSHTN, and LHTN. Results are expressed as mean + SEM (n=6–13 per group) and statistical analyses were performed with Student’s t-test. *P<0.05 vs KidVD- mice.
Attenuation of A2HTN, SSHTN, and LHTN by renal lymphangiogenesis in KidVD+ mice was associated with an improvement in renal sodium handling
To investigate how renal lymphangiogenesis impacted sodium handling, qPCR was conducted for sodium transporter mRNA expression. KidVD+ A2HTN and KidVD+ SSHTN mice exhibited a decrease in expression of Ncc, Nhe3, and Enac a (Figure 5A). These findings are consistent with the previous results from our laboratory for salt-challenged KidVD+ mice, but newly noted for attenuated SSHTN and A2HTN [24]. KidVD+ LHTN mice did not experience these changes, although Ncc expression trended toward a decrease (Figure 5A).
Figure 5. Renal lymphangiogenesis improves renal sodium handling.
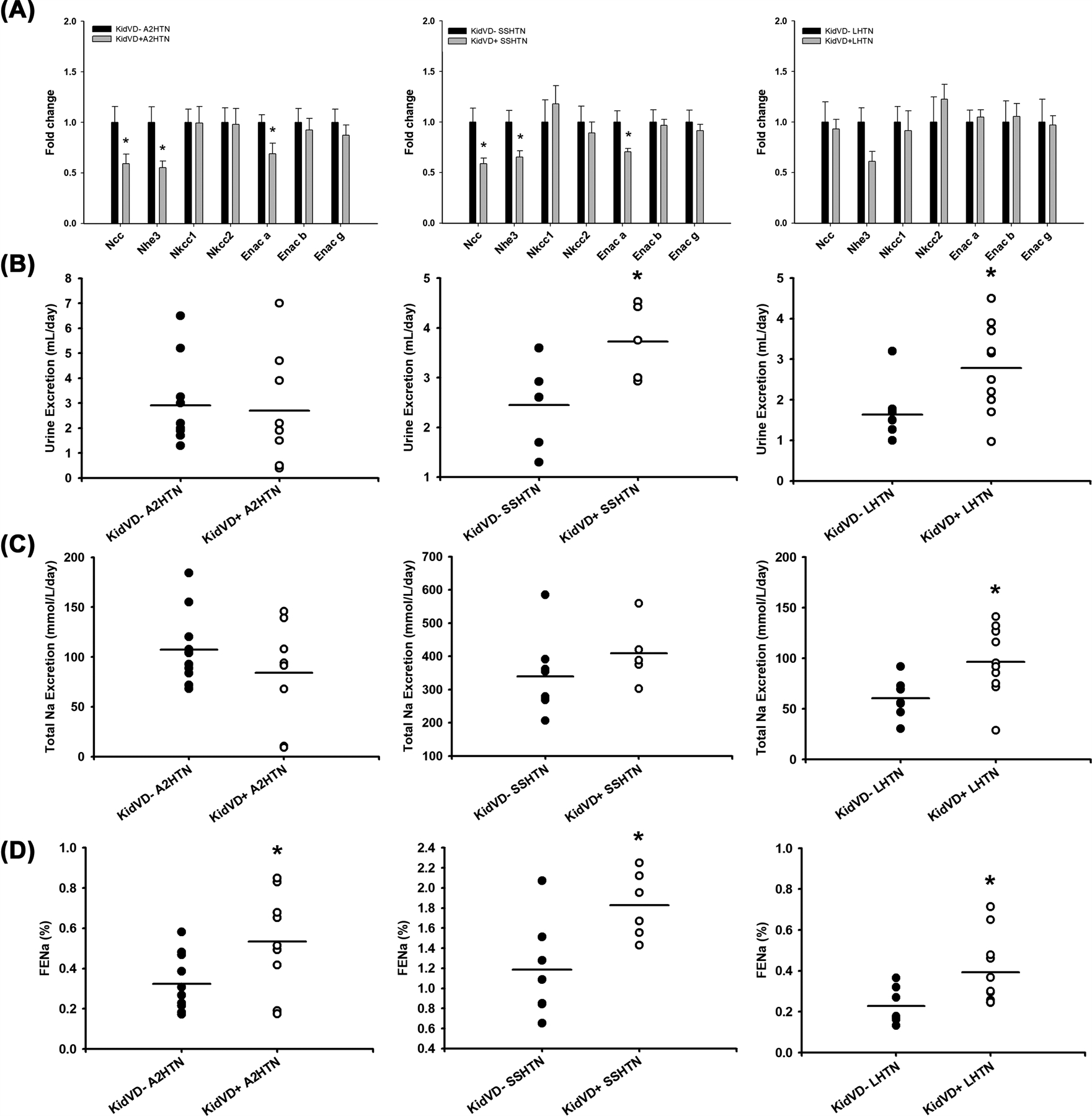
(A) mRNA expression of renal sodium transporters, (B) 24-h urine excretion, (C) 24-h sodium excretion, and (D) fractional excretion of sodium (FENa) in KidVD- and KidVD+ mice with A2HTN, SSHTN, and LHTN. Results are expressed as mean + SEM or dot plots with mean (n=6–11 per group) and statistical analyses were performed with Student’s t-test. *P<0.05 vs KidVD- mice.
Over 24 h, KidVD+ SSHTN and KidVD+ LHTN mice excreted a higher volume of urine than their KidVD- counterparts (Figure 5B), and KidVD+ LHTN mice had an increased sodium output (Figure 5C). KidVD+ mice in all hypertensive models had a significantly increased FENa (Figure 5D). There were no notable changes in potassium or sodium concentrations in the serum or urine in any groups (Supplementary Figure 8). KidVD+ A2HTN mice demonstrated an increase in serum creatinine concentration compared with KidVD- A2HTN mice (Supplementary Figure 10A). There were no differences in urinary creatinine between groups (Supplementary Figure 10B). KidVD+ SSHTN mice exhibited increased serum chlorine concentration compared with KidVD- SSHTN mice (Supplementary Figure 10C). There were no differences in urinary chlorine between groups (Supplementary Figure 10D). KidVD+ A2HTN mice demonstrated a significant decrease in creatinine clearance rate, but no other groups experienced a change (Supplementary Figure 11).
Discussion
In the present study, we utilized a genetically engineered mouse model to induce renal-specific lymphangiogenesis in male and female mice with three forms of established HTN. Three weeks of renal lymphangiogenesis significantly decreased SBP in mice with A2HTN, SSHTN, and LHTN, and this was accompanied by beneficial alterations in renal immune cell populations and a significant increase in FENa.
We have reported previously that renal lymphangiogenesis decreases SBP in mice with LHTN, and results from the current study support that finding [24]. Additionally, we have explored the SBP effects, which result from renal-specific lymphangiogenesis in other translational HTN models, along with potential mechanisms that caused the beneficial SBP effects. Together, these results suggest that inducing renal lymphangiogenesis may be a promising antihypertensive therapeutic strategy.
The immune system is a well-known participant in the establishment, progression, and maintenance of HTN. Under HTN conditions, immune cells infiltrate and accumulate in the kidney. Specific mechanisms regarding the immune system’s involvement in HTN remain obscure; however, it is known that certain antigen-presenting cells, such as activated DCs, are required for initiation and maintenance of HTN [25,26]. DCs play a large role in initiating T-cell activation and interacting with T cells to produce TEM cells. DCs are activated directly by pathogen molecules and indirectly by interactions with innate, cytokine-releasing immune cells; however, DCs can also be activated by sodium. Sodium enters DCs through amiloride-sensitive channels, including the α-subunit of the epithelial sodium channel (ENaCα), which eventually results in the formation of immunogenic isolevuglandin (IsoLG)-protein adducts [27]. As previously noted, HTN induces conditions of salt retention and in the present study, renal-specific lymphangiogenesis was associated with an increased FENa, decreased expression of Enac a, and decreased activated DCs. Therefore, it is possible that the observed decrease in retained sodium in KidVD+ mice could be directly related to the decrease in activated DCs.
In addition to the decrease in activated DCs, KidVD+ mice in all hypertensive groups had decreased myeloid cells and CD11blowCD11c- macrophages. Myeloid cells, including macrophages, DCs, and monocytes, have been known to accumulate in the kidney under hypertensive conditions and play important roles in the pathogenesis of HTN by secreting proinflammatory cytokines and altering renal solute transport [28–30]. Our laboratory, as well as many others, have observed decreased renal macrophage and DC populations in models of attenuated HTN previously [21,22,31–34]. KidVD+ A2HTN and KidVD+ SSHTN mice also demonstrated a decrease in TEM cells, which have been implicated in the development of HTN [35].
Despite not being labeled as part of the immune system, lymphatics play a vital role in modulating the immune response. Lymphatic endothelial cells mediate peripheral tolerance by archiving antigens for later presentation and directly interact with DCs to inhibit DC maturation and DC-mediated T-cell activation [36–39]. Lymphatic vessels enable peripheral tissues to communicate with the immune system by facilitating transport of antigens and activated immune cells to draining lymph nodes. At the lymph node, immunosurveillance is conducted and additional immune responses, such as lymphocyte activation, can be initiated. CCL21/CCL19-mediated chemotaxis allows leukocytes expressing chemokine receptor CCR7 to enter lymphatic vasculature for transport [40]. Myeloid cells, which were decreased in KidVD+ mice, largely express the chemokine receptor CCR7, which binds to its ligand CCL21 to initiate lymphatic trafficking of immune cells to lymph nodes. Expression of both Ccr7 and Ccl21 was found to be significantly elevated in the kidneys of KidVD+ mice from all hypertensive groups, further indicating improved immune cell trafficking in mice with augmented renal lymphatic vasculature. The observed decrease in left and right kidney weight-to-body weight ratios in all KidVD+ mice also support improved lymphatic-mediated immune cell trafficking and could support a decrease in inflammation. This is further evidenced by the significant increase in renal Tgfb1 and Tgfb3 expression, which may have potent renal anti-inflammatory and protective effects [41].
In the current study, A2HTN and SSHTN mice that underwent renal-specific lymphangiogenesis exhibited an increase in certain proinflammatory immune cells, such as M1 macrophages and cytotoxic T cells, and LHTN mice that underwent renal-specific lymphangiogenesis saw a decrease in anti-inflammatory immune cell populations, such as helper T cells. It is worth reiterating that renal lymphangiogenesis did not fully rescue HTN, but only attenuated SBP. Due to the heavy involvement of T lymphocytes and M1 macrophages in HTN, it is possible that these cell populations were not cleared to the same level as other immune cells. It would be of interest to observe longer-term effects of renal lymphangiogenesis on these cell populations.
It is well established that sodium retention is directly related to HTN. Increased dietary sodium is linked to cardiovascular disease, and it has been observed that decreasing sodium intake can lower mean arterial pressure in hypertensive adults [42,43]. Salt sensitivity, a condition in which kidneys react abnormally to increased salt intake and retain excess salt, is associated with insulin resistance, renal disease, left ventricle hypertrophy, and a higher risk for cardiovascular events in hypertensive patients [44–46]. Given the health risks of salt sensitivity, many studies have investigated causes and heritability of salt sensitivity, the mechanism by which it operates, comorbidities and associated factors, and potential treatments. The pressure natriuresis mechanism reported by Guyton and Coleman can be seen in the current study. KidVD+ mice with attenuated HTN had a concomitant increase in urine excretion, accompanied by an increase in FENa. This improvement in renal sodium handling could be a contributing factor to the decrease in SBP in mice with an augmented renal lymphatic network.
Limitations for the present study include the combination of males and females for each HTN group. We previously combined males and females in the case of LHTN and SSHTN, as these types of HTN do not produce sex-specific SBP effects or differences in HTN-induced renal lymphatic vessel density [21]. Female mice are generally better protected from A2HTN than male mice; however, they experience many of the same effects. Following 14 days of angiotensin II infusion at a rate of 1.5 mg/kg per day, Leite et al. did not observe a difference in mean arterial blood pressure between male and female mice [47]. A previous study from our laboratory reported that, despite females having a lower-average SBP, both male and female mice are severely hypertensive following 3 weeks of A2HTN and experienced a similar degree of renal lymphatic expansion [22]. Given this information, we opted to combine males and females to keep the n-values per group high enough to see trends specific to the disease condition and treatment. Combining males and females allowed us a more accurate comparison with the world’s current HTN population; however, there is a chance that combining males and females could obscure a sex-specific effect related to the blood pressure mechanism. Within the present study, only induced models of HTN were examined. As there is some heritability associated with high blood pressure, investigating a genetic model of HTN (i.e., BPH/2 mice) to see if similar effects are observed would be of interest and enhance future studies. The present study was also limited using a tail-cuff blood pressure system. We opted for tail-cuff measurements due to the noninvasive nature. It has been shown that tail-cuff measurement of SBP is reliable enough to determine the presence or absence of HTN when the operator is well-trained and aware of potential limitations [48–50]. We take great care in preparing and handling mice for these measurements to achieve the most accurate results possible.
In conclusion, genetically induced renal lymphatic augmentation was able to significantly decrease blood pressure, improve renal immune cell populations, and improve renal sodium handling in male and female mice with three different forms of established HTN. Targeting renal lymphatics may be an alternative strategy to reduce inflammation, improve renal health, and decrease HTN.
Supplementary Material
Clinical perspectives.
Currently available treatments for HTN are unable to stabilize and maintain blood pressure for all patients, leaving many to struggle with uncontrolled blood pressure.
Augmenting renal lymphatic vasculature in three hypertensive mouse models lowered blood pressure, improved renal sodium handling, and produced beneficial changes in renal immune cell populations.
These results identify renal-specific lymphangiogenesis as a potential therapy for hypertensive patients.
Acknowledgements
The authors would like to acknowledge Andrea Reyna, Heidi Creed, and Gaurav Baranwal for assistance with mouse husbandry and Braden Sims, Daniela Avilez, Marissa Henley, Miranda Albee, and Emma Pickup for technical assistance. The authors acknowledge UT Southwestern George M. O’Brien Kidney Research Core Center (NIH P30DK079328) for providing creatinine measurements and expertise, along with the Texas A&M Integrated Microscopy and Imaging Laboratory for providing access to microscopes and imaging expertise.
Funding
This work was supported by the (National Institutes of Health [grant number RO1 DK120493] and Texas A&M University Triads for Transformation T3 to B.M.M.) and the (National Institutes of Health [grant number RO1 DK119497] and receives support from DK120493 to J.M.R.).
Abbreviations
- A2HTN
angiotensin II-induced hypertension
- DC
dendritic cell
- FBS
fetal bovine serum
- FENa
fractional excretion of sodium
- HTN
hypertension
- KidVD
kidney-specific overexpression of VEGF-D
- LHTN
L-NAME-induced hypertension
- L-NAME
nitro-l-arginine methyl ester hydrochloride
- PDPN
podoplanin
- SBP
systolic blood pressure
- SSHTN
salt-sensitive hypertension
- TEM
effector memory T
- VEGF-D
vascular endothelial growth factor D
Footnotes
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
CRediT Author Contribution
Bethany L. Goodlett: Data curation, Formal Analysis, Investigation, Writing—original draft. Dakshnapriya Balasubbramanian: Data curation, Formal Analysis, Investigation. Shobana Navaneethabalakrishnan: Data curation, Formal Analysis, Investigation. Sydney E. Love: Data curation, Formal Analysis, Investigation. Emily M. Luera: Data curation, Formal Analysis, Investigation. Sunitha Konatham: Data curation, Formal Analysis, Investigation. Valorie L. Chiasson: Data curation, Formal Analysis, Investigation. Sophie Wedgeworth: Data curation, Formal Analysis, Investigation. Joseph M. Rutkowski: Conceptualization, Resources, Formal Analysis, Supervision, Project administration, Writing—review & editing. Brett M. Mitchell: Conceptualization, Supervision, Funding acquisition, Project administration, Writing—review & editing.
Data Availability
The data, analytic methods, and study materials that support the findings of the present study are available from the corresponding author upon reasonable request.
References
- 1.Whelton PK, Carey RM, Aronow WS, Casey DE Jr., Collins KJ, Dennison Himmelfarb C et al. (2018) 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 138, e484–e594 [DOI] [PubMed] [Google Scholar]
- 2.Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP et al. (2019) Heart disease and stroke statistics-2019 update: a report from the American Heart Association. Circulation 139, e56–e528, 10.1161/CIR.0000000000000659 [DOI] [PubMed] [Google Scholar]
- 3.CDC (2021) Hypertension Cascade: Hypertension Prevalence, Treatment and Control Estimates among US Adults Aged 18 Years and Older Applying the Criteria from the American College of Cardiology and American Heart Association’s 2017 Hypertension Guideline—NHANES 2015–2018, US Department of Health and Human Services, Atlanta, GA [Google Scholar]
- 4.Lippi G, Wong J and Henry BM (2020) Hypertension in patients with coronavirus disease 2019 (COVID-19): a pooled analysis. Pol. Arch. Intern. Med 130, 304–309 [DOI] [PubMed] [Google Scholar]
- 5.Dahl LK, Heine M and Thompson K (1974) Genetic influence of the kidneys on blood pressure. Evidence from chronic renal homografts in rats with opposite predispositions to hypertension. Circ. Res 34, 94–101, 10.1161/01.RES.34.1.94 [DOI] [PubMed] [Google Scholar]
- 6.Dahl LK and Heine M (1975) Primary role of renal homografts in setting chronic blood pressure levels in rats. Circ. Res 36, 692–696, 10.1161/01.RES.36.6.692 [DOI] [PubMed] [Google Scholar]
- 7.Bianchi G, Fox U, Di Francesco GF, Giovanetti AM and Pagetti D (1974) Blood pressure changes produced by kidney cross-transplantation between spontaneously hypertensive rats and normotensive rats. Clin. Sci. Mol. Med 47, 435–448, 10.1042/cs0470435 [DOI] [PubMed] [Google Scholar]
- 8.Cowley AW, Miller JP and Guyton AC (1971) Open-loop analysis of the renin-angiotensin system in the dog. Circ. Res 28, 568–581, 10.1161/01.RES.28.5.568 [DOI] [Google Scholar]
- 9.Guyton AC and Coleman TG (1967) Long-term Regulation of the Circulation: Interrelationships with Body Fluid Volumes, W B Saunders Co, Philadelphia, PA [Google Scholar]
- 10.Crowley SD, Song YS, Lin EE, Griffiths R, Kim HS and Ruiz P (2010) Lymphocyte responses exacerbate angiotensin II-dependent hypertension. Am. J. Physiol. Regul. Integr. Comp. Physiol 298, R1089–R1097, 10.1152/ajpregu.00373.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ozawa Y, Kobori H, Suzaki Y and Navar LG (2007) Sustained renal interstitial macrophage infiltration following chronic angiotensin II infusions. Am. J. Physiol. Renal. Physiol 292, F330–F339, 10.1152/ajprenal.00059.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodriguez-Iturbe B, Quiroz Y, Herrera-Acosta J, Johnson RJ and Pons HA (2002) The role of immune cells infiltrating the kidney in the pathogenesis of salt-sensitive hypertension. J. Hypertens. Suppl 20, S9–S14 [PubMed] [Google Scholar]
- 13.Rodriguez-Iturbe B, Quiroz Y, Nava M, Bonet L, Chavez M, Herrera-Acosta J et al. (2002) Reduction of renal immune cell infiltration results in blood pressure control in genetically hypertensive rats. Am. J. Physiol. Renal. Physiol 282, F191–F201, 10.1152/ajprenal.0197.2001 [DOI] [PubMed] [Google Scholar]
- 14.Kriska T, Cepura C, Magier D, Siangjong L, Gauthier KM and Campbell WB (2012) Mice lacking macrophage 12/15-lipoxygenase are resistant to experimental hypertension. Am. J. Physiol. Heart Circ. Physiol 302, H2428–H2438, 10.1152/ajpheart.01120.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S et al. (2007) Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J. Exp. Med 204, 2449–2460, 10.1084/jem.20070657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tian N, Gu JW, Jordan S, Rose RA, Hughson MD and Manning RD Jr (2007) Immune suppression prevents renal damage and dysfunction and reduces arterial pressure in salt-sensitive hypertension. Am. J. Physiol. Heart Circ. Physiol 292, H1018–H1025, 10.1152/ajpheart.00487.2006 [DOI] [PubMed] [Google Scholar]
- 17.Quiroz Y, Pons H, Gordon KL, Rincon J, Chavez M, Parra G et al. (2001) Mycophenolate mofetil prevents salt-sensitive hypertension resulting from nitric oxide synthesis inhibition. Am. J. Physiol. Renal. Physiol 281, F38–F47, 10.1152/ajprenal.2001.281.1.F38 [DOI] [PubMed] [Google Scholar]
- 18.Zhang Q, Lu Y, Proulx ST, Guo R, Yao Z, Schwarz EM et al. (2007) Increased lymphangiogenesis in joints of mice with inflammatory arthritis. Arthritis Res. Ther 9, R118, 10.1186/ar2326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uchiyama T, Takata S, Ishikawa H and Sawa Y (2013) Altered dynamics in the renal lymphatic circulation of type 1 and type 2 diabetic mice. Acta. Histochem. Cytochem 46, 97–104, 10.1267/ahc.13006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kneedler SC, Phillips LE, Hudson KR, Beckman KM, Lopez Gelston CA, Rutkowski JM et al. (2017) Renal inflammation and injury are associated with lymphangiogenesis in hypertension. Am. J. Physiol. Renal. Physiol 312, F861–F869, 10.1152/ajprenal.00679.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lopez Gelston CA, Balasubbramanian D, Abouelkheir GR, Lopez AH, Hudson KR, Johnson ER et al. (2018) Enhancing renal lymphatic expansion prevents hypertension in mice. Circ. Res 122, 1094–1101, 10.1161/CIRCRESAHA.118.312765 [DOI] [PubMed] [Google Scholar]
- 22.Balasubbramanian D, Gelston CAL, Lopez AH, Iskander G, Tate W, Holderness H et al. (2020) Augmenting renal lymphatic density prevents angiotensin ii-induced hypertension in male and female mice. Am. J. Hypertens 33, 61–69, 10.1093/ajh/hpz139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lammoglia GM, Van Zandt CE, Galvan DX, Orozco JL, Dellinger MT and Rutkowski JM (2016) Hyperplasia, de novo lymphangiogenesis, and lymphatic regression in mice with tissue-specific, inducible overexpression of murine VEGF-D. Am. J. Physiol. Heart Circ. Physiol 311, H384–H394, 10.1152/ajpheart.00208.2016 [DOI] [PubMed] [Google Scholar]
- 24.Balasubbramanian D, Baranwal G, Clark MC, Goodlett BL, Mitchell BM and Rutkowski JM (2020) Kidney-specific lymphangiogenesis increases sodium excretion and lowers blood pressure in mice. J. Hypertens 38, 874–885, 10.1097/HJH.0000000000002349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hevia D, Araos P, Prado C, Fuentes Luppichini E, Rojas M, Alzamora R et al. (2018) Myeloid CD11c(+) antigen-presenting cells ablation prevents hypertension in response to angiotensin II plus high-salt diet. Hypertension 71, 709–718, 10.1161/HYPERTENSIONAHA.117.10145 [DOI] [PubMed] [Google Scholar]
- 26.Kirabo A, Fontana V, de Faria AP, Loperena R, Galindo CL, Wu J et al. (2014) DC isoketal-modified proteins activate T cells and promote hypertension. J. Clin. Invest 124, 4642–4656, 10.1172/JCI74084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barbaro NR, Foss JD, Kryshtal DO, Tsyba N, Kumaresan S, Xiao L et al. (2017) Dendritic cell amiloride-sensitive channels mediate sodium-induced inflammation and hypertension. Cell Rep. 21, 1009–1020, 10.1016/j.celrep.2017.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rucker AJ, Rudemiller NP and Crowley SD (2018) Salt, hypertension, and immunity. Annu. Rev. Physiol 80, 283–307, 10.1146/annurev-physiol-021317-121134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Justin Rucker A and Crowley SD (2017) The role of macrophages in hypertension and its complications. Pflugers Arch. 469, 419–430, 10.1007/s00424-017-1950-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu X and Crowley SD (2020) Actions of immune cells in the hypertensive kidney. Curr. Opin. Nephrol. Hypertens 29, 515–522, 10.1097/MNH.0000000000000635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liao TD, Yang XP, Liu YH, Shesely EG, Cavasin MA, Kuziel WA et al. (2008) Role of inflammation in the development of renal damage and dysfunction in angiotensin II-induced hypertension. Hypertension 52, 256–263, 10.1161/HYPERTENSIONAHA.108.112706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fehrenbach DJ and Mattson DL (2020) Inflammatory macrophages in the kidney contribute to salt-sensitive hypertension. Am. J. Physiol. Renal. Physiol 318, F544–F548, 10.1152/ajprenal.00454.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang L, Wang A, Hao Y, Li W, Liu C, Yang Z et al. (2018) Macrophage depletion lowered blood pressure and attenuated hypertensive renal injury and fibrosis. Front. Physiol 9, 473, 10.3389/fphys.2018.00473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu X, Rudemiller NP, Privratsky JR, Ren J, Wen Y, Griffiths R et al. (2020) Classical dendritic cells mediate hypertension by promoting renal oxidative stress and fluid retention. Hypertension 75, 131–138, 10.1161/HYPERTENSIONAHA.119.13667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Itani HA, Xiao L, Saleh MA, Wu J, Pilkinton MA, Dale BL et al. (2016) CD70 exacerbates blood pressure elevation and renal damage in response to repeated hypertensive stimuli. Circ. Res 118, 1233–1243, 10.1161/CIRCRESAHA.115.308111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cohen JN, Guidi CJ, Tewalt EF, Qiao H, Rouhani SJ, Ruddell A et al. (2010) Lymph node-resident lymphatic endothelial cells mediate peripheral tolerance via aire-independent direct antigen presentation. J. Exp. Med 207, 681–688, 10.1084/jem.20092465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Christiansen AJ, Dieterich LC, Ohs I, Bachmann SB, Bianchi R, Proulx ST et al. (2016) Lymphatic endothelial cells attenuate inflammation via suppression of dendritic cell maturation. Oncotarget 7, 39421–39435, 10.18632/oncotarget.9820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maisel K, Sasso MS, Potin L and Swartz MA (2017) Exploiting lymphatic vessels for immunomodulation: rationale, opportunities, and challenges. Adv. Drug. Deliv. Rev 114, 43–59, 10.1016/j.addr.2017.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Podgrabinska S, Kamalu O, Mayer L, Shimaoka M, Snoeck H, Randolph GJ et al. (2009) Inflamed lymphatic endothelium suppresses dendritic cell maturation and function via Mac-1/ICAM-1-dependent mechanism. J. Immunol 183, 1767–1779, 10.4049/jimmunol.0802167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Forster R, Davalos-Misslitz AC and Rot A (2008) CCR7 and its ligands: balancing immunity and tolerance. Nat. Rev. Immunol 8, 362–371, 10.1038/nri2297 [DOI] [PubMed] [Google Scholar]
- 41.Sureshbabu A, Muhsin SA and Choi ME (2016) TGF-beta signaling in the kidney: profibrotic and protective effects. Am. J. Physiol. Renal. Physiol 310, F596–F606, 10.1152/ajprenal.00365.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Whelton PK, Appel LJ, Sacco RL, Anderson CA, Antman EM, Campbell N et al. (2012) Sodium, blood pressure, and cardiovascular disease: further evidence supporting the American Heart Association sodium reduction recommendations. Circulation 126, 2880–2889, 10.1161/CIR.0b013e318279acbf [DOI] [PubMed] [Google Scholar]
- 43.Graudal NA, Hubeck-Graudal T and Jurgens G (2020) Effects of low sodium diet versus high sodium diet on blood pressure, renin, aldosterone, catecholamines, cholesterol, and triglyceride. Cochrane Database Syst. Rev 12, CD004022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Laffer CL and Elijovich F (2013) Differential predictors of insulin resistance in nondiabetic salt-resistant and salt-sensitive subjects. Hypertension 61, 707–715, 10.1161/HYPERTENSIONAHA.111.00423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bihorac A, Tezcan H, Ozener C, Oktay A and Akoglu E (2000) Association between salt sensitivity and target organ damage in essential hypertension. Am. J. Hypertens 13, 864–872, 10.1016/S0895-7061(00)00253-3 [DOI] [PubMed] [Google Scholar]
- 46.Morimoto A, Uzu T, Fujii T, Nishimura M, Kuroda S, Nakamura S et al. (1997) Sodium sensitivity and cardiovascular events in patients with essential hypertension. Lancet 350, 1734–1737, 10.1016/S0140-6736(97)05189-1 [DOI] [PubMed] [Google Scholar]
- 47.Leite APO, Li XC, Hassan R, Zheng X, Alexander B, Casarini DE et al. (2021) Sex differences in angiotensin II-induced hypertension and kidney injury: role of AT1a receptors in the proximal tubule of the kidney. Clin. Sci. (Lond.) 135, 1825–1843, 10.1042/CS20201574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Whitesall SE, Hoff JB, Vollmer AP and D’Alecy LG (2004) Comparison of simultaneous measurement of mouse systolic arterial blood pressure by radiotelemetry and tail-cuff methods. Am. J. Physiol. Heart Circ. Physiol 286, H2408–H2415, 10.1152/ajpheart.01089.2003 [DOI] [PubMed] [Google Scholar]
- 49.Pfeffer JM, Pfeffer MA and Frohlich ED (1971) Validity of an indirect tail-cuff method for determining systolic arterial pressure in unanesthetized normotensive and spontaneously hypertensive rats. J. Lab. Clin. Med 78, 957–962 [PubMed] [Google Scholar]
- 50.Kurtz TW, Griffin KA, Bidani AK, Davisson RL, Hall JE, Aha Council on High Blood Pressure Research P et al. (2005) Recommendations for blood pressure measurement in animals: summary of an AHA scientific statement from the Council on High Blood Pressure Research, Professional and Public Education Subcommittee. Arterioscler. Thromb. Vasc. Biol 25, 478–479, 10.1161/01.ATV.0000153088.15433.8f [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data, analytic methods, and study materials that support the findings of the present study are available from the corresponding author upon reasonable request.


