Abstract
Salmonella can cause severe foodborne diseases. This study investigated the prevalence of Salmonella spp. in fresh foods in Hangzhou market and their harborage of antibiotic resistance and virulence genes, antibiotic susceptibility, and pathogenicity. A total of 500 samples (pork, n = 140; chicken, n = 128; vegetable, n = 232) were collected over a one-year period. Salmonella was found in 4.2% (21) of samples with the detection rate in pork, chicken and vegetables as 4.3% (6), 6.3% (8), and 3% (7), respectively. One Salmonella strain was recovered from each positive sample. The isolates were identified as six serotypes, of which S. Enteritidis (n = 7) and S. Typhimurium (n = 6) were the most predominant serotypes. The majority of isolates showed resistance to tetracycline (85.7%) and/or ciprofloxacin (71.4%). Tetracycline resistance genes showed the highest prevalence (90.5%). The occurrence of resistance genes for β-lactams (blaTEM-1, 66.7%; and blaSHV, 9.5%) and aminoglycosides (aadA1, 47.6%; Aac(3)-Ia, 19%) was higher than sulfonamides (sul1, 42.9%) and quinolones (parC, 38.1%). The virulence gene fimA was detected in 57.1% of isolates. Gene co-occurrence analysis implied that resistance genes were associated with virulence genes. Furthermore, selected S. Typhimurium isolates (n = 4) carrying different resistance and virulence genes up-regulated the secretions of cytokines IL-6 and IL-8 by Caco-2 cells in different degrees, suggesting that virulence genes may play a role in inflammatory transcription. In in vivo virulence test, microbiological counts in mouse feces and tissues showed that all included S. Typhimurium were able to infect mice, with one strain showing significantly higher virulence than others. In conclusion, this study indicates Salmonella contamination in fresh foods in Hangzhou market poses a risk to public health and it should be closely monitored to prevent and control foodborne diseases.
Introduction
Food safety challenged by accelerated economic globalization and trade liberalization has become a global public health issue prompting widespread concern [1]. Salmonella is a critical foodborne pathogen distributed globally among humans, animals, and open environments [2]. It is spread through various paths, including food and water resources, human-to-human, and human-animal contact. Recently, according to the food poisoning statistics of the National Health Insurance Administration, 50–60% of food poisoning is caused by microorganisms, among which, Salmonella has the highest detection rate [3–5]. Salmonella is a diverse gastrointestinal pathogen that can cause a variety of diseases, and its serotypes can be categorized into two main groups—typhoidal and non-typhoidal. Typhoidal serovars (TS) can cause systemic enteric fever, whereas non-typhoidal serovars (NTS) are associated with infections in a wide range of hosts, are usually zoonotic, cause acute and self-limiting gastroenteritis, and can lead to foodborne illness in humans [6, 7]. Salmonella enterica serotypes that cause human disease include S. Typhimurium and S. Enteritidis [8]. Salmonella has been frequently found in meat, especially chicken and pork. In addition, Salmonella infection varies with seasons, climates, years, and geographical locations [9]. Salmonella also is often multidrug-resistant and carries multiple virus-related genes. Drug resistance of Salmonella has become a significant public health concern worldwide [10]. Several reports have been recently published on Salmonella contamination in foods. In Shanghai, China, Ni et al. [11]found that 4.5% of lettuce sold in markets were tested positive for Salmonella. Salmonella detection rates in chicken and pork collected from Henan, China, were 45.2% and 29.2%, respectively, according to Xu et al. [12]. According to Chen et al. [13], Salmonella was found in 67% of pork and 46.2% of chicken samples. The majority of the studies were focused on chicken and pork products.
Antibiotics have been used for treatment, prevention, and growth promotion in animals for decades. However, their misuse has led to the selection for antibiotic-resistant bacteria (ARB) in animals and the environment [14]. ARBs can transfer resistance to bacteria infecting humans through the food chain, gene pools, phages, and DNA fragments [15]. In recent years, researchers found that Antibiotic Resistance Genes (ARGs) can be transmitted through gene levels and mobile elements with the transfer arrays like plasmid, insert sequence, transposon, integron, etc. Thus, ARGs in bacteria can cross the genus boundary and transmit within or between species, spreading between fungi and bacteria [16]. In addition, the pathogenicity of Salmonella in humans and animals is closely related to its virulence factors. Salmonella virulence factors include virulence islands, enterotoxins, fimbriae, and virulence plasmids [17, 18]. Most virulence genes are on virulence islands, and a few are on virulence plasmids [19]. For example, the invA gene is located on the virulence island and is involved in host recognition and invasion of intestinal mucosal epithelial cells [18]. The spvC gene is mainly located on the virulence plasmid and plays a role in the intracellular proliferation and survival of Salmonella within the host [17]. Caco-2 cells are an intestinal epithelial model widely used to study the organization and function of human intestinal cells in vitro [20]. Salmonella can adhere to and invade Caco-2 cells and induce the expression and upregulation of pro-inflammatory cytokines in the cells [21]. IL-6 regulates cell growth and differentiation, regulates immune responses, and is also involved in acute phase responses and hematopoiesis [22]. IL-8 is the major pro-inflammatory CXC chemokine secreted by intestinal epithelial cells in response to bacterial entry [23]. Studies have shown that the amount of intracellular bacteria in the Caco-2 cell line increases with increased inflammation during infection [24]. However, there is no detailed report on whether virulence genes carried by bacteria affect cytokine expression and whether the association between antibiotic resistance and virulence increases pathogenic risk.
In this study, samples of fresh food were collected from the retail markets in Hangzhou, China over a one-year period and detected for Salmonella. The recovered Salmonella isolates were determined for antibiotic resistance, virulence and presence of antibiotic resistance and virulence associated genes. The correlation between antibiotic resistance genes and virulence genes was analyzed.
Materials and methods
Sampling
From March 2020 to February 2021, 500 samples (140 raw minced pork, 128 raw chicken carcasses, and 232 vegetables including cilantro, lettuce, tomatoes, cucumbers, spinach and cabbage) were randomly collected from retail markets in Hangzhou, Zhejiang Province, China, and placed in sterile sampling bags/tubes. All samples were stored at 2–8°C and transported to the laboratory for processing within 24 hours.
Detection and isolation of Salmonella
Salmonella was detected using the method described previously [25]. Briefly, 25 g sample was added to 225 ml of buffered peptone water (BPW; HB4084, Hopebio, China). All samples were incubated at 37°C for 18 h for pre-enrichment. One mL of the pre-enrichment solution was transferred to 9 ml of selenite cysteine broth (SC; HB4085, Hopebio, China) and incubated at 37°C for 24 h for enrichment. SC broth cultures were subsequently taken and streaked onto XLD plates. The plates were incubated at 37°C for 24–48 h. Suspected Salmonella spp. colonies (red colonies with black centers) on the XLD plates were picked for subsequent experiments. Salmonella was initially screened by biochemical assays, namely indole, sugar fermentation assay, production of urease and H2S, and production of lysine and ornithine decarboxylase. Genomic DNA of Salmonella species was extracted by the boiling method described by Coo et al. [26] to be used as template DNA for PCR. The isolates were identified as Salmonella by PCR detection of the specific gene invA, as previously described [27]. A 25 μl of reaction mixture was prepared, containing 12.5 μl 2X Taq PCR Master Mix (manufacture information), 0.5 μl each primer (10 μM), 1.0 μl template DNA, and 10.5 μl ddH2O. PCR condition was as follows: pre-denaturation at 96°C for 5 min; 30 cycles of denaturation at 95°C for 30 s, annealing at 50°C for 90 s, and extension at 72°C for 60 s; extension at 72°C for 5 min; incubation at 12°C. After the program, the PCR product was stored at 4°C. Five μl of PCR amplification product was electrophoresed in a 1.5% agarose gel, visualized, and recorded in Gel Doc XR (Bio-Rad, USA).
Serotyping
The serotypes of the isolated Salmonella were identified by slide agglutination method, and the O antigen and H antigen types were determined, respectively. According to the product manual of Salmonella diagnostic serum and antigenic formulae of the salmonella serovars, the serotype of Salmonella was determined based on the antigenic formula [28].
Antibiotic susceptibility testing
Kirby Bauer disk diffusion method for antimicrobial susceptibility testing was performed on all isolates according to the Clinical and Laboratory Standards Institute guidelines [29]. The 12 antimicrobials tested included ciprofloxacin (5 μg), gentamicin (10 μg), streptomycin (10 μg), erythromycin (15 μg), ampicillin (10 μg), amoxicillin (25 μg), enrofloxacin (5 μg), kanamycin (30 μg), cefotaxime (30 μg), sulfamethoxazole (30 μg), cephalothin (30 μg), tetracycline (30 μg). Antibiotic disks were from Hangzhou Microbial Reagent Company (Zhejiang, China). Inoculated plates were incubated at 37°C for 24 h. Salmonella strains were assessed based on the size of the inhibition zone.
Detection and correlation analysis of ARGs and VGs
All Salmonella isolates were tested for antimicrobial resistance genes, including quinolones (parC), aminoglycosides (aadA1 and Aac(3)-Ia), sulfonamides (sul1), β -lactamase (blaTEM-1 and blaSHV), tetracycline (tet(A) and tet(B)) by PCR. The flagellin fliC gene, fimbrial toxin fimA gene, enterotoxin stn gene, and virulence plasmid spvC gene related to Salmonella pathogenicity were detected. The DNA extraction steps were as described above. PCR reagent concentrations and cycling conditions (including annealing temperature) were consistent with previous studies [30, 31]. Primers are shown in S1 Table.
Co-occurrence and contributor networks of ARGs and VGs were analyzed by R software (X64 3.5.1) with the package of "psych" and "vegan" based on Spearman’s rank correlations (P < 0.05 indicating a statistical significance) and visualized by Gephi.
In vitro virulence test
Caco-2 cell maintenance and preparation
Caco-2 cells are a human colonic epithelial cell line (ATCC, BFB, Shanghai). Cells were seeded into plastic Petri dishes. Cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM, Genomcell, China) supplemented with 10% fetal calf serum (FBS, Genomcell, China) and 1% antibiotics (penicillin/streptomycin, Genomcell) at 37°C. Once the cells reached 90% confluence, cells were trypsinized (0.05%, Genomcell, China) and seeded at the desired density in 96-well tissue culture plates (Nunc, France) containing 200 μL of complete medium per well. Cells were incubated at 37°C for at least 24 h after seeding in a humidified atmosphere containing 5% CO2 to achieve complete confluency.
Determination of Caco-2 invasion by Salmonella
The 96-well plate monolayer was rinsed twice with phosphate-buffered saline (PBS, pH 7.4). A strain of S. Typhimurium, which carried the highest number of types and drug resistance and virulence genes from each season was selected (spring, parC, sul1; summer, tet(A), blaTEM-1, fimA; autumn, aadA1, spvC; winter, aadA1). The suspension of different Salmonella strains was added to washed Caco-2 cells at an initial concentration of 106 CFU/mL. The plates were incubated at 37°C for 4 h in a 5% CO2 incubator. They were maintained for 1 h in DMEM (Genomcell, China) containing 50 μg/mL gentamicin per well to inactivate extracellular bacteria. After incubation, the infected cells were lysed by adding 1% Triton X-100 (Sigma). Lysates were plated on LB plates and incubated at 37°C for 24 h. The invasion rate was calculated as the percentage of invading bacteria versus initial bacterial addition.
Total RNA isolation, cDNA synthesis, and real-time PCR
Caco-2 cell maintenance and preparation and Salmonella infection of Caco-2 cells were performed as described above. According to the manufacturer’s instructions, total RNA was extracted from cells using RNAiso Plus (Takara, Japan). The RNA was reverse transcribed with a HiScript II Q RT SuperMix for qPCR (Vazyme, China). The resulting cDNA was quantified by RT-PCR using AceQ qPCR SYBR Green Master Mix (Vazyme, China). The RT-PCR reaction mixture contained 2 μl cDNA, 0.8 μl of different primers (concentration of primers), and 10 μl SYBR Green Master Mix, with a total volume of 20μl. The reaction program was as follows: 15 min at 95°C; followed by 40 cycles of 5 s at 95°C, 30 s at 60°C and 30 s at 72°C in 96-well plates with the ABI 9700HT Sequence Detection System (Applied Biosystems, USA). Using the human GAPDH gene as an internal reference, the relative expression of each related gene mRNA was calculated by the 2-ΔΔCt method. Primers are shown in S1 Table.
In vivo virulence test
Infection model
Six-week-old male mice were provided by Ziyuan Laboratory Animal Technology Co., Ltd (Hangzhou, China), free of pathogens and weighing 20–25 g. Before testing, mice were kept in a controlled environment with a 12 h light, 12 h dark cycle at 22°C, with food and water provided ad libitum. A total of 30 mice were divided into 5 groups, one group was given only sterile saline solution (0.9% w/v) and 4 experimental groups were inoculated with the target strain. Mice were fasted for 12 h before inoculation and subsequently, 1 ml of bacterial suspension (1x108 CFU/ml) was administered by the oral needle. After inoculation (day 0), food and water were provided ad libitum. On day 5, the mice were executed by breaking their necks. Immediately after the execution, the ileum, liver, and spleen were removed and microbiological analysis was performed. The breeding and use of mice were supervised by the Laboratory Animal Welfare and Ethics Committee of Hangzhou Normal University under the ethical approval number HSD20211201.
Clinical observations
Animal weights were measured every 24 h. To lessen the initial weight change for each animal, the baseline weight was recorded on day 0 and the value was deducted from the weight on the following days.
Microbiological analyses
Feces samples, liver, spleen, and ileal tissues were mixed with sterile saline-peptone solution (w/v, 1:10) and homogenized. A proportion of 0.2 ml of the dilution was applied to bright green agar (BGA) plates. Plates were incubated at 37°C for 24 h for bacterial counting.
Statistical analysis
Statistical analysis was conducted using SPSS 26.0. The differences between groups/strains were assessed by Chi-square test/ANOVA with p < 0.05 regarded as significant difference. Data was visualized using Origin 2021.
Results
Prevalence and serotypes of Salmonella
Salmonella contamination in different fresh foods collected at different seasons was investigated. A percentage of 4.2% samples (21/500) was found to have Salmonella (Table 1). One Salmonella isolate was recovered from each positive sample. The difference of Salmonella detection rate was not statistically different among food types or seasons. However, both pork (4.3%, 6/140) and chicken (6.3%, 8/128) showed numerically higher detection rate of Salmonella compared to vegetables (3%, 7/232). The detection rate of Salmonella in summer (5.9%, 8/135) was numerically higher than that in other seasons (spring, 4.2%, 5/120, autumn, 3.9%, 5/130, winter, 3/115).
Table 1. The prevalence of Salmonella.
| Pork | Chicken | Vegetable | Total | |
| Positive number | 6/140 | 8/128 | 7/232 | 21/500 |
| Positive rate (%) | 4.3 | 6.3 | 3.0 | 4.2 |
| Season | Month | Total number | Positive number (%) | |
| Spring | 2–5 | 120 | 5 (4.2) | |
| Summer | 6–8 | 135 | 8 (5.9) | |
| Autumn | 9–11 | 130 | 5 (3.9) | |
| Winter | 12–2 | 115 | 3 (2.6) | |
The distribution of Salmonella serotypes from different seasons, fresh meat, and vegetables is shown in Fig 1. Among 21 Salmonella isolates, 6 Salmonella serotypes were identified. The most predominant serotype was S. Enteritidis (n = 7, 33.3%), followed by S. Typhimurium (6, 28.6%), S. Derby (4, 19%), S. Lexington (2, 9.5%), S. Infantis (1, 4.8%), and S. Anatum (1, 4.8%). Of the 6 different Salmonella serotypes, 5, 5 and 3 types were isolated from pork, chicken and vegetables (Fig 1a), and 4, 5, 4 and 3 types were recovered from spring, summer, autumn and winter, respectively (Fig 1b).
Fig 1. Serotype distribution of the Salmonella isolates.

a) Fresh foods. b) Seasons.
The antibacterial susceptibility of 21 Salmonella strains is shown in Table 2. The strains showed varying degrees of resistance to antimicrobial drugs. The largest proportion of Salmonella showed resistance to tetracycline (85.7%) followed by ciprofloxacin (71.4%), streptomycin (66.7%), erythromycin (66.7%), gentamycin (52.4%), ampicillin (38.1%), enrofloxacin (33.3%), cephalothin (28.6%), cefotaxime (23.8%), sulfamethoxazole (19.1%), amoxicillin (19.1%) and kanamycin (19.1%).
Table 2. Salmonella antibiotic resistance.
| Antibiotic | Pork (n = 6) (%) | Chicken (n = 8) (%) | Vegetable (n = 7) (%) | Spring (n = 5) (%) | Summer (n = 8) (%) | Autumn (n = 5) (%) | Winter (n = 3) (%) | Total (%) |
|---|---|---|---|---|---|---|---|---|
| Ciprofloxacin | 5 (83.3) | 7 (87.5) | 3 (42.9) | 3 (60) | 7 (87.5) | 3 (60) | 2 (66. 7) | 15 (71.4) |
| Gentamycin | 4 (66. 7) | 5 (62.5) | 2 (28.6) | 2 (40) | 5 (62.5) | 3 (60) | 1 (33.3) | 11 (52.4) |
| Streptomycin | 2 (33. 3) | 6 (75) | 6 (85.7) | 3 (60) | 6 (75) | 3 (60) | 2 (66. 7) | 14 (66.7) |
| Erythromycin | 4 (66. 7) | 4 (50) | 6 (85.7) | 3 (60) | 6 (75) | 2 (40) | 3 (100) | 14 (66.7) |
| Ampicillin | 3 (50) | 2 (25) | 3 (42.9) | 1 (20) | 4 (50) | 1 (20) | 2 (66.7) | 8 (38.1) |
| Amoxicillin | 2 (33. 3) | 2 (25) | / | 1 (20) | 2 (25) | / | 1 (33.3) | 4 (28.6) |
| Enrofloxacin | 1 (16. 7) | 4 (50) | 2 (28.6) | 2 (40) | 3 (37.5) | 1 (20) | 1 (33.3) | 7 (33.3) |
| Kanamycin | 2 (33. 3) | 1 (12.5) | 1 (14.3) | 1 (20) | 2 (25) | 1 (20) | / | 4 (19.1) |
| Cefotaxime | 2 (33. 3) | 3 (37.5) | / | 1 (20) | 2 (25) | 1 (20) | 1 (33.3) | 5 (23.8) |
| Sulfamethoxazole | 2 (33. 3) | 1 (12.5) | 1 (14.3) | / | 2 (25) | 1 (20) | 1 (33.3) | 4 (19.1) |
| Cephalothin | / | 3 (37.5) | 3 (42.9) | 1 (20) | 3 (37.5) | 2 (40) | / | 6 (28.6) |
| Tetracycline | 4 (66.7) | 7 (87.5) | 7 (100) | 4 (80) | 7 (100) | 4 (80) | 3 (100) | 18 (85.7) |
PCR detection of ARGs and VGs
The resistance genes for tetracycline exhibited the highest detection rate (90.5%) in 21 Salmonella, which was consistent with the phenotypic characterization of antimicrobial resistance (Table 3). Resistance genes were observed to be higher for β-lactams and aminoglycosides than sulfonamides and quinolones (Fig 2).
Table 3. Antimicrobial resistance genes of the Salmonella isolates.
| Classification | Resistance gene | Pork (n = 6) (%) | Chicken (n = 8) (%) | Vegetable (n = 7) (%) | Spring (n = 5) (%) | Summer (n = 8) (%) | Autumn (n = 5) (%) | Winter (n = 3) (%) | Total (%) |
|---|---|---|---|---|---|---|---|---|---|
| β-lactams | bla TEM-1 | 5 (83.3) | 6 (75) | 2 (28.6) | 2 (40) | 6 (75) | 3 (60) | 2 (66.7) | 13 (66.7) |
| bla SHV | 1 (16.7) | 1 (12.5) | / | / | 1 (12.5) | / | 1 (33.3) | 2 (9.5) | |
| Aminoglycosides | aadA1 | 2 (33.3) | 6 (75) | 2 (28.6) | 2 (40) | 5 (62.5) | 2 (400) | 1 (33.3) | 10 (47.6) |
| Aac(3)-Ia | 1 (14.3) | 2 (25) | 1 (14.3) | / | 3 (37.5) | 1 (20) | / | 4 (19) | |
| Sulfa | sul1 | 5 (83.3) | 2 (25) | 2 (28.6) | / | 4 (50) | 3 (60) | 2 (66. 7) | 9 (42.9) |
| Tetracyclines | tet(A) | 4 (66.7) | 6 (75) | 6 (85.7) | 3 (60) | 7 (87.5) | 3 (60) | 3 (100) | 16 (76.2) |
| tet(B) | / | 2 (25) | 1 (14.3) | / | 1 (12.5) | 2 (40) | / | 3 (14.3) | |
| Quinolones | parC | 4 (66.7) | 4 (50) | / | 2 (40) | 4 (50) | 2 (40) | / | 8 (38.1) |
Fig 2. The detection rate of resistance genes.
Error bars correspond to the standard deviation of the means and letters indicate statistically significant differences between groups.
The virulence genes in Salmonella isolates are shown in Table 4. fimA was detected at the highest rate of 57.1%, followed by fliC (33.3%), spvC (23.8%), and stn (19.1%).
Table 4. Virulence genes of Salmonella isolates.
| Virulence genes | Pork (n = 6) (%) | Chicken (n = 8) (%) | Vegetable (n = 7) (%) | Spring (n = 5) (%) | Summer (n = 8) (%) | Autumn (n = 5) (%) | Winter (n = 3) (%) | Total (%) |
|---|---|---|---|---|---|---|---|---|
| fliC | 2 (33. 3) | 4 (50) | 1 (14.3) | 2 (40) | 3 (37.5) | 1 (20) | 1 (33. 3) | 7 (33. 3) |
| fimA | 3 (50) | 6 (75) | 3 (42.9) | 3 (60) | 5 (62.5) | 3 (60) | 1 (33.3) | 12 (57.1) |
| stn | 1 (16.7) | 3 (37.5) | / | / | 2 (25) | 1 (20) | 1 (33.3) | 4 (19.1) |
| spvC | 2 (33. 3) | 3 (37.5) | 1 (14.3) | 1 (20) | 3 (37.5) | 1 (20) | / | 5 (23.8) |
Co-occurrence analysis of ARGs and VGs
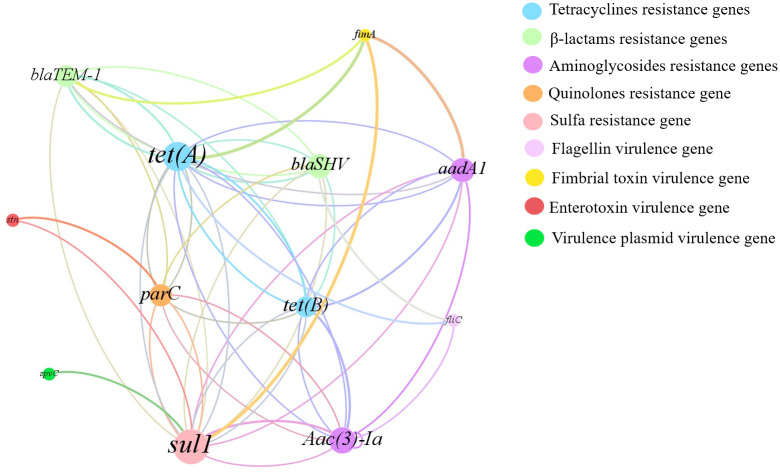
As shown in Fig 3, the gene co-occurrence analysis of the ARGs network showed a close correlation between ARGs and VGs (with a correlation of P < 0.05). In the figure, resistance genes were significantly associated with the virulence gene fimA. The fimA gene was mainly co-occurring with sul1, aadA1, tet(A) and blaTEM-1 genes for resistance to sulfonamides, β-lactams, tetracyclines and aminoglycosides, respectively. In addition, fliC co-occurred mainly with blaSHV, Aaca(3)-I and tet(A). Notably, all these resistance genes were detected at relatively high levels, especially sul1, aadA1, and tet(A), which were significantly associated with fimA. In addition, blaSHV and parC genes, which have relatively low detection rates, were also associated with VGs such as fliC and stn. In addition, the network showed the complexity of the linkage between ARGs of tetracyclines, β-lactams, sulfonamides, aminoglycosides, and quinolones (tet(A), tet(B), blaTEM-1, blaSHV, sul1, aadA1, Aaca(3)-I, parC, etc.).
Fig 3. Co-occurrence gene network of ARG subtypes and VGs in MDR isolates collected.
9 different colors represent 5 kinds of antibiotics and 4 virulence genes. Nodes belonging to the same kinds of VGs or resistant to the same class of antibiotics are presented in the same color. A connection represents a significant (P<0.05) correlation. The size of each node is proportional to the number of connections.
In vitro virulence
The invasive capacity ranged from 0.3% to 0.8% by spring, summer, autumn, and winter strains of S. Typhimurium. The ability to invade Caco-2 cells was the lowest for winter strain and the highest for summer strain (Fig 4).
Fig 4. Percentage of invasion of Caco-2 cells by Salmonella strain.
Error bars correspond to the standard deviation of the means and letters indicate statistically significant differences between groups.
Cytokine (IL-6, IL-8) production by Salmonella-infected Caco-2 cells was studied. As shown in Fig 5, Salmonella in samples from different seasons could induce inflammatory transcription in Caco-2 cells. The highest mRNA expression of IL-6 and IL-8 was observed in Salmonella-infected Caco-2 cells from summer strain. The least mRNA expression of IL-6 was observed in Salmonella-infected Caco-2 cells from winter strain (P<0.05). The data suggest that Caco-2 cells infected with Salmonella carrying different virulence genes may induce different levels of IL-6 and IL-8 secretion.
Fig 5. Expression of IL-6 and IL-8 mRNA was determined by real-time qPCR and normalized to GAPDH using the 2-ΔΔCt method.
Bars represent the mean of triplicates. Error bars correspond to the standard deviation of the means and letters indicate statistically significant differences between groups.
In vivo virulence
For body weight, on day 0, all mice lost an average of 1 g of body weight after fasting. The control group began to show rapid weight recovery on day 1 after the start of the experiment and continued to gain weight (Fig 6). The group of mice infected with spring, autumn, and winter strains of Salmonella performed similarly to the control group, with an increase in body weight on the fourth and fifth day after infection. In contrast, mice inoculated with Salmonella summer samples showed significant weight loss on all monitoring days, more severe on the fourth day with a value of -2.6 g (P<0.05).
Fig 6. Body weight observation of mice infected with Salmonella strains.
Data represent the mean of 6 mice per group. The means of weight in each group were statistically different (P<0.05).
The uninfected control group did not show the presence of Salmonella in the feces. The number of bacteria was found in all groups, with the highest amounts on day 3 and 5 (Fig 7a). On the first day after infection, more CFU was found in the feces of mice infected with the spring strain than that in the other groups (P<0.05). On day 3, the group infected with summer samples had more CFU, and on day 5, there was little difference between the mice of the different infected groups (P<0.05). Regarding the microbiological analysis of the ileum, liver, and spleen, the presence of Salmonella was observed in all three tissues of the infected animal groups (Fig 7b). The tissues of the Salmonella group infected with the summer sample showed the highest number of bacteria compared to the mice infected with the other sample strains (P<0.05).
Fig 7. Microbiological analysis of feces and tissues.
a) Colony counts in feces of infected mice (log10CFU/g). The mean number of feces in each group was statistically different (P< 0.05). b) Colony counts in tissues of infected mice (log10CFU/g). Means in each tissue were statistically different (P< 0.05).
Discussion
With rapid economic development, people’s awareness of food safety has gradually increased. Foodborne diseases and poisoning are mainly due to foodborne pathogenic bacteria [1]. Salmonella is a common cause of food poisoning worldwide, leading to epidemics in birds, reptiles, amphibians, and insects. Vegetable, fruit, and egg products are susceptible to Salmonella contamination in the production, processing, and marketing of such products, posing food safety concerns [32, 33]. Therefore, it is necessary to monitor the prevalence of Salmonella. The current study showed that the detection rate of Salmonella in different fresh foods reached 4.2%, and the detection rate in meat samples (5.2%) was slightly higher than that in vegetable samples (3.1%), which was consistent with the previous report by Li et al. [34], indicating that meat samples tend to be more susceptible to Salmonella contamination than vegetables. In this study, it was hypothesized that the rate of Salmonella contamination would be influenced by the season. This study found the detection rate of Salmonella was the highest in summer, and similar results have been reported in the relevant literature. In summer (June-August), both higher temperature and humidity in Hangzhou may provide a breeding ground for Salmonella. We identified 6 serotypes from 21 Salmonella strains, among which S. Enteritidis and S. Typhimurium were the most prevalent. Zhang et al. [35] found that among the clinically-isolated Salmonella from Shanghai, a city geographically close to Hangzhou, the two most abundant serotypes were also S. Typhimurium and S. Enteritidis.
Previous studies have shown that Salmonella is multidrug-resistant [36]. In this study, 21 Salmonella isolates were resistant to at least one antibacterial agent, and most isolates exhibited multi-resistance, mainly to tetracycline, ciprofloxacin, streptomycin, and erythromycin. According to previous reports [37, 38], Salmonella strains were mostly resistant to streptomycin, tetracyclines, and quinolones, which is consistent with our findings. Resistance genes for tetracyclines showed the highest detection rate, which was consistent with the resistance phenotype of Salmonella isolates. These data suggest that resistance to specific antimicrobial agents may be related to antimicrobial resistance genes.
The pathogenicity of Salmonella is related to the interaction between antibiotic and virulence factors [39]. Infections caused by antibiotic-resistant Salmonella with virulence genes have taken a long time to recover [40]. Salmonella fimbriae virulence gene fimA contributes to epithelial cell colonization [41]. In this study, the fimA virulence gene had a high detection rate and when tested with an invasion assay using intestinal epithelial (Caco-2) cells, they showed varying degrees of invasiveness, which is consistent with previously reported results [42]. In the current study, the highest fimA detection rate was found in both meat, vegetable, and different seasonal samples. The flagellin fliC gene is associated with biofilm formation, and Salmonella significantly enhances resistance to external pressure after biofilm formation [43]. Salmonella enterotoxin gene stn is a significant factor in gastroenteritis [44]. Salmonella virulence plasmid spvC is essential for systemic virulence [45]. The correlation between virulence factors and antibiotic resistance has been studied worldwide [46]. In the current study, the two genes with the highest detection rate, tet(A) and sul1 coexisted significantly with fimA. The virulence gene fimA mostly co-occurred with a total of 4 resistance genes (aadA1, sul1, tet(A), blaTEM-1), which were more than other virulence genes elements. After Salmonella invaded Caco-2, the summer samples had a very high invasion rate (0.8%), and their invasion ability was significantly higher than other samples (P<0.05). In addition, the mRNA levels of inflammatory factors IL-6 and IL-8 increased. The results showed that the mRNA expression of IL-6 and IL-8 was the highest in Caco-2 cells infected with Salmonella in summer strain and the least in winter strain, which was consistent with the detection rates of antibiotic resistance genes and virulence genes carried by Salmonella samples in different seasons. It is speculated that virulence genes may affect inflammatory transcription in the Caco-2 cell line. Evidence from in vivo experiments indicates that Salmonella infection of mice in summer exhibits significantly higher virulence effects than in other seasons, which is consistent with the results of in vitro experiments. In addition to this, when antibiotic resistance and virulence correlations are considered, isolates with strong antibiotic resistance and high virulence levels are at the highest risk. The combination of antibiotic resistance and virulence poses a major and alarming problem for food safety and public health. The mechanism behind the coupling is worth investigating.
Conclusion
In conclusion, this study showed that Salmonella contamination rates vary by season and source in different fresh foods, that antibiotic-resistant Salmonella isolates existed, and that isolates containing virulence genes exhibited virulence effects in vitro and in vivo, in addition to the combination of antibiotic resistance and virulence increasing the risk of Salmonella pathogenicity. This may pose potential risks to food safety and human health. Therefore, the fresh food market requires strict hygiene standards in different seasons to reduce the occurrence of Salmonella contamination, antibiotic resistance, and virulence characteristics. They are critical parameters in improving food safety, reducing the risk of foodborne illness, and selecting effective antibiotics to treat salmonellosis.
Supporting information
(XLSX)
Acknowledgments
We thank Dingting Xu, Jiankang Wang, Jianzhong Han and Daofeng Qu for practical support.
Data Availability
All relevant data are within the paper and its Supporting information files.
Funding Statement
Daofeng Qu, National Natural Science Foundation of China (32172188). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Gao W, Huang H, Zhu P, Yan X, Fan J, Jiang J, et al. Recombinase polymerase amplification combined with lateral flow dipstick for equipment-free detection of Salmonella in shellfish. Bioprocess and Biosystems Engineering. 2018;41(5):603–11. doi: 10.1007/s00449-018-1895-2 [DOI] [PubMed] [Google Scholar]
- 2.Zx A, Min WA, Cw A, Cz A, Jl A, Gg B, et al. The emergence of extended-spectrum β-lactamase (ESBL)-producing Salmonella London isolates from human patients, retail meats and chickens in southern China and the evaluation of the potential risk factors of Salmonella London. Food Control. 2021;128. doi: 10.1016/j.foodcont.2021.108187 [DOI] [Google Scholar]
- 3.Fagre AC, Pabilonia KL, Johnston MS, Morley PS, Burgess BA. Comparison of detection methods for Salmonella enterica shedding among reptilian patients at a veterinary teaching hospital. J Vet Diagn Invest. 2020;32(1):118–23. doi: 10.1177/1040638719886542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu W, Gao J, Zheng H, Yuan C, Hou J, Zhang L, et al. Establishment and Application of Polymerase Spiral Reaction Amplification for Salmonella Detection in Food. J Microbiol Biotechnol. 2019;29(10):1543–52. Epub 2019/10/28. doi: 10.4014/jmb.1906.06027 [DOI] [PubMed] [Google Scholar]
- 5.Yang Q, Domesle KJ, Ge B. Loop-Mediated Isothermal Amplification for Salmonella Detection in Food and Feed: Current Applications and Future Directions. Foodborne Pathogens Disease. 2018;15(6):309–31. doi: 10.1089/fpd.2018.2445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nair S, Patel V, Hickey T, Maguire C, Greig DR, Lee W, et al. Real-Time PCR Assay for Differentiation of Typhoidal and Nontyphoidal Salmonella. Journal of Clinical Microbiology. 2019;57(8): doi: 10.1128/JCM.00167-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scallan E, Griffin P, Angulo F, Tauxe R, Hoekstra R. Foodborne Illness Acquired in the United States—Unspecified Agents. Emerging Infectious Disease journal. 2011;17(1):16. doi: 10.3201/eid1701.091101p2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ed-dra A, Filali FR, Karraouan B, El Allaoui A, Aboulkacem A, Bouchrif B. Prevalence, molecular and antimicrobial resistance of Salmonella isolated from sausages in Meknes, Morocco. Microbial Pathogenesis. 2017;105:340–5. doi: 10.1016/j.micpath.2017.02.042 [DOI] [PubMed] [Google Scholar]
- 9.Rama EN, Bailey M, Kumar S, Leone C, den Bakker HC, Thippareddi H, et al. Prevalence and antimicrobial resistance of Salmonella in conventional and no antibiotics ever broiler farms in the United States. Food Control. 2021;30. doi: 10.1016/j.foodcont.2021.108738 [DOI] [PubMed] [Google Scholar]
- 10.Sun T, Liu Y, Qin X, Aspridou Z, Zheng J, Wang X, et al. The Prevalence and Epidemiology of Salmonella in Retail Raw Poultry Meat in China: A Systematic Review and Meta-Analysis. Foods. 2021;10(11):2757. doi: 10.3390/foods10112757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pe Ni, Xu Q, Yin Y, Liu D, Zhang J, Wu Q, et al. Prevalence and characterization of Salmonella serovars isolated from farm products in Shanghai. Food Control. 2018;85:269–75. doi: 10.1016/j.foodcont.2017.10.009 [DOI] [Google Scholar]
- 12.Xu X, Cui S, Zhang F, Luo Y, Gu Y, Yang B, et al. Prevalence and characterization of cefotaxime and ciprofloxacin co-resistant Escherichia coli isolates in retail chicken carcasses and ground pork, China. Microbial drug resistance. 2014;20(1):73–81. doi: 10.1089/mdr.2012.0224 [DOI] [PubMed] [Google Scholar]
- 13.Chen Z, Bai Jie, Zhang Xibin, Wang Shaojun, Chen Kaifeng, Lin Qijie, et al. Highly prevalent multidrug resistance and QRDR mutations in Salmonella isolated from chicken, pork and duck meat in Southern China, 2018–2019. International Journal of Food Microbiology. 2021. doi: 10.1016/j.ijfoodmicro.2021.109055 [DOI] [PubMed] [Google Scholar]
- 14.Lavilla Lerma L, Benomar N, Knapp CW, Correa Galeote D, Gálvez A, Abriouel H. Diversity, Distribution and Quantification of Antibiotic Resistance Genes in Goat and Lamb Slaughterhouse Surfaces and Meat Products. PLOS ONE. 2014;9(12):e114252. doi: 10.1371/journal.pone.0114252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park J-H, Kim Y-J, Binn K, Seo K-H. Spread of multidrug-resistant Escherichia coli harboring integron via swine farm waste water treatment plant. Ecotoxicology and Environmental Safety. 2018;149:36–42. doi: 10.1016/j.ecoenv.2017.10.071 [DOI] [PubMed] [Google Scholar]
- 16.Christaki E, Marcou M, Tofarides A. Antimicrobial resistance in bacteria: mechanisms, evolution, and persistence. Journal of molecular evolution. 2020;88(1):26–40. doi: 10.1007/s00239-019-09914-3 [DOI] [PubMed] [Google Scholar]
- 17.Webber B, Borges KA, Furian TQ, Rizzo NN, Tondo EC, Santos LRd, et al. Detection of virulence genes in Salmonella Heidelberg isolated from chicken carcasses. Revista do Instituto de Medicina Tropical de São Paulo. 2019;61. doi: 10.1590/S1678-9946201961036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tarabees R, Elsayed MS, Shawish R, Basiouni S, Shehata AA. Isolation and characterization of Salmonella Enteritidis and Salmonella Typhimurium from chicken meat in Egypt. The journal of infection in developing countries. 2017;11(04):314–9. doi: 10.3855/jidc.8043 [DOI] [PubMed] [Google Scholar]
- 19.Bahramianfard H, Derakhshandeh A, Naziri Z, Khaltabadi Farahani R. Prevalence, virulence factor and antimicrobial resistance analysis of Salmonella Enteritidis from poultry and egg samples in Iran. BMC Veterinary Research. 2021;17(1):1–8. doi: 10.1186/s12917-021-02900-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cencič A, Langerholc T. Functional cell models of the gut and their applications in food microbiology—A review. International Journal of Food Microbiology. 2010;141:S4–S14. doi: 10.1016/j.ijfoodmicro.2010.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saarinen M, Ekman P, Ikeda M, Virtala M, Grönberg A, Yu DTY, et al. Invasion of Salmonella into human intestinal epithelial cells is modulated by HLA‐B27. Rheumatology. 2002;41(6):651–7. doi: 10.1093/rheumatology/41.6.651 [DOI] [PubMed] [Google Scholar]
- 22.Choy EH, De Benedetti F, Takeuchi T, Hashizume M, John MR, Kishimoto T. Translating IL-6 biology into effective treatments. Nature Reviews Rheumatology. 2020;16(6):335–45. doi: 10.1038/s41584-020-0419-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moore BB, Kunkel SL. Attracting Attention: Discovery of IL-8/CXCL8 and the Birth of the Chemokine Field. The Journal of Immunology. 2019;202(1):3–4. doi: 10.4049/jimmunol.1801485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garrido D, Alber A, Kut E, Chanteloup NK, Lion A, Trotereau A, et al. The role of type I interferons (IFNs) in the regulation of chicken macrophage inflammatory response to bacterial challenge. Developmental & Comparative Immunology. 2018;86:156–70. doi: 10.1016/j.dci.2018.04.025 [DOI] [PubMed] [Google Scholar]
- 25.Sohail MN, Rathnamma D, Priya SC, Isloor S, Naryanaswamy HD, Ruban SW, et al. Salmonella from Farm to Table: Isolation, Characterization, and Antimicrobial Resistance of Salmonella from Commercial Broiler Supply Chain and Its Environment. BioMed Research International. 2021;2021:3987111. doi: 10.1155/2021/3987111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koo E-J, Kim D, Oh S-W. Comparison of DNA isolation methods for detection of foodborne pathogens by real-time PCR from foods. Korean Journal of Food Science and Technology. 2016;48(4):335–40. doi: 10.9721/kjfst.2016.48.4.335 [DOI] [Google Scholar]
- 27.Priya GB, Agrawal RK, Prince Milton AA, Mishra M, Mendiratta SK, Luke A, et al. Rapid and visual detection of Salmonella in meat using invasin A (invA) gene-based loop-mediated isothermal amplification assay. LWT. 2020;126:109262. doi: 10.1016/j.lwt.2020.109262 [DOI] [Google Scholar]
- 28.Organization WH. ANTIGENIC FORMULAE OF THE SALMONELLA SEROVARS 2007. https://www.pasteur.fr/sites/default/files/veng_0.pdf.
- 29.Clinical, Institute LS. Performance standards for antimicrobial susceptibility testing. Clinical and Laboratory Standards Institute Wayne, PA; 2017.
- 30.Kong-Ngoen T, Santajit S, Tunyong W, Pumirat P, Sookrung N, Chaicumpa W, et al. Antimicrobial Resistance and Virulence of Non-Typhoidal Salmonella from Retail Foods Marketed in Bangkok, Thailand. Foods. 2022;11(5):661. doi: 10.3390/foods11050661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bahramianfard H, Derakhshandeh A, Naziri Z, Khaltabadi Farahani R. Prevalence, virulence factor and antimicrobial resistance analysis of Salmonella Enteritidis from poultry and egg samples in Iran. BMC Veterinary Research. 2021;17(1):196. doi: 10.1186/s12917-021-02900-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Casanova-Higes A, Marín-Alcalá CM, Andrés-Barranco S, Cebollada-Solanas A, Mainar-Jaime RC. Weaned piglets: another factor to be considered for the control of Salmonella infection in breeding pig farms. Veterinary Research. 2019;50(1). doi: 10.1186/s13567-019-0666-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reza Farahani, Khaltabadi Nader, Shahrokhi Mina, et al. Salmonella Typhimurium in Iran: Contribution of molecular and IS200 PCR methods in variants detection. Plos One. 2019;14. doi: 10.1371/journal.pone.0213726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li J, Ma B, Fang J, Zhi A, Chen E, Xu Y, et al. Recombinase Polymerase Amplification (RPA) Combined with Lateral Flow Immunoassay for Rapid Detection of Salmonella in Food. Foods. 2020;9(1):27. doi: 10.3390/foods9010027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang J, Jin H, Hu J, Yuan Z, Shi W, Ran L, et al. Serovars and antimicrobial resistance of non-typhoidal Salmonella from human patients in Shanghai, China, 2006–2010. Epidemiology and Infection. 2014;142(4):826–32. Epub 2013/07/10. doi: 10.1017/S0950268813001659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ahmed AM, Shimabukuro H, Shimamoto T. Isolation and Molecular Characterization of Multidrug-Resistant Strains of Escherichia coli and Salmonella from Retail Chicken Meat in Japan. Journal of Food Science. 2009;74(7):M405–M10. doi: 10.1111/j.1750-3841.2009.01291.x [DOI] [PubMed] [Google Scholar]
- 37.Zhao X, Yang J, Zhang B, Sun S, Chang W. Characterization of Integrons and Resistance Genes in Salmonella Isolates from Farm Animals in Shandong Province, China. Frontiers in Microbiology. 2017;8. doi: 10.3389/fmicb.2017.01300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu Yan, Wu C-M, Wu G-J, Zhao H-Y, He T, Cao X-Y, et al. Prevalence of Antimicrobial Resistance Among Salmonella Isolates from Chicken in China. Foodborne Pathogens and Disease. 2011;8(1):45–53. doi: 10.1089/fpd.2010.0605 . [DOI] [PubMed] [Google Scholar]
- 39.Turki Y, Mehr I, Ouzari H, Khessairi A, Hassen A. Molecular typing, antibiotic resistance, virulence gene and biofilm formation of different Salmonella enterica serotypes. The Journal of General and Applied Microbiology. 2014;60(4):123–30. doi: 10.2323/jgam.60.123 [DOI] [PubMed] [Google Scholar]
- 40.Beshiru A, Igbinosa IH, Igbinosa EO. Prevalence of Antimicrobial Resistance and Virulence Gene Elements of Salmonella Serovars From Ready-to-Eat (RTE) Shrimps. Frontiers in Microbiology. 2019;10. doi: 10.3389/fmicb.2019.01613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Collinson SK, Liu SL, Clouthier SC, Banser PA, Doran JL, Sanderson KE, et al. The location of four fimbrin-encoding genes, agfA, fimA, sefA and sefD, on the Salmonella enteritidis and/or S. typhimurium XbaI-BlnI genomic restriction maps. Gene. 1996;169(1):75–80. Epub 1996/02/22. doi: 10.1016/0378-1119(95)00763-6 . [DOI] [PubMed] [Google Scholar]
- 42.Thung TY, Radu S, Mahyudin NA, Rukayadi Y, Zakaria Z, Mazlan N, et al. Prevalence, Virulence Genes and Antimicrobial Resistance Profiles of Salmonella Serovars from Retail Beef in Selangor, Malaysia. Frontiers in Microbiology. 2018;8. doi: 10.3389/fmicb.2017.02697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gupta P, Sarkar S, Das B, Bhattacharjee S, Tribedi P. Biofilm, pathogenesis and prevention—a journey to break the wall: a review. Archives of Microbiology. 2016;198(1):1–15. doi: 10.1007/s00203-015-1148-6 [DOI] [PubMed] [Google Scholar]
- 44.Nakano M, Yamasaki E, Ichinose A, Shimohata T, Takahashi A, Akada JK, et al. Salmonella enterotoxin (Stn) regulates membrane composition and integrity. Disease Models & Mechanisms. 2012;5(4):515–21. doi: 10.1242/dmm.009324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zuo L, Zhou L, Wu C, Wang Y, Li Y, Huang R, et al. Salmonella spvC Gene Inhibits Pyroptosis and Intestinal Inflammation to Aggravate Systemic Infection in Mice. Frontiers in Microbiology. 2020;11. doi: 10.3389/fmicb.2020.562491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hosseini RS, Rahimian G, Shafigh MH, Validi M, Khaledi M, Gholipour A. Correlation between clarithromycin resistance, virulence factors and clinical characteristics of the disease in Helicobacter pylori infected patients in Shahrekord, Southwest Iran. AMB Express. 2021;11(1):147. doi: 10.1186/s13568-021-01310-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting information files.