Abstract
In an effort to augment the anti-Helicobacter pylori effect of amoxicillin, mucoadhesive microspheres, which have the ability to reside in the gastrointestinal tract for an extended period, were prepared. The microspheres contained the antimicrobial agent and an adhesive polymer (carboxyvinyl polymer) powder dispersed in waxy hydrogenated castor oil. The percentage of amoxicillin remaining in the stomach both 2 and 4 h after oral administration of the mucoadhesive microspheres to Mongolian gerbils under fed conditions was about three times higher than that after administration in the form of a 0.5% methylcellulose suspension. The in vivo clearance of H. pylori following oral administration of the mucoadhesive microspheres and the 0.5% methylcellulose suspension to infected Mongolian gerbils was examined under fed conditions. The mucoadhesive microspheres and the 0.5% methylcellulose suspension both showed anti-H. pylori effects in this experimental model of infection, but the required dose of amoxicillin was effectively reduced by a factor of 10 when the mucoadhesive microspheres were used. In conclusion, the mucoadhesive microspheres more effectively cleared H. pylori from the gastrointestinal tract than the 0.5% methylcellulose suspension due to the prolonged gastrointestinal residence time resulting from mucoadhesion. A dosage form consisting of mucoadhesive microspheres containing an appropriate antimicrobial agent should be useful for the eradication of H. pylori.
Since the discovery of Helicobacter pylori in 1983 by Marshall and Warren (16), a great deal of attention has come to be focused on this organism and its association with gastric and duodenal ulcers (14, 20). In fact, it has become increasingly accepted that H. pylori is the major cause of peptic ulcers (13). In 1994, a National Institutes of Health Consensus Development Conference in the United States concluded that all patients with peptic ulcers and H. pylori infection should receive eradication therapy (18). However, clinical trials with single antimicrobial agents have not shown the complete eradication of H. pylori, although the organism is susceptible to many antimicrobial agents (8, 12).
One of the reasons for incomplete eradication may be the degradation of antimicrobial agents such as amoxicillin and clarithromycin by gastric acid (5). In an effort to overcome this problem, concomitant administration of antimicrobial agents and drugs which inhibit gastric acid secretion such as H2 receptor antagonists and proton pump inhibitors has been tried, but complete eradication has not been achieved (1, 6, 7). Therefore, the administration of high doses of antimicrobial agents on a daily basis is necessary for H. pylori eradication, and poor patient compliance due to adverse effects such as diarrhea, nausea, and retching is not unusual (21).
Another reason for incomplete eradication is probably that the residence time of antimicrobial agents in the stomach is so short that effective antimicrobial concentrations cannot be achieved in the gastric mucous layer or epithelial cell surfaces where H. pylori exists (12). Therefore, it is expected that if local delivery of antimicrobial agents from the gastric lumen into the mucous layer can be achieved, the H. pylori eradication rate will be increased. In fact, a 1-h treatment regimen developed by Kimura et al. (15) provided more complete eradication of H. pylori than conventional therapy due to the extended gastric residence times of the antimicrobial agents. However, no in vivo eradication trials with dosage forms that prolong the gastric residence times have been reported.
Akiyama et al. (4) developed mucoadhesive microspheres which are referred to as the Adhesive Micromatrix System and which consist of a drug and an adhesive polymer powder such as a cross-linked polyacrylic acid derivative dispersed in a waxy base. It has been confirmed that these mucoadhesive microspheres have the ability to adhere to the stomach wall in rats and thereby remain in the gastrointestinal tract for an extended period. It is expected that mucoadhesive microspheres containing anti-H. pylori agents will provide potent anti-H. pylori activity.
The purpose of this study was to design mucoadhesive microspheres containing amoxicillin as an anti-H. pylori agent and to evaluate the effectiveness of the mucoadhesive microspheres for H. pylori eradication therapy.
MATERIALS AND METHODS
Materials.
Hydrogenated castor oil (Lubri wax 101) was purchased from Freund Industrial Co. Ltd. (Tokyo, Japan). Carboxyvinyl polymer (HIVISWAKO 104) was purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). Amoxicillin was purchased from Beecham Pharmaceuticals Ltd. (Singapore). Curdlan, a β-1,3-glucan-type polysaccharide, was manufactured in-house. All other chemicals were of reagent grade.
Preparation of mucoadhesive microspheres.
Amoxicillin (0.15 g), curdlan (1.35 g), and carboxyvinyl polymer (1.0 g), which was used as a mucoadhesive polymer, were dispersed in melted hydrogenated castor oil (7.5 g) as a waxy base at 95°C. Mucoadhesive microspheres containing amoxicillin (amoxicillin-microspheres) were prepared by the spray-chilling method with a rotating aluminum disk of 15 cm in diameter (2). Amoxicillin-microspheres of 250 to 335 μm in diameter were obtained by sieving. Placebo mucoadhesive microspheres lacking amoxicillin (placebo-microspheres) were prepared by dispersing curdlan (1.35 g) and carboxyvinyl polymer (1.0 g) in melted hydrogenated castor oil (7.5 g) in the same manner.
In vivo evaluation of the mucoadhesiveness of amoxicillin-microspheres.
Amoxicillin-microspheres or amoxicillin suspended in a 0.5% aqueous solution of methylcellulose at a concentration of 1 mg/ml (amoxicillin suspension) was orally administered to 7-week-old male specific-pathogen-free Mongolian gerbils which were obtained from Seiwa Experimental Animal Ltd. (Fukuoka, Japan). The amoxicillin dose was 10 mg/kg of body weight. Amoxicillin-microspheres were administered as follows: amoxicillin-microspheres were placed in a polyethylene tube (Intramedic Polyethylene Tubing; inner diameter, 1.14 mm; outer diameter, 1.57 mm; Becton Dickinson and Company, Sparks, Md.), one end of which was covered with hydroxypropyl cellulose film, and were administered to each Mongolian gerbil with 0.2 ml of water by using the polyethylene tube attached to a gastric sonde (4).
At 2 or 4 h after administration, the stomach of each Mongolian gerbil was excised while the gerbil was under ether anesthesia, and the remaining amount of amoxicillin was evaluated; i.e., 40 ml of 1/15 M phosphate buffer (pH 7.2) was added to each stomach, and the amount of amoxicillin extracted was determined by a reversed-phase high-performance liquid chromatography (HPLC) method. The HPLC conditions were as follows. The mobile phase consisted of 95 parts of Kolthoff buffer (pH 8.0) and 5 parts of acetonitrile. The flow rate was 1.2 ml/min, and the detector was set at 230 nm. The remaining percentage of amoxicillin as an index of residence in the stomach, i.e., mucoadhesiveness, was calculated by the following equation: remaining percentage = (R/T) · 100, where R represents the amount of amoxicillin remaining in the stomach and T represents the amount of amoxicillin administered.
Concentration of amoxicillin in plasma.
Amoxicillin was orally administered to 7-week-old male specific-pathogen-free Mongolian gerbils at a dose of 30 mg/kg in the form of amoxicillin-microspheres or amoxicillin suspension in the same manner as described above for the in vivo evaluation of the mucoadhesiveness. Blood samples (1 ml), collected by cardiac puncture at 1, 2, 4, or 6 h after administration while the gerbils were under ether anesthesia, were centrifuged at 3,000 rpm for 15 min at 5°C. The plasma samples that were obtained were kept at −20°C until analysis. Five hundred microliters of a plasma sample, to which 500 μl of 1/15 M phosphate buffer (pH 7.2) and 1 ml of acetonitrile were added, was vortex mixed for 1 min and was then centrifuged at 3,000 rpm for 15 min at 5°C. The supernatant was evaporated to dryness at 30°C under a stream of nitrogen. The concentration of amoxicillin was determined by the HPLC method after reconstituting the residue with 200 μl of Kolthoff buffer (pH 8.0). The conditions for HPLC were as follows. The mobile phase consisted of 96 parts of Kolthoff buffer (pH 8.0) and 4 parts of acetonitrile. The flow rate was 1.0 ml/min, and the detector was set at 230 nm. The maximum concentration in plasma (Cmax) was obtained from individual plasma amoxicillin concentrations. The area under the plasma concentration-time curve from time zero to 6 h after administration (AUC0–6) was calculated by the trapezoidal method.
Bacteria.
The bacterial strain used in this study, TN2, was originally isolated from a human patient with gastric ulcer and was adapted to the gastric mucosae of Mongolian gerbils by four serial passages. The fourth-passage derivative strain of TN2 was named TN2GF4. The bacteria used to infect the Mongolian gerbils were grown in brucella broth (Becton Dickinson Microbiology Systems, Cockeysville, Md.) supplemented with 2.5% heat-inactivated fetal bovine serum in GasPak jars containing CampyPak with shaking at 37°C until the late logarithmic phase (approximately 24 h of growth), and the cultures were kept at −80°C until use.
Gastric infection procedure.
Four-week-old male specific-pathogen-free Mongolian gerbils were fasted for about 24 h, and 1 ml of broth containing 107.63 CFU of H. pylori TN2GF4 per ml was inoculated into the stomach of each gerbil via an orogastric tube.
In vivo clearance of H. pylori.
Fourteen days after infection, amoxicillin was orally administered twice a day for 3 consecutive days at a dose of 1, 3, 10, or 30 mg/kg in the form of amoxicillin-microspheres or amoxicillin suspension. Placebo-microspheres and a 0.5% aqueous methylcellulose solution, which were used as controls, were administered in the same manner. One day after administration of the final dose, the gerbils were killed and the stomachs were removed. Each stomach was homogenized with brucella broth (3 ml/stomach), and serial dilutions were plated on modified Skirrow’s medium. The plates were incubated for 4 days at 37°C under microaerobic conditions in GasPak jars. The viable cell counts for each gastric wall were calculated by counting the number of colonies on the agar plates.
Statistics.
Differences between the control-treated and the amoxicillin-treated groups in bacterial counts in the gastric wall were analyzed by Dunnett’s test. P values below 0.05 were considered statistically significant.
RESULTS
In vivo evaluation of mucoadhesiveness.
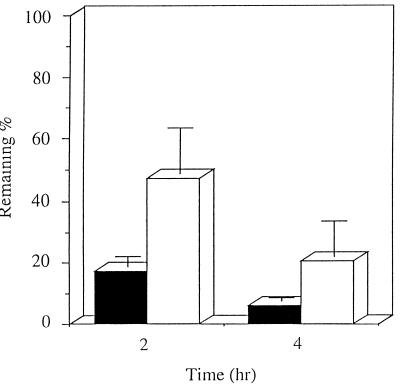
The in vivo gastric residence, i.e., adhesiveness, of the amoxicillin-microspheres was examined by using uninfected Mongolian gerbils under fed conditions. Adhesion of the amoxicillin-microspheres to the stomach wall was observed. The remaining percentage of amoxicillin 2 and 4 h after amoxicillin-microsphere administration, 47.3% ± 14.4% and 20.4% ± 11.5%, respectively, was about three times higher than that after amoxicillin suspension administration, 17.3% ± 3.2% and 6.2% ± 1.1%, respectively (Fig. 1).
FIG. 1.
Remaining percentage of amoxicillin in the stomachs of Mongolian gerbils 2 and 4 h after oral administration of amoxicillin suspension (■) and amoxicillin-microspheres (□). Mean and standard deviations are shown (n = 4).
Absorption of amoxicillin.
Cmax and AUC0–6 after oral administration of the amoxicillin-microspheres or amoxicillin suspension were examined. The AUC0–6 value after amoxicillin-microsphere administration was the same as that after amoxicillin suspension administration (13.1 and 13.9 μg · h/ml, respectively). No significant differences in Cmax were found between amoxicillin-microspheres and amoxicillin suspension (5.08 ± 1.14 and 7.19 ± 2.25 μg/ml, respectively [values are means ± standard deviations; n = 4]).
In vivo clearance of H. pylori.
The in vivo clearance of H. pylori after multiple administrations of amoxicillin-microspheres or the amoxicillin suspension under fed conditions (amoxicillin doses, 1, 3, 10, and 30 mg/kg) is presented in Table 1. The mean bacterial count after oral administration of the amoxicillin suspension decreased as the dose of amoxicillin increased; however, complete clearance of H. pylori was not obtained even with the highest dose. The mean bacterial count after 3 days of treatment with the amoxicillin suspension with an amoxicillin dose of 1.0 mg/kg (107.08 ± 0.07) was similar to that after vehicle administration (107.02 ± 0.15), and the values did not differ significantly (Dunnett’s test). On the other hand, the mean bacterial count after 3 days of treatment with amoxicillin-microspheres with an amoxicillin dose of 1.0 mg/kg (104.37 ± 0.98) was significantly lower than that after placebo-microsphere administration (106.44 ± 0.16) (Dunnett’s test), and complete clearance of H. pylori (clearance rate, 100%) was obtained after the administration of amoxicillin-microspheres with amoxicillin doses of 10 and 30 mg/kg. The amoxicillin-microspheres with an amoxicillin dose of 1.0 mg/kg provided the same clearance rate (20%) as the amoxicillin suspension with an amoxicillin dose of 10 mg/kg. This means that the amoxicillin-microspheres provided 10 times greater anti-H. pylori activity than the amoxicillin suspension.
TABLE 1.
Effect of repetitive oral administration of amoxicillin suspension and amoxicillin-microspheres against gastric infection caused by H. pylori TN2 in Mongolian gerbils
| Preparation | Dose (mg/kg)a | Clearance rate (no. of gerbils cleared of infection/total no. (%) | Bacterial recovery (log CFU/ gastric wall)b |
|---|---|---|---|
| Vehicle controlc | 0 | 0/5 (0) | 7.02 ± 0.15 |
| Amoxicillin suspension | 1 | 0/5 (0) | 7.08 ± 0.07 |
| 3 | 0/5 (0) | 5.53 ± 0.67 | |
| 10 | 1/5 (20) | 3.12 ± 0.65** | |
| 30 | 3/5 (60) | 1.76 ± 0.21** | |
| Placebo-microspheres | 0 | 0/5 (0) | 6.44 ± 0.16 |
| Amoxicillin-microspheres | 1 | 1/5 (20) | 4.37 ± 0.98* |
| 3 | 2/5 (40) | 3.32 ± 0.85** | |
| 10 | 5/5 (100) | NDd | |
| 30 | 5/5 (100) | ND |
Twice daily for 3 days as Amoxicillin.
Bacterial counts less than 101.48 CFU were considered to be 101.48 CFU to calculate the mean. Values are means ± standard error. *, P < 0.05; **, P < 0.01 (versus respective controls by Dunnett’s test).
The control was a 0.5% methylcellulose solution.
ND, not detected.
DISCUSSION
The present work is the first evidence demonstrating the in vivo usefulness of a dosage form, i.e., the mucoadhesive amoxicillin-microspheres, for the eradication of H. pylori. In vivo evaluation of amoxicillin-microspheres was carried out with an animal model, Mongolian gerbils infected with human H. pylori. The advantage of this evaluation method is that errors due to sampling site variation (23) can be avoided because the whole stomach is used to determine the bacterial cell count.
Amoxicillin has a low MIC for H. pylori (11, 17, 19) and luminal anti-H. pylori activity (22). However, compared with clarithromycin or metronidazole, it takes several hours for amoxicillin to kill H. pylori (10). On the other hand, the amoxicillin residence time in the stomach after oral administration of the conventional dosage form is expected to be short (9). Therefore, the resulting insufficient duration of contact with the gastric mucosa may be the reason for the incomplete eradication of H. pylori.
In this study, we found that amoxicillin resided in the stomach for a longer period of time when it was administered in the form of the mucoadhesive microspheres than when it was administered in a suspension. The amoxicillin-microspheres provided greater anti-H. pylori activity than the amoxicillin suspension. Considering that the amoxicillin-microspheres and amoxicillin suspension showed equivalent AUCs, these results indicate that the topical action of amoxicillin on the gastric mucus played an important role in the clearance of H. pylori.
According to microscopic findings, the gastric mucosae of Mongolian gerbils from which H. pylori had been eradicated (1 month after successful H. pylori clearance) with the amoxicillin-microspheres revealed no histological changes in the pyloric region. This indicates that the prolonged adhesion of the amoxicillin-microspheres to the mucosa of the stomach did not cause an unfavorable effect.
Akiyama et al. (3) reported that the mucoadhesive microspheres were found to have a prolonged gastrointestinal residence time when they were administered to humans in the form of capsules. Considering the higher levels of anti-H. pylori activity provided by the amoxicillin-microspheres, capsules filled with the microspheres are expected to show stronger anti-H. pylori effects than conventional capsules filled with amoxicillin powder in humans.
In conclusion, amoxicillin administered in the form of amoxicillin-microspheres more effectively cleared H. pylori than amoxicillin administered in the form of an amoxicillin suspension. There is a possibility that the use of amoxicillin-microspheres would allow the dose of amoxicillin to be reduced, which is important from the viewpoint of reducing adverse effects.
REFERENCES
- 1.Adamek R J, Opferkuch W, Pfaffenbach B, Wegener M. Cure of Helicobacter pylori infection: role of duration of treatment with omeprazole and amoxicillin. Am J Gastroenterol. 1996;91:98–100. [PubMed] [Google Scholar]
- 2.Akiyama Y, Yoshioka M, Horibe H, Hirai S, Kitamori N, Toguchi H. Novel oral controlled-release microspheres using polyglycerol esters of fatty acids. J Controlled Release. 1993;26:1–10. doi: 10.1002/jps.2600831116. [DOI] [PubMed] [Google Scholar]
- 3.Akiyama Y, Nagahara N, Nara E, Kitano M, Iwasa S, Ogawa Y, Yamamoto I, Azuma J. Evaluation of oral mucoadhesive microspheres in man on the basis of pharmacokinetics of furosemide and riboflavin, compounds with limited gastrointestinal absorption sites. J Pharm Pharmacol. 1998;50:159–166. doi: 10.1111/j.2042-7158.1998.tb06171.x. [DOI] [PubMed] [Google Scholar]
- 4.Akiyama Y, Nagahara N, Kashihara T, Hirai S, Toguchi H. In vitro and in vivo evaluation of mucoadhesive microspheres prepared for the gastrointestinal tract using polyglycerol esters of fatty acids and poly(acrylic acid) Pharm Res. 1995;12:397–405. doi: 10.1023/a:1016208703380. [DOI] [PubMed] [Google Scholar]
- 5.Axon A T. The role of acid inhibition in the treatment of Helicobacter pylori infection. Scand J Gastroenterol. 1994;29:16–23. [PubMed] [Google Scholar]
- 6.Bayerdorffer E, Miehlk S, Mannes G A, Sommer A, Hochter W, Weingart J, Heldwein W, Klann H, Simon T, Schmitt W, Bastlein E, Eimiller A, Hatz R, Lehn N, Dirschedl P, Stolte M. Double-blind trial of omeprazole and amoxicillin to cure Helicobacter pylori infection in patients with duodenal ulcers. Gastroenterology. 1995;108:1412–1417. doi: 10.1016/0016-5085(95)90689-4. [DOI] [PubMed] [Google Scholar]
- 7.Bell G D, Powell K U, Burridge S M, Spencer G, Bolton G, Purser K, Brooks S, Prosser S, Harrison G, Gant P W, Jones P H, Trowell J E. Omeprazole plus antimicrobial combination for the eradication of metronidazole-resistant Helicobacter pylori. Aliment Pharmacol Ther. 1992;6:751–758. doi: 10.1111/j.1365-2036.1992.tb00740.x. [DOI] [PubMed] [Google Scholar]
- 8.Chiba N, Rao B V, Rademaker J W, Hunt H. Metaanalysis of the efficacy of antimicrobial therapy in eradicating Helicobacter pylori. Am J Gastroenterol. 1992;87:1716–1727. [PubMed] [Google Scholar]
- 9.Cooreman M P, Krausgrill P, Hengels K J. Local gastric and serum amoxicillin concentrations after different oral application forms. Antimicrob Agents Chemother. 1993;37:1506–1509. doi: 10.1128/aac.37.7.1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flamm R K, Beyer J, Tanaka S K, Clement J. Kill kinetics of antimicrobial agents against Helicobacter pylori. J Antimicrob Chemother. 1996;38:719–725. doi: 10.1093/jac/38.4.719. [DOI] [PubMed] [Google Scholar]
- 11.Goodwin C S, Blake P, Blincow E. The minimum inhibitory and bactericidal concentrations of antimicrobials and anti-ulcer agents against Campylobacter pyloridis. J Antimicrob Chemother. 1986;17:309–314. doi: 10.1093/jac/17.3.309. [DOI] [PubMed] [Google Scholar]
- 12.Graham D Y, Borsch G M A. The who’s and when’s of therapy for Helicobacter pylori. Am J Gastroenterol. 1990;85:1552–1555. [PubMed] [Google Scholar]
- 13.Graham D Y. Helicobacter pylori: its epidemiology and its role in duodenal ulcer disease. J Gastroenterol Hepatol. 1991;6:105–113. doi: 10.1111/j.1440-1746.1991.tb01448.x. [DOI] [PubMed] [Google Scholar]
- 14.Hentschel E, Brandstatter G, Dragosics B, Hirschl A M, Nemec H, Schvtze K, Tanfer M, Wurzer H. Effect of ranitidine and amoxicillin plus metronidazole on the eradication of Helicobacter pylori and the recurrence of duodenal ulcer. N Engl J Med. 1993;328:308–312. doi: 10.1056/NEJM199302043280503. [DOI] [PubMed] [Google Scholar]
- 15.Kimura K, Ido K, Saifuku K, Taniguchi Y, Kihira K, Satoh K, Takimoto T, Yoshida Y. A 1-h topical therapy for the treatment of Helicobacter pylori infection. Am J Gastroenterol. 1995;90:60–63. [PubMed] [Google Scholar]
- 16.Marshall B J, Warren J R. Unidentified cured bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983;i:1273–1275. [PubMed] [Google Scholar]
- 17.McNulty C A M, Dent J, Wise R. Susceptibility of clinical isolates of Campylobacter pyloridis to 11 antimicrobial agents. Antimicrob Agents Chemother. 1985;28:837–838. doi: 10.1128/aac.28.6.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Institutes of Health Consensus Development Panel. NIH consensus development panel on Helicobacter pylori in peptic ulcer disease. JAMA. 1994;272:65–69. [PubMed] [Google Scholar]
- 19.Rauws E A J, Langenberg W, Houthoff H J, Zanzen H C, Tytgat G N J. Campylobacter pyloridis associated chronic active antral gastritis: a prospective study of its prevalence and the effects of antibacterial and antiulcer treatment. Gastroenterology. 1988;94:33–40. [PubMed] [Google Scholar]
- 20.Rauws E A J, Tytgat G N J. Cure of duodenal ulcer associated with eradication of Helicobacter pylori. Lancet. 1990;335:1233–1235. doi: 10.1016/0140-6736(90)91301-p. [DOI] [PubMed] [Google Scholar]
- 21.Thijs J C, Zwet A A, Moolenaar W, Wolfhagen M J H M, Huinink J B. Triple therapy vs. amoxicillin plus omeprazole for treatment of Helicobacter pylori infection: a multicenter, prospective, randomized, controlled study of efficacy and side effects. Am J Gastroenterol. 1996;91:93–97. [PubMed] [Google Scholar]
- 22.Tytgat G N J. Review article—treatments that impact favourably upon the eradication of Helicobacter pylori and ulcer recurrence. Aliment Pharmacol Ther. 1994;8:359–368. doi: 10.1111/j.1365-2036.1994.tb00303.x. [DOI] [PubMed] [Google Scholar]
- 23.Westblom T U, Duriex D E, Madan E, Belshe R B. Guinea pig model for antimicrobial transport across gastric mucosa: inhibitory tissue concentrations of clindamycin against Helicobacter pylori (Campylobacter pylori) following two separate dose regimens. Antimicrob Agents Chemother. 1990;34:25–28. doi: 10.1128/aac.34.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]