Abstract
1. Hosts and their parasites exist within complex ecological communities. However, the role that non-focal community members, species which cannot be infected by a focal pathogen, may play in altering parasite transmission is often only studied in the lens of the “diversity-disease” relationship by focusing on species richness. This approach largely ignores mechanistic species interactions and risks collapsing our understanding of the community ecology of disease down to defining the prominence of “amplification” vs. “dilution” effects.
2. However, non-focal species vary in their traits, densities, and types of interactions with focal hosts and parasites. Therefore, a community ecology approach based on the mechanisms underlying parasite transmission, host harm, and dynamic species interactions may better advance our understanding of parasite transmission in complex communities.
3. Using the concept of the parasite’s basic reproductive ratio, R0, as a generalizable framework, we examine several critical mechanisms by which interactions among hosts, parasites, and non-focal species modulate transmission and provide examples from relevant literature.
4. By focusing on the mechanism by which non-focal species impact transmission, we can emphasize the similarities among classic paradigms in the community ecology of disease, gain new insights into parasite invasion and persistence, community traits correlated with disease dilution or amplification, and the feasibility of biocontrol for parasites of conservation, agricultural, or human health concern.
Keywords: dilution effect, diversity-disease relationship, non-focal species, parasite transmission, species interactions
Parasite transmission in a community context
Parasites are ubiquitous and diverse, and they impact all scales of biological organization, from molecules to ecosystems (Poulin & Morand, 2000). Moreover, interactions among hosts, parasites, and other species can influence transmission (Johnson & Thieltges, 2010). While many studies investigate the effects of hosts of differing host competence as drivers of disease dynamics (LoGiudice, Ostfeld, Schmidt, & Keesing, 2003; Schmidt & Ostfeld, 2001), focusing on the specific impact of non-focal species on parasite transmission can improve mechanistic explanations for community-level disease ecology phenomena. We define non-focal species as any species that cannot be successfully infected by a focal parasite and that may interact with that parasite and/or its host species, with consequences for transmission.
Investigating the impact of non-focal species on disease dynamics is often done under the umbrella of examining the “dilution effect”, a phenomenon in which biodiversity is negatively correlated with parasite prevalence (Johnson, Ostfeld, & Keesing, 2015; LoGiudice et al., 2003; Ostfeld & Keesing, 2000). A large number of systems exhibit this pattern (Civitello et al., 2015), although there are counterexamples (Wood & Lafferty, 2013). However, this approach limits the scope in which non-focal species can impact disease dynamics. Specifically, it largely focuses on the importance of less competent hosts or fully resistant non-hosts through two mechanisms: susceptible host regulation and transmission interference (Ostfeld & Keesing, 2000). While these two mechanisms have strong, general support, other ecological interactions between non-focal species, hosts, and parasites remain underappreciated (Johnson, De Roode, & Fenton, 2015).
Overall, an increased focus on the functional ecological interactions between hosts, parasites, and non-focal species in a full community context is needed to broaden our understanding of the community ecology of infectious disease. Our review builds on seminal work by Keesing et al which introduced several mechanisms through which non-focal hosts could alter disease dynamics (Keesing et al., 2006). Here, we expand upon this work by reviewing recent literature that has examined these mechanisms, adding in other potential mechanisms such as the effect of non-focal hosts on both virulence and parasite productivity within hosts, and including environmentally transmitted parasites with complex life cycles. This approach acknowledges the potential impact of non-focal species on disease dynamics by including the diversity, abundance, and traits of species outside of a pre-defined trophic level, functional group, or arbitrarily defined set of potential hosts (e.g., the “horizontal community” sensu Vellend, 2016). Reemphasizing the abundance and functional traits of other species as drivers of disease dynamics moves beyond phenomenological characterization of diversity-disease relationships and instead represents the diverse suite of community ecological mechanisms influencing a host-parasite system. Most importantly, this functional approach to disease in communities can improve predictions for a variety of fundamental and applied problems because the effects of species on community and ecosystem dynamics are often driven by abundance and functional traits rather than mere presence or absence (Searle et al., 2016; Strauss, Bowling, Duffy, Cáceres, & Hall, 2018). Ultimately, this mechanistic community ecological view can enhance our knowledge of why some parasites thrive in complex communities while others are lost, of important traits related to transmission interference or amplification, or of new strategies to prevent or combat outbreaks of problematic parasites or maximize the effects of pathogens as biocontrol agents of pests.
We propose a functional categorization of the impact of non-focal species on disease dynamics through their effects on components of the parasite’s basic reproductive ratio, R0, which estimates the potential for transmission as a function of host and parasite traits. After categorizing these effects, we summarize the evidence, or lack thereof, for each mechanism by which non-focal species may affect disease dynamics. Last, we highlight key gaps and future directions to enhance our mechanistic understanding of disease in ecological communities.
R0 as a guiding tool
Pathogen dynamics are classically modeled using compartmental models with state variables representing discrete classes of hosts and parasites with ordinary differential equations that track their densities (Anderson & May, 1986). The basic reproductive ratio, R0, represents the expected number of infections produced from the first infection in a wholly susceptible population at its disease-free equilibrium. If R0 is greater than one, then a parasite can invade and spread (McCallum, Barlow, & Hone, 2001). R0 explicitly links changes in host and parasite traits and population densities with the potential for disease spread, and in doing so, R0 evaluates the net effects of simultaneous, potentially divergent changes to transmission.
Because the components of R0 are directly tied to the densities and traits of hosts and parasites, this value can be used as a framework to evaluate the influence of non-focal species on transmission by how they change these components. For example, R0 includes susceptible host density as well as parasite virulence: non-focal species have the potential to impact these values, likely via different mechanisms in different host-parasite systems. Exploring the effects of non-focal species on the components of R0, and then considering their potential synergism or antagonism, facilitates dissecting the vast complexity of how community ecology functionally impacts parasite transmission (Fig. 1).
Figure 1.

A schematic of components of R0 that non-focal species may influence, with choice examples illustrated. For parasites with environmental life stages and/or a complex life cycle, different life stages of the parasite will be influenced by different ecological interactions. (I) Non-focal species can alter the probability of a parasite encountering a susceptible host through intraspecific resource competition, predation of hosts and parasites, influencing host-host contact rates or host behavior, or by acting as decoys. (II) Non-focal species can influence the probability of infection given contact between a host and parasite by changing the resource quantity and quality available to hosts or causing physiologic stress to hosts, all of which can alter immune responses. (III) Non-focal species can impact parasite productivity
We illustrate how non-focal species influence R0 by referencing canonical R0 derivations for common host-parasite transmission modes, in the absence of other species (Box 1). Then we review the effects of non-focal species on the disease-driving processes reflected by the parameters of these reproductive ratios.
Box 1. Canonical R0 equations for common types of parasite transmission.
R0 can be obtained from simple models as the ratio of the transmission rate to the “removal rate”, evaluated at the disease-free equilibrium density of susceptible hosts, or, more formally, with the next generation matrix method1. While every host-parasite system has unique features that might require a system-specific derivation of R0, disease ecologists have derived canonical R0 equations that generally apply to common types of parasite transmission. Here we present the generalized R0 equations for a directly-transmitted, density-dependent microparasite (Eq. 1), an environmentally-iransmitted parasite (Eq. 2), a macroparasite (Eq. 3), and a vector-bome parasite (Eq. 4)2–4.
These models often assume logistic growth of hosts or vectors in the absence of disease, such that their disease-free equilibrium density depends on birth rate, b, death rate, d, and the strength of competition, c e.g., S* or H* −(b−d)(bc). Altematively, these densities are sometimes treated as a constant parameter. In all cases not involving vectors, we dissect the transmission rate, β, into a joint product of the exposure rate and the probability of infection given exposure5. For a vector-borne parasite, the exposure rate between host and parasite is dependent on the biting rate of the vector (C). There are also two probabilities of infection given exposure: the probability of a vector infecting a host (σ1), and a host infecting a vector (σ2)4.
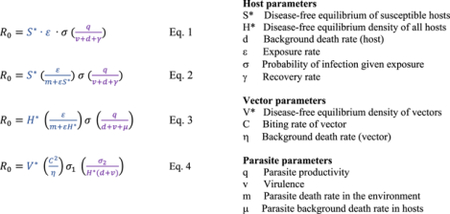
There is strong similarity in the general structure of R0 across these systems, which depends on three biologically-intuitive components: (i) the probability of a parasite encountering an infectious host or vector, which depends on both the exposure rate and the density of available hosts (or vectors) at their disease-free equilibrium (e.g.,S*,H*, or V*) (ii) the probability of infection given exposure, and (iii) the expected infectiousness (or productivity of parasites) over the lifetime of infection. Thus, non-focal species that perturb these three components could have predictable and consistent effects on disease dynamics, despite differences across systems.
I. The probability of a parasite encountering a susceptible host
Non-focal species may impact transmission via three general processes (Fig. 1): (I) the probability of a parasite encountering a susceptible host, (II) the probability of infection given contact, and (III) the number of parasites released during the infectious period. The probability of a parasite encountering a susceptible host depends on both the equilibrium density of susceptible hosts/vectors (S*, H*, or V* depending on the host-parasite system, see Box 1), encounter rates between hosts and parasites (ɛ), and the background death rate of parasites (m) (Civitello & Rohr, 2014). Non-focal species can alter any of these factors by directly interacting with hosts and parasites to change their densities (Greer, Briggs, & Collins, 2008), or through indirect mechanisms that impact the per capita rates at which host and parasite come into contact (Werner & Peacor, 2003). Often these mechanisms that drive the probability of a parasite encountering a susceptible host (host density, exposure rate, and parasite death rate) can operate simultaneously, but below we consider each separately, along with specific examples from the literature of how these effects can operate.
a. Susceptible host or vector density at the disease-free equilibrium (S*, H*, or V*)
Susceptible host density prior to parasite invasion is a function of maximum host birth rate, b, host background death rate, d¸ and the strength of competition, c (Box 1). Non-focal species can impact the birth and death rates, as well as the strength of competition, through multiple, overlapping mechanisms.
Resource competition results in species coexisting at lower densities than they would in isolation (Tilman, 1987). This can result in substitutive community assembly, a key mechanism of dilution effects (Halliday, Heckman, Wilfahrt, & Mitchell, 2019). In substitutive community assembly, increases in species richness are correlated with decreases in the relative abundance of a focal host species by both decreasing birth rates or increasing death rates. For example, field data and epidemiological modeling suggest that the prevalence of hantavirus in a focal host decreases with increasing resource competition from a resistant competitor (Peixoto & Abramson, 2006). Removing this competitor increased the relative abundance of hosts by 15%, with a concomitant two-fold increase in hantavirus seroprevalence (Suzán, Marcé, Giermakowski, Mills, & Ceballos, 2009), highlighting the potential for interspecific resource competition to regulate host densities with downstream effects on disease transmission.
Predation by non-focal species can increase host death rates, decreasing R0 by suppressing host or vector density, and these impacts are often most apparent when predators are removed (Johnson & Thieltges, 2010). The removal of a predator can increase prey density, facilitating parasite transmission. For example, in the kelp forests off the coast of Northern California, the sea urchin (Strongylocentrotus purpuratus) population has been historically regulated by predation from the spiny lobster, Panulirus interruptus (Lafferty, 2004). However, increased fishing of lobster suppressed this key regulator, triggering an urchin population boom that was soon followed by Vibrio sp. epidemics, an effect that was not observed in areas protected from lobster harvest. The maintenance of trophic diversity in complex food webs is an example of a concept in community ecology that likely impacts disease transmission in multiple systems, but which often remains uninvestigated (Johnson et al., 2015; Tanner et al., 2019).
Transmission of vectored parasites depends on vector density, V* (Box 1), and therefore predation on vectors can also alter transmission. Predator introductions have been correlated with decreased clinical dengue and malaria cases in some regions (Ghosh & Dash, 2007; Kay & Nam, 2005; Kittayapong et al., 2012). Predators of vectors can prevent pathogen establishment in naïve host populations, and the introduction of a vector predator into a host-vector-parasite system at equilibrium can extirpate pathogens through vector population regulation (Moore, Borer, & Hosseini, 2009). Non-consumptive effects of predators on the vector Culex can also decrease vector density by decreasing survival to pupation, adult size, and longevity (Meadows, Owen, & Snyder, 2017).
b. Exposure (ɛ)
Non-focal species can modulate the exposure rate between hosts and parasites (ɛ). For example, hantavirus transmission can also be interrupted by non-focal species decreasing host-host contact rates. Sin Nombre Virus (SNV) is a hantavirus of deer mice (Peromyscus maniculatus), transmitted through aggressive biting and fighting. Heterogeneity in host-host contact rates between deer mice can explain the variability in SNV transmission efficiency, suggesting that interactions “wasted” on non-focal species might interrupt transmission. In the presence of non-host species Ord’s kangaroo rat (Dipodomys ordii) and the Great Basin pocket mouse (Perognathus parvus), deer mice have fewer intraspecific encounters, irrespective of deer mouse density (Clay, Lehmer, St. Jeor, & Dearing, 2009). This effect caused reduced SNV prevalence at sites where the rate of intraspecific contact between hosts was most negatively impacted.
In addition to their impact on parasite and host death rates, predators can also impact parasite transmission indirectly by altering host and/or parasite traits such as behavior, morphology, or life history strategies, which may impact exposure to parasites (Werner & Peacor, 2003). Trait-mediated interactions can increase disease transmission due to conflicts between predator- and parasite avoidance behaviors. This trade-off arises for tadpoles (Anaxyrus americanus) previously exposed to odonate predator cues (Koprivnikar & Urichuk, 2017). Typically, tadpoles avoid free-swimming trematode parasites by increasing their activity and making rapid movements. However, tadpoles experimentally exposed to predator cues suppress their activity, thereby increasing their exposure to trematodes, even if a predator is no longer present. This behavioral shift, an example of “the ecology of fear”, results in high infection intensities and underscores the extreme impacts predation can have on transmission via behavior.
c. Death in the environment (m)
Parasites with free-living stages (Box 1, Eqs. 2&3) also face intentional or coincidental predation from non-focal species that can disrupt their transmission (Welsh, Liddell, Van Der Meer, & Thieltges, 2017). For example, dung beetles (Scarabaeinae) kill helminths and protozoans incidentally consumed while harvesting feces, and their presence can reduce prevalence of these parasites in ruminant hosts (Fincher, 1973). These effects are widespread in aquatic systems, in which many parasites have free-living, swimming life stages and are surrounded by a cadre of potential predators that they must evade to find their hosts (Johnson et al., 2010; Thieltges et al., 2013). For example, chorus frogs (Pseudacris regilla) are highly susceptible to Ribeiroia ondatrae, a trematode that infects amphibians and some fish. These frogs co-occur with non-focal species including the damselfly (Enallagma sp.), which preferentially predates Ribeiroia. In laboratory experiments, damselflies reduce transmission to frogs by nearly 50% (Orlofske, Jadin, Preston, & Johnson, 2012). In the case of Batrachochytrium dendrobatidis (Bd), a fungal pathogen that has devastated numerous species of amphibians, non-host microfauna will incidentally consume Bd spores, acting as a parasite sink that can reduce infection risk to hosts in certain environmental conditions (Searle, Mendelson, Green, & Duffy, 2013). These results exemplify that for parasites with free-living environmental stages, predation can significantly alter transmission dynamics, suggesting that predator density is a critical component of the community ecology of disease. Future work, both empirical and theoretical, on parasites with the ability or requirement to persist in the environment could reveal other roles that non-focal hosts play in these systems.
For free-living parasites, invading resistant non-host individuals is functionally equivalent to death in the environment because the parasite dies without encountering a susceptible host (King, Jokela, & Lively, 2011). Non-focal species that have similar properties to hosts act as “decoys” for parasites that have high host specificity but poor host-identification abilities. Decoy hosts are ecological traps from the parasite’s perspective: because they appear beneficial but are actually detrimental (Schlaepfer, Runge, & Sherman, 2002). This effect has been shown in some parasites with free-living life stages that seek out their host, such as schistosomes, and the presence of decoys can significantly decrease transmission to hosts in experimental trials (Johnson, Lund, Hartson, & Yoshino, 2009). However, the importance of this phenomenon in these systems at a landscape scale is unknown.
II. Probability of infection given contact (σ)
Infection probability, often equated with host susceptibility, is the probability that a given encounter between a host and parasite will result in an infection. In all R0 equations referenced in Box 1, infection probability is encompassed in the σ parameter. Many characteristics of the host, such as genotype or exposure history, can impact infection probability (Archie, Luikart, & Ezenwa, 2009; Read & Taylor, 2001). However, the influence of non-focal species on infection probability remains relatively unexplored compared to other mechanisms. One exception is the literature on defensive mutualists, which has been extensively examined and provides many examples of non-focal species decreasing disease transmission in the host (Clay, 2014; Doremus & Oliver, 2017; Flórez et al., 2017), and is being re-emphasized in the literature on symbiont-mediated vector-borne disease control (Mendiola, Civitello, & Gerardo, 2020). Other than defensive mutualists, the influence of non-focal hosts on infection probability is an area in which more work is warranted, especially because non-focal species can potentially increase or decrease infection probability in ways that may be difficult to predict.
a. Resource quantity and quality
When non-focal species act as resource competitors, the effect of decreased resource availability can modulate a host’s ability to mount an immune response to invading parasites. Immune responses are energetically costly (Lochmiller & Deerenberg, 2000; Paul, 2003), and limited access to resources in some cases leads to an increase in infection probability and therefore transmission. Tse-tse flies (Glossinia morsitans morsitans) are the intermediate hosts of Trypanosoma spp., a parasite genus that infects humans and other mammals. When flies were starved prior to Trypanosoma exposure, the probability of infection increased in the flies by 24% (Masumu et al., 2006). Similarly, food supplementation in wildlife has been shown to bolster the immune response in some species (Strandin, Babayan, & Forbes, 2018). Work in wild field voles (Microtus agrestis) has shown that voles supplemented with extra food in the resource-scarce winter mount a stronger immune response and have a lower prevalence of helminth infection (Forbes et al., 2016). However, both of these studies relied on experimental manipulation of resource availability. Future work on non-focal competitor species altering infection probability via changing quantity or quality of naturally available resources, in addition to their known impacts on susceptible host density, may enhance our knowledge of the competing impact of these mechanisms on R0.
When non-focal species are a food source for hosts, they can also alter parasite transmission if they contain medicinal properties. For example, monarch butterfly larvae (Danaus plexippus) consume milkweed (Asclepias) plants until pupation. Monarchs are hosts for the protozoal parasite Ophryocystis elektroscirrha, which is consumed by larvae feeding on milkweed leaves, reproduces within them and then develops into spores on the abdomen of adult butterflies (Gowler, Leon, Hunter, & de Roode, 2015). Milkweed species range in their concentration of cardenolides, chemicals that can decrease the transmission of O. elektroscirrha, and monarchs will preferentially lay their eggs on milkweeds with high levels of cardenolides to confer this advantage to their offspring (Gowler et al., 2015; Lefèvre, Oliver, Hunter, & De Roode, 2010). Such tri-tropic interactions between host, food source, and parasite underscore the fundamental role that non-focal species can play in host-parasite interactions, when the non-focal species is a direct food source to hosts.
b. Physiologic impacts of non-host species
Non-focal species may also affect infection probability through altering a focal host’s physiological state, usually resulting in an increase in infection probability. Predators or other species that are perceived as a threat by the host can have trait-mediated, non-consumptive effects, deemed the “ecology of fear” (Clinchy, Sheriff, & Zanette, 2013). In addition to altering host parasite avoidance behavior and changing the probability of exposure (Creel, Christianson, Liley, & Winnie, 2007), the presence of non-focal species may trigger physiological changes that are maladaptive for hosts in the presence of parasites. The acute stress response, characterized by an upregulation in circulating stress hormones such as glucocorticoids and catecholamines, can bolster immunity in many vertebrate species. Chronic stimulation of this pathway, however, results in dysregulation of immune functioning and has been shown to increase infection probability (Hing, Narayan, Thompson, & Godfrey, 2016). Non-focal species have been shown to contribute to chronic stress responses and immunosuppression in some species. The most commonly-investigated non-focal species invoking a stress response is Homo sapiens, which may lead to chronic stress in other species due to direct predation or indirect effects (e.g. altering local noise or light levels) (Keay, Singh, Gaunt, & Kaur, 2006). Work with the Great Tit (Parus major) has investigated the maladaptive impact humans can have on wildlife by altering disease transmission (Isaksson, Örnborg, Stephensen, & Andersson, 2005; Isaksson, Sepil, Baramidze, & Sheldon, 2013). In many avian species, the innate immune response relies heavily on the production of Reactive Oxygen Species (ROS) to attack invading parasites. However, similar immune effectors are also acutely produced in response to stress from human activity, leading to subsequent downregulation of the immune system to avoid cellular damage from excess ROS (Isaksson et al., 2005). This stress-mediated tug-of-war can increase these birds’ probability of infection by the avian malaria parasite (Plasmodium spp.) in urban environments (Isaksson et al., 2013). As in this example, these types of host/non-focal species interactions are anticipated to increase infection probability and therefore increase transmission, however they remain relatively unexplored.
III. Parasites released during the lifetime of an infection
This component of R0 represents the expected number of parasites released by a host during its infectious period, which is driven simultaneously by the production rate of parasites and the duration of infection. In our example R0 equations (Box 1), parasite productivity is represented by the parameter q, and the duration of infection is a function of host background mortality (d), the rate of recovery from infection (γ), and parasite virulence (v), in which virulence is defined as the parasite-induced per capita mortality rate in hosts (Real & Biek, 2007). In the case of macroparasites (Box 1, Eq. 3), parasite background death in the host (μ) is also explicitly tracked in the R0 equation. Increasing both productivity and duration of infection will lead to an increase in R0. However, there are empirically documented tradeoffs between these processes, which are critical for the evolution of parasite virulence (Alizon, de Roode, & Michalakis, 2013). Non-focal species have the potential to impact infection duration by modulating both the rate of recovery and parasite virulence.
a. Productivity within hosts (q) and rate of recovery (γ)
Hosts are often co-infected with more than one parasite in addition to a host of other microbial players in their microbiome, and many of the same ecological processes that describe host/non-focal species interactions outside of hosts are mirrored in within-host interspecific interactions (Lagrue & Poulin, 2008). For example, when two parasite species are exploiting the host as a common resource, competition can result in “bottom-up” regulation of one or multiple co-infecting species (Graham, 2008). Helminths that infect a host first and decrease the number of host red blood cells, for example, would deplete the available resource pool for any subsequent microparasite (Box 1, Eq. 1) that relied on these cells for replication, thereby decreasing focal parasite productivity (q). In a meta-analysis of laboratory co-infections of mice with helminths and microparasites, this bottom-up effect was shown in the microparasites examined that utilize host red blood cells: when co-infected simultaneously or after a helminth infection, the mean density of the microparasite was reduced (Graham, 2008).
Co-infecting non-focal species may also modulate the productivity of a focal parasite (q) and the host recovery rate (γ) if they induce cross-immunity in the host. Cross-immunity can be broadly defined as the host immune response to one parasite conferring some immunity against other co-infecting parasites, either of the same species but different genotype, or parasites of different species (Cox, 2001). As a result of cross-immunity, immune activation in response to parasitic infection can limit both the focal parasite and any other co-infecting parasites. Cross immunity induced by non-focal species could decrease R0 of a focal parasite by decreasing parasite production (q), decreasing host recovery time (γ), or through both mechanisms (Box 1). In species with adaptive immunity, this is in a large part due to memory T cells, and molecular evidence for this effect has been shown repeatedly, including cross-protection for Hepatitis C Virus in humans with previous exposure to Influenza A (Wedemeyer, Mizukoshi, Davis, Bennink, & Rehermann, 2001). In the same meta-analysis that examined “bottom-up” competition between helminths and microparasites, helminths were also shown to reduce microparasite density with an efficiency directly related to their stimulation of host production of the cytokine IFN-γ (Graham, 2008). The impact of cross-immunity across systems, however, is relatively understudied: coinfections that increase disease severity are easier to detect than those that lessen it, and in general coinfections remain understudied in non-human systems.
Furthermore, cross-immunity is not a generalized property of all co-infections. The opposite can also occur: a focal parasite may be facilitated by the presence of another co-infecting species. This could occur through several potential mechanisms that impact R0, such as by altering infection probability (σ), parasite productivity (q), or the duration of infection. One striking example can be found in Human Immunodeficiency Virus (HIV). HIV replicates within the immune cells of its host, and viral replication leads to cellular lysis, thereby directly reducing the functionality and density of host immune cells, creating a permissive environment for other parasites, such as Plasmodium, the causative agent of malaria, to replicate unchecked (AbuRaddad, Patnaik, & Kublin, 2006). Facilitation can also occur between parasites that do not directly infect immune cells. In helminths that infect white tailed deer (Odocoileus virginianus), co-infecting parasites have been found to facilitate focal parasites by either increasing infection probability or delaying parasite clearance, depending on the species (Park & Ezenwa, 2020). These findings in a wild mammal system are intriguing, and likely many other complex relationships exist between co-infecting parasites in wild species that have yet to be explored. Similar to the effects of non-focal species on infection probability, open questions on the impact of co-infections on disease transmission remain in many host-parasite systems.
b. Virulence (v)
When virulence is defined as host mortality due to infection (Alizon et al., 2013), increasing parasite virulence results in a decreased duration of infection (Box 1, Eqs. 1–4). While this singular effect would predict that an increase in virulence always decreases R0, empirical and theoretical work has shown that there is a trade-off between virulence and parasite transmission, often leading over evolutionary time to an intermediate level of virulence for parasites (Alizon, 2008; Alizon, Hurford, Mideo, & Van Baalen, 2009; De Roode, Yates, & Altizer, 2008; Fraser, Hollingsworth, Chapman, De Wolf, & Hanage, 2007). On an ecological timescale, virulence may still be impacted by non-focal host species, but the degree to which this occurs may be constrained by the evolutionary history of the parasite.
Predators can decrease both host and parasite population densities separately, but they can also act to functionally increase apparent parasite virulence if they preferentially predate infected hosts. We categorize selective predation of infected hosts as a type of functional virulence because it is host mortality that occurs as a result of parasite infection – even when the focal parasite itself may not be lethal. This mechanism underlies the “healthy herds hypothesis”, which posits that when prey species are infected by a parasite that makes them more susceptible to predation, predators simultaneously decrease both host and parasite density and therefore overall disease transmission in their prey populations (Hudson, Dobson, & Newborn, 1992; Packer, Holt, Hudson, Lafferty, & Dobson, 2003). Parasites have been shown to increase predator-induced mortality in several systems, and this effect has been empirically reversed in some cases by treating prey to reduce or eliminate their parasite burden (Hudson, Dobson, & Newborn, 1998). While evidence of this effect exists in microparasites (Packer et al., 2003), it is most pronounced in macroparasites (Box 1, Eq.3), which tend to aggregate such that proportionally few hosts carry the majority of the parasite population (Shaw & Dobson, 1995). Morbidity tends to increase with parasite burden, as does predation risk, resulting in predators removing a large proportion of parasites by preying upon the most heavily infected hosts (Johnson, Stanton, Preu, Forshay, & Carpenter, 2006).
When a non-focal species is a co-infecting parasite, it could potentially impact the virulence of the focal parasite either directly or by altering the host’s ability to mount a successful immune response. A review of the effect of co-infection on virulence evolution, however, found no consistent effect on virulence evolution across multiple host-parasite systems (Alizon et al., 2013). If the impact of a non-focal co-infecting species on a focal parasite’s virulence can have multiple potential outcomes for R0, then it may warrant particular attention in studies examining the overall effects of co-infection on population level transmission dynamics.
Multiple mechanisms impacting R0
The ways in which non-focal species impact R0 can also operate concurrently, sometimes resulting in outcomes that are difficult to predict. In combination, the effects of non-focal species can result in synergistic reduction of disease transmission or may exert opposite effects and result in unchanged or even increased transmission. One strength of an R0-based approach is that it provides a quantitative way to evaluate the net effects of non-focal species on the potential for disease spread.
Mechanisms can work in concert, such as in the case of predators simultaneously reducing host and parasite densities (i.e., “healthy herds hypothesis”) (Packer et al., 2003). This mechanism operates both by reducing host density and by effectively increasing parasite-induced mortality (virulence). Alternatively, a single non-focal species may increase R0 through one mechanism but decrease it through another. Intraguild predators, which consume both parasites and hosts, may have opposing impacts on parasite transmission by simultaneously decreasing parasite and susceptible host density. For example, one investigation of the impacts of predation in a frog-trematode system found that intraguild predators did not significantly reduce metacercarial infections in tadpoles (Rohr et al., 2015). This was due to both density and trait mediated effects: intra-guild predators reduced parasite density and host density through consumption, but their presence also decreased host behaviors associated with parasite avoidance (Koprivnikar & Urichuk, 2017; Rohr et al., 2015). Since predators of parasites are often considered as a method of biocontrol for parasites that impact human health, this example illustrates the importance of evaluating the multiple ways in which one species may impact R0 in the focal host.
Conclusion
Hosts and parasites live in complex ecological communities, and non-focal community members can play a large, while currently understudied, role in host-parasite dynamics. We emphasize that an approach based on understanding ecological interactions, rather than simply species richness, will improve our understanding of disease in complex communities. Much research is currently focused on quantifying dilution/amplification patterns and, e.g., how they depend on scale, host type, etc.(Cohen et al., 2016), but a correlative approach will likely always yield examples and counterexamples that cannot be extrapolated beyond a particular system. Explicitly incorporating mechanism allows for a more nuanced approach to the relative importance of non-focal species abundance and functional traits, rather than merely their presence or absence. One key takeaway of this framework is that the relative importance of the mechanisms discussed in this review can vary drastically between host-parasite systems, or even dynamically within a given system across spatiotemporal gradients, especially if the relative densities of hosts and non-focal species vary along these gradients (Hall et al., 2009; Levi, Keesing, Holt, Barfield, & Ostfeld, 2016). This variability supports the use of a mechanistic framework such as we propose: one that helps define the appropriate level of complexity needed to dissect the ecological factors driving each part of the transmission process and the downstream impact on disease dynamics.
Additionally, using this approach facilitates the ability of scientists to investigate the net effects, both positive and negative, that non-focal species may have and the resulting overall impact on transmission. This comprehensive approach is required for any parasite management plans that utilize non-focal species to control host or parasite densities in order to avoid these interventions failing or even backfiring. Finally, our conceptual framework provides a means by which to unify the subfields of community disease ecology, for example by integrating the simultaneous impacts of a non-focal species on resource competition and altered host-parasite encounter rates, an important step to advance the disease ecology field (Johnson et al., 2015). To approach these types of questions, we propose that collaborative work between scientists that specialize in numerous subfields, such as disease ecology, behavioral ecology, and landscape ecology, is necessary to gain a deeper understanding of the importance of non-focal community members in parasite transmission. Investigating parasite transmission at this level can yield important insights to guide management and intervention policies for parasites with relevance to human health and animal conservation.
Acknowledgements:
We thank Jaap de Roode and members of the Civitello Lab for their comments on this manuscript. KS was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number T32AI138952 and Award Number F31AI147611. DJC was supported by NIH 1R01 AI150774-01 and NSF IOS 1755002. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or National Science Foundation.
Footnotes
Conflict of Interest: The authors do not have any conflicts of interest.
References
- Abu-Raddad LJ, Patnaik P, & Kublin JG (2006). Dual Infection with HIV and Malaria Fuels the Spread of Both Diseases in Sub-Saharan Africa. Science, 314(5805), 1603 LP–1606. doi: 10.1126/science.1132338 [DOI] [PubMed] [Google Scholar]
- Alizon S (2008). Transmission-recovery trade-offs to study parasite evolution. AMERICAN NATURALIST, 172(3), E113–E121. doi: 10.1086/589892 [DOI] [PubMed] [Google Scholar]
- Alizon S, de Roode J, & Michalakis Y (2013). Multiple infections and the evolution of virulence. Ecology Letters, 16(4), 556–567. doi: 10.1111/ele.12076 [DOI] [PubMed] [Google Scholar]
- Alizon S, Hurford A, Mideo N, & Van Baalen M (2009). Virulence evolution and the tradeoff hypothesis: history, current state of affairs and the future. Journal of Evolutionary Biology, 22(2), 245–259. doi: 10.1111/j.1420-9101.2008.01658.x [DOI] [PubMed] [Google Scholar]
- Anderson RM, & May RM (1986). The invasion, persistence and spread of infectious diseases within animal and plant communities. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, Vol. 314, pp. 533–570. doi: 10.1098/rstb.1986.0072 [DOI] [PubMed] [Google Scholar]
- Archie EA, Luikart G, & Ezenwa VO (2009). Infecting epidemiology with genetics: a new frontier in disease ecology. Trends in Ecology & Evolution, 24(1), 21–30. doi: 10.1016/j.tree.2008.08.008 [DOI] [PubMed] [Google Scholar]
- Civitello DJ, Cohen J, Fatima H, Halstead NT, Liriano J, McMahon TA, … Rohr JR (2015). Biodiversity inhibits parasites: Broad evidence for the dilution effect. Proceedings of the National Academy of Sciences, 112(28), 8667–8671. doi: 10.1073/pnas.1506279112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civitello DJ, & Rohr JR (2014). Disentangling the effects of exposure and susceptibility on transmission of the zoonotic parasite Schistosoma mansoni. Journal of Animal Ecology, 83(6), 1379–1386. doi: 10.1111/1365-2656.12222 [DOI] [PubMed] [Google Scholar]
- Clay CA, Lehmer EM, Jeor S St., & Dearing MD (2009). Testing Mechanisms of the Dilution Effect: Deer Mice Encounter Rates, Sin Nombre Virus Prevalence and Species Diversity. EcoHealth, 6(2), 250–259. doi: 10.1007/s10393-009-0240-2 [DOI] [PubMed] [Google Scholar]
- Clay K (2014). EDITORIAL: Defensive symbiosis: a microbial perspective. Source: Functional Ecology, 28(2), 293–298. doi: 10.1111/1365-2435.12258 [DOI] [Google Scholar]
- Clinchy M, Sheriff MJ, & Zanette LY (2013). Predator-induced stress and the ecology of fear. Functional Ecology, 27(1), 56–65. doi: 10.1111/1365-2435.12007 [DOI] [Google Scholar]
- Cohen JM, Civitello DJ, Brace AJ, Feichtinger EM, Ortega CN, Richardson JC, … Rohr JR (2016). Spatial scale modulates the strength of ecological processes driving disease distributions. Proceedings of the National Academy of Sciences of the United States of America, 113(24), E3359–E3364. doi: 10.1073/pnas.1521657113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox FEG (2001). Concomitant infections, parasites and immune responses. Parasitology, 122(SUPPL.), S23–S38. doi: 10.1017/s003118200001698x [DOI] [PubMed] [Google Scholar]
- Creel S, Christianson D, Liley S, & Winnie JA (2007). Predation risk affects reproductive physiology and demography of elk. Science, 315(5814), 960. doi: 10.1126/science.1135918 [DOI] [PubMed] [Google Scholar]
- De Roode JC, Yates AJ, & Altizer S (2008). Virulence-transmission trade-offs and population divergence in virulence in a naturally occurring butterfly parasite. Proceedings of the National Academy of Sciences of the United States of America, 105(21), 7489–7494. doi: 10.1073/pnas.0710909105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doremus MR, & Oliver KM (2017). Aphid heritable symbiont exploits defensive mutualism. Applied and Environmental Microbiology, 83(8). doi: 10.1128/AEM.03276-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fincher GT (1973). Dung Beetles as Biological Control Agents for Gastrointestinal Parasites of Livestock. The Journal of Parasitology, 59(2), 396–399. doi: 10.2307/3278842 [DOI] [PubMed] [Google Scholar]
- Flórez LV, Scherlach K, Gaube P, Ross C, Sitte E, Hermes C, … Kaltenpoth M (2017). Antibiotic-producing symbionts dynamically transition between plant pathogenicity and insect-defensive mutualism. Nature Communications, 8(1), 1–9. doi: 10.1038/ncomms15172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes KM, Mappes T, Sironen T, Strandin T, Stuart P, Meri S, … Huitu O (2016). Food limitation constrains host immune responses to nematode infections. Biology Letters, 12(9), 20160471. doi: 10.1098/rsbl.2016.0471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser C, Hollingsworth TD, Chapman R, De Wolf F, & Hanage WP (2007). Variation in HIV-1 set-point viral load: Epidemiological analysis and an evolutionary hypothesis. Proceedings of the National Academy of Sciences of the United States of America, 104(44), 17441–17446. doi: 10.1073/pnas.0708559104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh SK, & Dash AP (2007, November). Larvivorous fish against malaria vectors: a new outlook. Transactions of the Royal Society of Tropical Medicine and Hygiene, Vol. 101, pp. 1063–1064. doi: 10.1016/j.trstmh.2007.07.008 [DOI] [PubMed] [Google Scholar]
- Gowler CD, Leon KE, Hunter MD, & de Roode JC (2015). Secondary Defense Chemicals in Milkweed Reduce Parasite Infection in Monarch Butterflies, Danaus plexippus. Journal of Chemical Ecology, 41(6), 520–523. doi: 10.1007/s10886-015-0586-6 [DOI] [PubMed] [Google Scholar]
- Graham AL (2008). Ecological rules governing helminth-microparasite coinfection. Proceedings of the National Academy of Sciences of the United States of America, 105(2), 566–570. doi: 10.1073/pnas.0707221105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer AL, Briggs CJ, & Collins JP (2008). Testing a key assumption of host-pathogen theory: Density and disease transmission. Oikos, 117(11), 1667–1673. doi: 10.1111/j.1600-0706.2008.16783.x [DOI] [Google Scholar]
- Hall SR, Becker CR, Simonis JL, Duffy MA, Tessier AJ, & Cáceres CE (2009). Friendly competition: evidence for a dilution effect among competitors in a planktonic host-parasite system. Ecology, 90(3), 791–801. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/19341148 [DOI] [PubMed] [Google Scholar]
- Halliday FW, Heckman RW, Wilfahrt PA, & Mitchell CE (2019). Past is prologue: host community assembly and the risk of infectious disease over time. Ecology Letters, 22(1), 138–148. doi: 10.1111/ele.13176 [DOI] [PubMed] [Google Scholar]
- Hing S, Narayan EJ, Thompson RCA, & Godfrey SS (2016, March 30). The relationship between physiological stress and wildlife disease: Consequences for health and conservation. Wildlife Research, Vol. 43, pp. 51–60. CSIRO. doi: 10.1071/WR15183 [DOI] [Google Scholar]
- Hudson PJ, Dobson AP, & Newborn D (1992). Do Parasites make Prey Vulnerable to Predation? Red Grouse and Parasites. Journal of Animal Ecology, 61(3), 681–692. doi: 10.2307/5623 [DOI] [Google Scholar]
- Hudson PJ, Dobson AP, & Newborn D (1998). Prevention of Population Cycles by Parasite Removal. Science, 282(5397), 2256 LP–2258. doi: 10.1126/science.282.5397.2256 [DOI] [PubMed] [Google Scholar]
- Isaksson C, Örnborg J, Stephensen E, & Andersson S (2005). Plasma glutathione and carotenoid coloration as potential biomarkers of environmental stress in great tits. EcoHealth, 2(2), 138–146. doi: 10.1007/s10393-005-3869-5 [DOI] [Google Scholar]
- Isaksson C, Sepil I, Baramidze V, & Sheldon BC (2013). Explaining variance of avian malaria infection in the wild: The importance of host density, habitat, individual life-history and oxidative stress. BMC Ecology, 13. doi: 10.1186/1472-6785-13-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PTJ, Dobson A, Lafferty KD, Marcogliese DJ, Memmott J, Orlofske SA, … Thieltges DW (2010). When parasites become prey: Ecological and epidemiological significance of eating parasites. Trends in Ecology and Evolution, Vol. 25, pp. 362–371. doi: 10.1016/j.tree.2010.01.005 [DOI] [PubMed] [Google Scholar]
- Johnson PTJ, Ostfeld RS, & Keesing F (2015). Frontiers in research on biodiversity and disease. Ecology Letters, 18(10), 1119–1133. doi: 10.1111/ele.12479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PTJ, Stanton DE, Preu ER, Forshay KJ, & Carpenter SR (2006). Dining on Disease: How Interactions Between Infection and Environment Affect Predation Risk. In Ecology (Vol. 87). John Wiley & Sons, Ltd. doi: 10.1890/0012-9658(2006)87[1973:DODHIB]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Johnson P, & Thieltges DW (2010). Diversity, decoys and the dilution effect: How ecological communities affect disease risk. Journal of Experimental Biology, 213(6), 961–970. doi: 10.1242/jeb.037721 [DOI] [PubMed] [Google Scholar]
- Johnson Pieter, De Roode JC, & Fenton A (2015, September 4). Why infectious disease research needs community ecology. Science, Vol. 349, p. 1259504. American Association for the Advancement of Science. doi: 10.1126/science.1259504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson Pieter, Lund PJ, Hartson RB, & Yoshino TP (2009). Community diversity reduces Schistosoma mansoni transmission, host pathology and human infection risk. Proceedings of the Royal Society B: Biological Sciences, 276(1662), 1657–1663. doi: 10.1098/rspb.2008.1718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay B, & Nam VS (2005). New strategy against Aedes aegypti in Vietnam. Lancet, 365(9459), 613–617. doi: 10.1016/S0140-6736(05)17913-6 [DOI] [PubMed] [Google Scholar]
- Keay JM, Singh J, Gaunt MC, & Kaur T (2006). Fecal Glucocorticoids and their Metabolites as Indicators of Stress in Various Mammalian Species: A Literature Review. Journal of Zoo and Wildlife Medicine, 37(3), 234–244. doi: 10.1638/05-050.1 [DOI] [PubMed] [Google Scholar]
- Keesing F, Holt RD, & Ostfeld RS (2006). Effects of species diversity on disease risk. Ecology Letters, 9(4), 485–498. doi: 10.1111/j.1461-0248.2006.00885.x [DOI] [PubMed] [Google Scholar]
- King KC, Jokela J, & Lively CM (2011). Trematode parasites infect or die in snail hosts. Biology Letters, 7(2), 265–268. doi: 10.1098/rsbl.2010.0857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittayapong P, Thongyuan S, Olanratmanee P, Aumchareoun W, Koyadun S, Kittayapong R, & Butraporn P (2012). Application of eco-friendly tools and eco-bio-social strategies to control dengue vectors in urban and peri-urban settings in Thailand. Pathogens and Global Health, 106(8), 446–454. doi: 10.1179/2047773212Y.0000000059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koprivnikar J, & Urichuk TMY (2017). Time-lagged effect of predators on tadpole behaviour and parasite infection. Biology Letters, 13(9). doi: 10.1098/rsbl.2017.0440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafferty KD (2004). Fishing for Lobsters Indirectly Increases Epidemics in Sea Urchins. Ecological Applications, 14(5), 1566–1573. doi: 10.1890/03-5088 [DOI] [Google Scholar]
- Lagrue C, & Poulin R (2008). Intra- and interspecific competition among helminth parasites: Effects on Coitocaecum parvum life history strategy, size and fecundity. International Journal for Parasitology, 38(12), 1435–1444. doi: 10.1016/J.IJPARA.2008.04.006 [DOI] [PubMed] [Google Scholar]
- Lefèvre T, Oliver L, Hunter MD, & De Roode JC (2010). Evidence for trans-generational medication in nature. Ecology Letters, 13(12), 1485–1493. doi: 10.1111/j.1461-0248.2010.01537.x [DOI] [PubMed] [Google Scholar]
- Levi T, Keesing F, Holt RD, Barfield M, & Ostfeld RS (2016). Quantifying dilution and amplification in a community of hosts for tick-borne pathogens. Ecological Applications, 26(2), 484–498. doi: 10.1890/15-0122 [DOI] [PubMed] [Google Scholar]
- Lochmiller RL, & Deerenberg C (2000). Trade-offs in evolutionary immunology: just what is the cost of immunity? Oikos, 88(1), 87–98. doi: 10.1034/j.1600-0706.2000.880110.x [DOI] [Google Scholar]
- LoGiudice K, Ostfeld RS, Schmidt KA, & Keesing F (2003). The ecology of infectious disease: Effects of host diversity and community composition on lyme disease risk. Proceedings of the National Academy of Sciences of the United States of America, 100(2), 567–571. doi: 10.1073/pnas.0233733100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masumu J, Marcotty T, Ndeledje N, Kubi C, Geerts S, Vercruysse J, … den Bossche P (2006). Comparison of the transmissibility of Trypanosoma congolense strains, isolated in a trypanosomiasis endemic area of eastern Zambia, by Glossina morsitans morsitans. PARASITOLOGY, 133(3), 331–334. doi: 10.1017/S0031182006000369 [DOI] [PubMed] [Google Scholar]
- McCallum H, Barlow N, & Hone J (2001). How should pathogen transmission be modelled? Trends in Ecology & Evolution, 16(6), 295–300. doi: 10.1016/S0169-5347(01)02144-9 [DOI] [PubMed] [Google Scholar]
- Meadows AJ, Owen JP, & Snyder WE (2017). Keystone nonconsumptive effects within a diverse predator community. Ecology and Evolution, 7. doi: 10.1002/ece3.3392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendiola SY, Civitello DJ, & Gerardo NM (2020, June 1). An integrative approach to symbiont-mediated vector control for agricultural pathogens. Current Opinion in Insect Science, Vol. 39, pp. 57–62. Elsevier Inc. doi: 10.1016/j.cois.2020.02.007 [DOI] [PubMed] [Google Scholar]
- Moore SM, Borer ET, & Hosseini PR (2009). Predators indirectly control vector-borne disease: Linking predator-prey and host-pathogen models. Journal of the Royal Society Interface, 7(42), 161–176. doi: 10.1098/rsif.2009.0131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlofske SA, Jadin RC, Preston DL, & Johnson P (2012). Parasite transmission in complex communities: Predators and alternative hosts alter pathogenic infections in amphibians. Ecology, 93(6), 1247–1253. doi: 10.1890/11-1901.1 [DOI] [PubMed] [Google Scholar]
- Ostfeld RS, & Keesing F (2000). Biodiversity and Disease Risk: the Case of Lyme Disease. Conservation Biology, 14(3), 722–728. doi: 10.1046/j.1523-1739.2000.99014.x [DOI] [Google Scholar]
- Packer C, Holt RD, Hudson PJ, Lafferty KD, & Dobson AP (2003). Keeping the herds healthy and alert: implications of predator control for infectious disease. Ecology Letters, 6(9), 797–802. doi: 10.1046/j.1461-0248.2003.00500.x [DOI] [Google Scholar]
- Park AW, & Ezenwa VO (2020). Characterising interactions between co-infecting parasites using age-intensity profiles. International Journal for Parasitology, 50, 23–26. doi: 10.1016/j.ijpara.2019.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul S-H (2003). Variation in immune defence as a question of evolutionary ecology. Proceedings of the Royal Society of London. Series B: Biological Sciences, 270(1513), 357–366. doi: 10.1098/rspb.2002.2265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peixoto ID, & Abramson G (2006). THE EFFECT OF BIODIVERSITY ON THE HANTAVIRUS EPIZOOTIC. In Ecology (Vol. 87). Retrieved from https://esajournals-onlinelibrary-wiley-com.proxy.library.emory.edu/doi/pdf/10.1890/0012-9658%282006%2987%5B873%3ATEOBOT%5D2.0.CO%3B2 [DOI] [PubMed] [Google Scholar]
- Poulin R, & Morand S (2000). The Diversity of Parasites. The Quarterly Review of Biology, 75(3), 277–293. Retrieved from http://www.jstor.org/stable/2665190 [DOI] [PubMed] [Google Scholar]
- Read AF, & Taylor LH (2001). The Ecology of Genetically Diverse Infections. Science, 292, 1099–1102. Retrieved from http://science.sciencemag.org/ [DOI] [PubMed] [Google Scholar]
- Real LA, & Biek R (2007). Infectious disease modeling and the dynamics of transmission. In Wildlife and Emerging Zoonotic Diseases: The Biology, Circumstances and Consequences of Cross-Species Transmission (pp. 33–49). doi: 10.1007/978-3-540-70962-6_2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohr JR, Civitello DJ, Crumrine PW, Halstead NT, Miller AD, Schotthoefer AM, … Beasley VR (2015). Predator diversity, intraguild predation, and indirect effects drive parasite transmission. PROCEEDINGS OF THE NATIONAL ACADEMY OF SCIENCES OF THE UNITED STATES OF AMERICA, 112(10), 3008–3013. doi: 10.1073/pnas.1415971112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaepfer MA, Runge MC, & Sherman PW (2002). Ecological and evolutionary traps. Trends in Ecology & Evolution, 17(10), 474–480. doi: 10.1016/S0169-5347(02)02580-6 [DOI] [Google Scholar]
- Schmidt KA, & Ostfeld RS (2001). BIODIVERSITY AND THE DILUTION EFFECT IN DISEASE ECOLOGY. In SYNTHESIS EMPHASIZING NEW IDEAS TO STIMULATE RESEARCH IN ECOLOGY Ecology (Vol. 82). Retrieved from https://esajournals-onlinelibrary-wiley-com.proxy.library.emory.edu/doi/pdf/10.1890/0012-9658%282001%29082%5B0609%3ABATDEI%5D2.0.CO%3B2 [Google Scholar]
- Searle CL, Cortez MH, Hunsberger KK, Grippi DC, Oleksy IA, Shaw CL, … Duffy MA (2016). Population Density, Not Host Competence, Drives Patterns of Disease in an Invaded Community. AMERICAN NATURALIST, 188(5), 554–566. doi: 10.1086/688402 [DOI] [PubMed] [Google Scholar]
- Searle CL, Mendelson JR, Green LE, & Duffy MA (2013). Daphnia predation on the amphibian chytrid fungus and its impacts on disease risk in tadpoles. Ecology and Evolution, 3(12), 4129–4138. doi: 10.1002/ece3.777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw DJ, & Dobson AP (1995). Patterns of macroparasite abundance and aggregation in wildlife populations: a quantitative review. Parasitology, 111(S1), S111. doi: 10.1017/S0031182000075855 [DOI] [PubMed] [Google Scholar]
- Strandin T, Babayan SA, & Forbes KM (2018). Reviewing the effects of food provisioning on wildlife immunity. Philosophical Transactions of the Royal Society B: Biological Sciences, Vol. 373. doi: 10.1098/rstb.2017.0088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss AT, Bowling AM, Duffy MA, Cáceres CE, & Hall SR (2018). Linking host traits, interactions with competitors and disease: Mechanistic foundations for disease dilution. Functional Ecology, 32(5), 1271–1279. doi: 10.1111/1365-2435.13066 [DOI] [Google Scholar]
- Suzán G, Marcé E, Giermakowski JT, Mills JN, & Ceballos G (2009). Experimental Evidence for Reduced Rodent Diversity Causing Increased Hantavirus Prevalence. PLoS ONE, 4(5), 5461. doi: 10.1371/journal.pone.0005461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner E, White A, Acevedo P, Balseiro A, Marcos J, & Gortázar C (2019). Wolves contribute to disease control in a multi-host system. Scientific Reports, 9(1). doi: 10.1038/s41598-019-44148-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thieltges DW, Amundsen P-A, Hechinger RF, Johnson P, Lafferty KD, Mouritsen KN, … Poulin R (2013). Parasites as prey in aquatic food webs: implications for predator infection and parasite transmission. OIKOS, 122(10), 1473–1482. doi: 10.1111/j.1600-0706.2013.00243.x [DOI] [Google Scholar]
- Tilman D (1987). The Importance of the Mechanisms of Interspecific Competition. The American Naturalist, 129(5), 769–774. doi: 10.1086/284672 [DOI] [Google Scholar]
- Vellend M (2016). The theory of ecological communities (MPB-57). Princeton University Press. [Google Scholar]
- Wedemeyer H, Mizukoshi Eishiro †, Davis AR, Bennink JR, & Rehermann B (2001). Cross-Reactivity between Hepatitis C Virus and Influenza A Virus Determinant-Specific Cytotoxic T Cells. JOURNAL OF VIROLOGY, 75(23), 11392–11400. doi: 10.1128/JVI.75.23.11392-11400.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh JE, Liddell C, Van Der Meer J, & Thieltges DW (2017). Parasites as prey: The effect of cercarial density and alternative prey on consumption of cercariae by four non-host species. Parasitology, 144(13), 1775–1782. doi: 10.1017/S0031182017001056 [DOI] [PubMed] [Google Scholar]
- Werner EE, & Peacor SD (2003). A Review of Trait-Mediated Indirect Interactions in Ecological Communities. Ecology, 84(5), 1083–1100. doi: 10.1890/0012-9658(2003)084[1083:AROTII]2.0.CO;2 [DOI] [Google Scholar]
- Wood CL, & Lafferty KD (2013). Biodiversity and disease: a synthesis of ecological perspectives on Lyme disease transmission. Trends in Ecology & Evolution, 28(4), 239–247. doi: 10.1016/j.tree.2012.10.011 [DOI] [PubMed] [Google Scholar]


