Sir,
Since most surveillance studies focus on candidaemia, non-Candida species such as Saccharomyces cerevisiae, Cryptococcus spp, Trichosporon spp, Rhodotorula spp, or Magnusiomyces spp, among others, have received little attention [1]. Emerging non-Candida spp might account for up to 2.8% of fungaemia episodes [2-6] and are characterised by diminished susceptibilities to systemic antifungal agents. We recently reported the epidemiology and antifungal susceptibility of Candida spp sourcing from blood cultures and intra-abdominal samples from patients under care at 16 hospitals in Madrid (Spain) from January 2019 to December 2022 (CANDIMAD study) [7]. Here we describe the non-Candida species distribution and their antifungal susceptibility collected in the CANDIMAD study. Isolates, one per species, patient, and compartment, were identified by molecular methods, and subjected to antifungal susceptibility testing to amphotericin B, azoles, echinocandins and ibrexafungerp according to the EUCAST E. Def 7.3.2 method [8].
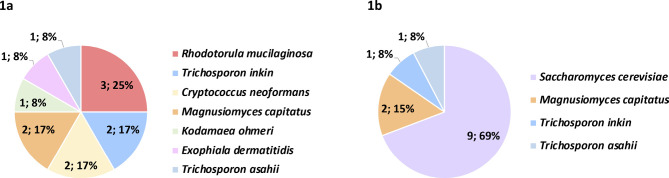
We detected a total of 25 non-Candida isolates sourcing from blood cultures (n=12) or intra-abdominal samples (n=13; peritoneal samples [n=11], liver samples [n=2]) that represented 1.1% (n=12/1,101) and 1.3% (n=13/1,031) of isolates from blood cultures and intra-abdominal samples, respectively (Figure 1). Non-Candida yeasts were found in 1.3 % (n=25/1,912) of the patients (Candida spp were simultaneously found in 8/25 patients). Interestingly, higher species diversity was found in isolates from blood cultures; the species found in intra-abdominal samples were also found in blood culture isolates except for S. cerevisiae, which was exclusively found in intra-abdominal samples. Blood-cultured isolates were mainly sourced from patients in medical (42%) or ICU wards (33%), whereas intra-abdominal isolates were mainly sourced from patients in surgery wards (77%). The number of isolates detected per year were 12 in 2019, 8 in 2020, and 5 in 2021.
Figure 1.
Non-Candida species found in blood cultures (1a) or intra-abdominal samples (1b)
Antifungal MIC ranges against the isolates were: amphotericin B, 0.125 – 4 mg/L; fluconazole 0.5 - >64 mg/L; voriconazole, 0.008 – 8 mg/L; posaconazole, 0.016 – 2 mg/L; isavuconazole, 0.016 - 2 mg/L; micafungin, 0.03 - >8 mg/L; anidulafungin, 0.016 - >8 mg/L; and ibrexafungerp, 0.25 - >8 mg/L. MIC distributions broken down per species is shown in Table 1. Clinical breakpoints to classify these species as resistant or susceptible according to EUCAST methodology are absent with the exception of amphotericin B against Cryptococcus neoformans; ECOFFS are only available for amphotericin B against Saccharomyces cerevisiae, and amphotericin B, voriconazole and posaconazole against Cryptococcus neoformans [9]. In those cases we did not find any resistant/non-wild type isolate but echinocandins and ibrexafungerp presented high MICs against the Trichosporon, Magnusiomyces, Rhodotorula, and Cryptococcus isolates studied (Table 1). If all isolates are considered intrinsically echinocandin-resistant, then the overall echinocandin resistance rate would rise from 0.5% to 1.5% (P<0.05) in blood cultures, and from 1.0% to 2.2% (P<0.05) in intra-abdominal samples. Likewise, adopting the non-species-specific fluconazole EUCAST breakpoint (resistant >4 mg/L) [10], a total of n=10 isolates should be considered fluconazole-resistant (Magnusiomyces capitatus [n=3], Rhodotorula mucilaginosa [n=3], Saccharomyces cerevisiae [n=3], and Trichosporon asahii [n=1]), and the overall fluconazole resistance rate would also rise slightly from 9.1% to 9.5% (P>0.05) in blood cultures, and from 8.2% to 8.4% (P>0.05) in intra-abdominal samples.
Table 1.
Minimum inhibitory concentration (MIC) distributions of the eight antifungal drugs used against the species tested
| MIC distributions (no.of isolates at each MIC,in mg/L) | No.isolates (%) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ≤0.016 | 0.03 | 0.06 | 0.125 | 0.25 | 0.5 | 1 | 2 | 4 | ≥8 | 16 | 32 | ≥64 | Non-wild type | Resistant | |
| Saccharomyces cerevisiae (n=9) | |||||||||||||||
| Amphotericin B | - | - | 0 | 4 | 3 | 2 | 0 | 0 | 0 | 0 | - | - | - | 0(0) | ND |
| Fluconazole | - | - | 0 | 0 | 0 | 0 | 1 | 2 | 3 | 1 | 1 | 0 | 1 | ND | ND |
| Voriconazole | 0 | 1 | 6 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | - | - | - | ND | ND |
| Posaconazole | 0 | 0 | 1 | 1 | 4 | 2 | 1 | 0 | 0 | 0 | - | - | - | ND | ND |
| Isavuconazole | 1 | 3 | 3 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | - | - | - | ND | ND |
| Micafungin | 0 | 1 | 1 | 6 | 1 | 0 | 0 | 0 | 0 | 0 | - | - | - | ND | ND |
| Anidulafungin | 1 | 0 | 2 | 5 | 1 | 0 | 0 | 0 | 0 | 0 | - | - | - | ND | ND |
| Ibrexafungerp | 0 | 0 | 0 | 0 | 1 | 6 | 2 | 0 | 0 | 0 | - | - | - | ND | ND |
| Trichosporon spp (n=5) | |||||||||||||||
| Amphotericin B | - | - | 0 | 0 | 0 | 1 | 0 | 2 | 2 | 0 | - | - | - | ND | ND |
| Fluconazole | - | - | - | 0 | 0 | 1 | 1 | 0 | 2 | 0 | 0 | 1 | 0 | ND | ND |
| Voriconazole | 2 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | - | - | - | ND | ND |
| Posaconazole | 0 | 0 | 3 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | - | - | - | ND | ND |
| Isavuconazole | 2 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | - | - | - | ND | ND |
| Micafungin | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | - | - | - | ND | ND |
| Anidulafungin | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | - | - | - | ND | ND |
| Ibrexafungerp | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 3 | 1 | - | - | - | ND | ND |
| Magnusiomyces capitatus (n=4) | |||||||||||||||
| Amphotericin B | - | - | 0 | 0 | 0 | 2 | 2 | 0 | 0 | 0 | - | - | - | ND | ND |
| Fluconazole | - | - | - | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 1 | 0 | ND | ND |
| Voriconazole | 0 | 0 | 0 | 3 | 0 | 1 | 0 | 0 | 0 | 0 | - | - | - | ND | ND |
| Posaconazole | 0 | 0 | 0 | 1 | 2 | 1 | 0 | 0 | 0 | 0 | - | - | - | ND | ND |
| Isavuconazole | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | - | - | - | ND | ND |
| Micafungin | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 3 | - | - | - | ND | ND |
| Anidulafungin | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 2 | 0 | - | - | - | ND | ND |
| Ibrexafungerp | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | - | - | - | ND | ND |
| Rhodotorula mucilaginosa (n=3) | |||||||||||||||
| Amphotericin B | - | - | 0 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | - | - | - | ND | ND |
| Fluconazole | - | - | - | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | ND | ND |
| Voriconazole | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 1 | - | - | - | ND | ND |
| Posaconazole | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 2 | 0 | 0 | - | - | - | ND | ND |
| Isavuconazole | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | - | - | - | ND | ND |
| Micafungin | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | - | - | - | ND | ND |
| Anidulafungin | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | - | - | - | ND | ND |
| Ibrexafungerp | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | - | - | - | ND | ND |
| Cryptococcus neoformans (n=2) | |||||||||||||||
| Amphotericin B | - | - | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | - | - | - | 0(0) | 0(0) |
| Fluconazole | - | - | - | 0 | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | ND | ND |
| Voriconazole | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | - | - | - | 0(0) | ND |
| Posaconazole | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | - | - | - | 0(0) | ND |
| Isavuconazole | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | - | - | - | ND | ND |
| Micafungin | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | - | - | - | ND | ND |
| Anidulafungin | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | - | - | - | ND | ND |
| Ibrexafungerp | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | - | - | - | ND | ND |
| Kodamaea ohmeri (n=1) | |||||||||||||||
| Amphotericin B | - | - | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | - | - | - | ND | ND |
| Fluconazole | - | - | - | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | ND | ND |
| Voriconazole | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | - | - | - | ND | ND |
| Posaconazole | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | - | - | - | ND | ND |
| Isavuconazole | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | - | - | - | ND | ND |
| Micafungin | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | - | - | - | ND | ND |
| Anidulafungin | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | - | - | - | ND | ND |
| Ibrexafungerp | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | - | - | - | ND | ND |
| Exophiala dermatitidis (n=1) | |||||||||||||||
| Amphotericin B | - | - | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | - | - | - | ND | ND |
| Fluconazole | - | - | - | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | ND | ND |
| Voriconazole | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | - | - | - | ND | ND |
| Posaconazole | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | - | - | - | ND | ND |
| Isavuconazole | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | - | - | - | ND | ND |
| Micafungin | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | - | - | - | ND | ND |
| Anidulafungin | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | - | - | - | ND | ND |
| Ibrexafungerp | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | - | - | - | ND | ND |
“-“, antifungal concentration not tested. “ND”, not determined as either breakpoints or ECOFFs were not available
Underlined values indicate non-wild-type isolates according to ECOFFs, and values in bold indicate resistant isolates [9]
In conclusion, we observed that non-Candida yeasts represented 1.1% and 1.3% of isolates from blood cultures and intra-abdominal samples, respectively. Considering these species as a cause of fungaemia will lead to increased rates of echinocandin resistance given the intrinsically diminished susceptibility of the species to these drugs. The future setting of ECOFF and clinical breakpoints may offer clinicians better guidance in the management of patients with invasive infections caused by non-Candida yeasts.
ACKNOWLEDGEMENTS
The CANDIMAD study group: Judith Díaz-García, Aina Mesquida, Ana Gómez, Marina Machado, Luis Alcalá, Elena Reigadas, Carlos Sánchez-Carrillo, Patricia Muñoz, Pilar Escribano, Jesús Guinea (Hospital General Universitario Gregorio Marañón); Ana Pérez-Ayala, Rosaura Pérez Muñoz, María del Carmen Vera González (Hospital Universitario 12 de Octubre); Elia Gómez-García De La Pedrosa (Hospital Universitario Ramón y Cajal); Fernando González Romo, Paloma Merino-Amador (Hospital Clínico San Carlos); María Soledad Cuétara, Oscar Manuel Muñoz Clemente, Víctor Antón Berenguer (Hospital Universitario Severo Ochoa); Aída SánchezGarcía (Hospital Universitario Infanta Sofía); Coral García-Esteban, Oscar Cuevas Lobato, Guadalupe Bernal (Hospital Universitario de Getafe); Nelly Zurita, Ainhoa Gutiérrez Cobos (Hospital Universitario de La Princesa); María Muñoz-Algarra, Isabel Sánchez Romero (Hospital Universitario Puerta de Hierro); Inmaculada Quiles-Melero, Florinda San Juan Delgado (Hospital Universitario La Paz); María Teresa Durán-Valle, Yolanda Gil Romero, Arturo Manuel Fraile Torres (Hospital Universitario de Móstoles).
ETHICAL CONSIDERATIONS
This study was approved by the Ethics Committee of the Gregorio Marañón Hospital (CEim; study no. MICRO. HGUGM.2019-001).
FUNDING
This study was supported by grants PI18/01155 and PI19/00074 from the Fondo de Investigación Sanitaria (FIS. Instituto de Salud Carlos III; Plan Nacional de I+D+I 2017-2020). The study was co-funded by the European Regional Development Fund (FEDER) ‘A way of making Europe.’ This study was partially funded by Scynexis, Inc., Jersey City, NJ, USA.
PE (CPII20/00015) is a recipient of a Miguel Servet contract supported by FIS. JG holds a permanent position at Fundación para Investigación Sanitaria del Hospital Gregorio Marañón. JD (FI19/00021) holds a predoctoral grant from the FIS.
CONFLICT OF INTEREST
JG has received funds for participating in educational activities organized on behalf of Gilead, Pfizer, Mundipharma, and MSD; he has also received research funds from FIS, Gilead, Scynexis, F2G, Mundipharma, and Cidara, outside the submitted work.
References
- 1.Pfaller MA, Diekema DJ, Turnidge JD, Castanheira M, Jones RN. Twenty Years of the SENTRY Antifungal Surveillance Program: Results for Candida Species From 1997-2016. Open Forum Infect Dis. 2019;6:S79-s94. DOI: 10.1093/ofid/ofy358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fernández-Ruiz M, Guinea J, Puig-Asensio M, Zaragoza Ó, Almirante B, Cuenca-Estrella M, et al. Fungemia due to rare opportunistic yeasts: data from a population-based surveillance in Spain. Med Mycol. 2017;55(2):125-36. DOI: 10.1093/mmy/myw055 [DOI] [PubMed] [Google Scholar]
- 3.Álvarez-Uría A, Muñoz P, Vena A, Guinea J, Marcos-Zambrano LJ, Escribano P, et al. Fungaemia caused by rare yeasts: incidence, clinical characteristics and outcome over 10 years. J Antimicrob Chem-other. 2018;73(3):823-5. DOI: 10.1093/jac/dkx436 [DOI] [PubMed] [Google Scholar]
- 4.Alp S, Gulmez D, Ayaz CM, Arikan-Akdagli S, Akova M. Fungaemia due to rare yeasts in a tertiary care university centre within 18 years. Mycoses. 2020;63(5):488-93. DOI: 10.1111/myc.13072 [DOI] [PubMed] [Google Scholar]
- 5.Díaz-García J, Mesquida A, Sánchez-Carrillo C, Reigadas E, Muñoz P, Escribano P, et al. Monitoring the Epidemiology and Antifungal Resistance of Yeasts Causing Fungemia in a Tertiary Care Hospital in Madrid, Spain: Any Relevant Changes in the Last 13 Years? Antimicrob Agents Chemother. 2021;65(4):e01827-20. DOI: 10.1128/aac.01827-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Risum M, Astvad K, Johansen HK, Schønheyder HC, Rosenvinge F, Knudsen JD, et al. Update 2016-2018 of the Nationwide Danish Fungaemia Surveillance Study: Epidemiologic Changes in a 15-Year Perspective. J Fungi. 2021;7(6). DOI: 10.3390/jof7060491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Díaz-García J, Gómez A, Machado M, Alcalá L, Reigadas E, Sánchez-Carrillo C, et al. Blood and intra-abdominal Candida spp. from a multicentre study conducted in Madrid using EU-CAST: emergence of fluconazole resistance in Candida parapsilosis, low echinocandin resistance and absence of Candida auris. J Antimicrob Chemother. 2022; 77(11):3102-3109 DOI: 10.1093/jac/dkac288 [DOI] [PubMed] [Google Scholar]
- 8.Arendrup MC, Meletiadis J, Mouton JW, Lagrou K, Hamal P, Guinea J. EUCAST Definitive document E.DEF 7.3.2. Method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for yeasts. 2020. [DOI] [PubMed]
- 9.Overview of antifungal ECOFFs and clinical breakpoints for yeasts, moulds and dermatophytes using the EUCAST E.Def 7.3, E.Def 9.4 and E.Def 11.0 procedures v 3.0, 2022. Available from: https://www.eucast.org. Accessed on May 19, 2023
- 10.Arendrup MC, Friberg N, Mares M, Kahlmeter G, Meletiadis J, Guinea J. How to interpret MICs of antifungal compounds according to the revised clinical breakpoints v. 10.0 European committee on antimicrobial susceptibility testing (EUCAST). Clin Microbiol Infect. 2020;26(11):1464-72. DOI: 10.1016/j.cmi.2020.06.007 [DOI] [PubMed] [Google Scholar]