Abstract
Sexually Transmitted Infections (STI) are a major public health problem. The problems inherent to their diagnosis, treatment and prevention have to do not only with their nature, but also with organizational issues and overlapping competencies of the different health authorities in Spain.
The real situation of STI in Spain, at present, is poorly known. For this reason, the Scientific Committee on COVID and Emerging Pathogens of the Illustrious Official College of Physicians of Madrid (ICOMEM) has formulated a series of questions on this subject which were distributed, not only among the members of the Committee, but also among experts outside it. The central health authorities provide very high and increasing figures for gonococcal infection, syphilis, Chlamydia trachomatis infection and lymphogranuloma venereum (LGV). Both HIV infection and Monkeypox are two important STI caused by viruses in our environment, to which it should be added, mainly, Herpes simplex
virus (HSV) and Human papillomavirus (HPV) infections. Emerging microorganisms such as Mycoplasma genitalium pose not only pathogenic challenges but also therapeutic problems, as in the case of N. gonohrroeae.
The pathways that patients with suspected STI follow until they are adequately diagnosed and treated are not well known in Spain. Experts understand that this problem is fundamentally managed in public health institutions, and that Primary Care and Hospital Emergency Services, together with some institutions that deal monographically with this problem, are the recipients of most of these patients. One of the most serious difficulties of STI lies in the availability of the microbiological tests necessary for their diagnosis, particularly in this era of outsourcing of microbiology services. Added to this is the increased cost of implementing the latest generation of molecular techniques and the difficulties of transporting samples.
It is clear that STI are not diseases to which the entire population is equally exposed and it is necessary to have a better knowledge of the risk groups where to focus the necessary interventions adapted to their characteristics. It should not be forgotten that STI are also a problem in the pediatric age group and that their presence can be a marker of sexual abuse with all that this implies in terms of health care and medico-legal activity.
Finally, STI are infections that are associated with a high cost of care for which we have very little information. The possibility of expanding the automatic performance of laboratory tests for STI surveillance through laboratory routines is encountering ethical and legal problems that are not always easy to solve.
Spain has created a ministerial area of specific attention to STI and there are plans to improve the diagnosis, treatment and prevention of these problems, but we still lack the necessary evidence on their impact. We cannot forget that these are diseases that transcend the individual and constitute a Public Health problem.
Keywords: Sexually Transmitted Infections, Sexually Transmitted Diseases, STI, STDs, HIV, Monkeypox, Herpes simplex virus, Human Papillomavirus, Mycoplasma genitalium, syphilis, Neisseria gonorrhoeae, Gonococcal infections, High risk groups, Public Health
Abstract
Las Infecciones de Transmisión Sexual (ITS) constituyen un problema de Salud Pública de primera magnitud. Los problemas inherentes a su diagnóstico, tratamiento y prevención tienen que ver no solo con la naturaleza de las mismas, sino también con problemas de organización y de solapamiento de competencias de las distintas autoridades sanitarias.
La situación real de las ITS en España no se conoce bien en el momento actual. Por este motivo, el Comité Científico sobre COVID y Patógenos emergentes del Ilustre Colegio Oficial de Médicos de Madrid (ICOMEM) se ha formulado una serie de preguntas sobre este tema que ha distribuido, no sólo entre los miembros del Comité, sino también entre expertos ajenos al mismo. Las autoridades ministeriales aportan cifras muy elevadas y crecientes de infección gonocócica, sífilis, infección por Chlamydia trachomatis y Linfogranuloma venéreo. Tanto la infección por VIH como Monkeypox son en nuestro medio dos importantes ITS causadas por virus a las que deben añadirse, principalmente, las infecciones por el Virus herpes simplex (VHS) y el Virus del Papiloma Humano (HPV). Emergen patógenos como Mycoplasma genitalium que plantean no sólo retos patogénicos si no también problemas terapéuticos, como ocurre en el caso de N. gonohrroeae.
Los caminos que siguen los pacientes con sospecha de ITS hasta su adecuado diagnóstico y tratamiento no se conocen bien en España. Los expertos entienden que este problema es fundamentalmente manejado en instituciones sanitarias de titularidad pública, y que los servicios de Atención Primaria y de Urgencias Hospitalarias, junto con algunas instituciones monográficamente destinadas a este problema, son los receptores de la mayor parte de estas enfermedades. Una de las dificultades más serias de las ITS estriba en la disponibilidad de las pruebas microbiológicas necesarias para su diagnóstico, particularmente en esta época de externalización de servicios de Microbiología. A ello se suma el aumento de costes de la implantación de técnicas moleculares de última generación y las dificultades del transporte de muestras.
Está claro que las ITS no son enfermedades a las que esté igualmente expuesta toda la población, por lo que es necesario conocer mejor los grupos de riesgo donde centrarse las necesarias intervenciones, adaptadas a su idiosincrasia. No hay que olvidar que las ITS son también un problema en la edad pediátrica y que su presencia puede ser un marcador de abuso sexual con lo que supone de actividad asistencial pero también medicolegal.
Las ITS son, finalmente, infecciones que se asocian a un elevado gasto de cuyas cifras disponemos de información muy escasa. La posibilidad de la realización automática de pruebas de laboratorio para su detección tropieza con problemas éticos y legales que no siempre tienen una fácil solución.
España ha creado un área ministerial de atención específica a las ITS y existen planes para mejorar el diagnóstico, tratamiento y prevención de estos problemas, pero carecemos todavía de la necesaria evidencia sobre el impacto de los mismos. No podemos olvidar que se trata de enfermedades que trascienden al individuo y constituyen un problema de Salud Pública.
Palabras clave: Infecciones de Transmisión Sexual, Enfermedades de transmisión Sexual, ITS, ETS, VIH, Monkeypox, Virus Herpes simplex, Papilomavirus Humano, Mycoplasma genitalium, sífilis, Neisseria gonorrhoeae, Gonococia, Grupos se alto riesgo, Salud Pública
INTRODUCTION
Sexually Transmitted Infections (STI) have accompanied mankind throughout its history and have been particularly prevalent in turbulent times. They are more frequent in periods of war or when major social movements and changes occur, and it can be said that this group of infections have transformed history by affecting, and sometimes limiting, the lives of many prominent people in the arts and sciences.
The revolution brought about by the discovery and introduction of antibiotics created the mirage of a rapid end to diseases such as syphilis or gonorrhea, but it was only a few decades before the great pandemic of Human Immunodeficiency Virus (HIV) infection appeared, with its tragic consequences for millions of people. The HIV pandemic showed that there were still causative agents of STI to be discovered. The darkest years of the pandemic were associated with a decline in other STI, which soon rebounded with the discovery of agents capable of controlling HIV infection and the consequent abandonment of some prevention measures.
The recent epidemic of Monkeypox Virus infections has shown once again that microorganisms that were not previously considered to be typically sexually transmitted can become so only through changes in human movements and habits. The recent Coronavirus pandemic has also taught us lessons in the STI universe by proving the impact that a state of confinement can have on human-to-human relationships.
What is happening with STI is poorly understood outside very specialized circles and, even within these circles, very important gaps in information and data recording are recognized. The professionals closest to this problem reflect their concern at the “chilling” figures of the increase in the incidence of these diseases, affecting not only groups at particular risk but also the general population.
For this reason, the Illustrious Official College of Physicians of Madrid (ICOMEM) has formulated a series of questions about the situation of STI in Spain, summoning to answer them, not only members of the COVID-19 and Emerging Pathogens Committee, but also experts from outside the Committee. The following pages attempt to answer some of these questions, without pretending to cover a vast area, but trying to clarify some aspects of what is happening, preferably in Spain, at the present time.
AT PRESENT, WHAT IS THE BEST DEFINITION OF “SEXUALLY TRANSMITTED INFECTION” AND WHICH ARE THE MOST PREVALENT, PARTICULARLY IN SPAIN?
STI are a group of diseases of infectious etiology that produce heterogeneous clinical pictures and whose reservoir is human. Transmission occurs mainly from person to person, during sexual intercourse, although many of the microorganisms that cause them can also have other transmission mechanisms such as the perinatal or parenteral route. The probability of transmission from an infected patient varies according to the STI and its stage.
One of the important problems of this group of infections is that, in general, suffering from them does not generate immunity and, therefore, reinfections are possible and frequent. There is also no mutual exclusion between them, since the same individual can have more than one STI at the same time.
Although there are several infections that can be transmitted sexually, not all of them constitute an STI. In addition to the route of sexual transmission, the epidemiological context related to each microorganism must always be taken into account, since this context defines the necessary prevention and control measures to be applied from the health point of view. We can therefore conclude that the concept of STI does not extend to all infections that can be transmitted during sexual intercourse, but only to those in which the sexual route is the main mechanism of transmission and, moreover, this route of transmission is of special epidemiological interest [1].
Table 1 shows data on the epidemiological surveillance of STI that are notifiable diseases for 2021, published in 2023 by the HIV, STI and hepatitis B and C Surveillance Unit of the National Epidemiology Center of the Carlos III Health Institute (Instituto de Salud Carlos III) [2]. The data establish incidence rates ranging from 1.66 episodes per 100,000 population for LGV to 48.36 episodes per 100,000 population for C. trachomatis infection. There is a clear predominance of males among reported cases and rates are particularly high among the population aged 20-24 years. National incidence data for other STI are not known, since they are not diseases subject to surveillance through the National Epidemiological Surveillance Network (RENAVE).
Table 1.
Epidemiological situation of gonococcal infection, syphilis, C. trachomatis infection and LGV in Spain 2021. Adapted from reference [2].
| Indicators | Gonococcal infection | Syphilis | C. trachomatis infection | Lymphogranuloma venereum |
|---|---|---|---|---|
| Number of Autonomous Communities that notify | 19 | 19 | 15 | 11 |
| Number of cases reported | 15,338 | 6,613 | 20,507 | 649 |
| Rate per 100,000 inhabitants* | 32.41 | 13.97 | 48.36 | 1.66 |
| Male: female ratio | 5.0 | 9.6 | 0.9 | 80.1 |
| Percentage of men | 83.0% | 86.6% | 49.8% | 98.8% |
| Percentage of cases in people under 25 years of age | 23.1% | 12.0% | 39.5% | 7.4% |
| Rate between 20-24 years per 100,000 inhabitants* | 110.02 | 28.02 | 251.49 | 2.28 |
Calculated for all the Autonomous Communities that have a surveillance system and that notified in 2021
TO WHAT EXTENT IS HIV STILL SEXUALLY TRANSMITTED IN SPAIN?
During the 1980s and 1990s, at the onset of the HIV pandemic, the most frequent mechanism of HIV transmission in Spain, unlike in most countries of the world, was not sexual transmission but injecting drug use (IDU). The measures adopted to prevent HIV transmission among IDUs, among other causes, decreased the incidence of infection associated with this risky practice and unprotected sex became the transmission mechanism for virtually all new infections since the mid-1990s.
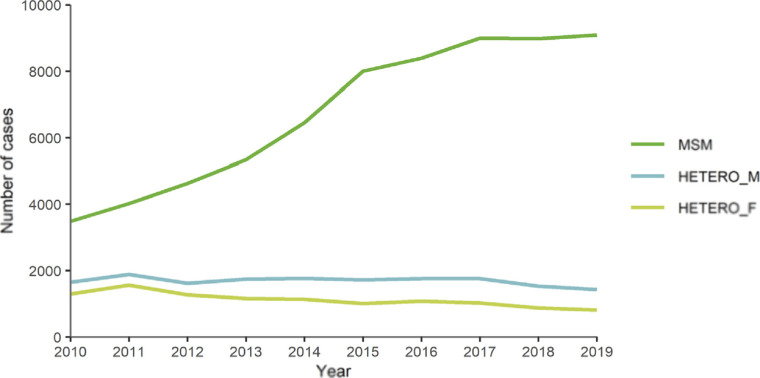
Official epidemiological surveillance in Spain confirms that this trend continues today [3]. In the latest report on HIV infection in Spain, 81.7% of new HIV diagnoses in 2021 were sexually transmitted and only 2.2% had another route of transmission documented (in 16% the route of transmission was not recorded). Transmission in men who have sex with men (MSM) was the most frequent, 56.3%, followed by heterosexual transmission, which accounted for 25.4%, overall. The importance of sexual transmission is maintained when broken down by sex. Among men, MSM transmission accounted for 65.4% of new HIV diagnoses and heterosexual transmission for 16.6%. Among women, heterosexual transmission constitutes the vast majority, accounting for 79.9% of new diagnoses.
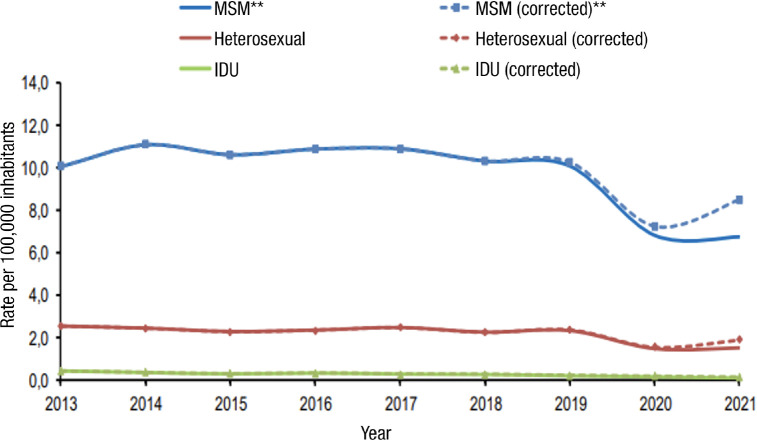
It is important to note that the sexually transmitted route has remained responsible for the highest number of new infections throughout the period 2013-2021, where all the infections reported by the Autonomous Communities are collected (Figure 1). The number of cases reported in this period was 35,019. Interestingly, both the overall rate and the rate by sex and by mode of transmission show a statistically significant downward trend. In summary, HIV infection is an infection that is mostly transmitted sexually. This fact is of enormous importance because it indicates that, without neglecting other forms of transmission, it is necessary to implement and reinforce effective actions to prevent transmission by this route. Within sexual transmission, the MSM group is a priority for prevention programs (Figure 1).
Figure 1.
Rates (per 100,000 population) of total annual new HIV diagnoses and by mechanism of transmission (2013-2021). Data corrected for delay in notification. Source: HIV, STI and hepatitis surveillance unit. Adapted from reference [3].
** Rate per 100.000 men, MSM: Men who have sex with men; IDU: injecting drug use
IS MONKEYPOX (MPOX) AN STI AND DO WE HAVE THE SPANISH FIGURES?
From the start of the current “Monkeypox” (Mpox pandemic, in June 2022, until March 1, 2023, WHO has reported a total of 86,231 cases of which 84,858 cases have been reported in countries that previously had not reported Mpox cases [4,5]. This incidence declines since the end of 2022 [5].
In Europe, 25,745 cases of Mpox have been reported to the ECDC from 47 countries as of February 28, 2023, through the European Surveillance System (TESSy). Ninety-eight percent were men, the vast majority (96%) being MSM. Thirty-eight percent were co-infected with HIV, 6% required hospitalization (6 in the ICU) and 5 people died as a direct result of this disease [6]. To date, WHO and ECDC have been informed of five cases of occupational exposure.
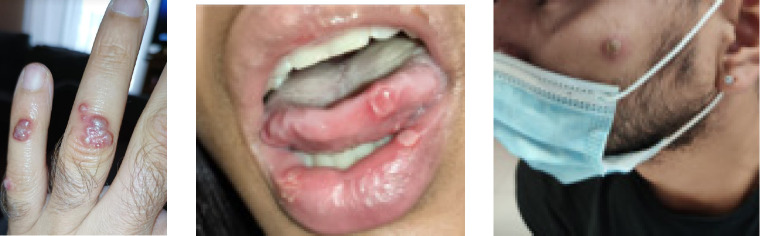
As of March 1, 2023, 7,541 cases of Mpox have been reported to the Spanish Ministry of Health’s Center for Health Alerts and Emergencies, in 17 Autonomous Communities (the most affected were Madrid, Catalonia, Andalusia, Valencia and the Basque Country). Ninety-eight percent were men with a median age of 37 years. Of the cases, 46.1% were born in Spain. Excluding cases with no information, 95.5% were MSM. Regarding the route of transmission, of the 5,725 reported cases, 82.6% were attributed to close contact in the context of sexual intercourse, 6.1% to non-sexual close contact (including cases in children) and 2 cases were due to occupational exposure in the healthcare setting. About 70% of the cases had general symptoms and in more than 60% the most common presentation was rash in the anogenital region (Figure 2). Overall, 9.1% presented complications, the most frequent being bacterial infections, pneumonitis, encephalitis, keratitis and oral ulcers. Three people have died: two cases due to meningoencephalitis and one case due to causes unrelated to Mpox. Since September 2022, there has been a clear decrease in the number of new cases of Mpox in Spain [7,8].
Figure 2.
Some examples of lesions caused by Mpox.
In Madrid, among the 435 first diagnosed cases of Mpox in an STI clinic (Centro Sandoval), 98% were MSM. Their median age was 37 years and 38% were co-infected with HIV. Thirty-eight percent of the Mpox patients were users of HIV Pre-Exposure Prophylaxis (PrEP) and 23% were HIV-uninfected MSM not receiving PrEP [9,10].
With respect to categorizing the Mpox pandemic as STI or non-STI, the epidemiological data reported in countries where data exist unquestionably confirm that it is an STI that has particularly affected MSM. The Mpox pandemic of 2022 would probably not have occurred in the absence of sexual transmission [4], although there is much evidence that not all cases have been transmitted in the context of sexual intercourse. The main route of spread has probably been different in previous outbreaks of Mpox.
Some public health officials and community representatives have expressed concern regarding the stigmatization of MSM by the spread of the large proportion of Mpox cases in that population. However, both the HIV and COVID-19 pandemics have demonstrated the need to provide clear and accurate information to the public as a preventive strategy (behavioral and vaccine) that raises awareness among individuals and the most affected population groups.
WHAT IS THE REALITY OF OTHER STI CAUSED BY VIRUSES IN SPAIN?
STI of viral etiology, in addition to HIV, include genital herpes and human papillomavirus (HPV) infection. Other viruses such as the causative agents of viral hepatitis, cytomegalo-virus (CMV) or poxvirus, although they can be sexually transmitted, are not genuinely STI.
Genital herpes is caused by herpes simplex virus (HSV), usually HSV2. Transmission occurs through sexual contact, either vaginally, anally or orally. Clinically it is characterized by the presence of vesicles on the vulva or vagina, penis, anus, rectum and, more rarely, in the mouth, which can lead to the appearance of very painful abrasions and ulcers. Many people with genital herpes infection have no symptoms, but transmission from asymptomatic individuals is possible.
It is estimated that between 400 and 500 million people between 15 and 49 years of age worldwide are carriers of HSV [11,12]. In Spain, experts estimate that between 10-15% of the adult population may be carriers of HSV. As for the incidence of the disease, since it is not a notifiable disease, the figures can easily be underestimated. It is the most common sexually transmitted viral disease. There has been an exponential increase since the 2010s, which seems to have stabilized at around 50,000 cases per year in Spain [13] (Figure 3). In a multicenter study carried out during the state of COVID-19 alarm in our country, in a population of 674 subjects with STI, microbiological results were obtained in 519, 10% corresponding to HSV [14]. As in the rest of STI, the risk of genital herpes is higher in MSM, sex workers and people with greater sexual promiscuity. Although the disease is more frequently linked to HSV-2, in recent years a progressive increase in HSV-1 infection is being observed in cases of genital herpes [15]. The diagnosis of genital herpes can be made clinically and confirmed by PCR techniques, replacing cell culture. A topic currently under discussion is the usefulness of performing serology to detect antibodies at least in the at-risk population. It would be indicated in couples in which one of them has a history or lesions of genital herpes and the serological status of the other is unknown. Also, in pregnant women with no history of genital herpes, whose partners do have it. Treatment is aimed at reducing symptoms and their duration, as well as preventing outbreaks. Acyclovir, valacyclovir or famciclovir may be used for treatment [16]. In patients with repeated outbreaks (more than 5 per year), preventive treatment, maintained for 12 months, is recommended, without the Cochrane review published in 2014 showing superiority of any of these drugs [17].
Figure 3.
Evolution of the incidence of genital herpes in Spain. Adapted from reference [13].
Anogenital HPV is the most common STI worldwide. The peak prevalence of HPV infection usually occurs between the ages of 15 and 25 years in most Western countries. It has been estimated that at least 80% of sexually active people are exposed to HPV at least once in their lifetime [18].
Most individuals exposed to HPV infection manage to clear the virus without developing lesions in less than two years [19]. Some of these viruses manage to integrate into the genome of the host cell and this fact contributes to oncologic transformation following the sequence of dysplasia, high-grade dysplasia and carcinoma. The role of HPV as a carcinogenic agent has been highlighted by the International Agency for Research on Cancer and the WHO [20]. Carcinogenic serotypes such as HPV-16 are the causative agents of multiple cancers (penis, anus, cervix, vagina, vulva, oropharynx, etc.) [20]. This makes it necessary in high-risk populations, such as people with HPV lesions in the anal canal, to carry out periodic diagnostic screening, as is done for the early detection of cervical carcinoma [21].
Routine HPV vaccination of adolescents and young adults is recommended in many countries. Following these vaccination programs, many studies have reported a decrease in the prevalence and incidence of HPV infection and HPV-related diseases [22-24].
WHAT ARE THE FIGURES FOR CHLAMYDIA SP INFECTION AS AN STI?
Chlamydia trachomatis is a bacterium that is the most common cause of bacterial STI in both men and women [25-28]. In the United States, it is the most frequent notifiable disease after SARS-CoV-2 infection (COVID-19).
The most common clinical manifestations of C. trachomatis infection are urethritis, cervicitis and proctitis (serotypes D-K). Lymphogranuloma venereum (LGV) (serotype L) is much less common. Local complications of these infections are orchi-epididymitis in males and tubaritis, ectopic pregnancy and pelvic inflammatory disease (PID) in females. They can also produce systemic complications, reactive arthritis being one of the best known. C. trachomatis infections can cause vertically transmitted infections during delivery that may manifest as conjunctivitis or pneumonia in the newborn.
Seventy percent of C. trachomatis infections in women and 50% in men are asymptomatic, which is a further complication in preventing transmission.
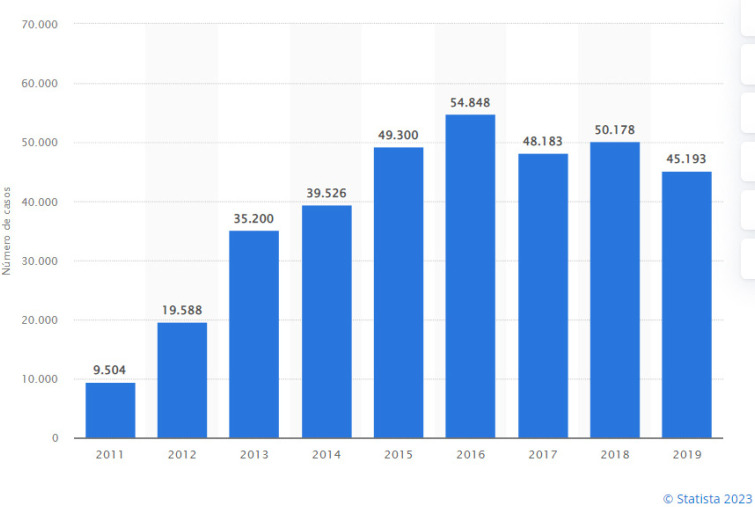
The incidence of C. trachomatis infection has increased in Europe over the past 20 years (Figure 4), where 434,184 cases were reported in 2019, of which two-thirds occurred in persons under 25 years of age. In 2019, 3,112 cases of LGV were reported, mainly (87%) from 4 countries: France, the Netherlands, the United Kingdom, and Spain [28bis]. These patients, almost without exception (>99%), were MSM and 64% were co-infected with HIV.
Figure 4.
Number of confirmed cases of C. trachomatis infection in the European Union (from countries reporting consistently). Obtained from reference [28bis].
In Spain, genital infection by C. trachomatis has shown a progressive increase in incidence in recent years (Figure 5) [3]. There were 17,718 cases reported in 2019, representing a rate of 44.18 episodes / 100,000 population (54.4% in women). In our country, more than 40% of cases are under 25 years of age [3]. As for LGV, 453 cases were reported in Spain in 2019. As in Europe, 99% were MSM and 70% of the cases have been reported in Catalonia (rate 1.24/ 100,000 inhabitants).
Figure 5.
Evolution of the number of cases and rates of C. trachomatis infection in Spain 2016-2019. Obtained from reference 2.
The treatment of choice for common C. trachomatis infections is the combination of doxycycline (100mg/12h x 7 days) and azithromycin (1g in single dose). In the case of LGV, the association of doxycycline (100mg/12h x 21 days) and azithromycin (1g/week x 3 weeks) is recommended.
WHICH MYCOPLASMAS PLAY A ROLE AMONG THE IMPORTANT STI IN SPAIN?
Among the Mycoplasmas, it is M. genitalium (MG) that is of increasing interest. Since 2015, it is considered by WHO as an emerging sexually transmitted pathogen. It was isolated for the first time in 1980 from specimens from patients with urethral syndrome [29]. The overall prevalence of MG is estimated to be between 1 and 3.3% of the general population, a frequency that increases in at-risk populations (35%) [30,31].
The infection initially appeared to be limited to a symptomatic or asymptomatic genitourinary condition, primarily urethritis (accounting for 40% of persistent or recurrent urethritis), but has been shown to cause cervicitis, pelvic inflammatory disease, and has been associated with preterm delivery, miscarriage and infertility. Its role as an agent of proctitis is under discussion at this time. Its presence in the pharynx is asymptomatic and does not appear to be associated with disease or subsequent complications [30-32].
Traditional microbiological methods are not useful for diagnosis. Culture is very difficult, requires a long incubation and has a low sensitivity (50%). Its detection is therefore based on the use of molecular techniques [30,32-34]. However, these techniques are not implemented in all laboratories, leading to a major problem of underdiagnosis.
MG is intrinsically resistant to antimicrobials acting at the cell wall level (beta-lactams, glycopeptides or fosfomycin), as it lacks a cell wall. The frequency of cure with doxycycline remains stable at 30-40%, although early studies showed in-vitro activity [35]. The first line of treatment is azithromycin at present, but mutations have been detected in 23S rRNA (polymorphism in 2058 and 2059) that produce changes in the 50S ribosomal subunit and confer a high level of resistance to macrolides. These mutations can appear immediately after treatment in 10% of the isolated microorganisms that were initially sensitive. The prevalence of azithromycin resistance is increasing significantly in recent years [36], although it differs greatly from one geographical area to another. Data collected in Spain place it between 20-35% [37]. Recent studies show that an extended regimen with an increased dose (1 g on the first day followed by 500 mg/d for 3 more days) achieves a higher response rate (85-95% in sensitive MG infections) and decreases the selection of resistance mutants [30,32,36-39].
Moxifloxacin (400 mg/d for 1 week) is the alternative treatment of choice in case of detection of macrolide resistance mutations. Resistance to quinolones has been associated, although less consistently, to mutations in the genes encoding DNA topoisomerase IV, mainly at parC level (S83 and D87), with a much lower prevalence [30, 32,37,39]. Coexistence of macrolide and quinolone resistance mutations leaves few treatment options [40].
A sequential treatment is recommended, starting with doxycycline to reduce the bacterial load, followed by azithromycin or moxifloxacin, according to the macrolide resistance study. This allows to have a greater response and decrease the selection of resistances [30,32,39]. Pristinamycin could be an alternative in case of failure [30,32,40,41].
WHAT IS HAPPENING WITH SYPHILIS IN THE WORLD, IN EUROPE AND IN SPAIN?
In 2020, WHO reported 7.1 million cases of syphilis worldwide. In 2016, approximately one million pregnant women had syphilis causing obstetric complications in more than 350,000 deliveries. All this despite the recommendation of rapid testing for syphilis screening in pregnant women, especially in pregnant women less than 28 weeks pregnant and during the third trimester in women with risk factors. WHO’s goal is to drastically reduce syphilis cases in any population group and particularly congenital syphilis.
In a communication from the European Centre for Disease Control in Stockholm [42], 35,039 new confirmed cases of syphilis were reported in 29 EU/EEA Member States in 2019, with a crude notification rate of 7.4 cases per 100,000 population. Reported syphilis rates were nine times higher in men than in women, peaking in the male age group 25-34 years (31 cases per 100,000 population). The majority (74%) of syphilis cases with transmission category information were reported in MSM. Between 2010 and 2017, the trend in syphilis notifications among men increased steadily, mainly due to an increase in the number of cases among MSM. However, this increase appears to have slowed in 2018 and 2019. During the same period, there were very small fluctuations in syphilis notifications among heterosexuals at the EU/EEA level. In 2019, the number of MSM cases with seropositive status decreased by 1%, while the number of MSM cases with seronegative status increased by 2% compared to 2018.
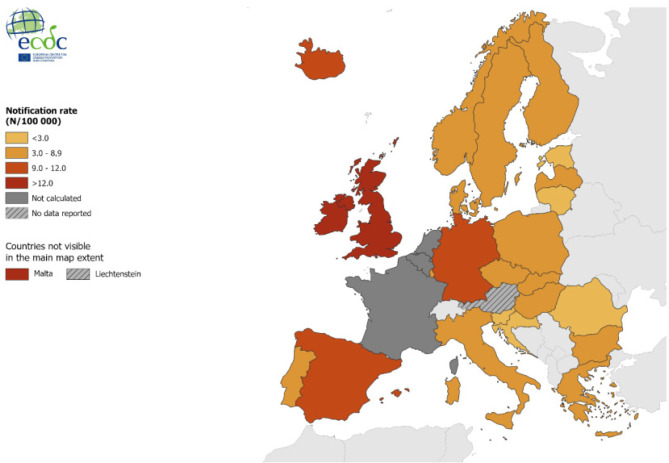
In Figure 6, the distribution of reported cases can be seen, in which Spain appears as one of the countries with the highest rates. In that European report, the evolution of the figures in the last decade can be appreciated, with a very significant increase in incidence attributable mainly to the MSM population that represents 74% of all episodes (Figure 7) [42]. In European data, HIV coinfection in patients with syphilis is 23%, which rises to 34% in MSM.
Figure 6.
Distribution of syphilis cases in Europe in 2019. Obtained from reference [42].
Figure 7.
Number of confirmed syphilis cases by gender, transmission, category and year in EU/EEA countries reporting consistently, 2010-2019. Obtained from reference [42].
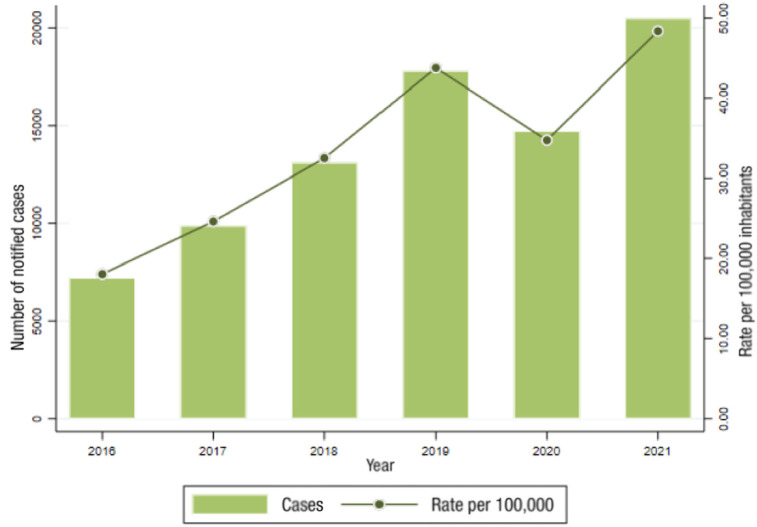
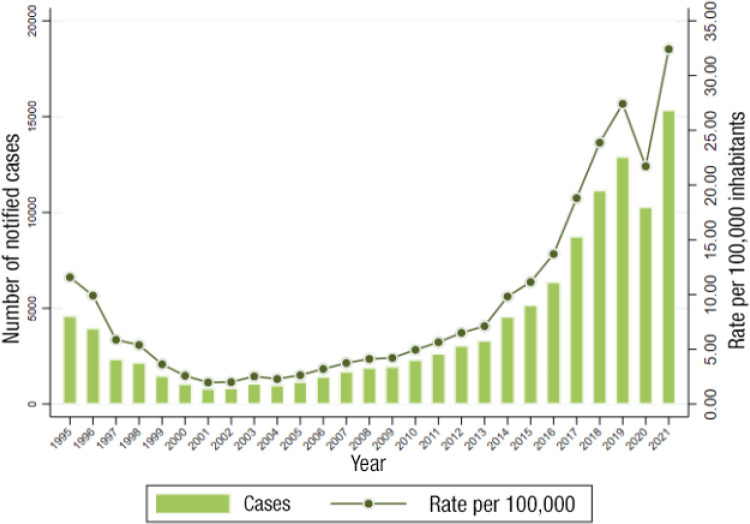
In Spain, syphilis data reported by the central health authority are 5,822 cases (88.7% male) in 2019, which is a rate of 13.29 cases per 100,000 population. The figures rise to 30.81 cases in the population aged between 20 and 24 years (Figure 8) [3]. This impressive increase may be due in part to improved data collection methods.
Figure 8.
Evolution of syphilis rates in Spain between 1995 and 2021. Adapted from reference [2].
WHAT ARE THE PROBLEMS POSED BY GONOCOCCAL INFECTION IN OUR ENVIRONMENT?
Neisseria gonorrhoeae (NG) infection remains a major public health problem today. It mainly affects the epithelium of the urethra, cervix, rectum, oropharynx and conjunctiva. In cases of genital involvement, it can ascend and cause PID in women and orchi-epididymitis and prostatitis in men. Other complications such as disseminated infection, bacteremia, skin lesions, arthritis and tenosynovitis, perihepatitis (Fitz-Hugh Curtis syndrome), meningitis or endocarditis are not frequent.
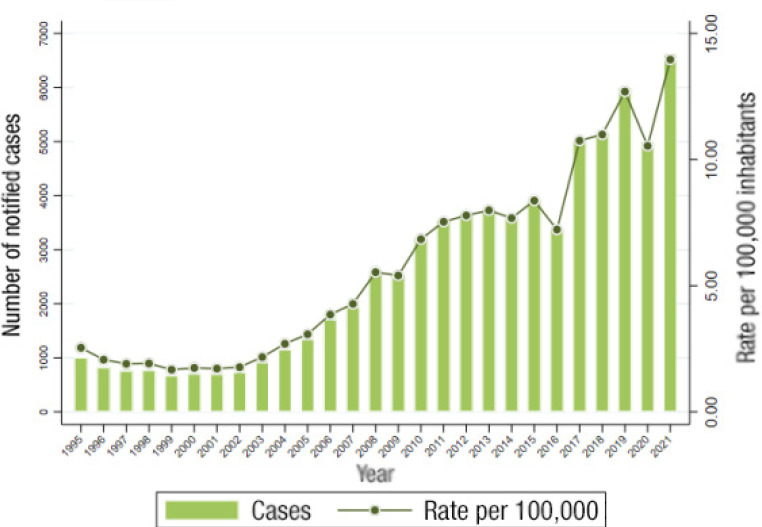
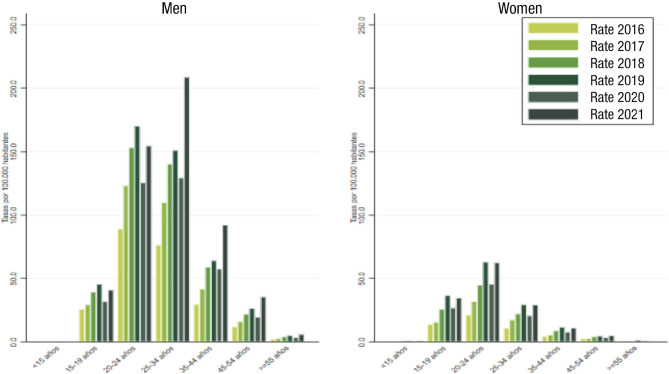
In recent years, we are experiencing a continuous increase in the incidence of gonococcal infection. The latest data published globally in Spain report a rate of 28.88/100,000 inhabitants in 2019, an increase of 25.2% over 2013 (Figure 9) [3]. The rate in men (79.7%) is higher than that observed in women. The trend is upward for both sexes, with an annual percentage change of 26.6% in men and 35.7% in women, and a significant increase in all age groups (Figure 10) [43]. The analysis of data from subsequent years will allow to assess the real impact that the COVID-19 pandemic has had on the incidence of gonococcal disease. In the experience of the Gregorio Marañón Hospital, a decrease in cases was observed in 2020 with respect to the previous year (x0.65) and a progressive increase thereafter (x1.63) (María Palomo, unpublished data).
Figure 9.
Evolution of the incidence of gonococcal infection in Spain between 1995 and 2021. Adapted from reference [2].
Figure 10.
Evolution of the incidence of gonococcal infection in Spain between 2016 and 2021 by age group and sex. Adapted from reference [2].
Therapeutically, NG is a microorganism that has become resistant to the different antimicrobial regimens used over time, with the result that treatment possibilities are increasingly reduced. In 2011, due to the appearance of the first cephalosporin-resistant strains, it was decided to add azithromycin to the cephalosporin regimen for uncomplicated gonococcal infections in order to optimize treatment and preserve sensitivity to these drugs. Surveillance studies in subsequent years have shown high rates of resistance to ciprofloxacin (between 30-70% in Spain), so it is not, any more, an option for empirical treatment, although it could be a good choice for susceptible strains [44,45]. Resistance rates to azithromycin have been progressively increasing in Spain, and are estimated at 5-30%. It is likely that its use is also related to the increased resistance of other microorganisms, including M. genitalium. Data on cephalosporin resistance in Spain remain low at present (<5%) [45,46].
In this context, the different international organizations recommend treatment with cephalosporins at an increased dose, although there is no established consensus. The United States guidelines opt for cephalosporins in monotherapy, as in the United Kingdom, although at higher doses; while the European and Australian guidelines continue to opt for doble therapy [45,47,48]. As an alternative, gentamicin can be used (in combination with azithromycin), which has been shown to be useful in patients allergic to beta-lactams, when there is no data on sensitivity to ciprofloxacin or the isolate is resistant to ciprofloxacin [32,44,47]. Recent studies have shown that ertapenem could be a therapeutic option in cases of resistance to cephalosporins [49-51].
The application of molecular techniques for the detection of resistance determinants is under development, but for the moment they have limitations and are not incorporated into daily practice [52]. It is therefore essential not to stop using bacteriological culture techniques in order to be able to carry out subsequent resistance studies.
Prevention is the basic pillar in the fight against this disease. There are no specific vaccines available, although studies carried out with the serogroup B meningococcal vaccine show promising results of cross-protection [52].
WHAT ARE THE GROUPS OF PEOPLE AT GREATEST RISK OF STI IN SPAIN?
DO ANY OF THESE GROUPS HAVE AN ORGANIZATION AS A PATIENT GROUP?
The groups with the highest risk of acquiring and transmitting an STI in our environment are MSM, sex workers and people with previous STI.
In Spain, there are several non-governmental organizations (NGOs) and community-based associations (ABC) that work to promote equal rights, the promotion of healthy sexuality without discrimination, and also to make LGBTQ+ people visible. Some of the most prominent are: Fundación Triángulo; COGAM (Colectivo de Lesbianas, Gays, Transexuales y Bisexuales de Madrid); FELGTB (Federación Estatal de Lesbianas, Gais, Trans y Bisexuales); Chrysallis (Asociación de Familias de Menores Transexuales); CESIDA (Coordinadora Estatal de VIH-SIDA); Spanish Red Cross; Gais Positius; Stop Sida; Apoyo Positivo; Barcelona Checkpoint and Sevilla - Checkpoint.
Historically, these entities have played a crucial role in the fight against HIV/AIDS. At the beginning of the epidemic, the organization of people to provide support and care to these patients played a fundamental and indispensable role in the progress and improvement of the global response to this disease. NGOs and ABCs play a vital role in the detection and prevention of HIV and STI. Their work, together and in collaboration with government and other institutions, lies in improving public health at the grassroots level, advocating for gender equality and promoting the elimination of discrimination [53].
The UNAIDS 2021-2026 strategy proposes that up to 30% of diagnostic testing and treatment services involve the community by 2026, with a focus on access to testing, linkage to treatment, and support for adherence and retention [54]. For this reason, the new Strategic Plan 2021 presented in Spain aims to work in a complementary manner with institutions and civil society organizations that promote actions that foster education, training, knowledge, and empowerment of people with HIV/STI infection and other population groups with risk practices in order to promote a positive, healthy sexuality and put an end to the HIV epidemic, its stigma and discrimination. From the National Public Health System, community participation has been granted in the decision-making processes on the response to the HIV epidemic and other STI, acquiring a cross-cutting and interdisciplinary approach: civil society, medical personnel, researchers and politicians, joining and adding tools to better address the response to this public health problem [53].
WHAT ARE THE PATHWAYS FOR A PATIENT WITH SUSPECTED OR CONFIRMED STI IN SPAIN?
Between 2013 and 2020, the Strategic Plan for the Prevention and Control of HIV Infection and other STI developed by the Ministry of Health has been in force in Spain [55]. In the 17 Autonomous Communities (AC) and the 2 Autonomous Cities (Ceuta and Melilla), there is a high degree of heterogeneity and variability in the development of local plans, with an insufficient level of development of specific actions for STI [55].
In most of the ACs, care for patients with suspected or confirmed STI in Spain is provided by different actors, in hospital and community settings (primary care and community services) and in clinical and non-clinical settings. Despite the heterogeneity of the Autonomous Communities, in general, the majority of registered STI cases are diagnosed through the public health network. In this health care network, primary care plays a special role and, due to its characteristics of accessibility and comprehensive treatment of the pathology, it is in a privileged position for the detection and clinical management of most STI cases and contacts. Clinical care for STI patients is provided within the primary care clinic, but not exclusively. STI cases are also seen in hospital care, mainly in the emergency department and during hospital admissions, in specialties such as gynecology, urology, dermatology, internal medicine, and through the infectious disease and microbiology services. Access to these centers can be at the patient’s own initiative or on demand from primary care or other community facilities. The STI care consultation will be carried out in the corresponding Specialized Care service, but not exclusively. There are also monographic care consultations, created within a Specialized Care service, dedicated exclusively to the health care of patients with STI. In addition to the conventional centers of the healthcare network, the Autonomous Regions have developed a series of centers for the prevention and early diagnosis of HIV and other STI, in clinical and non-clinical settings [56]. These devices are varied and include monographic community care centers (outpatient and/or inpatient) dedicated exclusively to STI health care, community STI rapid detection and counseling services, or mobile information or detection units. These facilities provide anonymous, confidential and free care, and have fewer administrative barriers than conventional health centers.
Community units have been designed for the most vulnerable people, who do not have regular contact with the health system and who have a high prevalence of STI and a higher risk of transmission (people who inject drugs, MSM, individuals with risky behaviors or practices, sex workers, immigrants from areas with a high prevalence of certain STI), and are attended by various agents, institutions or organizations that are distributed in centers or units of the community network. These units work with HIV and other STI prevention programs. Most of them operate in non-clinical settings (specific STI care centers, addiction care centers, dual pathology centers, immigrant shelters, day centers, NGOs, municipal health centers, mobile STI detection units, etc.), with or without links to or integration in the public health network. In Spain, this care circuit for people who are less linked to the health system is complex, with regional, provincial and even local differences. Among the intervention strategies of these community devices are direct intervention and clinical-therapeutic management or referral to other health resources, generally to primary care or to specialized units (STI referral center, hospital HIV units, emergency rooms or hospital clinics).
WHAT IS THE BURDEN OF STI IN THE EMERGENCY DEPARTMENT?
U.S. studies show that 1.3/1,000 of the diagnoses recorded in emergency care are for an STI [57]. A recent Spanish study conducted in 250 hospital emergency departments (ED), with a population coverage of around 45.7 million people and 19.4 million attendances, showed that approximately 71,000 of those attendances corresponded to a suspected STI, which means an incidence of 3.7/1,000 in our country [58]. A noteworthy aspect of this study is that only 36.4% of Spanish hospital EDs have a therapeutic approach protocol for STI, which is more frequent in large hospitals and in hospital EDs with a high influx of patients [58]. The protocolization of STI in the ED is important for 5 reasons: 1) clinical protocols improve the quality of care; 2) STI are a public health problem so their proper management has implications not only for the patient; 3) sample collection, transport and processing are especially important in some frequent STI such as gonococcal infection, as Neisseria gonorrhoeae is very sensitive to environmental conditions; 4) the successive emergence of antibiotic resistance to gonococcus has forced frequent modifications of empirical treatment guidelines for STI worldwide [59]; and 5) ensuring subsequent follow-up is important in order to ensure clinical cure, screen for other STI, interrupt the chain of transmission and implement preventive and sexual health promotion measures. Seventy percent of EDs frequently or almost always perform exudates for etiological diagnosis, 44% STI serology and 35% HIV serology [58].
Another important aspect of STI is their association with an increased likelihood of occult HIV infection [60]. It is known that 1 in 3 missed opportunities for HIV diagnosis occur in the ED, so they can be key in the fight against hidden infection and late diagnosis of this infection [61], being a strategy that multiple studies have shown to be efficient [62]. Despite the perceived difficulties in the ED in detecting patients with unknown infection, it is not possible to detect patients with unknown infection [63], EDs are getting involved in this task by encouraging requests for HIV serology for STI care and other entities routinely seen in the ED through the “dejatuhuella” program [64].
We have not found reliable data on the proportion of STI patient care provided by the private sector.
WHAT ARE THE PROBLEMS OF ETIOLOGICAL DIAGNOSIS OF STI?
Traditionally, the diagnosis of STI was performed in monographic consultations dealing with the so-called “venereal diseases”. In addition to analyzing the symptoms and lesions on the external genitalia and adjacent areas, microscopy techniques were used on the exudates of the lesions observed, including dark field, fresh slides and a few stains (Gram, Giemsa or silver stain) and culture for some of the microorganisms, essentially Neisseria gonorrhoeae. Most of these techniques were performed at the place of patient care or with the proximity of the laboratory to the people attending these consultations. Later, useful serological techniques were implemented for some of the pathogens (e.g., syphilis and HIV). More recently, molecular biology techniques were introduced, broadening the spectrum of microorganisms to be investigated, especially with the so-called syndromic or “multiplexed” panels that simultaneously investigate a multitude of pathogens and can be performed in individual formats or with automated high-throughput platforms [65,66].
This evolution has had the benefit of greater precision in microbiological diagnosis and an increase in the diagnosis of the pathogens involved. However, with the implementation of all these new techniques, and with some exceptions, STI monographic consultations have been disappearing in parallel in many centers, dispersing in many cases the care of these patients. Microbiological diagnosis has also been centralized in many cases, making it necessary to establish adequate transport systems and the development of methods independent of microbiological culture.
Microbiology services and laboratories respond in many cases to requests for STI studies in which they do not know the clinical symptoms, so multiplex platforms that cover numerous possibilities are used, without being able in many cases to optimize the available resources. However, this strategy has brought as a benefit the diagnosis of coinfections, in clear increase in recent years [67,68].
At present, the problems faced by Microbiology Services in the diagnosis of STI can be summarized as follows:
- Distance of the patient and the physician responsible for the laboratory, with loss and lack of clinical information, often necessary to guide and optimize the diagnosis.
- Need to organize adequate transport of samples to preserve the viability of microorganisms in case microbiological culture is necessary.
- Reduction of personnel and high rotation with loss of experience in conventional techniques such as culture, use of microscopy or interpretation of stains.
- Use of expensive molecular systems that increase the total costs of diagnosing STI and the laboratory in general.
- Increase in the number of requests due to the implementation of screening programs (HIV, hepatitis).
- Controls in persons with pre-exposure drugs to the HIV virus.
- Discontinuation and, if necessary, stock-outs of certain conventional diagnostic tests because their manufacturing costs are low and the economic margin for the diagnostic company is very small.
- Introduction of point-of-care systems, including self-diagnosis systems, which on the one hand facilitate rapid diagnosis, but steal the results from the patient’s or the laboratory’s registration systems, making it difficult to declare the case and follow it up [69].
Improvements in the diagnosis of STI are related to the solution of the aforementioned problems, for which the participation of the clinical microbiologist in the multidisciplinary teams caring for people with STI is important. This attitude facilitates not only the improvement of diagnosis, but also the transmission of results and the implementation of follow-up protocols.
HOW SHOULD CARE TEAMS FOR THESE PATIENTS BE STRUCTURED?
If anything has become clearer in recent decades, it is the need for a multidisciplinary approach to STI. These diseases have ceased to be the preserve of specific groups of specialists and have become almost a paradigm of the virtues of multidisciplinary care by well-coordinated teams. On the other hand, patients do not go to groups, but to physicians or other independent health professionals and, therefore, the interest, knowledge and culture about STI must be very widespread among all the components of the care chain.
STI care teams, whether in primary care or in hospitals, should be multidisciplinary and have immediate access to microbiological diagnostic tools. These units seem to us to be indispensable, at least in general and referral hospitals, with close links to emergency departments, with the capacity to provide care 24 hours a day, 7 days a week and linked to a well-established and coordinated network. STI units must have the facility to care for patients of any condition, without the need for “paperwork” and be able to administer treatments as immediately as possible. In addition, they must be very well connected with social and prevention services to turn each case into the index of a possible chain to be searched for and treated.
Very often, these units are serving a very vulnerable population where the specific STI is linked to social and personal problems of all kinds and physical or mental pathology that require a total response.
WHAT IMPACT IS PrEP HAVING ON STI INCIDENCE?
PrEP is the acronym for the use of antiretroviral drugs to reduce the likelihood of HIV infection in uninfected patients. Its preventive efficacy for sexual transmission of HIV is estimated to be around 99% when there is high adherence [70-73] and 74-84% in people who inject drugs [74,75].
PrEP also shows an impact on other facets of sexuality. It improves self-esteem, sexual satisfaction and reduces anxiety associated with sexual intercourse. In addition, in some cases, it can facilitate access to the health care system and sexual health care. [76].
Numerous studies have shown that the daily oral regimen (for men, women and adolescents), with a co-formulated tablet containing 200 mg of Emtricitabine (FTC) and 300 mg of Tenofovir Disoproxil Fumarate (TDF), is safe and effective in reducing the likelihood of acquiring HIV infection in men and women, adults and adolescents at risk for HIV infection [77]. For men and transgender women (adults and adolescents), daily oral PrEP with a co-formulated tablet containing 25 mg Tenofovir Alanine Fumarate (TAF) and 200 mg FTC (TAF/FTC, Descovy Ò) is also a recommended option for HIV prevention in adults and adolescents at risk for HIV infection. PrEP with FTC/TAF has not yet been studied in cisgender women and is therefore not recommended in them for this purpose.
On December 22, 2021, the FDA approved the use of Cabotegravir, for PrEP, in adults and adolescents with sexual or parenteral risk practices for HIV acquisition. Its dosage is one intramuscular injection every 8 weeks [78].
In Spain, the Ministry of Health guidelines for the implementation of PrEP, updated in December 2021, recommend its use in HIV-uninfected individuals aged 16 years or older who meet one or more of the following criteria [79]:
-MSM and transgender women presenting at least two of the following criteria: More than 10 different sexual partners/ year; Unprotected anal sex in the last year; Drug use associated with unprotected sex (chemsex) in the last year; Administration of post-exposure prophylaxis on more than one occasion in the last year; Any bacterial STI in the last year.
Cis or transgender women and male sex workers who report non-habitual condom use.
-Injection drug users with unsafe sexual practices.
Heterosexual women and men reporting non-habitual condom use, presenting at least two of the same criteria as MSM.
In developed countries, especially in the last 10 years, a continuous increase of bacterial STI in PrEP users has been detected. The causes generally related to this increase are: the loss of fear of AIDS due to the preventive efficacy of suppressive ART; the use of drugs for sexual intercourse (chemsex, slamsex,...) and the increased frequency of STI screening in people in these programs.
A study conducted in an STI clinic in Madrid found a significant decrease in condom use 2 years after starting PrEP. The factors that showed an independent association with the presence of an STI after multivariate analysis were: age under 30 years, practicing chemsex and having more than 10 sexual partners/month [80].
A meta-analysis of 17 observational studies conducted in Australia, which included 2,058 participants [81], found that PrEP use was associated with an increase in STI. Multivariate analysis showed that the highest incidence of STI (more than 3 per year) were concentrated in a subgroup of PrEP users, who possessed the following characteristics: being younger than 30 years, having a higher number of sexual partners (more than 10 partners in the last six months), and engaging in group sex [81]. The effect was greater for rectal infections, both for C. trachomatis and for other STI. Rates of repeat STI diagnoses during follow-up were high.
In contrast to the studies discussed above, other work in PrEP users found that before starting PrEP, the incidence of STI was high and increasing in these individuals, and after starting PrEP, STI rates remained high, although they did not increase [14,82].
WHAT IS THE ECONOMIC COST OF STI IN SPAIN?
It is difficult to estimate the cost of STI because they are a heterogeneous set of diseases that are often underreported, partly because they are often asymptomatic and patients do not always seek care for their management or do so outside the health system. An additional reason is that some STI become chronic and have long-term consequences, requiring prolonged follow-ups of patients to assess their costs. Therefore, it is not surprising that not even the Ministry of Health’s Plan for the prevention and control of HIV infection and STI 2021-2030 in Spain includes an evaluation of the costs of these processes [53]. We have not found it on the ECDC web-site dedicated to communicable disease surveillance either [83], although it contains very rich information on the magnitude and distribution of STI in Europe.
The only report we are aware of on this issue in Spain is the one carried out by the group in Public and Health Economics of the University of Cantabria with the sponsorship of Durex Ò [84,85] and Europe [86] questions the effectiveness of available measures, the intensity of their implementation, or both.
HOW IS THE FIGHT AGAINST THIS PROBLEM STRUCTURED AT THE STATE LEVEL? WHAT COULD BE IMPROVED?
Royal Decree 852/2021, of October 5th, modifies the basic organic structure of the Ministry of Health, creating the Division for the Control of HIV, STI, viral hepatitis and tuberculosis (DCVIHT), with the aim of providing an integrated response to these 4 diseases, in line with the international guidelines established by the WHO, UNAIDS and the ECDC. This new structure recognizes the functions assumed in recent years by the former Secretariat of the National AIDS Plan in the prevention and control of the aforementioned diseases and defines a new structure in three distinct areas: primary prevention and community response, secondary prevention and diagnostic innovation, and tertiary prevention and management of chronicity.
This new structure encompasses various functions transversally to all areas of work of the DCVIHT, highlighting the collaboration in epidemiological surveillance and monitoring of the response to these diseases, the implementation of the Social Pact for non-discrimination and equal treatment associated with HIV, as well as actions in teaching and collaboration with the various regional and national administrations, scientific societies and international organizations.
The lines of action for the prevention of STI are reflected in the Plan for the Prevention and Control of HIV infection and STI 2021-2030 in Spain, whose objective is to promote and coordinate actions for the elimination of HIV and STI as a public health problem by 2030, through prevention, early diagnosis and treatment of infections, attention to chronicity and improvement of quality of life, as well as addressing the stigma and discrimination associated with HIV and other STI.
Within strategic objective 1 “Promote the combined prevention of HIV and other STI”, in the first line of action, special emphasis is placed on the promotion of comprehensive sexual health from a positive approach, encouraging training, education and promotion of comprehensive sexual health aimed at both the general population and the vulnerable population, especially the young population, as well as the promotion of condom use through specific HIV and other STI prevention campaigns aimed at young people and other groups of special epidemiological interest. In this line, the DCVIHT has carried out collaborative actions with condom distribution companies to facilitate access to condoms among the population, and is currently studying various improvements to promote access to condoms among the most vulnerable sectors of the population and improve their acceptability.
On the other hand, in strategic objective 2 “Promote early diagnosis of HIV infection and other STI”, different strategies are addressed to improve the diagnosis of HIV and other STI in order to incorporate them into care and treatment early, cut the chains of transmission and favor the enjoyment of a full sexual life. In the field of secondary prevention of STI, the Ministry is promoting HIV self-testing in collaboration with community entities and important initiatives are being carried out in terms of regulatory changes to promote the implementation of self-testing for the diagnosis of STI in Spain in order to implement a self-care strategy that will promote healthy habits and try to improve the perception of risk of acquiring HIV and other STI, providing various preventive tools to the population to ensure the enjoyment of sexuality in a safe manner.
Prior to the implementation of the Strategic Plan, an integrated review of the HIV and other STI prevention and control plans of the different Autonomous Communities was carried out to determine the actions carried out in the last ten years by the autonomous administrations in the prevention and control of HIV and other STI and to determine the degree of alignment with the national strategy, as well as a characterization of the existing infrastructures dedicated to addressing STI at the national level in order to know the capacity to address STI in our country, as well as the diagnostic capacity of STI and the training of health professionals dedicated to STI care in Spain.
However, as aspects that could clearly be improved, coordination with the different Autonomous Communities for the implementation of measures for the prevention and control of HIV and other STI, the need to incorporate STI as a priority in the political agenda, identifying these pathologies as a public health problem that requires a joint response by the different health administrations, and improving investment in the facilities dedicated to STI care in the different territories to achieve an effective response.
WHAT CAN BE EXPECTED FROM A LARGE STATEWIDE PROGRAM TO CONTROL THESE INFECTIONS?
Actions at three different levels can be expected from a national strategic plan of the characteristics of the one we have mentioned. At the national level, one of the main objectives of the plan is to provide useful information to all the Autonomous Regions on the resources and the state of STI care at the national level in order to facilitate decision-making in the management of this type of pathology, as well as to undertake the necessary regulatory changes to ensure that innovations in the prevention and early diagnosis of these diseases can be implemented throughout the territory and are accessible to the public. At the regional level, this plan aims to provide each territory with a common framework for the development of the activities necessary to curb the STI epidemic in a manner adapted to the territorial particularities and characteristics of the population in each territory. At the community level, this plan aims to integrate the community response in the prevention and control of HIV and other STI, involving the third sector in activities aimed at curbing the different epidemics.
WHAT ARE THE MAIN PECULIARITIES OF STI IN CHILDREN AND ADOLESCENTS?
Their differential characteristics with respect to adults are due to the age of infection in pediatrics (from newborns to adolescents). They adopt various clinical forms of presentation and their morbidity, mortality and sequelae can be permanent. The most important thing to remember is that STI in children and adolescents can be secondary to sexual abuse, with all the personal, family, legal and juridical connotations that this entails.
It is difficult to know the real incidence of STI in children and adolescents because not all of them are notifiable, nor are they always recorded and reported by age group. Recently, on the occasion of the European Sexual Health Day (February 2022), Spanish pediatricians warned of the rising trend of STI in all population groups, including adolescents [53,87].
STI in newborns and infants are acquired through vertical transmission, in children born to infected mothers. The most frequent perinatal STI are syphilis and infections by N. gonorrhoeae and C. trachomatis. Their clinical forms are varied and complex [88] and sometimes debut with symptoms suggestive of congenital STI such as perinatal mucopurulent rhinitis in syphilis, persistent neonatal ophthalmia secondary to gonococcal infection or neonatal pneumonitis and conjunctivitis secondary to C. trachomatis. Early diagnosis and treatment is essential for cure and to avoid permanent sequelae. It is frequent that perinatal infection is the “index case” of STI in the family, with the mother being unaware of her infection and her role as a carrier of the infection [89,90].
The detection of STI in prepubertal children or adolescents who do not admit to having sexual relations leads to the suspicion of sexual abuse in the first place. It is also possible that cutaneous contact or self-inoculation mechanisms are involved, as in the case of anogenital warts [32]. Confirmation, in this age group, of infection by gonococcus, syphilis, HIV or C. trachomatis infection, once vertical transmission has been ruled out, is considered evidence of sexual abuse and the corresponding medical (complete personal and family history, diagnostic tests, treatment if necessary, etc.) and legal protocols must be applied [32,91].
Isolation of other STI such as genital herpes (especially HSV-type 2) or Trichomonas vaginalis infection are also suspected of sexual abuse and, even if such abuse is not evident, should be officially reported for information and follow-up [32,91].
Adolescents and young adults are currently the most vulnerable population for STI transmission. Globally, STI are the second most important cause of overall morbidity in women aged 15-44 years [90]. According to the report of the National Epidemiology Center between 2016-2019 the incidence of infection by gonococcus, C. trachomatis and syphilis have doubled in Spain among young people aged 15-19 years. Their current way of life, initiating early sexual relations with risk factors, and the lack of knowledge of protective measures, condition the increase of STI [92,93]. The main risk factors for contracting STI are: age under 25 years, sexual contact with people with STI, sexual relations with different partners, previous history of STI, drug and/or alcohol use especially associated with sexual relations, inconsistent use of condoms with casual partners, being prostitution professionals or their clients, or being a victim of sexual violence [92-94].
The adequate management of STI in pediatric age is based on five basic aspects: rapid diagnosis, adequate treatment, evaluation of sexual contacts, communication of cases and health education to detect and modify risky sexual behaviors. Treatment will be adjusted to current protocols according to age [89,91,95]. If the STI is secondary to sexual abuse, the protocols will be followed.
Suspected sexual abuse should always be handled as an emergency until the patient is stabilized and the risk is assessed. In all healthcare centers that deal with these cases, there should be protocols for the management of sexual abuse, accessible and known by the professionals, endorsed by the competent authority, which must be complied with for healthcare and legal reasons [94-97].
The American Academy of Pediatrics [97] recommends the progressive inclusion in sex education campaigns of children and adolescents with disabilities who survive their underlying pathology and are frequently able to have sexual relations. They are especially fragile and vulnerable to sexual abuse, STI and unwanted pregnancies.
The decrease and control of STI in children and adolescents will only be possible by addressing sex education programs based on knowledge and prevention. Training programs should be developed to be taught in schools, primary care clinics (pediatrics or family medicine). Training and support for families on this subject, and their inclusion in treatment and health education groups for adolescents will be a key element in the control of these diseases.
WHAT ARE THE MECHANISMS FOR APPROACHING THE SCREENING AND TREATMENT OF STI IN VICTIMS OF SEXUAL ASSAULT IN SPANISH HOSPITALS?
Sexual assault and abuse not only have an immediate and global effect on women’s lives and health, but also have a medium- and long-term impact on sexual life, among many others. One way in which this impact materializes is through the risk of transmission of STI.
In the Community of Madrid the VISEM code is followed [98]. It is a protocol that should be activated with women over 16 years of age who have suffered sexual violence in the form of sexual assault or abuse in the last 72 hours (3 days), or 168 hours (7 days) if there has been vaginal intercourse. Patient consent must be obtained for care and specimen collection.
The care is carried out in the ED, which must have the means to perform the care in a single act, in an urgent, coordinated and comprehensive manner: forensic medical examination, clinical care and specimen collection. The intervention will be governed by a code that standardizes as much as possible the procedures of all those involved with the guarantees of confidentiality and protection of the victim. Once hospital emergency care has been completed, continuity of care must be ensured in primary care, which is essential in the case of STI.
IS IT CURRENTLY NECESSARY TO REQUEST CONSENT FOR PERFORMING AUTOMATED STI DIAGNOSTIC TESTS IN CLINICAL LABORATORIES?
In Spain, the patient’s informed consent is a right derived from the fundamental right to information and is protected by Law 41/2002, of November 14, 2002, which regulates patient autonomy and the rights and obligations regarding clinical information and documentation [99]. Obtaining it is a legal requirement to perform any type of medical test, including HIV testing. In addition, in the specific case of HIV testing, the following regulations apply:
- The performance of HIV testing must be justified on clinical and epidemiological grounds, and must be prescribed by a physician.
- The patient must be adequately informed about the nature of the test, its purpose, the risks and benefits of the test and the possible results.
- The patient has the right to refuse to undergo the test, without affecting his or her medical care.
- Information obtained during HIV testing is protected by medical confidentiality, and can only be disclosed with the explicit consent of the patient, or in specific circumstances provided for by law (e.g., in the case of mandatory reporting of infectious diseases).
From an ethical point of view, it could be argued that the trust that presides over the ordinary doctor-patient relationship would justify the possibility for the physician to perform all the examinations necessary to establish the diagnosis, provided that they are not explicitly rejected or prohibited. This would be the case of the determination of the HIV test when making the diagnosis of the patient who comes for suspected STI. However, this interpretation of implied consent would not comply with the necessary free and voluntary consent required by the principle of personal autonomy. The ethical mandate still requires explicit consent to be obtained in these cases, with verbal consent being sufficient, which should be reflected in the patient’s clinical history.
Nor would it be appropriate from the ethical-legal point of view to perform routine HIV diagnosis by laboratories on the basis of algorithms or protocols, without the explicit request of the requesting physician.
In the event that the requesting physician includes in the clinical history the patient’s verbal consent for the performance of the appropriate examinations for the diagnosis of STI, including HIV, if necessary, the laboratory could extend the corresponding tests that the clinical protocol establishes, as in the case of invasive interventions where this is contemplated in the consent for the intervention itself (for example, in the donation of blood, cells, tissues or organs for transplantation).
However, in the event that the requesting physician records in the medical record the patient’s verbal consent for the performance of the appropriate tests for the diagnosis of a specific disease, for example an STI (which by protocol includes all diseases such as HIV infection), if necessary, the laboratory could extend the corresponding tests that the clinical protocol establishes, including HIV, as in the case of invasive procedures where it is contemplated in the consent for the procedure itself (for example, in the donation of blood, cells, tissues or organs for transplantation).
In summary, although the early detection of STI is important for public health, this does not automatically justify the performance of tests without adequate informed consent from the patient (explicit verbal and recorded in the clinical history), and it is advisable to regulate it within the different algorithms or clinical protocols for action.
In spite of this, efforts should be made in the future to standardize HIV diagnosis in our setting and subject it to the same regulations as the other complementary tests requested of the patient, considering the existence today of an effective treatment from which the patient will benefit to a greater extent the earlier the diagnosis is made, in addition to the obvious public health benefits of avoiding secondary cases. In this same sense, safe strategies that respect the patient’s rights should be promoted, so that microbiology services can transform situations of missed diagnostic opportunities into gained ones based on well-established clinical protocols.
FUNDING
None to declare
CONFLICTS OF INTEREST
The authors declare no conflicts of interest
References
- 1.Piedrola-Gil G. Medicina preventiva y salud pública. 12th Edition. Barcelona: Elsevier; 2015. [Google Scholar]
- 2.Unidad de vigilancia de VIH, ITS y hepatitis B y C. Vigilancia epidemiológica de las infecciones de transmisión sexual, 2021. Centro Nacional de Epidemiología, Instituto de Salud Carlos III/División de Control de VIH, ITS, Hepatitis virales y Tuberculosis, Dirección General de Salud Pública; 2023. https://www.sanidad.gob.es/eu/ciudadanos/enfLesiones/enfTransmisibles/sida/vigilancia/doc/Vigilancia_ITS_1995_2021.pdf
- 3.Sistema de Información sobre Nuevos Diagnósticos de VIH y Regis-tro Nacional de Casos de Sida. Ministerio de Sanidad. Madrid; noviembre 2022. Available at: https://www.sanidad.gob.es/ciudadanos/enfLesiones/enfTransmisibles/sida/vigilancia/docs/Informe_VIH_SIDA_2022_CCAA.pdf. [Google Scholar]
- 4.Peiró-Mestres A, Fuertes I, Camprubí-Ferrer D, Marcos M, Vilella A, Navarro M, et al. Frequent detection of monkeypox virus DNA in saliva, semen, and other clinical samples from 12 patients, Barcelona, Spain, May to June 2022. Euro Surveill. 2022;27(28). 10.2807/1560-7917.Es.2022.27.28.2200503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO . WHO Emergency Appeal: Monkeypox July 2022 – June 2023. Available at: https://www.who.int/publications/m/item/who-emergency-appeal--monkeypox---july-2022---june-2023
- 6.ECDC . Joint ECDC-WHO Regional Office for Europe Mpox Surveillance Bulletin. Available at: https://www.ecdc.europa.eu/en/mpox-monkeypox
- 7.Vigilancia Viruela del Mono . Situación epidemiológica de los casos de viruela del mono. Available at: https://www.isciii.es/QueHacemos/Servicios/VigilanciaSaludPublicaRENAVE/EnfermedadesTransmisibles/Paginas/Resultados_Vigilancia_Viruela-del-mono.aspx
- 8.Centro de Coordinación de Alertas y Emergencias Sanitarias . Ministerio de Sanidad. Informe de situación a 11 de octubre de 2022 Alerta sobre infección de viruela del mono en España y otros países no endémicos. 2022. Available at: https://www.isciii.es/QueHacemos/Servicios/VigilanciaSaludPublicaRENAVE/EnfermedadesTransmisibles/Documents/archivos%20A-Z/MPOX/SITUACION%20EPIDEMIOLOGICA%20DE%20LOS%20CASOS%20DE%20VIRUELA%20DEL%20MONO-11102022.pdf
- 9.Orviz E, Ayerdi O, Muñoz A, et al. Centro Sandoval, HCSC, IdISSC, Madrid. Presentación al Congreso Nacional GeSIDA Sitges, Noviembre 2022.
- 10.Orviz E, Negredo A, Ayerdi O, et al. Monkeypox outbreak in Madrid (Spain): Clinical and virological aspects. J Infect. 2022;85(4):412-7. 10.1016/j.jinf.2022.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.James C, Harfouche M, Welton NJ, Turner KM, Abu-Raddad LJ, Gottlieb SL, et al. Herpes simplex virus: global infection prevalence and incidence estimates, 2016. Bull World Health Organ. 2020;98(5):315-29. 10.2471/blt.19.237149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Looker KJ, Magaret AS, Turner KM, Vickerman P, Gottlieb SL, Newman LM. Global estimates of prevalent and incident herpes simplex virus type 2 infections in 2012. PLoS One. 2015;10(1):e114989. 10.1371/journal.pone.0114989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Statista Salud e Industria Farmacéutica . Número de casos de herpes genital registrados en España de 2011 a 2019. Available at https://es.statista.com/estadisticas/1046517/numero-de-casos-de-herpes-genital-en-espana/
- 14.Tarin-Vicente EJ, Sendagorta Cudos E, Servera Negre G, Falces Romero I, Ballesteros Martin J, Martin-Gorgojo A, et al. [Sexually Transmitted Infections During the First Wave of the COVID-19 Pandemic in Spain]. Actas Dermosifiliogr. 2022;113(2):115-22. 10.1016/j.ad.2021.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reina J, Gutiérrez O, Ruiz de Gopegui E, Padilla E. [Incidence of genital infections caused by herpes simplex virus type 1 (HSV-1) from 1995 to 2003]. Enferm Infecc Microbiol Clin. 2005;23(8):482-4. 10.1157/13078827 [DOI] [PubMed] [Google Scholar]
- 16.Ministerio de Sanidad, Grupo de expertos del grupo de estudio de SIDA de la SEIMC (GESIDA), Secretaria del plan nacional sobre el SIDA (SPNS), Grupo de estudio de ITS de la SEIMC (GEITS), Documento de consenso sobre diagnóstico y tratamiento de las infecciones de transmisión sexual en adultos, niños y adolescentes. Available at: Ministerio de Sanidad - Ciudadanos - Infecciones de Transmisión Sexual. 2017.
- 17.Le Cleach L, Trinquart L, Do G, Maruani A, Lebrun-Vignes B, Ravaud P, et al. Oral antiviral therapy for prevention of genital herpes outbreaks in immunocompetent and nonpregnant patients. Cochrane Database Syst Rev. 2014(8):Cd009036. 10.1002/14651858.CD009036.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention . Genital HPV Infection – Basic Fact Sheet. Available at: https://www.cdc.gov/std/hpv/std-fact-hpv.htm
- 19.Darragh TM, Winkler B. Anal cancer and cervical cancer screening: key differences. Cancer Cytopathol. 2011;119(1):5-19. 10.1002/cncy.20126 [DOI] [PubMed] [Google Scholar]
- 20.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans . Human papillomaviruses. IARC Monogr Eval Carcinog Risks Hum. 2007;90:1-636. [PMC free article] [PubMed] [Google Scholar]
- 21.Sanz Ortega G, J. GS. Practicum en coloproctología de la AECP: estrategias y técnicas para la práctica diaria: Tema 2. Capítulo 10; Neoplasia intraepitelial anal y proctoscopia de alta resolución. [Google Scholar]
- 22.Machalek DA, Chow EP, Garland SM, Wigan R, Cornall AM, Fairley CK, et al. Human Papillomavirus Prevalence in Unvaccinated Heterosexual Men After a National Female Vaccination Program. J Infect Dis. 2017;215(2):202-8. 10.1093/infdis/jiw530 [DOI] [PubMed] [Google Scholar]
- 23.Kahn JA, Widdice LE, Ding L, Huang B, Brown DR, Franco EL, et al. Substantial Decline in Vaccine-Type Human Papillomavirus (HPV) Among Vaccinated Young Women During the First 8 Years After HPV Vaccine Introduction in a Community. Clin Infect Dis. 2016;63(10):1281-7. 10.1093/cid/ciw533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baussano I, Tshomo U, Tenet V, Heideman DAM, Wangden T, Franc-eschi S, et al. Prevalence of Human Papillomavirus and Estimation of Human Papillomavirus Vaccine Effectiveness in Thimphu, Bhutan, in 2011-2012 and 2018 : A Cross-sectional Study. Ann Intern Med. 2020;173(11):888-94. 10.7326/m20-2849 [DOI] [PubMed] [Google Scholar]
- 25.Stamm WE. Chlamydia trachomatis infections of the adult. In: Holmes KK SP, Mardh PA, et al (Eds), editor. In: Sexually Transmitted Diseases, 4th Edition. New York. McGraw-Hill; 2008. p. 575. [Google Scholar]
- 26.Rodrigues R, Marques L, Vieira-Baptista P, Sousa C, Vale N. Therapeutic Options for Chlamydia trachomatis Infection: Present and Future. Antibiotics (Basel). 2022;11(11). 10.3390/antibiotics11111634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodrigues R, Sousa C, Vale N. Chlamydia trachomatis as a Current Health Problem: Challenges and Opportunities. Diagnostics (Basel). 2022;12(8). 10.3390/diagnostics12081795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brunham RC. Problems With Understanding Chlamydia trachomatis Immunology. J Infect Dis. 2022;225(11):2043-9. 10.1093/infdis/jiab610 [DOI] [PubMed] [Google Scholar]
- 28bis.European CDC . Chlamydia infection. Annual Epidemiological Report for 2019. Stocholm, september 2022. Available at: https://www.ecdc.europa.eu/sites/default/files/documents/chlamydia-annual-epidemiological-report-2019.pdf [Google Scholar]
- 29.Tully JG, Taylor-Robinson D, Cole RM, Rose DL. A newly discovered mycoplasma in the human urogenital tract. Lancet. 1981;1(8233):1288-91. 10.1016/s0140-6736(81)92461-2 [DOI] [PubMed] [Google Scholar]
- 30.Jensen JS, Cusini M, Gomberg M, Moi H, Wilson J, Unemo M. 2021 European guideline on the management of Mycoplasma genitalium infections. J Eur Acad Dermatol Venereol. 2022;36(5):641-50. 10.1111/jdv.17972 [DOI] [PubMed] [Google Scholar]
- 31.Taylor-Robinson D, Jensen JS. Mycoplasma genitalium: from Chrysalis to multicolored butterfly. Clin Microbiol Rev. 2011;24(3):498-514. 10.1128/cmr.00006-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Workowski K A, Bachmann LH, Chan PA, Johnston CM, Muzny CA, Park I, et al. Sexually Transmitted Infections Treatment Guidelines, 2021. MMWR Recomm Rep. 2021;70(4):1-187. 10.15585/mmwr.rr7004a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hamasuna R, Osada Y, Jensen JS. Isolation of Mycoplasma genitalium from first-void urine specimens by coculture with Vero cells. J Clin Microbiol. 2007;45(3):847-50. 10.1128/jcm.02056-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shipitsyna E, Savicheva A, Solokovskiy E, Ballard RC, Domeika M, Unemo M, et al. Guidelines for the laboratory diagnosis of mycoplasma genitalium infections in East European countries. Acta Derm Venereol. 2010;90(5):461-7. 10.2340/00015555-0929 [DOI] [PubMed] [Google Scholar]
- 35.Renaudin H, Tully JG, Bebear C. In vitro susceptibilities of Myco-plasma genitalium to antibiotics. Antimicrob Agents Chemother. 1992;36(4):870-2. 10.1128/aac.36.4.870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Machalek DA, Tao Y, Shilling H, Jensen JS, Unemo M, Murray G, et al. Prevalence of mutations associated with resistance to macrolides and fluoroquinolones in Mycoplasma genitalium: a systematic review and meta-analysis. Lancet Infect Dis. 2020;20(11):1302-14. 10.1016/s1473-3099(20)30154-7 [DOI] [PubMed] [Google Scholar]
- 37.Fernández-Huerta M, Barberá MJ, Serra-Pladevall J, Esperalba J, Martínez-Gómez X, Centeno C, et al. Mycoplasma genitalium and antimicrobial resistance in Europe: a comprehensive review. Int J STD AIDS. 2020;31(3):190-7. 10.1177/0956462419890737 [DOI] [PubMed] [Google Scholar]
- 38.Horner P, Ingle SM, Garrett F, Blee K, Kong F, Muir P, et al. Which azithromycin regimen should be used for treating Mycoplasma genitalium? A meta-analysis. Sex Transm Infect. 2018;94(1):14-20. 10.1136/sextrans-2016-053060 [DOI] [PubMed] [Google Scholar]
- 39.Read TRH, Fairley CK, Murray GL, Jensen JS, Danielewski J, Worthington K, et al. Outcomes of Resistance-guided Sequential Treatment of Mycoplasma genitalium Infections: A Prospective Evaluation. Clin Infect Dis. 2019;68(4):554-60. 10.1093/cid/ciy477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bissessor M, Tabrizi SN, Twin J, Abdo H, Fairley CK, Chen MY, et al. Macrolide resistance and azithromycin failure in a Mycoplasma genitalium-infected cohort and response of azithromycin failures to alternative antibiotic regimens. Clin Infect Dis. 2015;60(8):1228-36. 10.1093/cid/ciu1162 [DOI] [PubMed] [Google Scholar]
- 41.Read TRH, Jensen JS, Fairley CK, Grant M, Danielewski JA, Su J, et al. Use of Pristinamycin for Macrolide-Resistant Mycoplasma genitalium Infection. Emerg Infect Dis. 2018;24(2):328-35. 10.3201/eid2402.170902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.European Center for Disease Prevention and Control . Syphilis. Annual Epidemiological Report for 2019. Available at : https://www.ecdc.europa.eu/en/publications-data/syphilis-annual-epidemio-logical-report-2019.
- 43.Hernando RA, Ruiz-Algueró M, Diaz A, C. y la Red Nacional de Vigilancia Epidemiológica (RENAVE) Unidad de vigilancia de VIH ITS y hepatitis B. Vigilancia epidemiológica de las infecciones de transmisión sexual. Centro Nacional de Epidemiología, Instituto de Salud Carlos III/Plan Nacional sobre el SIDA, Dirección General de Salud Pública 2021.
- 44.Unemo M, Ross J, Serwin AB, Gomberg M, Cusini M, Jensen JS. 2020 European guideline for the diagnosis and treatment of gonorrhoea in adults. Int J STD AIDS. 2020:956462420949126. 10.1177/0956462420949126 [DOI] [PubMed] [Google Scholar]
- 45.Unemo M, Lahra MM, Escher M, Eremin S, Cole MJ, Galarza P, et al. WHO global antimicrobial resistance surveillance for Neisseria gonorrhoeae 2017-18: a retrospective observational study. Lancet Microbe. 2021;2(11):e627-e36. 10.1016/s2666-5247(21)00171-3 [DOI] [PubMed] [Google Scholar]
- 46.Salmerón P, Viñado B, Arando M, Alcoceba E, Romero B, Menéndez B, et al. Neisseria gonorrhoeae antimicrobial resistance in Spain: a prospective multicentre study. J Antimicrob Chemother. 2021;76(6):1523-31. 10.1093/jac/dkab037 [DOI] [PubMed] [Google Scholar]
- 47.Fifer H, Saunders J, Soni S, Sadiq ST, FitzGerald M. 2018 UK national guideline for the management of infection with Neisseria gonorrhoeae. Int J STD AIDS. 2020;31(1):4-15. 10.1177/0956462419886775 [DOI] [PubMed] [Google Scholar]
- 48.Australasian Society for HIV Viral Hepatitis and Sexual Health Medicine (ASHM) . Australian STI treatment Guidelines. Available at: https://www.ashm.org.au/.
- 49.Li X, Le W, Lou X, Genco CA, Rice PA, Su X. In Vitro Activity of Ertapenem against Neisseria gonorrhoeae Clinical Isolates with Decreased Susceptibility or Resistance to Extended-Spectrum Cephalosporins in Nanjing, China (2013 to 2019). Antimicrob Agents Chemother. 2022;66(5):e0010922. 10.1128/aac.00109-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gubenšek U, de Laat M, Foerster S, Boyd A, van Dam AP. Pharmaco-dynamics of Ceftriaxone, Ertapenem, Fosfomycin and Gentamicin in Neisseria gonorrhoeae. Antibiotics (Basel). 2022;11(3). 10.3390/antibiotics11030299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.De Vries HJC, de Laat M, Jongen VW, Heijman T, Wind CM, Boyd A, et al. Efficacy of ertapenem, gentamicin, fosfomycin, and ceftriaxone for the treatment of anogenital gonorrhoea (NABOGO): a randomised, non-inferiority trial. Lancet Infect Dis. 2022;22(5):706-17. 10.1016/s1473-3099(21)00625-3 [DOI] [PubMed] [Google Scholar]
- 52.Golparian D, Unemo M. Antimicrobial resistance prediction in Neisseria gonorrhoeae: current status and future prospects. Expert Rev Mol Diagn. 2022;22(1):29-48. 10.1080/14737159.2022.2015329 [DOI] [PubMed] [Google Scholar]
- 53.Plan de prevención y control de la infección por el VIH y las ITS 2021-2030 en España. Ministerio de Sanidad y Consumo. 2021. Available at: https://www.sanidad.gob.es/ciudadanos/enfLesiones/enfTransmisibles/sida/planNalSida/Plan_de_Prevencion_y_Control1.pdf
- 54.ONUSIDA . Estrategia mundial contra el sida 2021-2026: Acabar con las desigualdades. Acabar con el sida. 2021. Available at: https://www.unaids.org/es/Global-AIDS-Strategy-2021-2026
- 55.La prevención y el control de las ITS en España 2013-2020: una revisión integrativa. 2021. Ministerio de Sanidad Secretaría del Plan Nacional sobre el SIDA. Available at: https://www.sanidad.gob.es/ciudadanos/enfLesiones/enfTransmisibles/sida/ITS/Informe_Revision_Planes_ITS_CCAA_2021.pdf [Google Scholar]
- 56.Servicios de información: VIH (Virus de la Inmunodeficiencia Humana) ITS . Infecciones de Transmisión Sexual. Madrid; Comunidad de Madrid; 2022. Available at: https://www.comunidad.madrid/servicios/salud/vih-virus-inmunodeficiencia-humana-its-infecciones-transmision-sexual [Google Scholar]
- 57.Pearson WS, Peterman TA, Gift TL. An increase in sexually transmitted infections seen in US emergency departments. Prev Med. 2017;100:143-4. 10.1016/j.ypmed.2017.04.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Miró O, Miró E, García-Lamberechts EJ, Villamor Ordozgoiti A, González Del Castillo J. [Map of sexually transmitted disease care in Spanish emergency departments]. Rev Esp Quimioter. 2021;34(4):353-64. 10.37201/req/051.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Barberá MJ, Serra-Pladevall J. Gonococcal infection: An unresolved problem. Enferm Infecc Microbiol Clin (Engl Ed). 2019;37(7):458-66. 10.1016/j.eimc.2018.12.008 [DOI] [PubMed] [Google Scholar]
- 60.Ugarte A, Mallolas J. Detection of unknown cases of human immunodeficiency virus infection in emergency departments: a utopian ideal? Emergencias. 2021;33(4):249-50. [PubMed] [Google Scholar]
- 61.González Del Castillo J. Current role of hospital emergency departments in the fight against the human immunodeficiency virus infection pandemic. Emergencias. 2021;33(1):7-8. [PubMed] [Google Scholar]
- 62.González Del Castillo J, Fuentes Ferrer ME, Fernández Pérez C, Molina Romera G, Núñez Orantos MJ, Estrada Pérez V. Efficiency of screening for human immunodeficiency virus infection in emergency departments: a systematic review and meta-analysis. Emergencias. 2022;34(3):204-12. [PubMed] [Google Scholar]
- 63.Miró Ò, Miró E, García-Lamberechts EJ, Villamor A, González Del Castillo J. Screening for undiagnosed human immunodeficiency virus infection in Spanish emergency departments: current attitudes, inclination, and perception of obstacles related to the implementation of measures to improve detection. Emergencias. 2021;33(4):254-64. [PubMed] [Google Scholar]
- 64.González Del Castillo J, Burillo-Putze G, Cabello A, Curran A, Jaloud Saavedra E, Malchair P, et al. Recommendations for the early diagnosis of suspected human immunodeficiency virus infection in the emergency department and the referral of patients for follow-up: a consensus statement of the Spanish Society of Emergency Medicine (SEMES). Emergencias. 2020;32(6):416-26. [PubMed] [Google Scholar]
- 65.Karellis A, Naeem F, Nair S, Mallya SD, Routy JP, Gahagan J, et al. Multiplexed rapid technologies for sexually transmitted infections: a systematic review. Lancet Microbe. 2022;3(4):e303-e15. 10.1016/s2666-5247(21)00191-9 [DOI] [PubMed] [Google Scholar]
- 66.Tuddenham S, Hamill MM, Ghanem KG. Diagnosis and Treatment of Sexually Transmitted Infections: A Review. Jama. 2022;327(2):161-72. 10.1001/jama.2021.23487 [DOI] [PubMed] [Google Scholar]
- 67.Su X, Le W, Zhu X, Li S, Wang B, Madico G, et al. Neisseria gonorrhoeae Infection in Women Increases With Rising Gonococcal Burdens in Partners: Chlamydia Coinfection in Women Increases Gonococcal Burden. J Infect Dis. 2022;226(12):2192-203. 10.1093/infdis/jiac408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dionne-Odom J, Workowski K, Perlowski C, Taylor SN, Mayer KH, McNeil CJ, et al. Coinfection With Chlamydial and Gonorrheal Infection Among US Adults With Early Syphilis. Sex Transm Dis. 2022;49(8):e87-e9. 10.1097/olq.0000000000001605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Adamson PC, Loeffelholz MJ, Klausner JD. Point-of-Care Testing for Sexually Transmitted Infections: A Review of Recent Developments. Arch Pathol Lab Med. 2020;144(11):1344-51. 10.5858/arpa.2020-0118-RA [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Volk JE, Marcus JL, Phengrasamy T, Blechinger D, Nguyen DP, Follansbee S, et al. No New HIV Infections With Increasing Use of HIV Preexposure Prophylaxis in a Clinical Practice Setting. Clin Infect Dis. 2015;61(10):1601-3. 10.1093/cid/civ778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jenness SM, Goodreau SM, Rosenberg E, Beylerian EN, Hoover KW, Smith DK, et al. Impact of the Centers for Disease Control’s HIV Pre-exposure Prophylaxis Guidelines for Men Who Have Sex With Men in the United States. J Infect Dis. 2016;214(12):1800-7. 10.1093/infdis/jiw223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Owens DK, Davidson KW, Krist AH, Barry MJ, Cabana M, Caughey AB, et al. Preexposure Prophylaxis for the Prevention of HIV Infection: US Preventive Services Task Force Recommendation Statement. Jama. 2019;321(22):2203-13. 10.1001/jama.2019.6390 [DOI] [PubMed] [Google Scholar]
- 73.Chou R, Evans C, Hoverman A, Sun C, Dana T, Bougatsos C, et al. Preexposure Prophylaxis for the Prevention of HIV Infection: Evidence Report and Systematic Review for the US Preventive Services Task Force. Jama. 2019;321(22):2214-30. 10.1001/jama.2019.2591 [DOI] [PubMed] [Google Scholar]
- 74.Choopanya K, Martin M, Suntharasamai P, Sangkum U, Mock PA, Leethochawalit M, et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2013;381(9883):2083-90. 10.1016/s0140-6736(13)61127-7 [DOI] [PubMed] [Google Scholar]
- 75.Martin M, Vanichseni S, Suntharasamai P, Sangkum U, Mock PA, Leethochawalit M, et al. The impact of adherence to preexposure prophylaxis on the risk of HIV infection among people who inject drugs. Aids. 2015;29(7):819-24. 10.1097/qad.0000000000000613 [DOI] [PubMed] [Google Scholar]
- 76.Whitfield THF, Jones SS, Wachman M, Grov C, Parsons JT, Rendina HJ. The Impact of Pre-Exposure Prophylaxis (PrEP) Use on Sexual Anxiety, Satisfaction, and Esteem Among Gay and Bisexual Men. J Sex Res. 2019;56(9):1128-35. 10.1080/00224499.2019.1572064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mayer KH, Molina JM, Thompson MA, Anderson PL, Mounzer KC, De Wet JJ, et al. Emtricitabine and tenofovir alafenamide vs emtricitabine and tenofovir disoproxil fumarate for HIV pre-expo-sure prophylaxis (DISCOVER): primary results from a randomised, double-blind, multicentre, active-controlled, phase 3, non-inferiority trial. Lancet. 2020;396(10246):239-54. 10.1016/s0140-6736(20)31065-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.FDA Approves First Injectable Treatment for HIV Pre-Expo-sure Prevention . First-injectable-treatment-hiv-pre-expo-sure-prevention. 2021. Available at https://www.fda.gov/news-events/press-announcements/fda-approves-first-injectable-treatment-hiv-pre-exposure-prevention
- 79.Manual de implementación de un Programa de Profilaxis Preexposición al VIH en España . Ministerio de Sanidad. 2020. Available at: https://www.sanidad.gob.es/ciudadanos/enfLesiones/enfTransmisibles/sida/docs/MANUAL_PREP.pdf
- 80.Ayerdi Aguirrebengoa O, Vera García M, Arias Ramírez D, Gil García N, Puerta López T, Clavo Escribano P, et al. Low use of condom and high STI incidence among men who have sex with men in PrEP programs. PLoS One. 2021;16(2):e0245925. 10.1371/journal.pone.0245925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Traeger MW, Cornelisse VJ, Asselin J, Price B, Roth NJ, Willcox J, et al. Association of HIV Preexposure Prophylaxis With Incidence of Sexually Transmitted Infections Among Individuals at High Risk of HIV Infection. Jama. 2019;321(14):1380-90. 10.1001/jama.2019.2947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.McManus H, Grulich AE, Amin J, Selvey C, Vickers T, Bavinton B, et al. Comparison of Trends in Rates of Sexually Transmitted Infections Before vs After Initiation of HIV Preexposure Prophylaxis Among Men Who Have Sex With Men. JAMA Netw Open. 2020;3(12):e2030806. 10.1001/jamanetworkopen.2020.30806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.European Centre for Disease Prevention and Control . Infectiuos disease topics. 2022. Available at: https://www.ecdc.europa.eu/en/infectious-disease-topics
- 84.Cantarero Prieto D. Análisis económico y social de estrategias de prevención de infecciones de transmisión sexual sobre la utilización de los recursos sanitarios disponibles .2018. . Available at: https://web.unican.es/portal-investigador/Proyectos.
- 85.EpData . Enfermedades de transmisión sexual (ETS) en España, datos, gráficos y estadísticas. 2022. . Available at: https://www.epdata.es/datos/enfermedades-transmision-sexual-espana-graficos/666
- 86.Geretti AM, Mardh O, de Vries HJC, Winter A, McSorley J, Seguy N, et al. Sexual transmission of infections across Europe: appraising the present, scoping the future. Sex Transm Infect. 2022. 10.1136/sextrans-2022-055455 [DOI] [PubMed] [Google Scholar]
- 87.Día Europeo de la Salud Sexual 2022: Los pediatras alertan de que la incidencia de infecciones de transmisión sexual se ha duplicado entre los adolescentes mientras los recursos para abordar la salud sexual son cada vez más limitados. Asociación Española de Pediatría. 2022. Available at: https://www.aeped.es/sites/default/files/20220211_ndp_dia_europeo_salud_sexual.pdf [Google Scholar]
- 88.Hammerschlag MR, Rawstron SA, Bromberg K. Sexually transmitted diseases. . In Krugman’s Infectious Diseases of Children, 11th edition Mosby: 2004. p. 563-612. [Google Scholar]
- 89.Patiño Ortega H. Protocolo de diagnóstico y tratamiento de las infecciones de transmisión sexual (ITS) en adultos, niños y adolescentes. . PROA SESCAM, Gerencia de Atención Integrada Alcazar de San Juan, . 2020:2-24. [Google Scholar]
- 90.Andrés Domingo P. Infecciones de transmisión Sexual. . Pediatr Integral 2017;21: 323-33. [Google Scholar]
- 91.Kimberlin DW, Brady MT, Jackson MA, SS L. RED BOOK Enfermedades Infecciosas en Pediatría. Informe 2018-2021 del Comité de Enfermedades Infecciosas de la AAP. Enfermedades de transmisión sexual en adolescentes y niños. Ed Panamericana 2021.
- 92.Gannon-Loew KE, Holland-Hall C. A review of current guidelines and research on the management of sexually transmitted infections in adolescents and young adults. Ther Adv Infect Dis. 2020;7:2049936120960664. 10.1177/2049936120960664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ilskens K, Wrona KJ, Dockweiler C, Fischer F. An Evidence Map on Serious Games in Preventing Sexually Transmitted Infections Among Adolescents: Systematic Review About Outcome Categories Investigated in Primary Studies. JMIR Serious Games. 2022;10(1):e30526. 10.2196/30526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ayerdi Aguirrebengoa O, Vera Garcia M, Rueda Sanchez M, G DE, Chavero Méndez B, Alvargonzalez Arrancudiaga M, et al. Risk factors associated with sexually transmitted infections and HIV among adolescents in a reference clinic in Madrid. PLoS One. 2020;15(3):e0228998. 10.1371/journal.pone.0228998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sanz Santaeufemia FJ, García Talavera ME, F. SP. .Abuso sexual (prevención de las infecciones de transmisión sexual) (v.2.0/2020). Guía_ABE. Infecciones en Pediatría. Guía rápida para la selección del tratamiento antimicrobiano empírico. 2020. Available at: https://www.guia-abe.es.
- 96.Skjælaaen K, Nesvold H, Brekke M, Sare M, Landaas ET, Mdala I, et al. Sexually transmitted infections among patients attending a sexual assault centre: a cohort study from Oslo, Norway. BMJ Open. 2022;12(12):e064934. 10.1136/bmjopen-2022-064934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Houtrow A, Elias ER, Davis BE. Promoting Healthy Sexuality for Children and Adolescents With Disabilities. Pediatrics. 2021;148(1). 10.1542/peds.2021-052043 [DOI] [PubMed] [Google Scholar]
- 98.Protocolo de asistencia sanitaria urgente y coordinada a mujeres víctimas de violencia sexual en la Comunidad de Madrid. Comuni- dad de Madrid VISEM. 2023. Available at: https://gestiona3.madrid.org/bvirtual/BVCM050659.pdf
- 99.Guía de recomendaciones para el diagnóstico precoz del VIH en el ámbito sanitario. Ministerio de Sanidad y Consumo. 2014. Available at: https://www.sanidad.gob.es/ciudadanos/enfLesiones/enfTransmisibles/sida/docs/GuiaRecomendacionesDiagnosticoPrecozVIH.pdf