Abstract
Background
Liver fibrosis plays a key role in the progression of non-alcoholic fatty liver disease to cirrhosis. Considering weight change is known to be closely associated with increased risk of liver fibrosis, we aimed to address a gap in evidence regarding the existence of this association in patients with type 2 diabetes (T2D).
Methods
We included data on 622 T2D patients and 1618 non-T2D participants from the 2017-2018 cycle of the National Health and Nutrition Examination Survey (NHANES). We assessed liver fibrosis by the median values of liver stiffness measurement (LSM). According to the participants' body mass index (BMI) at age 25 (early adulthood), 10 years prior (middle adulthood), and at the 2017-2018 cycle (late adulthood), we categorised weight change patterns into stable non-obese, weight loss, weight gain, and stable obese. We applied logistic regression to association analysis and used population attributable fraction (PAF) to analyses hypothetical prevention regimens.
Results
The prevalence of liver fibrosis was higher in T2D patients (23.04%) than in non-T2D participants (6.70%), while weight change was associated with a greater risk of fibrosis in the former compared to the latter group. Compared with T2D patients in the stable non-obese group, stable obese individuals from 10 years prior to the 2017-2018 cycle had the highest risk of developing liver fibrosis, corresponding to an adjusted odds ratio (aOR) of 3.13 (95% confidence interval = 1.84-5.48). Absolute weight change patterns showed that the risk of liver fibrosis was highest (aOR = 2.94) when T2D patients gained at least 20 kg of weight from 10 years prior to 2017-2018 cycle.
Conclusions
Obesity in middle and late adulthood is associated with an increased risk of T2D complicated with liver fibrosis.
Non-alcoholic fatty liver disease (NAFLD) has become the most common form chronic liver disease in the USA [1]; it is closely relate to obesity, ectopic fat deposition, insulin resistance, high fructose, cholesterol intake, and genetic mutation [2,3]. About 38% of NAFLD patients will develop liver fibrosis or even liver cirrhosis and liver cancer [4], making early identification of liver fibrosis and its risk factors crucial.
The degree of fibrosis is the best predictor of future NAFDL-related clinical outcomes [5]. Notably, early identification of patients with liver fibrosis and risk factors can potentially prevent progression to decompensated cirrhosis, hepatocellular carcinoma (HCC), as well as liver-related and non-liver-related death [6]. Although liver biopsy is the gold standard for assessing liver fibrosis staging [7], it is expensive and invasive, with poor patient compliance. Current guidelines recommend the use of non-invasive tests to evaluate liver lesions, such as ultrasound transient elastography (TE) to screen for liver stiffness [8].
Weight change reflects health trajectories over an individual's life course. Previous studies have shown that weight gain from adolescence to mid or late adulthood is associated with increased morbidity and mortality risk from diabetes, cardiovascular disease (CVD), cancer, and all-cause mortality [9,10]. Surprisingly, only four studies examined the association of weight change in adulthood with NAFLD [11-14].
Previous studies have shown that type 2 diabetes (T2D) is closely related to the occurrence and progression of liver fibrosis [15,16], based on a pathological mechanism through which high blood glucose potentially has a direct toxic effect on liver cells, leading to liver cell damage and death. Although the association between weight change and liver fibrosis risk has been explored, it is unclear whether this risk also exists in patients with T2D, who tend to have a longer disease course and go through dynamic changes in weight. We aimed to address this gap by investigating the prevalence of liver fibrosis in T2D and non-T2D participants and comparing the relationship between weight change and the risk of liver fibrosis in these two groups.
METHODS
Study population
We conducted a cohort study using data from the 2017-2018 cycle of National Nutritional Health Survey (NHANES), which specifically assessed liver fibrosis with TE. We defined T2D according to any of the following criteria from the American Diabetes Association (ADA) [17]:
Self-reported diagnosis of diabetes;
Haemoglobin A1c (HbA1c) level ≥6.5% (48 mmol/mol);
Fasting plasma glucose (FPG) ≥126 mg/dL.
We defined T2D duration as the cumulative period from the age at which an individual was first diagnosed with T2D to the 2017-2018 cycle survey. Overall, 6401 participants underwent TE during the 2017-2018 cycle; we excluded 493 who did not fulfil the fasting requirement (n = 257), had <10 valid measurements (n = 129), or had an interquartile range/median liver stiffness measurements (LSM) value ≥30 (n = 107). We also excluded individuals positive for hepatitis B virus surface antigen and hepatitis C virus surface antibody RNA, and participants with significant alcohol consumption (n = 453). We estimated alcohol consumption based on self-reported data on the amount and frequency of alcohol use within the previous year. It was considered significant an individual consumed >1 drink per day (for females) and >2 drinks per day (for males = on average). We then excluded missing values for body mass index (BMI) (2017-2018 cycle, 10 years prior, age 25). After merging data, we could no longer match data on participants with information on drug use and dietary intake, because they were indirectly excluded when merging data on weight and diabetes. Finally, we enrolled a total of 2240 participants, 622 of whom were T2D patients and 1618 of whom were no-T2D (Figure 1). We followed the STROBE statement in conducting the study [43].
Figure 1.
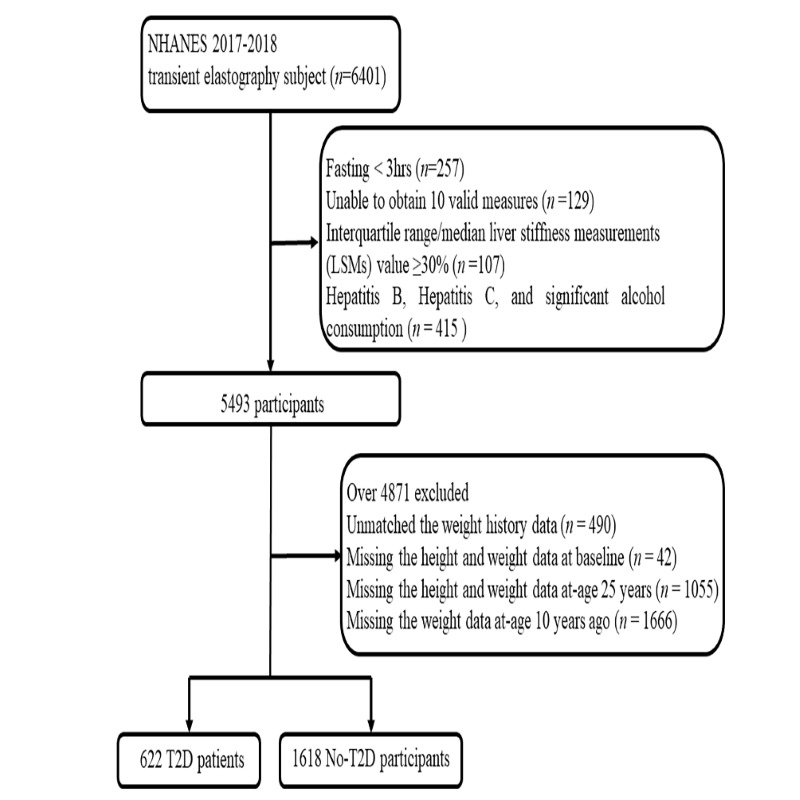
Data exclusion process.
Exposure
Respondents were asked to self-report their weight and height at age 25 (early adulthood) and their weight 10 years prior (mid-adulthood) the 2017-2018 cycle, while these values were measured via physical examination for the exact 2017-2018 cycle (late adulthood). We converted the said values to kgs (for weight) and m (for height) to calculate BMI (kg/m2) for each corresponding period.
We created patterns of weight change for three times intervals for BMI: age 25 to 10 years prior, age 25 to 2017-2018 cycle, and 10 years prior to 2017-2018 cycle [9]. We used four categories of BMI change patterns: stable non-obese (age 25 < 30 kg/m2 and 10 years prior <30 kg/m2), losing (age 25 ≥ 30 kg/m2 and 10 years prior <30 kg/m2), gaining (age 25 < 30 kg/m2 and 10 years prior ≥30 kg/m2), and stable obese (age 25 ≥ 30 kg/m2 and 10 years prior ≥30 kg/m2). We calculated the weight change categories for age 25 to 2017-2018 cycle and 10 years prior to 2017-2018 cycle as above [10]. We also divided the absolute weight change within each time interval into five groups: weight loss of at least 2.5 kg, weight change within 2.5 kg (reference group), weight gain of at least 2.5 kg but less than 10.0 kg, and weight gain of at least 10.0 kg but less than 20.0 kg, andf weight gain of at least 20.0 kg [9,18].
Outcome
FibroScan model 502 V2 Touch (Echosens®TM, Echosens, Paris, France) was performed, equipped with a medium (M) or extra-large (XL) probe. The M probe was used initially unless the machine indicated use of the XL probe. LSM scores were measured to detect liver fibrosis. A median LSM≥8.2 kPa was considered the threshold for significant fibrosis (≥F1), while values ≥9.7 kPa and ≥13.6 kPa were considered the threshold for advanced fibrosis (F2) and cirrhosis (F3), respectively [8].
Covariates
Covariate information was obtained at 2017-2018 cycle by physical examination, laboratory tests, and questionnaires. Sociodemographic characteristics including sex (males, females), age, education (high school below, high school education or equivalent, college degree or above), and marital status (married, divorce). Smoking, drinking, and physical activity (PA) were obtained through self-reporting. Physical examination measured systolic blood pressure (SBP), diastolic blood pressure (DBP), waist circumference (WC). Laboratory-checked levels of HbA1c, total cholesterol (TC), total triglycerides (TG), platelet count, alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyl transpeptidase (GGT), creatinine, albumin, globulin, high-sensitivity C-reactive protein (hs-CRP), and other assays have been detailed elsewhere [19]. Individuals’ comorbidities – chronic diseases, such as dyslipidaemia (DLP), hypertension (HTN), CVD (congestive heart failure, coronary heart disease, heart attack, stroke), and cancer, were obtained by physical examination, laboratory tests, or self-report [20].
Statistical analysis
We applied appropriate sampling weight analysis in our analysis. We expressed continuous variables as means with standard deviations (SDs) or medians with interquartile ranges (IQRs), testing for differences between groups using an independent sample t-test or non-parametric test. We presented categorical variables as numbers and percentages, and tested for differences between groups using χ2 test. We applied bivariate logistic regression to evaluate the association of weight change patterns and absolute weight change on the presence of fibrosis, which we reported through odds ratios (ORs) and 95% confidence intervals (CIs). We performed subgroup analyses based on sex, age (2017-2018 cycle), and T2D duration. Additionally, we employed a restricted cubic spline regression model with three knots (5th, 50th, and 95th percentiles) to estimate the linear dose-response relationship between BMI at three time points, and with absolute weight change and liver fibrosis risk.
We calculated population attributable fraction (PAF) [10,12] to estimate the percentage of liver fibrosis cases that could be prevented under four hypothetical scenarios (Table S7 in the Online Supplementary Document).The category-specific attributable fraction is the percentage of liver fibrosis cases that would be eliminated if individuals in that weight change category were moved to another lower-risk category, assuming causation. We also performed sensitivity analyses to evaluate the stability of the results.
We performed all statistical analyses in R, version 4.10 (R Core Team, Auckland, New Zealand) and Stata, version 14.0 (StataCorp, College Station, TX, USA), with the Stata package “punafcc” for calculating PAF. We considered a two-tailed value of P < 0.05 as statistically significant, but set the test level for interaction effects at P < 0.10.
RESULTS
Participants characteristics
LSM, CAP, age, BMI, WC, DBP, ALT, GGT, LDH, albumin to globulin ratio, hs-CRP, HbA1c, TC, TG values, and the prevalence of HTN, DLP, CVD, cancer were higher in T2D compared to non-T2D participants; this difference was statistically significant (Table S1 in the Online Supplementary Document).
Prevalence of liver fibrosis
Among 622 T2D patients, there were 131 with any degree of liver fibrosis, with an overall weighted prevalence (wp) of 23.04% (95% CI = 17.86-29.20). In this group, there were 39 patients with grade 1 (wp = 7.21%; 95% CI = 4.49-11.38), 51 with grade 2 (wp = 8.44%; 95% CI = 5.58-12.57), and 41 with grade 39 liver fibrosis (wp = 7.39%; 95%CI = 4.24-12.59). Among 1618 non-T2D participants, there were 150 patients with any degree of liver fibrosis (wp = 6.70%; 95% CI = 5.10-8.16), among whom 57 had grade 1 (wp = 2.05%; 95% CI = 1.35-3.10), 55 had grade 2 (wp = 2.55%; 95% CI = 1.69-3.83), and 38 had grade 3 liver fibrosis (wp = 2.10%; 95% CI = 1.15-3.81). The prevalence of liver fibrosis was significantly higher in T2D patients than in non-T2D participants (Table S2 in the Online Supplementary Document).
Relationship of weight change patterns with liver fibrosis
After adjusting for all covariates, the risk of liver fibrosis was highest for the stable obese group in both T2D patients and non-T2D participants (Table 1 and Table 2). However, the risk of liver fibrosis was higher in stable obese individuals with T2D (OR = 3.86; 95% CI = 1.78-16.53) than in their non-T2D peers (OR = 3.46; 95% CI = 1.46-8.18). T2D patients and the stable obese group of non-T2D participants had the highest risk in the 10 years period to 2017-2018 cycle; however, this risk was higher in T2D patients (OR = 3.13; 95%CI = 1.84-5.48) compared to the non-T2D participants (OR = 2.63; 95%CI = 1.40-4.42) (Tables S3-S4 in the Online Supplementary Document). However, weight loss was rare, and the association was not statistically significant among T2D patients. When evaluating the weight status at each time point (Figure 2), we found that the association of BMI with fibrosis risk at age 25 years, 10 years prior, and at 2017-2018 cycle was linear among T2D patients.
Table 1.
Association of weight changes with liver fibrosis risk among T2D patients
| Weight change patterns |
||||
|---|---|---|---|---|
|
Liver fibrosis
|
Stable non-obese (reference)
|
Weight loss, OR (95%CI)
|
Weight gain, OR (95%CI)
|
Stable obese, OR (95%CI)
|
| Events/total |
24/203 |
8/70 |
83/292 |
16/51 |
| Model 1* |
1.00 |
0.88 (0.38-2.05) |
2.96 (1.80-4.87) |
3.46 (1.65-7.07) |
| Model 2† |
1.00 |
0.85 (0.36-1.99) |
3.13 (1.84-5.00) |
3.41 (1.62-7.18) |
| Model 3‡ | 1.00 | 0.45 (0.34-6.16) | 3.24 (1.24-8.05) | 3.86 (1.78-16.53) |
BMI – body mass index, OR – odds ratios, CI –confidence interval, T2D – type 2 diabetes
*Crude model.
†Adjusted for sex and age.
‡Adjusted for sex, age, education, marital status, smoking, alcohol consumption, physical activity, SBP, DBP, WC, ALT, AST, GGT, LDH, hs-CRP, Uric acid to creatinine ratio, albumin to globulin ratio TC, TG, platelet count, dyslipidaemia, hypertension, CVD, and cancer.
Table 2.
Association of weight changes with liver fibrosis risk among no-T2D participants
| Weight change patterns |
||||
|---|---|---|---|---|
|
Liver fibrosis
|
Stable non-obese (reference)
|
Weight loss, OR (95%CI)
|
Weight gain, OR (95%CI)
|
Stable obese, OR (95%CI)
|
| Events/total |
57/940 |
8/117 |
76/512 |
9/49 |
| Model 1* |
1.00 |
1.14 (0.53-2.46) |
2.70 (1.88-3.88) |
3.49 (1.61- 7.54) |
| Model 2† |
1.00 |
1.04 (0.48-2.25) |
2.86 (1.98-4.13) |
3.68 (1.68-8.06) |
| Model 3‡ | 1.00 | 0.80 (0.33-1.93) | 3.23 (2.12-4.93) | 3.46 (1.46-8.18) |
BMI – body mass index, OR – odds ratios, CI –confidence interval, T2D – type 2 diabetes
*Crude model.
†Model 2: Adjusted for sex and age.
‡Model 3: Adjusted for sex, age, education, marital status, smoking, alcohol consumption, physical activity, SBP, DBP, WC, ALT, AST, GGT, LDH, hs-CRP, Uric acid to creatinine ratio, albumin to globulin ratio TC, TG, platelet count, dyslipidaemia, hypertension, CVD, and cancer.
Figure 2.
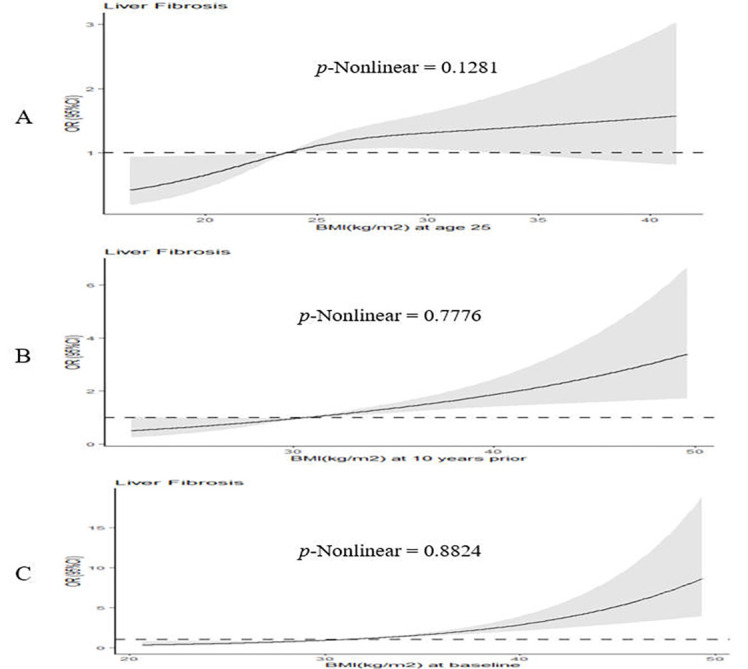
Dose-response curves of BMI at three time points associated with liver fibrosis risk among T2D patients.
Association of absolute weight change with liver fibrosis among T2D patients
Since the relationship between weight change and the risk of liver fibrosis was greater in T2D patients, we performed subgroup analyses of absolute weight change and demographic characteristics in T2D patients. When classified into categories (Table S5 in the Online Supplementary Document), the OR for liver fibrosis in the extreme weight gain (weight gain ≥20 kg) group were 2.94 (95% CI = 1.24-7.07) from 10 years prior to 2017-2018 cycle among T2D patients. However, weight loss of at least 2.5kg was not associated with increased liver fibrosis risk. The relationship between absolute weight change from 10 years prior to 2017-2018 cycle, with a nonlinear risk for liver fibrosis risk (P = 0.031) (Figure S1, panel C in the Online Supplementary Document).
Subgroup analysis among T2D patients
We found an association between weight change patterns and liver fibrosis within males (OR = 3.64; 95% CI = 1.79-7.42) and patients younger than 65 years (OR = 3.56; 95% CI = 1.59-7.95), while T2D patients maintained obesity from 10 years ago to 2017-2018 cycle (Table S6 in the Online Supplementary Document). Individuals who have been obese from age 25 to the 2017-2018 cycle and had a T2D duration of more than 10 years had a OR of 4.88 (95% CI = 1.38-17.35). We found an interactive effect of T2D duration and weight change when BMI changed from age 25 to 2017-2018 cycle (P = 0.037).
PAF analysis among T2D patients
In the weight loss scenario, 60.71% (95% CI = 39.75-64.83) of liver fibrosis events could have been if those who gained weight from early adulthood had lost weight in late adulthood (Table S7 in the Online Supplementary Document). With weight maintenance, 58.55% (95% CI = 37.05-72.71) of liver fibrosis events could have been if those who gained weight remained non-obese in adulthood. In partial prevention regimens, 53.70% (95% CI = 30.24-69.28) of liver fibrosis events could have been prevented if the T2D patients remained stable and non-obese (BMI<30 kg/m2) throughout life. However, as there were only 39 liver fibrosis cases with BMI<25 kg/m2 across the three lifespans in our analysis, we did not observe a protective effect of the comprehensive preventive regimen.
Sensitivity analysis among T2D patients
Risk of significant liver fibrosis and cirrhosis was highest in stable obesity from 10 years prior to 2017-2018 cycle, while the risk of advanced fibrosis was highest in stable obesity from age 25 to 2017-2018 cycle survey (Table S8 in the Online Supplementary Document).
DISCUSSION
Main findings
We found tha the prevalence of liver fibrosis was higher in T2D patients than in non-T2D participants and that weight change was more associated with fibrosis risk in T2D patients than in non-T2D participants. We also observed the highest risk of liver fibrosis in stable obese individuals from mid to late adulthood among T2D patients. These findings suggest that enhanced weight management in midlife could contribute to reduce the risk of liver fibrosis in adults with T2D.
Interpretation of the results
Previous studies have suggested that the mechanisms underlying the deleterious effects of weight change over certain lifespans may differ [9,21]. For example, in early adulthood, weight gain primarily results from the accumulation of fat mass [22-24], while in late adulthood, it is usually attributed to a decrease in lean body mass and an increase in fat mass [25,26]. Our results are therefore in line with previous studies showing that obesity in midlife exacerbates lipid deposition [27]. Imbalances in lipid metabolism lead to the formation of lipotoxic intermediates that contribute to cellular stress (ie, oxidative stress and endoplasmic reticulum stress), inflammasome activation, and apoptotic cell death, followed by inflammation, tissue regeneration, and fibrogenesis [28]. Previous evidence suggests that mild fibrosis (F1), considered an early stage of NAFLD, mostly does not progress to cirrhosis [29]. However, in the setting of obesity and T2D, many patients with fibrosis may be at risk for being “rapid progressors” to more severe liver disease [30,31]. We found that 23.04% of T2D patients had liver fibrosis, which supports the evidence that obese and diabetic patients are at highest risk and need more aggressive screening.
Besides obesity status, more research is now focusing on the association between weight change and health outcomes, as weight change is common throughout adulthood [32,33]. A large prospective cohort study found that obesity and weight gain were independently associated with an increased risk of liver fibrosis progression [34]. We also found that the effect of weight gain was generally stronger from young to late adulthood than from young to mid-adulthood and mid-adult to late adulthood. Weight gain in early adulthood was more strongly associated with adverse metabolic markers (e.g. adiponectin, C-peptide, HbA1c, and GGT) than weight gain in late adulthood [35]. Excessive free fatty acid transport to the liver and skeletal muscle leads to increased intrahepatic triglycerides and leads to hepatic fat accumulation, which further contributes to liver fibrotic lesions [36]. We found that extreme weight gain (≥20 kg) in the 10 years prior to 2017-2018 cycle was associated with a 194% increased liver fibrosis risk, but found no such association for low to moderate weight gain (ranging from 2.5 kg to 20 kg) or weight loss of at least 2.5 kg. We also found that only absolute weight change from 10 years prior to 2017-2018 cycle exhibited a J-shaped nonlinear association with liver fibrosis. Although the underlying mechanisms require further investigation, our findings suggest that controlling weight gain at middle age would be an effective strategy to reduce the risk of future liver fibrosis.
In contrast to our study, Ciardullo et al. found a significant effect of body fat distribution on liver fibrosis in women rather than in men [37]. A recent meta-analysis showed that men are at a higher risk of developing NAFLD compared to women [38]. Although we are unable to determine the reason for the differences between studies, sex hormones might play a role in the progression of liver fibrosis [39]. Previous studies have shown that with age, the number and volume of individual hepatocytes decreases as hepatic blood flow declines, which may increase liver stiffness [40]. Our subgroup analysis showed that individuals with T2D duration of more than 10 years who remained obese throughout adulthood had a higher risk of developing liver fibrosis than those 25 to 10 years prior and 10 years prior to 2017-2018 cycle. Considering the wide 95% CI (1.38-17.35), this might be related to the small number of events. This finding suggests that the longer the duration of T2D, the more obesity begins at a young age, and the higher the risk of liver fibrosis.
We also employed a PAF analysis to explore the potential impact of weight loss interventions and preventive measures against weight gain.
Sensitivity analysis showed that stable obesity in adulthood resulting in the highest risk of advanced liver fibrosis, which may be related to the smaller sample size. However, wo found that weight control is beneficial in reducing liver fibrosis risk in T2D patients.
Strengths and limitations
Our study had several strengths. It is the first to explore the association of weight change with liver fibrosis risk in adults with T2D using data from the NHANES. Our PAF analysis suggest that starting weight control in midlife is beneficial for reducing and preventing liver fibrosis risk.
Our study also had some limitations. We used recalled and self-reported weight data from 25 age years and 10 years prior, making misclassification bias possible. However, several studies suggest that recalling early life weight may be a valid indicator for use in life course epidemiological analyses [41,42]. We did not apply other non-invasive markers such as Fibrosis 4 score (FIB-4) to diagnose liver fibrosis because there were only 14 liver fibrosis events, which may lead to many liver fibrosis events being missed if this index was used. We also did not include drug treatments such as Glucagon-like peptide-1 receptor agonist (GLP-1) in the analysis due to unmatched data. Moreover, we did not adjust for covariates such as income or dietary intake in the regression models, as these variables could not be matched after through the inclusion and exclusion criteria. Finally, we did not evaluate changes in other obesity-related markers such as WC and fat mass in relation to liver fibrosis due to the lack of data at different time points.
CONCLUSIONS
We found that stable obesity in midlife and weight gain from young age were associated with an increased liver fibrosis risk in adults with T2D. Future studies are needed to determine the relationship between changes in body composition in adulthood with liver fibrosis.
Additional material
Acknowledgements
We thank all individuals at the National Center for Health Statistics of the Centers for Disease Control and Prevention who were responsible for the planning and administration of NHANES and for making the datasets of NHANES available on the website.
Ethics statement: The CDC Research Ethics Review Board approved the initial investigation, and written informed consent was obtained from all adult participants. This analysis was considered exempt from ethical approval by our institution's institutional review board because the dataset used in the analysis was fully de-identified.
Data availability: Detailed survey operation manuals, consent documents, and brochures are available on the NHANES website (https://www.cdc.gov/nchs/nhanes/about_nhanes.htm, accessed on 20 March 2023).
Footnotes
Funding: ZXL was supported by the National Social Science Foundation of China (Grant No. 18ZDA085). JF was supported by grant from the Fundamental Research Funds for the Central Universities (Grant No. YCJJ202201030).
Authorship contributions: Conceptualization, methodology, data curation, and analysis: Pan Ke. Original draft preparation: Minzhi Xu, Jie Feng, Qingfeng Tian, Yan He, Zuxun Lu. Validation, visualization, critical revision of the manuscript: Pan Ke, Minzhi Xu, Yan He, Kai Lu. Conceptualization, administrative support, supervision: Zuxun Lu, Kai Lu.
Zuxun Lu, Kai Lu are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Disclosure of interest: The authors completed the ICMJE Disclosure of Interest Form (available upon request from the corresponding author) and disclose no relevant interests.
REFERENCES
- 1.Sheka AC, Adeyi O, Thompson J, Hameed B, Crawford PA, Ikramuddin S.Nonalcoholic Steatohepatitis: A Review. JAMA. 2020;323:1175-83. 10.1001/jama.2020.2298 [DOI] [PubMed] [Google Scholar]
- 2.Dewidar B, Kahl S, Pafili K, Roden M.Metabolic liver disease in diabetes - From mechanisms to clinical trials. Metabolism. 2020;111S:154299. 10.1016/j.metabol.2020.154299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khan RS, Bril F, Cusi K, Newsome PN.Modulation of Insulin Resistance in Nonalcoholic Fatty Liver Disease. Hepatology. 2019;70:711-24. 10.1002/hep.30429 [DOI] [PubMed] [Google Scholar]
- 4.Argo CK, Caldwell SH.Epidemiology and natural history of non-alcoholic steatohepatitis. Clin Liver Dis. 2009;13:511-31. 10.1016/j.cld.2009.07.005 [DOI] [PubMed] [Google Scholar]
- 5.Lee J, Vali Y, Boursier J, Spijker R, Anstee QM, Bossuyt PM, et al. Prognostic accuracy of FIB-4, NAFLD fibrosis score and APRI for NAFLD-related events: A systematic review. Liver Int. 2021;41:261-70. 10.1111/liv.14669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maya-Miles D, Ampuero J, Gallego-Durán R, Dingianna P, Romero-Gómez M.Management of NAFLD patients with advanced fibrosis. Liver Int. 2021;41 Suppl 1:95-104. 10.1111/liv.14847 [DOI] [PubMed] [Google Scholar]
- 7.Lai M, Afdhal NH.Liver Fibrosis Determination. Gastroenterol Clin North Am. 2019;48:281-9. 10.1016/j.gtc.2019.02.002 [DOI] [PubMed] [Google Scholar]
- 8.Eddowes PJ, Sasso M, Allison M, Tsochatzis E, Anstee QM, Sheridan D, et al. Accuracy of FibroScan Controlled Attenuation Parameter and Liver Stiffness Measurement in Assessing Steatosis and Fibrosis in Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2019;156:1717-30. 10.1053/j.gastro.2019.01.042 [DOI] [PubMed] [Google Scholar]
- 9.Chen C, Ye Y, Zhang Y, Pan XF, Pan A.Weight change across adulthood in relation to all cause and cause specific mortality: prospective cohort study. BMJ. 2019;367:l5584. 10.1136/bmj.l5584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stokes A, Collins JM, Grant BF, Scamuffa RF, Hsiao CW, Johnston SS, et al. Obesity Progression Between Young Adulthood and Midlife and Incident Diabetes: A Retrospective Cohort Study of U.S. Adults. Diabetes Care. 2018;41:1025-31. 10.2337/dc17-2336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nguyen VH, Yeo YH, Zou B, Le MH, Henry L, Cheung RC, et al. Discrepancies between actual weight, weight perception and weight loss intention amongst persons with NAFLD. J Intern Med. 2021;289:840-50. 10.1111/joim.13203 [DOI] [PubMed] [Google Scholar]
- 12.Ding Y, Xu X, Tian T, Yu C, Ge X, Gao J, et al. Weight Change across Adulthood in Relation to Non-Alcoholic Fatty Liver Disease among Non-Obese Individuals. Nutrients. 2022;14:2140. 10.3390/nu14102140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nah BKY, Ng CH, Chan KE, Tan C, Aggarwal M, Zeng RW, et al. Historical Changes in Weight Classes and the Influence of NAFLD Prevalence: A Population Analysis of 34,486 Individuals. Int J Environ Res Public Health. 2022;19:9935. 10.3390/ijerph19169935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang L, Yi J, Guo J, Ren X.Weigh change across adulthood is related to the presence of NAFLD: results from NHANES III. J Transl Med. 2023;21:142. 10.1186/s12967-023-04007-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barb D, Repetto EM, Stokes ME, Shankar SS, Cusi K.Type 2 diabetes mellitus increases the risk of hepatic fibrosis in individuals with obesity and nonalcoholic fatty liver disease. Obesity (Silver Spring). 2021;29:1950-60. 10.1002/oby.23263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park J, Kwon HJ, Sohn W, Cho JY, Park SJ, Chang Y, et al. Risk of liver fibrosis in patients with prediabetes and diabetes mellitus. PLoS One. 2022;17:e0269070. 10.1371/journal.pone.0269070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.American Diabetes Association Professional Practice Committee 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2022. Diabetes Care. 2022;45:S17-S38. 10.2337/dc22-S002 [DOI] [PubMed] [Google Scholar]
- 18.Zheng Y, Manson JE, Yuan C, Liang MH, Grodstein F, Stampfer MJ, et al. Associations of Weight Gain From Early to Middle Adulthood With Major Health Outcomes Later in Life. JAMA. 2017;318:255-69. 10.1001/jama.2017.7092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey (NHANES). MEC Laboratory Procedures Manual. 2017. Washington, DC, U.S. Department of Health and Human Services. Available: https://wwwn.cdc.gov/nchs/data/nhanes/2017-2018/manuals/2017_MEC_Laboratory_Procedures_Manual.pdf. Accessed: 20 March 2023.
- 20.Peng Y, Liu F, Qiao Y, Wang P, Ma B, Li L, et al. Association of abnormal bowel health with major chronic diseases and risk of mortality. Ann Epidemiol. 2022;75:39-46. 10.1016/j.cgh.2018.05.059 [DOI] [PubMed] [Google Scholar]
- 21.Underland LJ, Schnatz PF, Wild RA, Saquib N, Shadyab AH, Allison M, et al. The impact of weight change and measures of physical functioning on mortality. J Am Geriatr Soc. 2022;70:1228-35. 10.1111/jgs.17626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim MN, Lo CH, Corey KE, Liu PH, Ma W, Zhang X, et al. Weight gain during early adulthood, trajectory of body shape and the risk of nonalcoholic fatty liver disease: A prospective cohort study among women. Metabolism. 2020;113:154398. 10.1016/j.metabol.2020.154398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jia G, Shu XO, Liu Y, Li HL, Cai H, Gao J, et al. Association of Adult Weight Gain With Major Health Outcomes Among Middle-aged Chinese Persons With Low Body Weight in Early Adulthood. JAMA Netw Open. 2019;2:e1917371. 10.1001/jamanetworkopen.2019.17371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kjøllesdal MKR, Ariansen I, Næss ØE.Early adulthood weight, subsequent midlife weight change and risk of cardiovascular disease mortality: an analysis of Norwegian cardiovascular surveys. Int J Obes (Lond). 2020;44:399-408. 10.1038/s41366-019-0467-0 [DOI] [PubMed] [Google Scholar]
- 25.Kodama S, Horikawa C, Fujihara K, Yoshizawa S, Yachi Y, Tanaka S, et al. Quantitative relationship between body weight gain in adulthood and incident type 2 diabetes: a meta-analysis. Obes Rev. 2014;15:202-14. 10.1111/obr.12129 [DOI] [PubMed] [Google Scholar]
- 26.Junno JA, Niskanen M, Maijanen H, Holt B, Sladek V, Niinimäki S, et al. The effect of age and body composition on body mass estimation of males using the stature/bi-iliac method. J Hum Evol. 2018;115:122-9. 10.1016/j.jhevol.2017.10.006 [DOI] [PubMed] [Google Scholar]
- 27.Taira S, Shimabukuro M, Higa M, Yabiku K, Kozuka C, Ueda R, et al. Lipid deposition in various sites of the skeletal muscles and liver exhibits a positive correlation with visceral fat accumulation in middle-aged Japanese men with metabolic syndrome. Intern Med. 2013;52:1561-71. 10.2169/internalmedicine.52.0521 [DOI] [PubMed] [Google Scholar]
- 28.Friedman SL, Neuschwander-Tetri BA, Rinella M, Sanyal AJ.Mechanisms of NAFLD development and therapeutic strategies. Nat Med. 2018;24:908-22. 10.1038/s41591-018-0104-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lomonaco R, Godinez Leiva E, Bril F, Shrestha S, Mansour L, Budd J, et al. Advanced Liver Fibrosis Is Common in Patients With Type 2 Diabetes Followed in the Outpatient Setting: The Need for Systematic Screening. Diabetes Care. 2021;44:399-406. 10.2337/dc20-1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pais R, Charlotte F, Fedchuk L, Bedossa P, Lebray P, Poynard T, et al. A systematic review of follow-up biopsies reveals disease progression in patients with non-alcoholic fatty liver. J Hepatol. 2013;59:550-6. 10.1016/j.jhep.2013.04.027 [DOI] [PubMed] [Google Scholar]
- 31.Bril F, Kalavalapalli S, Clark VC, Lomonaco R, Soldevila-Pico C, Liu IC, et al. Response to Pioglitazone in Patients With Nonalcoholic Steatohepatitis With vs Without Type 2 Diabetes. Clin Gastroenterol Hepatol. 2018;16:558-66.e2. 10.1016/j.cgh.2017.12.001 [DOI] [PubMed] [Google Scholar]
- 32.Liu D, Huang Y, Huang C, Yang S, Wei X, Zhang P, et al. Calorie Restriction with or without Time-Restricted Eating in Weight Loss. N Engl J Med. 2022;386:1495-504. 10.1056/NEJMoa2114833 [DOI] [PubMed] [Google Scholar]
- 33.Stephan Y, Sutin AR, Terracciano A.Change in weight and personality in middle-aged and older adults. Psychol Health. 2020;35:872-86. 10.1080/08870446.2019.1679372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim Y, Chang Y, Cho YK, Ahn J, Shin H, Ryu S.Obesity and Weight Gain Are Associated With Progression of Fibrosis in Patients With Nonalcoholic Fatty Liver Disease. Clin Gastroenterol Hepatol. 2019;17:543-50.e2. 10.1016/j.cgh.2018.07.006 [DOI] [PubMed] [Google Scholar]
- 35.Kværner AS, Hang D, Giovannucci EL, Willett WC, Chan AT, Song M.Trajectories of body fatness from age 5 to 60 y and plasma biomarker concentrations of the insulin-insulin-like growth factor system. Am J Clin Nutr. 2018;108:388-97. 10.1093/ajcn/nqy103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, Mynatt RL, et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17:179-88. 10.1038/nm.2279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ciardullo S, Oltolini A, Cannistraci R, Muraca E, Perseghin G.Sex-related association of nonalcoholic fatty liver disease and liver fibrosis with body fat distribution in the general US population. Am J Clin Nutr. 2022;115:1528-34. 10.1093/ajcn/nqac059 [DOI] [PubMed] [Google Scholar]
- 38.Balakrishnan M, Patel P, Dunn-Valadez S, Dao C, Khan V, Ali H, et al. Women Have a Lower Risk of Nonalcoholic Fatty Liver Disease but a Higher Risk of Progression vs Men: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2021;19:61-71.e15. 10.1016/j.cgh.2020.04.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wei S, Schmidt MD, Dwyer T, Norman RJ, Venn AJ.Obesity and menstrual irregularity: associations with SHBG, testosterone, and insulin. Obesity (Silver Spring). 2009;17:1070-6. 10.1038/oby.2008.641 [DOI] [PubMed] [Google Scholar]
- 40.Tokuhara D, Cho Y, Shintaku H.Transient Elastography-Based Liver Stiffness Age-Dependently Increases in Children. PLoS One. 2016;11:e0166683. 10.1371/journal.pone.0166683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De Rubeis V, Bayat S, Griffith LE, Smith BT, Anderson LN.Validity of self-reported recall of anthropometric measures in early life: A systematic review and meta-analysis. Obes Rev. 2019;20:1426-40. 10.1111/obr.12881 [DOI] [PubMed] [Google Scholar]
- 42.Jeffreys M, Lawlor DA, Galobardes B, McCarron P, Kinra S, Ebrahim S, et al. Lifecourse weight patterns and adult-onset diabetes: the Glasgow Alumni and British Women’s Heart and Health studies. Int J Obes (Lond). 2006;30:507-12. 10.1038/sj.ijo.0803161 [DOI] [PubMed] [Google Scholar]
- 43.Cuschieri S.The STROBE Guidelines. Saudi J Anaesth. 2019;13(Suppl 1):S31-S34. 10.1038/sj.ijo.0803161 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


