Abstract
IMPORTANCE:
Protein binding of valproate varies among ICU patients, altering the biologically active free valproate concentration (VPAC). Free VPAC is measured at few laboratories and is often discordant with total VPAC. Existing equations to predict free VPAC are either not validated or are inaccurate in ICU patients.
OBJECTIVES:
We designed this study to derive and validate a novel equation to predict free VPAC using data from ICU patients and to compare its performance to published equations.
DESIGN:
Retrospective cohort study.
SETTING:
Two academic medical centers.
PARTICIPANTS:
Patients older than 18 years old with concomitant free and total VPACs measured in the ICU were included in the derivation cohort if admitted from 2014 to 2018, and the validation cohort if admitted from 2019 to 2022.
MAIN OUTCOMES AND MEASURES:
Multivariable linear regression was used to derive an equation to predict free VPAC. Modified Bland-Altman plots and the rate of therapeutic concordance between the measured and predicted free VPAC were compared.
RESULTS:
Demographics, median free and total VPACs, and valproate free fractions were similar among 115 patients in the derivation cohort and 147 patients in the validation cohort. The Bland-Altman plots showed the new equation performed better (bias, 0.3 [95% limits of agreement, –13.6 to 14.2]) than the Nasreddine (–9.2 [–26.5 to 8.2]), Kodama (–9.7 [–30.0 to 10.7]), Conde Giner (–7.9 [–24.9 to 9.1]), and Parent (–9.9 [–30.7 to 11.0]) equations, and similar to Doré (–2.0 [–16.0 to 11.9]). The Doré and new equations had the highest therapeutic concordance rate (73%).
CONCLUSIONS AND RELEVANCE:
For patients at risk of altered protein binding such as ICU patients, existing equations to predict free VPAC are discordant with measured free VPAC. A new equation had low bias but was imprecise. External validation should be performed to improve its precision and generalizability. Until then, monitoring free valproate is recommended during critical illness.
Keywords: critical care, pharmacology, therapeutic drug monitoring, valproate, valproic acid
KEY POINTS
Objectives: We aimed to derive and validate a novel equation to predict free valproate concentration (VPAC) using data from ICU patients and to compare its performance to published predictive equations.
Findings: This study confirms that measured free VPAC is often discordant with predicted free VPAC in patients at risk of altered protein binding such as critically ill patients and suggests that published equations to predict free VPAC may not apply to these patients.
Meaning: Given the limited availability of free VPAC monitoring, an accurate equation-based model might facilitate better monitoring for this drug with a narrow therapeutic index. Further work is recommended to externally validate the newly derived equation and to test its performance during routine clinical practice.
Valproate is an anti-seizure drug approved to treat seizures with expanded indications for manic episodes in bipolar disorder and migraine prophylaxis (1–4). Clinical practice guidelines recommend therapeutic drug monitoring during valproate therapy due to its narrow therapeutic index, and doses are generally adjusted to maintain a total valproate concentration (VPAC) of 50–125 mg/L (1, 5).
Valproate is highly protein-bound to albumin, with the biologically active free (unbound) fraction expected to be 5–10% of the total concentration (6, 7). The protein binding of valproate is altered by hypoalbuminemia, uremia, increasing total VPAC, free fatty acid-containing therapies (e.g., propofol, clevidipine, IV fat emulsion), and medications (e.g., aspirin) resulting in an increased free fraction (6–8). ICU patients and others at risk for altered protein binding have consistently shown much higher free fractions ranging from 15% to 89% (9). The resultant therapeutic discordance may lead to erroneous dose adjustments when guided by total VPAC alone (9–11). Free VPAC monitoring is warranted in these patients given significant interindividual variability to prevent dose-related toxicities associated with increased free VPAC (6–8).
Despite free VPAC monitoring being increasingly advocated, access to these assays remains limited (12). A 2002 survey found that only 2% of laboratories that measured total VPAC also measured free VPAC (12). A recent 2021 survey of the American College of Clinical Pharmacy Practice and Research Networks found that only 20% of those select hospitals performed free valproate monitoring in-house, although this likely overestimates assay availability at all healthcare centers due to responder bias (D. Gagnon, unpublished observations). Limited access to free VPAC assays has spurred the development of equation-based models to predict free VPAC (Table 1) (13–17). However, these equations have yet to be externally validated or have already been shown to be inaccurate for ICU and other patients (9, 18, 19).
TABLE 1.
Equations to Predict Free Valproate Concentration
| References | Equation | Population | Previously Validated in ICU Patients? |
|---|---|---|---|
| Parent et al (13) | Y = Ae−BX | Twenty-nine samples from 22 patients in a single hospital | Therapeutic discordance 40% in 104 ICU patients (19), and 93% in 15 ICU patients (9) |
| Y = valproate free fraction % | |||
| A = 130.69 | |||
| B = 4.96 × 10–3 | |||
| X = serum albumin (μmol/L) | |||
| Kodama et al (14) | Forty-six samples from 29 adults in the outpatient setting receiving polytherapy for epilepsy | No | |
| Cf = free valproate concentration (μM) | |||
| Ct = total valproate concentration (μM) | |||
| K = 0.0281, population mean association constant | |||
| n(Pt) = 757, population mean total number of binding sites | |||
| Doré et al (15) | Y = 103.667 + (0.362 × X1) − (4.538 × X2 | Forty-one adult and pediatric outpatients or in a single hospital | No |
| Y = free valproate concentration (μmol/L) | |||
| X1 = total valproate concentration (μmol/L) | |||
| X2 = serum albumin (g/L) | |||
| Nasreddine et al (16) | Y = (0.0016 × X2) + (0.012 × x) + 0.4314 | Nine hundred two samples from 228 adult and pediatric patients who were participating in a trial of valproate monotherapy | No |
| Y = free valproate concentration (μg/mL) | |||
| X = total valproate concentration (μg/mL) | |||
| Conde Giner et al (17) | Y = 11.882 + (0.261 × X1) − (4.722 × X2) | Twenty-six samples from 20 adult patients hospitalized and outpatients with hypoalbuminemia (albumin < 3.5 g/dL) | No |
| Y = free valproate concentration (μg/mL) | |||
| X1 = total valproate concentration (μg/mL) | |||
| X2 = serum albumin (g/dL) | |||
| Fraser 2023 | Y = 10.74 + (0.34 × X1) − (4.60 × X2) + (0.02 × X3) + 2.14 (if propofol = yes) + 1.51 | Derivation cohort 115 adult ICU patients | Not available |
| Y = free valproate concentration (μg/mL) | Validation cohort 147 adult ICU patients | ||
| X1 = total valproate concentration (μg/mL) | |||
| X2 = serum albumin (g/dL) | |||
| X3 = serum blood urea nitrogen (mg/dL) |
To convert from μg/mL to μmol/L valproate, multiply by 6.93.
The objectives of this study were to derive a novel equation to predict free VPAC using ICU patient data, to validate the equation in a separate cohort, and to compare its performance to five previously published predictive equations.
MATERIALS AND METHODS
Study Design and Participants
This retrospective cohort study was conducted at Maine Medical Center (MMC) in Portland, Maine and Mayo Clinic in Rochester, MN. For the derivation and validation cohorts, consecutive patients older than 18 years old were included if they had samples for simultaneous free and total VPAC and albumin collected during their ICU stay. Patients were excluded if they had unmeasurable VPAC (< 3 μg/mL), and if they did not give consent for research authorization in the state of Minnesota (Mayo Clinic only). The derivation cohort included patients from January 2014 to December 2018 at Mayo Clinic and from September 2015 to December 2018 at MMC (free VPAC was not monitored before 2015 at MMC). The validation cohort included patients admitted from August 2019 to August 2022 at both centers. For patients with multiple sets of free and total VPAC, only the initial set of VPAC drawn during each patient’s ICU stay was evaluated. The protocol (“Total and Free Valproate Levels in Critically Ill Patients”) was approved by the Institutional Review Boards (IRBs) at Mayo Clinic (IRB number 18-011183) on February 14, 2019, and MMC (IRB number 1468271-1) on August 23, 2019. The IRBs provided a waiver of informed consent, and all study procedures were in accordance with the IRBs and the Helsinki Declaration of 1975.
Data Collection
Patient demographics included age, sex, race, weight, hospital length of stay, Charlson comorbidity index, Acute Physiology and Chronic Health Evaluation III (Mayo Clinic) or IV (MMC) scores, discharge disposition, and indication(s) for valproate therapy. At Mayo Clinic, total and free VPAC were analyzed using ultrafiltration and a homogeneous enzyme immunoassay (Roche Valproic Reagent; Roche Diagnostics, Indianapolis, IN) by Mayo Clinic Laboratories (Rochester, MN). At MMC, free VPACs was sent to Mayo Clinic in Rochester, MN, from September 2015 to October 2017. After that date, all total and free VPAC were assayed in-house at MMC using the same technique as Mayo Clinic. At both centers, total and free VPAC were collected together during the study period. Although the timing of valproate monitoring was at the discretion of the treatment team, it was our practice to avoid immediate post-load sampling.
Laboratory values were extracted within 72 hours of collecting the free and total VPACs. Serum albumin (g/dL), creatinine (mg/dL), blood urea nitrogen (BUN; mg/dL), and total bilirubin (mg/dL) obtained closest to when the free and total VPAC were recorded. Monitoring of these values was not protocolized during the study period. Data regarding administration of concomitant medications previously associated with valproate displacement from albumin binding sites were collected including aspirin, ibuprofen, ketorolac, clevidipine, propofol, and IV fat emulsion (9, 10). Medications were considered concomitant if they were administered during the 24 hours preceding free VPAC sample collection.
Published Predictive Equation Identification
PubMed was queried from database inception to December 31, 2022, using search terms “valproic acid OR valproate,” “equation,” “unbound,” and “free fraction” in humans and without language restrictions. Reference lists of identified papers were examined for possible additional citations. We identified five prediction equations derived in hospitalized noncritically ill patients and outpatients with epilepsy (Table 1).
Statistical Analysis
Continuous data are reported as median (interquartile range [IQR]) and frequencies as n (%). Free fraction of valproate was calculated by dividing the free VPAC by the total VPAC and multiplying by 100. For example, for a total VPAC of 100 μg/mL and a free VPAC of 9 μg/mL the resultant free fraction was calculated as follows: .
Using data from the derivation cohort of ICU patients, a new equation to predict free VPAC was determined with multivariable linear regression. Although valproate is reported to exhibit nonlinear pharmacokinetics due to saturable protein binding, we did not observe non-linearity between total and free VPAC for the ranges we observed in either the derivation or validation cohort (Fig. E1, http://links.lww.com/CCX/B260). Variables entered into the model included: total VPAC (1 μg/mL increase), serum albumin (1 g/dL increase), BUN (1 mg/dL increase), propofol or other lipid-containing therapy (clevidipine, IV fat emulsion) exposure (yes or no), and aspirin exposure (yes or no). These variables were determined a priori, but their respective weights were determined by the multivariable linear regression analysis.
The five previously published predictive equations and the derived equation were validated using clinical data from patients included in the validation cohort. Linear regression of predicted free VPAC was compared with measured free VPAC for each equation to generate Pearson correlation coefficients (r). Correlation was considered fair (0.3–0.5), moderate (0.51–0.8), or strong (> 0.8). The agreement between the predicted free VPAC and the measured free VPAC was compared for the five previously published equations and the new equation using a modified Bland-Altman plot, reporting the mean difference (“bias”) and corresponding 95% limits of agreement (LOA). Free VPAC was categorized according to its reference range as subtherapeutic (< 5 μg/mL), therapeutic (5–17 μg/mL), or supratherapeutic (> 17 μg/mL) (9). Predicted and measured free VPAC were therapeutically concordant if they were in the same category, and therapeutically discordant if they were in different categories. Therapeutic concordance for the new equation was compared with other equations using test for equality of proportions. Analyses were performed using SAS Version 9.4 (SAS Institute, Cary, NC) and R Version 4.0.3 (R Core Team; R Foundation for Statistical Computing, Vienna, Austria). A p value of less than 0.05 was considered statistically significant.
RESULTS
Demographics and Baseline Characteristics
A total of 262 ICU patients with simultaneous free and total VPAC and albumin measured were included, with 115 patients in the derivation cohort and 147 patients in the validation cohort. Baseline characteristics of the two cohorts were similar (Table E1, http://links.lww.com/CCX/B260). Median age was 55 years (IQR, 42–68 yr) in the derivation cohort and 62 years (48–68 yr) in the validation cohort, 79 (69%) and 93 (63%) patients were male, and indication for valproate was predominantly seizures (67%). The median total and free VPAC was 52 μg/mL (36–66 μg/mL) and 12 μg/mL (8–22 μg/mL) in the derivation cohort, and 54 μg/mL (26–71 μg/mL) and 13 μg/mL (8–23 μg/mL) in the validation cohort. The free fraction was in the “normal” reference range of 5–10% for three patients (3%) in the derivation cohort and three patients (2%) in the validation cohort (Table E1, http://links.lww.com/CCX/B260).
Derivation of a New Equation—The Fraser Equation
The novel equation to predict free VPAC from the derivation cohort was:
Only propofol was included in the adjustment variable as the number of patients on clevidipine or IV fat emulsion was low to absent (Table E1, http://links.lww.com/CCX/B260).
Validation of Published Equations
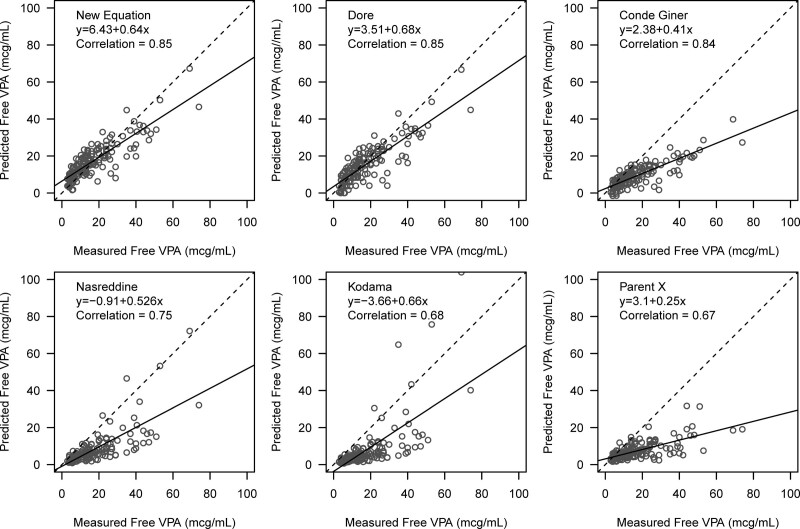
This new Fraser equation had a strong positive linear correlation with measured free VPAC (r = 0.85), compared with Doré (r = 0.85), Conde Giner (r = 0.84), Nasreddine (r = 0.75), Kodama (r = 0.68), and Parent (r = 0.67) (Fig. 1). The modified Bland-Altman plots showed that the Fraser equation overpredicted free VPAC (bias, 0.3 [95% LOA, –13.6 to 14.2]), whereas other equations underpredicted free VPAC such as Doré (–2.0 [–16.0 to 11.9]), Nasreddine (–9.2 [–26.5 to 8.2]), Kodama (–9.7 [–30.0 to 10.7]), Parent (–9.9 [–30.7 to 11.0]), and Conde Giner (–7.9 [–24.9 to 9.1]) (Fig. E2, http://links.lww.com/CCX/B260).
Figure 1.
Correlation of measured free valproate (VPA) concentration and predicted value from six equations. Dotted line represents 100% correlation between measured free VPA concentration and predicted value for each of the six equations. The solid black line is the trend line for each equation.
Therapeutic Concordance
The free VPAC predicted from the Fraser equation and the Doré equation had the highest therapeutic concordance with measured free VPAC (73%; p = 0.99 for difference between Doré and Fraser) which was significantly better than the Conde Giner (60%; p = 0.035), Parent (43%; p < 0.001), Nasreddine (43%; p < 0.001), and Kodama (29%; p < 0.001) equations (Table 2). The therapeutic discordance observed by the Conde Giner, Nasreddine, Kodama, and Parent equations was almost entirely due to underestimation of measured free VPAC. Most of the therapeutic discordance of the Doré equation was attributed to underestimation (54%) while the therapeutic discordance of the Fraser equation was mostly attributed to overestimation of measured free VPAC (78%).
TABLE 2.
Comparison of Predicted Free Valproate Concentration to Measured Values for Six Equations Among 147 ICU Patients
| Therapeutic Comparison | Fraser 2023 | Parent et al (13) | Kodama et al (14) | Doré et al (15) | Nasreddine et al (16) | Conde Giner et al (17) |
|---|---|---|---|---|---|---|
| Therapeutic comparison | ||||||
| Correlation | 0.85 | 0.67 | 0.68 | 0.85 | 0.75 | 0.84 |
| Bias (95% limits of agreement) | 0.3 (–13.6 to 14.2) | –9.9 (–30.7 to 11.0) | –9.7 (–30.0 to 10.7) | –2.0 (–16.0 to 11.9) | –9.2 (–26.5 to 8.2) | –7.9 (–24.9 to 9.1) |
| Concordancea | 107 (73%) | 64 (43%; p < 0.001) | 43 (29%; p < 0.001) | 108 (73%; p = 0.99) | 63 (43%; p < 0.001) | 89 (60%; p = 0.035) |
| Discordance | 40 (27%) | 83 (57%) | 104 (71%) | 39 (27%) | 84 (57%) | 58 (40%) |
| Overestimationb | 31 (78%) | 2 (2%) | 0 | 18 (46%) | 1 (1%) | 3 (5%) |
| Underestimationb | 9 (22%) | 81 (98%) | 104 (100%) | 21 (54%) | 83 (99%) | 55 (95%) |
p represents concordance for new equation compared with each subsequent equation using test for equality of proportions.
Percentages for overestimation and underestimation reflect fraction of discordant patients.
DISCUSSION
Therapeutic drug monitoring of total drug concentrations assumes that they consistently reflect the biologically active free concentrations (i.e., that free fraction is normal and constant such that the concentrations are therapeutically concordant). More than 40 years ago, this assumption was called into question for valproate (8) and was recently shown to not be true among ICU patients (9, 10). Total VPAC are discordant with measured free VPAC in 70–87% of ICU patients (9, 10). The limited availability of free VPAC assays has spurred the development of care equation-based models to predict free VPAC (13–17). However, none of them included ICU patients (13–17). This study evaluated the predictive performance of published equations and derived a new equation using a cohort of 115 ICU patients. Although the new Fraser equation demonstrated a lower bias compared with the five published equations, it should be used with caution due to its imprecision with a wide 95% LOA.
We found that the Parent, Kodama, Nasreddine, and Conde Giner equations underestimated the measured free VPAC by 7.9 to 9.9 μg/mL with LOA ranging from to 30.7 to 11 μg/mL. The mean difference between predicted and measured free VPAC across these four equations was 9.2 μg/mL. Because the reference range of free VPAC is narrow (5–17 μg/mL), a mean difference of 9.2 μg/mL represents 75% of the reference range. Beyond this concern is that the 95% LOA were wide with an absolute mean range of 42 μg/mL. Our results demonstrated that these four questions may have little utility in ICU patients.
The Doré equation performed similarly to the new Fraser equation in regard to mean bias difference and therapeutic concordance. Although the new equation had a lower bias, the overlap of CI and a similar absolute mean range (new equation 27.8 vs. Doré 27.9 μg/mL) suggested that the new equation did not perform better than the Doré equation. The Doré equation predicts free VPAC from total VPAC and serum albumin alone (15), while our model also included adjustment variables for aspirin, propofol, and BUN.
Prior evaluations have identified that antipyretic doses of aspirin may cause a four-fold increase in valproate free fraction, and a similar effect may be seen with lower cardio protective doses (7, 20, 21). Furthermore, concomitant administration of free fatty acids containing medications (i.e., propofol or clevidipine) or IV fat emulsion itself may increase valproate free fraction, due to valproate structural similarity to free fatty acids, in a concentration-dependent manner by 19–118% (7, 22). Similarly, uremic toxins may compete for valproate binding sites on albumin, thus increasing free fractions (7, 8). As a result of these previous findings, variables such as aspirin, propofol, and BUN were determined a priori in our model.
It is interesting to note that the inclusion of aspirin, propofol, and BUN did not improve our equation’s predictive performance compared with Doré’s. Our derivation cohort had normal BUN which was reflected in the small BUN coefficient of 0.02, indicating that BUN had little influence on free VPAC in this cohort. The new equation was not able to assess the concentration-dependent effect of free fatty acids since only 22% of our derivation cohort included patients receiving propofol, and we felt that incorporating a dose adjustment variable in our equation would result in overfitting. It is theoretically plausible that higher doses or concomitant use of propofol, clevidipine, and IV fat emulsion and greater elevations of BUN would result in a greater protein binding displacement of valproate. Continued efforts to enrich the cohort with a larger sample size of patients on propofol and/or aspirin and kidney dysfunction will be needed to test this hypothesis.
Currently, it appears that none of the published predictive equations including the new Fraser equation can predict free VPAC in ICU patients with minimal bias and imprecision. The Fraser and Doré equations had the lowest bias and may be useful as a surrogate marker while a measured free VPAC is pending. Given the imprecision, it may be prudent for the clinicians to not rely solely on these two equations for dose adjustments. When used, free VPAC should at least be intermittently obtained to confirm the performance of the equations in a given patient.
This study has several limitations. Our derivation and validation cohorts were of moderate size from ICUs at two institutions, but this is the only multicenter study deriving such a predictive equation, and with the exception of the study by Nasreddine et al (16) with 228 patients, our cohorts represent a much larger sample than the 20–41 patients from which the other four equations were derived (13–15, 17). This study represents an internal validation performed on a separate cohort but from the same institutions deriving the equation; external validation in different institutions and patient cohorts will strengthen generalizability. The retrospective design introduced a risk for bias and missed events. No protocol to guide the decision to measure free VPAC existed at either center at the time of the study, which may have introduced selection bias. Because we evaluated the initial VPAC drawn during each patient’s ICU stay, we were unable to ensure that steady state conditions were met, which may have resulted in higher free VPAC until metabolic pathways compensated (23). Future studies should accurately report the timing of concentrations (trough or peak) or if concentrations were drawn at steady state. Last, we also did not collect potential drug interactions (most notably phenytoin), which should be further explored.
It has been demonstrated that the valproate free fraction increases at higher total VPAC (> 100 μg/mL) due to saturable protein binding (6, 24). We did not observe any nonlinearity in our analysis; therefore, multivariable linear regression was used to derive the equation. We postulate that this is likely because our cohorts had lower total VPAC, below the threshold of saturable protein binding (6, 7). The predictive performance of our derived equation at higher total VPAC should be further investigated. We assessed therapeutic concordance based on a published reference range for free VPAC, and different reference ranges have been published (9, 11, 19, 25). In addition, reference ranges reflect laboratory reporting, while therapeutic ranges based on successful control of seizures and adverse effects may better guide personalized doses for each patient. Further studies are needed to better define the therapeutic range for free VPAC.
CONCLUSIONS
For patients at risk of altered protein binding such as ICU patients, existing equations to predict free VPAC are discordant with measured free VPAC. This new Fraser equation derived from ICU patients had low bias but was imprecise. External validation should be performed to evaluate its precision and generalizability. Until then, measuring the free VPAC during critical illness is recommended.
Supplementary Material
Footnotes
This study was partially funded by a research grant from the Mayo Midwest Pharmacy Research Committee.
Drs. Riker, May, Seder, and Gagnon are supported in part by a National Institute of General Medical Sciences grant (1P20GM139745). The remaining authors have disclosed that they do not have any potential conflicts of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
REFERENCES
- 1.Patsalos PN, Berry DJ, Bourgeois BFD, et al. : Antiepileptic drugs - best practice guidelines for therapeutic drug monitoring: A position paper by the subcommission on therapeutic drug monitoring, ILAE commission on therapeutic strategies. Epilepsia 2008; 49:1239–1276 [DOI] [PubMed] [Google Scholar]
- 2.Gagnon DJ, Fontaine GV, Smith KE, et al. : Valproate for agitation in critically ill patients: A retrospective study. J Crit Care 2017; 37:119–125 [DOI] [PubMed] [Google Scholar]
- 3.Tomson T, Battino D, Perucca E: Valproic acid after five decades of use in epilepsy: Time to reconsider the indications of a time-honoured drug. Lancet Neurol 2016; 15:210–218 [DOI] [PubMed] [Google Scholar]
- 4.Bowden CL: Anticonvulsants in bipolar disorders: Current research and practice and future directions. Bipolar Disord 2009; 11:20–33 [DOI] [PubMed] [Google Scholar]
- 5.Patsalos PN, Spencer EP, Berry DJ: Therapeutic drug monitoring of antiepileptic drugs in epilepsy: A 2018 update. Ther Drug Monit 2018; 40:526–548 [DOI] [PubMed] [Google Scholar]
- 6.Zaccara G, Messori A, Moroni F: Clinical pharmacokinetics of valproic acid—1988. Clin Pharmacokinet 1988; 15:367–389 [DOI] [PubMed] [Google Scholar]
- 7.Lin K, Cao VFS, Au C, et al. : Clinical pharmacokinetic monitoring of free valproic acid levels: A systematic review. Clin Pharmacokinet 2022; 61:1345–1363 [DOI] [PubMed] [Google Scholar]
- 8.Levy RH: Monitoring of free valproic acid levels? Ther Drug Monit 1980; 2:199–201 [DOI] [PubMed] [Google Scholar]
- 9.Riker RR, Gagnon DJ, Hatton C, et al. : Valproate protein binding is highly variable in ICU patients and not predicted by total serum concentrations: A case series and literature review. Pharmacotherapy 2017; 37:500–508 [DOI] [PubMed] [Google Scholar]
- 10.Brown CS, Liu JT, Riker RR, et al. : Evaluation of free valproate concentration in critically ill patients. Crit Care Explor 2022; 4:E0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drisaldi A, Weeda E, Neyens R, et al. : Accuracy of valproic acid concentration correction based on serum albumin. Neurocrit Care 2019; 30:301–306 [DOI] [PubMed] [Google Scholar]
- 12.Dasgupta A, Crossey MJ: Elevated free fatty acid concentrations in lipemic sera reduce protein binding of valproic acid significantly more than phenytoin. Am J Med Sci 1997; 313:75–79 [DOI] [PubMed] [Google Scholar]
- 13.Parent X, Marzullo C, Gutbub AM: Acide valproique: Estimation simple de la concentration serique libre. Ann Biol Clin (Paris) 1993; 51:649–650 [PubMed] [Google Scholar]
- 14.Kodama Y, Kuranari M, Kodama H, et al. : Comparison of two binding equations for prediction of the concentration of unbound valproic acid in the serum of adult epileptic polytherapy patients. J Pharm Pharmacol 1996; 48:1068–1072 [DOI] [PubMed] [Google Scholar]
- 15.Doré M, San Juan AE, Frenette AJ, et al. : Clinical importance of monitoring unbound valproic acid concentration in patients with hypoalbuminemia. Pharmacotherapy 2017; 37:900–907 [DOI] [PubMed] [Google Scholar]
- 16.Nasreddine W, Dirani M, Atweh S, et al. : Determinants of free serum valproate concentration: A prospective study in patients on divalproex sodium monotherapy. Seizure 2018; 59:24–27 [DOI] [PubMed] [Google Scholar]
- 17.Conde Giner S, Belles Medall MD, Ferrando Piqueres R: Design and validation of a predictive equation to estimate unbound valproic acid concentration. Eur J Hosp Pharm 2021; 30:293–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Riker RR, Gagnon D, May T, et al. : Valproate free serum concentrations: More complex than simple formulas. Seizure 2018; 60:155–156 [DOI] [PubMed] [Google Scholar]
- 19.Fisch U, Baumann SM, Semmlack S, et al. : Accuracy of calculated free valproate levels in adult patients with status epilepticus. Neurology 2021; 96:e102–e110 [DOI] [PubMed] [Google Scholar]
- 20.Sandson NB, Marcucci C, Bourke DL, et al. : An interaction between aspirin and valproate: The relevance of plasma protein displacement drug-drug interactions. Am J Psychiatry 2006; 163:1891–1896 [DOI] [PubMed] [Google Scholar]
- 21.Abbott FS, Kassam J, Orr JM, et al. : The effect of aspirin on valproic acid metabolism. Clin Pharmacol Ther 1986; 40:94–100 [DOI] [PubMed] [Google Scholar]
- 22.Patel IH, Levy RH: Valproic acid binding to human serum albumin and determination of free fraction in the presence of anticonvulsants and free fatty acids. Epilepsia 1979; 20:85–90 [DOI] [PubMed] [Google Scholar]
- 23.Cloyd JC, Dutta S, Cao G, et al. ; Depacon Study Group: Valproate unbound fraction and distribution volume following rapid infusions in patients with epilepsy. Epilepsy Res 2003; 53:19–27 [DOI] [PubMed] [Google Scholar]
- 24.Gómez Bellver MJ, García Sánchez MJ, Alonso González AC, et al. : Plasma protein binding kinetics of valproic acid over a broad dosage range: Therapeutic implications. J Clin Pharm Ther 1993; 18:191–197 [DOI] [PubMed] [Google Scholar]
- 25.Chan K, Beran RG: Value of therapeutic drug level monitoring and unbound (free) levels. Seizure 2008; 17:572–575 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.