Abstract
Background:
Excessive proliferation and migration of vascular smooth muscle cells (VSMCs) are the main causes of restenosis (RS) in diabetic lower extremity arterial disease (LEAD). However, the relevant pathogenic mechanisms are poorly understood.
Methods:
In this study, we introduced a "two-step injury protocol" rat RS model, which started with the induction of atherosclerosis (AS) and was followed by percutaneous transluminal angioplasty (PTA). Hematoxylin-eosin (HE) staining and immunohistochemistry staining were used to verify the form of RS. Two-step transfection was performed, with the first transfection of Lin28a followed by a second transfection of let-7c and let-7g, to explore the possible mechanism by which Lin28a exerted effects. 5-ethynyl-2΄-deoxyuridine (EdU) and Transwell assay were performed to evaluate the ability of proliferation and migration of VSMCs. Western blotting and quantitative real-time polymerase chain reaction (qRT-PCR) were performed to detect the expression of Lin28a protein and let-7 family members.
Results:
Using a combination of in vitro and in vivo experiments, we discovered that let-7c, let-7g, and microRNA98 (miR98) were downstream targets of Lin28a. More importantly, decreased expression of let-7c/let-7g increased Lin28a, leading to further inhibition of let-7c/let-7g. We also found an increased level of let-7d in the RS pathological condition, suggesting that it may function as a protective regulator of the Lin28a/let-7 loop by inhibiting the proliferation and migration of VSMCs.
Conclusion:
These findings indicated the presence of a double-negative feedback loop consisting of Lin28a and let-7c/let-7g, which may be responsible for the vicious behavior of VSMCs in RS.
Keywords: Restenosis, Lower extremity arterial disease, Vascular smooth muscle cells, Let-7, Lin28a
Introduction
Lower extremity arterial disease (LEAD) is characterized by vascular narrowing or occlusion in the lower limb arteries,[1] and is highly prevalent in individuals with diabetes. It is considered to be the major cause of foot ulceration and amputation among diabetic patients.[2,3] Percutaneous transluminal angioplasty (PTA) is the preferred treatment for diabetic LEAD and shows a high technical success rate.[4] However, restenosis (RS) limits the long-term clinical benefit of PTA.[5] Even with recent advances in the technical aspect of PTA, RS remains the most frequent cause of target lesion failure.[6] According to statistics, the occurrence of RS after PTA is between 40–60% after six months and nearly 100% after five years.[6] Despite the massive effort taken to resolve RS, no effective treatment has been found. More importantly, the pathogenetic mechanism underlying RS is still unclear. Further study is needed to understand its molecular details for the successful discovery of novel approaches to prevent RS.
Vascular smooth muscle cells (VSMCs) are highly differentiated cells that help to maintain the structure and function of blood vessels.[7] PTA triggers the migration of VSMCs from the middle layer to the inner layer of the arteries while remarkably promoting their proliferation. Excessive VSMC proliferation and migration are considered to be the main cause of RS.[8,9,10] Thus, preventing this VSMC dysregulation is a vital step to reduce the risk of RS after PTA.
Since atherosclerosis (AS) and RS were assumed to share similar pathogenic mechanisms, some of studies simply used models of AS to investigate RS.[11,12,13] However, the behavior of VSMCs shows notable differences between the two conditions. In AS, VSMCs behave appropriately, stabilizing the plaque.[14,15,16] In contrast, the "malignant" behavior of VSMCs is the main feature of RS, and it leads to rapid obstruction of the already atherosclerotic vessel after treatment. The speedy formation of RS plaques formed the background and foundation of our research, since focusing on the differences between RS and AS may provide a more effective route to identify the specific protein targets of RS and reveal the molecular mechanisms underlying the excessive migration and proliferation of VSMCs after PTA.
Lin28a is an RNA-binding protein that has been suggested to regulate the self-renewal of stem cells.[17] For RS, Lin28a has been shown to be a potent regulator of VSMCs.[18] Upregulation of Lin28a expression promotes the proliferation and migration of VSMCs. However, the exact mechanisms underlying the effects of Lin28a in RS require elucidation. Suppression of let-7 is the best-studied mechanism in the Lin28a regulation network; The let-7 family was one of the first tumor suppressor microRNAs (miRNAs) to be identified.[19,20] Recent studies have shown that let-7 is downregulated in the pathological process in some vascular diseases.[21] Yu et al[22] found that let-7d affects the proliferation of VSMCs in spontaneous hypertension through Kirsten rat sarcoma viral oncogene homolog (KRAS). However, in RS, the involvement of let-7 in the Lin28a signaling pathway is unknown. Furthermore, since the let-7 family contains multiple members (let-7a-g, let-7i, microRNA98 [miR98], and microRNA202 [miR202]),[23] Lin28a may selectively bind to distinct members of let-7 and exert different effects in a cell type-specific manner. We aimed to explore the relevant let-7 family members involved in Lin28a-mediated excessive VSMC migration and proliferation in RS.[24]
Methods
Ethics approval
Samples of human AS and RS arteries were collected during amputation surgery of individuals with severe RS or AS. All animal experiments and human samples were approved by the Ethical Committee of Shandong Provincial Qianfoshan Hospital (No.S0035) and patients have signed written informed consent.
Double-immunofluorescence staining
Double-immunofluorescence staining[25] was performed to detect the levels and locations of Lin28a and α-smooth muscle actin (α-SMA) in human RS and AS vessels. The human RS and AS tissue sections were incubated with mouse polyclonal anti-Lin28a (1:100; Santa Cruz Biotechnologies, Dallas, Texas, USA) and rabbit anti-α-SMA (1:200; Abcam, Cambridgeshire, Cambridge, UK) overnight at 4°C. After rinsing with phosphate-buffered saline (PBS) for three times, the primary antibodies were detected by appropriate Alexa Fluor 488-conjugated secondary antibodies (1:50; Invitrogen, Carlsbad, California, USA) or Alexa Fluor 594-conjugated secondary antibodies (1:150; Invitrogen), along with 4′,6-diamidino-2-phenylindole (DAPI; Solarbio, Beijing, China) staining to visualize the nuclei. After rinsing, the specimens were examined with a fluorescence microscope (OLYMPUS FSX100; OLYMPUS, Tokyo, Japan).
Rats
Male Sprague–Dawley (SD) rats (weight, ~120 g, 5 rats/group) were purchased from Beijing Huafukang Bioscience Co. Inc., China. All rats were bred and housed in specific pathogen-free conditions. The animals had free access to tap water and pelleted food throughout the study period.
Induction of diabetes
The rats were fed a high-fat diet (34.5% fat, 17.5% protein, and 48% carbohydrate; Keaoxieli FEEDS, Beijing, China) for four weeks, and then given a single intraperitoneal injection of streptozotocin (STZ; Sigma–Aldrich, Louis, Missouri, USA; 27.5 mg/kg in 0.1 mol/L acetate buffer at pH 4.5). Fasting glucose concentration was measured three days after STZ injection. Rats with fasting blood glucose (FBG) ≥11.1 mmol/L in three consecutive measurements were considered as diabetic models.[26,27,28]
Induction of RS and AS
Once a stable diabetic state was achieved, rats were randomly assigned to the RS and AS groups (5 rats/group). Rats in both groups were anesthetized using 1% pentobarbital sodium (40 mg/kg).
In the RS group, RS plaques in the right iliac artery were induced by a "two-step injury protocol"[16] [Supplementary Figure 1, http://links.lww.com/CM9/B619]. First, a 1.5-mm, wire-guided balloon catheter (Medtronic, Minnesota, USA) was inserted into the iliac artery through the femoral artery, which was followed by inflation and hauling from the iliac artery to the femoral artery to induce endothelial damage. Second, approximately four weeks after the first injury, the atherosclerotic plaques in the iliac artery were confirmed by ultrasound examination (FUJIFILM, Tokyo, Japan). PTA was then performed to induce RS. A 1.5-mm, wire-guided balloon catheter was inflated to 1215.9 kPa three times for 5 s each time at the stenosis site. After dilatation, the catheter was removed, and the artery was ligated.
In the AS group, rats received a sham surgery on the same day as the first surgical injury in the RS group. Four weeks later, AS plaques in the right femoral artery were induced by the same protocol as that used for the first-step injury in the RS group.
Immunohistochemical analysis
Rats were euthanized with an intraperitoneal injection of an overdose of pentobarbital (200 mg/kg). Immunohistochemical analysis was performed on 4 µm thick sections. Antigen retrieval was performed using citrate buffer. Sections were incubated with rabbit anti-α-SMA antibodies (1:400; Abcam) overnight at 4°C. After rinsing with PBS, the sections were incubated with Horseradish peroxidase (HRP)-conjugated Goat Anti-Rabbit immunoglobulin G (IgG) (#PV9001; ZSGB-BIO, Beijing, China) for 1 h at 37°C. Immunohistochemical staining was performed using the 3,3′-diaminobenzidine (DAB) detection system (ZLI-9018; ZSGB-BIO), and the sections were counterstained with hematoxylin.
Primary VSMCs culture
Primary VSMCs were obtained as described.[26] In brief, the adventitia and endarterium of the rat artery were removed by mechanical denudation. The remaining middle layers of the vessels were cut into small pieces (~1 mm3) and transferred into Dulbecco's modified Eagle medium (DMEM) containing 20% fetal bovine serum (FBS; Invitrogen), streptomycin (100 µg/mL), and penicillin (100 U/mL). The VSMCs migrated out of the tissue and were allowed to proliferate. These cells were maintained in DMEM (high glucose) + 10% FBS for subsequent experiments. All cultures were incubated at 37°C under a humidified atmosphere with 5% CO2.
Transfection of lentivirus vectors of Lin28a
Overexpressed plasmids and short-hairpin RNAs (shRNAs) of Lin28a were synthesized and cloned into GV493 (GeneChem, Shanghai, China). Lentiviral shRNAs targeting Lin28a (Lenti-Lin28a-shRNA) and lentivirus-carrying Lin28a complementary DNA (Lenti-Lin28a) were eventually obtained. Enhanced green fluorescent protein shRNA (Lenti-NC-shRNA) was used as the control. Lentiviral vectors and packing vectors were transfected into VSMCs using HitransG P Transfection Reagent (GeneChem). The medium was changed 12 h after transfection, and lentivirus-containing cells were collected 48 h later.
Transfection of mimic and inhibitor of let-7
Mimic and inhibitor oligos for let-7c, let-7d, let-7g, and miR98 were purchased from Genepharma Company (Shanghai, China); the sequences are shown in Supplementary Table 1 [http://links.lww.com/CM9/B619]. A negative control oligo (NC) was used as the control for let-7c, let-7d, let-7g, and miR98 mimic oligos, and microRNA inhibitor negative control oligos (inhibitor NC) was used as the control for let-7c, let-7d, let-7g, and miR98 inhibitor oligos. The let-7 mimics or inhibitors were transfected into the VSMCs using Lipofectamine 2000 (Invitrogen) in accordance with the manufacturer's instructions, and the medium was changed after 6 h. The cells were collected for further experiments 24 h after transfection.
The two-step transfection of Lin28a and let-7 was performed with a 48 h interval using the same cells. After the second transfection, cells were cultured for an additional 24 h, and were collected for further experiments.
Quantitative real-time polymerase chain reaction (qRT-PCR)
qRT-PCR was performed as described previously.[29] Briefly, total RNA was isolated using Takara RNAiso Plus (Cat. #9108, Takara, Dalian, China). First-strand cDNA was synthesized with a Prime Script RT reagent kit (Cat. #RR047A, Takara). qRT-PCR was then performed using SYBR Premix Ex Taq™ II (RR820A, Takara). β-actin and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were tested as internal controls while β-actin was selected for result normalization. The primers for Lin28a, GAPDH, and β-actin amplification were designed by and purchased from Takara. The 2-(ΔCt-sample - ΔCt-control) method was used to calculate the relative expression levels for each mRNA.
The let-7 expression ratios were calculated using the same methods, with microRNAs normalized to small nuclear RNA (U6). The primers for let-7 and U6 reverse transcription and amplification were designed by and purchased from GeneChem Company. The dashed line represents the normalized results for the control group, compared with the experimental group. All primer sequences are shown in Supplementary Table 2 [http://links.lww.com/CM9/B619].
Western blotting analyses
Total protein extraction from the cells was performed using lysis buffer (Beyotime Biotechnology, Shanghai, China) supplemented with protease inhibitors (Beyotime Biotechnology).[30] All samples were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE; 12%) and transferred onto polyvinylidene difluoride (PVDF) membranes, which were immunoprobed with rabbit anti-Lin28a (1:1000, ab124765; Abcam) and mouse anti-β-actin (1:1000, ZRB1312; Sigma–Aldrich). The bands were detected using an enhanced chemiluminescence reagent (Millipore, Billerica, Massachusetts, USA) and recorded with a FluorChem E enhanced chemiluminescence system (ProteinSimple, Silicon Valley, California, USA).
5-ethynyl-2΄-deoxyuridine (EdU) incorporation assay
Cell proliferation was tested by the EdU assay kit (C10310-1, RiboBio, Wuhan, China).[31,32] Cells were cultured in 96-well plates after transfection for 48 h, and then incubated with 50 µmol/L EdU for an additional 2 h at 37°C. After fixation with 4% formaldehyde in PBS and permeabilization with 0.5% Triton™ X-100, cells were incubated with 100 µL of 1× Apollo reaction cocktail for 30 min and washed with 0.5% TritonX-100. DNA was stained with Hoechst 33342 (RiboBio) for 30 min. The staining results were recorded by fluorescence microscopy (Olympus, Tokyo, Japan). The number of EdU-positive cells in five random fields was counted under a fluorescence microscope.
Transwell migration assay
Cell migration was assessed using 24-well Transwell plates (Corning–Costar, Corning, New York, USA).[33] After transfection, VSMCs in each group were resuspended in a serum-free culture medium, then transferred into the Transwell plate at a density of 2 × 104 cells/100 µL in the upper chamber, while 10% serum culture medium was placed in the bottom of the plates. After incubation with 5% CO2 at 37°C for 24 h, cells migrating through the membrane filter were fixed and stained with 0.5% crystal violet (C0121; Beyotime Biotechnology) and then counted using a microscope (Olympus) in five random fields.
In vivo transfection of let-7c/g using adeno-associated virus (AAV)
Adeno-associated virus 9 (AAV9) carrying let-7c (AAV9-let-7c) and let-7g (AAV9-let-7g) were purchased from GeneChem. An AAV9-empty vector (AAV9-EV) was used as a control.
Four weeks after the first-step injury, the rats were randomly divided into four groups (5 rats/group) for tail vein injection treatment: an AAV9-let-7c RS group (virus at a dose of 5 × 1011 viral genomes/rat), an AAV9-let-7g RS group (virus at a dose of 5 × 1011 viral genomes/rat), an AAV9-EV RS group (null virus at a dose of 5 × 1011 viral genomes/rat), and an RS group (equal volume of saline alone). After injection, all four groups of rats underwent PTA. Four weeks later, iliac arteries were collected for analyses.
The cDNA sequences used for in vivo gene targeting were as follows.
let-7c-5p: 5′-UGAGGUAGUAGGUUGUAUGGUU-3′;
let-7g-5p: 5′-UGAGGUAGUAGUUUGUACAGUU-3′.
Statistical analysis
All statistical analyses were performed using Prism 8.0 (GraphPad, La Jolla, CA, USA). Data were shown as the mean ± standard error of mean (SEM) when they passed the normality test and analyzed by the two-tailed Student's t-test or one-way analysis of variance (ANOVA) followed by Tukey's post hoc test. When the data did not pass the normality test, they were shown as median values and analyzed by Mann–Whitney test or Kruskal–Wallis test followed by Dunn's post hoc test. The level of statistical significance was determined to be P <0.05.
Results
RS plaques showed physiopathological characteristics distinct from those in AS
To understand the specific expression profiles of VSMCs in RS plaques, we performed α-SMA and Lin28a double-immunofluorescent staining of human RS and AS vessel samples obtained from amputation surgeries. We found that while the RS plaques exhibited a remarkable number of α-SMA-positive cells, the AS plaques showed much fewer α-SMA-positive cells. Moreover, α-SMA and Lin28a showed high colocalization in RS plaques (RS vs. AS, 32.42 ± 3.06% vs. 6.19 ± 1.34%, P = 0.0003; Figures 1A,B). Next, human RS and AS plaque samples were collected by TurboHawk Peripheral Plaque Excision System (Medtronic,Inc., Minneapolis, Minnesota 55432, USA) for gene expression studies, and the expression of let-7 family members was assessed. As shown in Figure 1C, the levels of let-7a, let-7b, let-7d, let-7e, let-7i, and miR202 were upregulated by 22.33-fold, 14.94-fold, 4.70-fold, 1.54-fold, 1.64-fold, and 8.81-fold, respectively (P <0.05), in RS plaques in comparison with those in AS plaques, while the levels of let-7c, let-7f, let-7g, and miR98 were downregulated to 0.71-fold, 0.13-fold, 0.52-fold, and 0.25-fold, respectively (P <0.05).
Figure 1.
Expression of Lin28a and the let-7 family in VSMCs in RS. (A) Expression and colocalization of Lin28a (red) and α-SMA (green) in human post-PTA RS and AS samples. α-SMA is the specific marker of VSMCs. The immunofluorescence image was obtained at magnification ×40 and the scale bar represents 500 µm; a magnification ×400 image is shown in the inset on the bottom right corner of the merged image; the scale bar represents 50 µm. (B) Quantification of Lin28a+ α-SMA+ cells in the neointima area. (C) The expression levels of let-7 miRNAs in human AS and RS (n = 5). P <0.05 vs. AS. (D) The immunohistochemical staining showed the expression of α-SMA in AS and RS plaques in rat models. The image was obtained at magnification ×100 and the scale bar represents 200 µm. (E) Quantification of positive staining for α-SMA in the AS and RS groups. (F) Expression of let-7 miRNAs in rats with AS and PTA-related RS. P <0.05 vs. AS. (G) Western blotting was performed to examine Lin28a expression after transfection with up- or down-regulating Lenti-virus in cultured primary VSMCs. (H) Statistical analysis of Western blotting results displayed by the bar graph. P <0.05 vs. Lenti-NC-shRNA group. qRT-PCR was performed to examine let-7 miRNAs after transfection with up-regulation (I) or down-regulating (J) Lin28a Lenti-virus in VSMCs. The results showed that let-7c, let-7g, and miR98 were negatively regulated by Lin28a. P <0.05 vs. Lenti-NC-shRNA group. α-SMA: α-smooth muscle actin; AS: Atherosclerosis; DAPI: 4′,6-diamidino-2-phenylindole; Lenti-NC-shRNA: Normal control short-hairpin RNA lentivirus; miRNA: microRNA; miR98: microRNA98; PTA: Percutaneous transluminal angioplasty; qRT-PCR: Quantitative real-time polymerase chain reaction; RS: Restenosis; shRNA: Short-hairpin RNA; VSMCs: Vascular smooth muscle cells.
We then constructed RS animal models using a "two-step injury protocol" [Supplementary Figure 1, http://links.lww.com/CM9/B619] in rats with STZ-induced hyperglycemia. Immunohistochemical staining revealed that the VSMCs in the RS samples were greater than those in AS plaques in rats (RS vs. AS, 56.32 ± 1.90% vs. 16.84 ± 2.38%, P = 0.0003) [Figures 1D,E]. The levels of let-7a, let-7b, let-7d, let-7e, and let-7f in RS samples increased by 3.44-fold, 2.80-fold, 2.45-fold, 2.85-fold, and 24.09-fold, respectively (P <0.05), in comparison with those in AS. Meanwhile, the levels of let-7c, let-7g, let-7i, miR98, and miR202 reduced to 0.51-fold, 0.43-fold, 0.37-fold, 0.13-fold, and 0.46-fold, respectively (P <0.05; Figure 1F).
Let-7 family members let-7c, let-7g, and miR98 were the downstream targets of Lin28a in VSMCs in RS
To study the relationship between Lin28a and the let-7 family at the onset of RS, we modulated Lin28a levels by lentivirus transfection in VSMCs. We found that Lin28a level increased by 6.88-fold in the Lenti-Lin28a group and decreased to 0.25-fold in the Lenti-Lin28a-shRNA group (vs. Lenti-NC-shRNA group, P <0.05; Figures 1G,H). In the Lenti-Lin28a group, the expressions of let-7a-c, let-7e-g, let-7i, miR98, and miR202 were statistically significantly lower than those in the Lenti-NC-shRNA group (P <0.05). However, let-7d expression was 1.56-fold higher than that in the Lenti-NC-shRNA group (P <0.05; Figure 1I). In contrast, the expressions of let-7c, let-7d, let-7g, and miR98 in the Lenti-Lin28a-shRNA group were significantly higher than those in the Lenti-NC-shRNA group (P <0.05), while the expressions of let-7a, let-7b, let-7e, let-7f, let-7i and miR202 were lower than those in the Lenti-NC-shRNA group (P <0.05; Figure 1J).
VSMC migration and proliferation were induced by let-7c, let-7g, and miR98 functioning as specific downstream effectors of Lin28a
We next focused on the roles of let-7c, let-7g, and miR98 in the subsequent studies. Rat primary VSMCs were treated with let-7c, let-7g, and miR98 mimic and inhibitor oligos [Figure 2A]. EdU analysis showed that let-7c, let-7g, and miR98 mimic oligos statistically significantly inhibited VSMC proliferation in comparison with that in the NC group (26.86 ± 2.08%, 24.64 ± 1.88%, and 27.52 ± 2.54% vs. 41.89 ± 1.49%, P <0.05). In contrast, the let-7c, let-7g, and miR98 inhibitor oligos statistically significantly promoted the proliferation of VSMCs (58.34 ± 2.50%, 60.47 ± 2.43%, and 49.75 ± 2.15% vs. 40.09 ± 2.69%; P <0.05) [Figures 2B,C]. Accordingly, the migration ability of VSMCs was also inhibited by let-7c, let-7g, and miR98 mimic oligos (33.67 ± 3.01, 31.80 ± 2.44, and 30.00 ± 1.93 vs. 44.20 ± 2.50; P <0.05), and stimulated by the inhibitor oligos (76.00 ± 3.39, 70.60 ± 2.89, and 68.20 ± 3.12 vs. 49.40 ± 3.60; P <0.05) [Figures 2D,E].
Figure 2.
Lin28a interacted with let-7c, let-7g, and miR98, and further regulates the proliferation and migration of VSMCs. (A–E) Let-7c, let-7g, miR98, NC and inhibitor NC oligos were transfected into VSMCs. (A) qRT-PCR validation of successful let-7c, let-7g or miR98 transfections. P <0.05 (let-7c, let-7g, miR98 mimic) vs. NC; P <0.05 (let-7c, let-7g, miR98 inhibitor) vs. inhibitor NC. (B) The EdU assay illustrated the proliferation of VSMCs. VSMCs were labeled with nucleoside analog EdU (red) and nuclei were labeled with Hoechst 33342 dye (blue). The colocalization of EdU (red) and Hoechst 33342 (blue) was shown as pink color, indicative of the proliferative VSMCs. (C) The quantification of the EdU-positive cells. (D) The Transwell assay illustrates the migration of VSMCs. The migrated VSMCs are presented in purple. (E) The quantification of the migration of VSMCs. (F–I) Let-7c, let-7g, miR98, NC and inhibitor NC oligo were transfected into the Lin28a Lenti-virus transfected VSMCs. (F,G) The EdU assay illustrated the proliferation of VSMCs. (H,I) The Transwell assay illustrated the migration of VSMCs. The images of EdU and Transwell assays were taken at magnification ×200 and the scale bar was 75 µm (n = 5). EdU: 5-ethynyl-2΄-deoxyuridine; miR98: microRNA98; NC: Normal control; qRT-PCR: Quantitative real-time polymerase chain reaction; shRNA: Short-hairpin RNA; VSMCs: Vascular smooth muscle cells.
Furthermore, we proved that the proliferation and migration of VSMCs were significantly influenced by the transfected Lenti-Lin28a. To test whether Lin28a stimulates VSMC proliferation and migration by suppressing let-7c, let-7g, and miR98 expression, we performed a two-step transfection, with the first transfection of Lin28a followed by a second transfection of let-7c, let-7g, or miR98. The EdU and Transwell assays [Figures 2F–I] showed that the co-transfection of let-7c, let-7g, and miR98 mimics inhibited the stimulatory effect of Lin28a on VSMC proliferation (31.40 ± 2.06%, 31.08 ± 2.05%, and 34.12 ± 1.97% vs. 57.32 ± 1.50%; P <0.05) and migration (58.80 ± 2.88, 47.80 ± 2.43, and 55.20 ± 2.76 vs. 125.60 ± 2.76, respectively; P <0.05). As expected, co-transfection of let-7c, let-7g, and miR98 inhibitor oligos suppressed the inhibitory effects of Lenti-Lin28a-shRNA on VSMC proliferation (60.06 ± 2.58%, 46.47 ± 1.40%, and 48.25 ± 2.23% vs. 25.57 ± 2.51%; P <0.05) and migration (88.40 ± 2.88, 92.20 ± 3.16, and 94.40 ± 3.59 vs. 46.00 ± 2.05; P <0.05).
Lin28a and let-7c/let-7g formed a double-negative feedback loop that triggers vicious VSMC migration and proliferation in RS
Since let-7 family members control the Lin28a level through a feedback loop in a tissue-specific manner, we next investigated the effects of the feedback loop of let-7c, let-7g, and miR98 on the expression of Lin28a in rat primary VSMCs. Figure 3A shows that Lin28a was significantly inhibited at the mRNA level by the overexpression of let-7c, let-7d, let-7g, or miR98 (vs. NC, P <0.05), while let-7c, let-7d, let-7g, and miR98 inhibitor oligos increased Lin28a mRNA levels (vs. inhibitor NC, P <0.05). Moreover, Western blotting analyses showed that Lin28a protein expression levels were suppressed by let-7c, let-7d and let-7g mimic oligos and increased by let-7c, let-7d and let-7g inhibitor oligos [Figures 3B,C]. However, miR98 did not statistically significantly affect Lin28a protein levels [Figures 3B,C].
Figure 3.
Lin28a and let-7c and let-7g interacted in a double-negative feedback loop. (A–C) Let-7c, let-7d, let-7g, miR98, and NC mimic and inhibitor oligos were transfected into VSMCs. (A) qRT-PCR analysis to validate Lin28a expression in mRNA level. P <0.05 (let-7c, let-7d, let-7g, miR98 mimic) vs. NC; P <0.05 (let-7c, let-7d, let-7g, miR98 inhibitor) vs. inhibitor NC. (B) Western blotting validation of Lin28a expression in protein level. (C) Quantification of the Lin28a protein levels by Western blotting. P <0.05 (let-7c, let-7d and let-7g mimic) vs. NC; P <0.05 (let-7c, let-7d, and let-7g inhibitor) vs. inhibitor NC. (D–G) Let-7c, let-7g individually or a mixture of let-7c and let-7g mimic or inhibitor oligos were transfected into the Lenti-Lin28a or Lenti-Lin28a-shRNA VSMCs. (D) The EdU assay illustrated the proliferation of VSMCs. (E) Quantification of the EdU-positive cells. (F) The Transwell assay illustrated the migration of VSMCs. (G) Quantification of the migration of VSMCs. Images the of EdU and Transwell assays were obtained at magnification ×200 and the scale bar was 75 µm (n = 5). EdU: 5-ethynyl-2΄-deoxyuridine; miR98: microRNA98; mRNA: Messenger RNA; NC: Normal control; qRT-PCR: Quantitative real-time polymerase chain reaction; shRNA: Short-hairpin RNA; VSMCs: Vascular smooth muscle cells.
To determine whether let-7c and let-7g had additive effects on Lin28a expression, we performed transfection of a mixture of let-7c and let-7g mimic oligos in Lin28a-overexpressing VSMCs (Lenti-Lin28a transfected) or Lin28a-knockdown VSMCs (Lenti-Lin28a-shRNA transfected). EdU and Transwell assays [Figures 3D–G] showed that the effects of the mixture of oligos on VSMC proliferation and migration were not significantly different from the effects of transfection of let-7c or let-7g mimic oligos alone.
Vicious migration and proliferation of VSMCs was restricted by let-7d through downregulation of Lin28a and upregulation of let-7g
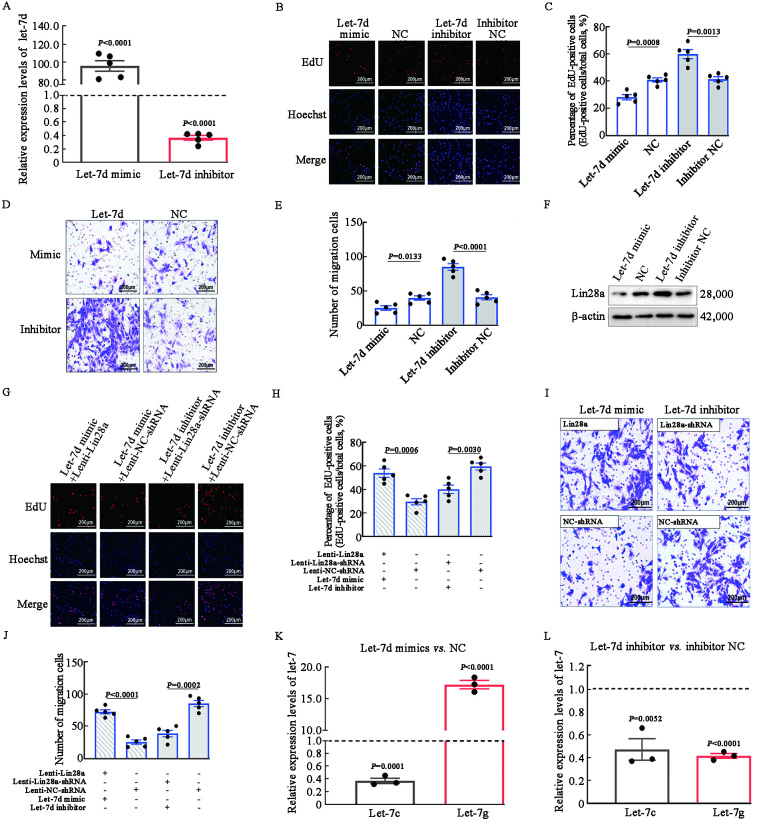
Unlike other let-7 family members, let-7d was overexpressed in both RS plaques and cultured rat primary VSMCs. After regulating the expression of let-7d, we performed EdU and Transwell assays to investigate the influence of let-7d on VSMC proliferation and migration. We found that overexpression of let-7d by mimic oligos (99.74-fold vs. NC, P <0.05) [Figures 4A] inhibited the proliferation of VSMCs (28.20 ± 1.97% vs. 41.09 ± 1.75%, P <0.05) [Figures 4B,C]. On the other hand, let-7d inhibitor oligos significantly stimulated VSMC proliferation (60.17 ± 2.61% vs. 41.69 ± 1.92%, P <0.05) [Figures 4B,C]. In addition, VSMC migration was inhibited by let-7d mimic oligos (25.00 ± 2.78 vs. 39.60 ± 2.53, P <0.05) and stimulated by let-7d inhibitor oligos (85.00 ± 3.01 vs. 40.60 ± 2.77, P <0.05) [Figures 4D,E].
Figure 4.
Let-7d restricted the proliferation and migration of VSMCs by downregulating Lin28a and upregulating let-7g. (A) Let-7d mimic and inhibitor oligo were transfected into VSMCs and their expression levels were validated by qRT-PCR. P <0.05 (let-7d mimic) vs. NC; P <0.05 (let-7d inhibitor) vs. inhibitor NC. (B) The EdU assay illustrated the proliferation of VSMCs. (C) Quantification of EdU-positive cells. (D) The Transwell assay illustrated the migration of VSMCs. (E) Quantification of the migration of VSMCs. (F) Western blotting validation of Lin28a expression in protein level. (G–J) Let-7d, NC, and NC inhibitor oligos were transfected into the Lin28a lentivirus-transfected VSMCs. (G,H) The EdU assay illustrated the proliferation of VSMCs. (I,J) The Transwell assay illustrated the migration of VSMCs. (K,L) qRT-PCR was performed to examine let-7c, let-7g levels with up- or down-regulated let-7d in VSMCs. Results showed that let-7g expression was promoted by let-7d. P <0.05 (let-7d mimic) vs. NC; P <0.05 (let-7d inhibitor) vs. inhibitor NC. The images of EdU and Transwell assays were taken at magnification ×200 and the scale bar was 75 µm (n = 5). EdU: 5-ethynyl-2΄-deoxyuridine; mRNA: Messenger RNA; NC: Normal control; qRT-PCR: Quantitative real-time polymerase chain reaction; shRNA: Short-hairpin RNA; VSMCs: Vascular smooth muscle cells.
We found that overexpression of let-7d by mimic oligos substantially decreased Lin28a protein levels [Figure 4F]. The interaction between let-7d and Lin28a in rat primary VSMCs was further investigated. We found that even after regulation with let-7d mimic oligos, Lin28a-overexpressing VSMCs showed increased EdU-positive cell rates (54.00 ± 2.67% vs. 29.60 ± 2.31%; P <0.05) and migration rates (72.20 ± 2.56 vs. 25.00 ± 2.58; P <0.05) than those of VSMCs transfected with Lenti-NC-shRNA. In contrast, the knockdown of Lin28a significantly reduced the proliferation rate (40.20 ± 2.66% vs. 59.67 ± 2.45%, P <0.05) and migration cell counts (38.20 ± 3.20 vs. 85.00 ± 3.11, P <0.05) with regulation using let-7d inhibitor oligos [Figures 4G–J]. These data suggest that Lin28a is the downstream effector of let-7d.
We next investigated whether let-7d modulated the levels of let-7c and let-7g. qRT-PCR analysis [Figures 4K,L] showed that let-7d inhibitor oligos significantly decreased the expression of let-7g (0.42-fold vs. inhibitor NC, P <0.05), and let-7d mimic oligos significantly increased the expression of let-7g (17.24-fold vs. NC, P <0.05). Intriguingly, both let-7d mimic and inhibitor oligos decreased the expression of let-7c (P <0.05).
Overexpression of let-7c and let-7g alleviated RS in vivo
To address the role of let-7c and let-7g in RS in vivo, we induced AAV9-mediated let-7c and let-7g overexpression in rat blood vessels. As shown in Figures 5A,B, let-7c and let-7g expressions were 9.90-fold and 7.63-fold (vs. AAV9-EV), respectively, in the RS plaques of AAV9-let-7c and AAV9-let-7g groups than in the AAV9-EV group (P <0.05). No significantly difference was found in the expression levels of let-7c/let-7g between RS plaques from the non-transfected and AAV9-EV groups. And the expression of Lin28a was significantly decreased in AAV9-let-7c and AAV9-let-7g groups compared with AAV9-EV RS group (P <0.05, Figure 5C).
Figure 5.
Let-7c and let-7g attenuated RS development by downregulating Lin28a in vivo. (A,B) qRT-PCR was performed to examine let-7c and let-7g expression after transfection with upregulation of AAV9. P <0.05 (AAV9-let-7c/g RS) vs. AAV9-EV RS. (C) qRT-PCR was performed to examine Lin28a expression after transfection with upregulating let-7c or let-7g AAV9 in RS rats. The results showed that Lin28a was negatively regulated by let-7c and let-7g. Data are presented as fold changes relative to the AAV9-EV RS group. P <0.05 (AAV9-let-7c/g RS) vs. AAV9-EV RS. (D) Representative HE staining of iliac arteries. The image of hematoxylin-eosin (HE) staining was taken at magnification ×100 and the scale bar represents 200 µm. (E) Quantitative analysis of lesion areas in arteries. (F) Quantitative analysis of neointima/media ratio. (G) The immumohistochemical staining was performed to examine the expression of α-SMA in different RS plaques. The image was taken at magnification ×100 and the scale bar represents 200 µm, and taken at magnification ×40 and the scale bar represents 50 µm. α-SMA: α-smooth muscle actin; AAV: Adeno-associated virus; AS: Atherosclerosis; EV: Empty vector; qRT-PCR: Quantitative real-time polymerase chain reaction; RS: Restenosis.
The stenosis rates were estimated by the ratio of the intimal area to the area bounded by the elastic lamina (luminal cross-sectional area narrowing). The stenosis rates were significantly lower in the RS plaques of the AAV9-let-7c (20.74 ± 2.21%, P <0.05) and AAV9-let-7g (18.79 ± 2.73%, P <0.05) groups than in the AAV9-EV group (82.07 ± 2.36%) [Figures 5D,E]. The neointima/media ratio was also lower in the RS plaques of the AAV9-let-7c (1.87 ± 0.48%, P <0.05) and AAV9-let-7g (2.08 ± 0.62%, P <0.05) groups than in the AAV9-EV group (4.03 ± 0.78%) [Figure 5F]. No significant difference was found in RS plaques from the non-transfected and AAV9-EV groups. The proportions of VSMCs in the plaques of the AAV9-let-7c and AAV9-let-7g groups were significantly lower than those in the AAV9-EV RS group [Figures 5G].
Discussion
RS, an occlusive vascular response, limits the effectiveness of PTA.[12] For many years, due to the lack of understanding of the pathogenic mechanisms underlying RS, efforts to prevent RS failed to achieve the desired effect. PTA-stimulated VSMC proliferation and migration has been shown to be the main cause of RS,[34] and VSMCs exhibit distinct molecular signaling in RS in comparison with that in AS.[14] More specifically, elevated Lin28a expression is critical for the formation of RS.[35]
Our results elucidated that the double-negative feedback loop consisting of Lin28a and let-7c/g contributes to the formation of RS. In the presence of Lin28a, let-7 precursors are uridylated and targeted for degradation, preventing their processing into mature let-7 miRNAs and decreasing let-7 target gene expression.[36] In this study, we found enhanced expression of Lin28a in the RS plaque samples in humans, which is associated with decreased expressions of let-7c, let-7f, let-7g, and miR98. The RS rat model also showed similar results. Moreover, while Lin28a overexpression resulted in the downregulation of let-7c, let-7g, miR98, and miR202, inhibition of Lin28a only restored the expression of let-7c, let-7g, and miR98. Thus, Lin28a exerts an inhibitory effect on let-7c, let-7g, and miR98 levels. Therefore, from all of the candidate let-7 miRNAs, we selected let-7c, let-7g, and miR98 to further investigate the specific roles of these molecules in RS.
First, to confirm whether let-7c, let-7g, and miR98 participate in Lin28a-controlled VSMC proliferation and migration, we altered the levels of these miRNAs in vitro. Our findings suggest that upregulation of let-7c, let-7g, and miR98 inhibits the proliferation and migration of VSMCs and vice versa. Next, we upregulated Lin28a while transfecting mimics of the above-mentioned miRNAs. As expected, mimics of let-7c, let-7g, and miR98 inhibited VSMC proliferation and migration even when Lin28a was upregulated. Transfection of inhibitors of these miRNAs restored the ability of VSMCs to proliferate and migrate while Lin28a was downregulated. Thus, let-7c, let-7g, and miR98 mediate the Lin28a-controlled pathological behavior of VSMCs in RS.[35]
Remarkably, while Lin28a has been reported to be a negative regulator of the let-7 family, the let-7 family has also been shown to be capable of repressing Lin28a expression.[17] Mature let-7 targets the Lin28a mRNA for posttranscriptional repression, further relieving the repression of pre-let-7 precursor processing and establishing a feedback loop that helps promote differentiation.[37,38] Our results suggested that Lin28a accumulation in VSMCs suppresses the levels of let-7c, let-7g and miR98 during RS. Meanwhile, both let-7c and let-7g can repress Lin28a expression. To be more specific, downregulation of let-7c and let-7g enhances Lin28a expression, which leads to further inhibition of let-7c and let-7g. Therefore, a double-negative feedback loop seems to exist between Lin28a and let-7c/let-7g.
PTA is the preferred treatment for LEAD.[35] However, while ballooning the blood vessel, PTA triggers the Lin28a-let7c/let-7g double-negative feedback loop. As a result, sustained high Lin28a and low let-7c/let-7g levels encourage massive VSMC proliferation and rapid development of RS. To date, while a few studies have focused on the poor prognosis of PTA-treated LEAD and the pathogenesis of RS, the mechanism underlying the vicious VSMC proliferation in RS remains unclear. The Lin28a-let7c/let-7g double-negative feedback loop may partly explain the molecular mechanism underlying the "malignant" behavior of VSMCs in RS.
On the other hand, our results showed that let-7c, let-7g, and miR98 inhibitors enhance proliferation and migration even in Lin28a-knockdown VSMCs, indicating the existence of other downstream effectors. Future studies exploring these effectors may help deepen our understanding of the mechanisms underlying RS. In addition, co-transfection of let-7c and let-7g caused no additive repression of Lin28a. Therefore, these two miRNAs seem to act independently in the regulation of Lin28a.
As mentioned in our results, we used both human tissue and rat models to observe the expression profile of let-7 family members. In humans, let-7f decreased while let-7i increased whereas rat models displayed an opposite pattern. We were also concerned whether it will affect the Lin28a/let-7 axis. Hence, we then modulated Lin28a in vitro to investigate whether let-7f or let-7i expression was regulated by Lin28a. Intriguingly, in rat primary VSMCs, let-7f and let-7i decreased independently Lin28a level. It seems that let-7f or let-7i was not affected by Lin28a expression. Therefore, the exact function of let-7f and let-7i was excluded from subsequent experiments. Given the conserved sequence, expression, and roles of let-7 in fundamental biological aspects across phylogeny, it is important for current medicine to better understand the let-7 function and its regulatory pathways. Further studies are required to fully elucidate the complex mechanism that controls the let-7f and let-7i expression and maturation.
Another notable finding of our study was the identification of a special let-7 family member let-7d. Intriguingly, while Lin28a could not regulate let-7d, the let-7d level was increased in both RS plaques and cultured primary VSMCs from RS rat models. We hypothesize that let-7d may be activated and acts as an ameliorator in RS. To obtain evidence for the first hypothesis, we tested the effects of let-7d on cultured VSMCs and found that overexpression of let-7d inhibited both the proliferation and migration of VSMCs. Let-7d also repressed the expression of Lin28a, which further inhibited Lin28a-promoted VSMC proliferation and migration. Moreover, the upregulation of let-7d increased the level of let-7g, suggesting that let-7d may be a key regulator to break the double-negative feedback loop in PTA-associated RS. In summary, while ballooning the blood vessel, PTA triggers the Lin28a-let7c/let-7g double-negative feedback loop. Sustained high Lin28a and low let-7c/let-7g levels encourage massive VSMCs proliferation, and rapid development of RS. We changed the let-7d level on cultured VSMCs and found that overexpression of let-7d increased the let-7g expression, suggesting that let-7d acts up-stream to let-7g. Let-7g decreased along with elevated Lin28a in the presence of increased let-7d, suggesting that Lin28a is a more dominant regulator in this axis. This is consistent with our observation in vivo where despite the increase in let-7d, RS plaque still developed due to the upregulation of Lin28a. Nevertheless, increased let-7d in vivo suggests a self-protective mechanism active, but not sufficient, during the pathogenesis of RS. In this situation, the effect of let-7d could not completely offset the decrease of let-7g caused by Lin28a.
To date, few studies have focused on LEAD in the field of cardiovascular diseases, and even fewer studies have investigated the mechanisms that contribute to the development of RS. Additionally, due to the wide use of atherosclerotic models to study RS, the pathogenic mechanisms unique to RS remain unclear. In light of the findings from analyses of human blood vessels, our study used a "two-step injury protocol" to induce RS plaques in rats, which is more similar to the pathogenesis of post-PTA RS in humans. We propose that the Lin28a/let-7 axis is an important signaling center in the formation and deterioration of RS; this axis triggers a double-negative feedback loop that maintains the vicious VSMC proliferation and migration. These results will pave the way for a deeper understanding of molecular signaling in VSMCs during RS and provide a foundation for the development of better therapies. The challenge we face is to apply this regulatory mechanism to clinical treatment. To achieve targeted arterial administration of lower extremity arteries and use the regulatory mechanism into small molecule drugs that are easy to absorb and have pharmacological activity is the major aim that needs in further research.
Data sharing
The raw data supporting the conclusion of this article will be made available by the authors, upon reasonable request.
Funding
This work was supported by the National Natural Science Foundation of China Grants (Nos. 82100891, 81670757, 82270888, 82170824, 81770822, 81800732, and 81900685), Natural Science Foundation of Shandong Province (No. ZR2017LH025), Shandong Provincial Medicine and Health Science and Technology Development Program (No. 2017WS461), and China Postdoctoral Science Foundation (No. 2021M691957).
Conflicts of interest
None.
Supplementary Material
Footnotes
How to cite this article: Zhang Q, Zhou XJ, Li XZ, Yao S, Jiang S, Zhang R, Zou ZW, Liao L, Dong JJ. Effect of down-regulation of let-7c/g on triggering a double-negative feedback loop and promoting restenosis. Chin Med J 2023;136:2484–2495. doi: 10.1097/CM9.0000000000002763
References
- 1.National Clinical Guideline Centre (UK) . Lower limb peripheral arterial disease: Diagnosis and management. London: National Institute for Health and Clinical Excellence: Guidance, 2012. [Google Scholar]
- 2.Low Wang CC Blomster JI Heizer G Berger JS Baumgartner I Fowkes FGR, et al. Cardiovascular and limb outcomes in patients with diabetes and peripheral artery disease: The EUCLID trial. J Am Coll Cardiol 2018;72: 3274–3284. doi: 10.1016/j.jacc.2018.09.078. [DOI] [PubMed] [Google Scholar]
- 3.Matsuzaki K, Hayashi R, Okabe K, Aramaki-Hattori N, Kishi K. Prognosis of critical limb ischemia: Major vs. minor amputation comparison. Wound Repair Regen 2015;23: 759–764. doi: 10.1111/wrr.12329. [DOI] [PubMed] [Google Scholar]
- 4.Cardaioli P Rigatelli G Dell'avvocata F Giordan M Lisato G Mollo F, et al. Endovascular treatment of diabetic foot syndrome: Results from a single center prospective registry using mixed coronary and peripheral techniques and equipment. J Interv Cardiol 2011; 24: 562–568. doi: 10.1111/j.1540-8183.2011.00676.x. [DOI] [PubMed] [Google Scholar]
- 5.Tepe G Zeller T Albrecht T Heller S Schwarzwälder U Beregi JP, et al. Local delivery of paclitaxel to inhibit restenosis during angioplasty of the leg. N Engl J Med 2008;358: 689–699. doi: 10.1056/NEJMoa0706356. [DOI] [PubMed] [Google Scholar]
- 6.Lee MS Rha SW Han SK Choi BG Choi SY Ali J, et al. Comparison of diabetic and non-diabetic patients undergoing endovascular revascularization for peripheral arterial disease. J Invasive Cardiol 2015;27: 167–171. [PubMed] [Google Scholar]
- 7.Metz RP, Patterson JL, Wilson E. Vascular smooth muscle cells: Isolation, culture, and characterization. Methods Mol Biol 2012; 843: 169–176. doi: 10.1007/978-1-61779-523-7_16. [DOI] [PubMed] [Google Scholar]
- 8.Chen LJ, Lim SH, Yeh YT, Lien SC, Chiu JJ. Roles of microRNAs in atherosclerosis and restenosis. J Biomed Sci 2012;19: 79. doi: 10.1186/1423-0127-19-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lange RA, Flores ED, Hillis LD. Restenosis after coronary balloon angioplasty. Annu Rev Med 1991;42: 127–132. doi: 10.1146/annurev.me.42.020191.001015. [DOI] [PubMed] [Google Scholar]
- 10.Kardesoglu E, Yalcin M, Isilak Z, Uz O, Atalay M. Inhibition of vascular smooth muscle cell proliferation: An indispensable target in treatment. Chin Med J 2012;125: 3600. doi: 10.3760/cma.j.issn.0366-6999.2012.19.049. [PubMed] [Google Scholar]
- 11.Iaconetti C De Rosa S Polimeni A Sorrentino S Gareri C Carino A, et al. Down-regulation of miR-23b induces phenotypic switching of vascular smooth muscle cells in vitro and in vivo. Cardiovasc Res 2015;107: 522–533. doi: 10.1093/cvr/cvv141. [DOI] [PubMed] [Google Scholar]
- 12.Marx SO, Totary-Jain H, Marks AR. Vascular smooth muscle cell proliferation in restenosis. Circ Cardiovasc Interv 2011;4: 104–111. doi: 10.1161/CIRCINTERVENTIONS.110.957332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Osman I, Segar L. Pioglitazone, a PPARγ agonist, attenuates PDGF-induced vascular smooth muscle cell proliferation through AMPK-dependent and AMPK-independent inhibition of mTOR/p70S6K and ERK signaling. Biochem Pharmacol 2016;101: 54–70. doi: 10.1016/j.bcp.2015.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou X Dong J Zhang L Liu J Dong X Yang Q, et al. Hyperglycemia has no effect on development of restenosis after percutaneous transluminal angioplasty (PTA) in a diabetic rabbit model. J Endocrinol 2015;224: 119–125. doi: 10.1530/JOE-14-0391. [DOI] [PubMed] [Google Scholar]
- 15.Zhou X Mou Y Shen X Yang T Liu J Liu F, et al. The role of atorvastatin on the restenosis process post-PTA in a diabetic rabbit model. BMC Cardiovasc Disord 2016;16: 153. doi: 10.1186/s12872-016-0324-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zou Z Zhou X Zhang R Zhang Q Jiang S Xu C, et al. Lin28a up-regulation is associated with the formation of restenosis via promoting proliferation and migration of vascular smooth muscle cells. J Cell Mol Med 2020;24: 9682–9691. doi: 10.1111/jcmm.15506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allahverdian S, Chaabane C, Boukais K, Francis GA, Bochaton-Piallat ML. Smooth muscle cell fate and plasticity in atherosclerosis. Cardiovasc Res 2018;114: 540–550. doi: 10.1093/cvr/cvy022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shyh-Chang N, Daley GQ. Lin28: Primal regulator of growth and metabolism in stem cells. Cell Stem Cell 2013;12: 395–406. doi: 10.1016/j.stem.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mayr C, Hemann MT, Bartel DP. Disrupting the pairing between let-7 and Hmga2 enhances oncogenic transformation. Science 2007; 315: 1576–1579. doi: 10.1126/science.1137999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xiao T, Yang X, Zhong N, Luo Z, Liu J. Role of let-7 family in the invasion and metastasis of osteosarcoma. Chin Med J 2023; 136: 120–122. doi: 10.1097/CM9.0000000000002070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bao MH, Feng X, Zhang YW, Lou XY, Cheng Y, Zhou HH. Let-7 in cardiovascular diseases, heart development and cardiovascular differentiation from stem cells. Int J Mol Sci 2013;14: 23086–23102. doi: 10.3390/ijms141123086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu ML Wang JF Wang GK You XH Zhao XX Jing Q, et al. Vascular smooth muscle cell proliferation is influenced by let-7d microRNA and its interaction with KRAS. Circ J 2011;75: 703–709. doi: 10.1253/circj.CJ-10-0393. [DOI] [PubMed] [Google Scholar]
- 23.Kuppusamy KT Jones DC Sperber H Madan A Fischer KA Rodriguez ML, et al. Let-7 family of microRNA is required for maturation and adult-like metabolism in stem cell-derived cardiomyocytes. Proc Natl Acad Sci U S A 2015;112: E2785–E2794. doi: 10.1073/pnas.1424042112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee H, Han S, Kwon CS, Lee D. Biogenesis and regulation of the let-7 miRNAs and their functional implications. Protein Cell 2016; 7: 100–113. doi: 10.1007/s13238-015-0212-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Donaldson JG. Immunofluorescence staining. Curr Protoc Cell Biol 2015;69: 4.3.1–4.3.7. doi: 10.1002/0471143030.cb0403s69. [DOI] [PubMed] [Google Scholar]
- 26.Geng FH Li GH Zhang X Zhang P Dong MQ Zhao ZJ, et al. Berberine improves mesenteric artery insulin sensitivity through up-regulating insulin receptor-mediated signalling in diabetic rats. Br J Pharmacol 2016;173: 1569–1579. doi: 10.1111/bph.13466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reed MJ Meszaros K Entes LJ Claypool MD Pinkett JG Gadbois TM, et al. A new rat model of type 2 diabetes: The fat-fed, streptozotocin-treated rat. Metabolism 2000;49: 1390–1394. doi: 10.1053/meta.2000.17721. [DOI] [PubMed] [Google Scholar]
- 28.Ti Y Xie GL Wang ZH Bi XL Ding WY Wang J, et al. TRB3 gene silencing alleviates diabetic cardiomyopathy in a type 2 diabetic rat model. Diabetes 2011;60: 2963–2974. doi: 10.2337/db11-0549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yin M Zhang J Wang Y Wang S Böckler D Duan Z, et al. Deficient CD4+CD25+ T regulatory cell function in patients with abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol 2010;30: 1825–1831. doi: 10.1161/ATVBAHA.109.200303. [DOI] [PubMed] [Google Scholar]
- 30.Hirano S. Western blot analysis. Methods Mol Biol 2012; 926: 87–97. doi: 10.1007/978-1-62703-002-1_6. [DOI] [PubMed] [Google Scholar]
- 31.Li D Liu X Lin L Hou J Li N Wang C, et al. MicroRNA-99a inhibits hepatocellular carcinoma growth and correlates with prognosis of patients with hepatocellular carcinoma. J Biol Chem 2011;286: 36677–36685. doi: 10.1074/jbc.M111.270561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shu YJ Weng H Ye YY Hu YP Bao RF Cao Y, et al. SPOCK1 as a potential cancer prognostic marker promotes the proliferation and metastasis of gallbladder cancer cells by activating the PI3K/AKT pathway. Mol Cancer 2015;14: 12. doi: 10.1186/s12943-014-0276-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo J, Wu G, Bao J, Hao W, Lu J, Chen X. Cucurbitacin B induced ATM-mediated DNA damage causes G2/M cell cycle arrest in a ROS-dependent manner. PLoS One 2014;9: e88140. doi: 10.1371/journal.pone.0088140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith SA, Newby AC, Bond M. Ending restenosis: Inhibition of vascular smooth muscle cell proliferation by cAMP. Cells 2019; 8: 1447. doi: 10.3390/cells8111447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aboyans V Ricco JB Bartelink MEL Björck M Brodmann M Cohnert T, et al. 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS): Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries Endorsed by: The European Stroke Organization (ESO) the Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur Heart J 2018;39: 763–816. doi: 10.1093/eurheartj/ehx095. [DOI] [PubMed] [Google Scholar]
- 36.Viswanathan SR Powers JT Einhorn W Hoshida Y Ng TL Toffanin S, et al. Lin28 promotes transformation and is associated with advanced human malignancies. Nat Genet 2009; 41: 843–848. doi: 10.1038/ng.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rybak A Fuchs H Smirnova L Brandt C Pohl EE Nitsch R, et al. A feedback loop comprising lin-28 and let-7 controls pre-let-7 maturation during neural stem-cell commitment. Nat Cell Biol 2008; 10: 987–993. doi: 10.1038/ncb1759. [DOI] [PubMed] [Google Scholar]
- 38.Thornton JE, Gregory RI. How does Lin28 let-7 control development and disease? Trends Cell Biol 2012;22: 474–482. doi: 10.1016/j.tcb.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]