Abstract
BACKGROUND:
Adult spinal deformity (ASD) represents a major cause of disability in the elderly population in the United States. Surgical intervention has been shown to reduce disability and pain in properly indicated patients. However, there is a small subset of patients in whom nonoperative treatment is also able to durably maintain or improve symptoms.
OBJECTIVE:
To examine the factors associated with successful nonoperative management in patients with ASD.
METHODS:
We retrospectively evaluated a cohort of 207 patients with nonoperative ASD, stratified into 3 groups: (1) success, (2) no change, and (3) failure. Success was defined as a gain in minimal clinically importance difference in both Oswestry Disability Index and Scoliosis Research Society-Pain. Logistic regression model and conditional inference decision trees established cutoffs for success according to baseline (BL) frailty and sagittal vertical axis.
RESULTS:
In our cohort, 44.9% of patients experienced successful nonoperative treatment, 22.7% exhibited no change, and 32.4% failed. Successful nonoperative patients at BL were significantly younger, had a lower body mass index, decreased Charlson Comorbidity Index, lower frailty scores, lower rates of hypertension, obesity, depression, and neurological dysfunction (all P < .05) and significantly higher rates of grade 0 deformity for all Schwab modifiers (all P < .05). Conditional inference decision tree analysis determined that patients with a BL ASD-frailty index ≤ 1.579 (odds ratio: 8.3 [4.0-17.5], P < .001) were significantly more likely to achieve nonoperative success.
CONCLUSION:
Success of nonoperative treatment was more frequent among younger patients and those with less severe deformity and frailty at BL, with BL frailty the most important determinant factor. The factors presented here may be useful in informing preoperative discussion and clinical decision-making regarding treatment strategies.
KEY WORDS: Adult spinal deformity, Spine surgery, Nonoperative management, Nonoperative, Spine, Thoracolumbar spine
ABBREVIATIONS:
- ASD
adult spinal deformity
- BL
baseline
- GAP
Global Alignment and Proportion
- MCID
minimal clinically importance difference
- LIV
lower instrumented vertebra
- MCS
mental component score
- ODI
Oswestry Disability Index
- PCS
pain catastrophizing scale
- SRS
Scoliosis Research Society
- SVA
sagittal vertical axis
- UIV
upper instrumented vertebra.
Adult spinal deformity (ASD) is a complex and increasingly prevalent spinal condition that may lead to substantial pain and disability.1 Despite providing meaningful clinical improvement, corrective deformity surgery is associated with a high risk of complications ranging from 10% to 40% in the literature.2-4 Furthermore, ASD often presents in patients who are physiologically frail and have other medical comorbidities.5,6 Surgeons must balance the potential for complications and other adverse events in an otherwise already high-risk population against the potential for meaningful recovery from what is often a high-intensity surgical intervention.7-9 When medical or surgical complications, as well as mechanical failure, occur after surgery, the ultimate trajectory and ceiling for clinical recovery could be compromised.10-12
As a result, without the presence of neurological symptoms or deficits at presentation, a nonoperative approach is generally considered the initial treatment strategy including spinal injections, physical therapy, exercise, bracing, and medication management.13-15 Among patients who undergo nonoperative treatment and fail to respond, a delay in spine surgery can cause protracted disability, discomfort, pain, and economic burden with increased resource utilization.15-17 Therefore, it becomes imperative to properly differentiate and recognize patients who would respond successfully to nonoperative management vs those for whom continued nonoperative care may prove futile.18,19 However, the factors at baseline (BL) which might inform such determinations are presently unknown.
In this context, we sought to determine patient characteristics at BL that would be informative of successful nonoperative management in the setting of ASD. To investigate this question, we used the multicenter clinical registry of the International Spine Study Group. This data set has previously been successfully used to study different aspects in the surgical management of ASD, including prognostic factors linked to outcomes.20-22 We hypothesized that patients with lower levels of frailty at BL, as well as those with less severe spinal deformity, would be more likely to achieve success with nonoperative management.
METHODS
Study Design, Setting, and Participants
This study was a retrospective analysis of a prospectively collected, multicenter database containing patients with ASD enrolled at 13 participating centers from 2009 to 2018. Institutional Review Board approval was obtained at each participating site, and all patients provided informed consent before enrollment. Patients enrolled in the database were older than 18 years, had a preliminary plan to undergo surgical correction within 6 months of enrollment dependent on success of nonoperative treatment consisting of patient-specific combinations of physical therapy, medication or injection pain management, and/or dietary counseling, and met at least one of the following radiographic criteria for ASD: coronal Cobb angle ≥ 20°, sagittal vertical axis ≥ 50 mm, pelvic tilt ≥ 25°, and thoracic kyphosis >60°. Patients with spinal deformity of neuromuscular etiology and those with active infections or malignancy were excluded from the database. All data collection at the participating sites was subject to rigorous quality control. Quarterly query reports were compiled and sent to designated research coordinators at participating sites to reconcile any missing or potentially erroneous data points.
Variables, Data Collection, and Radiographic Parameters
Demographic and surgical characteristics were obtained with standardized data collection forms. Patient-reported outcome measures administered at BL and follow-up intervals included The Oswestry Disability Index (ODI), Scoliosis Research Society Outcomes Questionnaire (SRS-22), and Short Form-36 questionnaire. Minimal clinically importance difference (MCID) thresholds were applied to evaluate improvement in outcomes using previously published values for ODI (12.8), Short Form-36 (4.9), SRS-Pain (0.587), SRS-Appearance (0.8), SRS-Activity (0.375), and SRS-Mental (0.42).
Whole-body free-standing lateral radiographs (36-inch cassette) were collected of the spine and assessed at BL and follow-up. Radiographics were analyzed by SpineView (ENSAM, Laboratory of Biomechanics) software according to standardized validated techniques as previously published in the literature. Spinopelvic radiographic parameters measured were pelvic tilt, pelvic incidence, sagittal vertical axis, and mismatch between pelvic incidence and lumbar lordosis.
Quantitative and Statistical Analysis
All statistical testing was performed using SPSS software (v21.0). Patients were stratified into 3 cohorts on the basis of outcome of nonoperative treatment: (1) success, (2) no change, and (3) failure. Success was defined as a gain in MCID in both ODI and SRS-Pain. Patients with a BL ODI less than or equal to 20 and those who also maintained an ODI less than or equal to 20 by 2-year follow-up were also considered nonoperative successes. Failure was defined as a loss in MCID in both ODI and SRS-Pain for those who continued nonoperative treatment or conversion to operative management at any time. Patients who did not meet the criteria for success or failure were characterized as having no change. Comparisons were made using the cohort experiencing failure as the referent. BL comparisons across demographic, clinical and radiographic characteristics were made using the χ2 and independent samples t tests for categorical and continuous outcome variables, respectively. Multivariable logistic regression analysis was used to identify factors independently associated with success after nonoperative management adjusting for confounders. Conditional inference decision trees established cutoffs for success according to frailty and sagittal vertical axis (SVA). Subanalysis was performed to assess the impact of frailty on successful vs unsuccessful nonoperative treatment based on findings of previous studies (Ref. D). Statistical significance was set to P < .05 with odds ratio (OR) and 95% CIs exclusive of 1.0 after regression testing.
RESULTS
Cohort Overview
We included 207 patients who received nonoperative treatment for ASD and who also had complete BL and 2-year follow-up clinical and radiographic data. The mean patient age was 52.6 ± 15.8 years, body mass index (BMI) of 25.4 ± 5.4 kg/m2, mean Adult Spinal Deformity Frailty Index (ASD-FI), was 1.9 ± 1.5, with 86% of patients being female. Of the included patients, 83.1% were older than 35 years.
BL Radiographic and Clinical Background
BL radiographic parameters for the nonoperative cohort were as follows: pelvic incidence minus lumbar lordosis of 4.4 ± 16.4, pelvic tilt of 19.6 ± 10.3, and SVA of 18.9 ± 51.4. Schwab-SRS classification deformity for pelvic tilt was 0 114, + 54, ++34; SVA: 0 143, +45, ++13; and pelvic incidence minus lumbar lordosis: 0 132, +31, ++39. By Global Alignment and Proportion (GAP) score, 27.7% of patients were proportioned (GAP‐proportioned), 29.7% were moderately disproportioned (GAP-moderately disproportioned), and 42.6% were severely disproportioned (GAP-severely disproportioned). By Ames cervical deformity cervical SVA classification, 70.5% of patients were classified as low deformity, 8.7% were classified as moderately deformed, and 20.8% were classified as highly deformed. BL radiographic parameters of successful vs failed nonoperative patients are presented in Table 1. Ames et al defined T1 Slope minus cervical lordosis criteria further stratified patients as 36.6% as low deformity, 20.7% as moderately deformed, and 42.7% as highly deformed. By Roussouly classification, 13.1% of patients were classified as Grade 1, 40.3% as Grade 2, 31.1% as Grade 3, and 15.5% as Grade 4. The most common comorbidities were arthritis (23%), hypertension (19%), depression (15%), osteoporosis (11%), and stomach ulcers (8%). Prior surgical history is presented in Table 2.
TABLE 1.
BL Radiographic Descriptors of Successful vs Failed Nonoperative Patients
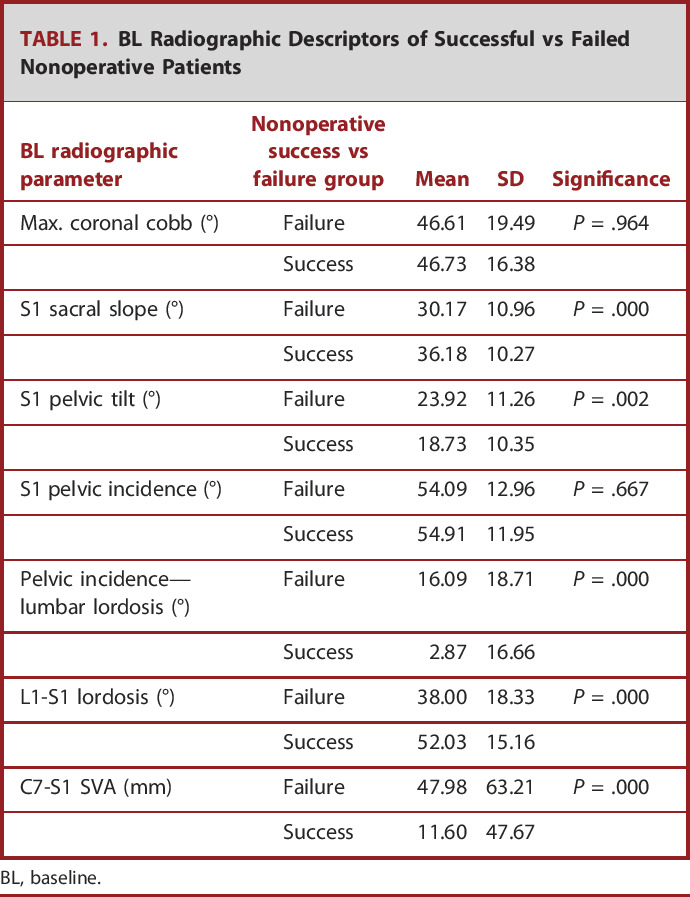
| BL radiographic parameter | Nonoperative success vs failure group | Mean | SD | Significance |
|---|---|---|---|---|
| Max. coronal cobb (°) | Failure | 46.61 | 19.49 | P = .964 |
| Success | 46.73 | 16.38 | ||
| S1 sacral slope (°) | Failure | 30.17 | 10.96 | P = .000 |
| Success | 36.18 | 10.27 | ||
| S1 pelvic tilt (°) | Failure | 23.92 | 11.26 | P = .002 |
| Success | 18.73 | 10.35 | ||
| S1 pelvic incidence (°) | Failure | 54.09 | 12.96 | P = .667 |
| Success | 54.91 | 11.95 | ||
| Pelvic incidence—lumbar lordosis (°) | Failure | 16.09 | 18.71 | P = .000 |
| Success | 2.87 | 16.66 | ||
| L1-S1 lordosis (°) | Failure | 38.00 | 18.33 | P = .000 |
| Success | 52.03 | 15.16 | ||
| C7-S1 SVA (mm) | Failure | 47.98 | 63.21 | P = .000 |
| Success | 11.60 | 47.67 |
BL, baseline.
TABLE 2.
Prior History of Surgical Management in Successful vs Failed Nonoperative Patients
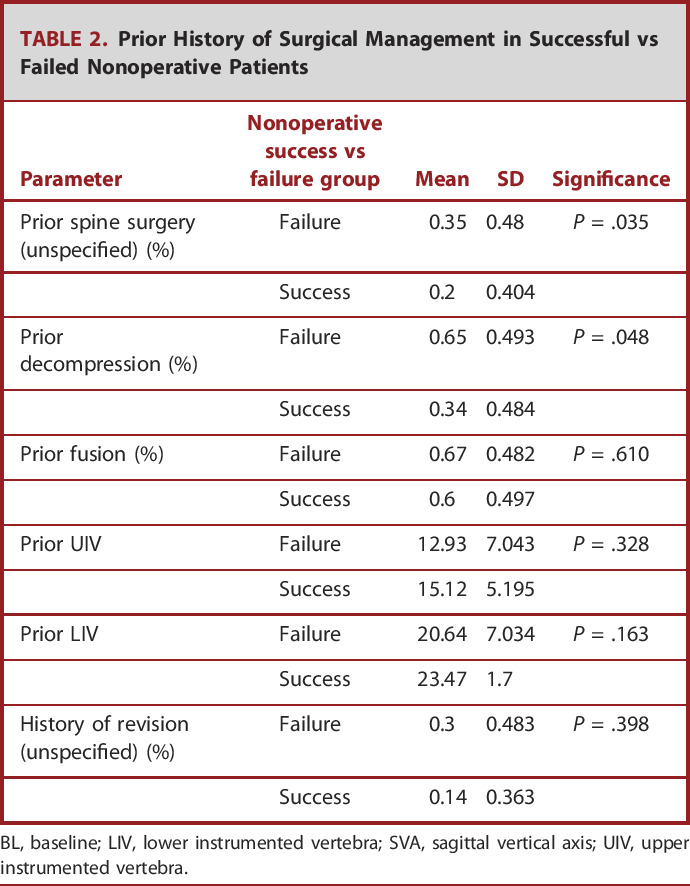
| Parameter | Nonoperative success vs failure group | Mean | SD | Significance |
|---|---|---|---|---|
| Prior spine surgery (unspecified) (%) | Failure | 0.35 | 0.48 | P = .035 |
| Success | 0.2 | 0.404 | ||
| Prior decompression (%) | Failure | 0.65 | 0.493 | P = .048 |
| Success | 0.34 | 0.484 | ||
| Prior fusion (%) | Failure | 0.67 | 0.482 | P = .610 |
| Success | 0.6 | 0.497 | ||
| Prior UIV | Failure | 12.93 | 7.043 | P = .328 |
| Success | 15.12 | 5.195 | ||
| Prior LIV | Failure | 20.64 | 7.034 | P = .163 |
| Success | 23.47 | 1.7 | ||
| History of revision (unspecified) (%) | Failure | 0.3 | 0.483 | P = .398 |
| Success | 0.14 | 0.363 |
BL, baseline; LIV, lower instrumented vertebra; SVA, sagittal vertical axis; UIV, upper instrumented vertebra.
Successful vs Failed Nonoperative Treatment
Overall at 2 years, 44.9% of patients met the parameters for our definition of successful nonoperative treatment, 22.7% exhibited no change, and 32.4% were classified as having failed nonoperative management (Figure 1).
FIGURE 1.
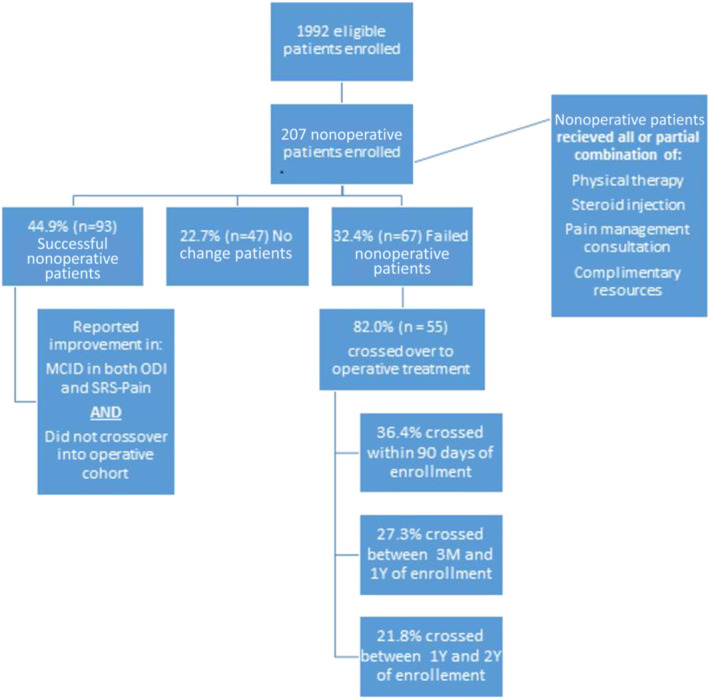
Flow chart demonstrating division of successful, no change, and failed nonoperative patients.
BL comparisons demonstrated that patients who achieved success after nonoperative management were significantly younger, had a lower BMI, decreased Charlson Comorbidity Index, less frail, and had lower rates of hypertension, obesity, depression, and neurological dysfunction at BL (all P < .05). With respect to the severity of deformity, successful nonoperative patients presented with significantly greater rates of grade 0 deformity for all SRS-Schwab Modifiers (all P < .05). Among patients who failed nonoperative treatment, 82% crossed over to operative treatment at an average of 377 days after initial enrollment. Patients who crossed over were significantly more likely to have lower Health-Related Quality of Life measures at BL, higher rates of lung disease, and higher frailty scores (all P < .05) (Table 3).
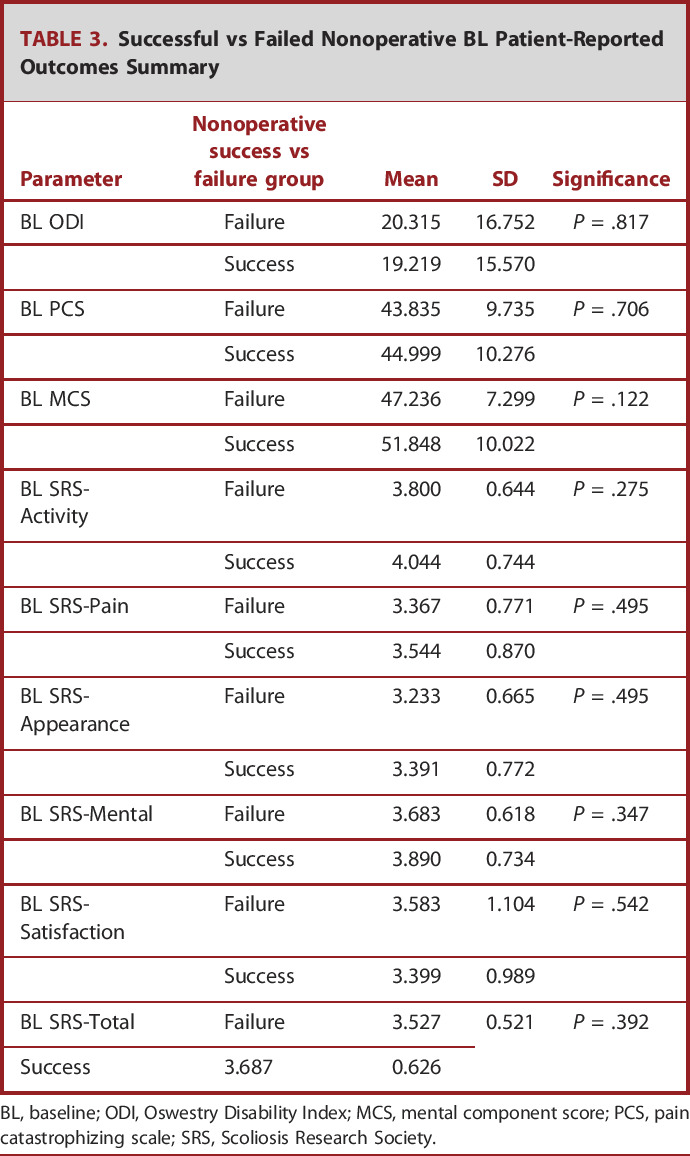
TABLE 3.
Successful vs Failed Nonoperative BL Patient-Reported Outcomes Summary
| Parameter | Nonoperative success vs failure group | Mean | SD | Significance |
|---|---|---|---|---|
| BL ODI | Failure | 20.315 | 16.752 | P = .817 |
| Success | 19.219 | 15.570 | ||
| BL PCS | Failure | 43.835 | 9.735 | P = .706 |
| Success | 44.999 | 10.276 | ||
| BL MCS | Failure | 47.236 | 7.299 | P = .122 |
| Success | 51.848 | 10.022 | ||
| BL SRS-Activity | Failure | 3.800 | 0.644 | P = .275 |
| Success | 4.044 | 0.744 | ||
| BL SRS-Pain | Failure | 3.367 | 0.771 | P = .495 |
| Success | 3.544 | 0.870 | ||
| BL SRS-Appearance | Failure | 3.233 | 0.665 | P = .495 |
| Success | 3.391 | 0.772 | ||
| BL SRS-Mental | Failure | 3.683 | 0.618 | P = .347 |
| Success | 3.890 | 0.734 | ||
| BL SRS-Satisfaction | Failure | 3.583 | 1.104 | P = .542 |
| Success | 3.399 | 0.989 | ||
| BL SRS-Total | Failure | 3.527 | 0.521 | P = .392 |
| Success | 3.687 | 0.626 |
BL, baseline; ODI, Oswestry Disability Index; MCS, mental component score; PCS, pain catastrophizing scale; SRS, Scoliosis Research Society.
Stratifying patients by BL disability because of observed diminishment in nonoperative success in severely frail patients, those with an ODI less than 40 had significantly higher rates of successful nonoperative treatment compared with patients with an ODI greater than 40 (P < .001). Comprehensive 2-year HRQL values are presented in Table 4. After multivariate regression analysis that accounted for confounders, increased frailty was the strongest predictor of failed nonoperative treatment (OR: 2.2 [1.6-3.0], P < .001). Even when excluding patients younger than 35 years and those with a prior history of revision surgery, and controlling for BL Charlson Comorbidity Index, C7-S1 SVA, T1PA, and Roussouly mismatch via backstep regression, only frailty remained a significant predictor of achieving nonoperative success in the studied cohort (P < .001). However, neither age nor a history of spine surgery was significant individual predictors in the model including all patients older than 18 years with the aforementioned controls in place (both P > .05). In addition, successful nonoperative patients were significantly more likely to present as GAP-proportioned at BL than their nonoperative failure counterparts (OR: 2.7 [1.1-6.3], P < .05).
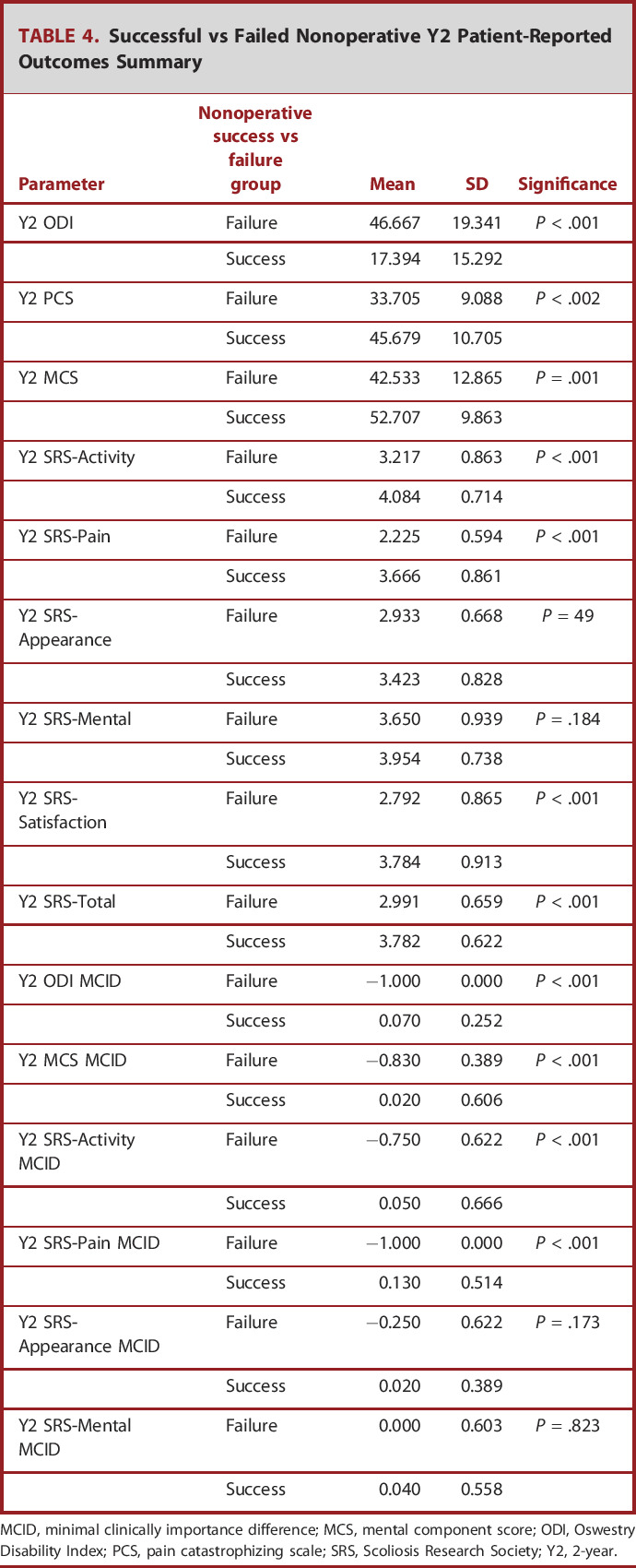
TABLE 4.
Successful vs Failed Nonoperative Y2 Patient-Reported Outcomes Summary
| Parameter | Nonoperative success vs failure group | Mean | SD | Significance |
|---|---|---|---|---|
| Y2 ODI | Failure | 46.667 | 19.341 | P < .001 |
| Success | 17.394 | 15.292 | ||
| Y2 PCS | Failure | 33.705 | 9.088 | P < .002 |
| Success | 45.679 | 10.705 | ||
| Y2 MCS | Failure | 42.533 | 12.865 | P = .001 |
| Success | 52.707 | 9.863 | ||
| Y2 SRS-Activity | Failure | 3.217 | 0.863 | P < .001 |
| Success | 4.084 | 0.714 | ||
| Y2 SRS-Pain | Failure | 2.225 | 0.594 | P < .001 |
| Success | 3.666 | 0.861 | ||
| Y2 SRS-Appearance | Failure | 2.933 | 0.668 | P = 49 |
| Success | 3.423 | 0.828 | ||
| Y2 SRS-Mental | Failure | 3.650 | 0.939 | P = .184 |
| Success | 3.954 | 0.738 | ||
| Y2 SRS-Satisfaction | Failure | 2.792 | 0.865 | P < .001 |
| Success | 3.784 | 0.913 | ||
| Y2 SRS-Total | Failure | 2.991 | 0.659 | P < .001 |
| Success | 3.782 | 0.622 | ||
| Y2 ODI MCID | Failure | −1.000 | 0.000 | P < .001 |
| Success | 0.070 | 0.252 | ||
| Y2 MCS MCID | Failure | −0.830 | 0.389 | P < .001 |
| Success | 0.020 | 0.606 | ||
| Y2 SRS-Activity MCID | Failure | −0.750 | 0.622 | P < .001 |
| Success | 0.050 | 0.666 | ||
| Y2 SRS-Pain MCID | Failure | −1.000 | 0.000 | P < .001 |
| Success | 0.130 | 0.514 | ||
| Y2 SRS-Appearance MCID | Failure | −0.250 | 0.622 | P = .173 |
| Success | 0.020 | 0.389 | ||
| Y2 SRS-Mental MCID | Failure | 0.000 | 0.603 | P = .823 |
| Success | 0.040 | 0.558 |
MCID, minimal clinically importance difference; MCS, mental component score; ODI, Oswestry Disability Index; PCS, pain catastrophizing scale; SRS, Scoliosis Research Society; Y2, 2-year.
After conditional inference decision tree analysis, we determined that patients presenting with a BL ASD-FI ≤ 1.6 (OR: 8.3 [4.0-17.5], P < .001) were significantly more likely to achieve success after nonoperative treatment.
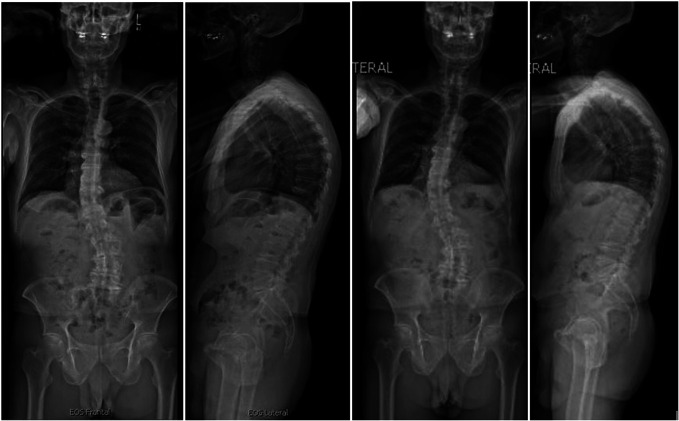
Case Example—Nonoperative Success
We present a 71-year-old man with a BMI of 23.0 kg/m2, with no history of prior fusion, and an ODI score of 18 (Figure 2). The patient was matched in SRS-Schwab pelvic tilt, SVA, and lumbar lordosis criteria and remained as Roussouly Grade 1 from BL to 2 years. At 2 years, the patient did not demonstrate evidence of radiographic deterioration nor significant complications, although no improvements in GAP or SRS-Schwab criteria were noted.
FIGURE 2.
A 71-one-year-old man with no history of prior thoracolumbar fusion exemplifying successful nonoperative treatment. No major complications or deterioration from baseline to 2 years postoperatively noted.
Case Example—Non-Operative Failure
We present a 73-year-old woman with a BMI of 22.6 kg/m2, with a history of arthritis, neurological dysfunction, gastric ulcers, and no prior fusion, and an ODI score of 48 (Figure 3). The patient was matched in SRS-Schwab SVA criteria and remained as Roussouly Grade 4 from BL to 1 year. At 225 days after study enrollment, the patient was deemed a failed nonoperative patient because of worsening pain and neuromotor function and required 10-level thoracolumbar fusion. At 2 years, the patient did not demonstrate evidence of PJK, PJF, or significant complications, although overall HRQLs decreased over time.
FIGURE 3.
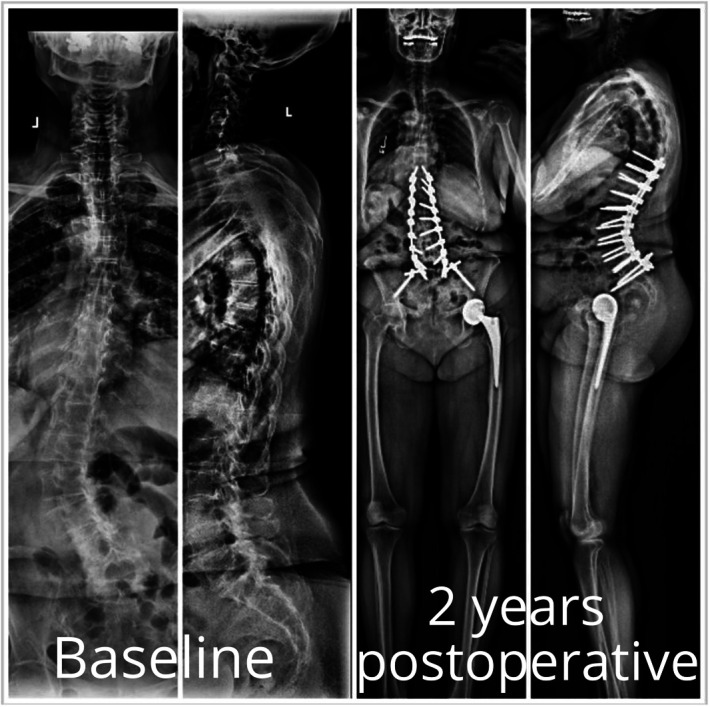
A 73-year-old woman with a history of arthritis, neurological dysfunction, gastric ulcers, and no prior fusion and an Oswestry Disability Index score of 48. The patient continued to experience pain and was deemed a candidate for surgery 225 after study enrollment. Ten-level thoracolumbar fusion was performed with no major complications noted at 2 years. The patient was deemed nonoperative failure.
DISCUSSION
Key Findings
Our study demonstrated that nonoperative treatment had higher prevalence of success in patients who were younger, with less severe deformity, a lower BMI, and lower rates of depression and neurological dysfunction at BL. Furthermore, patients who presented with a BL ODI below 40 were significantly more likely to experience success after nonoperative care. In our multivariable analysis, we determined that the most influential independent predictor of nonoperative success or failure appears to be frailty, with a cutoff threshold of 1.579 on the ASD-FI.
Limitations
There are some important limitations to this study. Many of these are inherent to retrospective work conducted using multicenter registries, including the potential for errors in data entry and misclassification. Furthermore, limited long-term follow-up, particularly 10 years or greater, precludes analysis of patients who demonstrate durable improvement without operative treatment, and future studies with longer follow-up are necessary. There is also the prospect of selection and indication bias and restricted clinical variation that cannot be fully addressed. Pursuant to this, although there are more than 200 patients in the cohort overall, smaller numbers are present in our subgroups and outcomes of interest, including treatment success and failure. This limits the capacity to effectively control for interactions and some of the prognostic factors identified here, such as age, severity of deformity and frailty, which may be colinear. Our statistical models should be viewed as hypothesis generating as opposed to prescriptive as a result. The factors identified here, as well as the frailty cutoffs generated, could be parochial to our cohort, and further testing in larger samples from other centers is likely necessary before these determinations can be considered definitively translatable. Furthermore, we recognize that the findings of this patient cohort may not be applicable to patients with complex deformity, as such individuals see greater risk by delaying operative treatment. Finally, we do recognize that our definitions of success and failure are study-specific to some degree, although we do believe that they have acceptable clinical and face validity. Surgeons or patients who would use different definitions of success from those applied here should adjust the application of our findings to those specific clinical contexts.
Interpretation and Generalizability
ASD has been shown to be increasingly prevalent within the aging populations of developed nations. Although advancements in surgical techniques and perioperative management have resulted in dramatic improvements in outcomes and reduced complications for surgical interventions used to correct ASD over the past 20 years, the fact remains that these are still highly intense surgical procedures.23 At the same time, given the nature of ASD, some patient complaints may be resistant to conventional nonoperative measures. In previous work, Passias et al14 suggested that there may be threshold of disability and malalignment where patients are at increased risk of crossing over from nonoperative to operative care. Surgeons must account for the risks of surgical intervention and balance these against a patient's capacity to benefit from surgery as opposed to nonoperative treatment.24,25 However, this paradigm is impaired to some degree at present, as current literature does not provide adequate information regarding the factors that may influence success after nonoperative treatment.26
As far as we are aware, this work is the first to consider clinical and radiographic characteristics that may be predictive of the success of nonoperative treatment in patients with ASD. Our work is advantaged by a relatively large sample, collected at multiple institutions over a narrow time frame that represents the modern period of spinal instrumentation and techniques used to treat ASD. We believe that the results are important for patients, surgeons, and health care systems. Specifically, our findings indicate that young patients, with lower levels of frailty and less severe spinal deformity likely should be considered for less invasive and less expensive nonoperative interventions, as a first-line treatment. Those who do not possess these characteristics might better be considered for a surgical intervention, if indicated, as a means to minimize health care expenditures and procedural exposure that may not be likely to result in meaningful improvements regarding care.
CONCLUSION
Success of nonoperative treatment was more frequent among younger patients and those with less severe deformity and frailty at BL. When controlling for all other confounders, BL frailty was the most important determinant of successful nonoperative management. The prognostic factors identified here may be useful in informing preoperative discussions and clinical decision-making regarding treatment strategies in patients presenting with ASD. Specifically, relatively young patients, with lower levels of frailty and less severe spinal deformity could be considered for less invasive and less expensive nonoperative interventions, before exploring surgical solutions. At the same time, those who do not possess any of the favorable characteristics informing success of nonoperative treatment might benefit from more direct consideration for surgery at the time of initial presentation. Additional study is also needed to better assess the threshold at which surgical benefit outweighs complication risk, especially for frail patients who are both at higher inherent risk of complications and may not tolerate their deformity and their less frail counterparts.
Contributor Information
Waleed Ahmad, Email: waleed.ahmad81@gmail.com.
Peter Tretiakov, Email: okrol90@gmail.com.
Oscar Krol, Email: petertretiakov@gmail.com.
Frank Segreto, Email: frankasegreto@gmail.com.
Renaud Lafage, Email: renaud.lafage@gmail.com.
Virginie Lafage, Email: virginie.lafage@gmail.com.
Alex Soroceanu, Email: alex.soroceanu@hotmail.com.
Alan Daniels, Email: alandanielsmd@gmail.com.
Jeffrey Gum, Email: jlgum001@gmail.com.
Breton Line, Email: breton.line@gmail.com.
Andrew J. Schoenfeld, Email: aschoenfeld@bwh.harvard.edu.
Shaleen Vira, Email: shalvira@gmail.com.
Robert Hart, Email: Robert.Hart@swedish.org.
Douglas Burton, Email: dburton@kumc.edu.
Justin S. Smith, Email: jss7f@virginia.edu.
Christopher P. Ames, Email: christopher.ames@ucsf.edu.
Christopher Shaffrey, Email: christopher.shaffrey@duke.edu.
Frank Schwab, Email: fschwab@att.net.
Shay Bess, Email: shay_bess@hotmail.com.
Funding
The ISSG supported this study. Andrew J. Shoenfeld has funding from NIH-NIAMS, US Department of Defense, and OREF. Justin S. Smith has funding from NREF and AOSpine.
Disclosures
Peter G. Passias: Allosource: Other financial or material support; Cervical Scoliosis Research Society: Research support; Globus Medical: Paid presenter or speaker; Medtronic: Paid consultant; Royal Biologics: Paid consultant; Spine: Editorial or governing board; SpineWave: Paid consultant; Terumo: Paid consultant; Zimmer: Paid presenter or speaker. Renaud Lafage: Nemaris: Stock or stock Options. Virginie Lafage: DePuy, A Johnson & Johnson Company: Paid presenter or speaker; European Spine Journal: Editorial or governing board; Globus Medical: Paid consultant; International Spine Study Group: Board or committee member; Nuvasive: IP royalties.
Scoliosis Research Society: Board or committee member; The Permanente Medical Group: Paid presenter or speaker; VFT Solution: present relationship; AO Spine Foundation, K2 Medical, Nemaris: past relationships. Alan Daniels: EOS: Paid consultant; Medicrea: Paid consultant; Medtronic Sofamor Danek: Paid consultant; Novabone: Paid consultant; Orthofix, Inc.: Paid consultant, Research support; Southern Spine: IP royalties; Spineart: IP royalties; Paid consultant; Springer: Publishing royalties, financial or material support; Stryker: Paid consultant. Jeffrey Gum: Acuity: IP royalties; Paid consultant; Alan L. & Jacqueline B. Stuart Spine Research: Research support; Biom’Up: Research support; Cerapedics: Research support; Cingulate Therapeutics: Stock or stock Options; DePuy, A Johnson & Johnson Company: Paid consultant; Empirical Spine, Inc.: Research Support; Global Spine Journal: Reviewer, Editorial or governing board; Medtronic: Board or committee member; Paid consultant; Research support, Patent; Norton Healthcare: Research support, Employee; Nuvasive: IP royalties; Paid consultant; Pfizer: Research support; Scoliosis Research Society: Research support; Spine Deformity: Reviewer, Editorial or governing board; Stryker: Paid consultant; Board or committee member, Research Support; Texas Scottish Rite Hospital: Research support; The Spine Journal—Reviewer: Editorial or governing board. Breton Line: ISSGF: Paid consultant; Financial relationships with DePuy Synthes Spine, Nuvasive, and Stryker/K2M. Andrew J. Schoenfeld: AAOS: Board or committee member; Journal of Bone and Joint Surgery—American: Editorial or governing board; North American Spine Society: Board or committee member; Springer Nature: Publishing royalties, financial or material support; Wolters Kluwer Health—Lippincott Williams & Wilkins: Publishing royalties, financial or material support.
Robert Hart: ISSG: Board; DePuy: Consulting, speaker, royalties, research support; Globus Medical: IP royalties; Paid consultant; Paid presenter or speaker; International Spine Study Group: Board or committee member; ISSLS Textbook of the Lumbar Spine: Editorial or governing board; Medtronic: Paid consultant; Paid presenter or speaker; SeaSpine: IP royalties; Paid Consultant; Spine Connect: Stock or stock Options; Advisory Board Member; MiRus: Paid Consultant. Douglas Burton: Globus and Blue Ocean Spine: financial relationships; Bioventus: Research support; DePuy, A Johnson & Johnson Company: IP royalties; Paid consultant; Research support; Pfizer: Research support; Progenerative Medical: Stock or stock Options; Scoliosis Research Society: Board or committee member; Spine Deformity: Editorial or governing board. Justin S. Smith: Alphatec Spine: Stock or stock Options; Carlsmed: Paid consultant; Cerapedics: Paid consultant; DePuy: Research support; DePuy, A Johnson & Johnson Company: Paid consultant; ISSG: Executive committee; Journal of Neurosurgery Spine: Editorial or governing board; Neurospine: Editorial or governing board; Neurosurgery: Editorial or governing board; Nuvasive: IP royalties; Paid consultant; Research support; Operative Neurosurgery: Editorial or governing board; Scoliosis Research Society: Board or committee member; SeaSpine: financial relationships; Spine Deformity: Editorial or governing board; Stryker: Paid consultant; Thieme: Publishing royalties, financial or material support; Zimmer: IP royalties; Paid consultant. Christopher P. Ames: Biomet Spine: IP royalties; DePuy, A Johnson & Johnson Company: IP royalties; Paid consultant; Research support; Global Spine Analytics—Director: Other financial or material support; International Spine Study Group (ISSG): Research support; International Spine Study Group (ISSG)—Executive Committee: Other financial or material support; K2M: IP royalties; Paid consultant; Medicrea: IP royalties; Paid consultant; Medtronic: Paid consultant; Next Orthosurgical: IP royalties; Nuvasive: IP royalties; Operative Neurosurgery—Editorial Board: Other financial or material support; Scoliosis Research Society (SRS)—Grant Funding: Other financial or material support; Stryker: IP royalties; Titan Spine: Research support. Christopher Shaffrey: AANS: Board or committee member; Cervical Spine Research Society: Board or committee member; DePuy, A Johnson & Johnson Company: Paid presenter or speaker; Research support; Globus Medical: Research support; Medtronic: Other financial or material support; Paid consultant; Medtronic Sofamor Danek: IP royalties; Paid presenter or speaker; Research support; Neurosurgery RRC: Board or committee member; Nuvasive: IP royalties; Paid consultant; Paid presenter or speaker; Research support; Stock or stock Options; Proprio, SI Bone: IP royalties; Spinal Deformity: Editorial or governing board; Spine: Editorial or governing board; Zimmer Biomet: financial relationship. Frank Schwab: DePuy, A Johnson & Johnson Company: Research support; Globus Medical: Paid consultant; Paid presenter or speaker; K2M: IP royalties; Paid consultant; Paid presenter or speaker; Medicrea: Royalties; Medtronic: Paid consultant; Medtronic Sofamor Danek: IP royalties; Paid presenter or speaker; Nuvasive: Research support; Scoliosis Research Society: Board or committee member; Spine Deformity: Editorial or governing board; See Spine: Shareholder/non-compensated; Stryker: Research support; VFT Solutions: Shareholder/non-compensated; VP of International Spine Society Group (ISSG): Board or committee member; Zimmer: IP royalties; Paid consultant; Paid presenter or speaker. Shay Bess: DePuy, A Johnson & Johnson Company: Research support; Alphatec/EOS; Paid consultant; Carlsmed: research support; Globus Medical: Research support; k2 medical: IP royalties; Paid consultant; Paid presenter or speaker; Research support; Medtronic Sofamor Danek: Research support; North American Spine Society: Board or committee member; Nuvasive: IP royalties; Research support; Orthofix, Inc.: Research support; Scoliosis Research Society: Board or committee member; Sea Spine: Research support; Stryker: IP royalties; Paid presenter or speaker. The International Spine Study Group (ISSG) is funded through research grants from DePuy Synthes and individual donations. The other authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
REFERENCES
- 1.Smith JS, Lafage V, Shaffrey CI, et al. Outcomes of operative and nonoperative treatment for adult spinal deformity: a prospective, multicenter, propensity-matched cohort assessment with minimum 2-year follow-up. Neurosurgery. 2016;78(6):851-861. [DOI] [PubMed] [Google Scholar]
- 2.Bridwell KH, Glassman S, Horton W, et al. Does treatment (nonoperative and operative) improve the two-year quality of life in patients with adult symptomatic lumbar scoliosis: a prospective multicenter evidence-based medicine study. Spine (Phila Pa. 1976). 2009;34(20):2171-2178. [DOI] [PubMed] [Google Scholar]
- 3.Smith JS, Kasliwal MK, Crawford A, Shaffrey CI. Outcomes, expectations, and complications overview for the surgical treatment of adult and pediatric spinal deformity. Spine Deformity. 2012;1(1):4-14. [Google Scholar]
- 4.Smith JS, Shaffrey CI, Glassman SD, et al. Risk-benefit assessment of surgery for adult scoliosis: an analysis based on patient age. Spine (Phila Pa. 1976). 2011;36(10):817-824. [DOI] [PubMed] [Google Scholar]
- 5.Li B, Meng X, Zhang X, Hai Y. Frailty as a risk factor for postoperative complications in adult patients with degenerative scoliosis administered posterior single approach, long-segment corrective surgery: a retrospective cohort study. BMC Musculoskelet Disord. 2021;22(1):333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yagi M, Fujita N, Okada E, et al. Impact of frailty and comorbidities on surgical outcomes and complications in adult spinal disorders. Spine (Phila Pa. 1976). 2018;43(18):1259-1267. [DOI] [PubMed] [Google Scholar]
- 7.Yagi M, Michikawa T, Hosogane N, et al. Risk, recovery, and clinical impact of neurological complications in adult spinal deformity surgery. Spine (Phila Pa. 1976). 2019;44(19):1364-1370. [DOI] [PubMed] [Google Scholar]
- 8.Jones MR, Brovman EY, Novitch MB, Rao N, Urman RD. Potential opioid-related adverse events following spine surgery in elderly patients. Clin Neurol Neurosurg. 2019;186:105550. [DOI] [PubMed] [Google Scholar]
- 9.Garreau de Loubresse C. Neurological risks in scheduled spinal surgery. Orthop Traumatol Surg Res. 2014;100(1 suppl):S85-S90. [DOI] [PubMed] [Google Scholar]
- 10.Cloyd JM, Acosta FL, Jr, Ames CP. Complications and outcomes of lumbar spine surgery in elderly people: a review of the literature. J Am Geriatr Soc. 2008;56(7):1318-1327. [DOI] [PubMed] [Google Scholar]
- 11.Nasser R, Yadla S, Maltenfort MG, et al. Complications in spine surgery. J Neurosurg Spine. 2010;13(2):144-157. [DOI] [PubMed] [Google Scholar]
- 12.Daubs MD, Lenke LG, Cheh G, Stobbs G, Bridwell KH. Adult spinal deformity surgery: complications and outcomes in patients over age 60. Spine (Phila Pa. 1976). 2007;32(20):2238-2244. [DOI] [PubMed] [Google Scholar]
- 13.Glassman SD, Berven S, Kostuik J, Dimar JR, Horton WC, Bridwell K. Nonsurgical resource utilization in adult spinal deformity. Spine (Phila Pa. 1976). 2006;31(8):941-947. [DOI] [PubMed] [Google Scholar]
- 14.Passias PG, Jalai CM, Line BG, et al. Patient profiling can identify patients with adult spinal deformity (ASD) at risk for conversion from nonoperative to surgical treatment: initial steps to reduce ineffective ASD management. Spine J. 2018;18(2):234-244. [DOI] [PubMed] [Google Scholar]
- 15.Slobodyanyuk K, Poorman CE, Smith JS, et al. Clinical improvement through nonoperative treatment of adult spinal deformity: who is likely to benefit? Neurosurg Focus. 2014;36(5):e2. [DOI] [PubMed] [Google Scholar]
- 16.Weinstein JN, Tosteson TD, Lurie JD, et al. Surgical versus nonoperative treatment for lumbar spinal stenosis four-year results of the Spine Patient Outcomes Research Trial. Spine (Phila Pa. 1976). 2010;35(14):1329-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weinstein JN, Tosteson TD, Lurie JD, et al. Surgical vs nonoperative treatment for lumbar disk herniation: the Spine Patient Outcomes Research Trial (SPORT): a randomized trial. JAMA. 2006;296(20):2441-2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beyer F, Geier F, Bredow J, et al. Non-operative treatment of lumbar spinal stenosis. Technol Health Care. 2016;24(4):551-557. [DOI] [PubMed] [Google Scholar]
- 19.Liu S, Diebo BG, Henry JK, et al. The benefit of nonoperative treatment for adult spinal deformity: identifying predictors for reaching a minimal clinically important difference. Spine J. 2016;16(2):210-218. [DOI] [PubMed] [Google Scholar]
- 20.Rushton A, Zoulas K, Powell A, Staal JB. Physical prognostic factors predicting outcome following lumbar discectomy surgery: systematic review and narrative synthesis. BMC Musculoskelet Disord. 2018;19(1):326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Passias PG, Segreto FA, Bortz CA, et al. Probability of severe frailty development among operative and nonoperative adult spinal deformity patients: an actuarial survivorship analysis over a 3-year period. Spine J. 2020;20(8):1276-1285. [DOI] [PubMed] [Google Scholar]
- 22.Daniels AH, Durand WM, Steinbaum AJ, et al. Examination of adult spinal deformity patients undergoing surgery with implanted spinal cord stimulators and intrathecal pumps. Spine (Phila Pa. 1976). 2022;47(3):227-233. [DOI] [PubMed] [Google Scholar]
- 23.Choi SH, Son SM, Goh TS, Park W, Lee JS. Outcomes of operative and nonoperative treatment in patients with adult spinal deformity with a minimum 2-year follow-up: a meta-analysis. World Neurosurg. 2018;120:e870-876. [DOI] [PubMed] [Google Scholar]
- 24.Sciubba DM, Scheer JK, Yurter A, et al. Patients with spinal deformity over the age of 75: a retrospective analysis of operative versus non-operative management. Eur Spine J. 2016;25(8):2433-2441. [DOI] [PubMed] [Google Scholar]
- 25.Núñez-Pereira S, Serra-Burriel M, Vila-Casademunt A, et al. The dynamics of satisfaction in surgical and non-surgical adult spinal deformity patients. Eur Spine J. 2021;30(5):1235-1246. [DOI] [PubMed] [Google Scholar]
- 26.Teles AR, Mattei TA, Righesso O, Falavigna A. Effectiveness of operative and nonoperative care for adult spinal deformity: systematic review of the literature. Global Spine J. 2017;7(2):170-178. [DOI] [PMC free article] [PubMed] [Google Scholar]