Abstract
Objective
In the acute stage of ruptured cerebral aneurysms, limited devices are available, making the treatment difficult. We aimed to evaluate the outcomes of the coil embolization with stenting for the ruptured cerebral aneurysms in the acute stage.
Methods
We assessed 22 cases treated with stenting among 134 of 169 consecutive patients with subarachnoid hemorrhages undergoing an endovascular treatment between April 2014 and December 2021, of which 134 underwent an embolization during the acute stage. A stent was used in the patients wherein the treatment with the balloon-assisted or double catheter technique was difficult. Stenting was performed under the loading of two or more antiplatelet agents.
Results
The mean age of the patients was 68.9 years, of which five were male and 14 (63.6%) had severe grade (World Federation of Neurosurgeons grade IV, V). The aneurysm site was the anterior communicating artery in four cases, internal carotid artery in nine, middle cerebral artery in two, vertebrobasilar artery in six, and posterior cerebral artery in one. The aneurysm shape was saccular in 13 cases, dissection in seven, and fusiform in two. Stents were used for wide-neck aneurysms in 12 cases, vascular preservation in seven, and rescue in three. The mean maximum diameter was 9.6 mm. The mean neck size was 6.4 mm. Complete occlusion and neck remnant were found in eight and seven cases, respectively. The perioperative complication rate was 45.5% (thromboembolism in five cases, stent occlusion in two, re-bleeding in two, and cerebral hemorrhage in one). The outcomes included modified Rankin Scale 0–2 in seven cases, 4–5 in five, and 6 in nine. Stent-related death occurred in one case. The rate of morbidity and mortality was 18.2%. Although stents were used in the acute stage of rupture, they were used for the right reasons. However, a high rate of complications occurred: two cases of re-bleeding, in which an incomplete occlusion was a factor.
Conclusion
Stent placement in patients with the acute ruptured cerebral aneurysms should be carefully determined and efforts should be made to reduce the embolic and hemorrhagic complications. However, it may be an effective treatment option when other options could be extremely difficult.
Keywords: stent-assisted coil embolization, ruptured aneurysms, acute stage, advantages and disadvantages
Introduction
Ruptured intracranial aneurysms (RIAs) should be treated during the acute stage to prevent re-rupture. However, in Japan, the number of devices that could be used during the acute stage is limited. Only platinum coils and the Woven EndoBridge (WEB; Microvention TERUMO, Tustin, CA, USA) could be used, with which treatment may be difficult. In addition, an early treatment is required in older people, as activities of daily living decline after 2 weeks of bed rest.
Stent-assisted coil embolization (SAC) for RIAs in the acute stage is one of the alternatives. However, all of these are still controversial, as there are concerns about thromboembolic and hemorrhagic complications when using antiplatelet agents. In addition, there are concerns about incomplete occlusion of aneurysms. Conversely, improved rate of complete occlusion (CO) of aneurysms, lower recurrence rates, and contribution to arteries preservation have been reported.1–12)
We believe that SAC for acute RIAs is a treatment for which absolutely necessary situations exist, and we wanted to explore its characteristics from the treatment we have performed. In this study, we retrospectively evaluated patients with RIAs treated with stenting in the acute stage.
Materials and Methods
We assessed 22 cases of stenting among 134 of 169 consecutive patients with subarachnoid hemorrhages (SAHs) treated with endovascular treatment (EVT) between April 2014 and December 2021: 134 underwent embolization during the acute stage.
The survey and analysis included data of age, sex, World Federation of Neurosurgeons (WFNS) grade (Gr), location, and type of aneurysm (saccular type, S; dissecting type, D; fusiform type, F). Data also included maximum diameter, neck diameter, reason for stent use, type of stent, occlusion status (CO, neck remnant [NR], body filling [BF]), complications, and outcome (modified Rankin Scale [mRS]).
Stents were used for the wide-necked (neck >4 mm and/or dome/neck ratio ≤2) saccular aneurysms or fusiform aneurysms that were difficult to treat with craniotomy and could not be treated without stenting. However, this is not the case for target embolization. It was also used when the parent artery occlusion (PAO) was necessary for the vertebrobasilar artery dissection and blood blister-like aneurysms that could not tolerate the PAO, or for the preservation of important branching arteries, such as the posterior communicating artery or the posterior inferior cerebellar artery. In addition, it was used for coil deviation, unraveling, and vascular dissection.
EVT was performed under general anesthesia with systemic heparinization and the activated clotting time was maintained above 250 s. If an embolization using a simple, balloon-assisted, or double-catheter technique was unsuccessful, SAC was performed with an informed consent from the family. Dual antiplatelet agents (aspirin 200 mg and clopidogrel 75 or 300 mg) were administered through nasal feeding tubes, at least 30 minutes before the stent deployment. After the procedure, 100 mg of aspirin and 75 mg of clopidogrel were given daily for 3 months. Depending on the severity of hydrocephalus, ventricular or lumbar drainage was performed before or after an embolization.
This treatment was approved by the Advanced Novel Medical Technology/Unapproved Novel Medical Devices Evaluation Committee and Ethical Review Board of Showa University (approval No. 01-22, F2019C88).
Results
Clinical and treatment characteristics are shown in Table 1. The 22 patients who underwent SAC in the acute stage for RIAs had an average age of 69.8 (38–89) years, including five males and 14 patients (63.6%) with poor grading (WFNS Gr: IV, V). Nine aneurysms were in the internal carotid artery (ICA), four in the anterior communicating artery, two in the middle cerebral artery, six in the vertebrobasilar artery, and one in the posterior cerebral artery. The aneurysms were type S in 13 cases (59.1%), type D in seven (31.8%), and type F in two (9.1%). The mean maximum diameter was 9.6 mm (4.2–32.0 mm). The mean neck diameter was 6.4 mm (3.0–13.0 mm). All patients received at least two antiplatelet agents. Stents were mainly used for wide-necked aneurysms in 12 cases, preservation of the arteries in seven, and rescue in three. The stents used were Enterprise VRD (Codman, Miami, FL, USA) and Enterprise 2 (Johnson & Johnson, Raynham, Miami, FL, USA) in six cases, Neuroform (NF) EZ (Stryker, Kalamazoo, MI, USA) in two, and NF Atlas (Stryker) in 14. The occlusion status was CO in eight cases and NR and BF in seven cases each.
Table 1. Clinical summary of patients with stenting for ruptured aneurysms in the acute stage.
| Case | Age | Sex | WFNS grading | Location | Type | Maximum size (mm) | Neck size (mm) | Device | Primary occlusion status | Ischemic complication related stent | Re-bleeding | mRS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 45 | F | V | VA | D | – | – | E VRD | BF | 3 | ||
| 2 | 38 | F | V | BA | D | 5.2 | 4.6 | E VRD | CO | 6 | ||
| 3 | 75 | F | V | ICA–ACh | S | 8.9 | 3.2 | NF EZ | NR | 2 | ||
| 4 | 86 | F | V | MCA | D | – | – | E2 | CO | ++ | 6 | |
| 5 | 77 | M | II | VA | S | 13 | 10.5 | E2 | CO | + | 4 | |
| 6 | 67 | F | V | IC (C1) | D | 4.2 | 3.8 | NF EZ | BF | + | 2 | |
| 7 | 45 | F | V | MCA | S | 32 | ≥4 | E2 × 2 | CO | #, ++ | 3 | |
| 8 | 77 | M | IV | PCA (P1) | S | 5.1 | 4.5 | NF Atlas | NR | 6 | ||
| 9 | 47 | F | V | VA | D | – | – | NF Atlas | CO | 6 | ||
| 10 | 66 | F | II | Acom | S | 4.6 | 4.6 | NF Atlas | BF | 6 | ||
| 11 | 60 | F | V | Acom | S | 19 | 3 | NF Atlas | CO | 6 | ||
| 12 | 52 | M | V | VA | D | – | – | NF Atlas | PAO (PICA spared) | # | 2 | |
| 13 | 88 | F | II | ICA–PCA | S | 6.1 | 5.1 | NF Atlas | NR | 5 | ||
| 14 | 73 | F | V | ICA–PCA | S | 7.6 | 7 | NF Atlas | BF | 6 | ||
| 15 | 81 | F | IV | ICA–PCA | S | 6 | 5 | NF Atlas | NR | ++ | 6 | |
| 16 | 52 | F | IV | Acom | S | 4.2 | 3.8 | NF Atlas | BF | 2 | ||
| 17 | 83 | F | II | ICA–PCA | F | 14.5 | 13 | NF Atlas | NR | + | 2 | |
| 18 | 89 | F | II | ICA–PCA | F | 6.5 | 6.1 | E2 | NR | ++ | 5 | |
| 19 | 80 | M | V | VA | D | 9 | 9 | NF Atlas | BF | 6 | ||
| 20 | 83 | F | III | ICA–PCA | S | 12.4 | 12 | NF Atlas | NR | 1 | ||
| 21 | 84 | M | II | Acom | S | 5 | 5 | NF Atlas | CO | 4 | ||
| 22 | 88 | F | II | ICA–PCA | S | 10.2 | 8.9 | NF Atlas | BF | 2 |
#: stent occlusion; +: cases with imaging findings only; ++: cases with a decrease in mRS of one or more; ACh: anterior choroidal artery; Acom: anterior communicating artery; BA: basilar artery; BF: body filling; CO: complete occlusion; D: dissecting type; E: Enterprise; F (in Sex): female; F (in Type): fusiform type; ICA: internal carotid artery; M: male; MCA: middle cerebral artery; mRS: modified Rankin Scale (at 90 days after the procedures); NF: Neuroform; NR: neck remnant; PAO: parent artery occlusion; PCA: posterior cerebral artery; S: saccular type; VA: vertebral artery; WFNS: World Federation of Neurosurgeons
Perioperative complications occurred in ten cases (45.5%), including thromboembolic complications in five cases, stent occlusions in two (acute phase and symptomatic in the case 7, chronic phase and asymptomatic in the case 12), re-ruptures (1 and 7 days after EVT), and intracerebral hemorrhage in one (abnormal coagulation capacity not related to stenting or antiplatelet therapy), with no complications related to drain insertion in the five cases. The outcomes were mRS of 0–2 in seven patients, 3 in one, 4–5 in five, and 6 in nine. The main cause of death was severe SAH in five cases, with one case of re-rupture and death after stenting. The morbidity and mortality rate related to stenting was 4/22 (18.2%).
Case Presentation
Case 6
The patient was a 67-year-old woman with a ruptured wide-necked aneurysm located in the anterior wall of the right ICA, with a suspected blood-blister-like aneurysm. She presented with sudden severe headache and coma (WFNS Gr V). Framing using the balloon-assisted technique (BAT) was performed after treatment with 200 mg of aspirin and 300 mg of clopidogrel. Coil embolization was performed after deployment of the NF Atlas 4.0 × 21 from the Scepter balloon catheter (Microvention TERUMO). The occlusion status was BF. Angiography performed 5 days after the initial embolization showed NR (Fig. 1). However, 7 days later, when the aneurysm re-ruptured, the shape of the aneurysm changed. Therefore, a coil embolization was performed, and two low-profile visualized intraluminal support stents (Microvention TERUMO) 4.5 × 18 were deployed. The patient was discharged with an mRS score of 2. Six months later, the aneurysm was CO (Fig. 2).
Fig. 1. (A) CT image on admission showing an SAH. (B and C) DSA of right ICA (working projection). The ruptured wide-neck aneurysm was located in the anterior wall of the right ICA with a suspected blood-blister-like aneurysm. (D) Cone-beam CT after stent deployment. Stent (NF Atlas) deployment from the balloon catheter after the first coil embolization using the BAT. (E and F) DSA just after embolization with a stent. The aneurysm was obliterated with a BF. BAT: balloon-assisted technique; BF: body filling; ICA: internal carotid artery; NF: neuroform; SAH: subarachnoid hemorrhage.

Fig. 2. (A) DSA 5 days after the initial embolization, showing an improvement from BF to NR. (B and C) Angiogram and DSA at re-rupture 7 days after the initial embolization, showing change in the aneurysm shape without in the coil shape. (D) Cone-beam CT just after re-treatment. Double stenting (low-profile visualized intraluminal support stent) deployment and additional coil embolization were performed. (E) DSA just after re-treatment. The aneurysm was obliterated with a small NR. (F) DSA 6 months after re-treatment, showing an improvement from NR to CO. BF: body filling; CO: complete occlusion; ICA: internal carotid artery; NR: neck remnant.
Case 22
The patient was an 88-year-old woman with a ruptured wide-necked aneurysm (maximum diameter, 10.2 mm and neck diameter, 8.9 mm) located in the right ICA–posterior communicating artery (Fig. 3). She presented with gait disturbance and right ptosis after a sudden headache (WFNS Gr Ⅰ). Since framing without stenting resulted in a severe coil deviation into the parent artery, aspirin 200 mg and clopidogrel 75 mg were administered, followed by a coil embolization with NF Atlas 4.5 × 21 deployed. The occlusion status was NR (Fig. 4). An asymptomatic embolism was present on imaging. The patient was discharged with an mRS of 2.
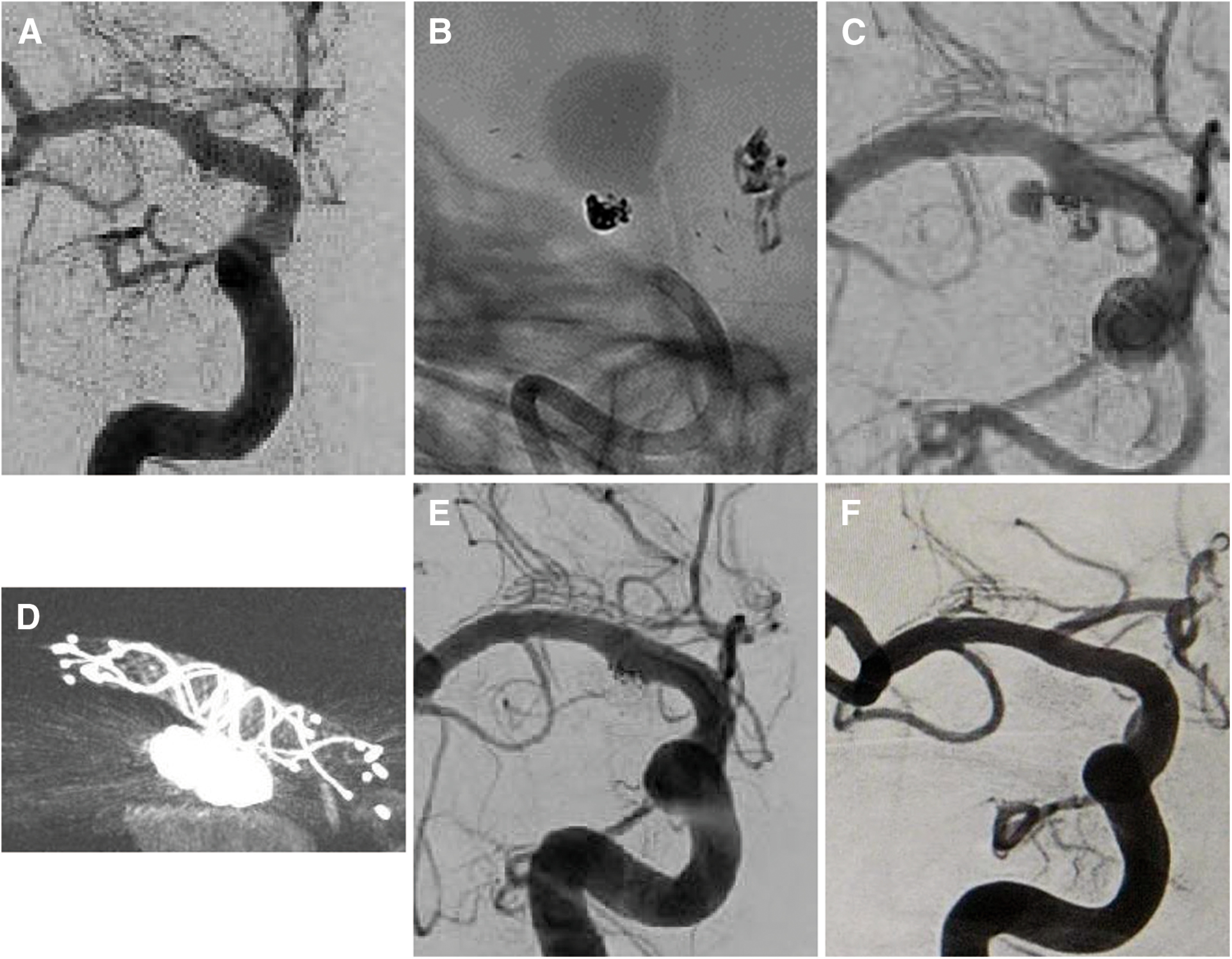
Fig. 3. (A and B) CT image on admission showing an SAH. (C and D) DSA of including the right ICA before embolization (working projection). The ruptured wide-neck aneurysm was located in the right ICA–posterior communicating artery (arrowhead). ICA: internal carotid artery; SAH: subarachnoid hemorrhage.
Fig. 4. (A and B) Angiogram after coil embolization using the stent-assisted technique after a challenge of using the BAT (working angle). (C and D) DSA just after embolization with a stent (NF Atlas). The aneurysm was obliterated with an NR. BAT: balloon-assisted technique; NF: neuroform; NR: neck remnant.
Discussion
Although stenting in the acute stage of RIAs is off-label in terms of insurance coverage, SAC may unavoidably be performed after obtaining an informed consent if there were no other treatment options. We used stents in 16.4% of the patients with SAH who underwent EVT. In all cases, we selected EVT based on the disease severity and difficulty in craniotomy. Since the reason for using stents was generally to cover the aneurysm neck or preserve the parent and branch arteries, we suggest that the use of stenting might be justified.
There were various reports on the administration of the antiplatelet agents, ranging from administration 2 hours before the procedure to administration immediately before or after the procedure. Also, one or two drugs, with or without loading, were used in each case. Antiplatelet agents were administered at least 30 minutes before the stent deployment in all of our cases, and the timing of administration was acceptable.2–12) Recently, we have frequently used the NF Atlas because it can be guided with a low-profile catheter,13) has a lower metal content than other stents, and can be deployed reliably with an excellent crimp adhesion to vessels. We also prefer to use the Scepter balloon catheter because of its rapid response in the event of rupture, the ability to attempt neck remodeling, and the eventual placement of the NF Atlas. However, the increased number of BFs could indicate that we must also consider the aneurysm shape and negative effects of stenting. For example, there are cases in which MC movement is restricted by stenting, which prevents adequate embolization, so it is necessary to devise such as a semi-jailing technique. In some cases, such as cases 12 and 17, stenting was necessary to preserve the important branch and parent arteries with satisfactory results.
Our results showed increased thromboembolic and hemorrhagic complications (31.8% and 13.6% in our study and 5.6%–25.5% and 6.9%–17.4% in previous reports)2,4,6–9) with a morbidity/mortality rate of 18.2% (22.2% in the previous report).8) Symptomatic cerebral infarction may be influenced by an increased intracranial pressure due to the severity of the SAH. Considering the large number of severe cases (59.1%), we considered the result of 40.9% for mRS of 0–3 to be reasonable.7)
The use of stents in the acute stage of RIAs may be an effective treatment option despite concerns about complications.1–12) However, SAC should be carefully selected after considering the risks and benefits of craniotomy and EVT with other techniques without stenting. Currently, we think that the use of two antiplatelet agents is essential in Japan for the treatment of RIAs with stents in the acute stage. Therefore, in embolization, tight packing is essential to ensure a complete disappearance of the rupture point on angiography. Consequently, we think that efforts and innovations are needed to increase the packing rate by using BAT for embolization and finally stenting, as well as by good framing and maintaining a good catheter position. In future, we expect to see the use of the WEB,14) approval for the use of GPIIb/IIIa receptor inhibitors,4,15) and t emergence of new devices, such as stents with antithrombogenic coatings.
Study limitation
This was a retrospective study with a small sample size. In addition, there were different patient backgrounds and an incomplete follow-up because many patients had a poor grade.
Conclusion
In the acute stage of RIAs, embolization with stenting was performed in patients who were considered to have a high risk of re-rupture and for whom craniotomy was difficult. Embolization with stenting was an effective treatment. However, tight packing was necessary to prevent rebleeding.
Disclosure Statement
The authors declare that they have no conflicts of interest.
References
- 1).Tähtinen OI, Vanninen RL, Manninen HI, et al. Wide-necked intracranial aneurysms: treatment with stent-assisted coil embolization during acute (<72 hours) subarachnoid hemorrhage–experience in 61 consecutive patients. Radiology 2009; 253: 199–208. [DOI] [PubMed] [Google Scholar]
- 2).Bodily KD, Cloft HJ, Lanzino G, et al. Stent-assisted coiling in acutely ruptured intracranial aneurysms: a qualitative, systematic review of the literature. AJNR Am J Neuroradiol 2011; 32: 1232–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).Li C, Li Y. Stent-assisted coiling of ruptured wide-necked intracranial aneurysms. Interv Neuroradiol 2013; 19: 283–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).Chung J, Lim YC, Suh SH, et al. Stent-assisted coil embolization of ruptured wide-necked aneurysms in the acute period: incidence of and risk factors for periprocedural complications. J Neurosurg 2014; 121: 4–11. [DOI] [PubMed] [Google Scholar]
- 5).Yang P, Zhao K, Zhou Y, et al. Stent-assisted coil placement for the treatment of 211 acutely ruptured wide-necked intracranial aneurysms: a single-center 11-year experience. Radiology 2015; 276: 545–552. [DOI] [PubMed] [Google Scholar]
- 6).Ryu CW, Park S, Shin HS, et al. Complications in stent-assisted endovascular therapy of ruptured intracranial aneurysms and relevance to antiplatelet administration: a systematic review. AJNR Am J Neuroradiol 2015; 36: 1682–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7).Zhao B, Tan X, Yang H, et al. Stent-assisted coiling versus coiling alone of poor-grade ruptured intracranial aneurysms: a multicenter study. J NeuroIntervent Surg 2017; 9: 165–168. [DOI] [PubMed] [Google Scholar]
- 8).Bechan RS, Sprengers ME, Majoie CB, et al. Stent-assisted coil embolization of intracranial aneurysms: complications in acutely ruptured versus unruptured aneurysms. AJNR Am J Neuroradiol 2016; 37: 502–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Choi HH, Cho YD, Han MH, et al. Antiplatelet premedication-free stent-assisted coil embolization in acutely ruptured aneurysms. World Neurosurg 2018; 114: e1152–e1160. [DOI] [PubMed] [Google Scholar]
- 10).Roh H, Kim J, Bae H, et al. Comparison of stent-assisted and no-stent coil embolization for safety and effectiveness in the treatment of ruptured intracranial aneurysms. J Neurosurg 2019; 30: 1–7. [DOI] [PubMed] [Google Scholar]
- 11).Bsat S, Bsat A, Tamim H, et al. Safety of stent-assisted coiling for the treatment of wide-necked ruptured aneurysm: a systematic literature review and meta-analysis of prevalence. Interv Neuroradiol 2020; 26: 547–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Goertz L, Liebig T, Pennig L, et al. Propensity score-adjusted analysis on stent-assisted coiling versus coiling alone for ruptured intracranial aneurysms. Sci Rep 2021; 11: 21742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).Russo R, Bradac GB, Castellan L, et al. Neuroform Atlas stent-assisted coiling of ruptured intracranial aneurysms: a multicenter study. J Neuroradiol 2021; 48: 479–485. [DOI] [PubMed] [Google Scholar]
- 14).Cortez GM, Akture E, Monteiro A, et al. Woven EndoBridge device for ruptured aneurysms: perioperative results of a US multicenter experience. J Neurointerv Surg 2021; 13: 1012–1016. [DOI] [PubMed] [Google Scholar]
- 15).Yan Y, He X, Fang Y, et al. The safety and efficacy of low-dosage tirofiban for stent-assisted coiling of ruptured intracranial aneurysms. Neurosurg Rev 2021; 44: 2211–2218. [DOI] [PubMed] [Google Scholar]




