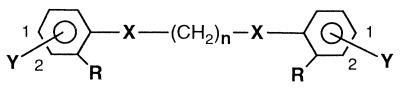Abstract
Twenty analogues of pentamidine, 7 primary metabolites of pentamidine, and 30 dicationic substituted bis-benzimidazoles were screened for their inhibitory and fungicidal activities against Candida albicans and Cryptococcus neoformans. A majority of the compounds had MICs at which 80% of the strains were inhibited (MIC80s) comparable to those of amphotericin B and fluconazole. Unlike fluconazole, many of these compounds were found to have potent fungicidal activity. The most potent compound against C. albicans had an MIC80 of ≤0.09 μg/ml, and the most potent compound against C. neoformans had an MIC80 of 0.19 μg/ml. Selected compounds were also found to be active against Aspergillus fumigatus, Fusarium solani, Candida species other than C. albicans, and fluconazole-resistant strains of C. albicans and C. neoformans. It is clear from the data presented here that further studies on the structure-activity relationships, mechanisms of action and toxicities, and in vivo efficacies of these compounds are warranted to determine their clinical potential.
In the last few years the incidence of fungal infections in the immunocompromised host has increased greatly. The emergence of these pathogens has been followed by both primary drug resistance and the secondary development of azole-resistant isolates of Candida albicans and Cryptococcus neoformans (11, 18, 32, 40). In some severely immunocompromised patients such as those with AIDS, the concept of lifelong suppressive therapy has been advocated. It is clear that new antifungal agents with potent and broad-spectrum fungicidal activity are needed for the effective management of these infections.
The search for new antifungal agents led to the examination of a series of dicationic aromatic compounds related to pentamidine (Table 1). This class of compounds has been shown to have excellent activity against a number of pathogenic organisms, including Giardia lamblia (4, 5), Toxoplasma gondii (24), Pneumocystis carinii (10, 34, 36, 38), Plasmodium falciparum (6), Leishmania mexicana amazonensis (6), and Trypanosoma brucei (21). In addition, pentamidine has previously been reported to have an inhibition effect on the growth of C. albicans (33) and C. neoformans (3).
TABLE 1.
Structures and in vitro activities of pentamidine analogues
| Compound | No. | Ya | Position Y | X | R |
C. albicans
|
C. neoformans
|
||
|---|---|---|---|---|---|---|---|---|---|
| MIC80b (μg/ml) | MFC (μg/ml) | MIC80 (μg/ml) | MFC (μg/ml) | ||||||
| Amphotericin B | 1.0 | NTc | 1.0 | NT | |||||
| Fluconazole | 0.25 | NAd | 2.0 | NA | |||||
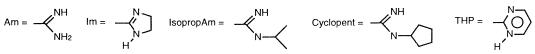
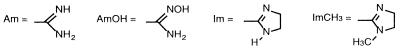
| Pentamidine | 5 | Am | 1 | O | H | 0.78 | 1.56 | 3.12 | 6.25 |
| 1 | 2 | Am | 1 | O | H | 3.12 | 100 | >100 | NT |
| 2 | 2 | Am | 1 | O | NH2 | 25 | NT | >100 | NT |
| 3 | 3 | Am | 1 | O | H | 3.12 | 3.12 | 6.25 | 6.25 |
| 4 | 3 | Am | 1 | O | OCH3 | 12.5 | 50 | 12.5 | 12.5 |
| 5 | 3 | AmOH | 1 | O | H | >100 | NT | >100 | NT |
| 6 | 3 | AmOH | 1 | O | OCH3 | >100 | NT | >100 | NT |
| 7 | 3 | Am | 2 | O | H | >100 | >100 | NT | NT |
| 8 | 3 | Am | 1 | N | H | 0.19 | 0.39 | 1.56 | 6.25 |
| 9 | 3 | Am | 1 | N | NH2 | 12.5 | >100 | 25 | 50 |
| 10 | 3 | Am | 1 | N | NO2 | 100 | 100 | 100 | 100 |
| 11 | 4 | Am | 1 | O | H | 25 | 50 | 3.12 | 6.25 |
| 12 | 4 | Am | 1 | O | OCH3 | >100 | >100 | 50 | >100 |
| 13 | 4 | Am | 1 | O | NH2 | 6.25 | NT | 12.5 | 50 |
| 14 | 4 | Im | 1 | O | H | 0.78 | 1.56 | 6.25 | 12.5 |
| 15 | 4 | ImCH3 | 1 | O | H | >100 | >100 | >100 | >100 |
| 16 | 4 | Am | 2 | O | H | 12.5 | 25 | 25 | 25 |
| 17 | 5 | Am | 1 | O | Cl | 3.12 | 6.25 | 1.56 | 3.12 |
| 18 | 5 | Am | 1 | N | H | ≤0.09 | 0.39 | 0.78 | 1.56 |
| 19 | 6 | Am | 2 | O | H | 1.56 | 12.5 | 6.25 | 12.5 |
| 20 | 6 | Am | 1 | N | NH2 | 0.78 | 3.12 | 0.78 | 3.12 |
MIC80, MIC at which 80% of isolates are inhibited.
NT, not tested.
NA, not active.
The current work describes the antifungal activities of analogues of pentamidine (Table 1), metabolites of pentamidine (Table 2), and a series of compounds derived from the highly potent anti-P. carinii bis-benzimidazoles (Table 3) (38). All of the compounds were screened for activity against C. albicans and C. neoformans. Selected compounds that showed high levels of activity against C. albicans and C. neoformans were tested for their activities against additional strains of these fungi as well as other important pathologic yeasts and clinically important molds (Table 4). The screening was carried out according to a broth macrodilution reference method for in vitro antifungal susceptibility testing of yeast by the National Committee for Clinical Laboratory Standards (29). Examination of the results from these studies reveal several compounds with potent and broad-spectrum antifungal activities.
TABLE 2.
Structures and in vitro activities of pentamidine metabolites
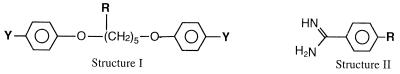
| Compound | Structure | Ya | R | Position R |
C. albicans
|
C. neoformans
|
||
|---|---|---|---|---|---|---|---|---|
| MIC80b (μg/ml) | MFC (μg/ml) | MIC80 (μg/ml) | MFC (μg/ml) | |||||
| Amphotericin B | 1.0 | NTc | 1.0 | NT | ||||
| Fluconazole | 0.25 | NAd | 2.0 | NA | ||||
| Pentamidine | I | Am | H | 0.78 | 1.56 | 3.12 | 6.25 | |
| 21 | I | Am | —OH | 2 | 6.25 | 12.5 | 6.25 | 12.5 |
| 22 | I | Am | —OH | 3 | 12.5 | 12.5 | 12.5 | 12.5 |
| 23 | I | AmOH | —H | >100 | NT | >100 | NT | |
| 24 | II | —OH | >100 | NT | >100 | NT | ||
| 25 | II | —O(CH2)4COOH | >100 | NT | >100 | NT | ||
| 26 | II | —O(CH2)4CH2OH | >100 | NT | >100 | NT | ||
| 27 | II | —O—(CH2)5—O—C6H5—AmOH | >100 | >100 | >100 | NT | ||
TABLE 3.
Structures and in vitro activities of benzimidazole compounds
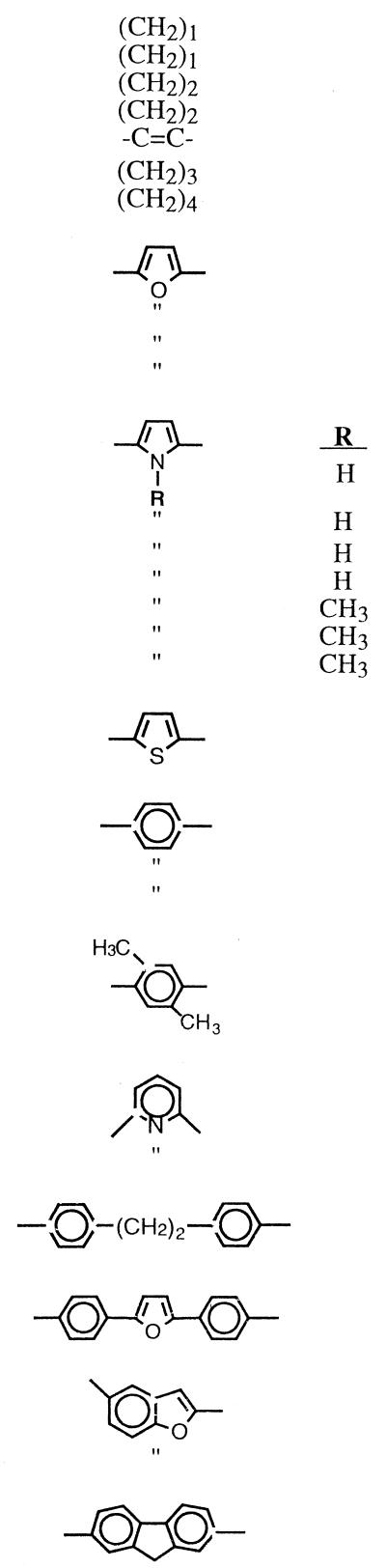
| Compound | X | Ya |
C. albicans
|
Cryptococcus neoformans
|
||
|---|---|---|---|---|---|---|
| MIC80b (μg/ml) | MFC (μg/ml) | MIC80 (μg/ml) | MFC (μg/ml) | |||
| 28 | 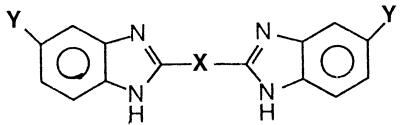 |
Am | >100 | NT | >100 | NT |
| 29 | Im | >100 | >100 | 100 | >100 | |
| 30 | Am | 12.5 | 50 | 6.25 | 25 | |
| 31 | Im | 100 | >100 | 25 | 50 | |
| 32 | Am | 6.25 | 12.5 | 1.56 | 3.12 | |
| 33 | Am | >100 | >100 | >100 | NT | |
| 34 | Im | >100 | >100 | >100 | >100 | |
| 35 | Am | 0.78 | 0.78 | 0.78 | 3.12 | |
| 36 | Im | 1.56 | 1.56 | 1.56 | 3.12 | |
| 37 | IsopropAm | >100 | NT | 6.25 | 50 | |
| 38 | CyclopentAm | 12.5 | >100 | 1.56 | >12.5 | |
| 39 | Am | 0.78 | 0.78 | 0.78 | 0.78 | |
| 40 | Im | 0.78 | 0.78 | 0.78 | 0.78 | |
| 41 | IsopropAm | 50 | NT | 6.25 | 25 | |
| 42 | CyclopentAm | 6.25 | 50 | 1.56 | 6.25 | |
| 43 | Am | 0.78 | 6.25 | 0.78 | 0.78 | |
| 44 | Im | 1.56 | 3.12 | 0.78 | 1.56 | |
| 45 | CyclopentAm | 3.12 | >25 | 0.19 | >1.56 | |
| 46 | IsopropAm | 0.78 | 0.78 | 0.19 | 0.19 | |
| 47 | Am | >100 | NTc | 0.39 | 0.39 | |
| 48 | IM | 0.78 | 6.25 | 0.78 | 1.56 | |
| 49 | THP | 1.56 | 3.12 | 0.78 | 1.56 | |
| 50 | Am | 25 | 100 | 25 | 25 | |
| 51 | Am | >100 | >100 | >100 | >100 | |
| 52 | Im | 1.56 | 1.56 | 1.56 | 1.56 | |
| 53 | IsopropAm | 1.56 | 6.25 | 0.39 | 0.78 | |
| 54 | CyclopentAm | 1.56 | 1.56 | 1.56 | 6.25 | |
| 55 | Am | 0.39 | 0.78 | 0.19 | 0.19 | |
| 56 | CyclopentAm | 0.78 | 0.78 | 0.78 | 0.78 | |
| 57 | IsopropAm | 0.78 | 1.56 | 0.78 | 0.78 | |
TABLE 4.
Extended antifungal spectrum of selected compounds
| Strain | Compound 39
|
Compound 57
|
||
|---|---|---|---|---|
| MIC80a (μg/ml) | MFC (μg/ml) | MIC80 (μg/ml) | MFC (μg/ml) | |
| Candida albicans A39 | 0.78 | 0.78 | 0.78 | 1.56 |
| Candida albicans 102.96b | 0.39 | 0.78 | 1.56 | 12.5 |
| Candida albicans 103.96b | 0.78 | 0.78 | 1.56 | 12.5 |
| Candida krusei 132.91b | NTc | NT | 0.78 | 0.78 |
| Candida lusitaniae 111.92 | NT | NT | 0.78 | 3.12 |
| Candida parapsilosis 111.96 | NT | NT | 0.78 | 0.78 |
| Candida tropicalis 110.96 | NT | NT | 0.78 | 1.56 |
| Torulopsis glabrata 142.91 | NT | NT | 0.78 | 0.78 |
| Cryptococcus neoformans H99 | 0.78 | 0.78 | 0.78 | 0.78 |
| Cryptococcus neoformans 114.96b | NT | NT | 0.78 | 0.78 |
| Aspergillus flavus 112.96 | >100 | NT | NT | NT |
| Aspergillus fumigatus 168.95 | 0.19 | 3.12 | NT | NT |
| Fusarium solani 152.89 | 0.39 | 0.39 | NT | NT |
MATERIALS AND METHODS
Compounds.
All compounds were synthesized in the laboratories of two of the authors (D.B. or R.R.T.). The synthesis and physical properties of the following compounds have been described previously: compounds 1 to 4, 7 to 14, and 16 to 20 (37); compounds 5 and 6 (18a); compound 15 (20); compounds 21 to 27 (8); compounds 28, 30, 32, and 47 (17, 35); and compounds 29, 31, 33, 34, and 48 to 50 (17). The remaining compounds, compounds 35 to 46 and 51 to 57, were newly synthesized, and brief descriptions of the synthesis as well as the physical properties of these compounds are given as follows.
Melting points (mp’s) were recorded with a Thomas Hoover (Uni-Melt) capillary melting point apparatus and are uncorrected. The proton (1H) and carbon (13C) nuclear magnetic resonance (NMR) spectra of the compounds were recorded with a Varian GX400 spectrometer, and chemical shifts (δ) are given in parts per million relative to tetramethylsilane, and coupling constants (J) are reported in hertz. Mass spectra (MS) were recorded on a VG Instruments 70-SE spectrometer (Georgia Institute of Technology, Atlanta). Infrared spectra were recorded with a Michelson 100 (Bomen, Inc.) instrument. Elemental analyses were performed by Atlantic Microlab Inc. (Norcross, Ga.) and are within ±0.4 of the theoretical values. All chemicals and solvents were purchased from Aldrich Chemical Co., St. Louis, Mo., or Fisher Scientific, Pittsburgh, Pa.
4-(N-Cyclopentylamidino)-1,2-phenylene diamine hydrochloride.
4-(N-Cyclopentylamidino)-1,2-phylene diamine hydrochloride is previously unreported and was used as an intermediate in the synthesis of compounds 38, 42, 45, 54, and 56. Distilled cyclopentylamine (1.83 g, 0.021 mol) was added to a stirred suspension of the imidate ester hydrochloride (4.91 g, 0.02 mol) formed from 4-cyano-2-nitroaniline under Pinner-type conditions in 30 ml of dry ethanol, and the mixture was stirred for 12 h at room temperature and for 1 h at 50°C. The solvent was removed under reduced pressure, and the residual thick oily mass was triturated with dry ether and dried under vacuum to yield 4.7 g (95%); mp, 168 to 179°C decompose (dec). 1H NMR (dimethyl sulfoxide [DMSO]-d6): 9.29 (br, 3H), 8.45 (d, 2H, J = 2.4 Hz), 803 (s, 2H), 7.77 (dd, 1H, J = 2.4 and 8.8 Hz), 4.20 (quintet, 1H, J = 6.0 Hz), 2.04 to 2.0 (m, 2H), 1.73 to 1.65 (m, 4H), 1.57 to 1.49 (m, 2H). 13C NMR (DMSO-d6): 160.4, 148.5, 134.1, 129.4, 127.3, 118.9, 114.6, 54.1, 31.2, 23.5. The 4-(N-cyclopentylamidino)-2-nitroaniline (5.0 g, 0.02 mol; mp, 238 to 240°C dec) was used directly without further characterization (5.0 g, 0.02 mol), and 1.0 g of 10% Pd/C in 130 ml of dry methanol was subjected to hydrogenation at 50 lb/in2 for approximately 1 h. The catalyst was filtered over Celite and washed with hot methanol, the solvent of the filtrate was removed under reduced pressure, the residue was triturated with dry ether, and the solid was filtered and dried under vacuum at 45°C for 24 h. The yield of light brown hygroscopic solid was 3.91 g (72%); mp, 170 to 178°C. 1H NMR (DMSO-d6): 8.97 (br s, 1H), 8.82 (br s, 1H), 8.64 (br s, 1H), 6.89 (s, 1H), 6.88 (d, 1H, J = 8.4 Hz), 6.59 (d, 1H, J = 8.4 Hz), 5.40 (br, 2H), 5.0 (br, 2H) 4.17 (m, 2H), 2.10 to 1.98 (m, 2H), 1.82 to 1.76 (m, 4H). 13C NMR (DMSO-d6): 162.4, 140.8, 134.0, 118.4, 115.6, 113.0, 112.5, 53.7, 31.3, 23.5. MS (fast atom bombardment [FAB]) 219 (M+ + 1). Analysis calculated for C12H18N4 · HCl · H2O: C, 52.84; H, 7.76; N, 20.54. Found: C, 53.10; H, 7.77; N, 20.72.
2,5-Bis[2-(5-amidino)benzimidazoyl]furan hydrochloride (compound 35).
A solution of furan-2,5-dicaboxaldehyde (28) (0.8 g 2 mmol), 4-amidino-1,2-phenylene diamino hydrochloride hydrate (0.8 g, 4 mmol), and 1,4-benzoquinone (0.432 g, 4 mmol) in ethanol (40 ml) was heated at reflux for 4 h (under nitrogen) (1). The reaction mixture was cooled to room temperature and the dark solid was collected by filtration, washed with cold ethanol and anhydrous ether, and dried to yield 0.55 g (71%) of the free base. This solid was dissolved slowly in hot ethanol (300 ml) and filtered. The filtrate volume was reduced to 70 ml and was acidified with HCl-saturated ethanol. After standing overnight in the refrigerator, the green solid was collected by filtration, washed with anhydrous ether, and dried under vacuum to yield 0.4 g (52%) yield of a solid; mp, >300°C. 1H NMR (DMSO-d6): 9.30 (s, 4H); 8.19 (2s, 2H), 7.81, (d, 2H, J = 8.8 Hz), 7.72 (d, 2H, J = 8.4 Hz), 7.60 (s, 2H). 13C NMR (DMSO-d6/D2O): 166.8, 146.4, 146.1, 142.2, 139.7, 123.4, 122.7, 117.1, 116.1, 115.4, MS (FAB) m/z 385 (M+ + 1); HRMS: calculated mass (free base), 385.1525 (M+ + 1); observed mass, 385.1535. Analysis calculated for C20H16N8O · 2HCl · 1.5 H2O: C, 49.59; H, 4.37; N, 23.14. Found: C, 49.40; H, 4.31; N, 22.96.
2,5-Bis{2-[5-(2-imidazolino)]benzimidazoyl}furan hydrochloride (compound 36).
A protocol similar to that described above was used for the condensation of 2,5-furandicarboxaldehyde (28) and 2-(3,4-diaminophenyl)imidazoline (17, 35) to give a 38% yield of a green powder; mp, <300°C. 1H NMR (DMSO-d6): 10.53 (s, 4H), 8.38 (s, 2H), 7.87 (d, 2H, J = 8.5 Hz), 7.83 (d, 2H, J = 8.2 Hz), 7.62 (s, 2H), 4.04 (s, 8H). 13C NMR (DMSO-d6/D2O): 166.3, 146.2, 146.1, 142.3, 139.8, 123.7, 117.6, 116.9, 116.1, 115.5, 45.0. MS (FAB) m/z 437 (M+ + 1); HRMS: calculated mass (free base), 437.1838 (M+ + 1); observed mass, 437.1832. Analysis calculated for C24H20N8O · 2HCl · 5H2O: C, 48.08; H, 5.38; N, 18.69. Found: C, 48.22; H, 5.25; N, 18.51.
2,5-Bis[2-(5-N-isopropylamidino)benzimidazoyl]furan hydrochloride (com pound 37).
A protocol similar to that described above (compound 35) was used for the condensation of 2,5-furandicarboxaldehyde (28) and 4-(N-isopropylamidino)-1,2-phenylene diamine (17, 35) to give a 54% yield of a yellow-green powder; mp, >300°C. 1H NMR (DMSO-d6): 9.60 (s, 1H), 9.58 (s, 1H), 9.45 (s, 2H), 9.04 (s, 2H), 8.06 (s, 2H), 7.82 (d, 2H, J = 8.4 Hz), 7.69 (s, 2H), 7.62 (d, 2H, J = 8.2 Hz), 4.09 (m, 2H, J = 7.0 Hz), 1.32 (d, 12H, J = 6.3 Hz). 13C NMR (DMSO-d6/D2O): 162.8, 145.9, 145.1, 140.9, 138.5, 124.5, 124.0, 116.9, 115.9, 115.8, 45.9, 21.7. MS (FAB) m/z 469 (M+ + 1); HRMS: calculated mass (free base), 469.2464 (M+ + 1); observed mass, 469.2475. Analysis calculated for C26H28N8O · 3HCl · 2.5H2O: C, 50.12; H, 5.83; N, 17.99. Found: C, 50.45; H, 5.76; N, 17.64.
2,5-Bis[2-(5-N-cyclopentylamidino)benzimidazoyl]furan hydrochloride (compound 38).
A protocol similar to that described above was used for the condensation of 2,5-furandicarboxaldehyde (28) and 4-(N-cyclopentylamidino)-1,2-phenylene diamine to give a 77% yield of a yellow-green powder, mp, 287 to 289°C dec. 1H NMR (DMSO-d6/D2O): 8.07 (s, 2H), 7.82 (d, 2H, J = 8.4 Hz), 7.66 (s, 2H), 7.63 (d, 2H, J = 8.4 Hz), 4.22 to 4.14 (m, 2H), 2.14 to 2.04 (m, 4H), 1.82 to 1.67 (m, 8H), 1.64 to 1.56 (m, 4H). 13C NMR (DMSO-d6): 163.0, 145.4, 144.5, 140.4, 137.7, 123.9, 116.4, 115.5, 115.2, 54.6, 31.5, 23.7. MS (FAB) m/z 521 (M+ + 1). Analysis calculated for C30H32N8O · 4HCl: C, 54.06; H, 5.44; N, 16.81. Found: C, 53.80; H, 5.51; N, 16.68.
2,5-Bis[2-(5-amidino)benzimidazoyl]pyrrole hydrochloride (compound 39).
A protocol similar to that described above was used for the condensation of 4-amidino-1,2-phenylene diamino hydrochloride hydrate (17, 35) with pyrrole-2,5-dicarboxaldehyde (27) to yield 0.83 g (76%) of a solid; mp, >300°C. 1H NMR (DMSO-d6): 9.48 (br s, 1H), 9.18 (br s, 1H), 8.25 (s, 2H), 7.87 (d, 2H, J = 8.4 Hz), 7.80 (dd, 2H, J = 8.8 and 0.8 Hz), 7.54 (s, 2H). MS (free base) m/e 384 (M+ + 1). Analysis calculated for C20H17N9 · 3HCl · 3H2O: C, 43.93; H, 4.73; N, 23.05. Found: C, 43.61; H, 4.62; N, 22.79.
2,5-Bis{2-[5-(2-imidazolino)]benzimidazoyl}pyrrole hydrochloride (com pound 40).
A protocol similar to that described above was used for the condensation of pyrrole-2,5-dicarboxaldehyde and 2-(3,4-diaminophenyl)imidazoline (17, 35) to give an 86% yield of a solid; mp, >300°C. 1H NMR (DMSO-d6): 10.71 (s, 1H), 8.44 (s, 2H), 7.92 (dd, 2H, J = 8.4 and 1.6 Hz), 7.86 (d, 2H, J = 8.8 Hz), 7.39 (s, 2H), 4.04 (s, 8H). MS (free base) m/e 436 (M+ + 1). Analysis calculated for C24H21N9 · 3HCl · 4H2O: C, 46.72; H, 5.23; N, 20.43. Found: C, 46.49; H, 5.11; N, 20.28.
2,5-Bis[2-(5-N-isopropylamidino)benzimidazoyl]pyrrole hydrochloride (compound 41).
A protocol similar to that described above was used for the condensation of pyrrole-2,5-dicarboxaldehyde and 4-(N-isopropylamidino)-1,2-phenylene diamine (17, 35) to yield 79% of a yellow-green solid; mp, 287 to 289°C dec. 1H NMR (DMSO-d6/D2O): 8.06 (s, 2H), 7.81 (d, 2H, J = 8.4 Hz), 7.65 (d, 2H, J = 8.4 Hz), 7.41 (s, 2H), 4.06 (septet, 2H, J = 6.4 Hz), 1.30 (d, 12H, J = 6.4 Hz). 13C NMR (DMSO-d6/D2O): 162.3, 145.8, 138.6, 135.7, 124.7, 124.2, 123.9, 115.7, 115.5, 114.9, 45.7, 21.4. MS (FAB) m/z 468 (M+ + 1). Analysis calculated for C26H29N9 · 4HCl: C, 50.90; H, 5.42; N, 20.55. Found: C, 51.54; H, 5.57; N, 20.30.
2,5-Bis[2-(5-N-cyclopentylamidino)benzimidazoyl]pyrrole hydrochloride (com pound 42).
A protocol similar to that described above was used for the condensation of 4-(N-cyclopentylamidino)-1,2-phenylene diamine with pyrrole-2,5-dicarboxaldehyde (27) to give a 71% yield of a blue-green solid; mp, 290 to 294°C. 1H NMR (DMSO-d6/D2O): 8.0 (s, 2H), 7.77 (d, 2H, J = 8.4 Hz), 7.60 (d, 2H, J = 8.4 Hz), 7.32 (s, 2H), 4.09 (br m, 2H), 2.11 to 1.97 (m, 4H), 1.77 to 1.62 (m, 8H), 1.61 to 1.50 (m, 4H). 13C NMR (DMSO-d6/D2O): 163.1, 145.7, 138.6, 135.6, 124.6, 124.3, 123.8, 115.8, 115.5, 115.1, 55.0, 31.7, 23.9. MS (FAB) m/z 520 (M+ + 1). Analysis calculated for C30H33N9 · 4HCl · 0.5 H2O: C, 53.42; H, 5.63; N, 18.68. Found: C, 53.90; H, 5.75; N, 18.16.
1-Methyl-2,5-bis[2-(5-amidino)benzimidazoyl]pyrrole hydrochloride (compound 43).
A protocol similar to that described above was used for the condensation of 4-amidino-1,2-phenylene diamine hydrochloride hydrate (17, 35) with 1-methylpyrrole-2,5-dicarboxaldehyde (12) to give a 70% yield of product; mp, >300°C. 1H NMR (DMSO-d6): 9.38 (br s, 1H), 9.11 (br s, 1H), 8.19 (s, 2H), 7.80 (d, J = 8.4 Hz), 7.73 (dd, 2H, J = 8 and 1.2 Hz), 7.33 (s, 2H), 4.72 (s, 3H). MS (free base) m/z 398 (M+ + 1). Analysis calculated for C21H19N9 · 3HCl · H2O: C, 48.06; H, 4.61; N, 24.02. Found: C, 48.16; H, 4.58; N, 23.93.
2,5-Bis{2-[5-(2-imidazolino)]benzimidazoyl}-1-methylpyrrole hydrochloride (compound 44).
A protocol similar to that described above was used for the condensation of 2-(3,4-diaminophenyl)imidazoline (17, 35) with 1-methylpyrrole-2,5-dicarboxaldehyde. A yield of 83% of a solid (mp, >300°C) was obtained. 1H NMR (DMSO-d6): 10.60 (s, 1H), 8.36 (s, 2H), 7.84 (dd, 4H, J = 8.4 and 8 Hz), 7.30 (s, 2H), 4.72 (s, 3H), 4.04 (s, 8H). MS (free base) m/e 450 (M+ + 1). Analysis calculated for C25H23N9 · 3HCl · 3H2O: C, 48.98; H, 5.26; N, 20.57. Found: C, 49.20; H, 4.79; N, 20.51.
2,5-Bis[2-(5-N-cyclopentylamidino)benzimidazoyl]-1-methylpyrrole hydrochlo ride (compound 45).
A protocol similar to that described above was used for the condensation of 4-(N-cyclopentylamidino)-1,2-phenylene diamine with 1-methyl-2,5-pyrrole dicarboxaldehyde (12) to give an 85% yield of a blue solid; mp, 324 to 326°C dec. 1H NMR (DMSO-d6/D2O): 8.0 (s, 2H), 7.73 (d, 2H, J = 8.4 Hz), 7.55 (d, 2H, J = 8.4 Hz), 7.13 (s, 2H), 4.57 (s, 3H), 4.14 (quintet, 2H, J = 5.2 Hz), 2.12 to 2.02 (m, 4H), 1.80 to 1.58 (m, 12H). MS (FAB) m/z 534 (M+ + 1). Analysis calculated for C31H35N9 · 3HCl · H2O: C: 56.32; H, 6.10; N, 19.07. Found: C, 56.90; H, 5.97; N, 18.83.
2,5-Bis[2-(5-N-isopropylamidino)benzimidazoyl]thiophene hydrochloride (compound 46).
A protocol similar to that described above was used for the condensation of 2,5-thiophenedicarboxaldehyde and 4-(N-isopropylamidino)-1,2-phenylene diamine (17, 35) to give a 75% yield of a green-yellow solid; mp, 290 to 292°C dec. 1H NMR (DMSO-d6/D2O): 8.18 (s, 2H), 8.05 (s, 2H), 7.77 (d, 2H, J = 8.4 Hz); 7.60 (d, 2H, J = 8.4 Hz), 4.11 (quintet, 2H, J = 6.4 Hz), 1.31 (d, 12H, J = 6.4 Hz). MS (FAB) m/z 485 (M+ + 1). Analysis calculated for C26H28N8S · 3HCl · H2O: C, 51.02; H, 5.43; N, 18.31; Cl, 17.38. Found: C, 51.56; H, 5.54; N, 18.09; Cl, 17.37.
2,6-Bis{2-[5-(2-imidazolino)]benzimidazoyl}pyridine hydrochloride (compound 51).
A protocol similar to that described above was used for the condensation of 2,6-pyridine carboxyaldehyde and 2-(3,4-diaminophenyl)imidazoline (17, 35) to give an 85% yield of a solid; mp, >300°C. 1H NMR (DMSO-d6): 10.71 (s, 1H), 8.51 to 8.49 (m, 4H), 8.30 (m, 1H), 7.96 (m, 4H), 4.05 (s, 8H). MS (free base) m/e 448 (M+ + 1). Analysis calculated for C25H21N9 · 3HCl · 3H2O: C, 49.15; H, 4.94; N, 20.63. Found: C, 49.14; H, 4.68; N, 20.51.
2,6-Bis[2-(5-amidino)benzimidazoyl]pyridine hydrochloride (compound 52).
A protocol similar to that described above was used to condense 2,6-pyridine dicarboxaldehyde with 4-amidino-1,2-phenylene diamine hydrochloride hydrate (17, 35) to give an 89% yield of a solid; mp, >300°C. 1H NMR (DMSO-d6): 9.45 (br s, 1H), 9.12 (br s, 1H), 8.51 (d, 2H, J = 8 Hz), 8.34 to 8.28 (m, 3H), 7.94 (d, 2H, J = 8.4 Hz), 7.79 (dd, 2H, J = 8.4 and 1.6 Hz), MS (free base) m/z 396 (M+ + 1). Analysis calculated for C21H17N9 · 3HCl · 3H2O: C, 45.13; H, 4.69; N, 22.56. Found: C, 45.16; H, 4.58; N, 22.45.
4,4′-Bis[2-(5-N-isopropylamidino)benzimidazoyl]-1,2-diphenylethane hydrochloride (compound 53).
1,2-Bis-(4-cyanophenyl)ethane was prepared in one step from 2,3-bis-(4-bromophenyl)propanoic acid (13) by the action of CuCN in dimethylformamide (15) in a 50% yield; mp, 195 to 197°C. 1H NMR (DMSO-d6): 7.68 (d, 4H, J = 8 Hz), 7.40 (d, 4H, J = 8 Hz), 3.01 (s, 4H). 13NMR (DMSO-d6): 146.7, 131.9, 129.3, 118.6, 108.6, 35.8. MS m/e 232 (M+ + 1). 1,2-Bis-(4-cyanophenyl)ethane was used without further characterization and on treatment with diisofutylaluminumhydride (DIBAL) gave a white crystalline solid (CHCl3-ether) of 76% of 1,2-bis-(4-formylphenyl)ethane; mp, 121 to 122°C. 1H NMR (DMSO-d6): 9.96 (s, 2H), 7.80 (d, 4H, J = 8 Hz), 7.44 (d, 4H, J = 8 Hz), 3.05 (s, 4H). 13C NMR (DMSO-d6/D2O): 192.0, 184.0, 134.2, 129.1, 128.8, 36.0. Analysis calculated for C16H14O2 · 0.1 H2O: C, 80.04; H, 5.96. Found: C, 80.09; H, 5.98. A protocol similar to that described above was used for the condensation of 1,2-bis-(4-formylphenyl)ethane and 4-(N-isopropylamidino)-1,2-phenylene diamine (17, 35) and gave 75% yield of a purple solid; mp, >320°C. 1H NMR (DMSO-d6/D2O): 8.09 (d, 4H, J = 8 Hz), 8.06 (s, 2H), 7.83 (d, 2H, J = 8.4 Hz), 7.65 (d, 2H, J = 8.4 Hz), 7.48 (d, 4H, J = 8 Hz), 4.03 (br m, 2H), 3.07 (br, 4H), 1.29 (d, 12H, J = 6 Hz). 13NMR (DMSO-d6/D2O): 162.4, 153.0, 146.6, 138.3, 135.4, 129.8, 127.9, 124.8, 124.1, 123.5, 115.5, 115.0, 45.6, 36.2, 21.2. MS (FAB) m/z 583 (M+ + 1) Analysis calculated for C36H38N8 · 4HCl · 0.5 H2O: C, 58.61; H, 5.87; N, 15.19. Found: C, 58.31; H, 5.79; N, 15.02.
4,4′-Bis[2-(5-N-cyclopentylamidino)benzimidazoyl]-2,5-diphenylfuran hydrochloride (compound 54).
2,5-Bis(4-formylphenyl)furan was prepared by reduction of 2,5-bis-(4-cyanophenyl)furan (2) (1.12 g 0.004 mol) with 1 M DIBAL in CH2Cl2 (1.83 g, 0.012 mol) to yield 0.77 g (70%) of a pale yellow solid; mp, 173 to 174 4°C (CHCl3-ether). 1H NMR (DMSO-d6/D2O) 10.0 (s, 2H), 8.05 (d, 4H, J = 7.5 Hz), 7.97 (d, 4H, J = 7.5 Hz), 7.37 (s, 2H). 13C NMR (DMSO-d6/D2O) 192.0, 125.7, 135.0, 134.6, 130.1, 123.9, 111.6. MS m/e 276. Analysis calculated for C18H12O3: C, 91.49; H, 5.12. Found: C, 91.22; H, 5.38.
A protocol similar to that described above was used for the condensation of 4-(N-cyclopentylamidino)-1,2-phenylene diamine with 2,5-bis(4-formylphenyl)furan to give a 77% yield of a yellow solid; mp 295 to 297°C dec. 1H NMR (DMSO-d6/D2O): 8.30 (d, 4H, J = 8.4 Hz), 8.05 (d, 4H, J = 8.4), 8.01 (s, 1H), 7.77 (d, 2H, J = 8.4 Hz), 7.56 (d, 2H, J = 8.4 Hz), 7.27 (s, 2H), 4.15 (br, 2H), 2.13 to 2.03 (m, 4H), 1.81 to 1.55 (m, 12H). 13C NMR (DMSO-d6/D2O): 163.7, 154.0, 153.1, 141.2, 138.6, 132.8, 127.9, 126.1, 124.6, 123.3, 123.1, 115.9, 115.7, 111.0, 55.7, 32.3, 24.4. MS (FAB) m/z 673 (M+ + 1). Analysis calculated for C42H40N8O · 4HCl: C, 61.61; H, 5.42; N, 13.69. Found: C, 62.28; H, 5.74; N, 13.62.
2,5-Bis[2-(5-amidino)benzimidazoyl]benzo[b]furan hydrochloride (compound 55).
A protocol similar to that described above was used for the condensation of benzo[b]furan-2,5-dicarboxaldehyde and 4-amidino-1,2-phenylene diamine hydrochloride hydrate (17, 35) to give a 70% yield of a blue-gray solid; mp, 338 to 340°C dec. 1H NMR (DMSO-d6/D2O): 8.6 (s, 1H), 8.27 (d, 1H, J = 8 Hz), 8.19 (d, 2H, J = 9.6 Hz), 7.89 (d, 1H, J = 8.8 Hz), 7.87 (s, 1H), 7.83 (d, 1H, J = 8.4 Hz), 7.78 (d, 1H, J = 8.4 Hz), 7.73 (d, 1H, J = 8.8 Hz), 7.68 (d, 1H, J = 8 Hz). 13C NMR (DMSO-d6/D2O): 166.4, 165.9, 156.6, 153.5, 147.4, 145.4, 141.6, 139.9, 139.1, 136.9, 128.6, 126.2, 123.3, 122.9, 122.6, 122.2, 122.1, 116.9, 115.8, 115.5, 115.0, 112.8, 108.6. MS (FAB) m/z 435 (M+ + 1). Analysis calculated for C24H18N8O · 3HCl · H2O: C, 51.30; H, 4.12; N, 19.94. Found: C, 51.72; H, 4.14; N, 15.64.
2,5-Bis[2-(5-N-cyclopentylamidino)benzimidazoyl]benzo[b]furan hydrochloride (compound 56).
2-Acetyl-5-bromobenzo[b]furan was prepared as reported in the literature (14) to give an 86% yield; mp, 110 to 111°C (literature mp, 108 to 111°C). 1H NMR (DMSO-d6):8.02 (d, 1H, J = 1.6 Hz), 7.79 (s, 1H), 7.68 (d, 1H, J = 9.2 Hz), 7.65 (dd, 1H, J = 9.2 and 1.6 Hz), 2.58 (s, 3H). 13C NMR (DMSO-d6): 181.6, 153.5, 152.9, 130.9, 128.8, 125.7, 115.9, 114.1, 112.9, 26.2. MS m/z 239 (M+). The acetyl compound was converted into 5-bromobenzo[b]furan-2-carboxylic acid in a 77% yield as reported previously (14); mp, 258 to 262°C (literature mp, 258 to 262°C). 1H NMR (DMSO-d6/D2O): 7.95 (d, 1H, J = 1.6 Hz), 7.64 (d, 1H, J = 8.8 Hz), 7.58 (dd, 1H, J = 8.8 and 1.6 Hz), 7.57 (s, 1H). 13C NMR (DMSO-d6/D2O): 159.5, 153.6, 147.3, 129.9, 128.9, 125.2, 115.8, 113.9, 112.5. MS m/z 241 (M+). 5-Bromobenzo[b]furan-2-carboxylic acid, which was used without further characterization, was converted by standard procedures via the acid chloride into 5-bromo-2-carboxamidobenzo[b]furan in an 81% yield; mp, 208 to 210°C. 1H NMR (DMSO-d6): 8.2 (br s, 1H), 7.99 (s, 1H), 7.77 (br s, 1H), 7.61 (d, 1H, J = 8.8 Hz), 7.57 (dd, 1H, J = 8.8 and 2 Hz), 7.51 (s, 1H). 13C NMR (DMSO-d6/D2O): 159.4, 153.0, 150.5, 129.4, 128.3, 125.2, 115.9, 113.9, 109.0. MS m/e 240 (M+). The amide, which was used without further characterization, was dehydrated with POCl3 to form 5-bromo-2-cyanobenzo[b]furan in an 88% yield; mp, 147 to 148°C. 1H NMR (DMSO-d6): 8.0 (s, 1H), 7.97 (s, 1H), 7.67 (br s, 2H). 13C NMR (DMSO-d6): 153.7, 131.1 127.2, 127.1, 125.2, 118.8, 116.7, 113.7, 111.1. MS m/e 222 (M+). 5-Bromo-2-cyanobenzo[b]furan, which was used without further characterization, was converted via a standard procedure (30) into 2,5-dicyanobenzo[b]furan in a 79% yield; mp, 166 to 167°C. 1H NMR (DMSO-d6): 8.39 (s, 1H), 8.14 (s, 1H), 7.97 (d, 1H, J = 8.8 Hz) 7.92 (d, 1H, J = 8.8 Hz). 13C NMR (DMSO-d6): 156.3, 131.5 128.5, 128.2, 125.8, 119.3, 117.9, 113.4, 110.8, 107.7. MS m/e 168 (M+). Analysis calculated for C10H4N2O: C, 71.42; H, 2.39; N, 16.16. Found: C, 71.78; H, 2.46; N, 16.51. 2,5-Diformylbenzo[b]furan was prepared by reduction of the bis-nitrile with DIBAL, which was added dropwise to a stirred solution of the bis-nitrile (1.68 g, 0.01 mol) in 150 ml of dry methylene chloride under nitrogen. The mixture was stirred for 15 min and allowed to reflux for 40 min, and the mixture was cooled and 100 ml of 1 M H2SO4 was added dropwise while maintaining the solution temperature below 25°C. The methylene chloride layer was separated, the aqueous layer was extracted with 100 ml of methylene chloride, and the combined organic phases were washed with 20% NaHCO3 and dried over Na2SO4. The solvent was removed under reduced pressure, the residue was triturated with ether-hexane (1:1), and the white solid was filtered and dried in a vacuum to yield 1.2 g (69%); mp, 141 to 142°C. 1H NMR (DMSO-d6): 10.1 (s, 1H), 9.92 (s, 1H), 8.48 (d, 1H, J = 0.8 Hz), 8.10 (s, 1H), 8.08 (dd, 1H, J = 0.8 and 8.88 Hz), 7.90 (d, 1H, J = 8.8 Hz). 13C NMR (DMSO-d6): 191.8, 180.6, 158.1, 153.6, 132.9, 128.9, 127.5, 126.9, 118.8, 113.1. Analysis calculated for C10H6N2 · 0.2 H2O: C, 67.75; H, 3.67. Found: C, 67.83; H, 3.59. A protocol similar to that described above was used for the condensation of 4-(N-cyclopentylamidino)-1,2-phenylene diamine with benzo[b]furan-2,5-dicarboxaldehyde to yield a gray solid in a 73% yield; mp, 290 to 292°C dec. 1H NMR (DMSO-d6/D2O): 8.62 (s, 1H), 8.27 (d, 1H, J = 8.8 Hz), 8.08 (s, 1H), 8.05 (s, 1H), 7.95 (d, 1H, J = 8.8 Hz), 7.90 (s, 1H), 7.84 (d, 1H, J = 8.8 Hz), 7.79 (d, 1H, J = 8.4 Hz), 7.64 (d, 1H, J = 8.4 Hz), 7.57 (d, 1H, J = 8.8 Hz), 4.14 (br 2H), 2.13 to 2.06 (m, 4H), 1.81 to 1.56 (m, 12H). 13C NMR (DMSO-d6/D2O): 163.4, 163.1, 156.9, 153.2, 147.6, 145.2, 141.2, 138.8, 136.2, 128.9, 126.5, 124.5, 124.1 123.9, 123.5 122.5, 122.4, 116.9, 116.1, 115.8, 115.7, 115.1, 113.2, 108.8, 54.9, 54.8, 31.7, 23.9. MS (FAB) m/z 571 (M+ + 1). Analysis calculated for C34H34N8O · 4HCl: C, 56.99; H, 5.34; N, 15.64. Found: C, 56.89; H, 5.34; N, 15.53.
2,7-Bis[2-(5-N-isopropylamidino)benzimidazoyl]fluorene hydrochloride (com pound 57).
A mixture of 2,7-dibromofluorene (6.48 g, 0.02 mol) and cuprous cyanide (5.37 g, 0.06 mol) in 35 ml of quinoline was heated under reflux for 2 h (followed by thin-layer chromatography with silica gel [Kodak] and a benzene mobile phase). The mixture was cooled and extracted twice with 150 ml of chloroform; and the organic layer was stirred with 200 ml of 2 M HCl for 2 h, washed with water, dried over anhydrous Na2SO4, filtered, dried and concentrated, redissolved in a minimum amount of chloroform, and chromatographed over neutral alumina. The column was first eluted with hexane-ether (2:1) to remove residual quinoline and finally with ether-CHCl3 (1:1) to afford a fluffy pale solid (2.72 g; 63%); mp 282 to 284°C of 2,7-dicyanofluorene. 1H NMR (DMSO-d6/D2O): 8.16 (d, 2H, J = 8.4 Hz), 8.04 (s, 2H), 7.83 (d, 2H, J = 8.4 Hz), 4.07 (s, 2H). 13C NMR (DMSO-d6/D2O): 144.2, 143.3, 130.8, 128.5, 121.7, 118.5, 110.1, 36.0. MS m/z 216 (M+). Analysis calculated for C15H8N2: C, 83.21; H, 3.72; N, 12.95. Found: C, 83.21; H, 3.78; N, 12.99. To a stirred solution of 2,7-dicyanofluorene (2.16 g, 0.01 mol) in 150 ml of dry CH2Cl2 was added DIBAL (1 M in cyclohexane [4.26 g, 0.03 mol]) under nitrogen at room temperature. The suspension was heated at 40°C for 1 h and cooled, 100 ml of 1 M H2SO4 was added dropwise, the mixture was stirred for 1 h, and the precipitated yellow solid was filtered and recrystallized from CHCl3-ether to yield 2,7-diformylfluorene as pale crystalline solid (1.6 g; 72%); mp, 218 to 220°C. 1H NMR (DMSO-d6/D2O): 10.08 (s, 2H), 8.16 (d, 2H, J = 8.0 Hz), 8.11 (s, 2H), 7.95 (d, 2H, J = 8.0 Hz), 4.10 (s, 2H). 13C NMR (DMSO-d6/D2O): 191.9, 145.0, 144.7, 135.6, 128.5, 125.4, 121.1, 36.0. MS m/e 222 (M+). Analysis calculated for C15H10O2 · 0.1 H2O: C, 80.41; H, 4.49. Found: C, 80.32; H, 4.63. A protocol similar to that described above was used for the condensation of 2,7-diformylfluorene and 4-(N-isopropylamidino)-1,2-phenylene diamine to give a 72% yield of a green solid; mp, 310 to 313°C dec. 1H NMR (DMSO-d6/D2O): 8.53 (s, 2H), 8.34 (d, 2H, J = 8.4 Hz), 8.22 (d, 2H, J = 8.4 Hz), 8.11 (s, 2H), 7.85 (d, 2H, J = 8.4 Hz), 7.65 (d, 2H, J = 8.4 Hz), 4.23 (s, 2H), 4.09 (quintet, 2H, J = 6.4 Hz), 1.33 (d, 12H, J = 6.4 Hz). 13C NMR (DMSO-d6/D2O) 162.2, 153.1, 144.9, 143.5, 138.7, 136.3, 126.9, 125.7, 124.3, 123.6, 121.7, 115.6, 114.7, 45.3, 36.7, 21.1. MS m/z (FAB) 567 (M+ + 1). Analysis calculated for C35H34N2 · 4HCl: C, 58.99; H, 5.37; N, 15.73. Found: C, 59.14; H, 5.59; N, 15.43.
Test organisms.
The fungi tested in the study included two reference strains, C. neoformans var. neoformans H99 and C. albicans A39, and the following clinical isolates: two strains of fluconazole-resistant C. neoformans var. neoformans (strains 135.95 and 114.96); two strains of C. neoformans var. gattii (strains 119.95 and 114.95); two strains of fluconazole-resistant C. albicans (strains 102.96 and 103.96); and one strain each of Torulopsis glabrata (strain 142.91), Candida parapsilosis (strain 111.96), Candida krusei (strain 132.91), Candida tropicalis (strain 110.96), Candida lusitaniae (strain 111.92), Fusarium solani (strain 152.89), Aspergillus fumigatus (strain 168.95), and Aspergillus flavus (strain 112.96).
Medium.
Antifungal susceptibility testing was performed with RPMI 1640 medium (Sigma Chemical Co., St. Louis, Mo.) with glutamine but without sodium bicarbonate and buffered at pH 7.0 with 0.165 M morpholinepropanesulfonic acid.
In vitro susceptibility testing.
Experiments for determination of MICs were performed by the broth macrodilution method according to the recommendations of the National Committee for Clinical Laboratory Standards (29). The only difference compared to the standardized method was the choice of drug dilutions, which ranged from 100 to 0.09 μg/ml. Briefly, this method specifies the use of an inoculum grown at 35°C and adjusted to a concentration of 0.5 × 103 to 2.5 × 103 CFU/ml, incubation of the culture at 35°C, and reading at 48 h for all yeasts except for C. neoformans, for which the results are interpreted at 72 h. The MIC was defined as the culture with the lowest drug concentration in which a visual turbidity less than or equal to 80% inhibition compared to that produced by the growth control tube was observed.
The methods used in experiments for determination of the minimum fungicidal concentration (MFC) were adapted from a method by McGinnis (26). Briefly, 10-μl aliquots from tubes with growth inhibition were plated onto Sabouraud agar plates. The lowest drug concentration that yielded three or fewer yeasts colonies was recorded as the MFC.
Molds were tested by the same method (29) but with the following modifications. Isolates were grown on Sabouraud dextrose agar at 30°C and were subcultured twice to ensure viability. After adequate sporulation occurred (after 4 to 12 days), conidia were harvested by flooding the colonies with a sterile solution of 0.85% NaCl and 0.05% Tween 80 in sterile distilled water. Inocula were prepared with a hemocytometer for counting and were then diluted with RPMI 1640 medium to obtain a final inoculum size of approximately 0.5 × 103 to 2.5 × 103 CFU/ml. The inoculum size was verified by plating an aliquot of the inoculum. The cultures were incubated at 30°C for 48 to 72 h or until growth in the control tube was visible.
In each experiment, the quality control included the testing of C. albicans A39 and C. neoformans var. neoformans H99 with fluconazole and amphotericin B.
RESULTS
The MICs of fluconazole and amphotericin B for C. albicans A39 and C. neoformans var. neoformans H99 were determined and served as positive controls for comparison with the experimental compounds. The MICs of fluconazole for C. albicans A39 and C. neoformans var. neoformans H99 were 0.25 and 2 μg/ml, respectively. The MIC of amphotericin B was 1 μg/ml for both isolates.
Pentamidine analogs.
The in vitro activities of pentamidine and its analogues are summarized in Table 1. Because the compounds appear to have fungicidal activity, both the MICs and the MFCs are reported in Table 1.
It is noteworthy that pentamidine exhibits inhibitory activity that is comparable to those of both amphotericin B and fluconazole against both C. albicans and C. neoformans. In addition, the MFCs of pentamidine for both organisms were determined and they were found to be in the low concentration range. The variation of the alkyl chain length of pentamidine (compounds 1 and 3) resulted in a decrease in activity against C. albicans. Likewise, the two-carbon analogue (compound 1) had significantly reduced activity against C. neoformans compared to that of the parent molecule. Substitution of methoxy groups meta to the amidine moieties (compare compound 3 to compound 4 and compound 11 to compound 12) produced a reduction in activity against both organisms. Likewise, substitution of amino groups meta to the amidino groups gave a reduction in activity for the two- and three-carbon-chain analogs (compare compound 1 to compound 2 and compound 3 to compound 9). Substitution of chloro groups meta to the amidine moieties of pentamidine (compound 17) resulted in a reduction in activity against C. albicans, while it afforded a modest increase in activity against C. neoformans. Moving the amidino groups from the para to the meta positions with regard to the ether link produced a marked reduction in activity against C. albicans for the three-carbon analogues (compare compounds 3 and 7). However, the same alteration in the four carbon analogues (compare compounds 11 and 16) resulted in modestly increased activity against C. albicans and a decrease in potency against C. neoformans. Perhaps the most striking structure-activity effects occurred when the oxygens of pentamidine were replaced by nitrogens (compound 18). The activities of the amino isosteres showed a marked improvement over that of pentamidine against C. albicans and some improvement over that of pentamidine against C. neoformans. A similar result was noted with the three-carbon-chain analogue (compare compounds 3 and 8) against C. albicans. A final observation for the pentamidine analogues was that the conversion of the cationic moieties from an amidino to an imidazolino group (compare compounds 11 and 14) produced an increase in antifungal activity against C. albicans.
Pentamidine metabolites.
Because pentamidine is rapidly metabolized in the body to at least seven primary metabolites, it was important to determine the antifungal effects of the known metabolites (Table 2). When compared to pentamidine, the chain-hydroxylated metabolites (compounds 21 and 22) have approximately 10-fold reductions in their inhibitory activities against C. albicans and a 2- to 4-fold reductions in their inhibitory activities against C. neoformans. However, hydroxylation of either one (compound 27) or both (compound 23) of the amidine groups results in no significant activity against either organism when the compounds are used at 100 μg/ml. Likewise, all metabolites resulting from the cleavage of an ether bond of pentamidine (compounds 24 to 26) have little or no activity at the highest dose tested.
Bis-benzimidazoles.
A number of dication-substituted bis-benzimidazole derivatives have been shown to have excellent activities against P. carinii pneumonia in the rat model of disease (20). For this reason we tested a large series of these compounds against C. albicans and C. neoformans (Table 3). Structural variations of the compound were accomplished by altering the bridge connecting the 2-benzimidazolyl moieties (X in Table 3) and changes in the structure of the cationic groups (Y in Table 3). Overall, several very potent compounds were found in this series. In general the bis-benzimidazole bridged by an alkane chain showed poor antifungal activity (compounds 28 to 34). Only the benzimidazole joined by ethene bridge (compound 32) exhibited activity within a log range of the control drugs. Connecting 5-amidino-2-benzimadazoyl groups with a 2,5-furanyl link (compound 35) produced a compound with potent inhibitory activity against both isolates. In addition, the MFCs of the compound for C. albicans and C. neoformans were 0.78 and 3.12 μg/ml, respectively. Alteration of the amidino cationic moieties (compounds 36 to 38) resulted in reduced activity. Isosteric replacement of the furan oxygen (compound 35) with nitrogen produced the corresponding pyrrole isostere (compound 39). Comparison of the isosteres (compounds 35 and 39) showed that they had very similar activities against both yeasts. In the case of the pyrrole-linked compounds, no difference was seen when the amidine was replaced by a 2-imidazoline moiety (compound 40). However, the other alterations of the cation groups (compounds 41 and 42) caused a decrease in activity. Methylation of the pyrrole nitrogen (compare compounds 39 and 43) had little effect on the activity against either organism. Interestingly, the cyclopentyl substitution of the amidino group (compound 45) on the N-methyl pyrrole-bridged compound produced one of the most potent inhibitors of C. neoformans (MIC, 0.19 μg/ml).
Only one thiophene-linked compound was included in this study (compound 46). However, this compound was highly effective against C. albicans (MIC, 0.78 μg/ml) and gave the highest activity of any of the compounds against C. neoformans (MIC, 0.19 μg/ml) and the fungicidal concentrations for these yeasts were the same as the MICs.
Bridging the benzimidazole rings with a 1,4-phenyl group produced compounds (compounds 47 to 49) with various antifungal activities. The most remarkable finding within this group was the highly selective activity of the amidino-substituted compound (compound 47) toward the two organisms. Compound 47 was highly effective against C. neoformans, while at 100 μg/ml it was ineffective against C. albicans. The addition of methyl groups to the phenyl bridge to give a p-xylene link (compound 50) resulted in decreased antifungal activity. Bridging of the benzimidazoles with a 2,6-pyridine linker gave two compounds (compounds 51 and 52) with interesting activities. At 100 μg/ml, the amidine-substituted analogue (compound 51) showed no activity against either organism, while at 1.56 μg/ml the imidazoline-substituted compound (compound 52) exhibited good activity against both organisms. This is in contrast to the furan- or pyrrole-bridged compounds, for which little difference in the activities of the imidazoline and amidine derivatives was noted.
The last five compounds in Table 3 (compounds 53 to 57) feature multiring bridges between the benzimidazole rings. All members of this group show good potency against both organisms. The most noteworthy of these compounds has a bridge consisting of a 2,5-benzo[b]furanyl group and amidino cationic moieties (compound 55). At concentrations under 1 μg/ml the compound exhibits excellent inhibitory and fungicidal activities against both yeasts.
Broad-spectrum activities of selected compounds.
In order to examine the spectrum of activity of these compounds, we selected, on the basis of compound activity and availability, compounds 39 and 57 for testing against other pathogenic fungi (Table 4). The results of the tests against filamentous fungi showed that compound 39 is highly effective against Aspergillus fumigatus, while it shows poor activity against Aspergillus flavus. Compound 39 also showed excellent in vitro activity against Fusarium solani, a mold with increasing clinical relevance and against which few treatment options exist. Moreover, these results indicate that compounds 39 and 57 have excellent breadths of antifungal activity against Candida species other than C. albicans and also against fluconazole-resistant strains of C. albicans and C. neoformans.
DISCUSSION
Examination of the in vitro antifungal data for the series of 57 compounds described here reveals a number of structures that have activities equal to or greater than those of the control drugs (fluconazole and amphotericin B). The MICs were determined by the standardized methods of the National Committee for Clinical Laboratory Standards. On the other hand, the method of determination of MFCs was adapted to the method of determination of MICs and, although it is not standardized, showed the apparent fungicidal activities of some compounds, even though fluconazole does not show fungicidal activity by this assay. The potential fungicidal properties of these compounds will need to be assessed with animal models. Furthermore, selected compounds also appear to have a wide spectrum of activity, including fungicidal activity against fluconazole-resistant strains of C. albicans and C. neoformans. The only data detailing the toxicity of this class of compounds in humans are based on the findings with pentamidine (22, 34, 39). While pentamidine has been associated with a number of adverse side effects, it has been proven to be effective in the treatment of the fungal infection pneumocystosis. It is encouraging that a large number of the pentamidine derivatives have been tested with animal models, and the findings indicate that the majority of the compounds are considerably less toxic than the parent compound (20, 23, 31, 34, 38). Furthermore, it has been shown in the studies cited above that host cell toxicity and animal toxicity are not linked to the same structural refinements that impart enhanced antimicrobial activity. Given the in vitro antifungal activity and recent toxicology findings, it is likely that dication-substituted compounds with an acceptable antifungal activity to toxicity ratio could be developed.
While pentamidine appears to be a potent in vitro antifungal agent, the development of pentamidine as a therapeutic drug may be limited due in part to its known toxic side effects in humans but may be even more likely to be limited due to metabolism of the parent drug. For example, it has been demonstrated that pentamidine is readily metabolized to at least seven primary metabolites (7). It can be seen from the data in Table 2 that all of the primary metabolites of pentamidine have reduced antifungal activity and that the metabolites resulting from the breaking of the ether bond or hydroxylation of an amidino nitrogen are devoid of activity. In addition to the loss of activity, recent work in our laboratory indicates that certain metabolites have increased toxicity (unpublished data). Unfortunately, metabolic breakup of the parent molecule may be a problem encountered with many of the direct pentamidine analogues, especially those that contain an ether bond in the bridge between the cationic moieties. An advantage to the bis-benzimidazole compounds would be the lack of a cleavage product resulting from primary metabolism.
A key to the further development of these antifungal compounds will be establishment of their mechanisms of action. It has been proposed that the activity of this class of compounds against G. lamblia is a result of their binding in the minor grooves of DNA followed by inhibition of topoisomerase II (4). While this mechanism appears to be likely for G. lamblia, no direct correlation was found between the anti-topoisomerase II inhibition of these compounds and their activity against Cryptosporidium parvum (9) or P. carinii (16). It has been hypothesized that the primary cellular target of pentamidine in the yeast Saccharomyces cerevisiae is the mitochondrion (25), and more recently, it has been suggested that these compounds have an effect on another fungus, P. carinii, due to their DNA binding followed by inhibition of an endo- or exonuclease (19). A major source of the problem in determining the mechanisms of action against P. carinii and C. parvum is the lack of effective in vitro culture systems. Such a problem would not exist for most classical fungal organisms since they are readily grown in vitro. Studies to determine the mechanisms of action of these compounds against selected fungi should have a high probability of success.
In conclusion, the data presented in this report indicate the potential of dicationic molecules as antifungal agents. A number of new molecules have been identified from this initial study of structure-function relationships, and these new molecules require future synthesis and antifungal testing. An example of this need to focus our attention is the development of a complete series of compounds with a thiophene as the linking group (compound 46). It is clear that further studies on the structure-activity relationships, mechanisms of action and toxicities, and in vivo efficacies of these compounds are warranted to determine their clinical potential.
ACKNOWLEDGMENTS
This work was supported by an NIH Program Project (NAIAD AI-33363) and awards from Pharm-Eco Pharmaceutical, Inc., Lexington, Mass., and Immtech International, Inc., Evanston, Ill.
We appreciate the clerical expertise and assistance of Vicki Wingate.
REFERENCES
- 1.Bajic M, Boykin D W. Synthesis of 2,4-bis[4-(5-amidino and 5-substituted amidino benzimidazoyl)]pyrimidines. Heterocycl Comm. 1995;1:225–230. [Google Scholar]
- 2.Bajic M, Kumar A, Boykin D W. Synthesis of 2,5-bis-(4-cyanophenyl)-furan. Heterocycl Comm. 1996;2:135–140. [Google Scholar]
- 3.Barchiesi F, Del Poeta M, Morbiducci V, Ancarani F, Scalise G. Effect of pentamidine on the growth of Cryptococcus neoformans. J Antimicrob Chemother. 1994;33:1229–1232. doi: 10.1093/jac/33.6.1229. [DOI] [PubMed] [Google Scholar]
- 4.Bell C A, Cory M, Fairley T A, Hall J E, Tidwell R R. Structure-activity relationships of pentamidine analogs against Giardia lamblia and correlation of antigiardial activity with DNA-binding affinity. Antimicrob Agents Chemother. 1991;35:1099–1107. doi: 10.1128/aac.35.6.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bell C A, Dykstra C C, Naiman N N, Cory M, Fairley T A, Tidwell R R. Structure-activity studies of dicationic substituted bis-benzimidazoles against Giardia lamblia: correlation of antigiardial activity with DNA binding affinity and giardial topoisomerase II inhibition. Antimicrob Agents Chemother. 1993;37:2668–2673. doi: 10.1128/aac.37.12.2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bell C A, Hall J E, Kyle D E, Grogl M, Ohemeng K A, Allen M A, Tidwell R R. Structure-activity relationships of analogs of pentamidine against Plasmodium falciparum and Leishmania mexicana amazonensis. Antimicrob Agents Chemother. 1990;34:1381–1386. doi: 10.1128/aac.34.7.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berger B J, Naiman N A, Hall J E, Peggins J, Brewer T G, Tidwell R R. Primary and secondary metabolism of pentamidine by rats. Antimicrob Agents Chemother. 1992;36:1825–1831. doi: 10.1128/aac.36.9.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berger B J, Reddy V V, Le S T, Lombardy R J, Hall J E, Tidwell R R. Hydroxylation of pentamidine by rat liver microsomes. J Pharmacol Exp Ther. 1991;256:883–889. [PubMed] [Google Scholar]
- 9.Blagburn B L, Sundermann C A, Lindsay D S, Hall J E, Tidwell R R. Inhibition of Cryptosporidium parvum in neonatal Hsd:(ICR)BR Swiss mice by polyether ionophores and aromatic amidines. Antimicrob Agents Chemother. 1991;35:1520–1523. doi: 10.1128/aac.35.7.1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boykin D W, Kumar A, Spychala J, Zhou M, Lombardy R J, Wilson W D, Dykstra C C, Hall J E, Jones S K, Tidwell R R, Laughton C, Neidle S. Dicationic diaryl furans as anti-Pneumocystis carinii agents. J Med Chem. 1995;38:912–916. doi: 10.1021/jm00006a009. [DOI] [PubMed] [Google Scholar]
- 11.Cameron M L, Schell W A, Bruch S, Bartlett J A, Waskin H A, Perfect J R. Correlation of in vitro fluconazole resistance of Candida isolates in relation to therapy and symptoms of individuals seropositive for human immunodeficiency virus type 1. Antimicrob Agents Chemother. 1993;37:2449–2453. doi: 10.1128/aac.37.11.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cresp T M, Sargent M V. Synthesis and paratropicity of heteroatom-bridged [17]annulenones. J Chem Soc Perkin Trans I. 1973;1:2961–2971. [Google Scholar]
- 13.Dann O, Bergen G, Demant E, Volz G. Trypanocide Diamidine des 2-Phenyl-Benzofurans, 2-Phenyl-Indens und 2-Phenyl-Indols. Liebigs Ann Chem. 1971;749:68–89. [Google Scholar]
- 14.Dann O, Fernback R, Pfeifer W, Demant E, Bergen G, Lang S, Lurding G. Trypanocide Diamidine mit drei Ringen in zwei isolierten Ringsystemen. Liebigs Ann Chem. 1972;760:37–87. doi: 10.1002/jlac.19727600105. [DOI] [PubMed] [Google Scholar]
- 15.Das B P, Boykin D W. Synthesis and antiprotozoal activity of 2,5-bis(4-guanylphenyl)furans. J Med Chem. 1977;20:531–536. doi: 10.1021/jm00214a014. [DOI] [PubMed] [Google Scholar]
- 16.Dykstra C C, McClernon D R, Elwell L P, Tidwell R R. Selective inhibition of topoisomerases from Pneumocystis carinii versus topoisomerases from mammalian cells. Antimicrob Agents Chemother. 1994;38:1890–1898. doi: 10.1128/aac.38.9.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fairley T, Tidwell R R, Donkor I, Naiman N, Ohemeng K, Lombardy R, Bentley J, Cory M. Structure, DNA minor groove binding, and base pair specificity of alkyl- and aryl-linked bis(amidinobenzimadazoles) and bis(amidinoindoles) J Med Chem. 1993;36:1746–1753. doi: 10.1021/jm00064a008. [DOI] [PubMed] [Google Scholar]
- 18.Fox R, Neal K R, Leen C L S, Ellis M E, Mandal B K. Fluconazole resistant Candida in AIDS. J Infect. 1991;22:201–204. doi: 10.1016/0163-4453(91)91767-r. [DOI] [PubMed] [Google Scholar]
- 18a.Hall J E, Kerrigan J E, Ramachandran K, Bender B C, Stanko J P, Jones S K, Patrick D A, Tidwell R R. Anti-Pneumocystis activities of aromatic diamidoxime prodrugs. Antimicrob Agents Chemother. 1998;42:666–674. doi: 10.1128/aac.42.3.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hildebrandt E F, Boykin D W, Tidwell R R, Dykstra C C. Identification and characterization of an endo/exonuclease in Pneumocystis carinii that is inhibited by dicationic diaryl furans with efficacy against pneumocystis pneumonia. J Eukaryot Microbiol. 1998;45:112–121. doi: 10.1111/j.1550-7408.1998.tb05078.x. [DOI] [PubMed] [Google Scholar]
- 20.Jones S K, Hall J E, Allen M A, Morrison S D, Ohemeng K A, Reddy V V, Geratz J D, Tidwell R R. Novel pentamidine analogs in the treatment of experimental Pneumocystis carinii pneumonia. Antimicrob Agents Chemother. 1990;34:1026–1030. doi: 10.1128/aac.34.6.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keku T O, Seed J R, Tidwell R R. The in vitro HLK-60 cell-Trypanosoma brucei rhodesiense culture system: a rapid in vitro drug screen. Trop Med and Parasitol. 1995;46:258–262. [PubMed] [Google Scholar]
- 22.Kovacs J A, Hiemenz J W, Macher A M, Stover D, Murray H W, Shelhammer J, Lane H C, Urmacher C, Honig C, Longo D L, Parker M M, Natanson C, Parrillo J E, Fauci A S, Pizzo P A, Masur H. Pneumocystis carinii pneumonia: a comparison between patients with the acquired immunodeficiency syndrome and patients with other immunodeficiencies. Ann Intern Med. 1984;100:663–671. doi: 10.7326/0003-4819-100-5-663. [DOI] [PubMed] [Google Scholar]
- 23.Kumar A, Boykin D W, Wilson W D, Jones S K, Bender B K, Dykstra C C, Hall J E, Tidwell R R. Anti-Pneumocystis carinii pneumonia activity of dicationic 2,4-diarylpyrimidines. Eur J Med Chem. 1996;31:767–773. doi: 10.1016/0223-5234(96)83970-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lindsay D S, Blagburn B L, Hall J E, Tidwell R R. Activity of pentamidine and pentamidine analogs against Toxoplasma gondii in cell cultures. Antimicrob Agents Chemother. 1991;35:1914–1916. doi: 10.1128/aac.35.9.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ludewig G, Marin J, Li Y, Staben C. Effects of pentamidine isethionate on Saccharomyces cerevisiae. Antimicrob Agents Chemother. 1994;38:1123–1128. doi: 10.1128/aac.38.5.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McGinnis M R. Susceptibility testing and bioassay procedure. New York, N.Y: Academic Press, Inc.; 1980. Susceptibility testing and bioassay procedure; p. 431. [Google Scholar]
- 27.Miller R, Olsson K. A convenient synthesis of pyrrole-2,5-dicarbonaldehyde. Acta Chem Scand Sect. 1981;35B:303–304. [Google Scholar]
- 28.Morikawa S. Synthesis of hydroxymethylfural and 2,5-furandicarboxaldehydes. Noguchi Kenkyusho Jiho. 1979;21:25–33. [Google Scholar]
- 29.National Committee for Clinical Laboratory Standards. Reference method for broth dilution susceptibility testing of yeasts. Document M27-T. 1995. Tentative standard. National Committee for Clinical Laboratory Standards, Wayne, Pa. [Google Scholar]
- 30.Newman M S, Boden J. N-Methylpyrrolidone as solvent for reaction of aryl halides with cuprous cyanide. J Org Chem. 1961;26:2525. [Google Scholar]
- 31.Patrick D A, Boykin D W, Wilson W D, Tanious F A, Spychala J, Bender B C, Hall J E, Dykstra C C, Ohemeng K A, Tidwell R R. Anti-Pneumocystis carinii pneumonia activity of dicationic carbazoles. Eur J Med Chem. 1997;32:781–793. [Google Scholar]
- 32.Pfaller M A, Rhine-Chalberg A J, Redding S W, Smith J, Farinacci G, Fothergill A W, Rinaldi M G. Variations in fluconazole susceptibility and electrophoretic karyotype among oral isolates of Candida albicans from AIDS and oral candidiasis. J Clin Microbiol. 1994;32:59–64. doi: 10.1128/jcm.32.1.59-64.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.St.-Germain G. Effects of pentamidine alone and in combination with ketoconazole or itraconazole on the growth of Candida albicans. Antimicrob Agents Chemother. 1990;34:2304–2306. doi: 10.1128/aac.34.12.2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tidwell R R, Bell C A. Pentamidine and related compounds in the treatment of Pneumocystis carinii infection. In: Walzer P D, editor. Pentamidine and related compounds in the treatment of Pneumocystis carinii Infection. New York, N.Y: Marcel Dekker, Inc.; 1993. pp. 561–583. [Google Scholar]
- 35.Tidwell R R, Geratz J D, Dann O, Volz G, Zeh D, Loewe H. Diarylamidine derivatives with one or both of the aryl moieties consisting of an indole or indole-like ring. Inhibitors of arginine-specific esteroproteases. J Med Chem. 1978;21:613–623. doi: 10.1021/jm00205a005. [DOI] [PubMed] [Google Scholar]
- 36.Tidwell R R, Jones S K, Geratz J D, Ohemeng K A, Bell C A, Berger B J, Hall J E. Development of pentamidine analogues as new agents for the treatment of Pneumocystis carinii pneumonia. Ann N Y Acad Sci. 1990;616:421–441. doi: 10.1111/j.1749-6632.1990.tb17862.x. [DOI] [PubMed] [Google Scholar]
- 37.Tidwell R R, Jones S K, Geratz J D, Ohemeng K A, Cory M, Hall J E. Analogues of 1,5-bis(4-amidinophenoxy)pentane (pentamidine) in the treatment of experimental Pneumocystis carinii pneumonia. J Med Chem. 1990;33:1252–1257. doi: 10.1021/jm00166a026. [DOI] [PubMed] [Google Scholar]
- 38.Tidwell R R, Jones S K, Naiman N A, Berger L C, Brake W B, Dykstra C C, Hall J E. Activity of cationically substituted bis-benzimidazoles against experimental Pneumocystis carinii pneumonia. Antimicrob Agents Chemother. 1993;37:1713–1716. doi: 10.1128/aac.37.8.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walzer P D, Perl D P, Krogstad D J, Rawson P G, Schultz M G. Pneumocystis carinii pneumonia in the United States. Epidemiologic, diagnostic, and clinical features. Ann Intern Med. 1974;80:83–93. doi: 10.7326/0003-4819-80-1-83. [DOI] [PubMed] [Google Scholar]
- 40.Willocks L, Leen C L S, Bretle R P, Urquhart D, Russel T B, Milne L J R. Fluconazole resistance in AIDS patients. J Antimicrob Chemother. 1991;28:937–939. doi: 10.1093/jac/28.6.937. [DOI] [PubMed] [Google Scholar]