Abstract
Background
Free radicals are very reactive molecules produced during oxidation events that in turn initiate a chain reaction resulting in cellular damage. Many degenerative diseases in humans, including cancer and central nervous system damage, are caused by free radicals. Scientific evidence indicates that active compounds from natural products can protect cells from free radical damage. As a result, the aim of this review is to provide evidence of the use of diverse Ethiopian medicinal plants with antioxidant properties that have been scientifically validated in order to draw attention and foster further investigations in this area.
Methods
The keywords antioxidant, radical scavenging activities, reactive oxygen species, natural product, Ethiopian Medicinal plants, and 2, 2-Diphenyl-1-picrylhydrazyl radical scavenging assay (DPPH) were used to identify relevant data in the major electronic scientific databases, including Google Scholar, ScienceDirect, PubMed, Medline, and Science domain. All articles with descriptions that were accessed until November 2022 were included in the search strategy.
Results
A total of 54 plant species from 33 families were identified, along with 46 compounds isolated. More scientific studies have been conducted on plant species from the Brassicaceae (19%), Asphodelaceae (12%), and Asteraceae (12%) families. The most used solvent and extraction method for plant samples are methanol (68%) and maceration (88%). The most examined plant parts were the leaves (42%). Plant extracts (56%) as well as isolated compounds (61%) exhibited significant antioxidant potential. The most effective plant extracts from Ethiopian flora were Bersama abyssinica, Solanecio gigas, Echinops kebericho, Verbascum sinaiticum, Apium leptophyllum, and Crinum abyssinicum. The best oxidative phytochemicals were Rutin (7), Flavan-3-ol-7-O-glucoside (8), Myricitrin (13), Myricetin-3-O-arabinopyranoside (14), 7-O-Methylaloeresin A (15), 3-Hydroxyisoagatholactone (17), β-Sitosterol-3-O-β-D-glucoside (22), Microdontin A/B (24), and Caffeic acid (39).
Conclusion
Many crude extracts and compounds exhibited significant antioxidant activity, making them excellent candidates for the development of novel drugs. However, there is a paucity of research into the mechanisms of action as well as clinical evidence supporting some of these isolated compounds. To fully authenticate and then commercialize, further investigation and systematic analysis of these antioxidant-rich species are required.
1. Introduction
The generation of reactive oxygen species (ROS) and other free radicals during metabolism is a natural activity that is adequately compensated for by an elaborate endogenous antioxidant defense mechanism [1]. Oxidative stress results from the overproduction of free radicals and an imbalance in their elimination. In diseases including cancer, cardiovascular disease, inflammatory disease, and cataract development, oxidative damage at the cellular or subcellular level is now considered a major event. Reactive oxygen radicals exert an adverse effect on cells due to their ability to promote lipid peroxidation in cellular membranes, which results in lipid peroxides that severely damage membranes and cause chromosomal damage through membrane contact [2, 3]. Hydrogen peroxide, superoxide anion, and hydroxyl radicals are examples of oxygen free radicals that have been linked to the development of several pathological disorders, including diabetes, atherosclerosis, ischemia, and inflammatory diseases. In many cases, the first stage of these disorders is endothelial cell damage. These oxidants can be immediately scavenged by the antioxidant enzymes superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPX), which are present intracellular or released into the extracellular milieu. They can also prevent these oxidants from becoming toxic species. It is well known that ROS and reactive metabolic intermediates produced by different chemical carcinogens play a significant role in cell damage as well as the beginning and development of carcinogenesis. In recent decades, there has been a growing understanding of the connection between nutrition and chronic diseases, particularly cancer and cardiovascular disorders. Many degenerative diseases, including cancer, cataract, type 2 diabetes, neurological diseases, cardiovascular diseases, and inflammatory diseases, as well as the natural aging process, are now thought to be primarily caused by oxidative stress. Consequently, there is currently a lot of interest in the potential role of natural antioxidants in delaying or suppressing oxidative stress [4, 5]. Exogenous antioxidants need to be consumed or taken as supplements to maintain the body's endogenous antioxidant system. It has been appreciated that both nutrient and non-nutrient-rich diet components have antioxidant capabilities and consequent potential benefits. There has been a growing interest in natural antioxidants found abundantly in plants [6, 7]. Since the dawn of human civilization, medicinal plants have been identified and customarily used throughout the world [8, 9].
Medicinal plants are a rich source of novel drugs that form the ingredients in traditional systems of medicine [10, 11]. Most developing countries rely on traditional medicinal plants for their healthcare. Therefore, it should come as no surprise that some of these plants contain chemical compounds that have therapeutic potential and could be utilized to treat serious diseases like malaria, cancer, and pathogenic microbes [12]. According to studies, more than 80% of Ethiopians use plant-based traditional medicine as their primary healthcare system. This high adoption rate can be largely ascribed to the fact that it draws on locally accessible wild plant resources [13, 14]. This is in part because the vast majority of rural residents cannot access modern medical services because of their high cost, lack of transportation, and scarcity of healthcare centers [15]. However, the limited number of medicinal plants has been the focus of the available reviews on the antioxidant potential of Ethiopian natural products [16]. In spite of this, there is a paucity of comprehensive ethnopharmacological research review on Ethiopian antioxidant medicinal herbs. This review examined the phytochemistry of the plants used in traditional Ethiopian medicine as well as numerous investigations that have been done to scientifically validate their antioxidant potential. This evaluation may pave the way for additional complementary studies as well as the development of some readily available and affordable antioxidant phytomedicines, in line with the objectives of the WHO's “Traditional Medicine Strategy” [17].
2. Methodology
This review was compiled from various databases, including Google Scholar, ScienceDirect, PubMed, Medline, and Science domain from September 2022 to November 2022, to identify natural products from Ethiopian flora and fauna with antioxidant potential. Each database search was done independently. Until November 2022, original studies about antioxidant plants that were published in peer-reviewed journals were included in the study databases. The keywords antioxidant, radical scavenging activities, antiaging principles, reactive oxygen species, free radicals, natural product, 2, 2-Diphenyl-1-picrylhydrazyl radical scavenging assay (DPPH), and reducing properties were used to identify relevant data. All valuable data previously published in English have been gathered. The reviewers found relevant articles and gathered the following information from them: plant species, plant family, parts of the plant used, extraction methods, extraction solvent, IC50 values, and isolated compounds.
2.1. Categorization of Antioxidant Activities
For evaluating the in vitro antioxidant potencies of natural compounds and extracts, many techniques have been developed. These techniques are based on two important chemical processes: electron transfer reactions and hydrogen atom reactions. Electron transfer reactions are used to measure the following parameters to determine the antioxidant potencies of extracts and compounds using hydrogen atom transfer mechanisms: ferric reducing antioxidant power (FRAP), diphenyl-2-picryl-hydroxyl radical scavenging assay (DPPH), Trolox equivalent antioxidant capacity (TEAC), hydroxyl radical scavenging assay, superoxide anion radical scavenging assay, and nitric oxide radical scavenging [18]. Despite the recent increase in interest in antioxidant studies, it has been difficult to evaluate research findings from various research groups due to a lack of standardized assays [19]. To increase the reliability of the antioxidant results, more than one protocol was used, and the antioxidant potencies of natural products reviewed in this study were classified into three groups based on previous studies: high or significant antioxidant capacity with IC50 < 50 μg/mL (extract) or IC50 < 10 μg/mL (compounds), moderate antioxidant capacity with 50 < IC50 < 100 μg/mL (extract) or 10 < IC50 < 20 μg/mL (compounds), and low antioxidant capacity with IC50 > 100 μg/mL (extract) or IC50 > 20 μg/mL (compounds) [16, 20]. All activity data were converted to IC50 values in μg/mL.
3. Result and Discussion
3.1. Promising Antioxidant Medicinal Plants from the Ethiopian Flora
The in vitro antioxidant activities of extracts from 54 plant species from 33 plant families were identified . Table 1 provides a summary of the plant species that were tested, their family, the portions of the plants that were utilized to generate the test samples, the solvent used during the extraction process, the assay methods, and their potencies based on the categorization/protocol used. This shows that Ethiopia has a diverse flora and that numerous people use several plant species for medicinal purposes [59]. Asteraceae 6 (19%), Brassicaceae 4 (12%), and Asphodelaceae 4 (12%) are the three plant families with the greatest antioxidant activity studied in Ethiopia (Figure 1 and Table 1).
Table 1.
Antioxidant potential of plant extracts from Ethiopian flora.
| Plant | Family | Plant part investigated | Extraction method | Solvents | Assay methods | Inhibition/IC50 | Antioxidant potential | Ref |
|---|---|---|---|---|---|---|---|---|
| Hypoestes forskaolii | Acanthaceae | Dried leaves | Maceration | Methanol | DPPH | 15.7 μg/mL | Significant | [21] |
| Achyranthes aspera | Amaranthaceae | Dried leaves | Maceration | Distilled water | DPPH | 13510 μg/mL | Low | [22] |
| Amaranthus hybridus | Amaranthaceae | Dried seeds | Maceration extraction | Methanol | DPPH | 197.22 μg/mL | Low | [23] |
| Crinum abyssinicum | Amaryllidaceae | Dried roots | Maceration extraction | DCM/methanol (1 : 1) | DPPH | 4.1 μg/mL | Significant | [24] |
| Apium leptophyllum | Apiaceae | Dried leaves | Hydrodistillation | Oil | DPPH | 4.3 μl/mL | Significant | [25] |
| Trachyspermum ammi | Apiaceae | Dried seeds | Maceration technique | Methanol | DPPH | 74.4 μg/mL | Moderate | [26] |
| Calotropis procera | Apocynaceae | Dried roots | Maceration extraction | Methanol | DPPH | 4.3 μg/mL | Significant | [24] |
| Gomphocarpus fruticosus | Apocynaceae | Dried leaves | Maceration extraction | Distilled water | DPPH | 1640 μg/mL | Low | [22] |
| Dracaena angustifolia | Asparagaceae | Dried leaves | Maceration extraction | Methanol | DPPH | 25.59 μg/mL | Significant | [27] |
| Aloe debrana | Asphodelaceae | Dried roots | Simultaneous distillation extraction | Distilled water and CH2Cl2 | DPPH, H2O2 | 48.65 and 51.97 μg/mL respectively | Significant, moderate | [28] |
| Aloe harlana | Asphodelaceae | Latex | — | — | DPPH | 14.21 μg/mL | Significant | [29] |
| Aloe pulcherrima | Asphodelaceae | Dried leaves | Maceration extraction | Distilled water | DPPH | 420 μg/mL | Low | [22] |
| Aloe schelpei | Asphodelaceae | Leaves' latex | — | — | DPPH | 25.3 μg/mL | Significant | [30] |
| Cineraria abyssinica | Asteraceae | Dried leaves | Maceration | Aqueous and methanol | DPPH | 6.73 and 5.78 μg/mL | Significant | [31] |
| Echinops kebericho | Asteraceae | Dried roots | Maceration extraction | Methanol crude extract and acetone fraction | DPPH | 5.89 and 4.11 μg/mL respectively | Significant | [32] |
| Haplocarpha rueppelii | Asteraceae | Dried leaves | Maceration extraction | Methanol | DPPH | 35.2 μg/mL | Significant | [23] |
| Haplocarpha schimperi | Asteraceae | Dried leaves | Maceration extraction | Methanol | DPPH | 64.52 μg/mL | Moderate | [23] |
| Laggera tomentosa | Asteraceae | Dried roots | Maceration extraction | EtOAc, and MeOH | DPPH | 9.4 and 29 μg/mL respectively | Significant | [33] |
| Solanecio gigas | Asteraceae | Dried stem bark | Maceration extraction | Methanol | DPPH | 4.2 μg/mL | Significant | [34] |
| Brassica carinata | Brassicaceae | Dried seeds | Maceration | Methanol | DPPH | 5.85 mg/mL | Significant | [35] |
| Eruca sativa | Brassicaceae | Dried leaves | Maceration technique | Methanol | DPPH | 150 μg/mL | Low | [36] |
| Erucastrum abyssinicum | Brassicaceae | Dried leaves | Maceration extraction | Methanol | DPPH | 100.58 μg/mL | Low | [23] |
| Raphanus sativus | Brassicaceae | Dried leaves, roots | Maceration technique | Methanol | DPPH | 160 and 450 μg/mL respectively | Low | [36] |
| Cucumis prophetarum | Cucurbitaceae | Dried roots | Maceration extraction | Methanol | DPPH | 28.9 μg/mL | Significant | [37] |
| Euclea racemosa | Ebenaceae | Dried leaves | Soxhlet | Acetone | DPPH | 11.3 μg/mL | Significant | [38] |
| Croton macrostachyus | Euphorbiaceae | Dried root barks | Maceration | Ethanol | DPPH | 128.6 μg/mL | Low | [39] |
| Albizia lebbeck | Fabaceae | Dried stem bark | Maceration extraction | Methanol | DPPH | 156 μg/mL | Low | [40] |
| Rhynchosia ferruginea | Fabaceae | Dried roots | Maceration extraction | CH2Cl2/CH3OH | DPPH | 17.7 μg/mL | Significant | [41] |
| Bersama abyssinica | Francoaceae | Dried leaves | Maceration extraction, Soxhlet | Methanol | DPPH | 5.35 and 7.5 μg/mL | Significant | [38, 42] |
| Salvia officinalis | Lamiaceae | Dried aerial parts | Hydrodistillation | Oil | DPPH | 4.65 μg/mL | Significant | [43] |
| Satureja punctata | Lamiaceae | Dried aerial parts | Maceration extraction | Distilled water | DPPH | 10 μg/mL | Significant | [22] |
| Thymus schimperi | Lamiaceae | Dried leaves | Maceration technique | Methanol | DPPH | 60.1 μg/mL | Moderate | [26] |
| Cadia purpurea | Leguminosae | Dried roots | Maceration extraction | Ethanol | DPPH | 12.9 μg/mL | Significant | [44] |
| Termitomyces schimperi | Lyophyllaceae | Dried leaves | Maceration extraction | Methanol | DPPH | 33.97 μg/mL | Significant | [27] |
| Hibiscus sabdariffa | Malvaceae | Dried seeds, calyces | Maceration technique | Methanol | DPPH | 430 and 140 μg/mL | Low | [36] |
| Maesa lanceolata | Myrsinaceae | Dried leaves | Maceration | Methanol | DPPH | 76.7 μg/mL | Moderate | [45] |
| Syzygium aromaticum | Myrtaceae | Dried flowers | Maceration extraction | Methanol | DPPH | 303.56 μg/mL | Low | [46] |
| Phytolacca dodecandra | Phytolaccaceae | Dried roots | Maceration extraction | Methanol | DPPH | 7.4 μg/mL | Significant | [47] |
| Piper capense | Piperaceae | Dried seeds | Maceration technique | Methanol | DPPH | 71.9 μg/mL | Moderate | [26] |
| Plumbago zeylanica | Plumbaginaceae | Dried leaves | Maceration extraction | Methanol | DPPH | 53.14 μg/mL | Moderate | [48] |
| Rumex nepalensis | Polygonaceae | Dried roots | Maceration | Ethanol | DPPH | 5.7 μg/mL | Significant | [49] |
| Cheilanthes farinosa | Pteridaceae | Dried aerial parts | Soxhlet | Methanol | DPPH | 52.5 μg/mL | Moderate | [38] |
| Clematis hirsuta | Ranunculaceae | Dried roots | Maceration | Methanol | DPPH | 590 μg/mL | Low | [50] |
| Clematis simensis | Ranunculaceae | Dried stem bark | Maceration extraction | Ethanol | DPPH | 42.35 mg/mL | Significant | [51] |
| Nigella sativa | Ranunculaceae | Dried seeds | Maceration technique | Methanol | DPPH | 94.1 μg/mL | Moderate | [26] |
| Ziziphus spina-christi | Rhamnaceae | Dried fruits | Soxhlet | Methanol | ABTS | 15480 μg/ml | Low | [52] |
| Hagenia abyssinica | Rosaceae | Dried leaves | Maceration extraction | Methanol | DPPH | 10.25 μg/mL | Significant | [53] |
| Rubus steudneri | Rosaceae | Dried roots | Maceration | Ethanol | DPPH | 5.8 μg/mL | Significant | [49] |
| Verbascum sinaiticum | Scrophulariaceae | Dried leaves | Maceration extraction | Methanol | DPPH | 1.70 μg/mL | Significant | [54] |
| Datura stramonium | Solanaceae | Dried roots, seeds | Maceration | Hydro methanol | DPPH | 13.47 and 11.95 μg/mL | Significant | [55, 56] |
| Gnidia involucrata | Thymelaeaceae | Dried root barks | Maceration extraction | EtOAc, methanol | DPPH | 7.9 and 17.7 μg/mL | Significant | [57] |
| Urtica simensis | Urticaceae | Dried leaves | Maceration extraction | Methanol | DPPH | 165.89 μg/mL | Low | [23] |
| Lippia adoensis | Verbenaceae | Dried leaves | Maceration technique | Methanol | DPPH | 49.2 μg/mL | Significant | [26] |
| Curcuma domestica | Zingiberaceae | Dried leaves | Maceration extraction | Methanol | DPPH | 96.98 μg/mL | Moderate | [27] |
| Dried rhizome | Hydrodistillation | Oil | DPPH | 23.05 μg/mL | Significant | [58] |
Figure 1.
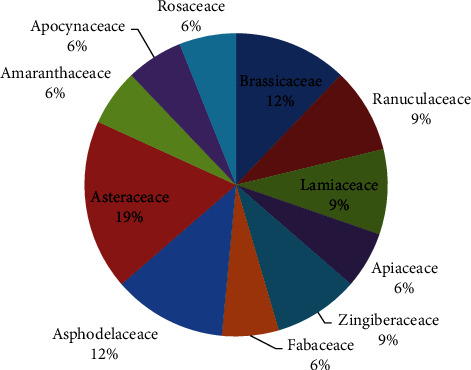
Percentage of the most well-investigated Ethiopian plant families for antioxidant activity.
The aforementioned family, which can be found in every floristic region of the country, may be the subject of this account [60]. Leaves 24 (42%) and roots 15 (26%) are the most investigated parts (Figure 2). This study indicates that using leaves for studies is crucial for medicinal plant conservation since, unlike with roots or whole plant collections, leaf harvesting may not be harmful to plants [61, 62].
Figure 2.
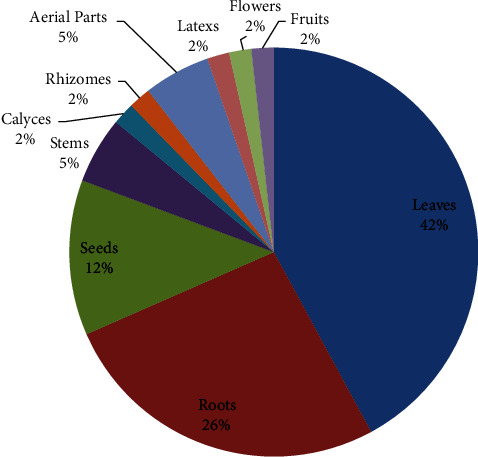
Plant parts investigated for their antioxidant potential.
Maceration (88%) is one of the most used plant sample extraction methods. Perhaps this is because solvent extraction, or more specifically, maceration, is one of the most popular and straightforward techniques for isolating plant antioxidants [63, 64]. Methanol is the most popular extraction solvent, although more polar solvents such as water and ethanol are frequently recommended in traditional preparations [65]. Surprisingly, in most studies, methanol (68%) plant extracts correlated with the antioxidant activity of the plant species studied. This is advantageous because it permits medicinal substances to absorb through the stomach lumen into the circulatory system, where they are required, following Lipinski's rules of 5 [66]. Therefore, active substances function through cell surface receptors, with polar components offering therapeutically significant potency in vivo. The antioxidant potential of plant extracts from 30 plants was significant (56%) (IC50 < 50 μg/mL). The antioxidant activity of eight plant extracts was moderate (15%), with IC50 values ranging from 50 to 100 μg/mL. With IC50 values greater than 100 μg/mL, 14 plant extracts showed low (26%) antioxidant activities, whereas two plant extracts exhibited both significant and moderate (2%) antioxidant activities. This implies that Ethiopian medicinal herbs were found to have strong antioxidant properties, indicating that, if thoroughly examined, they might produce valuable pharmaceutical drugs for the treatment of oxidative stress disease.
3.2. Promising Antioxidant Phytochemicals Derived from the Ethiopian Flora
More than 40 compounds from different chemical classes have so far been found in Ethiopian medicinal plants. Flavonoids 15 (32%), terpenoids 7 (15%), and organic acids 7 (15%) are the main components isolated from diverse plant species (Figure 3 and Table 2). Serial extraction, bioassay-guided extraction, successive fractionation using various polarity solvents, and column chromatography are the techniques used to isolate novel compounds for the plants of the species. The rising interest in using traditional medicine as an alternative and complementary therapy is encouraging activity-guided bioactive compound isolation to gain attention at the moment [70].
Figure 3.
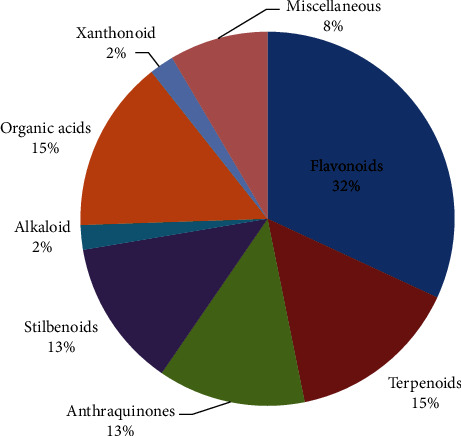
Percentage occurrence of antioxidant compounds isolated from Ethiopian medicinal plants.
Table 2.
Antioxidant compounds isolated from Ethiopian flora.
| Compounds | Plant species | Family | Plant part used | Solvent used | Isolation and identification Method | Assay method | IC50 (μg/mL) | Antioxidant potential | Ref |
|---|---|---|---|---|---|---|---|---|---|
| Flavonoid | |||||||||
| 7, 2′-Dihydroxy-4′-methoxy-6-(3″, 3″-dimethylallyl) isoflavan (1) | Rhynchosia ferruginea | Fabaceae | Roots | CH2Cl2/CH3OH | TLC, CC, NMR | DPPH | 32 | Low | [41] |
| 7-Hydroxy-2′, 4′ di-methoxy-8-(2‴, 3‴-dihydroxy-3‴-methylbutyl)-5′- (3″, 3″-dimethylallyl) isoflav-3-ene (2) | Rhynchosia ferruginea | Fabaceae | Roots | CH2Cl2/CH3OH | TLC, CC, NMR | DPPH | 64.5 | Low | [41] |
| Robustaflavone (3) | Rhus ruspolii | Anacardiaceae | Roots | CH2Cl2/MeOH | TLC, CC,NMR | DPPH | 7.90 | Significant | [67] |
| 3-(1-(2,4-Dihydroxyphenyl)-3,3-bis(4-hydroxyphenyl)-1-oxopropan-2-yl)-7-methoxy-4H-chromone-4-one (4) | Rhus ruspolii | Anacardiaceae | Roots | CH2Cl2/MeOH | TLC, CC,NMR | DPPH | 8.40 | Significant | [67] |
| 2′,4′,4″,2‴-Tetrahydroxy-4‴-methoxy-4-O-5‴-bichalcone (5) | Rhus ruspolii | Anacardiaceae | Roots | CH2Cl2/MeOH | TLC, CC,NMR | DPPH | 10.8 | Moderate | [67] |
| Rhuschalcone I (6) | Rhus ruspolii | Anacardiaceae | Roots | CH2Cl2/MeOH | TLC, CC,NMR | DPPH | 26.03 | Low | [67] |
| Rutin (7) | Cineraria abyssinica | Asteraceae | Leaves | Aqueous and methanol | TLC, PTLC, NMR | DPPH | 3.53 | Significant | [31] |
| Cheilanthes farinosa | Pteridaceae | Aerial parts | Methanol | TLC, CC, NMR | DPPH | 5.79 | Significant | [38] | |
| Euclea racemosa | Ebenaceae | Leaves | Acetone | TLC, CC, NMR | DPPH | 5.79 | Significant | [38] | |
| Flavan-3-ol-7-O-glucoside (8) | Hydnora johannis | Hydnoraceae | Roots | CH2Cl2/MeOH (1 : 1) | TLC, CC, NMR | DPPH | 0.190 | Significant | [68] |
| Hyperoside (9) | Bersama abyssinica | Francoaceae | Leaves | Methanol | TLC, CC, NMR | DPPH | 10.49 | Moderate | [38] |
| Quercetin-3-O-arabinopyranoside (10) | Bersama abyssinica | Francoaceae | Leaves | Methanol | TLC, CC, NMR | DPPH | 8.99 | Significant | [38] |
| Quercetin-3-O-diglucosylrhamnoside (11) | Cheilanthes farinosa | Pteridaceae | Aerial parts | Methanol | TLC, CC, NMR | DPPH | 11.59 | Moderate | [38] |
| Quercetrin (12) | Euclea racemosa | Ebenaceae | Leaves | Acetone | TLC, CC, NMR | DPPH | 12.33 | Moderate | [38] |
| Myricitrin (13) | Euclea racemosa | Ebenaceae | Leaves | Acetone | TLC, CC, NMR | DPPH | 6.59 | Significant | [38] |
| Myricetin-3-O-arabinopyranoside (14) | Euclea racemosa | Ebenaceae | Leaves | Acetone | TLC, CC, NMR | DPPH | 6.99 | Significant | [38] |
| 7-O-Methylaloeresin A (15) | Aloe harlana | Asphodelaceae | Leaves' latex | — | TLC, CC, PTLC, NMR | DPPH | 0.014 | Significant | [29] |
| Terpenoids | |||||||||
| β-Stigmasterol (16), | Laggera tomentosa | Asteraceae | Roots | Methanol | TLC, CC, NMR | DPPH | 1150 | Low | [33] |
| 3-Hydroxyisoagatholactone (17) | Cyphostemma cyphopetalum | Vitaceae | Roots | CH2Cl2/MeOH | TLC, CC, NMR | DPPH | 6.05 | Significant | [69] |
| β-Sitosterol (18) | Cyphostemma cyphopetalum | Vitaceae | Roots | CH2Cl2/MeOH | TLC, CC, NMR | DPPH | 2.72 | Significant | [69] |
| Hydnora johannis | Hydnoraceae | Roots | CH2Cl2/MeOH (1 : 1) | TLC, CC, NMR | DPPH | 14.668 | Moderate | [68] | |
| Cucurbitacin (19) | Cucumis prophetarum | Cucurbitaceae | Roots | Methanol | TLC, CC, NMR | DPPH | 80.2 | Low | [37] |
| α-Spinasterol (20) | Cucumis prophetarum | Cucurbitaceae | Roots | n-Hexane | TLC, CC, NMR | DPPH | 172.7 | Low | [37] |
| Spinasterol (21) | Calotropis procera | Apocynaceae | Roots | CH2Cl2/MeOH (1 : 1) | TLC, CC, NMR | DPPH | 0.3 | Significant | [24] |
| β-Sitosterol-3-O-β-D-glucoside (22) | Hydnora johannis | Hydnoraceae | Roots | CH2Cl2/MeOH | TLC, CC, NMR | DPPH | 0.014 | Significant | [68] |
|
| |||||||||
| Anthraquinone | |||||||||
| Aloin (23) | Aloe harlana | Asphodelaceae | Leaves' latex | — | TLC, CC, PTLC, NMR | DPPH | 41.84 | Low | [29] |
| Microdontin A/B (24) | Aloe schelpei | Asphodelaceae | Leaves' latex | — | PTLC, NMR | DPPH | 0.07 | Significant | [30] |
| Aloin A/B (25) | Aloe schelpei | Asphodelaceae | Leaves' latex | — | PTLC, NMR | DPPH | 0.15 | Significant | [30] |
| Aloinoside A/B (26) | Aloe schelpei | Asphodelaceae | Leaves' latex | — | PTLC, NMR | DPPH | 0.13 | Significant | [30] |
| Chrysophanol (27) | Laggera tomentosa | Asteraceae | Roots | Methanol | TLC, CC, NMR | DPPH | 6.2 | Significant | [33] |
| Emodin (28) | Laggera tomentosa | Asteraceae | Roots | Methanol | TLC, CC, NMR | DPPH | 3.8 | Significant | [33] |
|
| |||||||||
| Stilbenoids | |||||||||
| ε-Viniferin (29) | Cyphostemma cyphopetalum | Vitaceae | Roots | CH2Cl2/MeOH | TLC, CC, NMR | DPPH | 0.017 | Significant | [69] |
| Trans-Resveratrol (30) | Cyphostemma cyphopetalum | Vitaceae | Roots | CH2Cl2/MeOH | TLC, CC, NMR | DPPH | 0.052 | Significant | [69] |
| Gnetin H (31) | Cyphostemma cyphopetalum | Vitaceae | Roots | CH2Cl2/MeOH | TLC, CC, NMR | DPPH | 0.063 | Significant | [69] |
| ε-Viniferin Diol (32) | Cyphostemma cyphopetalum | Vitaceae | Roots | CH2Cl2/MeOH | TLC, CC, NMR | DPPH | 0.157 | Significant | [69] |
| Parthenostilbenin B (33) | Cyphostemma cyphopetalum | Vitaceae | Roots | CH2Cl2/MeOH | TLC, CC, NMR | DPPH | 0.025 | Significant | [69] |
|
| |||||||||
| Alkaloids | |||||||||
| 13-O-Pyrrolecarboxyl lupanine (34) | Cadia purpurea | Fabaceae | Roots | MeOH | TLC, CC, NMR | DPPH | 58.44 | Low | [44] |
| Organic acid | |||||||||
| Tetratriacontanyl caffeate (35) | Gnidia involucrata | Thymelaeoideae | Root barks | EtOAC | TLC, CC, NMR | DPPH | 73 | Low | [57] |
| 12-O-Dodeca-2,4-dienoylphorbol-13-acetate (36) | Gnidia involucrata | Thymelaeoideae | Root barks | EtOAC | TLC, CC, NMR | DPPH | 84.9 | Low | [57] |
| (E)-Octadec-7-enoic acid (37) | Crinum abyssinicum | Amaryllidaceae | Roots | CH2Cl2/MeOH (1 : 1) | TLC, CC, NMR | DPPH | 10.1 | Moderate | [24] |
| Myristic acid (38) | Cucumis prophetarum | Cucurbitaceae | Roots | n-Hexane | TLC, CC, NMR | DPPH | 232.3 | Low | [37] |
| Caffeic acid (39) | Cheilanthes farinosa | Pteridaceae | Aerial parts | Methanol | TLC, CC, NMR | DPPH | 4.19 | Significant | [38] |
| Chlorogenic acid (40) | Cheilanthes farinosa | Pteridaceae | Aerial parts | Methanol | TLC, CC, NMR | DPPH | 8.01 | Significant | [38] |
| 1, 3-Dilinoleoyl-2-stearoylglycerol (41) | Rhynchosia ferruginea | Fabaceae | Roots | CH2Cl2/CH3OH | TLC, CC, NMR | DPPH | 90.6 | Low | [41] |
|
| |||||||||
| Xanthonoid | |||||||||
| Mangiferin (42) | Bersama abyssinica | Francoaceae | Leaves | Methanol | TLC, CC, NMR | DPPH | 6.72 | Significant | [38] |
|
| |||||||||
| Miscellaneous | |||||||||
| Di-(2-methylheptyl) phthalate (43) | Cadia purpurea | Fabaceae | Roots | MeOH | TLC, CC, NMR | DPPH | 7.99 | Significant | [44] |
| Ethyl (E)-octadec-8-enoate (44) | Crinum abyssinicum | Amaryllidaceae | Roots | CH2Cl2/MeOH (1 : 1) | TLC, CC, NMR | DPPH | 3.3 | Significant | [24] |
| (4Z)-Dodec-4-en-1-ol (45) | Calotropis procera | Apocynaceae | Roots | CH2Cl2/MeOH (1 : 1) | TLC, CC, NMR | DPPH | 7.9 | Significant | [24] |
| Penicilloitins B (46) | Crinum abyssinicum | Amaryllidaceae | Roots | CH2Cl2/MeOH (1 : 1) | TLC, CC, NMR | DPPH | 8.4 | Significant | [24] |
The significant (IC50 < 10 μg/mL) antioxidant potential of 29 compounds was 61%. With IC50 values ranging from 10 to 20 μg/mL, the antioxidant activity of 5 compounds was moderate (11%), and one compound exhibited both significant and moderate (3%) antioxidant activities, while 12 compounds with IC50 values higher than 20 μg/mL exhibited low antioxidant activity (25%). The root of the plant species was frequently considered for investigation.
3.2.1. Flavonoids
From ten plant species, 15 compounds (1–15) were isolated. Table 2 summarizes them, and Figure 4 depicts their chemical structures. The most effective compounds were Rutin (7) from Cineraria abyssinica's aqueous and methanol leaf extracts, Flavan-3-ol-7-O-glucoside (8) from Hydnora johannis' CH2Cl2/MeOH (1 : 1) root extracts, and 7-O-Methylaloeresin A (15) from Aloe harlana's leaf latex, with IC50 values of 3.53, 0.19, and 0.014 μg/mL, respectively [29, 31, 68]. Flavonoids are the most abundant naturally occurring phenolic compounds well known for their antioxidant properties (Figure 5), which help in the prevention of a number of diseases including cancer, cardiovascular disease, and neurodegenerative diseases [71–74]. As a result, the presence of these significant compounds and the powerful antioxidant potential they exhibited indicate that, if rigorously screened, these compounds could provide medications of pharmaceutical relevance from those species.
Figure 4.
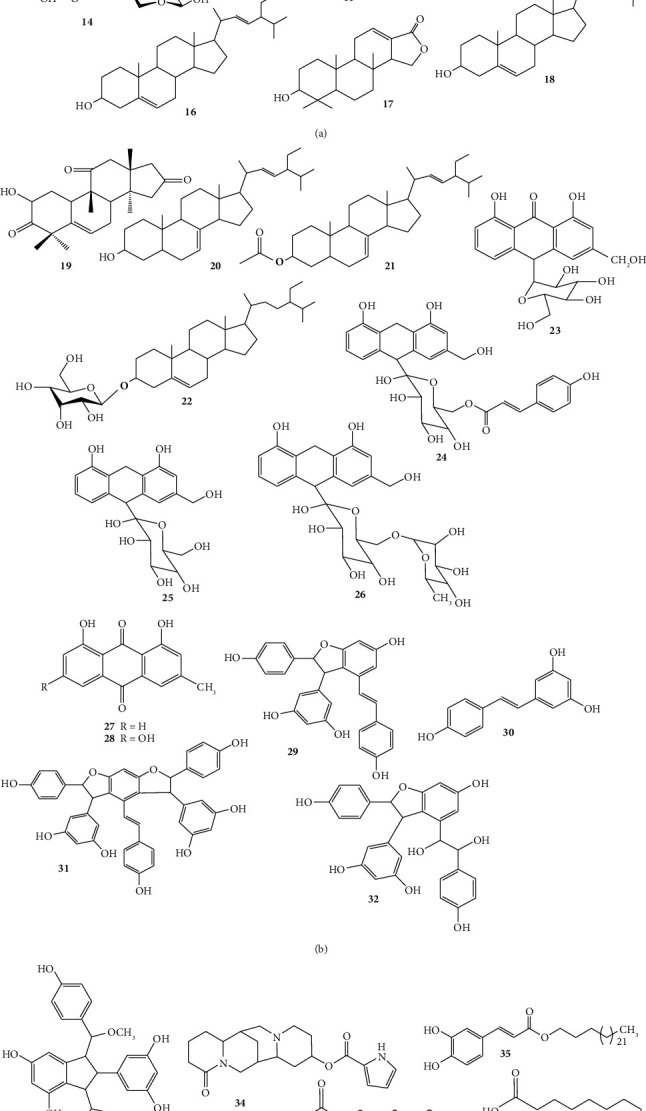
Antioxidant compounds isolated from Ethiopian flora.
Figure 5.
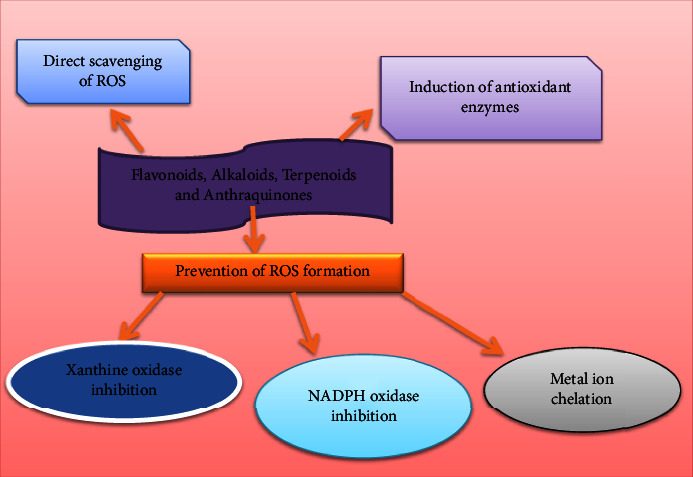
Mechanism of action of antioxidant effects of flavonoids, alkaloids, terpenoids, and anthraquinones. Flavonoids, alkaloids, terpenoids, and anthraquinones exert antioxidant effects by reactive oxygen species (ROS) scavenging, preventing ROS formation, and increasing production of antioxidant enzymes.
3.2.2. Terpenoids
Terpenoids represent the largest group of plant secondary metabolites [75]. There are tens of thousands of naturally occurring hydrocarbons, making them one of the classes of natural compounds with the most structural diversity. Terpenoids are categorized as hemiterpenes (C5), monoterpenes (C10), sesquiterpenes (C15), diterpenes (C20), triterpenes (C30), tetraterpenes or carotenoids (C40), and polyterpenes (Cn,n > 40) [75]. Numerous studies indicated that terpenoids and their derivatives exhibited antioxidant and antiaging properties (Figure 5), which help in the prevention of a number of diseases including cancer, cardiovascular disease, and neurodegenerative diseases [76–78]. Six plant species from Ethiopia's flora were studied for their antioxidant compounds. Seven compounds (16–22) were isolated, and 17, 18, 21, and 22 of those compounds demonstrated significant antioxidant properties with IC50 values of 6.05, 2.72, 0.3, and 0.014 μg/mL, respectively (Table 2 and Figure 4). The most effective compound (22), which is in line with the previous investigation, has been reported in the literature for its antioxidant activity [79–81].
3.2.3. Anthraquinone
Anthraquinones, also known as anthracene diones or dioxoanthracenes, are significant quinones that make up a wide range of structurally different compounds of the polyketide family. It is essentially an organic compound that is aromatic. There are around 700 members of this group in fungi, lichens, and plants [82]. Many of them possess antimicrobial, antioxidant, anti-inflammatory, and antiviral properties [83, 84]. The mechanism of action of anthraquinones' antioxidant properties is demonstrated in Figure 5. In Table 2, the most promising recently discovered antioxidant anthraquinones derived from Ethiopian flora have been included. These include Aloin (23), Microdontin A/B (24), Aloin A/B (25), Aloinoside A/B (26), Chrysophanol (27), and Emodin (28), whose chemical structures are depicted in Figure 4. Aloe harlana (Asphodelaceae) [29], Aloe schelpei (Asphodelaceae) [30], and Laggera tomentosa (Asteraceae) [33] species were used to isolate the compounds. Compounds 24–26, which had IC50 values of 0.07, 0.15, and 0.13 μg/mL, were isolated from Aloe schelpei leaves' latex and showed significant antioxidant activity [30]. Compounds 27 and 28 were obtained by extracting the roots of Laggera tomentosa in methanol, and they demonstrated significant antioxidant activity, with IC50 values of 6.2 and 3.8 μg/mL, respectively [33]. Compound 23 was derived from the leaves' latex of Aloe harlana, but it only has low antioxidant properties, with an IC50 value of 41.84 μg/mL [29].
3.2.4. Stilbenoids
Stilbenoids are a distinct class of phenolic compounds with C6-C2-C6 units as their basic structure [85]. Nowadays, natural stilbenoids are sold commercially as nutraceuticals [85]. According to a recent review, stilbenoids exhibited significant biological effects, including antioxidant, anti-inflammatory, cardioprotective, neuroprotective, antidiabetic, depigmentation, and cancer prevention and treatment [86–88]. Table 2 shows the most promising antioxidant stilbenoids from Ethiopian flora that have recently been published. Figure 4 illustrates the chemical structures of these compounds, which include ε-Viniferin (29), Trans-Resveratrol (30), Gnetin (31), ε-Viniferin Diol (32), and Parthenostilbenin (33). The compounds were isolated from the roots of Cyphostemma cyphopetalum (Vitaceae), and they demonstrated significant antioxidant activity with IC50 values ranging from 0.017 to 0.157 μg/mL [69].
3.2.5. Alkaloids
Alkaloids are secondary metabolites that were first described as pharmacologically active molecules largely made of nitrogen [89]. They are formed from lysine, tyrosine, and tryptophan, three of the few common amino acids. Plants have been shown to contain more than 12,000 alkaloids, representing more than 150 families, and about 20% of the “species of flowering plants” contain alkaloids [89]. The mechanism of action of alkaloids' antioxidant properties is demonstrated in Figure 5 [90]. Compound 33 was isolated from Cadia purpurea (Fabaceae), and it exhibits a low level of antioxidant activity, with an IC50 value of 58.44 μg/mL [44].
3.2.6. Organic Acid
Seven antioxidant organic acid compounds (35–41) that were isolated in the Ethiopian flora are listed in Table 2 along with a depiction of their chemical structure in Figure 4. Caffeic acid (39) and chlorogenic acid (40), two of such compounds, were isolated from the aerial parts of Cheilanthes farinosa (Pteridaceae), and they exhibited significant antioxidant activity with IC50 values of 4.19 and 8.01 g/mL, respectively [38].
3.2.7. Xanthonoid
A xanthonoid is a chemical natural phenolic compound formed from the xanthone backbone [91]. Mangiferin is the best example, as it is a powerful therapeutic agent for treating a variety of diseases [92–94]. The antioxidant compound mangiferin (42), which was isolated from the leaves of Bersama abyssinica, had a significant antioxidant activity with an IC50 value of 6.72 μg/mL [38].
3.2.8. Miscellaneous Compounds
From three different plant species, four different compounds have been isolated (Table 2 and Figure 4). Di-(2-methylheptyl) phthalate (43) was isolated from the roots of Cadia purpurea (Fabaceae) [44], Ethyl (E)-octadec-8-enoate (44) and Penicilloitins B (46) were isolated from the roots of Crinum abyssinicum (Amaryllidaceae), and (4Z)-dodec-4-en-1-ol (45) was isolated from the roots of Calotropis procera (Apocynaceae) [24]. With an IC50 value of 3.3 μg/mL, (4Z)-dodec-4-en-1-ol (45) exhibited the most significant antioxidant properties [24].
4. Conclusion and Future Prospects
Oxidative stress results from an excessive free radical formation that is out of balance with the elimination of those radicals. Oxidative stress has been linked to the etiology of cancer, inflammatory diseases, cardiovascular disease, and other serious diseases. Antioxidants are substances that impede oxidative processes, prolonging or suppressing oxidative stress in the process. Natural antioxidants that are present in plants are gaining popularity. From a safety perspective, herbs and spices are the most crucial objectives when looking for natural antioxidants. Strong antioxidant, anti-inflammatory, antimutagenic, and cancer-preventive properties are shared by a wide range of phenolic compounds found in spices that are frequently employed as food additives. The current review provides a summary of Ethiopian studies on potentially antioxidant-rich medicinal herbs. The article reviews draw attention to some active metabolites and plant extracts that have the potential to become brand-new drugs or improved plant medicines. A number of these natural products and secondary metabolites demonstrated and showed significant antioxidant properties. Based on the findings, the most effective oxidative plant extracts from Ethiopian flora were Bersama abyssinica, Solanecio gigas, Echinops kebericho, Verbascum sinaiticum, Apium leptophyllum, and Crinum abyssinicum. The best oxidative phytochemicals were rutin (7), flavan-3-ol-7-O-glucoside (8), myricitrin (13), myricetin-3-O-arabinopyranoside (14), 7-O-methylaloeresin A (15), 3-hydroxyisoagatholactone (17), beta-sitosterol (18), β-sitosterol-3-O-β-D-glucoside (22), microdontin A/B (24), aloin A/B (25), aloinoside A/B (26), chrysophanol (27), emodin (28), ε-viniferin (29), trans-resveratrol (30), gnetin H (31), ε-viniferin diol (32), parthenostilbenin B (33), and caffeic acid (39). It is hoped that competent researchers and interested individuals will investigate some of these plants and compounds further to provide a thorough verification and subsequently facilitate commercialization. The detailed isolation, characterization, mechanisms of action, safety investigations, quality control, and clinical trials on some of these herbs and their isolated compounds are far from satisfactory, although the majority of the studies examined are preliminary. Therefore, further in vivo studies on these species are needed, as well as a systematic analysis of these antioxidant-rich species.
Acknowledgments
The authors would like to acknowledge the Armauer Hansen Research Institute for providing access to various journal databases.
Contributor Information
Gashaw Nigussie, Email: gashawnigussie20@gmail.com.
Aman Dekebo, Email: amandeke@gmail.com.
Data Availability
The data used in this study are included within the article.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
GN and AS designed and conceived this study. RN, MA, ED, and AD acquired and analyzed the data. GN, AS, and AD wrote the manuscript. GN, AS, RN, and AD revised the manuscript. All authors have read and approved the final manuscript and agree to be accountable for all aspects of the work. GN and AS contributed equally to this work.
References
- 1.Aadesariya M. K., Gauni B. M., Duggirala S. M., Ram V. R., Vyas S. J. Antibacterial activity of Abutilon pannosum and Grewia tenax leaves extracts. World Journal of Pharmaceutical Research . 2017;6:1259–1274. doi: 10.20959/wjpr201716-10325. [DOI] [Google Scholar]
- 2.Ahmad M. M. Recent trends in chemical modification and antioxidant activities of plants-based polysaccharides: a review. Carbohydrate Polymer Technologies and Applications . 2021;2 doi: 10.1016/j.carpta.2021.100045.100045 [DOI] [Google Scholar]
- 3.de Alencar M. V. O. B., Islam M. T., dos Reis A. C., et al. Oxidative stress mediated cytogenotoxicological effects of phytol in wistar albino rats. Advances in Traditional Medicine . 2023;23(1):273–290. doi: 10.1007/s13596-022-00628-4. [DOI] [Google Scholar]
- 4.Park S.-J., Sharma A., Lee H.-J. A review of recent studies on the antioxidant activities of a third-millennium food: Amaranthus spp. Antioxidants . 2020;9(12):p. 1236. doi: 10.3390/antiox9121236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aunjum A., Biswas R., Nurunnabi T. R., et al. Antioxidant and antibacterial activity of three herbs belonging to Zingiber genus of Bangladesh. Advances in Traditional Medicine . 2020;20(3):343–350. doi: 10.1007/s13596-019-00403-y. [DOI] [Google Scholar]
- 6.Diniz do Nascimento L., Moraes A. A. B. D., Costa K. S. D., et al. Bioactive natural compounds and antioxidant activity of essential oils from spice plants: new findings and potential applications. Biomolecules . 2020;10(7):p. 988. doi: 10.3390/biom10070988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ravichandran Y. D., Yetayih M. M. The GC–MS analysis of the diethylether and ethylacetate fraction of the peel of Solanum incanum and the study of their antibacterial activity. Advances in Traditional Medicine . 2022;22(4):809–821. doi: 10.1007/s13596-021-00623-1. [DOI] [Google Scholar]
- 8.Nigussie G., Wale M. Medicinal plants used in traditional treatment of malaria in Ethiopia: a review of ethnomedicine, anti-malarial and toxicity studies. Malaria Journal . 2022;21(1):262–316. doi: 10.1186/s12936-022-04264-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agbodjento E., Klotoé J. R., Sacramento T. I., et al. Ethnobotanical knowledge of medicinal plants used in the treatment of male infertility in southern Benin. Advances in Traditional Medicine . 2021;21(4):655–673. doi: 10.1007/s13596-020-00473-3. [DOI] [Google Scholar]
- 10.Tola M. A., Ibrahim F., Melak H., Tafesse T., Alemayehu M., Nigussie G. Traditional herbal remedies in the management of metabolic disorders in Ethiopia: a systematic review of ethnobotanical studies and pharmacological activities. Evidence-based Complementary and Alternative Medicine . 2023;2023:15. doi: 10.1155/2023/1413038.1413038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Belayneh A., Bussa N. F., Demissew S., Bisrat D. Acute oral toxicity test from leaf exudates of 17 Aloe species from east and south of the great rift valley in Ethiopia. Advances in Traditional Medicine . 2021;21(4):713–724. doi: 10.1007/s13596-020-00497-9. [DOI] [Google Scholar]
- 12.Nigussie G., Tegegn M., Abeje D., Melak H. A comprehensive review of the ethnomedicine, phytochemistry, pharmacological activities of the genus Kniphofia. Pharmaceutical Biology . 2022;60(1):1177–1189. doi: 10.1080/13880209.2022.2085753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nigussie G. A review on traditionally used medicinal plants for scabies therapy in Ethiopia. Advances in Traditional Medicine . 2021;21(2):199–208. doi: 10.1007/s13596-020-00453-7. [DOI] [Google Scholar]
- 14.Tole T. T., Diriba E., Bahiru L. A. Bioactive compounds from Croton macrostachyus and Commiphora habessinica occurring in Ethiopia. Advances in Traditional Medicine . 2021;22(3):621–629. doi: 10.1007/s13596-021-00570-x. [DOI] [Google Scholar]
- 15.Teka A., Maryo M. Ethiopian medicinal plants used for respiratory tract disorders: ethnomedicinal review. Evidence-based Complementary and Alternative Medicine . 2023;2023:9. doi: 10.1155/2023/7612804.7612804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lawal B., Shittu O. K., Oibiokpa F. I., Berinyuy E. B., Mohammed H. African natural products with potential antioxidants and hepatoprotectives properties: a review. Clinical Phytoscience . 2017;2(1):23–66. doi: 10.1186/s40816-016-0037-0. [DOI] [Google Scholar]
- 17.Zhang Q., Sharan A., Espinosa S. A., Gallego-Perez D., Weeks J. The path toward integration of traditional and complementary medicine into health systems globally: the World Health Organization report on the implementation of the 2014–2023 strategy. Journal of Alternative and Complementary Medicine . 2019;25(9):869–871. doi: 10.1089/acm.2019.29077.jjw. [DOI] [PubMed] [Google Scholar]
- 18.Gulcin İ. Antioxidants and antioxidant methods: an updated overview. Archives of Toxicology . 2020;94(3):651–715. doi: 10.1007/s00204-020-02689-3. [DOI] [PubMed] [Google Scholar]
- 19.Ndhlala A. R., Moyo M., Van Staden J. Natural antioxidants: fascinating or mythical biomolecules? Molecules . 2010;15(10):6905–6930. doi: 10.3390/molecules15106905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuete V., Efferth T. Cameroonian medicinal plants: pharmacology and derived natural products. Frontiers in Pharmacology . 2010;1:p. 123. doi: 10.3389/fphar.2010.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wakene W., Asmamaw S., Kahaliw W. Evaluation of antidiabetic and antioxidant activity of leaf extract and solvent fractions of hypoestes forskaolii (Val)(Acanthaceae) in mice. Journal of Experimental Pharmacology . 2021;13:859–872. doi: 10.2147/jep.s318696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alelign T., Debella A., Petros B. Evaluations of antioxidant effects of selected medicinal plant extracts claimed to treat kidney stone disease. Free Radicals and Antioxidants . 2021;10(2):63–68. doi: 10.5530/fra.2020.2.12. [DOI] [Google Scholar]
- 23.Adamu E., Asfaw Z., Demissew S., Baye K. Antioxidant activity and anti-nutritional factors of selected wild edible plants collected from northeastern Ethiopia. Foods . 2022;11(15):p. 2291. doi: 10.3390/foods11152291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tegegn G., Melaku Y., Eswaramoorthy R., Endale Anni̇sa M. Pharmacokinetics, drug-likeness, antibacterial and antioxidant activity of secondary metabolites from the roots extracts of Crinum abyssinicum and Calotropis procera and in silico molecular docking study. International Journal of Secondary Metabolite . 2022;9(4):467–492. doi: 10.21448/ijsm.1107685. [DOI] [Google Scholar]
- 25.Asres K., Asamenew G., Tadesse S., Mazumder A., Bucar F. A study on the composition, antimicrobial and antioxidant activities of the leaf essential oil of Apium leptophylum (Pers.) Benth. growing in Ethiopia. Ethiopian Pharmaceutical Journal . 2009;26(2) doi: 10.4314/epj.v26i2.43040. [DOI] [Google Scholar]
- 26.Sasikumar J., Erba O., Egigu M. C. In vitro antioxidant activity and polyphenolic content of commonly used spices from Ethiopia. Heliyon . 2020;6(9) doi: 10.1016/j.heliyon.2020.e05027.e05027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tadesse D., Retta N., Girma M., et al. In vitro antioxidant activities of plant polyphenol extracts and their combined effect with flaxseed on raw and cooked breast muscle fatty acid content, lipid health indices and oxidative stability in slow-growing sasso chickens. Foods . 2022;12(1):p. 115. doi: 10.3390/foods12010115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Getahun T., Sharma V., Gupta N. Chemical composition and biological activity of essential oils from Aloe debrana roots. Journal of Essential Oil Bearing Plants . 2020;23(3):493–502. doi: 10.1080/0972060x.2020.1788996. [DOI] [Google Scholar]
- 29.Asamenew G., Bisrat D., Mazumder A., Asres K. In vitro antimicrobial and antioxidant activities of anthrone and chromone from the latex of Aloe harlana Reynolds. Phytotherapy Research . 2011;25(12):1756–1760. doi: 10.1002/ptr.3482. [DOI] [PubMed] [Google Scholar]
- 30.Teka T., Kassahun H. Characterization and Evaluation of Antioxidant Activity of Aloe schelpei Reynolds. Drug Design, Development and Therapy . 2020;14:1003–1008. doi: 10.2147/dddt.s241412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sintayehu B., Bucar F., Veeresham C., Asres K. Hepatoprotective and free radical scavenging activities of extracts and a major compound isolated from the leaves of Cineraria abyssinica sch. Bip. exA. Rich. Pharmacognosy Journal . 2012;4(29):40–46. doi: 10.5530/pj.2012.29.6. [DOI] [Google Scholar]
- 32.Yimer T., Emiru Y. K., Kifle Z. D., Ewunetei A., Adugna M., Birru E. M. Pharmacological evaluation of antipyretic and antioxidant activities of 80% methanol root extract and derived solvent fraction of Echinops kebericho M. (Asteraceae) in mice model. BioMed Research International . 2021;2021:8. doi: 10.1155/2021/6670984.6670984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Melaku Y., Getahun T., Addi̇su M., Tesso H., Eswaramoorthy R., Garg A. Molecular docking, antibacterial and antioxidant activities of compounds isolated from Ethiopian plants. International Journal of Secondary Metabolite . 2022;9(2):208–328. doi: 10.21448/ijsm.1023864. [DOI] [Google Scholar]
- 34.Molla Yitayeh M., Monie Wassihun A. Chemical composition and antibacterial and antioxidant activities of stem bark essential oil and extracts of Solanecio gigas. Biochemistry Research International . 2022;2022:10. doi: 10.1155/2022/4900917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alemu B. K., Ayalew Getahun K., Kahaliw W. In vitro Antioxidant and in vivo Wound Healing Activities of the 80% Methanol Extract and Solvent Fractions of Seeds of Brassica carinata A. Braun (Brassicaceae) in Mice. Journal of Experimental Pharmacology . 2020;12:463–474. doi: 10.2147/jep.s278622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Keyata E. O., Tola Y. B., Bultosa G., Forsido S. F. Phytochemical contents, antioxidant activity and functional properties of Raphanus sativus L, Eruca sativa L. and Hibiscus sabdariffa L. growing in Ethiopia. Heliyon . 2021;7(1) doi: 10.1016/j.heliyon.2021.e05939.e05939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Galma W., Endale M., Getaneh E., Eswaramoorthy R., Assefa T., Melaku Y. Antibacterial and antioxidant activities of extracts and isolated compounds from the roots extract of Cucumis prophetarum and in silico study on DNA gyrase and human peroxiredoxin 5. BMC chemistry . 2021;15(1):p. 32. doi: 10.1186/s13065-021-00758-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Asres K., Gibbons S., Bucar F. Radical scavenging compounds from Ethiopian medicinal plants. Ethiopian Pharmaceutical Journal . 2006;24(1):23–30. doi: 10.4314/epj.v24i1.35095. [DOI] [Google Scholar]
- 39.Gebremedhin G., Tuem K. B., Kahsu A., Balasubramanian R. In vitro Antioxidant and in vivo Hepatoprotective Activities of Root Bark Extract and Solvent Fractions of Croton macrostachyus Hochst. Ex Del. (Euphorbiaceae) on Paracetamol-Induced Liver Damage in Mice. Journal of Experimental Pharmacology . 2020;12:301–311. doi: 10.2147/jep.s259081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abriham H., Paulos B. In vitro antioxidant and antibacterial activity of albizia lebbeck (L) benth stem bark. Science, Technology and Arts Research Journal . 2015;4(2):204–206. [Google Scholar]
- 41.Hussei̇n K., Eswaramoorthy R., Melaku Y., Endale Anni̇sa M. Antibacterial and antioxidant activity of isoflavans from the roots of rhynchosia ferruginea and in silico study on DNA gyrase and human peroxiredoxin. International Journal of Secondary Metabolite . 2021;8(4):321–336. doi: 10.21448/ijsm.962120. [DOI] [Google Scholar]
- 42.Kifle Z. D., Enyew E. F. Evaluation of in vivo antidiabetic, in vitro α-amylase inhibitory, and in vitro antioxidant activity of leaves crude extract and solvent fractions of Bersama abyssinica fresen (melianthaceae) Journal of Evidence-Based Integrative Medicine . 2020;25 doi: 10.1177/2515690X20935827.2515690X20935827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Asamenew G., Asres K., Bisrat D., Mazumder A., Lindemann P. Studies on chemical compositions, antimicrobial and antioxidant activities of essential oils of salvia officinialis linn. Grown in two locations of Ethiopia. Journal of Essential Oil Bearing Plants . 2017;20(2):426–437. doi: 10.1080/0972060x.2017.1287008. [DOI] [Google Scholar]
- 44.Kiros T., Eswaramoorthy R., Melaku Y., Dekebo A. In vitro antibacterial and antioxidant activities and molecular docking analysis of phytochemicals from Cadia purpurea roots. Journal of Tropical Medicine . 2022;2022:13. doi: 10.1155/2022/4190166.4190166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ismael J., Dessalegn E., Fereja W. M. In Vitro antioxidant and antibacterial activity of leaf extracts of Measa lanceolata. International Journal of Food Properties . 2021;24(1):702–712. doi: 10.1080/10942912.2021.1917608. [DOI] [Google Scholar]
- 46.Temesgen S., Sasikumar J., Egigu M. C. Effect of extraction solvents on total polyphenolic content and antioxidant capacity of syzygium aromaticum L. Flower bud from Ethiopia. BioMed Research International . 2022;2022:9. doi: 10.1155/2022/4568944.4568944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meharie B. G., Tunta T. A. Phytolacca dodecandra (phytolaccaceae) root extract exhibits antioxidant and hepatoprotective activities in mice with CCl4-induced acute liver damage. Clinical and Experimental Gastroenterology . 2021;14:59–70. doi: 10.2147/ceg.s290859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Beyene B. B., Alem F. A., Ayana M. T. Determination of antioxidant and antibacterial activities of leaf extracts of Plumbago zeylanica (Amira) Cogent Chemistry . 2020;6(1) doi: 10.1080/23312009.2020.1831715.1831715 [DOI] [Google Scholar]
- 49.Tauchen J., Doskocil I., Caffi C., et al. In vitro antioxidant and anti-proliferative activity of Ethiopian medicinal plant extracts. Industrial Crops and Products . 2015;74:671–679. doi: 10.1016/j.indcrop.2015.05.068. [DOI] [Google Scholar]
- 50.Abdisa Z., Kenea F. Phytochemical screening, antibacterial and antioxidant activity studies on the crude root extract of Clematis hirsuta. Cogent Chemistry . 2020;6(1) doi: 10.1080/23312009.2020.1862389.1862389 [DOI] [Google Scholar]
- 51.Ayana M. T., Mehari Y. G., Beyene B. B. A phytochemical investigation and evaluation of the antioxidant and antibacterial activities of the stem bark extract of Clematis simensis (Yeazo hareg) Journal of Chemical Research . 2022;46(5) doi: 10.1177/17475198221127398.174751982211273 [DOI] [Google Scholar]
- 52.Belayneh T., Gebremichael S., Chinchkar A. V., Berhanu T., Singh A., Upadhyay A. Comparative study on chemical composition and antioxidant properties (GraphPad prism approach) of wild Ethiopian Z. spina-christi and Indian Z. jujube fruit species. Food Analytical Methods . 2022;15(8):2224–2237. doi: 10.1007/s12161-022-02274-7. [DOI] [Google Scholar]
- 53.Kifle Z. D., Debeb S. G., Belayneh Y. M. In vitro α-amylase and α-glucosidase inhibitory and antioxidant activities of the crude extract and solvent fractions of hagenia abyssinica leaves. BioMed Research International . 2021;2021:9. doi: 10.1155/2021/6652777.6652777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lulseged K., Akele M. Z., Abiye A. A., Abebe B., Assefa Huluka S. Wound healing and antioxidant properties of 80% methanol leaf extract of Verbascum sinaiticum (scrophulariaceae): an Ethiopian medicinal plant. Evidence-based Complementary and Alternative Medicine . 2022;2022:10. doi: 10.1155/2022/9836773.9836773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Belayneh Y. M., Birhanu Z., Birru E. M., Getenet G. Evaluation of in vivo antidiabetic, antidyslipidemic and in vitro antioxidant activities of hydromethanolic root extract of Datura stramonium L. (Solanaceae) Journal of Experimental Pharmacology . 2019;11:29–38. doi: 10.2147/jep.s192264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cherie Melaku B., Amare G. G. Evaluation of Antidiabetic and Antioxidant Potential of Hydromethanolic Seed Extract of Datura stramonium; Linn (Solanaceae) Journal of Experimental Pharmacology . 2020;12:181–189. doi: 10.2147/jep.s258522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kalbessa A., Dekebo A., Tesso H., Abdo T., Abdissa N., Melaku Y. Chemical constituents of root barks of Gnidia involucrata and evaluation for antibacterial and antioxidant activities. Journal of Tropical Medicine . 2019;2019:8. doi: 10.1155/2019/8486214.8486214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kebede B. H., Forsido S. F., Tola Y. B., Astatkie T. Free radical scavenging capacity, antibacterial activity and essential oil composition of turmeric (Curcuma domestica) varieties grown in Ethiopia. Heliyon . 2021;7(2) doi: 10.1016/j.heliyon.2021.e06239.e06239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Haile A. A., Tsegay B. A., Seid A., Adnew W., Moges A. A review on medicinal plants used in the management of respiratory problems in Ethiopia over a twenty-year period (2000–2021) Evidence-based Complementary and Alternative Medicine . 2022;2022:14. doi: 10.1155/2022/2935015.2935015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lulekal E., Asfaw Z., Kelbessa E., Van Damme P. Ethnomedicinal study of plants used for human ailments in ankober district, north shewa zone, amhara region, Ethiopia. Journal of Ethnobiology and Ethnomedicine . 2013;9:63–13. doi: 10.1186/1746-4269-9-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Megenase J. A., Tilahun Gelaye K., Kumar Dara P. Indigenous knowledge and practices on medicinal plants used by local communities of Gambella Region, South West Ethiopia. International Journal of Tropical Disease and Health . 2019;39(2):1–14. doi: 10.9734/ijtdh/2019/v39i230203. [DOI] [Google Scholar]
- 62.Birhan Y. S. Medicinal plants utilized in the management of epilepsy in Ethiopia: ethnobotany, pharmacology and phytochemistry. Chinese Medicine . 2022;17(1):129–137. doi: 10.1186/s13020-022-00686-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Musa K. H., Abdullah A., Jusoh K., Subramaniam V. Antioxidant activity of pink-flesh guava (Psidium guajava L.): effect of extraction techniques and solvents. Food Analytical Methods . 2011;4(1):100–107. doi: 10.1007/s12161-010-9139-3. [DOI] [Google Scholar]
- 64.Dhanani T., Shah S., Gajbhiye N. A., Kumar S. Effect of extraction methods on yield, phytochemical constituents and antioxidant activity of Withania somnifera. Arabian Journal of Chemistry . 2017;10:1193–1199. doi: 10.1016/j.arabjc.2013.02.015. [DOI] [Google Scholar]
- 65.Abubakar A. R., Haque M. Preparation of medicinal plants: basic extraction and fractionation procedures for experimental purposes. Journal of Pharmacy and BioAllied Sciences . 2020;12(1):p. 1. doi: 10.4103/jpbs.jpbs_175_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lipinski C. A. Lead-and drug-like compounds: the rule-of-five revolution. Drug Discovery Today: Technologies . 2004;1(4):337–341. doi: 10.1016/j.ddtec.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 67.Abebe Deresa D., Abdissa Z., Tadesse Gurmessa G., et al. Biflavonoids from the roots of Rhus ruspolii and evaluations of their antioxidant activities. Bulletin of the Chemical Society of Ethiopia . 2022;36(3):667–674. doi: 10.4314/bcse.v36i3.15. [DOI] [Google Scholar]
- 68.Degfie T., Endale M., Tafese T., Dekebo A., Shenkute K. In vitro antibacterial, antioxidant activities, molecular docking, and ADMET analysis of phytochemicals from roots of Hydnora johannis. Applied Biological Chemistry . 2022;65(1):76–13. doi: 10.1186/s13765-022-00740-8. [DOI] [Google Scholar]
- 69.Degfie T., Ombito J. O., Demissie T. B., Eswaramoorthy R., Dekebo A., Endale M. Antibacterial and antioxidant activities, in silico molecular docking, ADMET and DFT analysis of compounds from roots of Cyphostemma cyphopetalum. Advances and Applications in Bioinformatics and Chemistry . 2022;15:79–97. doi: 10.2147/aabc.s377336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.World Health Organization. Traditional Medicine Strategy: 2014-2023 . Geneva, Switzerland: World Health Organization; 2013. [Google Scholar]
- 71.Liu H., Guo X., Chu Y., Lu S. Heart protective effects and mechanism of quercetin preconditioning on anti-myocardial ischemia reperfusion (IR) injuries in rats. Gene . 2014;545(1):149–155. doi: 10.1016/j.gene.2014.04.043. [DOI] [PubMed] [Google Scholar]
- 72.Lee Y. J., Suh K. S., Choi M. C., et al. Kaempferol protects HIT-T15 pancreatic beta cells from 2-deoxy-D-ribose-induced oxidative damage. Phytotherapy Research . 2010;24(3):419–423. doi: 10.1002/ptr.2983. [DOI] [PubMed] [Google Scholar]
- 73.Banjarnahor S. D., Artanti N. Antioxidant properties of flavonoids. Medical Journal of Indonesia . 2015;23(4):239–244. doi: 10.13181/mji.v23i4.1015. [DOI] [Google Scholar]
- 74.Khan H., Ullah H., Tundis R., et al. Dietary flavonoids in the management of huntington’s disease: mechanism and clinical perspective. EFood . 2020;1(1):38–52. doi: 10.2991/efood.k.200203.001. [DOI] [Google Scholar]
- 75.Yazaki K., Arimura G.-I., Ohnishi T. “Hidden” terpenoids in plants: their biosynthesis, localization and ecological roles. Plant and Cell Physiology . 2017;58(10):1615–1621. doi: 10.1093/pcp/pcx123. [DOI] [PubMed] [Google Scholar]
- 76.Mohandas G. G., Kumaraswamy M. Antioxidant activities of terpenoids from thuidium tamariscellum (C. Muell.) bosch. And sande-lac. A moss. Pharmacognosy Journal . 2018;10(4):645–649. doi: 10.5530/pj.2018.4.106. [DOI] [Google Scholar]
- 77.Wang C.-Y., Chen Y.-W., Hou C.-Y. Antioxidant and antibacterial activity of seven predominant terpenoids. International Journal of Food Properties . 2019;22(1):230–238. doi: 10.1080/10942912.2019.1582541. [DOI] [Google Scholar]
- 78.Proshkina E., Plyusnin S., Babak T., et al. Terpenoids as potential geroprotectors. Antioxidants . 2020;9(6):p. 529. doi: 10.3390/antiox9060529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shahat A. A., Hidayathulla S., Khan A. A., et al. Phytochemical profiling, antioxidant and anticancer activities of Gastrocotyle hispida growing in Saudi Arabia. Acta Tropica . 2019;191:243–247. doi: 10.1016/j.actatropica.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 80.Sarkar S., Pal A., Chouni A., Paul S. A novel compound β-sitosterol-3-O-β-D-glucoside isolated from Azadirachta indica effectively induces apoptosis in leukemic cells by targeting G0/G1 populations. Indian Journal of Biochemistry & Biophysics . 2020;57(1):27–32. [Google Scholar]
- 81.Tahany M. A., Hegazy A. K., Sayed A. M., Kabiel H. F., El-Alfy T., El-Komy S. M. Study on combined antimicrobial activity of some biologically active constituents from wild Moringa peregrina Forssk. Journal of Yeast and Fungal Research . 2010;1(1):15–24. [Google Scholar]
- 82.Yang J., Huang Y., Xu H., et al. Optimization of fungi co-fermentation for improving anthraquinone contents and antioxidant activity using artificial neural networks. Food Chemistry . 2020;313 doi: 10.1016/j.foodchem.2019.126138.126138 [DOI] [PubMed] [Google Scholar]
- 83.Khanal P., Patil B. M., Chand J., Naaz Y. Anthraquinone derivatives as an immune booster and their therapeutic option against COVID-19. Natural products and bioprospecting . 2020;10(5):325–335. doi: 10.1007/s13659-020-00260-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Eom T., Kim E., Kim J.-S. In vitro antioxidant, antiinflammation, and anticancer activities and anthraquinone content from Rumex crispus root extract and fractions. Antioxidants . 2020;9(8):p. 726. doi: 10.3390/antiox9080726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shen T., Xie C. F., Wang X. N., Lou H. X. Stilbenoids. In: Ramawat K., Mérillon J. M., editors. Natural Products . Berlin, Heidelberg: Springer; 2013. pp. 1901–1949. [DOI] [Google Scholar]
- 86.Akinwumi B. C., Bordun K.-A. M., Anderson H. D. Biological activities of stilbenoids. International Journal of Molecular Sciences . 2018;19(3):p. 792. doi: 10.3390/ijms19030792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Koh Y.-C., Ho C.-T., Pan M.-H. Recent advances in health benefits of stilbenoids. Journal of Agricultural and Food Chemistry . 2021;69(35):10036–10057. doi: 10.1021/acs.jafc.1c03699. [DOI] [PubMed] [Google Scholar]
- 88.Dvorakova M., Landa P. Anti-inflammatory activity of natural stilbenoids: a review. Pharmacological Research . 2017;124:126–145. doi: 10.1016/j.phrs.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 89.Chen W., Ma Y., He W., et al. Structure units oriented approach towards collective synthesis of sarpagine-ajmaline-koumine type alkaloids. Nature Communications . 2022;13(1):p. 908. doi: 10.1038/s41467-022-28535-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Parvin M. S., Chlebek J., Hošťálková A., et al. Interactions of isoquinoline alkaloids with transition metals iron and copper. Molecules . 2022;27(19):p. 6429. doi: 10.3390/molecules27196429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Saha S., Sadhukhan P., Sil P. C. Mangiferin: a xanthonoid with multipotent anti-inflammatory potential. BioFactors . 2016;42(5):459–474. doi: 10.1002/biof.1292. [DOI] [PubMed] [Google Scholar]
- 92.Du S., Liu H., Lei T., et al. Mangiferin: an effective therapeutic agent against several disorders (Review) Molecular Medicine Reports . 2018;18(6):4775–4786. doi: 10.3892/mmr.2018.9529. [DOI] [PubMed] [Google Scholar]
- 93.Morozkina S. N., Nhung Vu T. H., Generalova Y. E., Snetkov P. P., Uspenskaya M. V. Mangiferin as new potential anti-cancer agent and mangiferin-integrated polymer systems—a novel research direction. Biomolecules . 2021;11(1):p. 79. doi: 10.3390/biom11010079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang M., Liang Y., Chen K., et al. The management of diabetes mellitus by mangiferin: advances and prospects. Nanoscale . 2022;14(6):2119–2135. doi: 10.1039/d1nr06690k. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used in this study are included within the article.


