Abstract
The mode of action of (1′S,2′R)-9-{[1′,2′-bis(hydroxymethyl)cycloprop-1′-yl]methyl}guanine (A-5021) against herpes simplex virus type 1 (HSV-1), HSV-2, and varicella-zoster virus (VZV) was studied. A-5021 was monophosphorylated at the 2′ site by viral thymidine kinases (TKs). The 50% inhibitory values for thymidine phosphorylation of A-5021 by HSV-1 TK and HSV-2 TK were comparable to those for penciclovir (PCV) and lower than those for acyclovir (ACV). Of these three agents, A-5021 inhibited VZV TK most efficiently. A-5021 was phosphorylated to a mono-, di-, and triphosphate in MRC-5 cells infected with HSV-1, HSV-2, and VZV. A-5021 triphosphate accumulated more than ACV triphosphate but less than PCV triphosphate in MRC-5 cells infected with HSV-1 or VZV, whereas HSV-2-infected MRC-5 cells had comparable levels of A-5021 and ACV triphosphates. The intracellular half-life of A-5021 triphosphate was considerably longer than that of ACV triphosphate and shorter than that of PCV triphosphate. A-5021 triphosphate competitively inhibited HSV DNA polymerases with respect to dGTP. Inhibition was strongest with ACV triphosphate, followed by A-5021 triphosphate and then (R,S)-PCV triphosphate. A DNA chain elongation experiment revealed that A-5021 triphosphate was incorporated into DNA instead of dGTP and terminated elongation, although limited chain extension was observed. Thus, the strong antiviral activity of A-5021 appears to depend on a more rapid and stable accumulation of its triphosphate in infected cells than that of ACV and on stronger inhibition of viral DNA polymerase by its triphosphate than that of PCV.
A-5021, or (1′S,2′R)-9-{[1′,2′-bis(hydroxymethyl)cycloprop-1′-yl]methyl}guanine, is a novel guanosine analog with potent antiviral activity against herpes simplex virus type 1 (HSV-1), HSV-2, varicella-zoster virus (VZV), and human cytomegalovirus (14, 23). The in vitro antiviral activity of A-5021 is higher than that of acyclovir (ACV) or penciclovir (PCV) against HSV-1, HSV-2, and VZV (14).
Nucleoside analogs having highly selective antiherpetic activity are selectively phosphorylated by viral thymidine kinases (TKs) in herpesvirus-infected cells (5). A-5021 shows reduced antiviral activity against TK-deficient strains of HSV, suggesting that this agent requires selective phosphorylation by herpesvirus TK, as is the case for ACV (11). The monophosphates of antiviral nucleosides are subsequently phosphorylated to triphosphates by cellular kinases (20, 21). These triphosphates accumulate in virus-infected cells and inhibit viral DNA polymerases in a competitive and chain-terminated fashion (7, 9, 10, 14, 18). Some nucleoside analogs like PCV have prolonged antiviral activities derived from the long intracellular half-lives of their triphosphates (2, 3, 7, 25). A-5021 also shows a prolonged in vitro antiviral effect (14).
In this study, we sought to clarify the mechanism of the highly potent and prolonged antiviral activity of A-5021 and examined its phosphorylation by viral TKs, the inhibition of viral DNA polymerases by A-5021 triphosphate, and the intracellular metabolism of this agent in herpesvirus-infected cells compared with those of ACV and PCV triphosphates.
MATERIALS AND METHODS
Cell culture and viruses.
MRC-5 cells (Dainippon Pharmaceutical Co. Ltd., Osaka, Japan) and human embryo lung cells were grown in Dulbecco’s modified Eagle’s medium supplemented with 10% inactivated fetal bovine serum. Vero C1008 cells (Dainippon) and BU25 TK− cells (Dainippon) were grown in Eagle’s minimum essential medium supplemented with 10% fetal bovine serum.
Stocks of HSV (KOS), HSV-2 (UW-268), and VZV (Kawaguchi) were prepared as described previously (14).
Compounds.
A-5021, ACV, and PCV were prepared at Ajinomoto Co., Inc. (Kawasaki, Japan) (13, 23, 24).
(1′-Methylene-3H)–A-5021 was prepared by reduction of (1′S,5′R)-9-[3′-oxa-2′-oxobicyclo(3.1.0)hex-1′-yl]methylguanine as the lactone precursor of A-5021 (radiochemical purity, >97%; chemical purity, >99%; specific activity, 15 Ci/mmol). (4′-3H)–PCV was prepared by reduction of 9-[3-(diethoxycarbonyl)propyl]guanine (radiochemical purity, >99%; chemical purity, >99%; specific activity, 63 Ci/mmol). ACV with a 2′-3H side chain (21.5 or 25.1 Ci/mmol) was obtained from NEN Research Products (Stevenage, United Kingdom). (1′,2′-3H)–dGTP (32 Ci/mmol), (methyl-[3H])thymidine (43 Ci/mmol), [α-32P]dCTP, and [γ-32P]ATP were obtained from Amersham International plc (Little Chalfont, Buckinghamshire, United Kingdom).
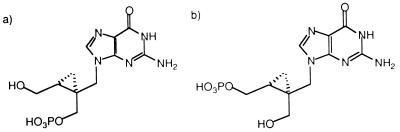
A-5021–2′-monophosphate (Fig. 2b) was prepared by phosphorylation of a regioselectively protected derivative. To 2.06 g (2.15 mmol) of (1′S,2′R)-6-ben- zyloxy-2-(4,4′-dimethoxyltrityl)amino-9-({[1′-(4,4′-dimethoxyltrityl)oxymethyl- 2′-hydroxymethyl]cycloprop-1′-yl}methyl)purine in dry tetrahydrofuran (THF) (4.3 ml) and pyridine (21.5 mmol; 1.74 ml) was added dibenzyl phosphochloridate (21.5 mmol) in THF (4.3 ml). After 1 h of stirring at room temperature, 20 ml of 50% aqueous pyridine was added. The mixture was concentrated in vacuo, and the residue was purified by silica gel column chromatography (0.5 to 1% methanol in dichloromethane). The dibenzyl phosphate was treated in 80% aqueous acetic acid for 2 h to remove the trityl groups, purified by silica gel column chromatography (1.5 to 4% methanol in dichloromethane), and then reduced by catalytic hydrogenation with 10% palladium/carbon in 80% aqueous acetic acid in a hydrogen atmosphere (4 atm) for 40 h. After purification by C18 reversed-phase chromatography, 230 mg (31%) of A-5021–2′-monophosphate was obtained. The purity of the compound was 96% by high-performance liquid chromatography (HPLC); 1H NMR (dimethyl sulfoxide [DMSO] d-6) δ 0.41 (t, J = 4.8 Hz, 1H), 0.93 (dd, J = 4.8, 8.7 Hz, 1H), 1.32 (m, 1H), 3.24 (d, J = 12.6 Hz, 1H), 3.38 (d, J = 12.6 Hz, 1H), 3.73 (d, J = 14.1 Hz, 2H), 3.85 (m, 2H), 4.12 (d, J = 14.1 Hz, 1H), 6.42 (bs, 2H), 7.67 (s, 1H), 10.54 (bs, 1H); high-resolution fast atom bombardment-mass spectrometry (FAB-MS) calculated m/z 346.0916 (MH+) for C11H17O6N5P; found m/z 346.0934 (MH+).
FIG. 2.
Structure of chemically synthesized A-5021 monophosphates. (a) A-5021–1′-monophosphate. (b) A-5021–2′-monophosphate.
As a reference, A-5021–1′-monophosphate (Fig. 2a) was prepared starting with (1′S,2′R)-6-benzyloxy-2-(4,4′-dimethoxyltrityl)amino-9-({[2′-(4,4′-dimeth- oxyltrityl)oxymethyl-1′-hydroxymethyl]cycloprop-1′-yl}methyl)purine as de- scribed above. After purification by C18 reversed-phase chromatography, A-5021–1′-monophosphate was obtained at a 19% yield with 97% purity. 1H NMR (DMSO d-6) δ 0.44 (m, 1H), 0.90 (dd, J = 5.1, 8.7 Hz, 1H), 1.28 (m, 1H), 3.38 (dd, J = 8.1, 11.7 Hz, 1H), 3.51 (dd, J = 6.3, 11.7 Hz, 1H), 3.78 (m, 2H), 3.88 (d, J = 14.1 Hz, 1H), 3.99 (d, J = 14.1 Hz, 1H), 6.38 (bs, 2H), 7.81 (s, 1H), 10.50 (bs, 1H); FAB-MS m/z 346 (MH+).
A-5021–2′-triphosphate was prepared by phosphorylation of A-5021–2′-monophosphate with pyrophosphate. A-5021–2′-monophosphate (34.56 mg; 0.1 mmol) suspended in 10 ml of 50% methanol in ethanol was mixed with an equimolar amount of tri(n-butyl)amine and heated at 80°C for 4 h. After concentration in vacuo, the residue was dissolved in 1 ml of N,N-dimethylformamide (DMF) and 81.1 mg (0.50 mmol) of 1,1′-carbonyldiimidazole in 1 ml of DMF was added. After the mixture was allowed to stand for 20 h at room temperature, 34 ml of methanol (0.80 mmol) was added, the mixture was stirred for 30 min, and then 181.7 mg (0.50 mmol) of tri(n-butyl)ammonium pyrophosphate in 5 ml of DMF was added. After 30 min of stirring, the mixture was filtered and 10 ml of methanol was added to the filtrate, which was concentrated in vacuo. The residue was desalted by C18 reversed-phase chromatography, eluted with water, purified by ion-exchange HPLC (TSK gel DEAE-2SW), and eluted with 0.1 to 0.5 M potassium phosphate buffer (pH 3) containing 20% acetonitrile. Finally, the triphosphate was desalted by DEAE Sephadex A-25 eluted with ammonium bicarbonate buffer and freeze-dried to afford 31.6 mg of the ammonium salt (54% yield as determined by absorbance at 255 nm [88% purity]). 1H NMR (D2O) δ 0.71 (t, J = 5.4 Hz, 1H), 1.11 (dd, J = 5.4, 8.7 Hz, 1H), 1.67 (m, 1H), 3.56 (s, 2H), 3.93 (ddd, J = 7.2, 8.7, 11.4 Hz, 1H), 4.11 (d, J = 14.7 Hz, 1H), 4.19 (d, J = 14.7 Hz, 1H), 4.23 (m, 1H), 8.18 (s, 1H); 31P NMR (D2O, pD = 4) d (H3PO4) −23.2 (t, J = 45.2 Hz), −11.3 (d, J = 52.8 Hz), −10.8 (d, J = 45.6 Hz); FAB-MS m/z 504 (M-H)−.
ACV triphosphate and (R,S)-PCV triphosphate were prepared as described by Dixit and Poulter (6) and Jarvest et al. (15), respectively. The final products were purified and desalted as described above, and the purities of the triphosphates were 95 and 70%, respectively.
Thymidine Sepharose was prepared as described by Kowal and Markus (16), except that ECH-Sepharose (12 to 16 mmol/ml; Pharmacia) was used as a support and galactosamine was used for blocking. The final loading of thymidine was 5.6 mmol/ml.
Purification and assay of viral TKs.
HSV-1 and HSV-2 TKs were obtained from HSV-1 (KOS)- and HSV-2 (UW268)-infected BU25 cells, respectively. Purification was performed as described by Cheng and Ostrander (4) and Fyfe (12) with some modifications. BU25 cells were infected with HSV-1 (KOS) or HSV-2 (UW-268). After 12 h of incubation, cells were harvested and washed three times with phosphate-buffered saline (PBS). Cells were disrupted with a Teflon homogenizer and sonicated in buffer A containing 10 mM Tris–HCl (pH 7.5), 150 mM KCl, 1 mM MgCl2, 1 mM 2-mercaptoethanol, 50 μM thymidine, 1 mM phenylmethylsulfonyl fluoride (PMSF), 5 μg of leupeptin per ml, 5 μg of pepstatin A per ml, and 10% glycerol. Then the disrupted cell suspension was centrifuged at 100,000 × g for 60 min at 4°C. To the supernatant was added 20% streptomycin at a final concentration of 1%, and the precipitate was removed by centrifugation. The supernatant was fractionated with ammonium sulfate in two steps (20 and 50%). The protein precipitated between 20 and 50% ammonium sulfate was suspended in buffer B, containing 20 mM Tris–HCl (pH 7.5), 3 mM dithiothreitol (DTT), 5 μg of leupeptin per ml, 5 μg of pepstatin A per ml, 1 mM PMSF, and 10% glycerol and was applied to the thymidine Sepharose affinity column. HSV-1 and HSV-2 TKs were eluted with buffer containing 200 and 800 μM thymidine, respectively. The enzymes were stored at −80°C and desalted with an NAP-5 (Pharmacia) gel filtration column on the day of use.
VZV TK was obtained by expression in Escherichia coli. The tk gene from VZV (Kawaguchi) was cloned into the expression vector pET28a(+) (Novagen) with a fragment obtained by PCR. The PCR was performed for 30 cycles with total DNA from MRC-5 cells infected with VZV (Kawaguchi) as the template, two 25-mer oligonucleotide primers (primer 1, 5′-TGTCTACAATACATATGTCAACGGA-3′; primer 2, 5′-AACACGTACACTCGAGTATGACAAT-3′) (modified from the method described by Lacey et al. [17]), and cloned Pfu DNA polymerase (Stratagene). The PCR fragment and pET28a(+) were digested with restriction enzymes NdeI and XhoI and ligated. The resultant plasmid (pETVZVTK) was used to transform E. coli BL21 (DE3). VZV TK was expressed by the induction of 100 μg of isopropyl-β-d-thiogalactopyranoside (IPTG), purified by His binding resin affinity chromatography according to the method in the Novagen manual, and further purified by thymidine Sepharose affinity chromatography. It was eluted with buffer containing 800 mM Tris–HCl (pH 7.5), 3 mM DTT, 0.4 mM thymidine, 1 mM ATP, 0.5 mg of bovine serum albumin per ml, and 10% glycerol. Then the enzyme was stored and desalted as described above.
A reaction mixture containing 50 mM Tris–HCl (pH 7.5), 2 mM ATP, 2 mM MgCl2, 9 mM NaF, 1 μM [methyl-3H]thymidine, and the purified enzyme was used to determine the 50% inhibitory concentration (IC50) of each nucleoside analog for thymidine phosphorylation. The reaction was performed at 37°C for 15 min, and then aliquots of 45 μl were spotted onto Whatman DEAE-81 filter paper disks. The filter was subsequently washed three times (for 10 min each time) in 1 mM ammonium formate and finally rinsed once in methanol. The disks were completely dried, and radioactivity was determined by scintillation counting.
For detection of the phosphorylation of A-5021 by viral TKs and determination of the phosphorylation site of A-5021, a reaction mixture containing 50 mM Tris–HCl (pH 7.5), 2 mM ATP, 2 mM MgCl2, 10 mM NaF, and the purified enzyme was used. The reaction mixture was analyzed by HPLC with a Whatman Partisil 10 SAX column (250 by 4.6 mm) and UV detection at 254 nm. Elution was performed with a linear gradient of potassium phosphate (pH 3.5) from 0 to 1 M at a flow rate of 1.5 ml/min.
Purification and assay of viral DNA polymerases.
HSV-1 and HSV-2 DNA polymerases were partially purified from HSV-1 (KOS)- and HSV-2 (UW-268)-infected Vero cells, respectively, by DEAE cellulose and phosphocellulose ion-exchange chromatography as described previously (22). VZV DNA polymerase was partially purified from VZV (Kawaguchi)-infected human embryo lung cells as described above.
Standard assay mixtures of HSV DNA polymerases contained 50 mM Tris–HCl (pH 8.0), 1 mM DTT, 80 μg of calf thymus-activated DNA per ml, 8 mM MgCl2, 200 mM KCl, 0.5 mg of bovine serum albumin per ml, 32 μM dATP, 32 μM dCTP, 32 μM TTP, 16 μM [3H]dGTP, and the purified enzyme. For kinetic experiments, concentrations of [3H]dGTP and the inhibitor were varied. Standard assay mixtures of VZV DNA polymerases contained 50 mM Tris–HCl (pH 8.0), 1 mM DTT, 20 μg of poly(dC) oligo(dG)12–18 per ml, 1 mM MgCl2, 0.5 mg of BSA per ml, 1 μM [3H]dGTP, and the purified enzyme. The reaction was performed at 37°C for 15 min and was terminated by adding an excess amount of ice-cold 10% trichloroacetic acid and 0.1% NaPPi. The precipitated DNA was filtered with Whatman GF/C filter disks. The filters were washed twice with 5% trichloroacetic acid and 0.02% NaPPi, washed twice with 50% EtOH, washed once with 99% EtOH, and then dried. Radioactivity was determined by scintillation counting.
DNA elongation assay.
For the DNA elongation assay with internal labeling (Fig. 8), M13mp18 single-stranded DNA was annealed to a universal primer (17 mer) and used as a template primer. Reaction mixtures (6 μl), comprising 30 mM Tris–HCl (pH 7.5), 1 mM DTT, 3 mM MgCl2, 50 mM KCl, 33.3 μM dATP, 33.3 μM TTP, 33.3 μM dGTP, 3.3 μM dCTP [α-32P]dCTP, 38 μg of template-primer per ml, various concentrations of inhibitor (A-5021 triphosphate or ACV triphosphate), and HSV-2 DNA polymerase, were incubated at 37°C for 10 min. The reaction was stopped by adding sequencing dye (4 μl) to the mixture, and then an aliquot was applied to 6% sequencing gel.
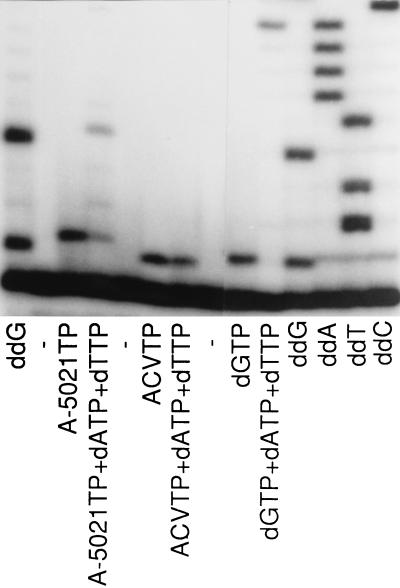
FIG. 8.
DNA chain extension by HSV-2 DNA polymerase. The reaction was performed with M13mp18 single-stranded DNA and a 5′ 32P-end-labeled specific oligomer (17 mer) as the template and the primer, respectively, in the presence or absence of 50 μM dATP, dTTP, and/or inhibitor as shown. Lanes ddG, ddA, ddT, and ddC, dideoxy sequencing with ddGTP, ddATP, ddTTP, and ddCTP, respectively. Samples were analyzed on a 15% sequencing gel, followed by autoradiography.
For the DNA elongation assay with end labeling, M13mp18 single-stranded DNA was annealed to a 5′ end-labeled universal primer (17 mer) and used as a template primer. Reaction mixtures (6 μl), comprising 30 mM Tris–HCl (pH 7.5), 1 mM DTT, 3 mM MgCl2, 50 mM KCl, 33.3 μM dATP, 33.3 μM TTP, 33.3 μM dGTP, 33.3 μM dCTP, 38 μg of template-primer per ml, 30 mM inhibitor [A-5021 triphosphate or ACV triphosphate, and HSV-2 DNA polymerase, were incubated at 37°C for 10 min. Natural deoxynucleoside triphosphates (dNTPs) were omitted in accordance with the purpose of the assay. The reaction was stopped by adding sequencing dye (4 μl) to the mixture, and then an aliquot was applied to 15% sequencing gel.
The control sequence was confirmed by dideoxy sequencing with Sequenase version 2.0 (United States Biochemical, Cleveland, Ohio). The radioactive bands were detected by an image analyzer (model BAS2000; Fuji Photo Film Co., Ltd., Tokyo, Japan).
Measurement of intracellular phosphate esters.
To study the formation of intracellular phosphate ester, monolayers of MRC-5 cells in 25-cm2 flasks were infected with HSV-1 (KOS) or HSV-2 (UW-268) at a multiplicity of infection of 0.01 PFU/cell and cultured for 20 h as described by Vere Hodge and Perkins (25), except that the virus solution was absorbed for 1 h. For VZV, monolayers of MRC-5 cells in 25-cm2 flasks were infected with VZV-infected MRC-5 cells (exhibiting a minimum 70% cytopathic effect) at a ratio of 1:6 (infected/uninfected) and incubated for 48 h. At the start of drug treatment, the medium was replaced with 3 ml of fresh medium containing the 3H-labeled compound.
To study the intracellular stability of the phosphate esters, monolayers of MRC-5 cells in 25-cm2 flasks were infected with HSV-1 (KOS) or HSV-2 (UW-268) at a multiplicity of infection of 2 PFU/cell. After 1 h of absorption of the virus solution, the cells were treated with 3 ml of medium containing radioactive compounds and incubated for 5 h. VZV-infected cells (which had been cultured for 48 h after infection) were incubated with 3H-labeled compounds for 6 h. After incubation, the cells were washed three times with ice-cold PBS and incubated with prewarmed fresh drug-free medium (50 ml).
At each designated time, the medium was removed, cells were washed three times with ice-cold PBS, and the nucleotide analog phosphates were extracted with 3 ml of 50% ethanol containing 10% PBS (23). The extracts were evaporated under reduced pressure and resolved in 300 μl of distilled water. Samples were stored at −20°C until HPLC analysis with a Whatman Partisil 10 SAX column (250 by 4.6 mm). Elution was performed with a linear gradient of 0 to 1 M potassium phosphate (pH 3.5) at a flow rate of 1.5 ml/min. Radioactivity was monitored by a β-RAM radiodetector (IN/US Systems Inc. Tampa, Fla.). The amounts of the phosphates were calculated from the peak area with SCINTIFLOW software (IN/US Systems Inc.). A-5021 monophosphate, diphosphate, and triphosphate were eluted at about 7.5, 16.5, and 28 min, respectively. ACV monophosphate, diphosphate, and triphosphate were eluted at about 8, 17.5, and 29.5 min, respectively. PCV monophosphate, diphosphate, and triphosphate were eluted at about 7.5, 15, and 27 min, respectively.
RESULTS
Phosphorylation of A-5021 by viral TK.
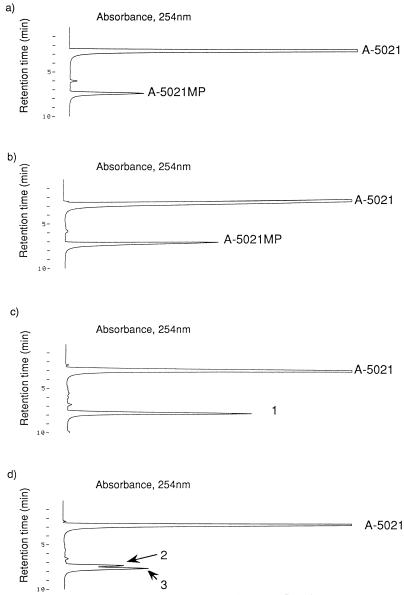
When 200 μM A-5021 was incubated with HSV-1 TK or VZV TK, an A-5021 monophosphate peak was easily detected by HPLC (Fig. 1a and b), but further phosphorylation of A-5021 monophosphate could not be detected (data not shown). The phosphorylation site of A-5021 was also revealed by HPLC. A-5021 has two free hydroxymethyl (-OH) groups on a pseudosugar moiety in its chemical structure. Two regioisomers of A-5021 monophosphate were synthesized in order to determine the site of A-5021 phosphorylation by viral TK reaction (Fig. 2). The retention time of the monophosphates was distinguished by HPLC with a SAX column. Because the retention time of A-5021 monophosphate derived from the viral TK reaction coincided with that of synthetic A-5021–2′-monophosphate (Fig. 1c and d), the -OH group at position 2′ was apparently phosphorylated by the viral TKs.
FIG. 1.
Phosphorylation of A-5021 by viral TKs and determination of the site of phosphorylation. A-5021 was incubated with HSV-1 (a) or VZV TK (b) at 37°C for 24 h. A-5021 monophosphate (A-5021MP) was detected by HPLC. The reaction solution of VZV TK was mixed with chemically synthesized A-5021–2′-monophosphate (MP) (C) or A-5021–1′-MP (d) and was analyzed by HPLC. Peak number 1, peak containing chemically synthesized A-5021–2′-MP and A-5021MP from viral TK; peak number 2, A-5021–1′-MP; peak number 3, A-5021MP from viral TK.
Inhibitory effect of A-5021 on thymidine phosphorylation by viral TKs.
The inhibitory effect of A-5021 on viral TKs was compared with those of ACV and PCV. The IC50 of A-5021 for phosphorylation of 1 μM thymidine by HSV-1 or HSV-2 TK was similar to that of PCV and much lower than that of ACV (by HSV-1, 95-fold; by HSV-2, 6.7-fold) (Table 1). In the case of VZV TK, A-5021 was the most potent inhibitor of these three agents (Table 1).
TABLE 1.
IC50s for viral TKs
| TK | IC50 (μM) ofa:
|
||
|---|---|---|---|
| A-5021 | ACV | PCV | |
| HSV-1 | 7.7 ± 1.1 | 734 ± 135 | 6.4 ± 0.8 |
| HSV-2 | 73 ± 5 | 488 ± 63 | 83 ± 1 |
| VZV | 659 ± 34 | >1,000 | >1,000 |
IC50s for 1 μM [3H]thymidine phosphorylation are the means ± standard errors of three determinations.
Formation of A-5021 phosphates in virus-infected MRC-5 cells.
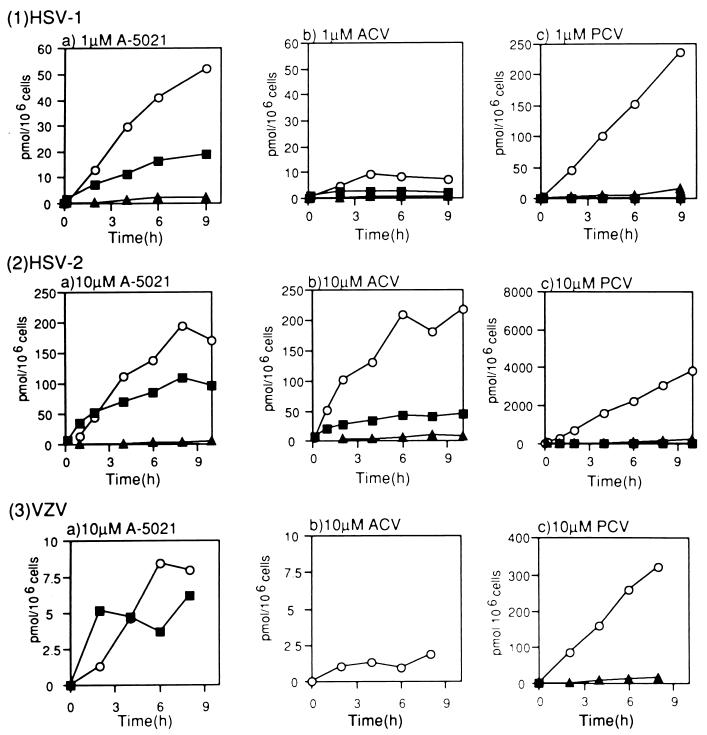
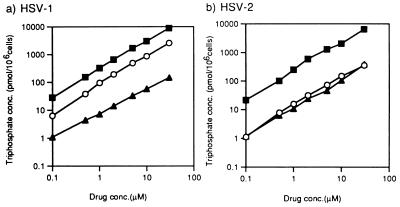
The intracellular concentrations of phosphates in infected cells were measured after incubation with A-5021, ACV, or PCV at 1 μM (HSV-1) and 10 μM (HSV-2 and VZV). Formation of A-5021 monophosphate, diphosphate, and triphosphate was detected in the infected MRC-5 cells (Fig. 3). In HSV-1- and VZV-infected cells, the rate of formation and the amount of A-5021 triphosphate exceeded those of ACV triphosphate (Fig. 4), while these parameters were equivalent for the two compounds in HSV-2-infected MRC-5 cells. However, more PCV triphosphate than A-5021 triphosphate formed and accumulated in all virus-infected MRC-5 cells. A-5021 triphosphate could not be detected in uninfected cells under the same conditions (data not shown). After continuous exposure for 6 h, the intracellular concentrations of the phosphates of these three agents in HSV-1- and HSV-2-infected MRC-5 cells showed a linear correlation with the drug concentrations from 0.1 to 30 μM (Fig. 5).
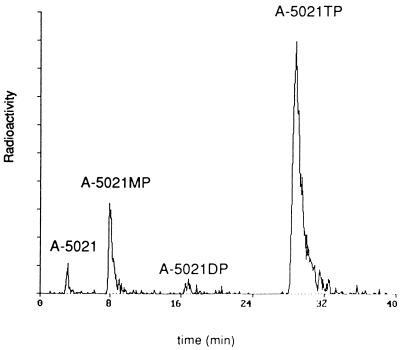
FIG. 3.
HPLC analysis of A-5021 and its phosphate esters in HSV-1-infected MRC-5 cells. HSV-1-infected MRC-5 cells were treated with 1 mM [3H]A-5021 for 6 h, and the extract was analyzed by HPLC. MP, monophosphate; DP, diphosphate; TP, triphosphate.
FIG. 4.
Formation of phosphate esters of nucleoside analogs in virus-infected MRC-5 cells. MRC-5 cells infected with HSV-1 (1), HSV-2 (2), or VZV (3) were treated with [3H]A-5021 (a), [3H]ACV (b), or [3H]PCV (c) at the indicated concentrations. At the designated times, cells were extracted, and the extracts were analyzed by HPLC. Open circles, triphosphate; closed triangles, diphosphate; closed squares, monophosphate.
FIG. 5.
Concentration dependency of formation of triphosphate esters of nucleoside analogs in virus-infected MRC-5 cells. MRC-5 cells infected with HSV-1 (a) or HSV-2 (b) were treated with various concentrations of [3H]A-5021, 3H[ACV], or [3H]PCV for 6 h. Then cells were extracted, and the extracts were analyzed by HPLC. Open circles, A-5021 triphosphate; closed triangles, ACV triphosphate; closed squares, PCV triphosphate. Conc, concentration.
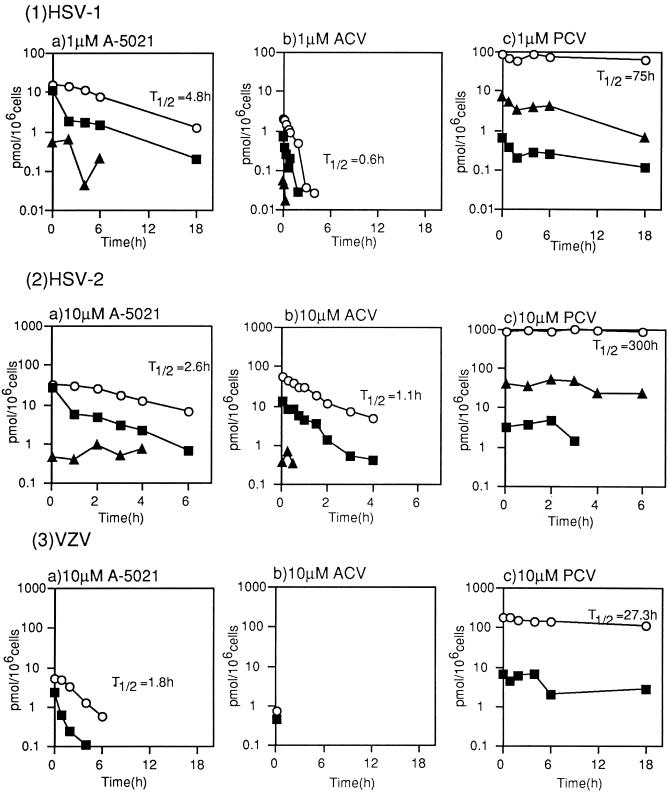
Intracellular stability of A-5021 triphosphate in virus-infected MRC-5 cells.
The changes in the intracellular concentrations of A-5021 triphosphate, ACV triphosphate, and PCV triphosphate in infected cells were measured after incubation with these agents for 5 to 6 h at 1 μM (HSV-1) or 10 μM (HSV-2 and VZV), followed by exposure to drug-free medium. A-5021 triphosphate was more stable than ACV triphosphate and had a longer half-life in MRC-5 cells infected with HSV-1 and HSV-2 (Fig. 6). In VZV-infected MRC-5 cells, the half-life of ACV triphosphate could not be measured because of low accumulation. The half-life of A-5021 triphosphate was shorter than that of PCV triphosphate in HSV-1-, HSV-2-, and VZV-infected MRC-5 cells.
FIG. 6.
Intracellular stability of phosphate esters of nucleoside analogs in virus-infected MRC-5 cells. MRC-5 cells infected with HSV-1 (1), HSV-2 (2), or VZV (3) were treated with [3H]A-5021 (a) [3H]ACV (b), or [3H]PCV (c) at the concentrations shown for 5 h (HSV-1 and HSV-2) or 6 h (VZV). After extracellular compounds were removed, the levels of intracellular phosphate esters were analyzed by HPLC. Open circles, triphosphate; closed triangles, diphosphate; closed squares, monophosphate. T1/2, half-life.
Inhibitory effect of A-5021 triphosphate on viral DNA polymerases.
A-5021 triphosphate inhibited HSV-1 DNA polymerase in a competitive manner with respect to dGTP (data not shown). A-5021 showed strong inhibition of this viral DNA polymerase, with the Ki value for HSV-1 DNA polymerase being 12-fold lower than the Km value for dGTP (Table 2). This Ki value was 185-fold lower than that for (R,S)-PCV triphosphate and 3.9-fold higher than that for ACV triphosphate (Table 2). The same order of inhibitory effects was observed among these three triphosphates in the case of HSV-2 (Table 2) and VZV DNA polymerases [IC50s of A-5021TP, ACVTP, and (R,S)-PCVTP were 0.014, 0.0053, and 3.6 μM, respectively].
TABLE 2.
Inhibition of HSV DNA polymerases by A-5021 triphosphatea
| Polymerase | Km (μM)b dGTP |
Ki (μM) for indicated triphosphatec:
|
||
|---|---|---|---|---|
| A-5021 | ACV | (R,S)PCVd | ||
| HSV-1 (KOS) | 0.24 ± 0.01 | 0.020 ± 0.002 | 0.0051 ± 0.0012 | 3.7 ± 0.1 |
| HSV-2 (UW-268) | 0.18 ± 0.01 | 0.017 ± 0.003 | 0.0067 ± 0.0007 | 3.0 ± 0.2 |
Activated calf thymus DNA was used as the template primer. Values are the means ± standard errors of three determinations.
Km from Lineweaver-Burk plots.
Ki from secondary plots of Lineweaver-Burk plots.
All triphosphates of nucleoside analogs were chemically synthesized. (R,S)-PCV triphosphate was the racemic form, while the biosynthesized form in HSV-1-infected cells was almost entirely the (S)-enantiomer (>95%). In HSV-2-infected cells, 90% of the triphosphate was (S)- and 10% was the (R)-enantiomer (26).
DNA extension assay.
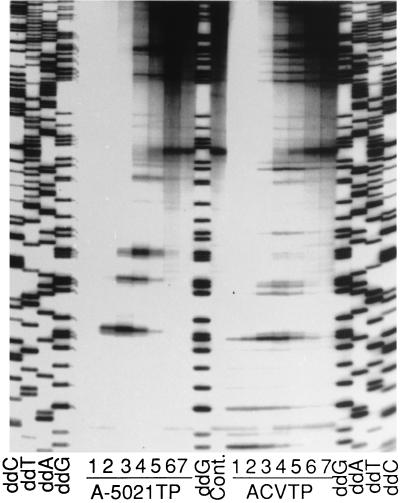
The mechanism of DNA chain termination by A-5021 triphosphate was studied with HSV-2 DNA polymerase. M13mp18 single-stranded DNA and universal primer were used as the template and primer, respectively. Figure 7 shows the inhibition of DNA synthesis by A-5021 triphosphate and ACV triphosphate. DNA synthesis by HSV-2 DNA polymerase was inhibited by both of these triphosphates in a concentration-dependent manner. Chain-terminated bands were detected at G sites in both cases, but the patterns of the gel bands were different. A-5021 triphosphate caused strong termination at tandem G repeats and weak termination at single G sites, but this tendency was marginal in the case of ACV triphosphate. Figure 8 shows the pattern of the DNA elongation adjacent to the primer. Both A-5021 triphosphate and ACV triphosphate were incorporated at the first G site when natural dNTP was omitted. A-5021-incorporated DNA had a slightly higher migration rate on the gel. When dATP, dCTP, and dTTP were added, a second chain-terminated band appeared at the site of the second G in the case of A-5021 triphosphate, but not with ACV triphosphate. This result was evidence that A-5021 triphosphate did not cause absolute chain termination the way ACV triphosphate did.
FIG. 7.
Chain termination by A-5021 triphosphate (A-5021TP) or ACV triphosphate (ACVTP). HSV-2 DNA polymerase was reacted with M13mp18 single-stranded DNA and specific oligomer (17 mer) as a template and a primer, respectively, in the presence of fixed concentrations of natural dNTPs and various concentrations of A-5021TP or ACVTP (lane 1, 1 mM; lane 2, 300 μM; lane 3, 100 μM; lane 4, 30 μM; lane 5, 10 μM; lane 6, 3 μM; lane 7, 1 μM). Cont., no chain terminator. Lanes ddG, ddA, ddT, and ddC, dideoxy sequencing with ddGTP, ddATP, ddTTP, and ddCTP, respectively. Samples were analyzed on a 6% sequencing gel, followed by autoradiography.
DISCUSSION
In this study, we characterized the mode of action of A-5021 and obtained data possibly explaining its superior antiviral activity relative to those of ACV and PCV against HSV-1, HSV-2, and VZV.
A-5021 has only weak antiviral activity against TK-deficient strains of HSV-1 and HSV-2, suggesting that it is phosphorylated mainly by viral TKs (14). The present study clearly showed that A-5021 was monophosphorylated by purified HSV-1 and VZV TKs at the 2′-OH site (Fig. 1). However, A-5021 monophosphate was not phosphorylated to the diphosphate form by the viral kinases. In the case of ACV monophosphate, there is phosphorylation to the triphosphate forms by cellular kinases (20, 21). A-5021–2′-monophosphate was diphosphorylated by GMP kinase and triphosphorylated by nucleoside diphosphate kinase in a cell-free system (22a), so it is thought to be phosphorylated to the triphosphate form by cellular kinases in HSV-and VZV-infected cells.
The IC50s of A-5021 for HSV-1 and HSV-2 TK were almost the same as those for PCV but were much lower than those for ACV (Table 1). Of these three agents, A-5021 had the lowest IC50 for VZV TK (Table 1). These results suggest that A-5021 was phosphorylated far more efficiently than ACV.
Our data showed that A-5021 triphosphate acted as a potent inhibitor of viral DNA polymerases. The apparent Ki of A-5021 triphosphate for HSV-1 DNA polymerase was lower than the Km for dGTP (Table 2). The apparent Ki of A-5021 triphosphate for HSV-1 DNA polymerase was comparable to that for HSV-2 DNA polymerase and was slightly higher than that of ACV triphosphate (Table 2). The IC50 of A-5021 triphosphate for VZV DNA polymerase was also slightly higher than that of ACV triphosphate, whereas the apparent Kis (or IC50s) values of A-5021 triphosphate for viral DNA polymerases were much lower than those of (R,S)-PCV triphosphate (Table 2), suggesting that the strong affinity of A-5021 triphosphate for viral DNA polymerases contributes to the potency of the antiviral activity of A-5021 compared with that of PCV.
A-5021 was reported to show 25-fold-more-potent antiviral activity than ACV against HSV-1 (KOS) and 5-fold-more-potent antiviral activity than ACV against VZV (Kawaguchi) but was only 2-fold more potent than ACV against HSV-2 (UW-268) in MRC-5 cells (14). In the present study, we found that the rate of A-5021 triphosphate formation and its level of accumulation were higher than those of ACV triphosphate in HSV-1- and VZV-infected MRC-5 cells but were similar in HSV-2-infected MRC-5 cells (Fig. 4 and 5). It seems likely that the high accumulation of A-5021 triphosphate in HSV-1- and VZV-infected cells contributes to its more potent antiviral activity against HSV-1 and VZV compared with that of ACV. However, it was not clear why A-5021 showed a stronger antiviral activity than did ACV against HSV-2 (UW-268). On the other hand, the accumulation of PCV triphosphate was much higher than that of A-5021 triphosphate in all of the virus-infected cells that we studied (Fig. 4 and 5). However, PCV triphosphate was a weak inhibitor of viral DNA polymerases, while A-5021 and ACV triphosphates were strong inhibitors (Table 2), so A-5021 was more potent than PCV against HSV-1, HSV-2, and VZV.
We previously showed that A-5021 has prolonged antiviral activities against HSV-1 and HSV-2 in in vitro virus yield experiments with short periods of drug treatment (14). The prolonged antiviral activity of A-5021 is greater than that of ACV but less than that of PCV. In this study, we found that A-5021 triphosphate was more stable than ACV triphosphate in HSV-1- and HSV-2-infected cells but less stable than PCV triphosphate (Fig. 6). This result correlates with the extent of prolonged antiviral effect. The intracellular stability of the triphosphate forms of PCV and (R)-9-[hydroxy-2-(hydroxymethyl)-butyl]guanine (H2G) is thought to contribute their prolonged antiviral effect (1, 7, 19, 25). The relatively high intracellular stability of A-5021 triphosphate may also contribute to its prolonged antiviral activity.
Lineweaver-Burk plots revealed that A-5021 triphosphate acted as a competitive inhibitor of dGTP by HSV DNA polymerases. In the DNA elongation study with HSV-2 DNA polymerase, highly processive DNA synthesis was inhibited by A-5021 triphosphate in a dose-dependent manner, and this triphosphate was incorporated into DNA at G sites and terminated elongation. However, the mode of chain termination was different from that of ACV. There are two -OH groups in the pseudosugar moiety of A-5021; the -OH at the 2′ site was phosphorylated by viral TKs (Fig. 1), so the other -OH at the 1′ site may act as the 3′-OH of dGTP. We found that further DNA elongation could occur even after incorporation of A-5021 monophosphate at the 3′ end of DNA (Fig. 8). These observations suggested that A-5021 was a pseudo-chain terminator like PCV. Interestingly, A-5021 triphosphate strongly terminated DNA elongation at tandem G repeats (Fig. 7). It is possible that tandem incorporation of A-5021 monophosphate into the 3′ end of DNA causes greater conformational distortion than incorporation of a single monophosphate, thereby limiting further DNA elongation, particularly after the tandem G repeat. Conformational distortion was shown to be actually induced in ganciclovir monophosphate-incorporated DNA by a study with solution-state nuclear magnetic resonance (8). The base of ganciclovir normally shows hydrogen binding to complementary dCMP, but significant distortion is observed at or near the site of ganciclovir incorporation.
The antiviral activity of nucleoside analogs is principally dependent on both the intracellular concentration of the triphosphates achieved in infected cells and the inhibitory effect of the triphosphates on viral DNA polymerases. Although A-5021 was not always superior to ACV or PCV in these individual factors, A-5021 is the most effective of these three antiviral agents (14). The superior antiviral activity of A-5021 against HSV-1 and VZV may well be explained by the integrated effect of these two factors. The prolonged antiviral effect of A-5021 could also be explained by the stability of its triphosphate in virus-infected cells.
ACKNOWLEDGMENT
We thank T. Tsurumi for helpful discussions.
REFERENCES
- 1.Abele G, Cox S, Bergman S, Lindbergh N, Vissgarden A, Karlstorm A, Harmenberg J, Wahren B. Antiviral activity against VZV and HSV type 1 and type 2 of the (+) and (−) enantiomers of (R,S)-9-/4-hydroxy-2-(hydroxymethyl)butyl/guanine, in comparison to other closely related acyclic nucleosides. Antivir Chem Chemother. 1991;2:163–169. [Google Scholar]
- 2.Bacon T H, Gilbart J, Howard B A, Standring-Cox R. Inhibition of varicella-zoster virus by penciclovir in cell culture and mechanism of action. Antivir Chem Chemother. 1996;7:71–78. [Google Scholar]
- 3.Boyd M R, Bacon T H, Sutton D, Cole M. Antiherpesvirus activity of 9-(4-hydroxy-3-hydroxy-methylbut-1-yl)guanine (BRL 39123) in cell culture. Antimicrob Agents Chemother. 1987;31:1238–1242. doi: 10.1128/aac.31.8.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng Y C, Ostrander M. Deoxythymidine kinase induced in HeLa TK− cells by herpes simplex virus type I and type II. II. Purification and characterization. J Biol Chem. 1976;251:2605–2610. [PubMed] [Google Scholar]
- 5.Darby, G. 1994. A history of antiherpes research. Antivir. Chem. Chemother. 5(Suppl. 1):3–9.
- 6.Dixit V M, Poulter C D. Convenient syntheses of adenosine 5′-diphosphate, adenosine 5′-methylenediphosphonate, and adenosine 5′-triphosphate. Tetrahedron Lett. 1984;25:4055–4056. [Google Scholar]
- 7.Earnshaw D L, Bacon T H, Darlison S J, Edmonds K, Perkins R M, Vere Hodge R A. Mode of antiviral action of penciclovir in MRC-5 cells infected with herpes simplex virus type 1 (HSV-1), HSV-2, and varicella-zoster virus. Antimicrob Agents Chemother. 1992;36:2747–2757. doi: 10.1128/aac.36.12.2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foti M, Marshalko S, Schurter E, Kumar S, Beardsley G P, Schweitzer B I. Solution structure of a DNA decamer containing the antiviral drug ganciclovir: combined use of NMR, restrained molecular dynamics, and full relaxation matrix refinement. Biochemistry. 1997;36:5336–5345. doi: 10.1021/bi962604e. [DOI] [PubMed] [Google Scholar]
- 9.Furman P A, St. Clair M H, Fyfe J A, Rideout J L, Keller P M, Elion G B. Inhibition of herpes simplex virus-induced DNA polymerase activity and viral DNA replication by 9-(2-hydroxyethoxymethyl)guanine and its triphosphate. J Virol. 1979;32:72–77. doi: 10.1128/jvi.32.1.72-77.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Furman P A, de Miranda P, St. Clair M, Elion G B. Metabolism of acyclovir in virus-infected and uninfected cells. Antimicrob Agents Chemother. 1981;20:518–524. doi: 10.1128/aac.20.4.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fyfe J A, Keller P M, Furman P A, Miller R L, Elion G B. Thymidine kinase from herpes simplex virus phosphorylates the new antiviral compound, 9-(2-hydroxyethoxymethyl)guanine. J Biol Chem. 1978;253:8721–8727. [PubMed] [Google Scholar]
- 12.Fyfe J A. Differential phosphorylation of (E)-5-(2-bromovinyl)-2′-deoxyuridine monophosphate by thymidine kinase from herpes simplex viruses type 1 and 2 and varicella zoster virus. Mol Pharmacol. 1982;21:432–437. [PubMed] [Google Scholar]
- 13.Geen G R, Grinter T J, Kincey P M, Jarvest R L. The effect of the C-6 substituent on the regioselectivity of N-alkylation of 2-aminopurines. Tetrahedron. 1990;46:6903–6914. [Google Scholar]
- 14.Iwayama S, Ono N, Ohmura Y, Suzuki K, Aoki M, Nakazawa H, Oikawa M, Kato T, Okunishi M, Nishiyama Y, Yamanishi K. Antiherpesvirus activities of (1′S,2′R)-9-{[1′,2′-bis(hydroxymethyl)cycloprop-1′-yl]methyl}guanine (A-5021) in cell culture. Antimicrob Agents Chemother. 1998;42:1666–1670. doi: 10.1128/aac.42.7.1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jarvest R L, Barnes R D, Earnshaw D L, O’Toole K J, Sime J T, Vere Hodge R A. Synthesis of isotopically chiral [13C]penciclovir (BRL 39123) and its use to determine the absolute configuration of penciclovir triphosphate formed in herpes virus infected cells. J Chem Soc Chem Commun. 1990;7:555–556. [Google Scholar]
- 16.Kowal E P, Markus G. Affinity chromatography of thymidine kinase from a rat colon adenocarcinoma. Prep Biochem. 1976;6:369–385. doi: 10.1080/00327487608061625. [DOI] [PubMed] [Google Scholar]
- 17.Lacey S F, Suzutani T, Powell K L, Purifoy D J, Honess R W. Analysis of mutations in the thymidine kinase genes of drug-resistant varicella-zoster virus populations using the polymerase chain reaction. J Gen Virol. 1991;72:623–630. doi: 10.1099/0022-1317-72-3-623. [DOI] [PubMed] [Google Scholar]
- 18.Larsson A, Stenberg K, Ericson A C, Haglund U, Yisak W A, Johansson N G, Oberg B, Datema R. Mode of action, toxicity, pharmacokinetics, and efficacy of some new antiherpesvirus guanosine analogs related to buciclovir. Antimicrob Agents Chemother. 1986;30:598–605. doi: 10.1128/aac.30.4.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lowe D M, Alderton W K, Ellis M R, Parmar V, Miller W H, Roberts G B, Fyfe J A, Gaillard R, Ertl P, Snowden W, Littler E. Mode of action of (R)-9-[4-hydroxy-2-(hydroxymethyl)butyl]guanine against herpesviruses. Antimicrob Agents Chemother. 1995;39:1802–1808. doi: 10.1128/aac.39.8.1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller W H, Miller R L. Phosphorylation of acyclovir (acycloguanosine) monophosphate by GMP kinase. J Biol Chem. 1980;255:7204–7207. [PubMed] [Google Scholar]
- 21.Miller W H, Miller R L. Phosphorylation of acyclovir diphosphate by cellular enzymes. Biochem Pharmacol. 1982;31:3879–3884. doi: 10.1016/0006-2952(82)90305-7. [DOI] [PubMed] [Google Scholar]
- 22.Nishiyama Y, Suzuki S, Yamauchi M, Maeno K, Yoshida S. Characterization of an aphidicolin-resistant mutant of herpes simplex virus type 2 which induces an altered viral DNA polymerase. Virology. 1984;135:87–96. doi: 10.1016/0042-6822(84)90119-3. [DOI] [PubMed] [Google Scholar]
- 22a.Ono, N. Unpublished observations.
- 23.Sekiyama T, Hatsuya S, Tanaka Y, Uchiyama M, Ono N, Iwayama S, Oikawa M, Suzuki K, Okunishi M, Tsuji T. Synthesis and antiviral activity of novel acyclic nucleosides: discovery of a novel cyclopropyl nucleoside with potent inhibitory activity against herpesviruses. J Med Chem. 1998;41:1284–1298. doi: 10.1021/jm9705869. [DOI] [PubMed] [Google Scholar]
- 24.Siragami H, Koguchi Y, Tanaka Y, Takamatsu S, Uchida Y, Ineyama T, Izawa K. Synthesis of 9-(2-hydroxyethoxy methyl) guanine (acyclovir) from guaninosine. Nucleosides Nucleotides. 1995;14:337–340. [Google Scholar]
- 25.Vere Hodge R A, Perkins R M. Mode of action of 9-(4-hydroxy-3-hydroxymethylbut-1-yl)guanine (BRL 39123) against herpes simplex virus in MRC-5 cells. Antimicrob Agents Chemother. 1989;33:223–229. doi: 10.1128/aac.33.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vere Hodge R A, Darlison S J, Earnshaw D L, Readshaw S A. Use of isotopically chiral [4′-13C]penciclovir and 13C NMR to determine the specificity and absolute configuration of penciclovir phosphate esters formed in HSV-1- and HSV-2-infected cells and by HSV-1-encoded thymidine kinase. Chirality. 1993;5:583–588. doi: 10.1002/chir.530050804. [DOI] [PubMed] [Google Scholar]