Abstract
Purpose of Review
The purpose of this review is to provide a comprehensive analysis of heterotopic ossification (HO) in pediatric patients, including an in-depth examination of the risk factors associated with this condition, current prophylactic measures, and available management strategies.
Recent Findings
HO is a medical disorder in which bone tissue inexplicably develops in soft tissues such as muscles and tendons. It involves the formation of mature, lamellar bone in extra-skeletal soft tissue, and its formation is influenced by oxygen tension, pH, the availability of micronutrients, and mechanical stimulation. HO has many cellular origins, with the most common theory being multipotent cells in local tissue. The diagnosis of HO is typically made based on exam, radiographs, and CT. Management includes both prophylactic nonsurgical options and surgical resection for severe or recalcitrant cases.
Summary
The review highlights the incidence, risk factors, and management strategies associated with HO in pediatric patients. HO is a rare condition in children, with severe neurologic injury being the most common cause. Pediatric patients most commonly develop HO following severe neurologic injury, followed by trauma and surgery. Current prophylactic measures, include nonsteroidal anti-inflammatory drugs and radiation therapy though limited literature on their use in the pediatric population exists. For recalcitrant symptomatic cases, wide surgical resection can be considered but has a higher risk profile and associated morbidity. This review highlights the need for further pediatric specific research to inform guidelines and management strategies for this debilitating condition.
Keywords: Pediatric heterotopic ossification, Pediatric heterotopic ossification prophylaxis, Pediatric heterotopic ossification management, Pediatric heterotopic ossification risk factors
Introduction
Heterotopic ossification (HO) is a medical disorder in which bone tissue develops in soft tissues like muscles and tendons [1••]. HO involves the formation of mature, lamellar bone in extra-skeletal soft tissue [1••, 2, 3, 4••, 5, 6]. The most frequent causes of heterotopic ossification include joint replacement, heat injury, fractures of the elbow and acetabulum, spinal cord injury, traumatic brain injury, and blast trauma [5, 7]. While some HO lesions may be minor and clinically insignificant, others may cause significant morbidity [4••]. This review aims to provide a comprehensive analysis of the incidence of HO in pediatric patients, as well as an in-depth examination of the risk factors associated with this condition, current prophylactic measures, and available management strategies.
Epidemiology
Heterotopic ossification (HO) exhibits a lower incidence in children compared to adults, with reports ranging between 4 and 22% [5, 8, 9•, 10]. In a comprehensive single institutional study of 643 patients admitted for neuropediatric rehabilitation, HO was diagnosed in 5% (n = 32) of patients, with the hip joint most frequently involved (n = 17) followed by the shoulder (n = 9), elbow (n = 9), and knee (n = 6) [5]. Rarely, a patient will develop multiple sites of HO [5, 11, 12]. Unlike in adults, there is no male predominance in pediatric HO, though increased age may be associated with higher likelihood [5, 9•, 10, 13, 14]. Pediatric patients most commonly develop HO following severe neurologic injury, followed by trauma and surgery [5].
Risk Factors
Heterotopic ossification can be a disabling complication for severe central nervous system insults, including traumatic and anoxic brain injury, encephalitis, and spinal cord injury [5, 10, 15]. Kluger et al. reported the development of HO in 7.9% of severe cases of traumatic brain injury and 3.8% of near-drowning cases [5]. Preexisting neurologic conditions, such as cerebral palsy, may predispose patients to HO development depending on the degree of impairment [13, 16].
Traumatic burn injury is a well-documented cause of HO with incidence as high as 35% when patients are screened, although only 0.25% cause significant impairment [11, 17]. The risk is tightly associated with total burn surface area with greatest prevalence in the elbow [11, 12, 17]. Besides burns on TBI, other traumatic events such as fractures, dislocations, and ligament sprains can also precede HO [3, 18–21].
Surgery has also been seen as a risk factor for development of HO in pediatrics and adults [22]. HO has been reported in pediatric internal fracture fixation and elbow arthroscopy [9•, 23–27]. Feroe et al. reported HO in 4.5% of pediatric patients within 2 years of hip arthroscopy, most often pericapsular [9•, 28]. Elbow arthroscopy appears to be a less common cause of HO, although this may change as procedures become more complex [24, 25]. The use of mechanical hardware has also been implicated in the development of HO, particularly in rigid intramedullary fixation for diaphyseal femoral shaft fractures with a protruding proximal rod [29–31]. Sutphen et al. found HO in 24% of patients with rigid femoral nails versus 5.9% with submuscular plate and none with flexible nail [27]. While adults have reported HO related to traction pins, no such cases have been reported in pediatric use [26]. Though very uncommonly performed in the pediatric population, total joint arthroplasty is a common cause of HO in adults that has additionally been well studied and documented [4••].
In extremely rare cases, HO can be attributed to a genetic cause. Fibrodysplasia ossificans progressiva (FOP), for instance, is a condition with a prevalence of 1.36 per million where polytopic HO is typically preceded by other congenital malformations [32–34]. Progressive osseous heteroplasia bears resemblance to FOP but emerges in infancy and is characterized by dermal ossifications that progress into deeper tissue [6]. Additionally, symptomatic HO has been associated with Albright hereditary osteodystrophy and ankylosing spondylitis [35, 36].
Clinical Presentation and Imaging
The onset of HO is variable, presenting within 3 weeks to 20 months after an inciting injury, with typical presentation within 4 months [5, 11, 12, 18, 19]. Though HO progresses more rapidly in children, pediatric cases are more likely to follow an indolent course [11, 12]. Severe HO predominantly manifests with sharp pain, decreased range of motion, and localized soft tissue swelling. Rare cases of bilateral joint involvement have been reported in neurologic and burn injury [12, 15, 37]. Clinical presentation can be further influenced by the location and size of the lesion, with findings including fever, palpable mass, neurovascular compression, and gait abnormalities [3, 11, 12, 15, 37] (Fig. 1).
Fig. 1.
A Computed tomography 3D reconstructions and B intra-operative fluoroscopy demonstrating heterotopic ossification of the right femur and ischium in a 16-year male who suffered severe traumatic brain injury from a motor vehicle accident. C Intra-operative fluoroscopy demonstrating resection of the prior ischial heterotopic ossification
Radiography is the primary modality for diagnosing HO, demonstrated as a peripheral zone of ossification which matures to a well-defined cancellous bone [38••]. HO typically only affects soft tissue, but it can also adhere to the surface of the bone (also termed parosteal HO) [4••]. To its disadvantage, radiography cannot detect HO until 4 to 8 weeks after symptom onset [12, 39]. For early diagnosis, triphasic nuclear bone scans and ultrasound are more sensitive [5, 17, 39]. Ultrasound is particularly advantageous due to bedside application, lack of radiation, and ability to quantitatively monitor progression through gray-scale variability [40]. Serum alkaline phosphatase can be elevated in the setting of HO, though was only seen increased above age-related normal levels in 16% (4/24) of patients by Kluger et al [5]. In magnetic resonance imaging (MRI) studies, the acute phase of HO has increased tissue vascularization and density [38••]. When HO is mature, it manifests as hyperintense cancellous fat that is bordered by hypointense cortical bone on T1 and T2 weighted imaging [41]. Computed tomography provides the most precise localization of HO and its relationship to surrounding tissues and can be useful for surgical planning [5, 12, 17, 42].
Histology
While the exact etiology of HO formation is unclear, studies suggest that HO results from the transformation of progenitor cells into osteogenic precursor cells as a result of cell-mediated interactions with the local tissue environment, which is influenced by oxygen tension, pH, the availability of micronutrients, and mechanical stimulation [7, 42–44]. Tissues of HO are usually disorganized and inhomogeneous [43]. HO has been thought to have many cellular origins including satellite cells, smooth muscle cells, and even endothelial cells, with the most common theory it being multipotent cells in local tissue [38••, 45–47]. An osteogenic precursor, an inducing agent, and an environment that is favorable for osteogenesis are all prerequisites for the creation of HO, which will eventually lead to proliferation and the formation of bone [38••, 41, 43].
According to histological investigations, both the endochondral process, which needs a cartilage template, and the intramembranous process, which does not necessitate cartilage production, can result in heterotopic ossification [48]. HO is defined by zonal distribution of proliferating fibroblasts that are surrounded by metaplastic trabecular bone [4••, 43, 49]. Early lesions have a high mitotic index with plump fibroblasts [44, 50]. Lesions can be several centimeters in size, and can comprise of exuberant, cellular granulation tissue that can mimic a sarcoma [7]. The presence of osteoblasts distinguish HO from dystrophic calcification [43].Within 6 weeks, these lesions develop a characteristic zonal pattern with the outer portion of the mass populated with dense lamellar bone arranged as a pseudocortex with spicules of bone are progressively thinner toward the center of the lesion. After 6 months to a year, the lesions develop into an orderly arrangement of thick, mature trabecular bone [50].
Prophylaxis
Nonsteroidal Anti-Inflammatory Drugs
Though the indications, role, and efficacy of pharmacologic therapy for HO prophylaxis is debated, the primary treatment modality is nonsteroidal anti-inflammatory drugs (NSAIDs) [5, 8]. NSAIDs are inhibitors of COX-2 which can inhibit HO formation through two proposed mechanisms. (1) Inhibition of COX-2 prevents it facilitating the differentiation of mesenchymal cells into osteoblasts and (2) COX inhibition can prevent prostaglandin formation which plays a role in angiogenesis needed for endochondral ossification during bone formation [51]. In adults and adolescents older than 15 years, the historical gold standard is indomethacin 25 mg three times a day for 6 weeks after surgery. This was popularized by Ritter et al., who demonstrated significant decrease in the incidence of HO in “high-risk” total hip arthroplasty candidates after institutional policy to treat all “high-risk” patients prophylactically with indomethacin [52]. No data regarding dosing of indomethacin in pediatric patients for HO exists; however, for children between the ages of 2 and 15, indomethacin has been tolerated in patients with inflammatory arthropathies at 1 to 2 mg/kg/day in 3 to 4 divided doses with maximum daily dose of 4 mg/kg/day or 200 mg/day, whichever is less [53].
Currently, there are no consensus guidelines for use of NSAIDs for prophylaxis against HO in the pediatric population. In general, most providers will consider pharmacologic prophylactic therapy in pediatric patients at high-risk as outlined above such as those who recently suffered traumatic brain injury admitted to neuropediatric rehabilitation unit and those who have suffered traumatic burn injuries involving an at-risk joint. In Kluger et al., they remark on a shift in institutional policy between 1995 and 1998 to begin using salicylates for patients in coma or persistent vegetative state with warm and painful swelling of a joint [5]. Though they are unable to offer conclusive proof of its efficacy, they comment that in the 231 patients they admitted for neuropediatric rehabilitation over this period, they observed no new cases of heterotopic ossification compared with 32 patients out of 643 admitted between 1985 and 1994 [5].
Radiation
Low-dose radiation has been well studied in the adult literature as a prophylactic modality in the context of hip arthroplasty for primary HO occurrence or HO recurrence following resection [4••, 54]. For high-risk patients (those with hypertrophic osteoarthritis, ankylosing spondylitis, diffuse idiopathic skeletal hyperostosis, or prior HO), a single fraction of 700 cGy can be given either 24 h preoperatively or up to 72 h postoperatively with doses given outside this temporal window demonstrating a greater rate of HO [4••, 55, 56]. Radiation similarly to NSAIDs is thought to prevent osteoblast differentiation of mesenchymal pluripotent stem cells [57]. Though proven efficacious in limiting development of HO in the adult population in various settings such as post-op acetabular fracture fixation, it has a higher risk profile than indomethacin including arthrofibrosis, oncogenesis, and nonunion in the setting of fracture [4••, 58, 59]. Given these associated risks and the lack of data regarding safety and efficacy in the pediatric population, it is our understanding that the radiation is not routinely used for HO prophylaxis (Table 1).
Table 1.
Considerations for prophylaxis of pediatric heterotopic ossification in high-risk* patients
| Intervention | Dosing | Advantages | Limitations |
|---|---|---|---|
| Indomethacin | 1 to 2 mg/kg/day in 3 to 4 divided doses with maximum daily dose of 4 mg/kg/day or 200 mg/day, whichever is less | Ease of administration, proven efficacy in adult populations | Limited data within pediatric populations, relative contraindications include presence of kidney disease, blood coagulation deficiencies, and dyscrasias |
| Salicylates | 60 mg/kg of body weight, divided to maintain a mid-dose serum drug level of 15 to 20 mg/mL | Ease of administration, documented use in pediatric populations |
Limited high-level data regarding efficacy in pediatric populations Relative contraindications include presence of kidney disease, blood coagulation deficiencies, and dyscrasias |
| Radiation | A single fraction of 700 cGy can be given either 24 h preoperatively or up to 72 h | Proven efficacy in adult populations |
Not routinely used in pediatric populations High-risk profile including arthrofibrosis, oncogenesis, and nonunion in the setting of fracture |
*Examples of high-risk patients include those with traumatic brain injury, severe traumatic burn injury, hypertrophic osteoarthritis, ankylosing spondylitis, diffuse idiopathic skeletal hyperostosis, or prior heterotopic ossification
Surgical Interventions
Indications to proceed with surgical management are painful and restricted motion or overlying skin changes limiting function with many patients having tried non-operative modality to limit burden of symptoms. Ideal timing of surgical resection is debated though some waiting 1 year to allow osseous maturation and excision as surgical resection within 6 months has been associated with an increased risk of HO recurrence [60]. Advocates for earlier resection argue that it may prevent functional deficits seen from prolonged limited motion as HO matures [61]. Though uncommon, positron emission tomography (PET) CT with radiolabeled glucose (FDG) has been used in monitor HO flare-ups in the setting of genetic HO and can be considered a long with serum alkaline phosphatase to try and estimate HO maturity [62]. Goals of surgical management include complete resection which can involve extensile incision and wide exposures as incomplete resection of HO has been associated with recurrence [60]. After resection, early active range of motion is recommended to maintain range of motion after resection. As discussed above post-operative radiation or NSAIDs can be considered to prophylactically prevent recurrence of HO. Given the extensile approach and wide resection involved, excision of HO can carry a higher risk of blood loss and transfusion, infection, wound problems, and damage to surrounding neurovascular structures [4••, 63] (Fig. 2).
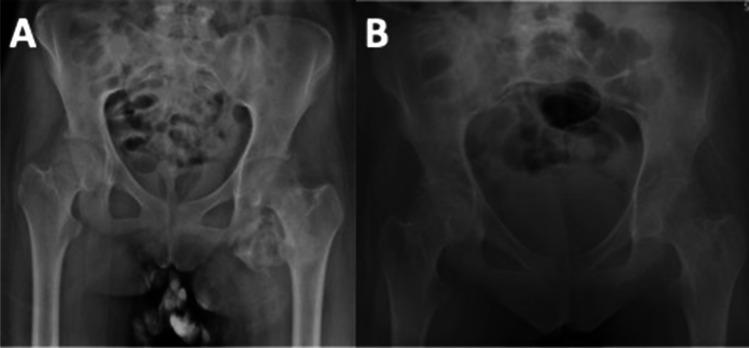
Fig. 2.
A AP pelvis radiographs demonstrating left hip heterotopic ossification in a 17-year-old female who 4 months prior had been involved in a motor vehicle accident suffering severe traumatic brain injury and B AP pelvis radiographs taken of the same patient in Fig. 2a after operative heterotopic ossification wide resection
Conclusion
Heterotopic ossification is a disabling condition that can occur in children. Risk factors for HO in pediatric patients include history of traumatic brain injury, trauma, and surgery. Diagnosis is made by physical exam and confirmed with imaging including radiographs and CT scan. Prophylaxis can be considered for high-risk surgical cases such as elbow or hip surgery, and it is typically comprised on indomethacin. However, the dosing and duration for prophylaxis in pediatric patients is unclear. We typically utilize 25 mg, three times a day for 5 days after higher risk procedures. Radiation is rarely considered in children but is an option in patients with significant risk. Surgical management is indicated for symptomatic HO, and typically occurs at least a year after onset to allow for maturation of the lesion and decrease risk of recurrence.
Declarations
Competing Interests
The authors declare no competing interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Alexander R. Markes, Email: Alexander.Markes@ucsf.edu
Nikit Venishetty, Email: nikit.venishetty@ttuhsc.edu.
Andrew Gatto, Email: agatto2@student.touro.edu.
Ishaan Swarup, Email: ishaan.swarup@ucsf.edu.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.•• Sun E, Hanyu-Deutmeyer AA. Heterotopic ossification. StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 [cited 2023 Mar 7]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK519029/. (Book chapter reviewing the etiology, presentation, and management options of patients with heterotopic ossification)
- 2.Cholok D, Chung MT, Ranganathan K, Ucer S, Day D, Davis TA, et al. Heterotopic ossification and the elucidation of pathologic differentiation. Bone. 2018;109:12–21. doi: 10.1016/j.bone.2017.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dua K, Barsi JM. Heterotopic ossification of the peroneus brevis tendon in a pediatric patient. J Foot Ankle Surg. 2017;56:1316–9. doi: 10.1053/j.jfas.2017.05.031. [DOI] [PubMed] [Google Scholar]
- 4.Meyers C, Lisiecki J, Miller S, Levin A, Fayad L, Ding C, et al. Heterotopic ossification: a comprehensive review. JBMR Plus. 2019;3:e10172. doi: 10.1002/jbm4.10172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kluger G, Kochs A, Holthausen H. Heterotopic ossification in childhood and adolescence. J Child Neurol. 2000;15:406–13. doi: 10.1177/088307380001500610. [DOI] [PubMed] [Google Scholar]
- 6.Kaplan FS, Shore EM. Progressive osseous heteroplasia. J Bone Miner Res. 2000;15:2084–94. doi: 10.1359/jbmr.2000.15.11.2084. [DOI] [PubMed] [Google Scholar]
- 7.Ranganathan K, Loder S, Agarwal S, Wong VW, Forsberg J, Davis TA, et al. Heterotopic ossification: basic-science principles and clinical correlates. J Bone Joint Surg Am. 2015;97:1101–11. doi: 10.2106/JBJS.N.01056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mital MA, Garber JE, Stinson JT. Ectopic bone formation in children and adolescents with head injuries: its management. J Pediatr Orthop. 1987;7:83–90. doi: 10.1097/01241398-198701000-00017. [DOI] [PubMed] [Google Scholar]
- 9.Feroe AG, Hassan MM, Flaugh RA, Maier SP, Cook DL, Yen Y-M, et al. Incidence and risk factors for heterotopic ossification in a matched cohort adolescent population undergoing hip arthroscopy. J Pediatr Orthop. 2022;42:e331–5. doi: 10.1097/BPO.0000000000002072. [DOI] [PubMed] [Google Scholar]
- 10.Hurvitz EA, Mandac BR, Davidoff G, Johnson JH, Nelson VS. Risk factors for heterotopic ossification in children and adolescents with severe traumatic brain injury. Arch Phys Med Rehabil. 1992;73:459–62. [PubMed] [Google Scholar]
- 11.Koch BM, Wu CM, Randolph J, Eng GD. Heterotopic ossification in children with burns: two case reports. Arch Phys Med Rehabil. 1992;73:1104–6. [PubMed] [Google Scholar]
- 12.Kornhaber R, Foster N, Edgar D, Visentin D, Ofir E, Haik J, et al. The development and impact of heterotopic ossification in burns: a review of four decades of research. Scars Burns Heal. 2017;3:2059513117695659. doi: 10.1177/2059513117695659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inan M, Chan G, Dabney K, Miller F. Heterotopic ossification following hip osteotomies in cerebral palsy: incidence and risk factors. J Pediatr Orthop. 2006;26:551–6. doi: 10.1097/01.bpo.0000217725.16417.af. [DOI] [PubMed] [Google Scholar]
- 14.Nuovo MA, Norman A, Chumas J, Ackerman LV. Myositis ossificans with atypical clinical, radiographic, or pathologic findings: a review of 23 cases. Skeletal Radiol. 1992;21:87–101. doi: 10.1007/BF00241831. [DOI] [PubMed] [Google Scholar]
- 15.Mohanty SS, Rao NN, Dash KK, Nashikkar PS. Postencephalitic bilateral heterotopic ossification of the hip in a pediatric patient. J Pediatr Orthop Part B. 2015;24:299–303. doi: 10.1097/BPB.0000000000000130. [DOI] [PubMed] [Google Scholar]
- 16.Lee M, Alexander MA, Miller F, Steg NL, McHugh BA. Postoperative heterotopic ossification in the child with cerebral palsy: three case reports. Arch Phys Med Rehabil. 1992;73:289–92. [PubMed] [Google Scholar]
- 17.Gaur A, Sinclair M, Caruso E, Peretti G, Zaleske D. Heterotopic ossification around the elbow following burns in children: results after excision. J Bone Joint Surg Am. 2003;85:1538–43. doi: 10.2106/00004623-200308000-00016. [DOI] [PubMed] [Google Scholar]
- 18.Nielson JH, Palathumpat SM. Heterotopic ossification after acromioclavicular separation in an adolescent: a case report. Curr Sports Med Rep. 2015;14:359–60. doi: 10.1249/JSR.0000000000000197. [DOI] [PubMed] [Google Scholar]
- 19.Kennedy MA, Sama AE, Sigman M. Tibiofibular syndesmosis and ossification. Case report: sequelae of ankle sprain in an adolescent football player. J Emerg Med. 2000;18:233–40. [DOI] [PubMed]
- 20.Sferopoulos NK, Anagnostopoulos D. Ectopic bone formation in a child with a head injury: complete regression after immobilisation. Int Orthop. 1998;21:412–4. doi: 10.1007/s002640050197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Susnjar T, Biocić M, Pogorelić Z. Traumatic heterotopic ossification of the elbow in children–a case report. Acta Chir Belg. 2010;110:246–9. doi: 10.1080/00015458.2010.11680611. [DOI] [PubMed] [Google Scholar]
- 22.Intravia J, Acevedo DC, Chung W-LJ, Mirzayan R. Complications of elbow arthroscopy in a community-based practice. Arthrosc J Arthrosc Relat Surg. 2020;36:1283–90. [DOI] [PubMed]
- 23.Lespasio MJ, Guarino A. Awareness of heterotopic ossification in total joint arthroplasty: a primer. Perm J. 2020;24:19.211. [DOI] [PMC free article] [PubMed]
- 24.Micheli LJ, Luke AC, Mintzer CM, Waters PM. Elbow arthroscopy in the pediatric and adolescent population. Arthrosc J Arthrosc Relat Surg Off Publ Arthrosc Assoc N Am Int Arthrosc Assoc. 2001;17:694–9. doi: 10.1053/jars.2001.25338. [DOI] [PubMed] [Google Scholar]
- 25.Micheloni GM, Tarallo L, Negri A, Giorgini A, Merolla G, Porcellini G. Pediatric elbow arthroscopy: clinical outcomes and complications after long-term follow-up. J Orthop Traumatol Off J Ital Soc Orthop Traumatol. 2021;22:55. doi: 10.1186/s10195-021-00619-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Specht LM, Gupta S, Egol KA, Koval KJ. Heterotopic ossification of the quadriceps following distal femoral traction: a report of three cases and a review of the literature. J Orthop Trauma. 2004;18:241–6. doi: 10.1097/00005131-200404000-00010. [DOI] [PubMed] [Google Scholar]
- 27.Sutphen SA, Mendoza JD, Mundy AC, Yang JG, Beebe AC, Samora WP, et al. Pediatric diaphyseal femur fractures: submuscular plating compared with intramedullary nailing. Orthopedics. 2016;39:353–8. doi: 10.3928/01477447-20160719-03. [DOI] [PubMed] [Google Scholar]
- 28.Kurz AZ, LeRoux E, Riediger M, Coughlin R, Simunovic N, Duong A, et al. Heterotopic ossification in hip arthroscopy: an updated review. Curr Rev Musculoskelet Med. 2019;12:147–55. doi: 10.1007/s12178-019-09543-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Galpin RD, Willis RB, Sabano N. Intramedullary nailing of pediatric femoral fractures. J Pediatr Orthop. 1994;14:184–9. doi: 10.1097/01241398-199403000-00010. [DOI] [PubMed] [Google Scholar]
- 30.Beaty JH, Austin SM, Warner WC, Canale ST, Nichols L. Interlocking intramedullary nailing of femoral-shaft fractures in adolescents: preliminary results and complications. J Pediatr Orthop. 1994;14:178–83. doi: 10.1097/01241398-199403000-00009. [DOI] [PubMed] [Google Scholar]
- 31.Letts M, Jarvis J, Lawton L, Davidson D. Complications of rigid intramedullary rodding of femoral shaft fractures in children. J Trauma. 2002;52:504–16. doi: 10.1097/00005373-200203000-00015. [DOI] [PubMed] [Google Scholar]
- 32.Baujat G, Choquet R, Bouée S, Jeanbat V, Courouve L, Ruel A, et al. Prevalence of fibrodysplasia ossificans progressiva (FOP) in France: an estimate based on a record linkage of two national databases. Orphanet J Rare Dis. 2017;12:123. doi: 10.1186/s13023-017-0674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pignolo RJ, Baujat G, Brown MA, De Cunto C, Hsiao EC, Keen R, et al. The natural history of fibrodysplasia ossificans progressiva: a prospective, global 36-month study. Genet Med. 2022;24:2422–33. doi: 10.1016/j.gim.2022.08.013. [DOI] [PubMed] [Google Scholar]
- 34.Kaplan FS, Xu M, Glaser DL, Collins F, Connor M, Kitterman J, et al. Early diagnosis of fibrodysplasia ossificans progressiva. Pediatrics. 2008;121:e1295–300. doi: 10.1542/peds.2007-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salemi P, Skalamera JM, Olson, Dickson LE, Germain-Lee EL. Ossifications in Albright hereditary osteodystrophy: role of genotype, inheritance, sex, age, hormonal status, and BMI. J Clin Endocrinol Metab. 2017;103:158–68. [DOI] [PMC free article] [PubMed]
- 36.Yu T, Zhang J, Zhu W, Wang X, Bai Y, Feng B, et al. Chondrogenesis mediates progression of ankylosing spondylitis through heterotopic ossification. Bone Res. 2021;9:19. doi: 10.1038/s41413-021-00140-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nóbrega JPG, Jordão P, Arcângelo J. Bilateral hip heterotopic ossification with sciatic nerve compression on a paediatric patient–an individualized surgical approach: a case report. World J Orthop. 2022;13:768–74. doi: 10.5312/wjo.v13.i8.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mujtaba B, Taher A, Fiala MJ, Nassar S, Madewell JE, Hanafy AK, et al. Heterotopic ossification: radiological and pathological review. Radiol Oncol. 2019;53:275–84. doi: 10.2478/raon-2019-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yeh T-S, Wu C-H, Chen W-S, Wang T-G. Serial ultrasonography for early detection and follow-up of heterotopic ossification in stroke. J Med Ultrasound. 2012;20:119–24. doi: 10.1016/j.jmu.2012.04.009. [DOI] [Google Scholar]
- 40.Wang Q, Zhang P, Li P, Song X, Hu H, Li X, et al. Ultrasonography monitoring of trauma-induced heterotopic ossification: guidance for rehabilitation procedures. Front Neurol. 2018;9:771. doi: 10.3389/fneur.2018.00771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang X, Jie S, Liu T, Zhang X. Acquired heterotopic ossification in hips and knees following encephalitis: case report and literature review. BMC Surg. 2014;14:74. doi: 10.1186/1471-2482-14-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Muhamad Effendi F, Nam Y, Shin CH, Cho T-J, Yoo WJ, Cheon J-E, et al. Postinfectious heterotopic ossification of the ilium involving the iliacus muscle. J Pediatr Orthop B. 2018;27:407–11. doi: 10.1097/BPB.0000000000000477. [DOI] [PubMed] [Google Scholar]
- 43.Shimono K, Uchibe K, Kuboki T, Iwamoto M. The pathophysiology of heterotopic ossification: current treatment considerations in dentistry. Jpn Dent Sci Rev. 2014;50:1–8. doi: 10.1016/j.jdsr.2013.07.003. [DOI] [Google Scholar]
- 44.Rath E, Sherman H, Sampson TG, Ben Tov T, Maman E, Amar E. The incidence of heterotopic ossification in hip arthroscopy. Arthrosc J Arthrosc Relat Surg Off Publ Arthrosc Assoc N Am Int Arthrosc Assoc. 2013;29:427–33. doi: 10.1016/j.arthro.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 45.Wosczyna MN, Biswas AA, Cogswell CA, Goldhamer DJ. Multipotent progenitors resident in the skeletal muscle interstitium exhibit robust BMP-dependent osteogenic activity and mediate heterotopic ossification. J Bone Miner Res. 2012;27:1004–17. doi: 10.1002/jbmr.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cairns DM, Liu R, Sen M, Canner JP, Schindeler A, Little DG, et al. Interplay of Nkx3.2, Sox9 and Pax3 regulates chondrogenic differentiation of muscle progenitor cells. Xiao Q, editor. PLoS One. 2012;7:e39642. [DOI] [PMC free article] [PubMed]
- 47.Lounev VY, Ramachandran R, Wosczyna MN, Yamamoto M, Maidment AD, Shore EM, et al. Identification of progenitor cells that contribute to heterotopic skeletogenesis. J Bone Jt Surg-Am Vol. 2009;91:652–63. [DOI] [PMC free article] [PubMed]
- 48.Chalmers J, Gray DH, Rush J. Observations on the induction of bone in soft tissues. J Bone Joint Surg Br. 1975;57-B:36–45. [PubMed]
- 49.Ohlmeier M, Krenn V, Thiesen DM, Sandiford NA, Gehrke T, Citak M. Heterotopic ossification in orthopaedic and trauma surgery: a histopathological ossification score. Sci Rep. 2019;9:18401. doi: 10.1038/s41598-019-54986-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McCarthy EF, Sundaram M. Heterotopic ossification: a review. Skeletal Radiol. 2005;34:609–19. doi: 10.1007/s00256-005-0958-z. [DOI] [PubMed] [Google Scholar]
- 51.Liu H, Zhao J-G, Li Y, Xia J, Zhao S. Non-steroidal anti-inflammatory drugs for preventing heterotopic bone formation after hip arthroplasty. Cochrane Bone, Joint and Muscle Trauma Group, editor. Cochrane Database Syst Rev [Internet]. 2017 [cited 2023 Mar 11]; Available from: https://doi.wiley.com/10.1002/14651858.CD012861
- 52.Ritter MA, Sieber JM. Prophylactic indomethacin for the prevention of heterotopic bone formation following total hip arthroplasty. Clin Orthop. 1985;217–25. [PubMed]
- 53.Weiss PF. Diagnosis and treatment of enthesitis-related arthritis. Adolesc Health Med Ther. 2012;2012:67–74. doi: 10.2147/AHMT.S25872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moore KD, Goss K, Anglen JO. Indomethacin versus radiation therapy for prophylaxis against heterotopic ossification in acetabular fractures: a randomised, prospective study. J Bone Joint Surg Br. 1998;80:259–63. doi: 10.1302/0301-620X.80B2.0800259. [DOI] [PubMed] [Google Scholar]
- 55.Popovic M, Agarwal A, Zhang L, Yip C, Kreder HJ, Nousiainen MT, et al. Radiotherapy for the prophylaxis of heterotopic ossification: a systematic review and meta-analysis of published data. Radiother Oncol J Eur Soc Ther Radiol Oncol. 2014;113:10–7. doi: 10.1016/j.radonc.2014.08.025. [DOI] [PubMed] [Google Scholar]
- 56.Milakovic M, Popovic M, Raman S, Tsao M, Lam H, Chow E. Radiotherapy for the prophylaxis of heterotopic ossification: a systematic review and meta-analysis of randomized controlled trials. Radiother Oncol J Eur Soc Ther Radiol Oncol. 2015;116:4–9. doi: 10.1016/j.radonc.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 57.Craven PL, Urist MR. Osteogenesis by radioisotope labelled cell populations in implants of bone matrix under the influence of ionizing radiation. Clin Orthop. 1971;76:231–43. doi: 10.1097/00003086-197105000-00030. [DOI] [PubMed] [Google Scholar]
- 58.Hamid N, Ashraf N, Bosse MJ, Connor PM, Kellam JF, Sims SH, et al. Radiation therapy for heterotopic ossification prophylaxis acutely after elbow trauma: a prospective randomized study. J Bone Joint Surg Am. 2010;92:2032–8. doi: 10.2106/JBJS.I.01435. [DOI] [PubMed] [Google Scholar]
- 59.Mazonakis M, Berris T, Lyraraki E, Damilakis J. Cancer risk estimates from radiation therapy for heterotopic ossification prophylaxis after total hip arthroplasty. Med Phys. 2013;40:101702. doi: 10.1118/1.4820366. [DOI] [PubMed] [Google Scholar]
- 60.Pavey GJ, Polfer EM, Nappo KE, Tintle SM, Forsberg JA, Potter BK. What risk factors predict recurrence of heterotopic ossification after excision in combat-related amputations? Clin Orthop. 2015;473:2814–24. doi: 10.1007/s11999-015-4266-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tsionos I, Leclercq C, Rochet JM. Heterotopic ossification of the elbow in patients with burns. Results after early excision. J Bone Joint Surg Br. 2004;86:396–403. [DOI] [PubMed]
- 62.Eekhoff EMW, Botman E, Coen Netelenbos J, de Graaf P, Bravenboer N, Micha D, et al. [18F]NaF PET/CT scan as an early marker of heterotopic ossification in fibrodysplasia ossificans progressiva. Bone. 2018;109:143–6. doi: 10.1016/j.bone.2017.08.012. [DOI] [PubMed] [Google Scholar]
- 63.Polfer EM, Forsberg JA, Fleming ME, Potter BK. Neurovascular entrapment due to combat-related heterotopic ossification in the lower extremity. J Bone Jt Surg. 2013;95:e195. doi: 10.2106/JBJS.M.00212. [DOI] [PubMed] [Google Scholar]