Abstract
Purpose of Review
Scheuermann’s kyphosis (SK) is a developmental deformity of the spine that affects up to 8% of children in the US. Although, the natural progression of SK is noted to be gradual over years, severe deformity can be associated with significant morbidity. Thorough clinical examination and interpretation of relevant imaging help differentiate and confirm this diagnosis. Treatment includes both operative and nonoperative approaches. The purpose of this article is to provide an updated overview of the current theories of its pathogenesis, as well as the principles of diagnosis and treatment of SK.
Recent Findings
Although a definitive, unified theory continues to be elusive, numerous reports in the past decade provide insight into the pathophysiology of SK. These include alterations in mechanical stress and/or hormonal disturbances. Candidate genes have also been identified to be linked to the inheritance of SK. Updates to nonoperative treatment include the effectiveness of dedicated exercise programs, as well as the types and duration of orthotic treatment. Advances in surgical technique can be observed with a trend toward a posterior-only approach, with supporting evidence for careful evaluation of both the sagittal and coronal planes to determine fusion levels in order to avoid postoperative junctional pathologies.
Summary
SK is an important cause of structural or rigid kyphosis. It can lead to significant morbidity in severe cases. Treatment is based on curve magnitude and symptoms. Nonoperative treatment consists of physical therapy in symptomatic patients, and bracing can be added for skeletally mature patients. Operative management can be considered in patients with large, progressive, and symptomatic deformity. Future studies can benefit from a focused investigation into patient-reported outcomes after undergoing appropriate treatment.
Keywords: Scheuermann’s kyphosis, Adolescent kyphosis, Hyperkyphosis, Spine deformity
Introduction
Kyphosis refers to the curve in the back in the sagittal plane and typically measures 20–40° [1, 2]. Increased kyphosis can result from various etiologies ranging from trauma to infectious disease [3]. Increased kyphosis is generally classified as structural versus non-structural. Structural, or rigid, kyphosis involves morphological deformities to the bones and soft tissue and is thus less flexible and easily corrected than examples of non-structural kyphosis, such as postural kyphosis, which can be effectively improved with exercise and muscle strengthening [4–6]. There exist many manifestations of structural kyphosis, including congenital kyphosis, which is caused by the failure of the spine to form, failure of the anterior segmentation of the vertebrae, or both, spondylolisthesis, and degenerative diseases like osteoporosis that cause vertebral fractures [7–9]. However, one of the most common types of structural kyphosis, particularly among adolescents, is Scheuermann’s kyphosis (SK) [10].
SK was first defined by Danish surgeon and radiologist Horgel Welfer Scheuermann in 1920 [11]. This form of developmental kyphosis is defined radiographically as the anterior wedging of ≥ 5° of at least three consecutive vertebral bodies with or without endplate irregularities and Schmorl’s nodes [12–14]. The incidence of SK in the US is 0.4–8% and is most often first diagnosed in children between the ages of 10 and 12, with a positive correlation between age and kyphosis angle [3, 15]. In terms of sex, the male to female ratio in SK ranges between 2:1 and 7:1 [3, 11, 14]. The purpose of this study is to (1) review the etiology of SK, (2) review pertinent methods of diagnosis including physical exam and imaging, and (3) discuss current concepts relating to its management.
Etiology and Natural History
Etiology
There are several theories regarding the etiology and pathogenesis of Scheuermann’s kyphosis. Genetics have been increasingly investigated as a possible contributor to the development of SK. Zaidman et al. performed both clinical and genetic investigations that demonstrate an autosomal dominant inheritance associated with a mutant major gene, while also identifying candidate genes such as IHH, PAX 1, and SOX9 [16]. Another etiology is the theory of altered biomechanics, with regards to the complexity of the spine and the intricacies of the surrounding ligamentous and muscular structures[17•]. Many studies have elucidated the effects of downward forces, vertebral compressive loading, and congruent posture on a bipedal spine and their relationship to disk pathology, vertebral fracture, spondylolisthesis, and increased kyphosis [18–20]. For example, Peleg et al. assessed the spino-pelvic orientation and noted that SK patients had a significantly more horizontally oriented sacrum than the control group [21]. While the pathophysiology of SK is unclear, the radiologic criteria of thoracic kyphosis of > 40°, irregular vertebral endplates, Schmorl’s nodes, and the loss of disk space bring into the question the relationship between SK and highly prevalent disease processes such as osteoporosis and disk herniation [22]. Findings from Liu et al. reinforce this relationship showing radiographic resemblances between the thoracolumbar disk herniation cohort and the signs of SK [23]. In addition, Lopez et al. demonstrated the mean bone-mineral density being significantly reduced in kyphotic patients compared to controls [24]. With more investigation into these associations, proper preventative management could improve prognosis and quality of life for SK patients.
Natural History
The progression of SK varies between the two types of curve patterns, as the thoracolumbar pattern is more likely to become symptomatic after skeletal maturity when compared to the thoracic pattern [17•]. Overall, it is understood that SK has a relatively benign natural history, with most patients reporting minimal progression of their curvature and few negative outcomes affecting daily activities and the quality of life. Murray et al. was able to corroborate these findings by investigating the outcome of 67 SK patients over an extended period of time [25]. Although pain and fatigue are commonly associated with increasing kyphosis, symptoms such as neurological deficits, hamstring tightness and persistent back pain with rotational movements vary from patient to patient [25]. In more severely kyphotic patients, respiratory function may be affected. Patients with a cobb angle greater than 100° and a thoracic apex within T1–T8 demonstrated signs of restrictive lung disease [25, 26]. Vera et al. more recently supported these findings by demonstrating reduced pre-operative pulmonary function tests in SK patients with an improvement 2 years after surgical intervention in patients with kyphosis greater than 75° [26].
Diagnosis
Presentation
Majority of patients with SK will present initially due to progressive deformity, cosmetic reasons, or pain [27]. It is rare to present with any neurologic complaints [28, 29]. Patients will often present in adolescence, with thoracic or thoracolumbar kyphosis; however, some patients may present into adulthood [13]. The differential diagnosis for structural kyphosis includes vertebral compression fractures, infection, or tumor, and these must all be ruled out.
Exam
Physical examination starts with inspection; patients typically have thoracic hyperkyphosis with compensatory lumbar and cervical lordosis, forward protrusion of the head and neck (“goose-neck deformity”), pigmentation of the skin around the apex caused by spinous process skin abrasion, and tightness of the iliopsoas, hamstrings, pectoralis, and anterior shoulder [13, 17•, 25, 28]. Flexibility can be assessed by physical examination with forced flexion and extension. This can aid in distinguishing between postural and rigid kyphosis. In SK, the kyphotic deformity is rigid and will not passively correct with extension. The physical exam should include a full neurovascular examination including an assessment of reflexes.
Imaging
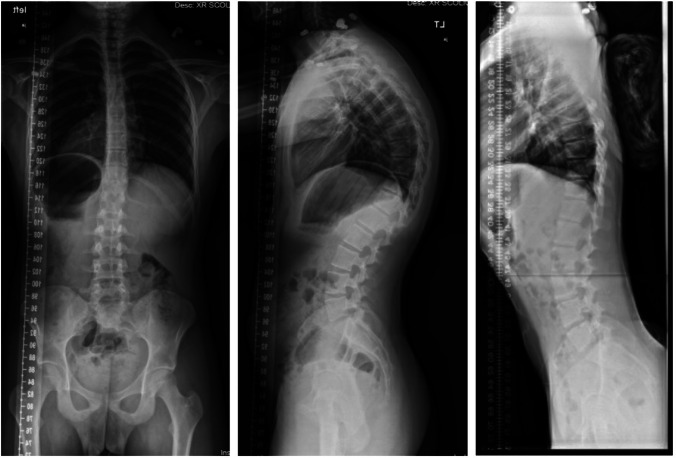
Standard spine radiographs are necessary to evaluate patients with Scheuermann’s kyphosis (Fig. 1). This includes standing full-spine radiographs with posterior–anterior and lateral views. Diagnostic criteria are largely radiographic and include anterior wedging in the sagittal plane greater than 5° across three consecutive vertebrae, as first defined by Sørensen et al in 1964 [30]. Additionally, a forced hyperextension lateral radiograph may be obtained to distinguish rigid versus flexible postural kyphotic deformities. This is achieved with a supine radiograph with a bolster placed beneath the kyphotic apex. Structural kyphosis will generally have little to no correction.
Fig. 1.
Standing preoperative radiographs of the posterior–anterior (PA). standing lateral, and maximum hyperextension lateral of a patient with Scheuermann kyphosis
Kyphosis and vertebral body wedging can be measured using the Cobb method on the lateral view [31]. Sagittal balance can be measured from a plumb line drawn from C7 to the sacral end plate [32].
Other findings include endplate irregularities, Schmorl’s nodes, and disc degeneration [33, 34]. Compensatory hyperlordosis in the cervical or lumbar spine may also be present.
Advanced imaging can be considered in SK patients. Magnetic resonance imaging (MRI) is indicated for any patients with an atypical history or neurologic findings on exam. An MRI may also be useful in patients undergoing surgical management. Findings on an MRI may include Schmorl’s nodes or disc herniations or degeneration (Fig. 2). A study of 22 adult SK patients found that 64% had Schmorl’s nodes on MRI compared to 8% of normal control patients [35•].
Fig. 2.
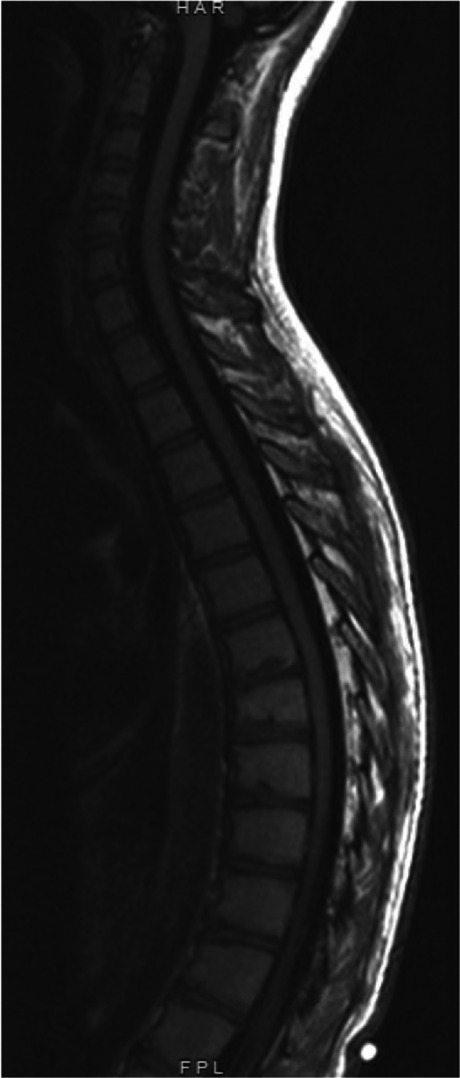
Thoracic MRI and sagittal T2 view of a patient with Scheuermann kyphosis. Representative findings of endplate irregularities and Schmorl’s nodes are seen, particularly in T6-12
Classification
SK is classified by severity and location. Specifically, there are two types of SK that have been described: type I (typical) and type II (atypical) [17•]. In type I SK, the apex of the deformity is mostly in the thoracic region (T7–T9) with an associated narrowing of the disc space and hyperlordosis of the cervical and lumbar spine; in contrast, in type II SK, the apex of the deformity is in the thoraco-lumbar or lumbar region with an associated reduction of disc space [3, 17•, 36, 37]. The severity is based on the magnitude of the Cobb angle.
Management and Complications
General Considerations
The treatment for SK is largely based on the magnitude of the deformity and symptoms. While the impact of untreated milder forms of SK are largely unknown, more severe deformity has been associated with progression and pain [38]. The treatment of SK is largely based on the natural history of the deformity, risks of intervention, and the aforementioned clinical findings [28]. Regardless of approach, the goals of treatment are restoration and stabilization of mechanical alignment, alleviation of pain, resolution of neurologic impairment, and improvement of cosmesis. Currently, both nonoperative and operative treatments have been adopted with satisfactory results [39••, 40].
Nonoperative Treatment
Indications for nonoperative treatment include thoracic kyphosis of 50 to 80°, or kyphosis greater than 45° with presence of radiographic stigmata such as wedged vertebrae or Schmorl’s nodes. The mainstay of orthopedic treatment in this category includes physiotherapy and orthotics [41–43].
A formal exercise program with emphasis on thoracic extensor muscle strengthening has been shown to improve function [43]. Abdominal muscle strengthening and pectoralis muscles stretching may be beneficial in improving posture. In addition, hamstring stretching can be an effective intervention to relieve lower extremity contractures associated with the compensatory increase in lumbar lordosis [44]. Physiotherapy is recommended for flexible deformity only, and to date, there is no conclusive research demonstrating significant lasting improvement in kyphosis with exercise alone.
Skeletally immature patients with SK benefit from similar exercise programs, but require the addition of a spinal orthosis. There is a reproducible and overall successful result in patients with kyphosis between 50 and 80° if initiated before skeletal maturity [41]. Bracing in SK focuses on improving thoracic kyphosis, with the goal of vertebral remodeling in skeletally immature patients [6, 45]. Bracing also has secondary effects on improved lumbar lordosis. Several types of orthosis can be used. Milwaukee brace extends from the pelvis up to the neck, helping with the extension of the torso while keeping the head centered relative to the pelvis. Boston braces such as thoracolumbosacral orthosis and other modern braces such as Gschwend and Kyphologic braces apply three-point corrective pressure against the deformity [42]. Functional bracing using Jewett hyperextension orthosis is also an option, and it is our preferred brace for this condition. Regardless of the type of brace used, compliance is paramount in its effectiveness. A minimum of 16 h per day is required until correction is achieved [46]. Although studies on efficacy and long-term results are still lacking, current reports on the outcome after brace treatment demonstrate a trend of initial correction averaging 50%, followed by some loss of correction after termination of the brace treatment [47]. Significant reduction in pain has also been reported with brace treatment.
Operative Treatment
Surgical indications are variable and must be individualized. Progressive deformity refractory to bracing, worsening pain, neurologic deficit, and significant deformity in skeletally mature patients are common indications for surgical management. Although no definitive curve magnitude threshold has been empirically determined, kyphosis greater than 70–80° is generally considered to be approaching the surgical range [41, 48]. Lower self-appearance scores, higher perception of pain, and higher body mass index have been shown to correlate with the decision for operative treatment [39••]. Surgical goals should be tailored based on patient biomechanics, with primary focus on restoring global spinal and spinopelvic balance. This restoration must be carried with caution against overcorrection due to its possible contribution to acute neurologic intolerance or subsequent junctional pathology [49, 50]. A correction of 40 to 50°, or within 50% of preoperative kyphosis, is advisable to achieve adequate correction while avoiding junctional kyphosis.
Fusion level selection is an important determinant of long term surgical success. Inclusion of the entire curvature as defined by the Cobb vertebrae is generally accepted as a minimum requirement needed for fusion [41]. Proximal junctional integrity is generally more problematic in SK treatment compared to the disruption at the distal junction [49]. Therefore, particular attention is paid to ensure proper selection of upper instrumented vertebra at or proximal to the Cobb vertebrae. Studies have shown that preoperative curve magnitude is directly correlated to the development of PJK, and a greater pelvic incidence is correlated with a higher magnitude of PJK [12, 51]. These findings may prompt the treating surgeon to extend the construct to include one neutral vertebra above the proximal end Cobb vertebra. Distally, fusion to include the sagittal stable vertebra (SSV), defined as the most proximal touched vertebra by the posterior sacral vertical line (PSVL), is generally accepted as the desirable level to minimize the risk for DJK [51–53]. We also typically assess the first lordotic disc to ensure there is no kyphosis at the lowest instrumented vertebra.
Advances in surgical technique and instrumentation have led to a shift in operative approach. A combined anterior-posterior (AP) approach was initially favored for the treatment of SK. More recently, however, the development of thoracic segmental pedicle screw instrumentation, combined with wide adoption of multilevel corrective osteotomies, now allow surgeons to achieve comparable sagittal correction through a posterior-only approach [39••, 41]. Ultimately, the advantages of a single approach call into question the utility of the anterior apical release. Indeed, most modern studies demonstrate that the posterior-only approach provides adequate correction with significantly less morbidity [54, 55]. A summary of surgical techniques is provided in Table 1.
Table 1.
Summary of available literature on the operative outcomes for SK
| Article | Year | Study design | Surgical technique |
|---|---|---|---|
| Bradford et al [55] | 1975 | Case series | Posterior spinal fusion alone using Harrington compression instrumentation, n = 22. High rate (73%) of loss of correction after surgery. |
| Herndon et al [56] | 1981 | Case series | Good relief of pain in 12 of 13 patients treated with combined anterior and posterior fusion. |
| Otsuka et al [57] | 1990 | Case series | Used heavier Harrington compression rods n = 10 patients and reported good correction of kyphosis |
| Lowe and Kasten [38] | 1994 | Case series | n = 24 patients treated with staged anterior release and posterior fusion with l-rod instrumentation. Around 75% had good reduction in pain. |
| Wenger and Frick [28] | 1999 | Review | Review of all surgical techniques and the historical refining of techniques with time. |
| Lee et al [54] | 2006 | Retrospective cohort | Posterior-only vs antero-posterior fusion, n = 39. Posterior-only achieved and maintained better correction and had significantly less complications. |
| Koller et al [51] | 2014 | Case series | Anterior release and posterior spinal fusion (combined approach), n = 111 patients at a single center. Results showed comparable correction and outcomes. |
| Huq et al [39••] | 2020 | Systematic review | Found that posterior-only approach is gaining in popularity, provides greater correction, and has smaller complication profile. |
| Tsirikos et al [58••] | 2021 | Prospective cohort study | Posterior spinal fusion with closing wedge osteotomies and hybrid instrumentation, n = 88 patients. Results showed satisfactory correction, improvements in physical and mental health, and a high degree of patient-reported satisfaction |
| Sebaaly et al [17•] | 2022 | Review | Review of surgical treatments found that the combined A/P approach was considered the gold standard for the surgical treatment of this disease, but there is an increasing trend toward posterior-only approaches especially with the use of segmental fixation and osteotomy. |
Maintenance of facet joints, paraspinal musculature, and ligamentous structures between fused and unfused segments is an important technique to avoid junctional pathology. The creation of a transitional “soft landing” at the two ends of the fusion construct is desirable; biomechanical studies have confirmed the benefits of a gradual transition from relative immobility at the fused segments to normalize motion at the unfused segments [59]. This transition can be achieved with hooks, wires, ligament augmentation, or tethers used at the top of the posterior fusion construct.
Multilevel posterior column osteotomies (Schwab type 2 or Ponte osteotomies) are used across the apex of the deformity [58••, 60]. These osteotomies provide 5 to 10° of sagittal correction per level and improve overall spinal flexibility prior to correction. Higher grades of osteotomies with advanced three-column resection are indicated for rigid and sharp focal deformity but rarely necessary for SK [41, 58••, 61].
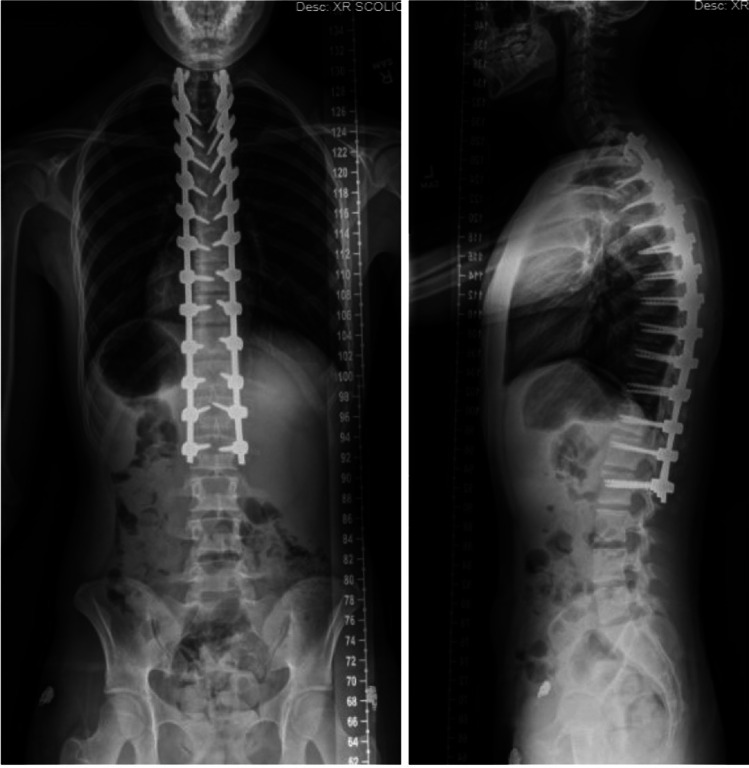
A corrective maneuver is generally sufficient with the cantilever technique. The principle of this technique is to achieve reduction by securing a pre-bent rod to pedicle screws proximal to the apex of the deformity following multilevel osteotomies as described above [50]. As the rod is sequentially connected to the pedicle screws distal to the apex of the deformity, the proximal portion of the rod will act as a cantilever that pulls the underlying segments posteriorly, correcting the hyperkyphosis (Fig. 3). A minimum of eight fixation points above and below the apex of deformity has been recommended by some studies, while others advocate for the inclusion of apical fixation [54, 61]. The size and material of the chosen implants should be carefully considered in light of individual osseous biology in order to achieve optimal balance between the maintenance of correction and minimizing the risk of junctional kyphosis [41, 51]. A representative patient is shown postoperatively in Fig. 4.
Fig. 3.
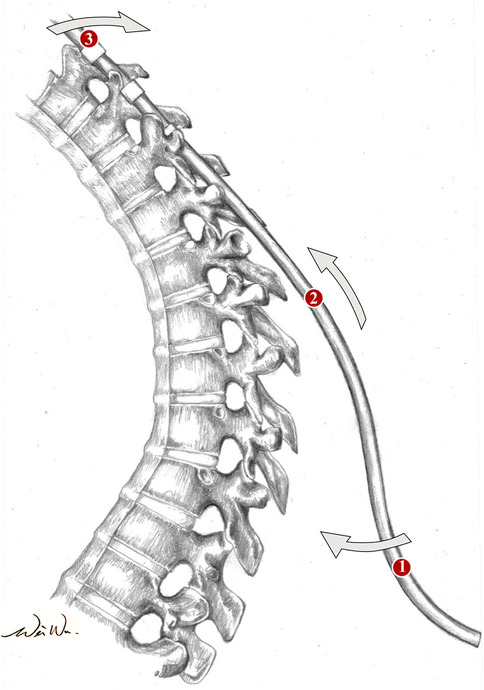
Illustration of the cantilever effect which is produced with the reduction of the kyphosis during rod placement
Fig. 4.
Standing postoperative radiographs of the posterior–anterior (PA) and standing lateral of the patient from Fig. 1
Complications
Complications in SK treatment are mainly associated with operative treatment. Within these patients, overall incidence of complications is significantly less in the posterior-only approach compared to anterior-posterior procedures [55, 62]. Most commonly reported complications are wound infection, neurologic complications, and junctional kyphosis [41, 63].
Neurologic Complications
The incidence of acute neurologic compromise is reported 1.9 to 2.1%, including both nerve root and spinal cord injuries [64]. Previous reports have found that neurologic complications may be caused by disc herniation after deformity correction [65]. A multimodal intraoperative neuromonitoring is recommended during surgical correction of SK. A true intraoperative loss of neuromonitoring signals should prompt partial or complete release of correction as well as optimization of arterial pressure; these measures will often lead to signal return, with no sustained neurologic deficit.
Proximal Junctional Kyphosis
PJK often manifests radiographically as a kyphotic change in the disk above the fusion in pediatric patients with SK. It is one of the most commonly reported complications for the posterior-only approach, with an incidence between 13% and 32% for PJK greater than 10° after PSF for SK [49, 50]. The primary cause of PJK is failure to include the proximal end vertebra, highlighting the importance of careful analysis of high-quality preoperative radiographs during preoperative planning. Other risk factors include the disruption of the junctional ligamentum flavum during surgical dissection for the posterior approach, overcorrection of thoracic kyphosis, particularly in the setting of preoperative high pelvic incidence [41].
Distal Junctional Kyphosis
The overall incidence of DJK is reportedly between 14% and 24% [66]. Similar to PJK, overcorrection of thoracic kyphosis is associated with the development of postoperative DJK. A recent meta-analysis found a risk reduction of 86% when fusion level is carried distally to include the SSV compared to constructs ending proximal to the SSV [67]. Compared to patients with thoracic kyphosis alone, SK patients with thoracolumbar kyphosis who underwent fusion with a construct proximal to the SSV are particularly at risk of developing DJK [52]. The decision to extend fusion must be carefully weighed against the preservation of motion segments in younger populations [68, 69].
Although patients with SK have overall higher postoperative complications rates than patients with adolescent idiopathic scoliosis, the majority of patients do well with appropriate management and close monitoring [12, 58••, 70•].
Conclusion
SK is an important cause of structural or rigid kyphosis. It is more common in boys than girls, and it likely has a genetic etiology. Over time, SK may progress in skeletally immature patients and eventually lead to pain and restrictive lung disease in severe cases. Diagnosis of SK is based on a careful physical exam and radiographs that classically show apical wedging of three consecutive vertebrae on the lateral view. Treatment is based on magnitude of deformity and symptoms. Nonoperative treatment consists of physical therapy in symptomatic patients, and bracing can be added for skeletally mature patients. Operative management can be considered in patients with larger deformity, progressive deformity, and symptoms. Several techniques have been described for the management of SK; however, posterior instrumentation and multi-level osteotomies allow for the appropriate correction of the deformity. There are several complications associated with surgical management which underscore the importance of level selection and avoiding over-correction of the kyphosis.
Data Availability
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Declarations
Conflicts of Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jennifer M. O’Donnell, Email: Jennifer.odonnell@ucsf.edu
Wei Wu, Email: wu.wei.k@gmail.com.
Alex Youn, Email: Alex.Youn@ucsf.edu.
Angad Mann, Email: mann2481@chsu.edu.
Ishaan Swarup, Email: Ishaan.Swarup@ucsf.edu.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Fon G, Pitt M, Thies A. Thoracic kyphosis: range in normal subjects. Am J Roentgenol. 1980;134(5):979–983. doi: 10.2214/ajr.134.5.979. [DOI] [PubMed] [Google Scholar]
- 2.Boseker EH, Moe JH, Winter RB, Koop SE. Determination of “normal” Thoracic Kyphosis: a roentgenographic study of 121 “normal” children. J Pediatr Orthop. 2000;20(6):796. doi: 10.1097/01241398-200011000-00019. [DOI] [PubMed] [Google Scholar]
- 3.Yaman O, Dalbayrak S. Kyphosis and review of the literature. Turk Neurosurg. Published online 2013. 10.5137/1019-5149.JTN.8940-13.0. [DOI] [PubMed]
- 4.Czaprowski D, Stoliński Ł, Tyrakowski M, Kozinoga M, Kotwicki T. Non-structural misalignments of body posture in the sagittal plane. Scoliosis Spinal Disord. 2018;13(1):6. doi: 10.1186/s13013-018-0151-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kamali F, Shirazi SA, Ebrahimi S, Mirshamsi M, Ghanbari A. Comparison of manual therapy and exercise therapy for postural hyperkyphosis: a randomized clinical trial. Physiother Theory Pract. 2016;32(2):92–97. doi: 10.3109/09593985.2015.1110739. [DOI] [PubMed] [Google Scholar]
- 6.Pizzutillo PD. Nonsurgical treatment of kyphosis. Instr Course Lect. 2004;53:485–491. [PubMed] [Google Scholar]
- 7.Montgomery SP, Hall JE. Congenital kyphosis. Spine. 1982;7(4):360–364. doi: 10.1097/00007632-198207000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Mizobuchi S, Tadokoro N, Takaya S, Kiyasu K, Takemasa R, Ikeuchi M. Nontraumatic Spondylolisthesis of the axis with cervical kyphosis. Case Rep Orthop. 2020;2020:1–3. doi: 10.1155/2020/6859474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCarthy J, Davis A. Diagnosis and management of vertebral compression fractures. afp. 2016;94(1):44–50. [PubMed]
- 10.Lowe TG. Scheuermann’s kyphosis. Neurosurg Clin N Am. 2007;18(2):305–315. doi: 10.1016/j.nec.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 11.Scheuermann HW. Kyphosis dorsalis juvenilis. Ugeskr Laeger. 1920;82:385–393. [Google Scholar]
- 12.Lonner BS, Newton P, Betz R, et al. Operative management of Scheuermannʼs kyphosis in 78 patients: radiographic outcomes, complications, and technique. Spine. 2007;32(24):2644–2652. doi: 10.1097/BRS.0b013e31815a5238. [DOI] [PubMed] [Google Scholar]
- 13.Wood KB, Melikian R, Villamil F. Adult Scheuermann kyphosis: evaluation, management, and new developments. J Am Acad Orthop Surg. 2012;20(2):113–121. doi: 10.5435/JAAOS-20-02-113. [DOI] [PubMed] [Google Scholar]
- 14.Blumenthal SL, Roach J, Herring JA. Lumbar Scheuermann’s. A clinical series and classification. Spine (Phila Pa 1976). 1987;12(9):929-932. [PubMed]
- 15.Urrutia J, Narvaez F, Besa P, Meissner-Haecker A, Rios C, Piza C. Scheuermann’s disease in patients 15–40 years old: a study to determine its prevalence and its relationship with age and sex using chest radiographs as screening tool. J Orthop Sci. 2019;24(5):776–779. doi: 10.1016/j.jos.2018.12.024. [DOI] [PubMed] [Google Scholar]
- 16.Zaidman AM, Zaidman MN, Strokova EL, et al. The mode of inheritance of Scheuermann’s disease. BioMed Res Int. 2013;2013:1–9. doi: 10.1155/2013/973716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sebaaly A, Farjallah S, Kharrat K, Kreichati G, Daher M. Scheuermann’s kyphosis: update on pathophysiology and surgical treatment. EFORT Open Rev. 2022;7(11):782–791. doi: 10.1530/EOR-22-0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stokes IAF, Windisch L. Vertebral height growth predominates over intervertebral disc height growth in adolescents with scoliosis. Spine. 2006;31(14):1600–1604. doi: 10.1097/01.brs.0000222008.15750.1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jaumard NV, Welch WC, Winkelstein BA. Spinal facet joint biomechanics and mechanotransduction in normal, injury and degenerative conditions. J Biomech Eng. 2011;133(7):071010. doi: 10.1115/1.4004493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bruno AG, Anderson DE, D’Agostino J, Bouxsein ML. The effect of thoracic kyphosis and sagittal plane alignment on vertebral compressive loading. J Bone Miner Res. 2012;27(10):2144–2151. doi: 10.1002/jbmr.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peleg S, Dar G, Steinberg N, Masharawi Y, Hershkovitz I. Sacral orientation and Scheuermann’s kyphosis. SpringerPlus. 2016;5(1):141. doi: 10.1186/s40064-016-1772-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mansfield JT, Bennett M. Scheuermann disease. In: StatPearls. StatPearls Publishing; 2023. Accessed March 17, 2023. http://www.ncbi.nlm.nih.gov/books/NBK499966/. [PubMed]
- 23.Liu N, Chen Z, Qi Q, Shi Z. The relationship of symptomatic thoracolumbar disc herniation and Scheuermann’s disease. Eur Spine J. 2014;23(5):1059–1066. doi: 10.1007/s00586-013-3108-7. [DOI] [PubMed] [Google Scholar]
- 24.Lopez RA, Burke SW, Levine DB, Schneider R. Osteoporosis in Scheuermannʼs disease. Spine. 1988;13(10):1099–1103. doi: 10.1097/00007632-198810000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Murray PM, Weinstein SL, Spratt KF. The natural history and long-term follow-up of Scheuermann kyphosis. JBJS. 1993;75(2):236. doi: 10.2106/00004623-199302000-00011. [DOI] [PubMed] [Google Scholar]
- 26.Vera P, Lorente A, Burgos J, et al. Cardiorespiratory function of patients undergoing surgical correction of Scheuermann’s hyperkyphosis. Sci Rep. 2021;11(1):20138. doi: 10.1038/s41598-021-99674-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tribus CB. Scheuermann’s kyphosis in adolescents and adults: diagnosis and management. J Am Acad Orthop Surg. 1998;6(1):36–43. doi: 10.5435/00124635-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 28.Wenger DR, Frick SL. Scheuermann kyphosis. Spine. 1999;24(24):2630. doi: 10.1097/00007632-199912150-00010. [DOI] [PubMed] [Google Scholar]
- 29.Bradford DS. Juvenile kyphosis. Clin Orthop Relat Res. 1977;128:45–55. [PubMed] [Google Scholar]
- 30.Sørensen K. Scheuermann’s juvenile kyphosis. Clinical appearances, radiography, aetiology, and prognosis. Munksgard. Published online 1964. 10.1302/0301-620X.47B1.203-a.
- 31.Cobb J. Outline for the study of scoliosis. Instr Course Lect. 1948;5:261–275. [Google Scholar]
- 32.Mac-Thiong JM, Labelle H, Roussouly P. Pediatric sagittal alignment. Eur Spine J. 2011;20(S5):586–590. doi: 10.1007/s00586-011-1925-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scoles PV, Latimer BM, DiGIOVANNI BF, Vargo E, Bauza S, Jellema LM. Vertebral alterations in Scheuermann’s kyphosis. Spine. 1991;16(5):509. doi: 10.1097/00007632-199105000-00004. [DOI] [PubMed] [Google Scholar]
- 34.Bradford DS, Moe JH. Scheuermann’s juvenile kyphosis. A histologic study. Clin Orthop Relat Res. 1975;(110):45–53. [PubMed]
- 35.Ristolainen L, Kettunen JA, Danielson H, Heliövaara M, Schlenzka D. Magnetic resonance imaging findings of the lumbar spine, back symptoms and physical function among male adult patients with Scheuermann’s disease. J Orthop. 2020;21:69–74. doi: 10.1016/j.jor.2020.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jansen RC, Van Rhijn LW, Van Ooij A. Predictable correction of the unfused lumbar lordosis after thoracic correction and fusion in Scheuermann kyphosis. Spine. 2006;31(11):1227–1231. doi: 10.1097/01.brs.0000217682.53629.ad. [DOI] [PubMed] [Google Scholar]
- 37.Bezalel T, Carmeli E, Been E, Kalichman L. Scheuermann’s disease: current diagnosis and treatment approach. BMR. 2014;27(4):383–390. doi: 10.3233/BMR-140483. [DOI] [PubMed] [Google Scholar]
- 38.Lowe TG, Kasten MD. An analysis of sagittal curves and balance after Cotrel-Dubousset instrumentation for kyphosis secondary to Scheuermann’s disease. A review of 32 patients. Spine (Phila Pa 1976). 1994;19(15):1680-1685. doi:10.1097/00007632-199408000-00005. [DOI] [PubMed]
- 39.Huq S, Ehresman J, Cottrill E, et al. Treatment approaches for Scheuermann kyphosis: a systematic review of historic and current management. J Neurosurg Spine. 2020;32(2):235–247. doi: 10.3171/2019.8.SPINE19500. [DOI] [PubMed] [Google Scholar]
- 40.Bradford DS, Moe JH, Montalvo FJ, Winter RB. Scheuermann’s kyphosis and roundback deformity Results of Milwaukee brace treatment. J Bone Joint Surg Am. 1974;56(4):740–758. doi: 10.2106/00004623-197456040-00009. [DOI] [PubMed] [Google Scholar]
- 41.Sardar ZM, Ames RJ, Lenke L. Scheuermannʼs kyphosis: diagnosis, management, and selecting fusion levels. J Am Acad Orthop Surg. 2019;27(10):e462–e472. doi: 10.5435/JAAOS-D-17-00748. [DOI] [PubMed] [Google Scholar]
- 42.Weiss HR, Turnbull D, Bohr S. Brace treatment for patients with Scheuermann’s disease - a review of the literature and first experiences with a new brace design. Scoliosis. 2009;4:22. doi: 10.1186/1748-7161-4-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weiss HR, Ddeckmann J, Gerner HJ. Effect of intensive rehabilitation on pain in patients with Scheuermann’s disease. Res Into Spinal Deformities 3. 2002;88:254–257. 10.3233/978-1-60750-932-5-254. [PubMed]
- 44.Fisk J, Baigent M. Hamstring tightness and Scheuermann’s disease: a pilot study. Am J Phys Med Rehabil. 1981;60(3):122–125. [PubMed] [Google Scholar]
- 45.Lowe TG, Line BG. Evidence based medicine: analysis of Scheuermann kyphosis. Spine. 2007;32(Supplement):S115–S119. doi: 10.1097/BRS.0b013e3181354501. [DOI] [PubMed] [Google Scholar]
- 46.Gutowski WT, Renshaw TS. Orthotic results in adolescent kyphosis. Spine. 1988;13(5):485–489. doi: 10.1097/00007632-198805000-00009. [DOI] [PubMed] [Google Scholar]
- 47.Riddle EC, Bowen JR, Shah SA, Moran EF, Lawall H. The duPont kyphosis brace for the treatment of adolescent Scheuermann kyphosis. J South Orthop Assoc. 2003;12(3):135–140. [PubMed] [Google Scholar]
- 48.Diaremes P, Braun S, Meurer A. Morbus Scheuermann. Orthopäde. 2022;51(4):339–348. doi: 10.1007/s00132-022-04239-4. [DOI] [PubMed] [Google Scholar]
- 49.Cho SK, Kim YJ, Lenke LG. Proximal junctional kyphosis following spinal deformity surgery in the pediatric patient. J Am Acad Orthop Surg. 2015;23(7):408–414. doi: 10.5435/JAAOS-D-14-00143. [DOI] [PubMed] [Google Scholar]
- 50.Yanik HS, Ketenci IE, Polat A, et al. Prevention of proximal junctional kyphosis after posterior surgery of Scheuermann kyphosis: an operative technique. J Spinal Disord Tech. 2015;28(2):E101–E105. doi: 10.1097/BSD.0000000000000157. [DOI] [PubMed] [Google Scholar]
- 51.Koller H, Juliane Z, Umstaetter M, Meier O, Schmidt R, Hitzl W. Surgical treatment of Scheuermann’s kyphosis using a combined antero-posterior strategy and pedicle screw constructs: efficacy, radiographic and clinical outcomes in 111 cases. Eur Spine J. 2014;23(1):180–191. doi: 10.1007/s00586-013-2894-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhu W, Sun X, Pan W, et al. Curve patterns deserve attention when determining the optimal distal fusion level in correction surgery for Scheuermann kyphosis. Spine J. 2019;19(9):1529–1539. doi: 10.1016/j.spinee.2019.04.007. [DOI] [PubMed] [Google Scholar]
- 53.Kim HJ, Nemani V, Boachie-Adjei O, et al. Distal fusion level selection in Scheuermann’s kyphosis: a comparison of lordotic disc segment versus the sagittal stable vertebrae. Global Spine J. 2017;7(3):254–259. doi: 10.1177/2192568217699183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee SS, Lenke LG, Kuklo TR, et al. Comparison of Scheuermann kyphosis correction by posterior-only thoracic pedicle screw fixation versus combined anterior/posterior fusion. Spine. 2006;31(20):2316–2321. doi: 10.1097/01.brs.0000238977.36165.b8. [DOI] [PubMed] [Google Scholar]
- 55.Bradford DS, Moe JH, Montalvo FJ, Winter RB. Scheuermann’s kyphosis. Results of surgical treatment by posterior spine arthrodesis in twenty-two patients. JBJS. 1975;57(4):439. [PubMed]
- 56.Herndon WA, Emans JB, Micheli LJ, Hall JE. Combined anterior and posterior fusion for Scheuermann’s kyphosis. Spine. 1981;6(2):125–30. doi: 10.1097/00007632-198103000-00003. [DOI] [PubMed] [Google Scholar]
- 57.Otsuka NY, Hall JE, Mah JY. Posterior fusion for Scheuermann’s kyphosis. Clin Orthop Relat Res. 1990;1(251):134–9. [PubMed] [Google Scholar]
- 58.•• Tsirikos AI, Carter TH. The surgical treatment of severe Scheuermann’s kyphosis: posterior spinal fusion with closing wedge osteotomies and hybrid instrumentation gives excellent results. Bone Joint J. 2021;103-B(1):148–156. 10.1302/0301-620X.103B1.BJJ-2020-1279.R2. (Recent article expanding on surgical techniques which have proved successful in the surgical management of SK.) [DOI] [PubMed]
- 59.Lange T, Schmoelz W, Gosheger G, et al. Is a gradual reduction of stiffness on top of posterior instrumentation possible with a suitable proximal implant? A biomechanical study. Spine J. 2017;17(8):1148–1155. doi: 10.1016/j.spinee.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 60.Schwab F, Blondel B, Chay E, et al. The comprehensive anatomical spinal osteotomy classification. Neurosurgery. 2014;74(1):112–120. doi: 10.1227/NEU.0000000000000182o. [DOI] [PubMed] [Google Scholar]
- 61.Tsirikos AI, Jain AK. Scheuermann’s kyphosis; current controversies. J Bone Joint Surg Br. 2011;93-B(7):857–864. 10.1302/0301-620X.93B7.26129. [DOI] [PubMed]
- 62.Lonner BS, Toombs CS, Guss M, et al. Complications in operative Scheuermann kyphosis: do the pitfalls differ from operative adolescent idiopathic scoliosis? Spine. 2015;40(5):305–311. doi: 10.1097/BRS.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 63.Sarwahi V, Hasan S, Koutsogiannis P, et al. Scheuermann kyphosis patients have a similar revision and infection rate to adolescent idiopathic scoliosis patients. Spine. 2022;47(7):E290–E295. doi: 10.1097/BRS.0000000000004233. [DOI] [PubMed] [Google Scholar]
- 64.Coe JD, Smith JS, Berven S, et al. Complications of spinal fusion for Scheuermann kyphosis: a report of the scoliosis research society morbidity and mortality committee. Spine. 2010;35(1):99–103. doi: 10.1097/BRS.0b013e3181c47f0f. [DOI] [PubMed] [Google Scholar]
- 65.Bradford DS, Garica A. Neurological complications in Scheuermann’s disease. A case report and review of the literature. J Bone Joint Surg Am. 1969;51(3):567–572. [PubMed]
- 66.Ghasemi A, Stubig T, A. Nasto L, Ahmed M, Mehdian H. Distal junctional kyphosis in patients with Scheuermann’s disease: a retrospective radiographic analysis. Eur Spine J. 2017;26(3):913–920. 10.1007/s00586-016-4924-3. [DOI] [PubMed]
- 67.Gong Y, Yuan L, He M, et al. Comparison between stable sagittal vertebra and first lordotic vertebra instrumentation for prevention of distal junctional kyphosis in Scheuermann disease: Systematic Review and Meta-analysis. Clin Spine Surg. 2019;32(8):330–336. doi: 10.1097/BSD.0000000000000792. [DOI] [PubMed] [Google Scholar]
- 68.Dikici F, Akgul T, Sariyilmaz K, et al. Selection of distal fusion level in terms of distal junctional kyphosis in Scheuermann kyphosis. A comparison of 3 methods. Acta Orthop Traumatol Turc. 2018;52(1):7–11. 10.1016/j.aott.2017.11.012. [DOI] [PMC free article] [PubMed]
- 69.Luzzi A, Sardar Z, Cerpa M, et al. Risk of distal junctional kyphosis in scheuermann’s kyphosis is decreased by selecting the LIV as two vertebrae distal to the first lordotic disc. Spine Deform. 2022;10(6):1437–1442. doi: 10.1007/s43390-022-00542-4. [DOI] [PubMed] [Google Scholar]
- 70.Debnath UK, Quraishi NA, McCarthy MJH, et al. Long-term outcome after surgical treatment of Scheuermann’s kyphosis (SK) Spine Deform. 2022;10(2):387–397. doi: 10.1007/s43390-021-00410-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.