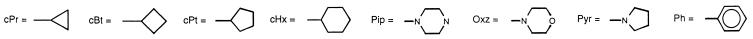Abstract
Aromatic dicationic compounds possess antimicrobial activity against a wide range of eucaryotic pathogens, and in the present study an examination of the structures-functions of a series of compounds against fungi was performed. Sixty-seven dicationic molecules were screened for their inhibitory and fungicidal activities against Candida albicans and Cryptococcus neoformans. The MICs of a large number of compounds were comparable to those of the standard antifungal drugs amphotericin B and fluconazole. Unlike fluconazole, potent inhibitory compounds in this series were found to have excellent fungicidal activities. The MIC of one of the most potent compounds against C. albicans was 0.39 μg/ml, and it was the most potent compound against C. neoformans (MIC, ≤0.09 μg/ml). Selected compounds were also found to be active against Aspergillus fumigatus, Fusarium solani, Candida species other than C. albicans, and fluconazole-resistant strains of C. albicans and C. neoformans. Since some of these compounds have been safely given to animals, these classes of molecules have the potential to be developed as antifungal agents.
The incidence of fungal infections in the immunocompromised population has significantly increased over the past two decades. Frequent infections caused by molds which may be primarily resistant to azoles and azole-resistant isolates of Candida albicans and Cryptococcus neoformans, which have developed recently, have increasingly been reported (7, 13, 23, 30). In light of these developments, new antifungal agents with various mechanisms of action and fungicidal activities are needed for the effective management of these clinically important infections.
Recently, we reported on the antifungal activities of analogues and metabolites of pentamidine and a series of dicationic substituted bis-benzimidazoles (11). Those in vitro studies uncovered a number of compounds with potent activity against C. albicans and C. neoformans. Several compounds were found to have inhibitory activity against these two fungi more potent than that of either fluconazole or amphotericin B. In addition, the dicationic molecules, unlike fluconazole, proved to have potent fungicidal activity, with the most potent compounds having minimum fungicidal concentrations (MFCs) below 1.0 μg/ml. These initial studies also found that dicationic molecules were effective against Aspergillus fumigatus, Fusarium solani, several Candida species other than C. albicans, and fluconazole-resistant strains of C. albicans and C. neoformans.
On the basis of the initial promising results reported above, the current work expands our studies on the antifungal activities of dication-substituted molecules by screening 67 additional compounds against C. albicans and C. neoformans. The criteria used to choose the structures for the current studies were based on years of testing dicationic molecules against the fungus Pneumocystis carinii in a rat model of disease (5, 14, 17, 18, 24, 26–28). Compounds in the current studies include molecules with the cationic moieties linked by carbazole, furan, and benzimidazole bridges. In addition to screening all compounds against C. albicans and C. neoformans, selected compounds were tested against other yeasts, molds, and azole-resistant strains of C. albicans and C. neoformans.
MATERIALS AND METHODS
Compounds.
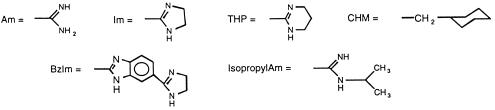
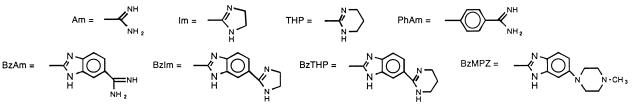
All compounds tested in this study were synthesized in the laboratories of two of the authors (D.W.B. or R.R.T.). Stock solutions of 10,000 μg/ml were made in sterile distilled water or dimethyl sulfoxide (DMSO), as recommended by the manufacturer, filter sterilized by passage through a 0.22-μm-pore-size Millex Durapore membrane filter, and stored at −70°C until use. The synthesis and physical properties for the following compounds have been or will be described in the indicated publications: compounds 1 to 15 (22); compounds 16 and 17 (10); compounds 18 (4); compounds 19 and 25 (29); compounds 20 to 24 (6); compounds 29, 32, 39, 42, 45, and 47 (31); compounds 43, 44, and 46 (16); compounds 48 to 63 (9); and compounds 64 to 67 (25). The remaining compounds (compounds 33 to 38, 40, and 41) have not been described in previous publications, and brief descriptions of the synthesis and physical properties of these novel compounds are given here, as follows.
Melting points (mp’s) were recorded with a Thomas Hoover (Uni-Melt) capillary melting point apparatus or a Fisher-Johns apparatus and are uncorrected. 1H nuclear magnetic resonance (NMR) and 13C NMR spectra were recorded with a Varian GX400 spectrometer, chemical shifts (δ) are in parts per million relative to tetramethylsilane, and coupling constants (J) are reported in hertz. Mass spectra (MS) were recorded on a VG Instrument 70-SE spectrometer (Georgia Institute of Technology, Atlanta). Infrared spectra were recorded with a Michelson 100 instrument (Bomem, Inc.). Elemental analyses were performed by Atlantic Microlab Inc. (Norcross, Ga.) and are within ±0.4 of the theoretical values. All chemicals and solvents were purchased from Aldrich Chemical Co. or Fisher Scientific (4, 10).
General procedure for synthesis of compounds 33 to 37.
To a suspension of 2,5-bis[4-carboxyphenyl]furan diacid chloride (6) (0.69 g, 0.002 mol) in 75 ml of dry CH2Cl2 a substituted diamine (0.002 mol) was added, and the mixture was stirred at room temperature for 6 h. The solvent was removed by distillation, the residue was treated with ice water, the pH of the mixture was adjusted to 10 with 2 M NaOH, and the separated solid was filtered, washed with cold water, and crystallized from ether-CHCl3 or ether-methanol to yield pale yellow solids in yields of 75 to 85%. The free base was converted to its HCl salt by treating the free base with saturated ethanolic-HCl, with yields of 75 to 90%.
2,5-Bis-{4-[3-(N-morpholinopropyl)carbamoyl]phenyl}furan dihydrochloride (compound 33).
An 83% yield of a yellow crystalline solid (mp, 140 to 142°C decompose [dec]) was obtained by the procedure described above. 1H NMR (DMSO-d6/D2O): 7.93 (d, 4H, J = 8.4 Hz), 7.86 (d, 4H, J = 8.4 Hz), 7.15 (s, 2H), 3.86 (br m, 8H), 3.38 (br m, 12H), 3.16 (t, 4H, J = 7.6 Hz), 2.0 (quintet, 4H, J = 7.6 Hz). 13C NMR (DMSO-d6/D2O): 166.5, 152.7, 133.1, 132.4, 128.0, 123.4, 110.0, 63.3, 54.3, 51.3, 36.6, 23.4. MS m/e 560 (M+). Analysis calculated for C32H40N4O5 · 2HCl: C, 60.65; H, 6.68; N, 8.84. Found: C, 60.59; H, 6.77; N, 8.87.
2,5-Bis[4-(2-N,N-dimethylaminoethylcarbamoyl)phenyl]furan dihydrochloride (compound 34).
An 80% yield of pale yellow crystalline solid (mp, 189 to 191°C dec) was obtained by the procedure described above. 1H NMR (DMSO-d6/D2O): 7.94 (d, 4H, J = 8.4 Hz), 7.84 (d, 4H, J = 8.4 Hz), 7.12 (s, 2H), 3.66 (t, 4H, J = 6.0 Hz), 3.30 (t, 4H, J = 6.0 Hz), 2.84 (s, 12H). 13C NMR (DMSO-d6/D2O): 167.4, 152.9, 132.9, 132.7, 128.4, 123.7, 110.4, 56.6, 43.1, 34.9. MS m/e 448 (M+). Analysis calculated for C26H32N4O3 · 2HCl: C, 59.88; H, 6.57; N, 10.74. Found: C, 59.78; H, 6.66; N, 10.64.
2,5-Bis[4-(3-N,N-dimethylaminopropylcarbamoyl)phenyl]furan dihydrochloride (compound 35).
A 75% yield of yellow hygroscopic solid (mp, 205 to 208°C dec) was obtained by the procedure described above. 1H NMR (DMSO-d6): 10.91 (br, 2H), 8.80 (b s 2H), 8.03 (d, 4H, J = 8.4 Hz), 7.92 (d, 4H, J = 8.4 Hz), 7.23 (s, 2H), 3.40 (q, 4H, J = 6 Hz), 3.15 (quintet, 4H, J = 5.2 Hz), 2.76 (s, 6H), 2.75 (s, 6H), 2.02 (quintet, 4H, J = 6.8 Hz). 13C NMR (DMSO-d6/D2O): 165.6, 152.4, 132.8, 131.9, 127.7, 122.9, 109.6, 54.3, 41.7, 36.2, 23.8. MS m/e 476 (M+). Analysis calculated for C28H36N4O3 · 2HCl · 0.5 H2O: C, 60.21; H, 7.03; N, 10.03. Found: C, 60.51; H, 7.13; N, 9.95.
2,5-Bis[4-(3-N-methyl-3-N-phenylaminopropylcarbamoyl)phenyl]furan dihydrochloride (compound 36).
A 78% yield of yellow solid (mp, 85 to 88°C dec) was obtained by the procedure described above. 1H NMR (DMSO-d6/D2O): 7.93 (d, 4H, J = 8.4 Hz), 7.89 (d, 4H, J = 8.4 Hz), 7.58 (d, 2H, J = 8.8 Hz), 7.55 to 7.48 (m, 6H), 7.37 (S, 2H), 7.18 (s, 2H), 3.59 (m, 4H), 3.35 (t, 4H, J = 7.2 Hz), 3.16 (s, 6H), 1.83 (quintet, 4H, J = 7.2 Hz). 13C NMR (DMSO-d6/D2O): 166.4, 152.7, 133.1, 132.4, 130.1, 130.0, 128.0, 123.4, 119.7, 109.9, 55.3, 43.6, 36.5, 25.3. MS m/e 601 (M+). Analysis calculated for C38H40N4O3 · 2HCl · 0.75 H2O: C; 66.37; H, 6.34; N, 8.15. Found: C, 66.43; H, 6.34; N, 8.21.
2,5-Bis[4-(3-N,N8,N11-trimethylaminopropylcarbamoyl)phenyl]furan dihydro chloride (compound 37).
An 85% yield of a yellow solid (mp, 245 to 247°C dec) was obtained by the procedure described above. 1H NMR (DMSO-d6/D2O): 7.82 (d, 4H, J = 8 Hz), 7.56 (d, 4H, J = 8 Hz), 7.07 (s, 2H), 3.5 to 3.48 (m, 4H), 3.03 (brm, 4H), 2.94 (s, 6H), 2.76 (s, 12H), 1.98 (quintet, 4H, J = 7.6 Hz). 13C NMR (DMSO-d6/D2O): 171.2, 152.8, 135.4, 131.2, 127.9, 123.8, 109.7, 54.9, 42.7, 38.7, 22.4. MS m/e 504 (M+). Analysis calculated for C30H40N4O3 · 2HCl · 0.5 H2O: C, 61.42; H, 7.38; N, 9.45. Found: C, 61.11; H, 7.27; N, 9.61.
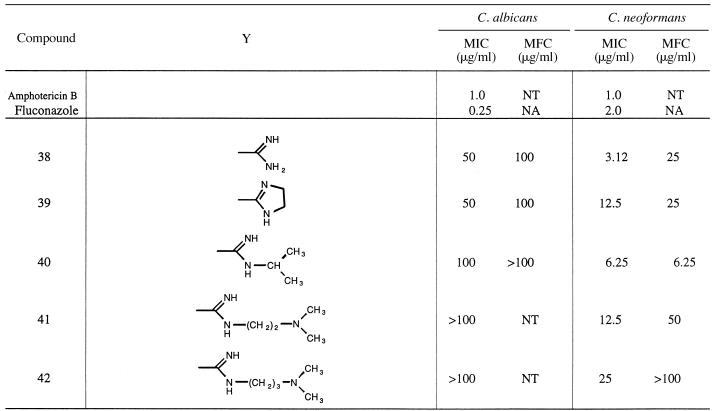
2,5-Bis[3-amidinophenyl]furan dihydrochloride (compound 38).
A suspension of the imidate ester dihydrochloride (0.87 g, 0.002 mol), prepared from 2,5-bis[3-cyanophenyl]furan (31), in 30 ml of absolute ethanol was saturated with dry ammonia at 0 to 5°C, and the mixture was stirred at room temperature for 2 h. The solvent was removed; the solid was treated with ice water, filtered, and basified with cold aqueous 2 M NaOH; and the off-white precipitate which formed was filtered. The solid was washed with water and dried to yield 0.49 g (80.6%; mp, 198 to 200°C). The free base (0.3 g, 0.001 mol) was converted into the HCl salt by treating it with ethanolic HCl to yield 0.35 g (90%; mp 221 to 224°C dec). 1H NMR (DMSO-d6/D2O) 8.18 (br s, 2H), 8.10 (d, 2H, J = 7.6 Hz), 7.68 (t, 2H, J = 7.6 Hz), 7.64 (d, 2H, J = 7.6 Hz), 7.14 (s, 2H). 13C NMR (DMSO-d6): 166.1, 152.5, 131.3, 130.6, 129.3, 129.0, 127.4, 123.3, 110.5. MS (fast atom bombardment [FAB]) m/e 305 (M+ + 1). Analysis calculated for C18H16N4O · 2HCl · 0.25 H2O: C, 56.55; H, 4.88; N, 14.67. Found: C, 56.75; H, 4.84; N, 14.26.
2,5-Bis-[3-(N-isopropylamidino)amidinophenyl]furan dihydrochloride (compound 40).
The imidate ester hydrochloride (0.435 g, 0.001 mol), prepared from 2,5-bis(3-cyanophenyl)furan (31), and isopropyl amine (0.124 g, 0.0022 mol) in 10 ml of ethanol were stirred for 12 h and yielded, after standard workup, 0.13 g (80%) of beige free base (mp, 180 to 181°C; crystallized from hexane-ether [3:1]). On treatment with saturated ethanolic HCl, the free base yielded 0.29 g (75%) of a crystalline solid (mp, 225 to 227°C dec). 1H NMR (DMSO-d6): 8.18 to 8.12 (m, 4H), 7.71 to 7.62 (m, 4H), 7.27 (s, 2H), 4.09 (quintet, 2H, J = 6.4 Hz), 1.30 (d, 12H, J = 6.4 Hz). 13C NMR (DMSO-d6): 161.6, 152.1, 130.4, 129.9, 129.7, 127.9, 127.3, 123.3, 110.1, 45.2, 21.2. MS (FAB) m/e 339 (M+ + 1). Analysis calculated for C24H28N4O · 2HCl · 0.25 H2O: C, 61.86; H, 6.59; N, 12.02. Found: C, 61.82; H, 6.27; N, 11.76.
2,5-Bis-3[(N-(2-dimethylaminoethyl)amidino]phenylfuran dihydrochloride (compound 41).
A mixture of imidate ester hydrochloride (0.435 g, 0.001 mol), prepared from 2,5-bis[3-cyanophenyl]furan (31), and 2-dimethylaminoethylamine (0.185 g, 0.0021 mol) in 10 ml of ethanol was stirred at room temperature for 12 h. On standard workup 0.33 g (74%) of crystalline solid (mp, 75 to 77°C) was obtained. On treatment with saturated ethanolic HCl, the free base (0.30 g, 0.00072 mol) gave 0.35 g (82%) hydrochloride salt (very hygroscopic), which was dried at 75°C for 24 h (mp, 260 to 263°C dec). 1H NMR (DMSO-d6): 8.28 (brs,2H), 8.12 (d, 2H, J = 8 Hz), 7.72 (d, 2H, J = 8 Hz), 7.65 (t, 2H, J = 8 Hz), 7.21 (s, 2H), 3.9 (t, 4H, J = 4.2 Hz), 3.50 (t, 3H, J = 4.2 Hz), 2.89 (s, 12H). 13C NMR (DMSO-d6): 164.1, 152.4, 130.9, 130.3, 129.6, 128.8, 127.6, 123.7, 110.5, 54.5, 43.2, 38.4. MS (FAB) m/e 447 (M+ + 1). Analysis calculated for C26H34N6O · 4HCl: C, 52.70; H, 6.46; N, 14.19. Found: C, 52.43; H, 6.42; N, 13.99.
Test organisms.
The fungi tested included two quality control strains, C. neoformans var. neoformans H99 and C. albicans A39, and the following clinical isolates: four strains of C. neoformans var. neoformans (strains 167.95, 135.95, 114.96, and 133.95), of which three strains (strains 135.95, 114.96, and 133.95) were fluconazole resistant (MICs, >64 μg/ml); two strains of C. neoformans var. gattii (strains 119.95 and 114.95); two strains of fluconazole-resistant C. albicans (MICs, >64 μg/ml; strains 102.96 and 103.96); and one strain each of Candida parapsilosis (strain 111.96), Candida krusei (strain 132.91), Candida tropicalis (strain 110.96), Candida lusitaniae (strain 111.92) Candida glabrata (strain 142.91), Rhizopus arrhizus (strain 117.89), Fusarium solani (strain 152.89), Aspergillus fumigatus (strain 168.95), Aspergillus flavus (strain 112.96), and Paecilomyces lilacinus (strain 137.90).
Medium.
Antifungal susceptibility testing was performed in RPMI 1640 medium (Sigma, St. Louis, Mo.) with glutamine but without sodium bicarbonate and buffered at pH 7.0 with 0.165 M morpholinopropanesulfonic acid.
In vitro susceptibility testing.
Experiments for determination of MICs were performed by the broth macrodilution method following the recommendations of the National Committee for Clinical Laboratory Standards (21). The only difference compared to the standardized method was the choice of drug dilutions, which ranged from 100 to 0.09 μg/ml. Briefly, this method specifies the use of an inoculum grown at 35°C and adjusted to a concentration of 0.5 × 103 to 2 × 103 CFU/ml, incubation at 35°C, and reading of the endpoints at 48 h for all yeasts other than C. neoformans, for which the endpoints are read at 72 h. The MIC was defined as the lowest drug concentration which produced a visual turbidity indicating less than or equal to 80% inhibition compared with that in the growth control tube.
Experiments for determination of MFCs were performed by a method adapted from that of McGinnis (20). Briefly, 10-μl aliquots from tubes with growth inhibition were plated onto Sabouraud agar plates. The lowest drug concentration that yielded three or fewer yeasts colonies was recorded as the MFC. Several drugs were also rescreened by subculturing 100-μl aliquots from tubes with no growth, and there was no change in the MFCs.
Molds were tested by the same method (21) but with the following modifications. Isolates were grown on Sabouraud dextrose agar at 30°C and were subcultured twice to ensure viability. After adequate sporulation occurred (after 4 to 12 days), the conidia were harvested by flooding the colonies with a sterile solution of 0.85% NaCl–0.05% Tween 80 in distilled water. Inoculum suspensions were prepared by a hemocytometric procedure and were then diluted with RPMI 1640 medium to obtain an inoculum size of approximately 0.5 × 103 to 2 × 103 CFU/ml. The inoculum size was verified by plating an aliquot of the inoculum. The tubes were incubated at 30°C for 48 to 72 h or until growth in the control tube was visible. The reproducibility of the method was quality controlled by testing fluconazole and amphotericin B against C. albicans A39 and C. neoformans var. neoformans H99 in each experiment. The MICs of fluconazole for C. albicans A39 and C. neoformans var. neoformans var. neoformans H99 were 0.25 and 2 μg/ml, respectively. The MIC of amphotericin B was 1 μg/ml for both isolates.
RESULTS
Carbazoles.
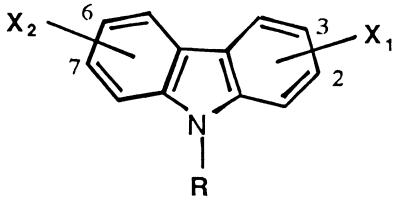
A series of 15 carbazoles (Table 1) were chosen for antifungal screening on the basis of their high levels of activity against P. carinii pneumonia (22). When the carbazoles were considered as a group, they appeared to have more potent activity against C. neoformans than against C. albicans. Examination of the data from Table 1 indicates that 2,7-substituted analogues (compounds 10, 11, and 12) were significantly more effective than the corresponding 3,6-substituted analogues (compounds 1, 3, and 5) against C. albicans. The only exception to this observation was in the increased activity of the 3,6-benzimidazole-substituted carbazole (compound 9) compared to the activity of the corresponding 2,7-substituted analogue (compound 13). In compounds 9 and 13 the distance separating the cationic moieties is greatly increased, and these compounds cannot be compared structurally with analogues having the cationic groups attached directly to the carbazole ring. The most potent carbazole against C. albicans was compound 10, which had an MIC of 0.78 μg/ml and an MFC of 1.56 μg/ml. The MIC of this compound was in the same range as that of fluconazole.
TABLE 1.
Structures and in vitro activities of dibenzocarbazoles
| Compound | X1a | X2a | Position X | R |
C. albicans
|
C. neoformans
|
||
|---|---|---|---|---|---|---|---|---|
| MIC (μg/ml) | MFC (μg/ml) | MIC (μg/ml) | MFC (μg/ml) | |||||
| Amphotericin B | 1.0b | NTc | 1.0b | NT | ||||
| Fluconazole | 0.25 | NAd | 2.0 | NA | ||||
| 1 | Am | Am | 3,6 | H | 25 | 25 | 12.5 | 25 |
| 2 | Am | Am | 3,6 | CH3 | 100 | >100 | 25 | 50 |
| 3 | Im | Im | 3,6 | H | >100 | >100 | 3.12 | 25 |
| 4 | IsopropAm | IsopropAm | 3,6 | H | >100 | >100 | >100 | >100 |
| 5 | Im | Im | 3,6 | CH3 | 50 | >100 | 1.56 | 1.56 |
| 6 | Im | Im | 3,6 | CHM | 25 | 25 | 1.56 | 3.12 |
| 7 | THP | THP | 3,6 | CHM | 100 | >100 | 6.25 | 6.25 |
| 8 | THP | THP | 3,6 | CH3 | >100 | NT | 25 | 50 |
| 9 | BzIm | BzIm | 3,6 | H | 1.56 | 6.25 | 3.12 | 6.25 |
| 10 | Am | Am | 2,7 | H | 0.78 | 1.56 | 6.25 | 50 |
| 11 | Im | Im | 2,7 | H | 3.12 | 3.12 | 1.56 | 3.12 |
| 12 | Im | Im | 2,7 | CH3 | 12.5 | 50 | 1.56 | 3.12 |
| 13 | BzIm | BzIm | 2,7 | H | 100 | >100 | 25 | 50 |
| 14 | Im | OCH3 | 2,7 | H | >100 | >100 | >100 | NT |
| 15 | Im | OCH3 | 2,7 | CH3 | >100 | >100 | >100 | NT |
100% inhibition.
NT, not tested.
NA, no activity.
Several of the carbazole derivatives were found to have activities against C. neoformans comparable to those of the control drugs. The only dicationic carbazole devoid of activity at 100 μg/ml against C. neoformans was the analogue with an isopropyl group-substituted amidine (compound 4). The most potent derivatives were compounds with imidazolines as the cationic groups (compounds 3, 5, 6, 11, and 12). It is important that replacement of one of the cationic moieties with a methoxy group resulted in a significant reduction or loss of antifungal activity against both organisms (compounds 14 and 15).
Furans.
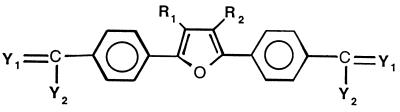
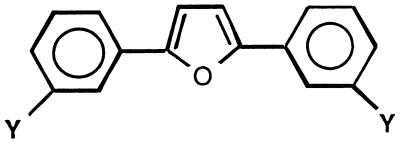
The series of compounds in which the cationic moieties were substituted in the para position on a phenyl ring attached to a 2,5-furanyl bridge (Table 2) was also selected for testing against fungi on the basis of the excellent anti-pneumocystis activities exhibited by selected members of the class (5, 6). Several members of this series exhibited good activity against C. albicans, but none of the compounds were found to have activity equal to or better than that of amphotericin B or fluconazole. Ten of the compounds in this series were devoid of activity against C. albicans at the highest dose tested (100 μg/ml). This series exhibited greater potency against C. neoformans than against C. albicans, with the MICs of four of the compounds (compounds 20, 21, 23, and 31) being lower than the MICs of the control drugs. The MIC of the most potent compound of the series (compound 31) against C. neoformans was more than 10 times greater than that of amphotericin B and 20 times greater than that of fluconazole. The only compounds devoid of activity against C. neoformans were the compounds with an amido moiety (compounds 33 to 37) and a compound with methoxy group-substituted amidines (compound 18).
TABLE 2.
Structures and in vitro activities of para-substituted symmetrical furans
| Compound | Y1 | Y2a | R1 | R2 |
C. albicans
|
C. neoformans
|
||
|---|---|---|---|---|---|---|---|---|
| MIC (μg/ml) | MFC (μg/ml) | MIC (μg/ml) | MFC (μg/ml) | |||||
| Amphotericin B | 1.0b | NTc | 1.0b | NT | ||||
| Fluconazole | 0.25 | NAd | 2.0 | NA | ||||
| 16 | NH | NH2 | H | H | 6.25 | 25 | 12.5 | 25 |
| 17 | NH | NH2 | CH3 | CH3 | 6.25 | 6.25 | 3.12 | 3.12 |
| 18 | NOCH3 | NH2 | H | H | >100 | NT | >100 | NT |
| 19 | NH | NH-cPr | H | H | 25 | 25 | 3.12 | 50 |
| 20 | NH | NH-cBt | H | H | 6.25 | >50 | 0.78 | 6.25 |
| 21 | NH | NH-cPt | H | H | 6.25 | 25 | 0.78 | 6.25 |
| 22 | NH | NH-cHx | H | H | 12.5 | 12.5 | 1.56 | 6.25 |
| 23 | NH | NH-CH2-cPr | H | H | 6.25 | 25 | 0.78 | 6.25 |
| 24 | NH | NH-CH2)2CH3 | H | H | 12.5 | 12.5 | 1.56 | 6.25 |
| 25 | NH | NH-CH(CH3)2 | H | H | 25 | 50 | 6.25 | 50 |
| 26 | NH | NH-CH(CH2CH3)2 | H | H | 100 | 100 | 12.5 | 12.5 |
| 27 | NH | NH-CH2-CH(CH3)2 | H | H | 12.5 | NT | 3.12 | 12.5 |
| 28 | NH | NH-(CH2)2-CH(CH3)2 | H | H | 12.5 | 12.5 | 6.25 | 12.5 |
| 29 | NH | NH-(CH2)3-Pyr | H | H | 100 | NT | 6.25 | 25 |
| 30 | NH | NH-(CH2)3PipCH3 | H | H | >100 | NT | 12.5 | 50 |
| 31 | NH | NH-(CH2)4-NH(CH3)2 | H | H | 12.5 | 25 | ≤0.09 | 0.19 |
| 32 | NH | NH-(CH2)6-NH(CH3)2 | H | H | >100 | NT | 1.56 | 3.12 |
| 33 | ONH-(CH2)3Oxz | H | H | >100 | NT | >100 | NT | |
| 34 | ONH-(CH2)2-NH(CH3)2 | H | H | >100 | NT | >100 | NT | |
| 35 | ONH-(CH2)3-NH(CH3)2 | H | H | >100 | NT | >100 | NT | |
| 36 | ONH-(CH2)3-NHCH3Ph | H | H | >100 | NT | >100 | NT | |
| 37 | ONCH3-(CH2)3-NH(CH3)2 | H | H | >100 | NT | >110 | NT | |
The meta analogues of the compounds described above (Table 3) exhibited only modest or no activity against C. albicans. A significant increase in activity is noted for the para-substituted compounds over the meta-substituted analogues (compare compounds 16 and 38 and compounds 25 and 40). The meta analogues are more potent against C. neoformans than against C. albicans. Against C. neoformans the meta analogues were as potent or slightly more potent than their para counterparts.
TABLE 3.
Structures and in vitro activities of meta-substituted symmetrical furansa
See footnote b of Table 1 for explanation of amphotericin B MICs. Abbreviations: NT, not tested. NA, no activity.
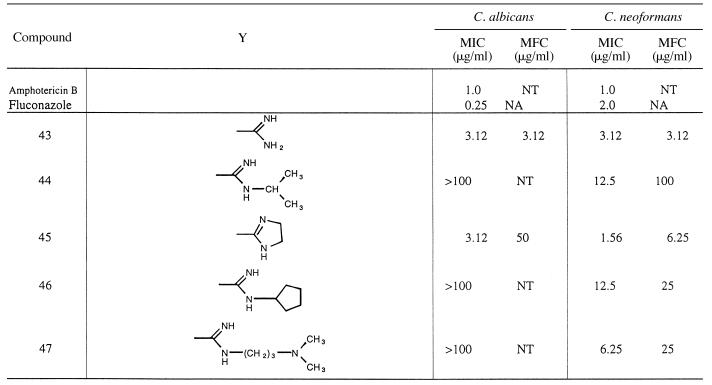
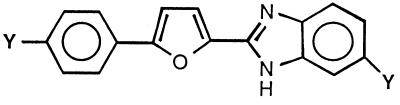
Like the compounds described above, those in Table 4 have the furan ring in the bridge between the cation-containing rings. However, these compounds are asymmetrical and have one cation on a benzimidazole ring, while the second cation is attached to a phenyl ring. Only two of these compounds (compound 43 and 45) exhibited significant activity against C. albicans. All five of the compounds of this series had good activity against C. neoformans. The two most potent compounds against C. neoformans (compounds 43 and 45) were the only two structures showing significant activity against C. albicans.
TABLE 4.
Structures and in vitro activities of asymmetrical furansa
See footnote b of Table 1 for explanation of amphotericin B MICs. Abbreviations: NT, not tested. NA, no activity.
Benzimidazoles.
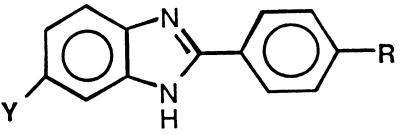
The compounds depicted in Table 5 are all characterized by having a 2-phenylbenzimidazole as the central linker between the cationic moieties. Molecules in this group have previously been studied for their DNA minor groove binding (8, 9) and protease inhibition (25) properties. Because we have associated the ability of these molecules to bind in the minor groove of DNA with their antimicrobial activity (1, 24), compounds 48 to 63 were logical choices for antifungal testing. In addition to being studied for antitrypsin potency, compounds 64 to 67 were determined to be excellent DNA binding agents and to have potent anti-P. carinii activities (23a). Eleven of the compounds in this series were found to have activity against C. albicans approaching the same level as those of amphotericin B and fluconazole. The MICs of three of the compounds (compounds 53, 64, and 65) for C. albicans were 0.39 μg/ml. Ten of the compounds in this group had potencies equal to or greater than those of the control drugs against C. neoformans, with two compounds (compounds 53 and 65) exhibiting a 10-fold greater potency than amphotericin B. In addition, seven compounds (compounds 53, 54, 56, and 64 to 67) demonstrated excellent activity against both fungi compared to the activities of amphotericin B and fluconazole.
TABLE 5.
Structures and in vitro activities of benzimidazoles
| Compound | Ya | Ra |
C. albicans
|
C. neoformans
|
||
|---|---|---|---|---|---|---|
| MIC (μg/ml) | MFC (μg/ml) | MIC (μg/ml) | MFC (μg/ml) | |||
| Amphotericin B | 1.0b | NTc | 1.0b | NT | ||
| Fluconazole | 0.25 | NAd | 2.0 | NA | ||
| 48 | BzIm | OH | 3.12 | NT | 3.12 | NT |
| 49 | BzIm | OCH3 | 0.78 | NT | 3.12 | 3.12 |
| 50 | BzIm | OC2H5 | 0.78 | 12.5 | 1.56 | 1.56 |
| 51 | BzTHP | OH | 3.12 | 25 | 1.56 | 6.25 |
| 52 | BzAm | OH | 0.78 | 6.25 | 3.12 | 6.25 |
| 53 | BzAm | Am | 0.39 | 1.56 | ≤0.09 | 0.78 |
| 54 | BzIm | Im | 0.78 | 1.56 | 0.39 | 0.39 |
| 55 | BzAm | OCH3 | >100 | NT | 3.12 | >25 |
| 56 | BzTHP | THP | 0.78 | 1.56 | 0.19 | 0.39 |
| 57 | BzAm | OC2H5 | 0.78 | 1.56 | 1.56 | 1.56 |
| 58 | BzMPZ | OH | 25 | 50 | 100 | >100 |
| 59 | Im | OCH3 | 25 | NT | 50 | 100 |
| 60 | Am | Am | 100 | >100 | 25 | 50 |
| 61 | Am | OH | >100 | NT | >100 | >100 |
| 62 | Im | Im | 50 | >100 | 50 | NT |
| 63 | THP | THP | 50 | 100 | 25 | 50 |
| 64 | Am | O(CH2)3OPhAm | 0.39 | 0.78 | 0.19 | 0.78 |
| 65 | Am | O(CH2)4OPhAm | 0.39 | 3.12 | ≤0.09 | 1.56 |
| 66 | Am | O(CH2)5OPhAm | 0.78 | 1.56 | 0.39 | 6.25 |
| 67 | Am | OPhAm | 0.78 | 0.78 | 0.78 | 6.25 |
Broad-spectrum activities of compounds 10, 21, and 53.
Three compounds were selected for extended in vitro susceptibility testing to determine their spectra of antifungal activity. Compound 10 was chosen as an example of the carbazole group on the basis of the excellent activity of the compound against P. carinii (22) and Cryptosporidium parvum (3) and the low level of toxicity of the compound in rats (22). Compound 21 was chosen on the basis of its good in vitro activity against C. albicans and C. neoformans and the availability of the compound. Compound 53 was chosen from the benzimidazole group because of its excellent activity against C. albicans and C. neoformans. The compounds were tested against Candida strains other than C. albicans including fluconazole-resistant isolates, several C. neoformans isolates including serotype B and C isolates, and some clinically important molds. All three compounds exhibited broad-spectrum activity (Table 6). Compound 10 was active against all strains of Candida and Cryptococcus tested, with excellent potency against C. tropicalis. Compound 10 showed good activity against A. fumigatus but only modest activity against A. flavus, F. solani, P. lilacinus, and R. arrhizus. Compound 21 showed a pattern of activity very similar to that of compound 10. A notable difference between the two compounds was that compound 10 tended to be more effective against Candida strains, while compound 21 exhibited more potency toward Cryptococcus strains. Compound 21 was observed to be active against fluconazole-resistant strains of C. albicans. Compound 53 showed excellent activity against all strains tested with the exceptions of A. flavus, P. lilacinus, and R. arrhizus. This compound was highly potent against A. fumigatus and F. solani and all strains of Cryptococcus and Candida.
TABLE 6.
Extended antifungal spectra of selected compounds
| Strain | Compound 10
|
Compound 21
|
Compound 53
|
|||
|---|---|---|---|---|---|---|
| MIC (μg/ml) | MFC (μg/ml) | MIC (μg/ml) | MFC (μg/ml) | MIC (μg/ml) | MFC (μg/ml) | |
| C. albicans A39 | 0.78 | 1.56 | 6.25 | 25 | 0.39 | 1.56 |
| C. albicans 102.96a | NTb | NT | 12.5 | 12.5 | NT | NT |
| C. albicans 103.96a | NT | NT | 12.5 | 12.5 | NT | NT |
| C. krusei 132.91 | 1.56 | 12.5 | 12.5 | >100 | 0.39 | 0.39 |
| C. lusitaniae 111.92 | 3.12 | 25 | 6.25 | 25 | 0.39 | 1.56 |
| C. parapsilosis 111.96 | 0.78 | >6.25 | 6.25 | 100 | 0.39 | 0.39 |
| C. tropicalis 110.96 | ≤0.09 | 0.19 | 3.12 | 12.5 | 0.39 | 0.39 |
| C. glabrata 142.91 | 0.78 | >12.5 | 0.78 | >6.25 | ≤0.09 | 0.78 |
| C. neoformans H99 | 6.25 | >50 | 0.39 | 6.25 | ≤0.09 | 0.78 |
| C. neoformans 114.95c | 6.25 | 12.5 | 0.78 | 3.12 | 0.19 | 0.39 |
| C. neoformans 119.95d | 1.56 | 6.25 | 0.39 | 1.56 | 0.19 | 0.39 |
| C. neoformans 133.95a | NT | NT | 0.39 | 1.56 | NT | NT |
| C. neoformans 135.95a | 3.12 | 6.25 | 0.39 | 1.56 | ≤0.09 | 0.19 |
| C. neoformans 167.95 | NT | NT | 0.39 | 0.78 | NT | NT |
| C. neoformans 114.96a | 6.25 | 25 | 0.78 | 1.56 | ≤0.09 | 0.19 |
| A. flavus 112.96 | 50 | 50 | 25 | NT | >100 | NT |
| A. fumigatus 168.95 | 3.12 | 25 | 1.56 | NT | ≤0.09 | NT |
| F. solani 152.89 | 50 | 100 | >100 | NT | 0.19 | NT |
| P. lilacinus 137.90 | 100 | NT | >100 | NT | >100 | NT |
| R. arrhizus 117.89 | 25 | 50 | 25 | NT | >100 | NT |
Fluconazole-resistant isolates.
NT, Not tested.
Serotype B.
Serotype C.
DISCUSSION
The antifungal data from Tables 1 to 5 indicate that a number of the 67 compounds tested exhibit activities equal to or greater than those of fluconazole and amphotericin B. As we reported earlier (11), this class of compounds is not only inhibitory but also shows in vitro fungicidal activity.
In addition to the activities of the compounds against several isolates of C. neoformans and C. albicans, it was encouraging to find that selected molecules exhibited activity against other yeasts and molds. Particularly exciting was compound 53, which was found to have excellent activity against C. albicans, C. neoformans, A. fumigatus, and F. solani.
Comparison of the reported DNA binding data for these compounds (5, 6, 8, 22) with the corresponding antifungal activity reveals no direct correlation between affinity for the minor groove of DNA and their antifungal activities. However, the only compounds in this series that did not bind in the minor groove of DNA (compounds 14 and 15) (22) were also devoid of antifungal activity at the highest dose tested, 100 μg/ml. These data are supportive of our hypothesis that minimal DNA binding is necessary for, but not an assurance of, antimicrobial activity (22). We have theorized that the binding of compounds to the DNA results in competitive inhibition of key DNA-directed enzymes. Therefore, the strength of DNA binding is not as important as the site of binding in the minor groove (2, 11, 22). Previously, we had reported that there was a strong correlation between the antigiardial activity of dicationic molecules and their ability to bind in the minor groove and to inhibit topoisomerase II (2). Our present data suggest that topoisomerase I is probably not the target of these compounds in C. neoformans, as for camptothecin and its congeners (12). It has been hypothesized that the primary cellular target of a dicationic compound, pentamidine, in S. cerevisiae is the mitochondrion (19), and more recently, it has been suggested that these compounds have an effect on P. carinii due to DNA binding followed by inhibition of an endo- or exonuclease (15). A key to the further development of these dicationic molecules as antifungal agents will be to establish their mechanisms of action. The ability to readily grow these fungi in vitro compared to the ability to grow giardia, cryptosporidium, and pneumocystis in vitro bodes well for future studies into determining the mechanism(s) of antifungal activity of these dicationic molecules.
In addition to determining the mechanisms of action of dicationic molecules and developing a rational approach to drug design, lead compounds (i.e., compound 53) will be resynthesized for testing in animal models of fungal disease. If it can be determined that dicationic molecules are effective in vivo, the enthusiasm for this class of compounds as clinically useful antifungal agents will be greatly increased.
ACKNOWLEDGMENTS
This work was supported by an NIH Program Project (NAIAD AI-33363) and awards from Pharm-Eco Pharmaceutical, Inc., Lexington, Mass., and Immtech International, Inc., Evanston, Ill.
We appreciate the clerical expertise and assistance of Vicki Wingate and Mary Ann Howard.
REFERENCES
- 1.Bell C A, Cory M, Fairley T A, Hall J E, Tidwell R R. Structure-activity relationships of pentamidine analogs against Giardia lamblia and correlation of antigiardial activity with DNA-binding affinity. Antimicrob Agents Chemother. 1991;35:1099–1107. doi: 10.1128/aac.35.6.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bell C A, Dykstra C C, Naiman N N, Cory M, Fairley T A, Tidwell R R. Structure-activity studies of dicationic substituted bis-benzimidazoles against Giardia lamblia: correlation of antigiardial activity with DNA binding affinity and giardial topoisomerase II inhibition. Antimicrob Agents Chemother. 1993;37:2668–2673. doi: 10.1128/aac.37.12.2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blagburn, B. L., K. L. Drain, T. M. Land, P. H. Moore, D. S. Lindsay, A. Kumar, J. Shi, D. W. Boykin, and R. R. Tidwell. Dicationic furans inhibit development of Cryptosporidium parvum in HSD/ICR suckling swiss mice. Submitted for publication. [PubMed]
- 4.Boykin D W, Kumar A, Hall J E, Bender B C, Tidwell R R. Anti-pneumocystis activity of bis-amidoximes and bis-o-alkylamidoximes prodrugs. Biorg Med Chem Lett. 1996;6:3017–3020. [Google Scholar]
- 5.Boykin D W, Kumar A, Spychala J, Zhou M, Lombardy R J, Wilson W D, Dykstra C C, Hall J E, Jones S K, Tidwell R R, Laughton C, Neidle S. Dicationic diaryl furans as anti-Pneumocystis carinii agents. J Med Chem. 1995;38:912–916. doi: 10.1021/jm00006a009. [DOI] [PubMed] [Google Scholar]
- 6.Boykin D W, Kumar Xiao A G, Wilson W D, Bender B C, McCurdy D R, Hall J E, Tidwell R R. 2,5-Bis[4-(N-alkylamidino) phenyl]furans as anti-Pneumocystis carinii agents. J Med Chem. 1998;41:124–129. doi: 10.1021/jm970570i. [DOI] [PubMed] [Google Scholar]
- 7.Cameron M L, Schell W A, Bruch S, Bartlett J A, Waskin H A, Perfect J R. Correlation of in vitro fluconazole resistance of Candida isolates in relation to therapy and symptoms of individuals seropositive for human immunodeficiency virus type 1. Antimicrob Agents Chemother. 1993;37:2449–2453. doi: 10.1128/aac.37.11.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Czarny A, Boykin D W, Wood A A, Nunn C M, Neidle S, Zhao M, Wilson W D. Analysis of van der Waals and electrostatic contributions in the interactions of minor groove binding benzimidazoles with DNA. J Am Chem Soc. 1995;117:4716–4717. [Google Scholar]
- 9.Czarny A, Wilson W D, Boykin D W. Synthesis of mono-cationic and dicationic analogs of Hoechst 33258. J Heterocycl Chem. 1996;33:1393–1397. [Google Scholar]
- 10.Das B P, Boykin D W. Synthesis and antiprotozoal activity of 2,5-bis(4-guanylphenyl)furans. J Med Chem. 1977;20:531–536. doi: 10.1021/jm00214a014. [DOI] [PubMed] [Google Scholar]
- 11.Del Poeta M, Schell W A, Dykstra C C, Jones S K, Tidwell R R, Czarny A, Bajic M, Bajic M, Kumar A, Boykin D, Perfect J R. Structure-in vitro activity relationships of pentamidine analogues and dicationic substituted bis-benzimidazoles as new antifungal agents. Antimicrob Agents and Chemother. 1998;42:2495–2502. doi: 10.1128/aac.42.10.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Del Poeta, M., W. A. Schell, and J. R. Perfect. 1998. Personal communication.
- 13.Fox R, Neal K R, Leen C L S, Ellis M E, Mandal B K. Fluconazole resistant Candida in AIDS. J Infect. 1991;22:201–204. doi: 10.1016/0163-4453(91)91767-r. [DOI] [PubMed] [Google Scholar]
- 14.Hall J E, Kerrigan J E, Ramachandran K, Bender B C, Stanko J P, Jones S K, Patrick D A, Tidwell R R. Anti-Pneumocystis activities of aromatic diamidoxime prodrugs. Antimicrob Agents Chemother. 1998;42:666–674. doi: 10.1128/aac.42.3.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hildebrandt E F, Boykin D W, Tidwell R R, Dykstra C C. Identification and characterization of an endo/exonuclease in Pneumocystis carinii that is inhibited by dicationic diaryl furans with efficacy against pneumocystis pneumonia. J Eukaryot Microbiol. 1998;45:112–121. doi: 10.1111/j.1550-7408.1998.tb05078.x. [DOI] [PubMed] [Google Scholar]
- 16.Hopkins, K. T., A. Kumar, M. Bajic, B. C. Bender, J. E. Hall, R. R. Tidwell, D. W. Boykin, and W. D. Wilson. Anti-Pneumocystis carinii activity of extended aryl furan amidines. Submitted for publication.
- 17.Jones S K, Hall J E, Allen M A, Morrison S D, Ohemeng K A, Reddy V V, Geratz J D, Tidwell R R. Novel pentamidine analogs in the treatment of experimental Pneumocystis carinii pneumonia. Antimicrob Agents Chemother. 1990;34:1026–1030. doi: 10.1128/aac.34.6.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumar A, Boykin D W, Wilson W D, Jones S K, Bender B K, Dykstra C C, Hall J E, Tidwell R R. Anti-Pneumocystis carinii pneumonia activity of dicationic 2,4-diarylpyrimidines. Eur J Med Chem. 1996;31:767–773. doi: 10.1016/0223-5234(96)83970-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ludewig G, Marin J, Li Y, Staben C. Effects of pentamidine isethionate on Saccharomyces cerevisiae. Antimicrob Agents Chemother. 1994;38:1123–1128. doi: 10.1128/aac.38.5.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McGinnis M R. Laboratory handbook of medical mycology. New York, N.Y: Academic Press, Inc.; 1980. Susceptibility testing and bioassay procedure; p. 431. [Google Scholar]
- 21.National Committee for Clinical Laboratory Standards. Reference method for broth dilution susceptibility testing of yeasts. Document M27-T. Tenative standard. Wayne Pa: National Committee for Clinical Laboratory Standards; 1995. [Google Scholar]
- 22.Patrick D A, Boykin D W, Wilson W D, Tanious F A, Spychala J, Bender B C, Hall J E, Dykstra C C, Ohemeng K A, Tidwell R R. Anti-Pneumocystis carinii pneumonia activity of dicationic carbazoles. Eur J Med Chem. 1997;32:781–793. [Google Scholar]
- 23.Pfaller M A, Rhine-Chalberg A J, Redding S W, Smith J, Farinacci G, Fothergill A W, Rinaldi M G. Variations in fluconazole susceptibility and electrophoretic karyotype among oral isolates of Candida albicans from AIDS and oral candidiasis. J Clin Microbiol. 1994;32:59–64. doi: 10.1128/jcm.32.1.59-64.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23a.Tidwell, R. R. Unpublished data.
- 24.Tidwell R R, Bell C A. Pentamidine and related compounds in the treatment of Pneumocystis carinii infection. In: Walzer P D, editor. Pentamidine and related compounds in the treatment of Pneumocystis carinii Infection. New York, N.Y: Marcel Dekker, Inc.; 1993. pp. 561–583. [Google Scholar]
- 25.Tidwell R R, Geratz J D, Dann O, Volz G, Zeh D, Loewe H. Diarylamidine derivatives with one or both of the aryl moieties consisting of an indole or indole-like ring. Inhibitors of arginine-specific esteroproteases. J Med Chem. 1978;21:613–623. doi: 10.1021/jm00205a005. [DOI] [PubMed] [Google Scholar]
- 26.Tidwell R R, Jones S K, Geratz J D, Ohemeng K A, Bell C A, Berger B J, Hall J E. Development of pentamidine analogues as new agents for the treatment of Pneumocystis carinii pneumonia. Ann NY Acad Sci. 1990;616:421–441. doi: 10.1111/j.1749-6632.1990.tb17862.x. [DOI] [PubMed] [Google Scholar]
- 27.Tidwell R R, Jones S K, Geratz J D, Ohemeng K A, Cory M, Hall J E. Analogues of 1,5-bis(4-amidinophenoxy)pentane (pentamidine) in the treatment of experimental Pneumocystis carinii pneumonia. J Med Chem. 1990;33:1252–1257. doi: 10.1021/jm00166a026. [DOI] [PubMed] [Google Scholar]
- 28.Tidwell R R, Jones S K, Naiman N A, Berger L C, Brake W B, Dykstra C C, Hall J E. Activity of cationically substituted bis-benzimidazoles against experimental Pneumocystis carinii pneumonia. Antimicrob Agents Chemother. 1993;37:1713–1716. doi: 10.1128/aac.37.8.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trent J O, Clark G R, Neidle S, Kumar A, Wilson W D, Boykin D W, Hall J E, Tidwell R R, Blagburn B L. Targeting the minor groove: crystal structures of two complexes between furan derivatives of berenil and the DNA dodecamer. J Med Chem. 1996;39:4554–4562. doi: 10.1021/jm9604484. [DOI] [PubMed] [Google Scholar]
- 30.Willocks L, Leen C L S, Bretle R P, Urquhart D, Russel T B, Milne L J R. Fluconazole resistance in AIDS patients. J Antimicrob Chemother. 1991;28:937–939. doi: 10.1093/jac/28.6.937. [DOI] [PubMed] [Google Scholar]
- 31.Xiao G, Li K, Tigl C T, Kumar A, Boykin D W, Wilson W D. Inhibition of HIV-1 Rev-RRE complex: structure-inhibition analysis with unfused aromatic cations. 1998. Unpublished data. [DOI] [PubMed] [Google Scholar]