Abstract
The objective of this study was to evaluate the in vitro and in vivo efficacy of clavulanic acid (C/A) in combination with tazobactam against clinical strains of carbapenem-resistant Acinetobacter baumannii. The MIC of 24 clinical strains of A. baumannii was determined, and a checkerboard assay and time-kill curve analysis were performed in selected strains to determine the synergy between C/A and tazobactam. The efficacy of C/A in monotherapy and in combination with tazobactam was evaluated in vitro in cell culture experiments and in a murine peritoneal sepsis model. The C/A and C/A plus tazobactam MIC50 were 128 and <1 mg/L, respectively. The checkerboard assay showed that tazobactam (4 and 8 mg/L) demonstrated synergy with C/A against A. baumannii Ab40, an OXA-24 producer strain, and Ab293, a lacking OXA β-lactamase strain. The time-kill curve assay showed both bactericidal and synergistic effects against Ab40 and Ab293, with C/A 1xMIC and tazobactam (4 and 8 mg/L) at 24 h. In the murine peritoneal sepsis model with Ab293 strain, the combination of C/A and tazobactam reduced bacterial loads in tissues and blood by 2 and 4 log10 CFU/g or mL compared with C/A alone. Combining C/A with tazobactam could be considered as a potential alternative strategy to treat A. baumannii in some cases, and future work with more strains is needed to confirm this possibility.
Keywords: Acinetobacter baumannii, Clavulanic acid, Tazobactam, Combination
Introduction
Acinetobacter baumannii is a multidrug-resistant (MDR) Gram-negative bacterium that is responsible for a large number of hospital-acquired infections, such as ventilator-associated pneumonia, bloodstream infections, burn and soft tissue infections, meningitis, and osteomyelitis [1]. The first-line therapeutic options for this pathogen have included broad-spectrum β-lactams (BLs), such as carbapenems, for several years [2, 3]. However, A. baumannii has developed mechanisms that confer resistance to these antibiotics, including decreased outer membrane permeability, efflux pumps, penicillin-binding protein modification, and the production of β-lactamases [4].
The emergence of MDR A. baumannii has made it difficult to find an effective antimicrobial treatment for infections caused by this pathogen. In response, the World Health Organization has designated A. baumannii resistant to carbapenems as a critical priority pathogen that poses a significant threat to human health and for which new antibiotics are urgently needed [5].
To overcome the loss of BL antibiotic activity, β-lactamase inhibitors (BLIs) were developed to be used in combination with BLs in order to inhibit β-lactamase and allow BLs to act unhindered [4, 6]. In general, commercial BLIs have low antibiotic activity against A. baumannii and are not used as single antimicrobial agents. However, sulbactam has demonstrated good antibacterial activity both in vitro and in animal models against A. baumannii [6–8]. This antibacterial activity is mediated through the inhibition of the penicillin-binding proteins (PBPs) PBP1 and PBP3 [9].
Clavulanic acid (C/A), sulbactam, and tazobactam are irreversible “suicide inhibitors” that can permanently inactive β-lactamase through secondary chemical reactions in the enzyme’s active site. These inhibitors have a high affinity for many class A β-lactamases but do not provide protection against class B, C, and D β-lactamases [10–12]. A. baumannii carbapenem-hydrolyzing class D β-lactamase (CHDLs) such as OXA-23, OXA-24, and OXA-58 are the main cause of carbapenem resistance, which are recalcitrant to inhibition against most commercially available inhibitors [13].
Although the activity of C/A and tazobactam against A. baumannii is lower than that of sulbactam, two studies have reported a range of minimum inhibitory concentrations (MICs) of C/A from 2 to 256 mg/L, with MICs of ≤8 mg/L for 29 and 40.9% of the A. baumannii strains tested, respectively [6, 14]. In vivo, C/A has demonstrated therapeutic efficacy against carbapenem-susceptible A. baumannii by reducing the bacterial loads in the lungs by 2–2.5 log CFU/g and increasing the frequency of sterile blood cultures [14]. On the other hand, tazobactam has been reported to have antibacterial activity against A. baumannii, with a MIC of 16 mg/L in vitro and a 1-log CFU reduction in a murine lung infection model [15]. In a study of 54 MDR A. baumannii strains, sulbactam had a MIC range from 16 to 256 mg/L, while tazobactam had a MIC range from 32 to 512 mg/L [16].
The combination of BLIs is being developed for the treatment of A. baumannii infections. Studies have shown potent in vitro activity of sulbactam/durlobactam, sulbactam/avibactam, and sulbactam/LN-1-255 against clinical isolates of A. baumannii in China, Argentina, and Spain, respectively [17–19] and in vitro and in vivo activity of sulbactam/ETX2514 against carbapenem-resistant A. baumannii [20, 21]. Additionally, in vitro and in vivo activity of sulbactam/YTR830H (another β-lactamase inhibitor) has been observed against A. calcoaceticus [22]. Durlobactam, avibactam, ETX2514, LN-1-255, and YTR830H significantly increased the susceptibility of clinical isolates of A. baumannii to sulbactam. To date, there is no reported data on the susceptibility data for C/A in combination with tazobactam against carbapenem-intermediate and carbapenem-resistant A. baumannii. Thus, the main aim of this study was to determine the efficacy of C/A alone and in combination with tazobactam against two selected carbapenem-resistant A. baumannii strains.
Material and methods
Bacterial strains
A total of 24 clinical strains of carbapenem-intermediate (n=4) and carbapenem-resistant (n=20) A. baumannii were collected from the “II Spanish Study of A. baumannii GEIH-REIPI 2000-2010” multicenter study (Genbank Bioproject PRJNA422585) for use in this study. The strains were chosen due to their carbapenem and C/A non-susceptible profiles.
Antimicrobial agents and in vitro susceptibility testing
Standard laboratory powders of C/A (Sigma, Spain) and tazobactam (Sigma, Spain) were used. The MICs of C/A alone and in combination with tazobactam were determined against 24 clinical strains of carbapenem-intermediate and carbapenem-resistant A. baumannii in two independent experiments using the broth microdilution method, in accordance with the standard guidelines of the European Committee on Antimicrobial Susceptibility Testing (EUCAST) [23]. A 5×105 CFU/mL inoculum of each strain was cultured in Mueller Hinton Broth (MHB) and added to U bottom microtiter plates (Deltlab, Spain) containing C/A alone and C/A and 4 mg/L of tazobactam. The plates were incubated for 18 h at 37°C. A. baumannii ATCC 17978 was used as a control strain. The MIC50 and MIC90, which represent the concentrations that were effective against ≥50 and ≥90% of the isolates tested, were determined.
Checkerboard assay
To determinate the synergistic activity between C/A and tazobactam, two strains of carbapenem-resistant A. baumannii (Ab40, an OXA-24 producer strain, and Ab293, a lacking OXA β-lactamase strain) were selected for further studies. The assay was performed in duplicate using a 96-well plate as described previously [24]. C/A (from 0 to 64 mg/L) was serially diluted 2-fold along the x axis, while tazobactam (from 0 to 64 mg/L) was serially diluted 2-fold along the y axis to create a matrix of different combinations of both agents at different concentrations. Bacterial cultures grown overnight were diluted in saline to a 0.5 McFarland turbidity and further diluted 1:50 in MHB before being inoculated into each well to achieve a final concentration of approximately 5.5×105 CFU/mL. The 96-well plates were then incubated at 37 °C for 18 h and examined for visible turbidity. The fractional inhibitory concentration (FIC) of C/A was calculated by dividing the MIC of C/A in the presence of tazobactam by the MIC of C/A alone. Similarly, the FIC of tazobactam was calculated by dividing the MIC of tazobactam in the presence of C/A by the MIC of tazobactam alone. The FIC index (FICI) was the sum of both FIC values. FICI values of ≤0.5 and >0.5 were interpreted as synergistic and additive, respectively.
Time-kill kinetic assays
In order to determine the bactericidal and synergistic activity, time-kill curves of the Ab40 and Ab293 strains were performed in duplicate as previously described [24]. An initial inoculum of 1×106 CFU/mL was prepared in MHB in the presence of 1xMIC of C/A alone or in combination with 4 and 8 mg/L of tazobactam. A drug-free broth was evaluated in parallel as a control. Tubes of each condition were incubated at 37°C with shaking, and viable counts were determined by serial dilution at 0, 2, 4, 8, and 24 h. Viable counts were determined by plating 100 µL of the control, test cultures, or the respective dilutions at the indicated times onto sheep blood agar plates (Thermo Fisher, Spain). Plates were incubated for 24 h at 37 °C, and after colony counts, the log10 of viable cells (CFU/mL) was determined. Synergy was defined as a reduction of ≥ 2 log10 CFU/mL with the combination compared to the more active drug [24]. Therefore, tazobactam was considered synergistic when, in combination with C/A, it reduced the bacterial concentration by ≥ 2 log10 CFU/mL compared to C/A alone. Bactericidal activity was defined as a reduction of ≥3 log10 CFU/mL from the initial inoculum [25].
Human cell culture
HeLa cells were grown in 24-well plates in DMEM supplemented with 10% heat-inactivated fetal bovine serum (FBS), vancomycin (50 mg/L), gentamicin (20 mg/L), amphotericin B (0.25 mg/L) (Invitrogen, Spain), and 1% HEPES in a humidified incubator with 5% CO2 at 37°C. HeLa cells were routinely passaged every 3 or 4 days. Immediately before infection, HeLa cells were washed three times with prewarmed PBS and further incubated in DMEM without FBS and antibiotics [26].
Adhesion-invasion assays
HeLa cells were infected with 1×108 CFU/mL of A. baumannii Ab40 and Ab293 strains in the absence and presence of 1xMIC of C/A alone or in combination with 4 and 8 mg/L of tazobactam at a multiplicity of infection (MOI) of 100 for 2 h with 5% CO2 at 37°C. Subsequently, infected HeLa cells were washed five times with prewarmed PBS and lysed with 0.5% Triton X-100. Diluted lysates were plated onto LB agar (Merck, Spain) and incubated at 37°C for 24 h for enumeration of developed colonies and then the determination of the number of bacteria that attached-invaded to HeLa cells [26].
Animals
Female C57BL/6 mice weighing 18 to 20 g and considered immunocompetent were obtained from the University of Seville. The mice were certified as pathogen-free and genetically authenticated and were housed in regulated cages with access to food and water ad libitum. This study was conducted in accordance with the UK Animals (Scientific Procedures) Act 1986 and associated guidelines, as well as the European Communities Council Directive of 24 November 1986 (86/609/EEC). The animal model of this study was approved by the Committee on the Ethics of Animal Experiments at the University Hospital of Virgen del Rocio in Seville, Spain. All surgeries were performed under sodium thiopental anesthesia, and measures were taken to minimize animal suffering.
A. baumannii peritoneal sepsis models
A murine peritoneal sepsis model caused by the carbapenem-resistant A. baumannii Ab40 OXA-24 producer was established by intraperitoneal inoculation of the bacteria in immunocompetent mice [24]. The Minimal Bacterial Lethal Dose 100 (MLD100) was determined by inoculating two groups of 6 mice each with 0.5 mL of the decreasing amounts of bacterial inoculum from 9 to 8 log10 CFU/mL and monitoring the survival of the mice for 7 days.
Therapeutic effect of clavulanic acid in monotherapy and in combination with tazobactam in a murine model of peritoneal sepsis
A murine peritoneal sepsis model was established by intraperitoneal inoculation of mice with the carbapenem-resistant A. baumannii Ab40 strain and treated with either C/A monotherapy or C/A combined with tazobactam. The mice were infected with 0.5 mL of the MLD100 of Ab40 strain (9 log CFU/mL) and randomly ascribed to the following groups: (i) controls (no treatment); (ii) C/A administered intraperitoneally at 13 mg/kg/4 h starting 4 h after bacterial inoculation, for 24 h [14]; (iii) C/A administered intraperitoneally at 13 mg/kg/4 h plus tazobactam administered intraperitoneally at 32 mg/kg/4 h starting 4 h after bacterial inoculation, for 24 h [27]. At the end of the experiment, after the mice died or were sacrificed, aseptic thoracotomies were performed, blood samples were obtained by cardiac puncture, and the spleen and lungs were aseptically removed and homogenized (Stomacher 80; Tekmar Co., USA) in 2 mL of sterile 0.9% NaCl solution. Tenfold dilutions of the homogenized spleen and lungs and blood were plated onto sheep blood agar for quantitative cultures.
Statistical analysis
Group data is presented as the mean ± standard error of the mean (SEM). Differences in bacterial concentrations in the spleen, lung, and blood (mean ± SEM log10 CFU/g or log10 CFU/mL) were analyzed using analysis of variance (ANOVA) and post hoc Dunnett’s and Tukey’s tests. P values less than 0.05 were considered significant. The statistical analysis was performed using SPSS version 21.0 (SPSS Inc.).
Results
In vitro activity of clavulanic acid alone and in combination with tazobactam
C/A alone and in combination with tazobactam were tested against 24 clinical strains of carbapenem-intermediate and carbapenem-resistant A. baumannii. The results of the MICs tests are displayed in Table 1. The MICs ranged from 16 to >256 mg/L for C/A and from <1 to 16 mg/L for the combination with tazobactam. The control strain ATCC 17978 present an MIC of 32 mg/L for C/A and <1 mg/L for the combination with tazobactam. The MIC50 and MIC90 concentrations, which represent the concentration effective for 50 and 90% of the isolates tested, respectively, for C/A alone were 128 and >256 mg/L, respectively. However, the MIC50 and MIC90 for C/A in combination with tazobactam were <1 and 16 mg/L, respectively.
Table 1.
MIC determination of clavulanic acid alone and in combination with tazobactam against clinical isolates of A. baumannii
| Strain | IMP | MPM | C/A | C/A + 4 mg/L of TAZ |
|---|---|---|---|---|
| ATCC 17978* | 0.5 | 0.5 | 32 | <1 |
| Ab16 | 64 | >64 | 32 | <1 |
| Ab17 | 8 | 8 | 64 | <1 |
| Ab19 | 16 | 8 | 256 | 8 |
| Ab37 | 16 | 8 | 16 | <1 |
| Ab40 | 64 | >64 | 16 | <1 |
| Ab53 | 64 | 16 | 128 | 32 |
| Ab286 | 2 | 16 | 32 | <1 |
| Ab288 | 4 | 32 | 64 | <1 |
| Ab289 | 2 | 8 | 32 | <1 |
| Ab293 | 16 | 8 | 32 | <1 |
| Ab295 | 8 | 4 | 128 | 4 |
| Ab298 | 16 | 8 | 128 | 8 |
| Ab299 | 16 | 8 | >256 | 16 |
| Ab303 | 32 | 16 | 128 | 16 |
| Ab399 | 64 | >64 | 256 | 2 |
| Ab405 | 32 | 16 | 64 | 16 |
| Ab410 | 32 | 16 | 128 | <1 |
| Ab414 | 32 | 16 | 64 | <1 |
| Ab416 | 32 | 16 | 32 | <1 |
| Ab417 | 16 | 8 | 128 | <1 |
| Ab440 | 2 | 8 | 256 | <1 |
| Ab441 | 2 | 8 | 128 | <1 |
| Ab448 | 8 | 8 | 128 | 1 |
| Ab453 | 4 | 4 | 128 | <1 |
IMP imipenem, MPM meropenem, C/A clavulanic acid, TAZ tazobactam
*ATCC 17978: control strain
The checkerboard assay indicated that tazobactam at concentrations of 4 and 8 mg/L had a synergistic effect with C/A against Ab40, an OXA-24 producer strain, and Ab293, a lacking OXA β-lactamase strain. When combined with C/A, tazobactam at 4 and 8 mg/L enhanced the activity of C/A against the Ab40 strain, resulting in an FIC index (FICI) of 0.275. Similarly, the combination of tazobactam at 4 and 8 mg/L with C/A increased the activity of C/A against the Ab93 strain, yielding an FICI of 0.25 (Table 2). In contrast, the combination of tazobactam at lower concentrations, such as 1 and 2 mg/L, with C/A caused additive effects instead of synergy, yielding an FICI > 0.5 for both strains (data not shown). As a result, the optimal concentrations for further experiments were determined to be 4 and 8 mg/L of tazobactam.
Table 2.
MIC determination of clavulanic acid and tazobactam alone or in combination against carbapenem-resistant A. baumannii strains
| Strain | MIC mg/L | ||||
|---|---|---|---|---|---|
| C/A | TAZ | C/A in the presence of 4 mg/L of TAZ | FICI | Fold change in C/A | |
| Ab40 | 16 | 16 | <1 | 0.275 | >16 |
| Ab293 | 32 | 16 | <1 | 0.25 | >32 |
The MICs of combined C/A with tazobactam were equal at 4 or 8 mg/L of tazobactam
C/A clavulanic acid, TAZ tazobactam, FICI fractional inhibitory concentration index
Time-kill curves
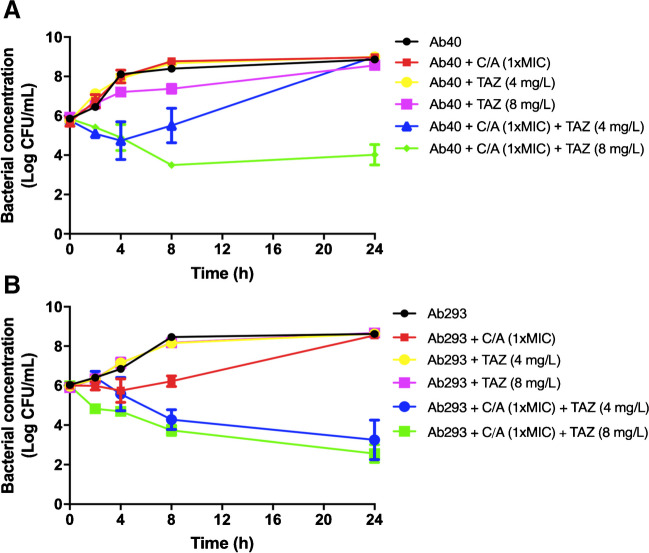
Using time-course assays, we evaluated the bactericidal activity of tazobactam in combination with C/A against Ab40 and Ab293 strains. Figure 1A illustrates that the combination of 8 mg/L tazobactam with 16 mg/L C/A (1xMIC for the Ab40 strain) exhibited a synergistic and bactericidal effect after 8 h, reducing the bacterial count by over 3 log10 CFU/mL compared to C/A alone. These bactericidal and synergistic effects persisted until 24 h. In contrast, the combination of 4 mg/L tazobactam with 1xMIC C/A only showed synergistic activity at 4 h in comparison to C/A alone. For the Ab293 strain, 4 and 8 mg/L tazobactam in combination with 32 mg/L C/A (1xMIC for Ab293 strain) demonstrated a synergistic effect after 8 and 24 h by reducing the bacterial count by over 2 and 3 log10 CFU/mL, respectively, compared to C/A alone. This effect was intensified at 24 h, where the two concentrations of tazobactam combined with C/A showed a bactericidal effect (Fig. 1B).
Fig. 1.
Tazobactam potentiates clavulanic acid activity against two selected carbapenem-resistant A. baumannii. Time-kill curves of A. baumannii Ab40 OXA-24 producer strain (A) and Ab293 strain (B) in the presence of 4 and 8 mg/L tazobactam and 1xMIC clavulanic acid, alone or in combination with tazobactam for 24 h. C/A, clavulanic acid; TAZ, tazobactam. Data are represented as mean ± SEM from two independent experiments
Effect of clavulanic acid in combination with tazobactam on the bacterial adherence invasion to host cells
To evaluate the effect of C/A in combination with tazobactam in A. baumannii interaction with host cells, we studied the adherence invasion of Ab40 and Ab293 strains on HeLa cells for 2 hours in the presence of C/A with or without tazobactam. We showed that treatment with C/A at 1xMIC plus tazobactam at 4 and 8 mg/L reduced the counts of adherent-invasive Ab40 to HeLa cells by 17 and 32% (P<0.05), respectively, when compared with C/A monotherapy. Of note, a more enhanced reduction has been observed with the Ab293 strain, for which the two combination treatments reduced its adherent-invasive counts by 54% (P<0.05) and 57% (P<0.05), respectively, when compared with C/A monotherapy (Fig. 2).
Fig. 2.
Effect of clavulanic acid alone or in combination with tazobactam on A. baumannii interaction with host cells. HeLa cells were pretreated with C/A (1xMIC) without or with tazobactam (4 and 8 mg/L) and infected with carbapenem-resistant A. baumannii Ab40 OXA-24 producer and Ab293 strains. The assay of A. baumannii adherence invasion to HeLa cells for 2 h was performed as described in “Material and methods.” C/A, clavulanic acid; TAZ, tazobactam. Data are represented as mean ± SEM from three independent experiments. *P < 0.05 vs. the clavulanic acid treatment group
In vivo activity of clavulanic acid alone and in combination with tazobactam against A. baumannii infection
To verify the in vitro synergistic effect of C/A and tazobactam against carbapenem-resistant A. baumannii and to study this synergy in a living organism, we conducted an experiment using a vertebrate model of infection by this pathogen. First, we determined the MLD100 of the Ab40 strain. Mice exposed intraperitoneally to 0.5 mL of the Ab40 strain culture at a dose of 9 log CFU/mL experienced 100% mortality, whereas 8 log CFU/mL resulted in only 33% mortality.
In a murine model of peritoneal sepsis, C/A (13 mg/kg/4 h, i.p.) was combined with tazobactam (32 mg/kg/4 h, i.p.) and administered to mice 4 h after intraperitoneal exposure to the 0.5 mL of Ab40 strain culture at a dose of 9 log CFU/mL, which caused 100% mortality. The combination treatment was found to significantly reduce the bacterial load in the lung and spleen by 2.27 and 2.46 log10 CFU/g (P < 0.05) and in the blood by 4.01 log10 CFU/mL (P < 0.05), compared to control groups. In contrast, treatment with C/A alone did not significantly decrease the bacterial load in the lung and spleen relative to control groups. In blood, slight statistically significant decrease has been observed (Fig. 3).
Fig. 3.

Therapeutic effect of clavulanic acid alone and in combination with tazobactam in vivo against carbapenem-resistant A. baumannii. Bacterial load in tissues and blood in the murine peritoneal sepsis model with 0.5 mL of A. baumannii Ab40 OXA-24 producer strain at 9 log CFU/mL. *P < 0.05 vs. control; #P < 0.05 vs. the C/A treatment group. C/A, clavulanic acid; TAZ, tazobactam
Discussion
The emergence of broad-spectrum antibiotic resistance in A. baumannii species has led to the search for new therapeutic alternatives. Due to the expression of resistance genes, bacteria have become resistant to BL antibiotics [28, 29], as well as to the combination of several BL-BLI [30–35]. This has prompted us to suggest that the combination of several BLIs could be an effective solution to this problem. In this study, we showed that the BLI C/A in combination with another BLI, tazobactam, acts synergistically against clinical isolates of A. baumannii. According to previous studies, none of both BLI have antibacterial activity in monotherapy against carbapenem-resistant A. baumannii. In this study, C/A alone had no activity against clinical isolates of A. baumannii and had a MIC range from 16 to >256 mg/L. The addition of avibactam did not increase the activity of C/A (data not shown). This result is in line with previous studies that showed that Acinetobacter spp. are largely resistant to ceftazidime-avibactam [32, 33]. In contrast, the combination of C/A with tazobactam in this study showed very promising results. The MICs for this combination range from <1 to >256 mg/L. It is noteworthy that the MIC50 of C/A in combination with tazobactam for the 24 analyzed carbapenem-intermediate and resistant strains decreased to 16 mg/L, 8-fold lower than the MIC50 of C/A alone. In next studies, these promising findings should be validated in large collections of clinical A. baumannii strains.
The results from microdilution assays were confirmed by checkerboard assays and time-kill curves. Combining 4 and 8 mg/L of tazobactam with 1xMIC of C/A showed synergy and bactericidal activity against Ab40, an OXA-24 producer strain, and Ab293, a lacking OXA β-lactamase strain. According to EUCAST guidelines, tazobactam is used with a fixed concentration of 4 mg/L for susceptibility testing. Our results suggest that increasing the concentration of tazobactam from 4 to 8 mg/L enhances the activity of C/A against clinical isolates of A. baumannii. This could be due to the similar spectrum of activity between C/A and tazobactam [3, 33].
The mode of action of the combination of C/A and tazobactam remains unknown. Like other β-lactams, such as sulbactam, whose mechanism of action is associated with binding to PBPs in A. baumannii [9], C/A has been demonstrated to bind to PBPs in Escherichia coli and other Gram-negative pathogens [36, 37]. However, there is currently no available information on tazobactam’s effect on PBPs. It is possible that the synergy observed in this study between C/A and tazobactam could be the result of a certain degree of additive effect in inhibiting PBPs.
Furthermore, with reference to the study by Fernandez et al. (2012), which assessed the expression of OXA-24 and OXA-10 in E. coli, it was demonstrated that the presence of these enzymes was linked to a low level of cross-linked peptidoglycan and longer sugar chains. This observation suggests that the expression of specific β-lactamases might be connected to alterations in the cell wall structure, potentially resulting in a diminished fitness both in vitro and in vivo [38]. This information could prove relevant for the consideration of future studies involving the Ab40 strain.
C/A is a broad-spectrum inhibitor that can inhibit most class A β-lactamases, including ESBLs and common TEM and SHV enzymes [3, 14]. Similarly, tazobactam has activity against many Ambler class A β-lactamases (TEM, SHV, and CTX-M-type) and some class C (AmpC-type) β-lactamases [14]. Both C/A and tazobactam inhibit most Ambler class A β-lactamases (excluding carbapenemases like KPC-2), but not those from Ambler classes B, C, or D [39].
On the other hand, our previous studies showed that A. baumannii relies adherence invasion to host cells as an initial and crucial step in causing infections [26, 40, 41]. However, no data have been reported on the combined effect of C/A and tazobactam on A. baumannii’s interaction with host cells. To our knowledge, this study provides the first evidence for the enhanced effect of tazobactam on C/A in reducing A. baumannii’s adherence invasion to host cells. Moreover, this effect is more pronounced against the Ab293 strain than the Ab40 strain, consistent with time-kill curve data indicating that C/A plus tazobactam is more bactericidal against Ab293, a lacking OXA β-lactamase strain, than Ab40, an OXA-24 producer strain.
Animal infection models are useful for studying the potential uses of β-lactamase inhibitors both as monotherapy and in combination. For example, the efficacy of ETX2514 in combination with sulbactam reduced the bacterial loads more than monotherapy with ETX1514 or sulbactam in neutropenic mouse thigh infection model by carbapenem-resistant A. baumannii [21]. In this study, the combination of C/A and tazobactam was effective against carbapenem-resistant A. baumannii in a murine peritoneal sepsis model. The combination reduced the bacterial load by around 2.5 log10 CFU/g in tissues and 4 log10 CFU/mL in blood, while there was no significant difference between C/A monotherapy and the control group. Tazobactam has been also showed to enhance the activity of colistin in murine pneumoniae model caused by a virulent A. baumannii strain [27].
The animal model of this study was not used beyond 24 h because of the high number of C/A doses that had to be administered to the animals to reach a serum concentration above the MIC for at least 40% of the time between doses [14]. In spite of this, combination treatment showed significant decrease in bacterial loads. The in vivo data shown do not exhibit a significant correlation with host survival. Naturally, numerous other factors come into play here that could lead to mortality, such as the excessive stimulation and promotion of proinflammatory cytokines like IL-6 and TNF-alpha [42, 43]. Nevertheless, the results are intriguing and offer a noteworthy strategy to enhance the effectiveness of existing β-lactams, while not excluding the consideration of other adjuvants or alternative therapies concurrently. Furthermore, this study possesses certain limitations due to the relatively low number of tested strains. We are of the opinion that the next focal point should involve expanding the bacterial collections to corroborate the therapeutic efficacy of the C/A combination with tazobactam.
Conclusions
This study provides new insights on the use of BLIs against carbapenem-resistant A. baumannii clinical isolates. Exploring novel combinations may offer new options to treat A. baumannii infections, which have limited treatment options.
Acknowledgements
We thank the members of Grupo de Estudio de Infecciones Hospitalaria-Grupo de Estudio de los Mecanismos de Acción y de la Resistencia a los Antimicrobianos (GEIH-GEMARA) (Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica [SEIMC]) and Red Española de Investigación de Patologías Infecciosas (REIPI) participating in the GEIH-Ab 2010 project.
Funding
Funding for open access publishing: Universidad Pablo de Olavide/CBUA This work was cofunded by the Consejería de Universidad, Investigación e Innovación de la Junta de Andalucía (grant ProyExcel_00116), and funded by the Instituto de Salud Carlos III, Subdirección General de Redes y Centros de Investigación Cooperativa, Ministerio de Economía, Industria y Competitividad (grant PI19/01009), cofinanced by the European Development Regional Fund (A way to achieve Europe, Operative Program Intelligent Growth 2014 to 2020). Funding for open access publishing: Universidad Pablo de Olavide / CBUA.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval
The animal model of this study was approved by the Committee on the Ethics of Animal Experiments at the University Hospital of Virgen del Rocio in Seville, Spain.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.McConnell MJ, Actis L, Pachón J. Acinetobacter baumannii: human infections, factors contributing to pathogenesis and animal models. FEMS Microbiol Rev. 2013;37(2):130–155. doi: 10.1111/j.1574-6976.2012.00344.x. [DOI] [PubMed] [Google Scholar]
- 2.Doi Y. Treatment Options for carbapenem-resistant Gram-negative bacterial infections. Clin Infect Dis. 2019;69(Suppl 7):S565–S575. doi: 10.1093/cid/ciz830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bush K, Bradford PA. β-Lactams and β-lactamase inhibitors: an overview. Cold Spring Harb Perspect Med. 2016;6(8):a025247. doi: 10.1101/cshperspect.a025247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drawz SM, Bonomo RA. Three decades of beta-lactamase inhibitors. Clin Microbiol Rev. 2010;23(1):160–201. doi: 10.1128/CMR.00037-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tacconelli E, Carrara E, Savoldi A, et al. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis. 2018;18(3):318–327. doi: 10.1016/S1473-3099(17)30753-3. [DOI] [PubMed] [Google Scholar]
- 6.Higgins PG, Wisplinghoff H, Stefanik D, Seifert H (2004) In vitro activities of the beta-lactamase inhibitors clavulanic acid, sulbactam, and tazobactam alone or in combination with beta-lactams against epidemiologically characterized multidrug-resistant Acinetobacterbaumannii strains. Antimicrob Agents Chemother 48(5):1586–1592 [DOI] [PMC free article] [PubMed]
- 7.Yokoyama Y, Matsumoto K, Ikawa K, et al. Pharmacokinetic/pharmacodynamic evaluation of sulbactam against Acinetobacter baumannii in vitro and murine thigh and lung infection models. Int J Antimicrob Agents. 2014;43(6):547–552. doi: 10.1016/j.ijantimicag.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 8.Rodríguez-Hernández MJ, Cuberos L, Pichardo C, et al. Sulbactam efficacy in experimental models caused by susceptible and intermediate Acinetobacter baumannii strains. J Antimicrob Chemother. 2001;47(4):479–82. doi: 10.1093/jac/47.4.479. [DOI] [PubMed] [Google Scholar]
- 9.Penwell WF, Shapiro AB, Giacobbe RA, et al. Molecular mechanisms of sulbactam antibacterial activity and resistance determinants in Acinetobacter baumannii. Antimicrob Agents Chemother. 2015;59(3):1680–1689. doi: 10.1128/AAC.04808-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bush K, Bradford PA. Interplay between β-lactamases and new β-lactamase inhibitors. Nat Rev Microbiol. 2019;17(5):295–306. doi: 10.1038/s41579-019-0159-8. [DOI] [PubMed] [Google Scholar]
- 11.Beceiro A, Fernández-Cuenca F, Ribera A, et al. False extended-spectrum beta-lactamase detection in Acinetobacter spp. due to intrinsic susceptibility to clavulanic acid. J Antimicrob Chemother. 2008;61(2):301–308. doi: 10.1093/jac/dkm461. [DOI] [PubMed] [Google Scholar]
- 12.Suh B, Shapiro T, Jones R, Satishchandran V, Truant AL. In vitro activity of beta-lactamase inhibitors against clinical isolates of Acinetobacter species. Diagn Microbiol Infect Dis. 1995;21(2):111–114. doi: 10.1016/0732-8893(95)00020-B. [DOI] [PubMed] [Google Scholar]
- 13.Mendes RE, Bell JM, Turnidge JD, Castanheira M, Jones RN. Emergence and widespread dissemination of OXA-23, -24/40 and -58 carbapenemases among Acinetobacter spp. in Asia-Pacific nations: report from the SENTRY Surveillance Program. J Antimicrob Chemother. 2008;63:55–59. doi: 10.1093/jac/dkn434. [DOI] [PubMed] [Google Scholar]
- 14.Beceiro A, López-Rojas R, Domínguez-Herrera J, et al. In vitro activity and in vivo efficacy of clavulanic acid against Acinetobacter baumannii. Antimicrob Agents Chemother. 2009;53(10):4298–4304. doi: 10.1128/AAC.00320-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Monogue ML, Sakoulas G, Nizet V, Nicolau DP. Humanized exposures of a β-lactam-β-lactamase inhibitor, tazobactam, versus non-β-lactam-β-lactamase inhibitor, avibactam, with or without colistin, against Acinetobacter baumannii in murine thigh and lung infection models. Pharmacology. 2018;101(5–6):255–261. doi: 10.1159/000486445. [DOI] [PubMed] [Google Scholar]
- 16.Marie MA, Krishnappa LG, Alzahrani AJ, Mubaraki MA, Alyousef AA. A prospective evaluation of synergistic effect of sulbactam and tazobactam combination with meropenem or colistin against multidrug resistant Acinetobacter baumannii. Bosn J Basic Med Sci. 2015;15(4):24–29. doi: 10.17305/bjbms.2015.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang Q, Xu Y, Jia P, et al. In vitro activity of sulbactam/durlobactam against clinical isolates of Acinetobacter baumannii collected in China. J Antimicrob Chemother. 2020;75(7):1833–1839. doi: 10.1093/jac/dkaa119. [DOI] [PubMed] [Google Scholar]
- 18.Rodriguez CH, Brune A, Nastro M, Vay C, Famiglietti A. In vitro synergistic activity of the sulbactam/avibactam combination against extensively drug-resistant Acinetobacter baumannii. J Med Microbiol. 2020;69(7):928–931. doi: 10.1099/jmm.0.001211. [DOI] [PubMed] [Google Scholar]
- 19.Lasarte-Monterrubio C, Vázquez-Ucha JC, Maneiro M, et al. Activity of Imipenem, meropenem, cefepime, and sulbactam in combination with the β-lactamase inhibitor LN-1-255 against Acinetobacter spp. Antibiotics (Basel) 2021;10(2):210. doi: 10.3390/antibiotics10020210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Durand-Réville TF, Guler S, Comita-Prevoir J, et al. ETX2514 is a broad-spectrum β-lactamase inhibitor for the treatment of drug-resistant Gram-negative bacteria including Acinetobacter baumannii. Nat Microbiol. 2017;2:17104. doi: 10.1038/nmicrobiol.2017.104. [DOI] [PubMed] [Google Scholar]
- 21.Barnes MD, Kumar V, Bethel CR, et al. Targeting multidrug-resistant Acinetobacter spp.: sulbactam and the diazabicyclooctenone β-lactamase inhibitor ETX2514 as a novel therapeutic agent. mBio. 2019;10(2):e00159–19. doi: 10.1128/mBio.00159-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Obana Y, Nishino T. In-vitro and in-vivo activities of sulbactam and YTR830H against Acinetobacter calcoaceticus. J Antimicrob Chemother. 1990;26(5):677–682. doi: 10.1093/jac/26.5.677. [DOI] [PubMed] [Google Scholar]
- 23.EUCAST (2021) European committee on antimicrobial susceptibility testing. European committee on antimicrobial susceptibility testing, Växjö, Sweden
- 24.Miró-Canturri A, Ayerbe-Algaba R, Villodres ÁR, Pachón J, Smani Y. Repositioning rafoxanide to treat Gram-negative bacilli infections. J Antimicrob Chemother. 2020;75(7):1895–1905. doi: 10.1093/jac/dkaa103. [DOI] [PubMed] [Google Scholar]
- 25.Souli M, Rekatsina PD, Chryssouli Z, et al. Does the activity of the combination of imipenem and colistin in vitro exceed the problem of resistance in metallo-β-lactamase-producing Klebsiella pneumoniae isolates? Antimicrob Agents Chemother. 2009;53:2133–2135. doi: 10.1128/AAC.01271-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parra-Millán R, Guerrero-Gómez D, Ayerbe-Algaba R, et al. Intracellular trafficking and persistence of Acinetobacter baumannii requires Transcription Factor EB. mSphere. 2018;3(2):e00106–18. doi: 10.1128/mSphere.00106-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Melchers MJ, Mavridou E, van Mil AC, Lagarde C, Mouton JW. Pharmacodynamics of ceftolozane combined with tazobactam against Enterobacteriaceae in a neutropenic mouse thigh model. Antimicrob Agents Chemother. 2016;60(12):7272–7279. doi: 10.1128/AAC.01580-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Doi Y (2019) Treatment options for carbapenem-resistant Gram-negative bacterial infections. Clin Infect Dis 69:565–575 [DOI] [PMC free article] [PubMed]
- 29.Papp-Wallace KM. The latest advances in β-lactam/β-lactamase inhibitor combinations for the treatment of Gram-negative bacterial infections. Expert Opin Pharmacother. 2019;20(17):2169–2184. doi: 10.1080/14656566.2019.1660772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fröding I, Vondracek M, Giske CG. Rapid EUCAST disc diffusion testing of MDR Escherichia coli and Klebsiella pneumoniae: inhibition zones for extended-spectrum cephalosporins can be reliably read after 6 h of incubation. J Antimicrob Chemother. 2017;72(4):1094–1102. doi: 10.1093/jac/dkw515. [DOI] [PubMed] [Google Scholar]
- 31.Schuetz AN, Reyes S, Tamma PD. Point-counterpoint: piperacillin-tazobactam should be used to treat infections with extended-spectrum-beta-lactamase-positive organisms. J Clin Microbiol. 2018;56(3):e01917–17. doi: 10.1128/JCM.01917-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fraile-Ribot PA, Del Rosario-Quintana C, López-Causapé C, Gomis-Font MA, Ojeda-Vargas M, Oliver A. Emergence of resistance to novel β-lactam-β-lactamase inhibitor combinations due to horizontally acquired AmpC (FOX-4) in Pseudomonas aeruginosa sequence type 308. Antimicrob Agents Chemother. 2019;64(1):e02112–19. doi: 10.1128/AAC.02112-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Y, Wang J, Wang R, Cai Y. Resistance to ceftazidime-avibactam and underlying mechanisms. J Glob Antimicrob Resist. 2020;22:18–27. doi: 10.1016/j.jgar.2019.12.009. [DOI] [PubMed] [Google Scholar]
- 34.Lomovskaya O, Sun D, Rubio-Aparicio D, et al. Vaborbactam: spectrum of beta-lactamase inhibition and impact of resistance mechanisms on activity in Enterobacteriaceae. Antimicrob Agents Chemother. 2017;61(11):e01443–17. doi: 10.1128/AAC.01443-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Papp-Wallace KM, Mack AR, Taracila MA, Bonomo RA. Resistance to novel β-lactam-β-lactamase inhibitor combinations: the “price of progress”. Infect Dis Clin North Am. 2020;34(4):773–819. doi: 10.1016/j.idc.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spratt BG, Jobanputra V, Zimmermann W. Binding of thienamycin and clavulanic acid to the penicillin-binding proteins of Escherichia coli K-12. Antimicrob Agents Chemother. 1977;12(3):406–409. doi: 10.1128/AAC.12.3.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Finlay J, Miller L, Poupard J. A review of the antimicrobial activity of clavulanate. J Antimicrob Chemother. 2003;52(1):18–23. doi: 10.1093/jac/dkg286. [DOI] [PubMed] [Google Scholar]
- 38.Fernández A, Pérez A, Ayala JA, et al. Expression of OXA-type and SFO-1 β-lactamases induces changes in peptidoglycan composition and affects bacterial fitness. Antimicrob Agents Chemother. 2012;56(4):1877–1884. doi: 10.1128/AAC.05402-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zasowski EJ, Rybak JM, Rybak MJ. The β-lactams strike back: ceftazidime-avibactam. Pharmacotherapy. 2015;35(8):755–770. doi: 10.1002/phar.1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smani Y, Docobo-Pérez F, López-Rojas R, et al. Platelet-activating factor receptor initiates contact of Acinetobacter baumannii expressing phosphorylcholine with host cells. J Biol Chem. 2012;287(32):26901–10. doi: 10.1074/jbc.M112.344556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smani Y, Dominguez-Herrera J, Pachón J. Association of the outer membrane protein Omp33 with fitness and virulence of Acinetobacter baumannii. J Infect Dis. 2013;208(10):1561–1570. doi: 10.1093/infdis/jit386. [DOI] [PubMed] [Google Scholar]
- 42.Smani Y, Domínguez-Herrera J, Ibáñez-Martínez J, Pachón J. Therapeutic efficacy of lysophosphatidylcholine in severe infections caused by Acinetobacter baumannii. Antimicrob Agents Chemother. 2015;59(7):3920–3924. doi: 10.1128/AAC.04986-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parra Millán R, Jiménez Mejías ME, Sánchez Encinales V, et al. Efficacy of lysophosphatidylcholine in combination with antimicrobial agents against Acinetobacter baumannii in experimental murine peritoneal sepsis and pneumonia models. Antimicrob Agents Chemother. 2016;60(8):4464–4470. doi: 10.1128/AAC.02708-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.