Abstract
Purpose
Patients with BRAFV600E-mutant metastatic colorectal cancer (mCRC) have a dismal prognosis. The best strategies in these patients remain elusive. Against this background, we report the clinical course of patients with BRAFV600E-mutant mCRC to retrieve the best treatment strategy.
Patients and methods
Clinico-pathological data were extracted from the electronic health records. Kaplan–Meier method was used to estimate overall (OS) and progression-free survival (PFS). Objective response rate (ORR) was assessed according to RECIST 1.1.
Results
In total, 51 patients were enrolled. FOLFOXIRI was administered to 12 patients; 29 patients received FOLFOX or FOLFIRI as first-line treatment. Median OS was 17.6 months. Median PFS with FOLFOXIRI (13.0 months) was significantly prolonged (HR 0.325) as compared to FOLFOX/FOLFIRI (4.3 months). However, this failed to translate into an OS benefit (p = 0.433). Interestingly, addition of a monoclonal antibody to chemotherapy associated with superior OS (HR 0.523). A total of 64.7% patients received further-line therapy, which included a BRAF inhibitor in 17 patients. Targeted therapy associated with very favourable OS (25.1 months).
Conclusion
Patients with BRAFV600E-mutated mCRC benefit from the addition of an antibody to first-line chemotherapy. Further-line treatment including a BRAF inhibitor has a dramatic impact on survival.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00432-023-05141-y.
Keywords: Metastatic colorectal cancer, BRAFV600E, Chemotherapy, Encorafenib
Introduction
Colorectal cancer (CRC) is the third most prevalent cancer and the second leading cause of cancer related death in western countries (Bray et al. 2018). Resection of the primary tumour with or without systemic adjuvant chemotherapy is a curative option in patients with local or locally advanced disease. However, up to 50% of patients initially present with distant metastases or develop distant relapse. Systemic chemotherapy is the primary choice of treatment for patients not amenable to curative surgery or metastasectomy. A comprehensive molecular profiling including mutational analysis of KRAS (Kirsten rat sarcoma virus), NRAS (neuroblastoma RAS viral oncogene homolog), BRAF (RAS-associated factor B) and dMMR/MSI (deficient mismatch repair/microsatellite instability) is recommended before initiation of systemic palliative treatment (Benson et al. 2021). In addition, primary tumour localization (right sided vs left sided) has an impact on prognosis and efficacy of several drugs and should be considered before the initiation of first-line systemic treatment (Arnold et al. 2017). Mutations of the KRAS or NRAS oncogene are detected in up to 55% of all mCRCs and are strong negative predictors for the efficacy of monoclonal antibodies targeting the epidermal growth factor receptor (EGFR) (Stintzing et al. 2017; Venook et al. 2017). Thus, patients with KRAS- or NRAS-mutant tumours should be treated with a chemotherapy doublet (FOLFOX or FOLFIRI) or triplet (FOLFOXIRI) in combination with the anti-angiogenic antibody bevacizumab targeting the vascular endothelial growth factor A (VEGFA) in first line. Patients with mCRC without KRAS or NRAS mutations (all RAS wildtype) should be treated with a chemotherapy doublet in combination with an EGFR antibody (cetuximab or panitumumab) rather than with bevacizumab in first line. However, efficacy of EGFR antibodies seems to be restricted to patients whose primary tumour is located on the left site of the colon, whilst patients harbouring a tumour situated proximal from the left splenic flexure should rather receive bevacizumab in first line (Arnold et al. 2017).
Mutations of the BRAF oncogene, primarily BRAFV600E, are detected in up to 10% of patients with colorectal cancer (Fransen et al. 2004; Souglakos et al. 2009; Roth et al. 2010; Yokota et al. 2011; Clancy et al. 2013; Sinicrope et al. 2015). Colorectal cancers with BRAFV600E mutations are clinically different from tumours without BRAF mutations and also from tumours harbouring non-V600 mutations (Jones et al. 2017). BRAFV600E-mutant tumours are more frequently observed in female patients and situated right-sided (Loupakis et al. 2016). Additionally, these tumours are associated with a poor histopathological grading, MSIhigh/dMMR status, larger primary tumour size, higher rate of nodal metastases and peritoneal carcinomatosis. Patients with BRAFV600E-mutant metastatic CRC have a dismal prognosis with a reduced response to chemotherapy and dramatically reduced overall survival time of only 1 year in clinical trials (Cremolini et al. 2015; Modest et al. 2016; Stintzing et al. 2017).
The best treatment strategy in this poor prognostic subgroup of patients with BRAFV600E-mutant mCRC remains elusive. Current clinical guidelines recommend a triplet chemotherapy with FOLFOXIRI in combination with bevacizumab as preferred first-line chemotherapy in fit patients (Van Cutsem et al. 2016). However, these recommendations were based on a small retrospective subgroup analysis of 28 patients treated within the TRIBE study (Cremolini et al. 2015). In this study, the triplet chemotherapy with FOLFOXIRI plus bevacizumab was superior to the doublet with FOLFIRI plus bevacizumab. Recently, pooled analyses of 5 clinical trials which tested the triplet FOLFOXIRI plus bevacizumab versus a doublet chemotherapy with FOLFOX or FOLFIRI plus bevacizumab showed comparable median overall survival with the triplet (13.6 months) or the doublet chemotherapy (14.5 months) in at least 115 patients with BRAFV600E-mutated metastatic CRC (Cremolini et al. 2020). In a real-world setting, a substantial number of patients do not qualify for the triplet chemotherapy with FOLFOXIRI due to age, reduced performance status or comorbidities. The efficacy of EGFR directed antibodies (cetuximab, panitumumab) in BRAFV600E-mutated mCRC has been discussed controversially (Pietrantonio et al. 2015; Rowland et al. 2015). Retrospective post-hoc meta-analyses of large clinical trials did not show conclusive results. However, the recently published FIRE4.5 study demonstrated that the triplet chemotherapy with a modified FOLFOXIRI plus the EGFR antibody cetuximab was inferior to FOLFOXIRI plus bevacizumab in first-line setting (Stintzing et al. 2021). Interestingly, after failure of cytotoxic chemotherapy, the combination of the BRAF inhibitor encorafenib and the monoclonal EGFR antibody cetuximab is effective in patients with BRAFV600E-mutant mCRC. In the randomized phase III BEACON trial, this combination was superior to a standard systemic chemotherapy and became the new standard for previously treated patients (Kopetz et al. 2019).
In the above-reported prospective clinical trials, only patients in good performance status without significant comorbidities were enrolled. The results of these trials are thus not fully applicable to patients treated in a real-world setting. Treatment strategies in patients not fulfilling generic study inclusion criteria have to be defined. Against this background, we report the clinical course of a cohort of 51 patients with BRAFV600E-mutant mCRC who consulted our centre and received palliative treatment irrespective of predefined capability criteria such as performance status, age, comorbidities or laboratory values as required for clinical trial inclusion. The aim of this retrospective study is to define the best treatment strategy in this poor prognostic patient population. Further, we wanted to evaluate the impact of the introduction of BRAF inhibitor treatment in a real-world setting.
Materials and methods
Study design
Patients with histologically confirmed BRAF-mutant CRC diagnosed between 2008 and 2020 at the West German Cancer Center, Essen, Germany, were retrospectively identified and enrolled into this study. Patients were evaluable for analyses if they had a BRAF p.V600E mutation and were treated in the palliative setting (relapse or distant metastases) at our centre. Patients with atypical BRAF mutations and patients who were only diagnosed or had surgery at our centre but did not receive palliative treatment, or had no clinical follow-up data, were excluded (Suppl. Figure 1). Palliative treatment decision was made by the multidisciplinary tumour board (MTB), and chemotherapy regimen was selected by the treating oncologist based on the current clinical guidelines, performance status, comorbidities and patient wish. Follow-up data were routinely assessed and documented in the electronic health record (EHR). Clinical parameters and applied chemotherapy protocols including efficacy data were also retrieved from the EHR. Personal patient data were anonymized in the data base, and the data were analysed by a blinded researcher. The study was approved by the Ethics Committee of the Medical Faculty of the University Duisburg-Essen (Project No. 05-2882).
Assessments
Tumour staging was performed according to the American Joint Committee on Cancer (AJCC)/International Union against Cancer (UICC) TNM classification (7th Edition). Clinical staging was routinely based on computed tomography (CT) or magnetic resonance imaging (MRI) before start of palliative chemotherapy and subsequently every 8–12 weeks according to the institutional guidelines. Objective response rate (ORR) was evaluated according to the Response Evaluation Criteria in Solid Tumours 1.1 (RECIST 1.1). Patients were eligible for ORR assessment if they had a baseline radiological examination and at least one examination during the palliative chemotherapy. ORR was defined as the proportion of patients with complete or partial remission, the disease control rate (DCR) was defined as the proportion of patients with complete or partial remission or sustained disease stabilization at first radiological follow-up. Progression-free survival (PFS) was defined as time from start of chemotherapy to date of radiological or clinical progression or death. Overall survival was defined as time from start of palliative therapy to death. Patients were censored at the time of last visit at our centre, if time of death was not documented.
Statistical analysis
All analyses were conducted using SPSS Statistics (V27, IBM, Armonk, NY, USA). Correlation analyses were performed using Pearson’s Chi-square test. Kaplan–Meier calculations with the log-rank test were used for analysis of OS and PFS. For follow-up time, the reverse Kaplan–Meier method was used. Univariate and multivariate analyses were performed by a Cox proportional-hazard model. Hazard ratio (HR) and 95% confidence intervals (CI) were indicated. Overall, p values ≤ 0.05 were regarded statistically significant.
Results
Patients’ characteristics
In total, 81 colorectal cancer patients with BRAF mutations were identified in our data base. For 23 of these patients, no clinical data were available and they were only diagnosed at our centre. Two of the 58 remaining patients had an atypical BRAF mutation (p.G469A and p.D594N) and were excluded from our analyses. Additional 5 patients were treated in curative intent and had no relapse or developed metastases till the data cut-off. The study population thus contained 51 patients with BRAF p.V600E-mutant mCRC, receiving palliative treatment at out centre. The median follow-up time was 68.4 months (range 2.7–99.3). Baseline characteristics are summarized in Table 1 and suppl. table 1. Median age at diagnosis was 61.9 years (range 40.5–88.4) and 62.2 years (range 41.4–88.4) at time of relapse; 54.9% patients were female, and 72.5% had a primary tumour located at the right side of the colon. Most patients had stage IV disease at time of diagnosis (72.5%), the remaining 14 patients (27.5%) developed metachronous metastases. The primary tumour was resected in 74.5% of all patients, and 62.3% of patients with initially non-metastatic disease received an adjuvant chemotherapy. The relapse-free survival (RFS) in those patients without synchronous metastases was 12 months (95% CI 7.6–16.4) (suppl. Figure 2).
Table 1.
Patients’ characteristics
| N | % | |
|---|---|---|
| Gender | ||
| Male | 23 | 45.1 |
| Female | 38 | 54.9 |
| Age at diagnosis | ||
| Median (range) | 61.9 years (40.5–88.4) | |
| Age at metastases/relapse | ||
| Median (range) | 62.2 years (41.4–88.4) | |
| Tumour localization | ||
| Left sided | 12 | 23.5 |
| Right sided | 37 | 72.5 |
| Unknown/both | 2 | 3.9 |
| M-stage | ||
| Synchronous M1 | 37 | 72.5 |
| Metachronous M1 | 14 | 27.5 |
| Neoadjuvant therapy | ||
| Yes | 3 | 5.9 |
| No | 48 | 94.1 |
| Resection of primary tumour | ||
| Yes | 38 | 74.5 |
| No | 13 | 25.5 |
| Adjuvant CTX (only resected with metachronous metastases; N = 14) | ||
| Yes | 9 | 62.3 |
| No | 5 | 35.7 |
| Type of adjuvant CTX | ||
| FOLFOX/CAPOX | 6 | 66.6 |
| 5-FU/Capecitabin | 3 | 33.3 |
Palliative first-line therapy
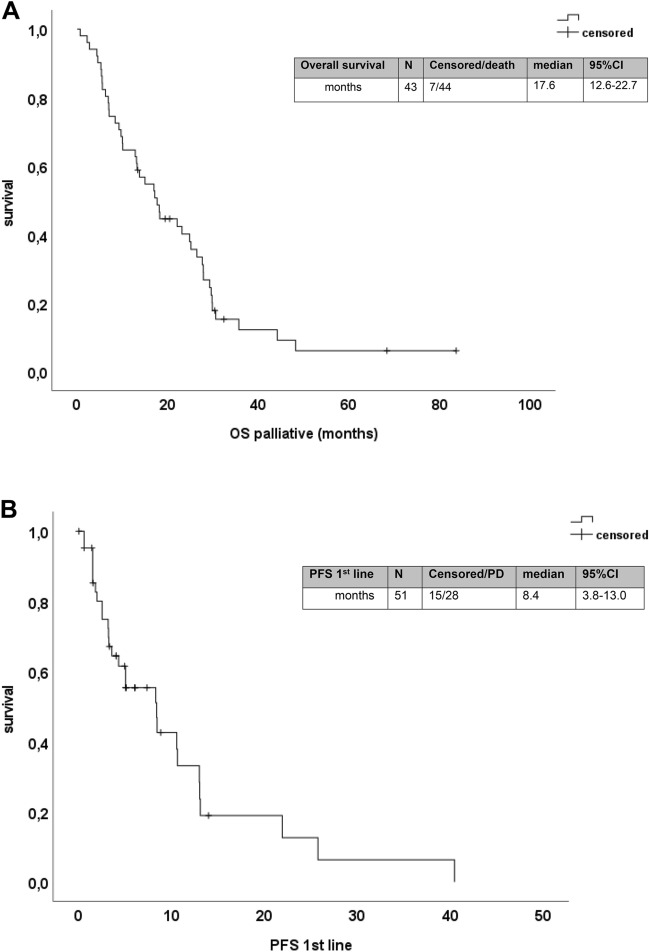
Palliative systemic treatment was administered to 48 patients (94.1%), whereas 3 (5.9%) patients only received best supportive care (BSC). Median number of therapy lines in the palliative setting was 2 (range 0–5); 33 (64.7%), 23 (45.1%), 7 (13.7%) and 3 (5.9%) of patients received second-, third-, fourth- or fifth-line therapy, respectively (Tables 2, 3, Fig. 1 and suppl. table 2). In total, 12 patients (23.5%) received triplet chemotherapy with FOLFOXIRI, 19 patients (37.3%) received oxaliplatin-based doublet chemotherapy, and 10 patients (19.6%) received irinotecan-based first-line therapy. A monoclonal antibody was added to first-line therapy in 31 patients (60.8%), 21 patients (41.2%) received bevacizumab, and 10 patients (19.6%) received an EGFR antibody (cetuximab or panitumumab). Objective response rate (ORR) according to RECIST 1.1 was evaluable for 39 patients. None of these patients had a complete remission (CR), 13 patients (33.3%) had a partial remission (PR), 10 patients (25.6%) had stable disease (SD), and 16 patients (41.0%) had a progressive disease (PD). Thus, the ORR was 33.3%, and the disease control rate (DCR) was 59.0% upon first-line therapy (Table 4). The median PFS of all enrolled patients (N = 51) was 8.4 months (95% CI 3.8–13.0), and the median OS from start of palliative treatment was 17.6 months (95% CI 12.6–22.7) (Fig. 2a, b).
Table 2.
Palliative first-line therapy
| N | % | |
|---|---|---|
| 1st CTX received | ||
| Yes | 48 | 94.1 |
| No (BSC) | 3 | 5.9 |
| Lines of therapy | ||
| 0 | 3 | 5.9 |
| 1 | 15 | 29.4 |
| 2 | 10 | 19.6 |
| 3 | 16 | 31.4 |
| > 3 | 4 | 7.8 |
| Median | 2 (range 0–5) | |
| Triplet CTX (FOLFOXIRI) | ||
| Yes | 12 | 23.5 |
| No | 38 | 74.5 |
| Unknown | 1 | 2.0 |
| First-line monoclonal antibody | ||
| Bevacizumab | 21 | 41.2 |
| EGFR (Panitumumab/Cetuximab) | 10 | 19.6 |
| None | 19 | 37.3 |
| Unknown | 1 | 2.0 |
| First-line chemotherapy backbone | ||
| Oxaliplatin-based (FOLFOX/CAPOX) | 19 | 37.3 |
| Irinotecan-based (FOLFIRI, Irinotecan) | 10 | 19.6 |
| FOLFOXIRI | 12 | 23.5 |
| Fluoropyrimidin mono | 3 | 5.9 |
| Other | 2 | 3.9 |
| Encorafenib | 1 | 2.0 |
| BSC | 3 | 5.9 |
| n.d. | 1 | 2.0 |
Table 3.
Systemic second-line therapy
| N | % | |
|---|---|---|
| Second-line CTX received | ||
| Yes | 33 | 64.7 |
| No (BSC) | 18 | 35.3 |
| Second-line monoclonal antibody (N = 33) | ||
| Anti-angiogenic (Bev/Afli/Ram) | 11 | 33.3 |
| EGFR (Panitumumab/Cetuximab) | 13 | 39.4 |
| None | 9 | 27.3 |
| Chemotherapy backbone | ||
| Oxaliplatin-based (FOLFOX/CAPOX) | 6 | 18.2 |
| Irinotecan-based (FOLFIRI, Irinotecan) | 10 | 30.3 |
| FOLFOXIRI | 3 | 9.1 |
| Fluoropyrimidin mono | 3 | 9.1 |
| Encorafenib | 10 | 30.3 |
| Pembrolizumab | 1 | 3.0 |
Fig. 1.
Funnel chart of palliative treatment regimen and conversion in first, second, third, fourth and fifth treatment line. FOLFOXIRI 5-fluorouracil, folinic acid, oxaliplatin, irinotecan, BRAFi BRAF inhibitor, BSC best supportive care
Table 4.
Efficacy of first-line therapy
| All patients % (N) |
Triplet (N = 12) % (N) |
No triplet % (N) |
moABX (N = 31) % (N) |
No moABX (N = 20) % (N) |
|
|---|---|---|---|---|---|
| ORR (N = 39) | 33.3 (13) | 72.7% (8) | 17.9% (5) | 42.3% (11) | 15.4% (2) |
| Odds ratio (95% CI) |
OR = 12.267 (2.375–63.360) p* = 0.002 |
OR = 4.033 (0.740–21.983) p* = 0.151 | |||
| DCR | 59.0 (23) | 81.8% (9) | 50% (14) | 73.1% (19) | 30.8% (4) |
| Odds ratio (95% CI) |
OR = 4.500 (0.821–24.679) p* = 0.086 |
OR = 6.107 (1.415–26.356) p* = 0.017 |
|||
| Median PFS (months) (N = 43) (95% CI) | 8.4 (3.8–13.0) | 13.0 (12.9–13.1) | 4.3 (2.3–6.3) | 10.5 (8.0–13.1) | 3.1 (1.5–4.8) |
| Hazard ratio (95% CI) |
p# = 0.018 HR = 0.325 (0.121–0.873); p = 0.026 |
p# = 0.063 HR = 0.474 (0.210–1.068); p = 0.072 |
|||
| Median OS (months) (N = 51) (95% CI) | 17.6 (12.6–22.7) | 22.1 (14.7–29.4) | 15.0 (9.9–20.1) | 24.8 (19.3–30.3) | 9.7 (3.4–16.0) |
| Hazard ratio (95% CI) |
p# = 0.404 HR = 0.730 (0.347–1.534); p = 0.406 |
p# = 0.040 HR = 0.523 (0.279–0.981); p = 0.043 |
|||
*Two sided Fisher’s exact test; OR = odds ratio
#Log rank test; HR: hazard ratio
Fig. 2.
a Kaplan–Meier analysis for overall survival from start of palliative treatment. b Kaplan–Meier analysis for progression-free survival to first-line therapy
An intensive first-line chemotherapy with the triplet FOLFOXIRI was administered to n = 12 patients (Table 4). The ORR was significantly higher in patients treated with triplet chemotherapy (72.7%) than in those treated with other chemotherapy backbones (17.9%, p = 0.002). In line, the median PFS was significantly longer in patients receiving triplet chemotherapy (13.0 months vs 4.3 months; p = 0.018). However, this higher efficacy of FOLFOXIRI in first line did not translate into higher OS (p = 0.404) (Table 4 and Fig. 3). The addition of a monoclonal antibody to the palliative first-line chemotherapy backbone was observed for 31 patients (21 bevacizumab and 10 cetuximab or panitumumab). The use of an antibody was associated with a higher chance to achieve a disease control (73.1% vs. 30.8%, p = 0.017) (Table 4). The median PFS was numerical longer with the use of a monoclonal antibody (10.5 months vs 3.1 months). However, this difference was not statistically significant (p = 0.063). Interestingly, patients receiving an antibody had an unexpectedly high median OS of 24.8 months compared to patients without antibody treatment (9.7 months; p = 0.040) (Fig. 3). Surprisingly, this effect was independent of the class of monoclonal antibody: Patients treated with bevacizumab in first line had a median OS of 23.1 months (95% CI 13.5–32.7), and patients who received an EGFR antibody had a median OS of 26.4 months (95% CI 19.6–33.2) (p = 0.533). In multivariate Cox regression analyses with other known prognostic factors including age, primary tumour sidedness, sex and time of metastases (synchronous vs metachronous), we identified the use of a triplet chemotherapy as independent prognostic factor for PFS and the addition of a monoclonal antibody to first-line chemotherapy as independent prognostic factor for OS (suppl. Figure 3).
Fig. 3.
Forrest plot for odds ratios for overall response rate (ORR) and disease control rate (DCR) and hazard ratios for progression-free (PFS) and overall survival (OS) for patients which received a triplet chemotherapy in first line and a monoclonal antibody (mAbx), respectively
Palliative second-line therapy
Upon disease progression on or after first-line therapy, 33 patients (64.7%) received a palliative second-line therapy. As encorafenib/cetuximab received EMA approval only in late May 2020, most patients were treated with an irinotecan- or oxaliplatin-based doublet chemotherapy in combination with an anti-angiogenic agent (bevacizumab, aflibercept or ramucirumab). However, 10 patients in our population already received a targeted therapy with the BRAF inhibitor encorafenib in combination with the EGFR antibody cetuximab as second line (Fig. 1 and suppl. table 2). The ORR to second line was only moderate with 8.7%, the DCR was 60.9% (suppl. table 3). The median PFS was 4.0 months (95% CI 3.8–4.3), and the median OS from start of palliative second line was 10.7 months (95% CI 6.3–15.0) (suppl. Figure 4A and B). The DCR upon encorafenib in combination with cetuximab was numerically higher with 87.5% compared to a classical cytotoxic chemotherapy (46.7%) (Table 4B). This difference did not reach statistical significance (p = 0.086) given the low number of patients. Interestingly, median PFS was comparable between patients receiving encorafenib or chemotherapy (4.1 vs 4.0 months; p = 0.697) (Table 5), while the median OS from start of the encorafenib-based therapy was almost twice as long as the median OS upon a cytotoxic chemotherapy (13.4 months vs 6.8 months; p = 0.329).
Table 5.
Efficacy of second line with encorafenib and cetuximab
| Second line encorafenib (N = 10) % (N) |
Second line w/o encorafenib (N = 23) % (N) |
|
|---|---|---|
| ORR to second-line therapy (N = 23) | 0% (0) | 13.3% (2) |
|
p* = 0.526 OR = 0.867 (95% CI 0.711–1.057) |
||
| DCR to second-line therapy (N = 23) | 87.5% (7) | 46.7% (7) |
|
p* = 0.086 OR = 8.000 (95% CI 0.780–82.052) |
||
| Median PFS (N = 21) (95% CI) | 4.1 months (1.4–6.9) | 4.0 months (2.7–5.3) |
|
p# = 0.697 HR = 0.810 (0.280–2.344); p = 0.698 |
||
| Median OS from second line (N = 33) (95% CI) | 13.4 months (9.7–17.1) | 6.8 months (2.7–11.0) |
|
p# = 0.329 HR = 0.625 (0.275–1.548); p = 0.332 |
||
*Two sided Fisher´s exact test; OR = odds ratio
#Log rank test; HR: hazard ratio
In addition to the 10 patients receiving encorafenib/cetuximab as second-line treatment, 7 patients received a BRAF inhibitor-based therapy (encorafenib, dabrafenib or vemurafenib) beyond second line, and one patient was treated with encorafenib and cetuximab in the first-line setting (suppl. table 2 and 4A). Summing up, a total of 18 patients (35.3%) of our cohort received a BRAF inhibitor during their clinical course. The median OS of these patients from start of palliative treatment was highly promising with 25.1 months (95% CI 15.1–35.1) compared to only 13.1 months (95% CI 4.9–21.3) in those patients, who received cytotoxic chemotherapy only (p = 0.196; HR 0.656; 95% CI 0.344–1.249; p = 0.199) (Suppl. Table 4 and suppl. Figure 5). To exclude potential confounding factors, we compared the baseline characteristics of both patient populations. We found no difference regarding age, primary tumour location, time of metastases (synchronous vs metachronous), resection of primary tumour or received adjuvant chemotherapy between patients, which were treated with or without a BRAF inhibitor (Suppl. Table 5). The decisive factor for receiving BRAF-targeted treatment was an initial diagnosis in the years since 2010, which allowed patient inclusion in clinical trials or administration of targeted treatment as an approved drug.
Discussion
Here, we report the clinical outcome of unselected patients with BRAFV600E-mutant mCRC, treated with palliative systemic therapy at a large comprehensive cancer centre. In contrast to prospective clinical trials in mCRC, we included patients irrespective of age, EOCG performance status, laboratory abnormalities or comorbidities in this retrospective analysis. The aim of this study was to identify the best treatment strategies for this poor prognostic patient population. As previously reported, patient characteristics were enriched with female sex, right-sided primary tumour, higher TNM stage at diagnosis and poor grading (G3) (Roth et al. 2010; Yamauchi et al. 2012; Clancy et al. 2013; Sinicrope et al. 2015; Wang et al. 2019). Most patients received a palliative doublet chemotherapy with FOLFOX or FOLFIRI in combination with bevacizumab as first line. Only a quarter (23.5%) of our real-world patients qualified for the intensive triplet FOLFOXIRI chemotherapy plus bevacizumab and were treated according to the current guidelines. These findings underline the challenges posed by the actual guidelines in a real-world patient population. However, those patients who received FOLFOXIRI had a median PFS of 13.0 months, which was comparable to the median PFS reported in the pivotal TRIBE study and significantly higher than in those patients who received a different first-line therapy (Cremolini et al. 2015). Furthermore, the ORR of 72.7% achieved with FOLFOXIRI was comparable to the control arm with FOLFOXIRI plus bevacizumab in the recently presented FIRE4.5 study (Stintzing et al. 2021). The median OS of 22.1 months in those FOLFOXIRI-treated patients was encouraging but it was not significantly higher compared to patients, receiving a different first-line therapy. This is in line with the recently published meta-analyses of 5 prospective randomized clinical trials in patients with mCRC, which investigated the additional benefit of the triplet chemotherapy with FOLFOXIRI plus bevacizumab versus a doublet with FOLFOX or FOLFIRI plus bevacizumab (Cremolini et al. 2020). In the subgroup of patients with BRAFV600E−mutant mCRC, the median OS was identical in patients who received a triplet or doublet first-line chemotherapy (13.6 vs. 14.5 months). Thus, the optimal first-line therapy in this poor prognostic patient population remains elusive. A doublet chemotherapy seems to be an appropriate alternative to FOLFOXIRI, in particular since the majority of patients in a real-world setting do not qualify for an intensive triplet chemotherapy. However, we recommend the addition of bevacizumab to the first-line chemotherapy backbone based on the results we observed in our patient population. Patients who received a monoclonal antibody had a higher ORR, median PFS and median OS compared to patients treated with chemotherapy only.
Interestingly, the median OS of 17.6 months in our unselected patient population was markedly higher than in the reported pooled analysis of Cremolini et al. and comparable to the recently reported data from the control arm with FOLFOXIRI plus bevacizumab of the FIRE4.5 trial (17.1 months) (Cremolini et al. 2020; Stintzing et al. 2021). This unexpectedly high median OS could be explained by the high number of patients, who had access to targeted therapies after progression following first-line therapy at our centre within clinical trials, as off-label use or later after the approval of encorafenib and cetuximab. In total, one third of our patients (18 out of 51) received a BRAF inhibitor in combination with an EGFR antibody or a different second targeted agent during the course of therapy. The median PFS in patients who received a BRAF inhibitor was 4.1 months and thus comparable to the median PFS observed in the pivotal BEACON trial with encorafenib and cetuximab (4.3 months) (Kopetz et al. 2019). Interestingly, the median PFS of 4.0 months in those patients receiving a standard chemotherapy in second-line in our cohort was comparable to the median PFS with encorafenib and cetuximab and markedly higher than the reported PFS of the control arm with cetuximab and irinotecan or FOLFIRI in the BEACON trial. This could be explained by the fact that only 3 patients in our cohort received an EGFR antibody in combination with cytotoxic chemotherapy and most patients were rather treated with a chemotherapy doublet in combination with an anti-angiogenic agent (bevacizumab or aflibercept) in second-line. A retrospective post-hoc molecular subgroup analyses of patients with BRAFV600E-mutated mCRC treated in the RAISE study, which evaluated the addition of the anti-angiogenic antibody ramucirumab to FOLFIRI in second line, also showed a median PFS of 5.7 months with a median OS of 9.0 months (Yoshino et al. 2019). Thus, cytotoxic chemotherapy should be rather combined with an anti-angiogenic agent rather than with an EGFR antibody in BRAFV600E-mutant mCRC patients. This was recently confirmed by the FIRE4.5 study which showed a superiority of bevacizumab versus cetuximab in first-line therapy with FOLFOXIRI.
Notably, in our cohort patients who received a BRAF inhibitor in combination with an EGFR antibody or a different targeted second agent had an extraordinarily high median OS of 25.1 months from start of their palliative treatment compared to only 13.1 months in those patients who received cytotoxic chemotherapy only. Thus, this treatment strategy should implicitly be integrated in the treatment algorithm of patients with BRAFV600E-mutant mCRC.
Conclusion
In conclusion, only a small number of patients with BRAFV600E-mutant mCRC in a real-world setting qualify for the guideline recommended intensive first-line chemotherapy with FOLFOXIRI and are rather treated with a doublet chemotherapy in combination with bevacizumab. Even though the PFS in first line is prolonged with the triplet chemotherapy compared to other less intensive protocols, it has no significant impact on OS and a doublet chemotherapy with FOLFOX or FOLFIRI in combination with bevacizumab is an appropriate alternative. After progress to cytotoxic chemotherapy, the integration of a BRAF inhibitor in combination with an EGFR antibody into the treatment strategy in a real-world setting is feasible and highly recommended. Encouraging median OS times could be achieved in a real-world setting if patients have early access to these molecular targeted therapies.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
MZ contributed to formal analysis; investigation; methodology; project administration; resources; validation; visualization; roles/writing—original draft; and writing—review and editing. MG contributed to investigation; methodology; resources; validation; and writing—review and editing. TH contributed to investigation; methodology; resources; validation; and writing—review and editing. JT, PM, BS, DA, AR, VR, GZ, KK, MP, JT, and HS contributed to resources; validation; and writing—review and editing. ST contributed to investigation; methodology; resources; validation; and writing—review and editing. RS contributed to resources and writing—review and editing. MW and MS contributed to data curation; formal analysis; investigation; methodology; resources; supervision; validation; visualization; roles/writing—original draft; and writing—review and editing. SK and IV contributed to data curation; formal analysis; investigation; methodology; project administration; resources; supervision; validation; visualization; roles/writing—original draft; and writing—review and editing.
Funding
Open Access funding enabled and organized by Projekt DEAL. This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data availability
The authors confirm that the data supporting the presented results of this study are available within in the article and its supplemeentary material. Raw data that support the findings are available from the corresponding author, upon reasonable request.
Declarations
Competing interests
Martin Schuler received honoraria as consultant (compensated) from Amgen, AstraZeneca, BIOCAD, Boehringer Ingelheim, Bristol-Myers Squibb, GlaxoSmithKline, Janssen, Merck Serono, Novartis, Roche, Sanofi, Takeda, Honoraries for CME presentations from Amgen, Boehringer Ingelheim, Bristol-Myers Squibb, Janssen, Novartis and Research funding to institution from AstraZeneca, Bristol Myers-Squibb Stefan Kasper received honoraria from Merck Serono, MSD, Novartis, BMS, Amgen, Roche, Sanofi-Aventis, Servier, Incyte, Pierre Fabre and Lilly; research funding from Merck Serono, Lilly, BMS, Roche. Isabel Virchow received travel support from Pierre Fabre. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Conflict of interest
MS received honoraria as consultant (compensated) from Amgen, AstraZeneca, BIOCAD, Boehringer Ingelheim, Bristol-Myers Squibb, GlaxoSmithKline, Janssen, Merck Serono, Novartis, Roche, Sanofi, Takeda, Honoraries for CME presentations from Amgen, Boehringer Ingelheim, Bristol-Myers Squibb, Janssen, Novartis and Research funding to institution from AstraZeneca, Bristol Myers-Squibb. SK received honoraria from Merck Serono, MSD, Novartis, BMS, Amgen, Roche, Sanofi-Aventis, Servier, Incyte, Pierre Fabre and Lilly; research funding from Merck Serono, Lilly, BMS, Roche. IV received travel support from Pierre Fabre. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Arnold D et al (2017) Prognostic and predictive value of primary tumour side in patients with RAS wild-type metastatic colorectal cancer treated with chemotherapy and EGFR directed antibodies in six randomized trials. Ann Oncol 28(8):1713–1729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson AB et al (2021) Colon cancer, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 19(3):329–359 [DOI] [PubMed] [Google Scholar]
- Bray F et al (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68(6):394–424 [DOI] [PubMed] [Google Scholar]
- Clancy C et al (2013) BRAF mutation is associated with distinct clinicopathological characteristics in colorectal cancer: a systematic review and meta-analysis. Colorectal Dis 15(12):e711-718 [DOI] [PubMed] [Google Scholar]
- Cremolini C et al (2015) FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: updated overall survival and molecular subgroup analyses of the open-label, phase 3 TRIBE study. Lancet Oncol 16(13):1306–1315 [DOI] [PubMed] [Google Scholar]
- Cremolini C et al (2020) Individual patient data meta-analysis of FOLFOXIRI plus bevacizumab versus doublets plus bevacizumab as initial therapy of unresectable metastatic colorectal cancer. J Clin Oncol JCO2001225(28):3314–3324. [DOI] [PubMed]
- Fransen K et al (2004) Mutation analysis of the BRAF, ARAF and RAF-1 genes in human colorectal adenocarcinomas. Carcinogenesis 25(4):527–533 [DOI] [PubMed] [Google Scholar]
- Jones JC et al (2017) (Non-V600) BRAF mutations define a clinically distinct molecular subtype of metastatic colorectal cancer. J Clin Oncol 35(23):2624–2630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopetz S et al (2019) Encorafenib, binimetinib, and cetuximab in BRAF V600E-mutated colorectal cancer. N Engl J Med 381(17):1632–1643 [DOI] [PubMed] [Google Scholar]
- Loupakis F et al (2016) Clinico-pathological nomogram for predicting BRAF mutational status of metastatic colorectal cancer. Br J Cancer 114(1):30–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modest DP et al (2016) Outcome according to KRAS-, NRAS- and BRAF-mutation as well as KRAS mutation variants: pooled analysis of five randomized trials in metastatic colorectal cancer by the AIO colorectal cancer study group. Ann Oncol 27(9):1746–1753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrantonio F et al (2015) Predictive role of BRAF mutations in patients with advanced colorectal cancer receiving cetuximab and panitumumab: a meta-analysis. Eur J Cancer 51(5):587–594 [DOI] [PubMed] [Google Scholar]
- Roth AD et al (2010) Prognostic role of KRAS and BRAF in stage II and III resected colon cancer: results of the translational study on the PETACC-3, EORTC 40993, SAKK 60-00 trial. J Clin Oncol 28(3):466–474 [DOI] [PubMed] [Google Scholar]
- Rowland A et al (2015) Meta-analysis of BRAF mutation as a predictive biomarker of benefit from anti-EGFR monoclonal antibody therapy for RAS wild-type metastatic colorectal cancer. Br J Cancer 112(12):1888–1894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinicrope FA et al (2015) Molecular markers identify subtypes of stage III colon cancer associated with patient outcomes. Gastroenterology 148(1):88–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souglakos J et al (2009) Prognostic and predictive value of common mutations for treatment response and survival in patients with metastatic colorectal cancer. Br J Cancer 101(3):465–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stintzing S et al (2017) Impact of BRAF and RAS mutations on first-line efficacy of FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab: analysis of the FIRE-3 (AIO KRK-0306) study. Eur J Cancer 79:50–60 [DOI] [PubMed] [Google Scholar]
- Stintzing S et al (2021) Randomized study to investigate FOLFOXIRI plus either bevacizumab or cetuximab as first-line treatment of BRAF V600E-mutant mCRC: the phase-II FIRE-4.5 study (AIO KRK-0116). J Clin Oncol 39(15_suppl):3502–3502 [Google Scholar]
- Van Cutsem E et al (2016) ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol 27(8):1386–1422 [DOI] [PubMed] [Google Scholar]
- Venook AP et al (2017) Effect of first-line chemotherapy combined with cetuximab or bevacizumab on overall survival in patients with KRAS wild-type advanced or metastatic colorectal cancer: a randomized clinical trial. JAMA 317(23):2392–2401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J et al (2019) Clinicopathological significance of BRAF(V600E) mutation in colorectal cancer: an updated meta-analysis. J Cancer 10(10):2332–2341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi M et al (2012) Assessment of colorectal cancer molecular features along bowel subsites challenges the conception of distinct dichotomy of proximal versus distal colorectum. Gut 61(6):847–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota T et al (2011) BRAF mutation is a powerful prognostic factor in advanced and recurrent colorectal cancer. Br J Cancer 104(5):856–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino T et al (2019) Biomarker analysis beyond angiogenesis: RAS/RAF mutation status, tumour sidedness, and second-line ramucirumab efficacy in patients with metastatic colorectal carcinoma from RAISE-a global phase III study. Ann Oncol 30(1):124–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that the data supporting the presented results of this study are available within in the article and its supplemeentary material. Raw data that support the findings are available from the corresponding author, upon reasonable request.