Abstract
Purpose: We aimed to investigate the prognosis and impact of postoperative acute kidney injury (AKI) in acute Stanford type A aortic dissection (ATAAD) patients, and to analyze the predictors of short- and medium-term survival.
Methods: A total of 192 patients who underwent ATAAD surgery were included between May 2014 and May 2019. Perioperative data of these patients were analyzed. All of the discharged patients were followed up for 2 years.
Results: Postoperative AKI was identified in 43 of 192 patients (22.4%). The two-year survival rate of patients with AKI after discharge was 88.2% and that without AKI was 97.2%.The difference was statistically significant (χ2 = 5.355, log-rank P = 0.021). Cox hazards regression showed that age (hazard ratio [HR], 1.070; P = 0.002), cardiopulmonary bypass (CPB) time (HR, 1.026; P = 0.026), postoperative AKI (HR, 3.681; P = 0.003), and red blood cell transfusion (HR, 1.548; P = 0.001) were independent risk factors for the short- and medium-term total mortality of ATAAD patients.
Conclusion: The incidence of postoperative AKI is high in ATAAD, and the mortality of patients with AKI increases significantly within 2 years. Age, CPB time, and red blood cell transfusion were also independent risk factors for short-and medium-term prognoses.
Keywords: aortic dissection, acute kidney injury, risk factors, mortality
Introduction
Acute type A aortic dissection is a surgical emergency with rapid progress. Unless there is continuous coma or severe intestinal ischemia, most of them need emergency surgery. Although the cardiovascular surgery technique is constantly improving, the Sun’s procedure has been performed in many grass roots hospitals in China, the perioperative complication rate and mortality rate of acute Stanford type A aortic dissection (ATAAD) patients are still the highest in cardiac surgery, and the incidence of multiple organ dysfunction is still high.
Acute kidney injury (AKI) is one of the main complications after cardiac surgery, especially for patients with ATAAD. The incidence of postoperative AKI of these patients is higher than that of other cardiac surgery, and up to 15%–25% or even higher because of the emergency surgery, large surgical trauma, and non-physiological process of deep hypothermic circulatory arrest. Postoperative AKI significantly increases the in-hospital mortality.1–4) Moreover, AKI requiring continuous renal replacement therapy (CRRT) significantly increases the incidence of other postoperative complications and increases the hospital mortality, up to 17%–35%.5–7) However, there are few studies on the impact of postoperative AKI on the medium- and long-term prognoses of ATAAD patients.
Patients and Methods
A retrospective cohort study was conducted among patients with ATAAD who underwent surgical treatment in the heart center of our hospital between May 2014 and May 2019, including 139 males and 53 females, aged 28–85 years, with an average age of 53.3 ± 11.4 years. All patients were diagnosed with Stanford type A aortic dissection by computed tomography (CT) angiography of thoracoabdominal aorta. Exclusion criteria were 1) history of chronic renal insufficiency, 2) seriously impaired function of important organs before operation, or 3) patient died during operation or within 24 hours after operation.
Surgical technique
Standard longitudinal median sternotomy was performed in the operation. Cardiopulmonary bypass was established by cannulation of right atrium or separately the superior and inferior vena cava. Axillary or femoral arterial cannulation was established. Most of the patients underwent axillary arterial cannulation. Selective anterograde cerebral perfusion was performed in patients with deep hypothermic circulatory arrest, and bilateral cerebral perfusion was performed in most patients. Cerebral oxygen saturation was monitored during the operation. There were 147 patients with deep hypothermic circulatory arrest and 45 patients with moderate hypothermia during cardiopulmonary bypass (CPB).
Variables analyzed and follow-up
The perioperative data of all the patients were recorded and analyzed: gender, age, body mass index, past history (hypertension, diabetes, coronary heart disease, chronic obstructive pulmonary disease, history of cerebrovascular disease, history of cardiac surgery), Marfan syndrome, preoperative serum creatinine level, preoperative left ventricular ejection fraction, malperfusion syndrome (MPS), pericardial tamponade/shock, time from onset to admission, whether concomitant coronary artery bypass grafting, aortic root procedure, aortic arch replacement, CPB time, aortic cross-clamp time, deep hypothermic circulatory arrest time, intraoperative and 24-hour postoperative red blood cell transfusion volume, plasma transfusion, postoperative AKI, and postoperative permanent neurological dysfunction (PND) and postoperative mechanical ventilation time. Outcome measures were as follows: the primary end point was mortality, and the secondary end point was AKI incidence. All patients received telephone or outpatient follow-up within 2 years after discharge, and were followed up at 1, 6, 12, 18, and 24 months after discharge.
Definition of important variables
Emergency surgery was defined as surgical treatment within 24 hours after admission. The diagnosis and staging criteria of AKI are based on the recommendations of the Kidney Disease: Improving Global Outcomes (KDIGO).8) AKI is defined as any of the following (not graded): increase in SCr by ≥0.3 mg/dL (≥26.5 μmol/L) within 48 hours or increase in SCr to ≥1.5 times baseline, which is known or presumed to have occurred within the prior 7 days; or urine volume <0.5 ml/kg/h for 6 hours. It is divided into 1, 2, and 3 stages according to its stage standard. Postoperative PND is defined as postoperative neurological deficits that include new onset of coma, sensory or motor impairment, and any neurological damage symptoms with positive manifestations of cranial magnetic resonance imaging or CT that did not completely disappear before discharge.9) Preoperative MPS is defined as the loss of blood supply to a vital organ or limb caused by aortic branch arterial obstruction, with clinical features, laboratory findings, and radiographic findings, leading to tissue/organ damage such as necrosis and/or end-organ dysfunction.10)
Statistical analysis
Statistical analysis was carried out by using Statistical Package of the Social Sciences 19.0 software. Continuous data with a normal distribution were presented as mean ± standard deviation and data with nonnormal distribution as median values with an interquartile range. Categorical data were presented as percentages. Independent samples t-test was used to compare the means of two groups in normally distributed continuous data, and the chi-squared test or Fisher’s exact test was used for analysis of categorical data. Kaplan–Meier and log-rank tests were used to analyze the influence of postoperative AKI on prognosis, and the survival curve was drawn. The multivariate Cox regression model was used to analyze the independent risk factors for total mortality during hospitalization and follow-up. A P value of less than 0.05 was considered statistically significant.
Results
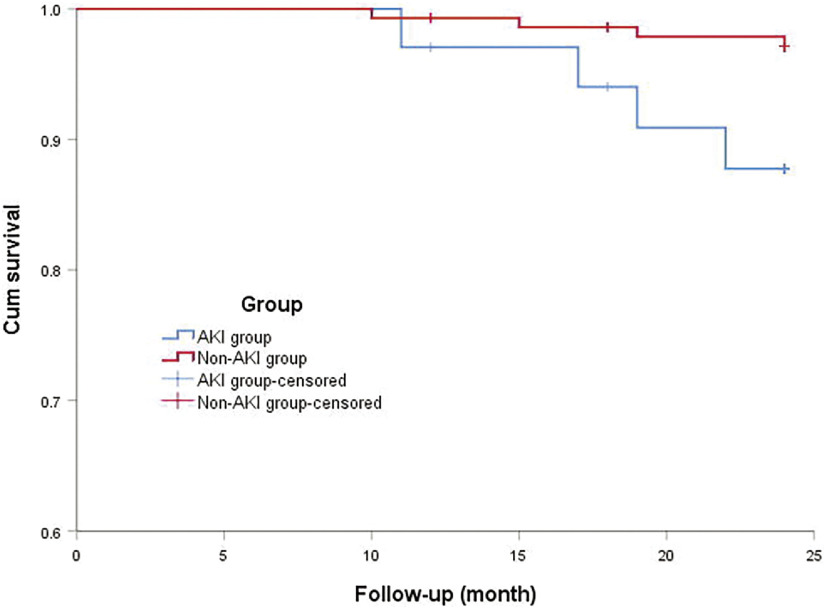
According to the KDIGO criteria, 43 (22.4%) patients developed postoperative AKI, 14 patients (7.3%) were in stage 1, 12 patients (6.3%) were in stage 2, and 17 patients (8.9%) were in stage 3. Nine patients in the postoperative AKI group died during hospitalization, including two cases of massive cerebral infarction or cerebral hemorrhage, five cases of multiple organ failure, one case of intestinal necrosis, and one case of severe septic shock. Six patients in the non-AKI group died during hospitalization. The discharged patients were followed up, and nine patients were lost to follow-up (5.1%); the mean follow-up time was (23.4 ± 2.4) months. Eight patients died during follow-up, including four patients in the AKI group and four patients in the non-AKI group. In the AKI group, one patient died of multiple organ failure, one patient died of massive cerebral infarction, one patient died of severe pulmonary infection, and one patient died of vascular graft infection. The 2-year survival rate of patients with AKI after discharge was 88.2% and that without AKI after discharge was 97.2%. The Kaplan–Meier survival analysis and log-rank test showed that the difference was statistically significant (χ2 = 5.355, log-rank P = 0.021) (Fig. 1). Univariate analysis showed that the mortality in patients with stage 1 AKI within 2 years after operation was higher than that in patients without AKI, but there was no significant difference (χ2 = 1.077, P = 0.299), while the mortality of patients with stage 2 AKI and stage 3 AKI increased significantly within 2 years compared with patients without AKI, the difference was statistically significant (χ2 = 9.913, P = 0.002; χ2 = 19.718, P <0.001). The mortality increased gradually with the aggravation of AKI, and the mortality increased from 14.3% of stage 1 AKI to 41.2% of stage 3 AKI (Table 1), but there was no statistical difference.
Fig. 1. Survival curve of patients with and without AKI. AKI: acute kidney injury.
Table 1. Comparison of mortality among ATAAD patients in different stages within 2 years.
| KDIGO stage | Total patients | Mortality (n, %) | Relative risk | 95% CI | χ2 | P value |
|---|---|---|---|---|---|---|
| No AKI | 149 | 10 (6.7) | – | – | – | – |
| 1 | 14 | 2 (14.3) | 2.317 | 0.454–11.809 | 1.077 | 0.299 |
| 2 | 12 | 4 (33.3) | 6.950 | 1.782–27.106 | 9.913 | 0.002 |
| 3 | 17 | 7 (41.2) | 9.730 | 3.051–31.026 | 19.718 | <0.001 |
AKI: acute kidney injury; ATAAD: acute Stanford type A aortic dissection; CI: confidence interval; KDIGO: Kidney Disease: Improving Global Outcomes
Risk factors analysis of total mortality within 2 years
Univariate analysis showed that there were statistical differences (P <0.05) (Tables 2 and 3) between the death group and the survival group in terms of age, preoperative pericardial tamponade/shock, preoperative MPS, CPB time, concomitant coronary artery bypass grafting, red blood cell transfusion intraoperatively and in 24 hours postoperatively, postoperative AKI, postoperative PND, and postoperative mechanical ventilation time. The results of multivariate Cox risk regression model analysis showed that there was 2.7 times increase in the mortality rate of patients with AKI (risk ratio = 3.681, 95% confidence interval: 1.579–8.582, P = 0.003). In addition, age, CPB time, and red blood cell transfusion intraoperatively and in 24 hours postoperatively were independent risk factors for 2-year total mortality after surgery in patients with ATAAD (P <0.05) (Table 4).
Table 2. Demographic and preoperative data of patients.
| Preoperative variables | Death group (n = 23) | Survival group (n = 169) | t/χ2 | P value |
|---|---|---|---|---|
| Age (years) | 60.9 ± 10.5 | 52.3 ± 11.2 | 3.494 | 0.001 |
| Female, n (%) | 6 (26.1) | 47 (27.8) | 0.030 | 0.862 |
| Body mass index (kg/m2) | 25.1 ± 1.4 | 24.6 ± 1.5 | 1.539 | 0.125 |
| Hypertension, n (%) | 15 (65.2) | 123 (72.8) | 0.573 | 0.449 |
| Diabetes n (%) | 2 (8.7) | 12 (7.1) | 0.076 | 0.783 |
| Chronic obstructive pulmonary disease, n (%) | 2 (8.7) | 14 (8.3) | 0.004 | 0.947 |
| Smoking, n (%) | 7 (30.4) | 41 (24.3) | 0.412 | 0.521 |
| CAD history, n (%) | 3 (13.0) | 9 (5.3) | 2.058 | 0.151 |
| Marfan’s syndrome, n (%) | 1 (4.3) | 5 (3.0) | 0.129 | 0.719 |
| Cardiac surgery history, n (%) | 1 (4.3) | 9 (5.3) | 0.039 | 0.843 |
| LVEF (%) | 54.3 ± 3.0 | 55.4 ± 2.8 | 1.615 | 0.108 |
| Preoperative serum creatinine (μmol/L) | 103.2 ± 19.0 | 97.2 ± 13.5 | 1.888 | 0.060 |
| Pericardial tamponade/shock, n (%) | 4 (17.4) | 10 (5.9) | 3.943 | 0.047 |
| MPS, n (%) | 10 (43.5) | 39 (23.1) | 4.433 | 0.035 |
| Emergency operation, n (%) | 21 (91.3) | 159 (94.1) | 0.267 | 0.606 |
CAD: coronary artery disease; LVEF: left ventricular ejection fraction; MPS: malperfusion syndrome
Table 3. Intraoperative and postoperative variables of patients.
| Variables | Death group (n = 23) | Survival group (n = 169) | t/χ2 | P value |
|---|---|---|---|---|
| Concomitant CABG, n (%) | 4 (17.4) | 10 (5.9) | 3.943 | 0.047 |
| Aortic arch replacement, n (%) | 15 (65.2) | 106 (62.7) | 0.054 | 0.816 |
| Cardiopulmonary bypass time (min) | 197.2 ± 20.4 | 182.7 ± 17.2 | 3.710 | <0.001 |
| Cross-clamp time (min) | 115.9 ± 13.2 | 111.9 ± 8.8 | 1.892 | 0.060 |
| Deep hypothermic circulatory arrest time (min) | 32.7 ± 18.4 | 31.4 ± 17.8 | 0.330 | 0.741 |
| Minimum rectal/bladder temperature (°C) | 23.2 ± 1.3 | 23.5 ± 1.1 | 1.054 | 0.293 |
| Intraoperative and 24-hour postoperative red blood cell transfusion volume (U) | 9.4 ± 1.8 | 7.8 ± 1.4 | 4.668 | <0.001 |
| Intraoperative and 24-hour postoperative blood plasma volume (mL) | 923.9 ± 191.2 | 868.9 ± 163.9 | 1.478 | 0.141 |
| PND, n (%) | 4 (17.4) | 10 (5.9) | 3.943 | 0.047 |
| AKI, n (%) | 13 (56.5) | 30 (17.8) | 17.509 | <0.001 |
| Re-exploration for bleeding, n (%) | 2 (8.7) | 11 (6.5) | 0.153 | 0.695 |
| Mechanical ventilation time (hours) | 92.0 ± 28.4 | 74.9 ± 38.6 | 2.055 | 0.041 |
| ICU stay length (h) | 137.5 ± 26.1 | 126.6 ± 35.7 | 1.415 | 0.159 |
AKI: acute kidney injury; CABG: coronary artery bypass grafting; ICU: intensive care unit; PND: permanent neurological dysfunction
Table 4. Cox proportional hazards regression model analysis for two-year mortality.
| Variables | HR | 95% CI | P value |
|---|---|---|---|
| Age | 1.070 | 1.026–1.116 | 0.002 |
| Cardiopulmonary bypass time | 1.026 | 1.003–1.050 | 0.026 |
| Intraoperative and 24-hour postoperative red blood cell transfusion volume | 1.548 | 1.183–2.026 | 0.001 |
| Postoperative AKI | 3.681 | 1.579–8.582 | 0.003 |
AKI: acute kidney injury; CI: confidence interval; HR: hazard ratio
Discussion
The previous studies of our research team have identified the independent risk factors of postoperative AKI in our center. Based on our own study and other research,3) we continue to carry out follow-up studies to explore its impact on the short-term and medium-term prognoses, and to analyze independent risk factors affecting the short-term and medium-term prognoses. This study showed that postoperative AKI was an independent risk factor for the total mortality of ATAAD patients within 2 years after surgery. With the aggravation of renal function injury, the total mortality of patients within 2 years gradually increased and the total mortality of patients with stage 3 AKI reached 41.2%. Tsai et al.’s study shows that AKI after aortic dissection surgery is an independent risk factor for the 1-year mortality of such patients, and the survival rate is strongly related to the severity of AKI (according to Risk, Injury, Failure, Loss, and End-stage renal failure classification standard).11)
AKI is a common serious complication after cardiac surgery, in which 10%–20% of patients need CRRT with a mortality of 40%–90%.12) The kidney is very sensitive to hypoxia and ischemia. Patients with ATAAD may have poor renal perfusion due to the influence of false lumen or renal artery thrombosis or stenosis before operation. Ischemia and ischemia-reperfusion injury after CPB or even deep hypothermic circulatory arrest will further aggravate the renal function injury, cause the disorder of the internal environment, and eventually lead to multiple organ function damage, thus increasing the postoperative mortality of patients. At the beginning of our own clinical work, the timing of CRRT for some AKI patients after operation was too conservative, so it led to excessive volume load and high venous pressure, which led to the aggravation of renal congestion, the acute aggravation of renal function injury, and even irreversible injury. Hyperkalemia increased the occurrence of malignant arrhythmia, and the disorder of the internal environment such as metabolic acidosis led to the occurrence of multiple organ dysfunction, so the mortality of such patients increased significantly. Therefore, it is necessary to fully evaluate the internal environment status of such patients, make comprehensive analysis, and select CRRT treatment in time. Especially before serious complications occur, it can also play a therapeutic role in fluid management, inflammation control, and toxin clearance. Thus, it can effectively curb the deterioration of renal function, and reduce the mortality and other complications.
At present, there are many studies on the analysis of postoperative risk factors associated with short-term mortality after ATAAD surgery. Most of the current studies show that factors such as advanced age, preoperative pericardial tamponade or shock, malperfusion, and postoperative stroke are directly related to the postoperative mortality.13,14) However, there are few studies about the long-term prognosis of postoperative AKI. In addition to postoperative AKI, this study also confirmed that age, CPB time, and red blood cell infusion were independent risk factors for overall mortality in the short and medium terms.
The study shows that age is an independent risk factor of short- and medium-term postoperative mortality of ATAAD. Age is a risk factor for cardiovascular disease. Elderly patients usually have variety of coexisting diseases, such as diabetes, hypertension, and renal insufficiency. Respiratory and circulatory system functions, general physical condition, and body compensatory ability of them decrease compared with young people, so their postoperative complications and mortality may be higher than young people.15) Jussli-Melchers et al. found that the 5-year postoperative survival rate of ATAAD patients over 70 years old was significantly lower than that of ATAAD patients under 70 years old (63% vs 79%, P = 0.008). This might be related to more preoperative comorbidities, higher incidence of pericardial tamponade, and severe neurological injury in elderly patients.16) Wang et al.’s study has shown that elderly ATAAD patients have a higher incidence of renal insufficiency and a lower long-term survival rate.17)
Prolonged CPB time is an independent risk factor of postoperative multiple organ dysfunction for patients with aortic dissection, which can lead to an increase in the incidence of complications such as postoperative hypoxemia and renal insufficiency.18,19) It can lead to systemic inflammatory response syndrome (SIRS) and oxidative stress injury. The occurrence of SIRS, hypoperfusion, and ischemia-reperfusion injury during CPB can lead to multiple organ dysfunction syndrome, which significantly increases the mortality. Xu et al. studied 115 patients with Debakey type I aortic dissection undergoing emergency surgery, and it was shown that the incidence of postoperative renal insufficiency would increase by 17.1% with every 10 minutes of prolonged CPB time.18) Zhang et al.’s research showed that CPB time was an independent risk factor of short-term postoperative adverse event (mortality within 30 days and stroke) by a retrospective analysis of 258 ATAAD patients undergoing total arch and elephant trunk surgery, and it was shown that the incidence of postoperative adverse events increased by 10.1% with every 10 minutes of prolonged CPB time.20) Our study also confirmed that prolonged CPB time was an independent risk factor for the short- and medium-term mortality of ATAAD patients after surgery. Therefore, the CPB time should be reduced as much as possible. In addition to systematic training to improve the surgical skills of surgeons and collaboration ability of the entire surgical team, the temperature can be lowered to about 25°C or even higher, so as to reduce the cooling and rewarming time to shorten the CPB time. Perioperative anti-inflammatory agents such as ulinastatin can be used to mitigate the effects of systemic inflammatory response. For some elderly patients, the debranching technique can be performed according to the patient’s pathological condition to shorten or even avoid CPB.
Cardiovascular surgery is the surgery with the most blood transfusion in all surgical operations. The transfusion of red blood cells can timely supplement blood volume, correct anemia, and improve oxygen supply. However, weakened deforming ability of stored red blood cell suspension and red blood cell fragments can cause the occurrence of inflammatory reaction and oxidative stress injury, cause the injury of various organs, and lead to immune suppression and the increase of mortality. Vlot et al. found that intraoperative red blood cell transfusion could increase the 30-day mortality by three times through the study of 2933 adult patients undergoing coronary artery surgery from a single center.21) On the other hand, the larger the amount of intraoperative blood transfusion, indicating the larger the amount of intraoperative blood loss and the operation may be more complicated, the operation time and the CPB time may be longer. A large number of blood loss will lead to ischemia and hypoxia injury of tissues and organs and at the same time. A large number of bleeding and blood transfusion will cause coagulation mechanism disorder, resulting in the occurrence of multiple organ dysfunction, thus increasing mortality. Therefore, perioperative blood protection should be strengthened to reduce bleeding. Preoperative autologous blood reserve can be used to avoid excessive blood dilution, and autologous blood recovery devices can be used as much as possible. At the same time, restrictive blood transfusion strategies may also be used. Garg et al. conducted a randomized clinical study on 4531 high-risk patients undergoing cardiovascular surgery on CPB from 73 centers of 19 countries and found that the amount of red blood cell transfusion was reduced by 38% in restrictive blood transfusion strategy (blood transfusion when hemoglobin <7.5 g/dL) group compared with the free blood transfusion method (blood transfusion when hemoglobin <9.5 g/dL) group, but the incidence of AKI did not increase.22)
Limitations
There are some limitations in our study that require emphasis. First, the patient population enrolled in this study was just in a single institution, so it is relatively small; therefore, the conclusions might not be applicable to other centers. Second, the follow-up time is short, and the follow-up data collection is not comprehensive. Prospective study can be performed according to this study and patients’ actual condition. We should expand the sample size, continue to prolong the follow-up time, and increase the collection of follow-up data.
Conclusion
The incidence of postoperative AKI in patients with ATAAD is still high, and it is an independent risk factor for patient’s short- and medium-term mortality. In addition, age, CPB time, and red blood cell transfusion volume are also independent risk factors for overall mortality in the short and medium terms. Therefore, we should try to shorten the CPB time and reduce red blood cell transfusion in order to reduce perioperative AKI. For elderly patients, hybrid techniques such as the debranching procedure can be used to shorten or even avoid CPB according to individual-specific condition, so as to reduce the mortality of these patients.
Ethics Committee Approval
This study was conducted in accordance with the principles of the Declaration of Helsinki and was approved by the Ethics Committee of Qingdao Municipal Hospital (No. 2021-045). Written informed consent was obtained from all study participants.
Funding
This work was supported by the project “Medical Science Research Guidance Plan of Qingdao (No: 2019-WJZD012)”.
Disclosure Statement
The authors declare no conflicts of interest.
References
- 1).Zhang K, Shang J, Chen Y, et al. The prognosis and risk factors for acute kidney injury in high-risk patients after surgery for type A aortic dissection in the ICU. J Thorac Dis 2021; 13: 4427–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2).Li L, Zhou J, Hao X, et al. The incidence, risk factors and in-hospital mortality of acute kidney injury in patients after surgery for acute type A aortic dissection: a single-center retrospective analysis of 335 patients. Front Med (Lausanne) 2020; 7: 557044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).Leballo G, Moutlana HJ, Muteba MK, et al. Factors associated with acute kidney injury and mortality during cardiac surgery. Cardiovasc J Afr 2021; 32: 308–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).Kim WH, Lee JH, Kim E, et al. Can we really predict postoperative acute kidney injury after aortic surgery? Diagnostic accuracy of risk scores using Gray Zone approach. Thorac Cardiovasc Surg 2016; 64: 281–9. [DOI] [PubMed] [Google Scholar]
- 5).Jiao R, Liu N. Prognostic factors for in-hospital mortality in patients with acute kidney injury requiring continuous renal replacement therapy undergoing surgery for acute Stanford type A aortic dissection. Zhonghua Wai Ke Za Zhi 2017; 55: 270–3. (in Chinese) [DOI] [PubMed] [Google Scholar]
- 6).Helgason D, Helgadottir S, Ahlsson A, et al. Acute kidney injury after acute repair of type A aortic dissection. Ann Thorac Surg 2021; 111: 1292–8. [DOI] [PubMed] [Google Scholar]
- 7).Nakamura T, Mikamo A, Matsuno Y, et al. Impact of acute kidney injury on prognosis of chronic kidney disease after aortic arch surgery. Interact Cardiovasc Thorac Surg 2020; 30: 273–9. [DOI] [PubMed] [Google Scholar]
- 8).Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract 2012; 120: c179–84. [DOI] [PubMed] [Google Scholar]
- 9).Yu Y, Lyu Y, Jin L, et al. Prognostic factors for permanent neurological dysfunction after total aortic arch replacement with regional cerebral oxygen saturation monitoring. Brain Behav 2019; 9: e01309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Norton EL, Khaja MS, Williams DM, et al. Type A aortic dissection complicated by malperfusion syndrome. Curr Opin Cardiol 2019; 34: 610–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Tsai HS, Tsai FC, Chen YC, et al. Impact of acute kidney injury on one-year survival after surgery for aortic dissection. Ann Thorac Surg 2012; 94: 1407–12. [DOI] [PubMed] [Google Scholar]
- 12).Sun S, Ma F, Li Q, et al. Risk model for deaths and renal replacement therapy dependence in patients with acute kidney injury after cardiac surgery. Interact Cardiovasc Thorac Surg 2017; 25: 548–54. [DOI] [PubMed] [Google Scholar]
- 13).Huo Y, Zhang H, Li B, et al. Risk factors for postoperative mortality in patients with acute Stanford type A aortic dissection. Int J Gen Med 2021; 14: 7007–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).Conzelmann LO, Weigang E, Mehlhorn U, et al. Mortality in patients with acute aortic dissection type A: analysis of pre- and intraoperative risk factors from the German Registry for Acute Aortic Dissection Type A (GERAADA). Eur J Cardiothorac Surg 2016; 49: e44–52. [DOI] [PubMed] [Google Scholar]
- 15).Salem M, Friedrich C, Thiem A, et al. Risk factors for mortality in acute aortic dissection type A: a centre experience over 15 years. Thorac Cardiovasc Surg 2021; 69: 322–8. [DOI] [PubMed] [Google Scholar]
- 16).Jussli-Melchers J, Panholzer B, Friedrich C, et al. Long-term outcome and quality of life following emergency surgery for acute aortic dissection type A: a comparison between young and elderly adults. Eur J Cardiothorac Surg 2017; 51: 465–71. [DOI] [PubMed] [Google Scholar]
- 17).Wang Z, Ge M, Chen T, et al. Risk factors and long-term outcomes of elderly patients complicating with acute kidney injury after type A acute aortic dissection surgery: a retrospective study. J Thorac Dis 2020; 12: 5833–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18).Xu S, Liu J, Li L, et al. Cardiopulmonary bypass time is an independent risk factor for acute kidney injury in emergent thoracic aortic surgery: a retrospective cohort study. J Cardiothorac Surg 2019; 14: 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19).Ge H, Jiang Y, Jin Q, et al. Nomogram for the prediction of postoperative hypoxemia in patients with acute aortic dissection. BMC Anesthesiol 2018; 18: 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20).Zhang K, Pan XD, Dong SB, et al. Cardiopulmonary bypass duration is an independent predictor of adverse outcome in surgical repair for acute type A aortic dissection. J Int Med Res 2020; 48: 300060520968450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21).Vlot EA, Verwijmeren L, van de Garde EMW, et al. Intra-operative red blood cell transfusion and mortality after cardiac surgery. BMC Anesthesiol 2019; 19: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22).Garg AX, Badner N, Bagshaw SM, et al. Safety of a restrictive versus liberal approach to red blood cell transfusion on the outcome of AKI in patients undergoing cardiac surgery: a randomized clinical trial. J Am Soc Nephrol 2019; 30: 1294–304. [DOI] [PMC free article] [PubMed] [Google Scholar]