Abstract
A 56-year-old man with a history of left nephrectomy for Wilms’ tumor on chronic hemodialysis underwent aortic valve neocuspidization using autologous pericardium (Ozaki procedure) for aortic stenosis (AS) due to a bicuspid aortic valve 6 years ago. The AS gradually progressed and a decrease in the left ventricular ejection fraction was observed. Because of this, we decided to perform reoperative aortic valve replacement using a mechanical valve. Intraoperative findings showed severe calcification at the site where the autologous pericardium was sutured to the annulus. However, the degeneration of the valve leaflets themselves was mild. While excellent mid-term results have been reported for the Ozaki procedure, the long-term results are still unclear. In this case, the annulus was severely calcified, which reduced the mobility of the leaflet. We report the first case of AS progression requiring reoperation in the long-term period after the Ozaki procedure.
Keywords: aortic valve reconstruction, autologous pericardium, Ozaki procedure
Introduction
In recent years, surgical procedures for valvular heart disease have been increasingly required, not only to cure but also to improve postoperative quality of life. Thus, valvuloplasty and neocuspidization are increasingly preferred over valve replacement. In 2011, the results of aortic valve neocuspidization using autologous pericardium (Ozaki procedure) were reported1); follow-up studies have shown excellent early to mid-term results.2) However, the long-term results are still unknown. In addition, there are presently no case reports in which aortic stenosis (AS) was observed in the long-term period after the Ozaki procedure. Herein, we report the first case of AS that necessitated reoperation due to disease progression after aortic valve neocuspidization using autologous pericardium (Ozaki procedure).
Case Report
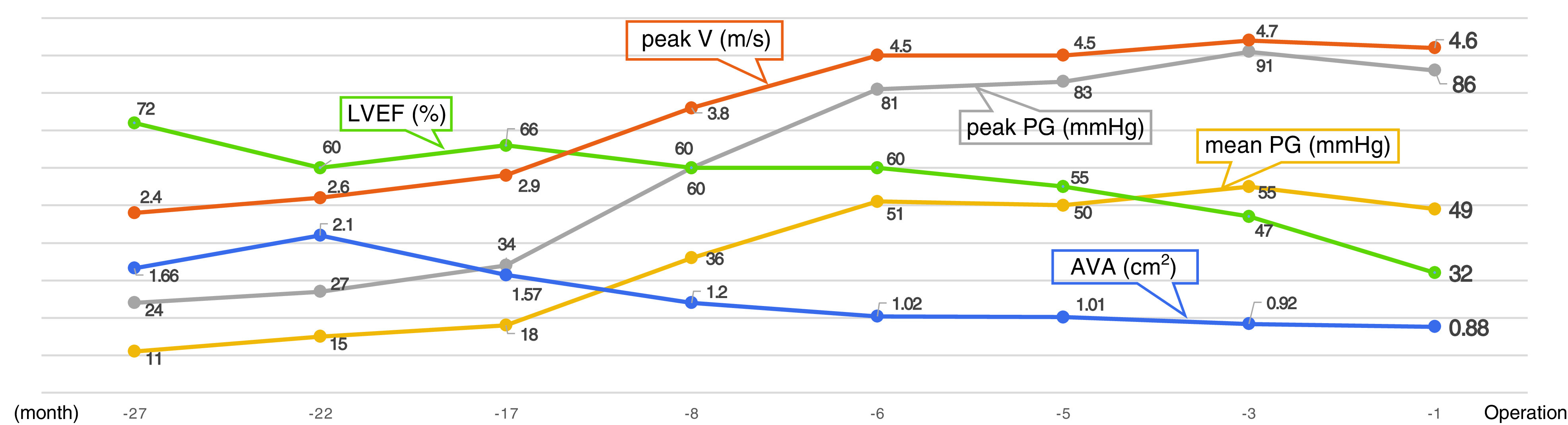
This is the case of a 56-year-old man who previously underwent left nephrectomy for Wilms’ tumor in early childhood and has been on chronic hemodialysis for 26 years. Six years ago, aortic valve neocuspidization using autologous pericardium (Ozaki procedure) was performed at another institution for AS due to a bicuspid aortic valve. Five years ago, mastectomy and chemoradiotherapy were performed for male breast cancer on the right. Approximately 4 years after the Ozaki procedure, AS was again detected by transthoracic echocardiography (Fig. 1A). His preoperative laboratory data were as follows: lactate dehydrogenase 223 U/L, hemoglobin 11.2 g/dL, N-terminal pro-brain natriuretic peptide >35000 pg/mL, white blood cell count 2940/μL, C-reactive protein 0.05 mg/dL, calcium 8.5 mg/dL, phosphorous 4.9 mg/dL, and parathyroid hormone 533.7 pg/mL (reference: 9.5–65.5 pg/mL). He had secondary hyperparathyroidism and was receiving oral sevelamer hydrochloride at 6.0 g/day for hyperphosphatemia. Hemolysis and increased inflammatory response were not observed. Preoperative transthoracic echocardiography showed a left ventricular ejection fraction (LVEF) of 32% and diffuse severe hypokinetic wall motion. The aortic valve findings were as follows: aortic valve area 0.88 cm2 (Fig. 1B), mean pressure gradient 49 mmHg, and peak velocity 4.64 m/s. No aortic valve regurgitation was observed. The left ventricular end diastolic and systolic diameters were 56 mm and 44 mm, respectively. The thicknesses of the interventricular septum and the posterior left ventricular wall were 13 mm and 14 mm, respectively. Mitral valve regurgitation and tricuspid valve regurgitation were moderate and mild, respectively. Preoperative computed tomography showed severe calcification in the aortic annulus (Fig. 1C). Since progressive AS and a decrease in LVEF were observed (Fig. 2), we decided to perform reoperative aortic valve replacement.
Fig. 1. Preoperative transthoracic echocardiography and computed tomography. (A) Aortic valve short-axis image on transthoracic echocardiography taken 2 years before reoperation (4 years after aortic valve reconstruction using autologous pericardium). The AVA is measured to be 2.10 cm2 (blue area). (B) Aortic valve short-axis image on transthoracic echocardiography before reoperation. Mobility of the aortic valve leaflet created from autologous pericardium is severely restricted. The AVA is measured to be 0.88 cm2 (dotted area). (C) Axial slice computed tomography before reoperation. Severe calcification is observed in the aortic annulus (arrowhead). AVA: aortic valve area.
Fig. 2. The patient’s preoperative transthoracic echocardiographic data through time, including LVEF, AVA, mean PG, peak PG, and peak V. LVEF: left ventricular ejection fraction; AVA: aortic valve area; PG: pressure gradient; peak V: peak velocity.
Surgery was performed via median resternotomy. Cardiopulmonary bypass was established by cannulating the common femoral artery and vein. Cardiac arrest was achieved by injecting antegrade and retrograde cardioplegia. The ascending aorta was incised and the aortic valve was inspected. There was mobility and only mild degeneration of the aortic valve leaflet created using autologous pericardium (Fig. 3A). However, the areas of the aortic annulus where the autologous pericardium was sutured to were severely calcified, which restricted the mobility of the valve leaflet (Figs. 3B–3D). After resection of the valve leaflet, the calcification at the annulus was removed as much as possible using a Cavitron ultrasonic surgical aspirator (Integra LifeSciences Corporation, Plainsboro, NJ, USA). After valve sizing, aortic valve replacement with a 21-mm RegentTM aortic mechanical heart valve (Abbott, Santa Clara, CA, USA) in the supra- annular position using a non-everting mattress suturing technique with pledgets was performed. The operative time, cardiopulmonary bypass time, and aortic cross-clamping time were 430 min, 210 min, and 133 min, respectively. Pathological examination of the aortic valve leaflet created using autologous pericardium revealed severe calcification of the annular attachment, while the leaflets themselves showed mild vitrification on hematoxylin–eosin staining (Fig. 3E).
Fig. 3. Intraoperative findings. (A) Intraoperative image of the aortic valve made from autologous pericardium. Severe calcification is observed in the annulus. (B–D) Resected aortic valve leaflets made from autologous pericardium: RCC (B), NCC (C), and LCC (D). Severe calcification is observed where the leaflets are sutured to the annulus (arrows). (E) Pathologic findings of the resected aortic valve using hematoxylin–eosin staining. There is severe calcification of the annular attachment, while the leaflets themselves showed mild vitrification. No other inflammatory findings were observed. RCC: right coronary cusp; NCC: non-coronary cusp; LCC: left coronary cusp.

Postoperative transthoracic echocardiography revealed an LVEF of 49%. The effective orifice area index of the aortic valve was 1.168 cm2/m2. There was trivial to mild paravalvular leakage from the left coronary cusp side. However, hemolysis was not observed. The postoperative course was uneventful, and the patient was discharged. Written informed consent was obtained from the patient for the anonymous use of his data.
Discussion
In this case, reoperation was necessary for AS observed after aortic valve neocuspidization using autologous pericardium (Ozaki procedure). The surgical findings showed only mild degeneration of the autologous pericardial leaflet. However, severe calcification was observed at the sutured areas of the annulus. Severe annular calcification restricted the mobility of the autologous pericardial leaflet and led to AS. This is the first case report of a patient undergoing hemodialysis who required reoperation 6 years after aortic valve neocuspidization with the Ozaki procedure.
Traditionally, valve replacement has been the basis of treatment for valvular heart disease. Although valve replacement is a completely curative treatment, mechanical valves require lifelong anticoagulant therapy. Durability, meanwhile, is considered to be limited in bioprosthetic valves. In recent years, aortic valvuloplasty or aortic valve neocuspidization procedures have been continuously updated, and their durability has gradually improved. Because of this, the surgical treatment for valvular heart disease has shifted to valvuloplasty or valve neocuspidization more than valve replacement. Aortic valve neocuspidization using autologous pericardium was first reported by Duran et al.,3) and long-term results have also been published.4) Although results were favorable, there has been no report on hemodialysis patients. In 2011, the Ozaki procedure, which involves aortic valve neocuspidization using autologous pericardium, was reported.1) In this procedure, autologous pericardium was collected, treated with glutaraldehyde to prevent calcification, and trimmed using a special sizer to form a valve leaflet. The trimmed autologous pericardium was sutured to the aortic annulus to reconstruct the aortic valve. Because of the ease in performing aortic valve neocuspidization using the patient’s own tissues, several studies on the surgical procedure have been performed and reported.5,6) Excellent early to mid-term results have likewise been reported in 850 cases.2) According to this report, there were 15 patients who required reoperation, including 13 patients with infective endocarditis, one patient with tears in the formed cusp, and one patient who had suture breakage. There are currently no reports of reoperation due to postoperative AS or aortic regurgitation after the Ozaki procedure. Moreover, the long-term results of this procedure have not been reported yet. To the best of our knowledge, there are very few reports on reoperation after aortic valve neocuspidization with the Ozaki procedure.7,8)
Based on the surgical findings, severe calcification observed in the aortic annulus was considered to be the cause of the AS progression in this case. Pathologic findings of the aortic valve leaflet made with autologous pericardium showed only mild degeneration of the valve leaflet itself, with slightly restricted pliability. However, severe calcification had progressed from the aortic annulus to the leaflets, resulting in restricted mobility of the aortic valve and leading to AS. In this case, hemodialysis was considered to be the cause of rapid calcification progression. It has been reported that calcification is the primary mechanism of arteriosclerosis in hemodialysis patients.9,10) Ectopic calcification due to secondary hyperparathyroidism in patients with chronic renal failure is known as one of the causes.11,12) Since calcification also develops in the aortic valve, it has been reported that AS is likely to occur in patients undergoing hemodialysis.13) In this case, the parathyroid hormone level was high, and he was considered to be in a state of secondary hyperparathyroidism. In addition, the patient was on medication for hyperphosphatemia. Therefore, ectopic calcification may have been caused by secondary hyperparathyroidism due to chronic renal failure. We believe that the major reason for ectopic calcification was secondary hyperparathyroidism due to hemodialysis. The mild degeneration of the aortic valve leaflets was attributed to treatment with glutaraldehyde to prevent calcification. However, since the aortic valve annulus to which the leaflets were sutured was not treated with glutaraldehyde, the area became severely calcified after surgery, leading to AS. However, to prevent calcification, the aortic annulus cannot be treated with glutaraldehyde. Based on this case, it is necessary to prevent postoperative calcification of the aortic annulus after the Ozaki procedure in patients undergoing hemodialysis or with secondary hyperparathyroidism to further improve durability. Mid-term results in 54 hemodialysis patients who underwent the Ozaki procedure have also been reported.14) In this report, the mean follow-up period was 28.2 ± 13.7 months, with only one patient requiring reoperation due to infective endocarditis and no patient requiring reoperation due to AS. The longest follow-up period in this report was 5 years; therefore, further long-term studies are required.
It has been reported that the life expectancy of hemodialysis patients undergoing aortic valve replacement is 50% in 5 years.11) In addition, it has been reported that structural valve disease of the bioprosthetic valve for patients undergoing hemodialysis was 29% in 5 years.15) A meta-analysis on aortic valve replacement in hemodialysis patients showed that mechanical valves were associated with better long-term survival, durability, and a non-inferior risk of bleeding compared to bioprosthetic valves.16) A comparison of the outcomes of aortic valve neocuspidization and aortic valve replacement using a mechanical valve in hemodialysis patients may be a future research subject of great interest.
Conclusion
We encountered the first case of AS after aortic valve neocuspidization using autologous pericardium (Ozaki procedure). Based on the findings of the resected aortic valve leaflet made from autologous pericardium, calcification of the annulus was the cause of AS progression.
Disclosure Statement
The authors disclose no conflicts of interest or funding for this study.
References
- 1).Ozaki S, Kawase I, Yamashita H, et al. Aortic valve reconstruction using self-developed aortic valve plasty system in aortic valve disease. Interact Cardiovasc Thorac Surg 2011; 12: 550–3. [DOI] [PubMed] [Google Scholar]
- 2).Ozaki S, Kawase I, Yamashita H, et al. Midterm outcomes after aortic valve neocuspidization with glutaraldehyde-treated autologous pericardium. J Thorac Cardiovasc Surg 2018; 155: 2379–87. [DOI] [PubMed] [Google Scholar]
- 3).Duran CM, Gometza B, Kumar N, et al. Aortic valve replacement with freehand autologous pericardium. J Thorac Cardiovasc Surg 1995; 110: 511–6. [DOI] [PubMed] [Google Scholar]
- 4).Al Halees Z, Al Shahid M, Al Sanei A, et al. Up to 16 years follow-up of aortic valve reconstruction with pericardium: a stentless readily available cheap valve? Eur J Cardiothorac Surg 2005; 28: 200–5 discussion 205. [DOI] [PubMed] [Google Scholar]
- 5).Ngo HT, Nguyen HC, Nguyen TT, et al. Reconstruction of aortic valve by autologous pericardium (Ozaki’s procedure): single center experience in Vietnam. Asian Cardiovasc Thorac Ann 2021; 29: 394–9. [DOI] [PubMed] [Google Scholar]
- 6).Reuthebuch O, Koechlin L, Schurr U, et al. Aortic valve replacement using autologous pericardium: single centre experience with the Ozaki technique. Swiss Med Wkly 2018; 148: w14591. [DOI] [PubMed] [Google Scholar]
- 7).Bernhardt L, Sogomonian R, Sood A, et al. Ozaki procedure complicated by postpericardiotomy syndrome and cardiac tamponade. Future Cardiol 2021; 17: 301–7. [DOI] [PubMed] [Google Scholar]
- 8).Nguyen T, Vo A, Nguyen D, et al. Progressive left ventricular hypertrophy after Ozaki procedure: a case report. Heart Surg Forum 2020; 23: E740–2. [DOI] [PubMed] [Google Scholar]
- 9).Suzuki C, Nakamura S, Ishibashi-Ueda H, et al. Evidence for severe atherosclerotic changes in chronic hemodialysis patients: comparative autopsy study against cardiovascular disease patients without chronic kidney disease. Ther Apher Dial 2011; 15: 51–7. [DOI] [PubMed] [Google Scholar]
- 10).Moe SM, Chen NX. Pathophysiology of vascular calcification in chronic kidney disease. Circ Res 2004; 95: 560–7. [DOI] [PubMed] [Google Scholar]
- 11).Kuroda Y, Marui A, Arai Y, et al. Impact of dialysis in patients undergoing bioprosthetic aortic valve replacement. Interact Cardiovasc Thorac Surg 2021; 33: 348–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Hekimian G, Boutten A, Flamant M, et al. Progression of aortic valve stenosis is associated with bone remodelling and secondary hyperparathyroidism in elderly patients–the COFRASA study. Eur Heart J 2013; 34: 1915–22. [DOI] [PubMed] [Google Scholar]
- 13).Perkovic V, Hunt D, Griffin SV, et al. Accelerated progression of calcific aortic stenosis in dialysis patients. Nephron Clin Pract 2003; 94: c40–5. [DOI] [PubMed] [Google Scholar]
- 14).Kawase I, Ozaki S, Yamashita H, et al. Aortic valve reconstruction with autologous pericardium for dialysis patients. Interact Cardiovasc Thorac Surg 2013; 16: 738–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Kuroda Y, Marui A, Arai Y, et al. Impact of dialysis in patients undergoing bioprosthetic aortic valve replacement. Interact Cardiovasc Thorac Surg 2021; 33: 348–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16).Chi KY, Chiang MH, Kang YN, et al. Mechanical or biological heart valve for dialysis-dependent patients? a meta-analysis. J Thorac Cardiovasc Surg 2020, S0022–5223(20)31532-4. [DOI] [PubMed] [Google Scholar]



