Abstract
Purpose: This study aimed to illustrate how percutaneous balloon mitral valvuloplasty (PBMV) and mitral valve (MV) surgeries influence women of childbearing age with rheumatic mitral valve diseases (RMVDs) from two aspects, including clinical outcomes and their postoperative childbearing performances.
Methods: Female patients with RMVD who were of childbearing age and underwent MV interventions between 2007 and 2019 at Beijing Anzhen Hospital were identified. Outcomes included all-cause deaths, repeated MV interventions, and atrial fibrillation. A survey about childbearing attempts and complications during pregnancy was also performed during follow-up.
Results: A total of 379 patients were involved in this study, consisting of 226 cases of mitral valve replacements, 107 cases of mitral valve repairs (MVrs), and 46 cases of PBMVs. PBMV was associated with higher possibilities of repeated MV interventions (P <0.05). Postoperative childbearing attempts were more frequently observed among bioprosthesis, MVr, and PBMV (P <0.05). However, PBMV and MVr showed a higher incidence of cardiac complications during pregnancy as compared to prosthesis replacement (P <0.05).
Conclusions: MVr and PBMV are not recommended to young female patients for higher incidences of postoperative complications. Safe pregnancy is more likely to be present among patients with biological prosthesis.
Keywords: rheumatic mitral valve disease, women, childbearing performances, mitral valve surgery, percutaneous balloon mitral valvuloplasty
Introduction
Rheumatic mitral valve diseases (RMVD) are prevalent in developing countries.1) There are at least 1 million patients with RMVD in China.2) Approximately 70% of all patients with RMVD are women.3) In recent years, more focuses have been placed on the management of cardiac diseases during pregnancy or of pregnant women with cardiac diseases.4–7) However, studies on postoperative subsequent childbearing treatments have rarely been reported, especially in young female patients with RMVD.
Before percutaneous balloon mitral valvuloplasty (PBMV) was recommended as the primary treatment,8) mitral valve (MV) surgeries, including mitral valve replacement (MVR) or mitral valve repair (MVr), were the mainstream treatments for RMVD. Outcomes of these three treatments in overall population had been discussed before,9) while female patients, especially women of childbearing ages, were rarely considered separately in such studies.
This study aimed to illustrate how PBMV and MV surgeries influence women of childbearing age with RMVD from two aspects, including clinical outcomes and their childbearing performances after MV interventions.
Patients and Methods
Patient selection
Women of childbearing age (15 to 49 years) with RMVD who received successful MVR, MVr, or PBMV in our center from 2007 to 2019 were referred. The exclusion criteria were as follows: 1) women with prior PBMV or cardiac surgeries; 2) women with a history of reproductive surgeries, including hysterectomy or tubal ligation; and 3) women with confirmed reproductive diseases that may lead to infertility; 4) women with contaminant aortic or aortic valve surgeries and bypass surgeries; and 5) patients died in hospital after MV interventions. In addition to the fundamental clinical information, patients identified in this study underwent a detailed review of their obstetric history before MV interventions, which included pregnancy history, delivery history, and previous abortions. This study was approved by the Institutional Review Board of Anzhen Hospital (No. 202111x). Consents were obtained from all patients of this study.
Echocardiography evaluations
All patients received two-dimensional echocardiography and Doppler color flow imaging (IE33; Philips Medical Systems, Andover, MA, USA) perioperatively. The preoperative echocardiographic evaluations were performed within 1 month before mitral interventions. Wilkins score was used for the comprehensive evaluations of mitral leaflet mobility, thickness, calcification, and subvalvular areas. Clinicians could choose the candidates suitable for PBMV or open cardiac surgeries according to patients’ Wilkins scores.
Surgical procedures
All surgical procedures were performed through median sternotomy under routine extracorporeal circulations. Mitral repair techniques mainly consisted of mitral commissurotomy, leaflet treatments, chordae treatments, and ring implantations, as we described before.10) Mitral commissurotomy was performed by dissecting the fused commissures to acquire a larger MV orifice. Leaflet thinning evolved as effective techniques for restoring the flexibility and mobility of leaflets. Edge to edge and artificial chordae replacements were conducted to manage mitral regurgitation (MR). Autologous pericardial augmentation was performed for severe leaflet contracture. Chordae tendineae shortening was treated by the release of the subvalvular apparatus. Additional ring implantations were used to prevent future annulus dilations. The different surgical techniques were based on cardiac surgeons’ discretion and the anatomic features of MV diseases. According to 2017 ESC/EACTS Guidelines for the management of valvular heart disease, bioprosthetic valves were also recommended to patients in the MVR group who had a desire for pregnancy after being properly informed. The patient’s inability or wish not to take anticoagulants also resulted in implantation of a tissue valve.11,12) Details on the contaminant tricuspid valve repair and Cox-Maze procedures are described in our previous study.13)
PBMV procedures
PBMV procedures were performed by clinician teams composed of interventional cardiologists, cardiac surgeons, and echocardiologists. All procedures were performed through a femoral approach under general anesthesia without tracheal intubation in hybrid operation rooms. A successful PBMV procedure was defined as an increase in the postoperative mitral valve area (MVA) to more than 1.5 cm2 without the existence of severe MR.
Anticoagulant and antiplatelet therapies
For patients with mechanical prosthesis, whole lifelong vitamin K antagonist therapy was needed. For patients with biological prosthesis and those who underwent MVr, vitamin K antagonist therapy was required for 3 months after surgery and then any oral anticoagulant therapies were eliminated. At least 1-year antiplatelet therapy was needed after the PBMV procedure. Once patients were diagnosed with atrial fibrillation (AF) during follow-up, vitamin K antagonist therapy was required. When patients with mechanical prostheses or AF were confirmed to be pregnant received, heparin replacement therapy was recommended during their pregnant periods.
Cardiac outcomes during follow-up
All-cause death, repeated MV interventions, and AF were evaluated as cardiac outcomes in this study. Repeated MV interventions were performed when patients suffered from restenosis (MVA <1.5 cm2) or aggravation of MR (>moderate). All patients experienced visits including echo evaluations scheduled at 1, 3, and 12 months after the procedure and every 1 year thereafter. Indicators for repeated MV interventions were mainly defined using the 2017 ESC/EACTS guidelines on valvular heart diseases, in which echocardiography results and clinical symptoms were both involved.11,14,15)
Childbearing outcomes during follow-up
A detailed survey on patients’ childbearing performance after MV interventions was carried out at the same time, consisting of childbearing attempts (successful or not), live-birth rates, maternal complications, and fetal complications during each pregnancy. Adverse cardiac events, the use of cardiac medications, and anticoagulant strategies during pregnancy were also recorded.
Statistics
Baseline variables were presented as medians (interquartile ranges) for continuous variables and as percentages for categorical variables. The chi-squared test and Fisher’s test were used to test unadjusted associations between treatment variables and outcomes. One-way ANOVA and the Kruskal–Wallis H test were used to test the differences in continuous variables among the three groups before adjustments.
Propensity scores (PSs), which were assumed to be as the probability that an individual with pretreatment characteristics X received treatment t, were acquired through the generalized boosted model. After the PSs were acquired, multiple treatment comparisons were performed through inverse probability of treatment weighting (IPTW) for causal effects.16,17) Important baseline characteristics were considered for the construction of IPTW, which was composed of age, New York Heart Association (NYHA) class, AF history, routine echocardiographic features, and anatomic characteristics in terms of MV assessed by echocardiography. Details of the variables in IPTW are shown in Supplementary Table 1 (all supplementary files are available online). The average treatment effect on the population was used to summarize the individual effects across populations, which answered the question of how, on average, the outcome of interest would change if everyone in the population of interest had been assigned to a particular treatment relative to if they had all received another single treatment. The absolute standardized mean difference was used to measure the difference between two univariate distributions of a single pretreatment variable. A value ≥0.20 was considered an indicator of imbalance.18) Doubly robust estimates were used if imbalance was still present after adjusting the baseline variables, which was also defined as an augmented inverse propensity weighted (AIPW).19) In this study, AIPW was composed of IPTW and confounding factors adjustments. Selection of confounding factors were mainly based on stepwise regression (Supplementary Table 2). R version 3.5.2 was used for all statistical analyses, and the Twang R package was used.
Results
Baseline characteristics
Two cases of in-hospital death were observed among MVR (0.9%): one case was observed among MVr (0.9%) and no in-hospital deaths were observed among PBMV in hospital. Excluding these in-hospital deaths, a total of 379 patients were involved in this study, consisting of 226 patients who underwent MVR, 107 patients who underwent MVr, and 46 patients who underwent PBMV. In the MVR group, mechanical prostheses were used in 155 patients (68.5%) and biological prostheses were used in 71 patients (31.4%). Concomitant tricuspid valvuloplasty was performed in 42 (18.6%) MVR and 20 (18.7%) MVr patients. Cox-Maze procedures were performed in 46 (20.35%) patients in the MVR group and 20 (18.7%) in the MVr group. As shown in Table 1, patients who received PBMV were younger than those who underwent open heart surgeries. Besides other clinical features, patients who underwent PBMV had smaller left atrium and ventricle when they were compared to those who underwent open heart surgeries (P <0.05). When compared to patients in open heart surgery groups, either over moderate MR or tricuspid regurgitation (TR) was more rarely observed among patients in the PBMV group (P <0.05). Differences in terms of Wilkin score among these four groups were not significant (Table 1).
Table 1. Baseline characteristics before mitral interventions.
| Characteristics | Mechanical prosthesis (n = 155) | Biological prosthesis (n = 71) | MVr (n = 107) | PBMV (n = 46) | Overall P value |
|---|---|---|---|---|---|
| Age (years), mean (SD) | 40.58 (5.17) | 39.69 (8.58) | 40.13 (7.05) | 36.3 (6.70)*,†,¶ | 0.002 |
| With AF history, n (%) | 29 (18.89) | 17 (23.94) | 20 (18.69) | 7 (15.2) | 0.682 |
| NYHA level, n (%) | |||||
| 1 | 2 (1.29) | 0 (0) | 0 (0) | 0 (0) | 0.768 |
| 2 | 145 (93.54) | 71 (100)* | 82 (76.64)*,† | 43 (93.45)*,† | <0.001 |
| 3 | 8 (5.16) | 0 (0) | 25 (23.36)* | 3 (6.52)*,† | <0.001 |
| BMI, mean (SD) | 22.12 (3.34) | 21.62 (3.16) | 21.57 (3.17) | 22.28 (3.52) | 0.673 |
| BSA, mean (SD) | 1.66 (0.13) | 1.68 (0.14) | 1.69 (0.12)* | 1.52 (0.19) | 0.401 |
| Left atrium thrombus, n (%) | 3 (1.94) | 0 (0) | 9 (8.41)*,† | 0 (0)¶ | 0.006 |
| CCR (mL/min), mean (SD) | 93.33 (12.31) | 92.20 (13.07) | 94.11 (12.86) | 92.87 (17.15) | 0.816 |
| Tobacco use, n (%) | 2 (1.29) | 0 (0) | 2 (1.87) | 1 (2.17) | 0.681 |
| Alcohol use, n (%) | 2 (1.29) | 0 (0) | 2 (1.87) | 0 (0) | 0.893 |
| Anticoagulated therapy, n (%) | 17 (10.97) | 10 (14.08) | 12 (11.21) | 4 (7.41)*,† | 0.853 |
| EuroSCORE II predicted mortality, median (IQR) | 0.0115 (0.0087) | 0.1002 (0.0056) | 0.0112 (0.0101) | 0.0151 (0.0141) | 0.133 |
| LAD (mm), mean (SD) | 58.29 (10.82) | 52.23 (7.95)* | 48.87 (8.22)* | 44.94 (4.98)*,†,¶ | <0.001 |
| LVEDD (mm), mean (SD) | 46.26 (6.25) | 47.18 (5.09) | 47.59 (6.78) | 44.62 (3.67)†,¶ | 0.040 |
| LVESD (mm), mean (SD) | 30.76 (5.16) | 31.27 (4.16) | 30.29 (4.30) | 28.72 (4.59)*,† | 0.022 |
| LVEF (mm), mean (SD) | 61.46 (7.03) | 61.72 (6.48) | 61.68 (6.44) | 63.17 (5.36) | 0.498 |
| MVA (mm), median (IQR) | 0.90 (0.40) | 1.2 (0.58)* | 1.10 (0.50)* | 0.90 (0.30)†,¶ | <0.001 |
| MR grade, n (%) | |||||
| Trivial | 28 (18.06) | 19 (26.76) | 18 (16.82) | 18 (39.13) | 0.832 |
| Mild and moderate | 69 (44.52) | 21 (29.58)* | 43 (40.19) | 28 (60.87)†,¶ | 0.008 |
| Moderate | 30 (19.35) | 24 (33.80)* | 21 (19.63)† | 0 (0)*,†,¶ | <0.001 |
| Severe | 32 (18.06) | 7 (9.86)* | 25 (23.36)† | 0 (0)*,†,¶ | <0.001 |
| TR grade, n (%) | |||||
| Trivial | 10 (6.45) | 9 (12.68) | 5 (4.67) | 22 (47.83)*,†,¶ | <0.001 |
| Mild and moderate | 116 (74.84) | 51 (71.83) | 82 (76.64) | 21 (45.65)*,†,¶ | <0.001 |
| Moderate | 9 (5.81) | 6 (8.45) | 12 (11.21) | 3 (6.52) | 0.452 |
| Severe | 20 (12.90) | 7 (9.86) | 8 (7.48) | 0 (0)* | 0.031 |
| Wilkins Score, mean (SD) | 8.35 (1.12) | 8.32 (1.13) | 8.22 (1.24) | 8.26 (1.22) | 0.160 |
Values are presented as n (%), mean (SD), or median (IQR). P-values may not be interpreted as confirmatory but rather descriptive.
*P <0.05 vs. mechanical prosthesis;
†P <0.05 vs. biological prosthesis, and
¶P <0.05 vs. MVr.
SD: standard deviation; IQR: interquartile range; MVr: mitral valve repair; PBMV: percutaneous balloon mitral valvuloplasty; AF: atrial fibrillation; BMI: body mass index; BSA: body surface area; CCR: creatinine clearance rate; LAD: left atrial diameter; NYHA: New York Heart Association; LVEDD: left ventricular end diastolic diameter; LVESD: left ventricular end systolic diameter; LVEF: left ventricular ejection fraction; MVA: mitral valve area; MR: mitral regurgitation; TR: tricuspid regurgitation; EuroSCORE: European System for Cardiac Operative Risk Evaluation
Cardiac outcomes after MV interventions
After a mean follow-up time of 5.4 years, there were 7 (2.1%) and 1 (2.2%) death in the patients who underwent MV surgeries and PBMV, respectively. No cases were lost to follow-up in this study. Among patients who underwent MV surgeries, six cases of death were observed in patients with mechanical prosthesis, one case of death was observed in the MVr group, and no deaths were observed in patients with biologic prosthesis.
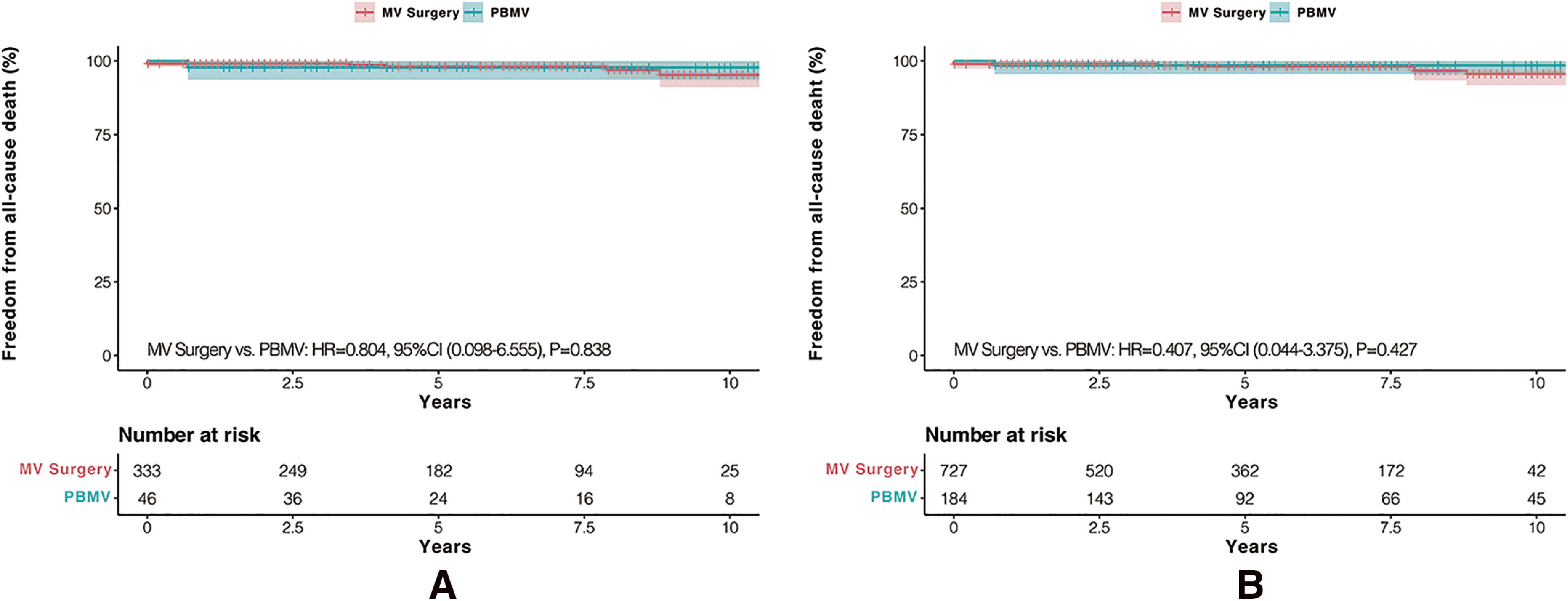
Among the deaths in the MVR group, two of six patients died due to cerebral hemorrhages, two of six patients died due to mechanical valve thrombosis, and the remaining two patients died due to malignant tumors and accidents. Deaths in MVr and PBMV groups were both due to accidents. No significant differences in terms of mortality were observed between MV surgeries and PBMV before or after IPTW adjustments (Figs. 1A and 1B).
Fig. 1. Kaplan–Meier analysis in terms of all-cause death: (A) freedom from all-cause death before IPTW adjustment and (B) freedom from all-cause death after IPTW adjustment. IPTW: inverse probability of treatment weighting; MV: mitral valve; PBMV: percutaneous balloon mitral valvuloplasty; HR: hazard ratio; CI: confidence interval.
After MV interventions, vitamin K antagonist therapy was present in all patients with mechanical prosthesis. Among patients who underwent biological prosthesis implantation and MVr, reuses of warfarin were observed in 25 of 178 patients for AF during follow-up. A total of 12 cases of warfarin uses were observed in the PBMV group for the same reason.
A total of 55 (14.5%) patients were diagnosed with AF during follow-up. Before adjustment, patients who underwent surgical repair or PBMV showed a higher incidence of AF than those with mechanical prosthesis (repair vs. mechanical: P = 0.019; PBMV vs. mechanical: P = 0.003). However, such differences were not present when they were compared to those with biological prosthesis (repair vs. biological: P = 0.266; PBMV vs. biological: P = 0.091). Among patients who underwent MVR, differences were also not significant between two different prostheses (biological vs. mechanical: P = 0.382) (Fig. 2A).
Fig. 2. Kaplan–Meier analysis in terms of main outcomes: (A) freedom from AF before IPTW adjustment, (B) freedom from AF after IPTW adjustment, (C) freedom from repeated MV interventions before IPTW adjustment, and (D) freedom from repeated MV interventions after IPTW adjustment. AF: atrial fibrillation; IPTW: inverse probability of treatment weighting; MV: mitral valve; PBMV: percutaneous balloon mitral valvuloplasty; HR: hazard ratio; CI: confidence interval.
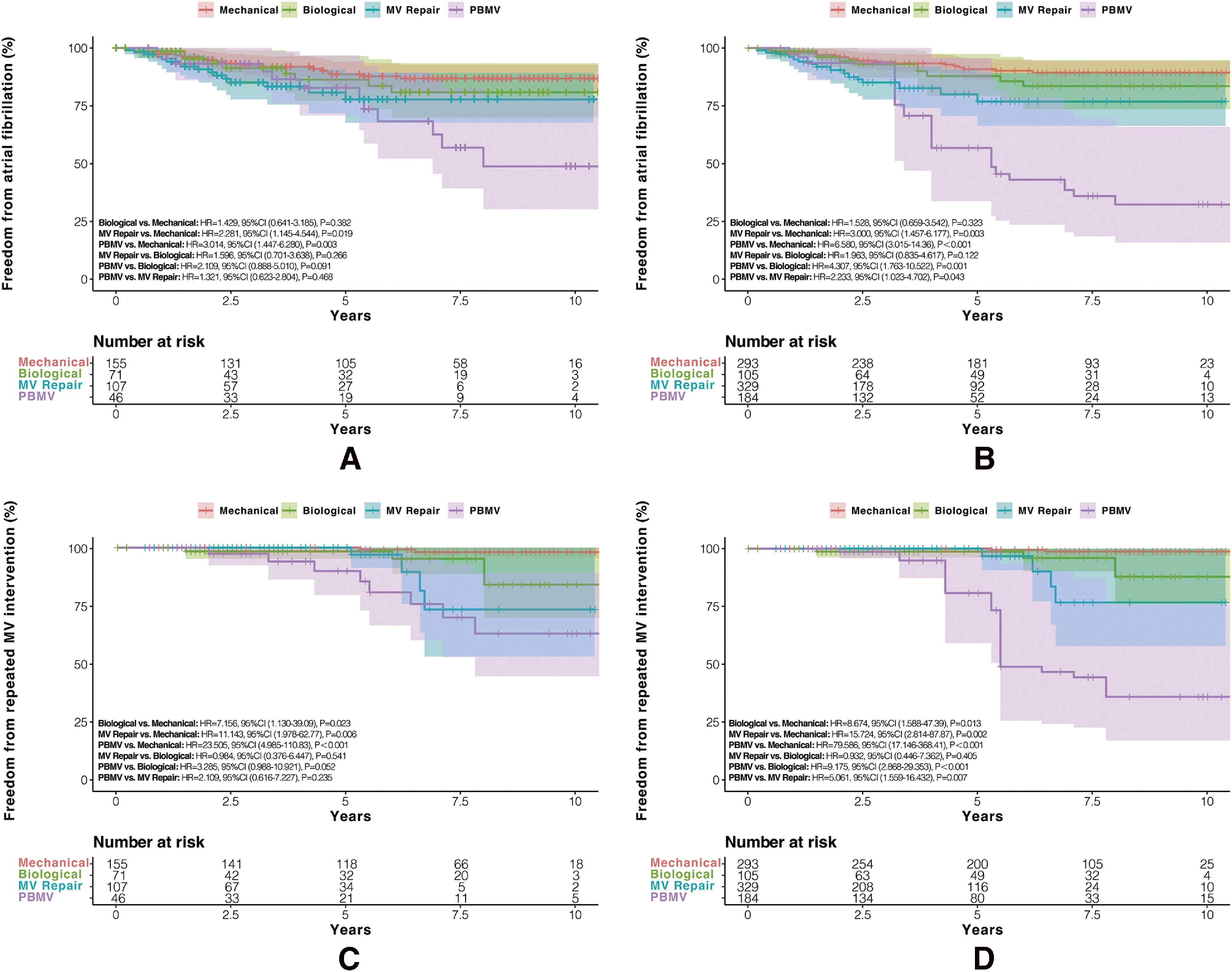
However, after IPTW adjustment, patients in the PBMV group showed the highest incidence of AF among overall populations (PBMV vs. mechanical: P <0.001, PBMV vs. biological: P = 0.001, PBMV vs. repair: P = 0.043). When compared to patients who underwent mechanical prosthesis implantation, patients in the MVr group were also more likely to suffer from AF during follow-up (repair vs. mechanical: P = 0.003). While no significant differences were observed between MVr and biological prosthesis groups, as well as between biological and mechanical groups (repair vs. biological: P = 0.122, biological vs. mechanical: P = 0.323) (Fig. 2B).
A total of 18 cases of repeated MV interventions were observed during follow-up, while no repeated MV interventions were observed during pregnancy. Before adjustment, patients who underwent surgical repair or PBMV were associated with a higher risk of repeat surgeries when compared to those with mechanical prostheses (PBMV vs. mechanical: P <0.001, repair vs. mechanical: P = 0.006). Only two cases of repeated MV surgeries were observed among patients with mechanical prosthesis during follow-up, which were both due to prosthetic valve endocarditis. However, no significant differences were observed when they were compared to the patients with biological prosthesis (PBMV vs. biological: P = 0.541, repair vs. biological: P = 0.052). Even among patients who similarly underwent valve replacements, patients with biological prosthesis still had a higher incidence of repeat surgeries than those with mechanical prosthesis (P = 0.023) (Fig. 2C).
After IPTW adjustment, patients with mechanical prosthesis were still significantly more likely to be free from repeated surgeries after the initial MV interventions. Among the other three groups, patients in the PBMV group seemed to be associated with the highest risk of repeated surgeries (PBMV vs. biological: P <0.001, PBMV vs. repair: P = 0.007), but no significant differences were observed between biological prosthesis and surgical repair (repair vs. biological: P = 0.405) (Fig. 2D).
In addition to IPTW adjustments, AIPW was used to test the reliability of the results after IPTW, and the significant differences remained unchanged in the AIPW model (Supplementary Table 3).
Echocardiography outcomes after MV interventions
The shrinkages of left atrium were observed among all groups right after MV interventions, though the left atrial diameter (LAD) did not show significant differences when compared to the baseline data. However, in the latest echo evaluations, patients who underwent MVRs showed more ferocious reduction of LAD. When compared to the in-hospital results, the latest mean LADs of patients with mechanical and biological prostheses were reduced from 56.62 ± 11.71 mm to 47.81 ± 10.42 mm and from 50.65 ± 8.26 mm to 45.22 ± 7.53 mm, respectively. Conversely, the shrinkages of the left atrium were slighter in patients who underwent repair procedures and PBMV (MVr: from 47.24 ± 7.21 mm to 45.66 ± 7.79 mm; PBMV: from 43.52 ± 5.23 mm to 42.27 ± 6.15 mm). After MV interventions, all patients showed significant reductions of MV mean gradient, and the mean MVA also got effective increasements as opposed to the baseline data (P <0.05). However, during follow-up, the MV mean gradient of patients who underwent PBMV (5.19 ± 0.76 mmHg) was significantly higher than that of patients with mechanical prosthesis (3.91 ± 0.72 mmHg) and those who underwent repair procedures (3.81 ± 1.08 mmHg). Similarly, the mean MVA of patients in the PBMV group during follow-up was 1.61 ± 0.45 cm2, which was much smaller than those who underwent open surgeries.
In the latest echo results, when compared to those with MV prosthesis (0.76%), cases with over moderate MR were more frequently seen among patients who underwent repair procedures and PBMV, which accounted for 19.81% and 32.61%, respectively. Such patients also showed higher percentages of over moderate TR during follow-up (MVr: 13.08%, PBMV: 32.61%), which were significantly higher than that of patients with MV prosthesis (6.11%). All details on echocardiography results are shown in Supplementary Table 4.
Childbearing performances after MV interventions
Before MV interventions, a total of 303 patients had pregnancy histories, which accounted for 79.9% of the overall population. Women with pregnant or delivery histories were more rarely observed in the PBMV group when they were compared to those who underwent surgical MV interventions, especially when compared to those who underwent mechanical prosthesis implantations (P <0.05). Similarly, women with biological prostheses or women who underwent PBMV showed a higher proportion of positive childbearing intentions when compared to those who received other surgical interventions (P <0.05) (Table 2).
Table 2. Childbearing performances before and after mitral interventions.
| Mechanical prostheses (n = 155) | Biological prostheses (n = 71) | MVr (n = 107) | PBMV (n = 46) | Overall P value | |
|---|---|---|---|---|---|
| Before MV interventions | |||||
| With pregnant history, no. (%) | 131 (84.52) | 55 (77.46) | 84 (78.50) | 33 (71.74)* | 0.225 |
| With delivery history, no. (%) | 122 (78.71) | 48 (67.61) | 78 (72.90) | 28 (60.86)* | 0.071 |
| Previous abortions, no. (%) | 15 (9.68) | 7 (9.86) | 11 (10.28) | 9 (19.57) | 0.278 |
| After MV interventions | |||||
| Childbearing attempts, n (%) | 7 (4.52) | 20 (28.17)* | 13 (12.15)*,† | 13 (28.26)*,¶ | <0.001 |
| Women positive about childbearing, n (%) | 9 (5.81) | 24 (33.80)* | 21 (19.63)*,† | 14 (30.43)*,¶ | <0.001 |
Values are presented as n (%). P-values may not be interpreted as confirmatory but rather descriptive.
*P <0.05 vs. mechanical prostheses,
†P <0.05 vs. biological prostheses, and
¶P <0.05 vs. MVr.
MV: mitral valve; MVr: mitral valve repair; PBMV: percutaneous balloon mitral valvuloplasty
After MV interventions, all patients experienced complete in-hospital rehabilitations and cardiopulmonary endurance assessment. No patients with NYHA class III or IV were observed. A total of 53 women attempted pregnancy during follow-up, accounting for 11.4% of overall population. In patients with mechanical prosthesis, only seven cases of childbearing attempts (4.5%) were observed, which was significantly rarer than those who underwent other treatment methods (P <0.05). When compared to other treatments, patients who underwent biological prosthesis implantations and PBMV before were more likely to attempt to be pregnant (P <0.05). However, differences in terms of childbearing between these two treatment methods were not significant (Table 2).
Once confirmed to be pregnant, patients who were taking vitamin K antagonist therapy all received heparin replacement therapy. No anticoagulant therapies were observed among patients who underwent other MV treatments. Patients who had actual childbearing attempts showed similar maternal ages (P = 0.913). Among these pregnant women, a total of 45 (84.9%) successful deliveries were observed. Higher incidences of cardiac medication (uses of diuretic and beta-blockers) were observed in women who underwent MVr and PBMV, when they were compared to those who received MVR (P <0.001). Women in the MVr group and PBMV group were also more likely to suffer from acute heart failure during pregnancy than those who underwent MVR (P <0.001). When compared to patients with biological prosthesis, a higher incidence of arrhythmia (including AF, atrial flutter, and supraventricular tachycardia) during pregnancy was observed among patients in the MVr group. No differences in terms of fetal complications were observed among patients excluding intrauterine growth retardation (IGR). The incidences of IGR were significantly higher than in those who underwent biological prosthesis implantations and PBMV (Table 3).
Table 3. Details during childbearing after mitral interventions.
| Characteristics | Mechanical prostheses (n = 7) | Biological prostheses (n = 20) | MVr (n = 13) | PBMV (n = 13) | Overall P value |
|---|---|---|---|---|---|
| Maternal age (years), median (IQR) | 31 (3) | 30 (5) | 31 (3) | 30 (3) | 0.913 |
| Live birth, n (%) | 4 (57.14) | 19 (95) | 11 (84.62) | 11 (84.62) | 0.573 |
| Cardiac medication, n (%) | |||||
| Digoxin | 0 (0) | 0 (0) | 2 (15.38) | 1 (7.69) | 0.200 |
| Diuretic | 0 (0) | 1 (5) | 8 (61.54)*,† | 10 (76.92)*,† | <0.001 |
| Beta-blockers | 0 (0) | 1 (5) | 8 (61.54)*,† | 9 (69.23)*,† | <0.001 |
| Anticoagulants | 7 (100) | 0 (0)* | 1 (7.69)* | 0 (0)* | <0.001 |
| Anti-hypertensive | 0 (0) | 0 (0) | 2 (15.38) | 1 (7.69) | 0.200 |
| Causes of abortion, n (%) | |||||
| Artificial | 1 (14.29) | 0 (0) | 2 (15.38) | 1 (7.69) | 0.249 |
| Spontaneous | 2 (28.57) | 1 (5) | 0 (0)* | 1 (7.69) | 0.140 |
| Obstetric complications, n (%) | |||||
| PROM | 0 (0) | 3 (15) | 3 (23.08) | 2 (15.38) | 0.700 |
| Placental abruption | 0 (0) | 1 (5) | 0 (0) | 2 (15.38) | 0.566 |
| Threatened abortion | 2 (28.57) | 2 (10) | 2 (15.38) | 1 (7.69) | 0.522 |
| Acute heart failure | 0 (0) | 1 (5) | 4 (30.77)*,† | 6 (46.15)*,† | 0.011 |
| Arrhythmia | 1 (14.29) | 0 (0) | 3 (23.8)† | 1 (7.69) | 0.100 |
| Fetal complications, n (%) | |||||
| Premature delivery | 3 (42.86) | 6 (30) | 6 (46.15) | 7 (53.85) | 0.448 |
| Low birth weight | 3 (42.86) | 6 (30) | 4 (30.77) | 3 (23.08) | 0.846 |
| IGR | 2 (28.57) | 0 (0)* | 1 (7.69) | 0 (0)* | 0.025 |
Values are presented as n (%) or median (IQR). P-values may not be interpreted as confirmatory but rather descriptive. *P <0.05 vs. mechanical prostheses and †P <0.05 vs. biological prostheses. IQR: interquartile range; MVr: mitral valve repair; PBMV: percutaneous balloon mitral valvuloplasty; PROM: premature rupture of membranes; IGR: intrauterine growth retardation.
Discussion
To the best of our knowledge, our study is the first study to investigate the different impacts that MV surgeries and PBMV may have on young female patients with RMVD including their cardiac outcomes and postoperative childbearing performances. Our main findings suggested that a higher risk of repeated MV interventions and AF was present among patients who underwent MV surgical repair and PBMV. Women could get more encouragement of childbearing attempts from biological prosthesis and PBMV. However, when compared to patients with valve prosthesis, patients who underwent PBMV and MVr may be at a higher risk of cardiac insufficiency during pregnancy.
PBMV versus open MV surgeries
PBMV has long been verified to be the recommended treatment for rheumatic mitral stenosis (RMS).1,20) Previous studies suggested that PBMV may yield a higher incidence of reoperations, while no significant differences were found in survival prognosis between PBMV and surgical procedures9,21); these findings are in accordance with the results of our study. However, as Song et al. reported in their study,9) PBMV was associated with a higher rate of redo PBMV or open heart surgery during follow-up, which was in accordance with the main investigations in this study. According to the baseline clinical features, though over moderate MR or TR was more frequently seen among patients who chose open MV surgeries, the fact proved that a complete replacement was associated with a better prognosis. Moreover, the contaminant surgical techniques to manage TR and AF, which frequently accompany MS, are important prognostic factors during long-term follow-up.9)
MVr versus MVR
MVr may be better than MVR when referring to treatments for degenerative mitral diseases according to current guidelines.1) It was not clear whether MVr or MVR was better for patients with RMVD. Fu et al., who were from the same center as ours, reported that the difference between MVr and MVR regarding repeat operations had no statistical significance among the entire population.10) However, it has to be figured out that the population in this study is not the same as that in Fu et al.’ s article. In this study, even when compared to those with biological prosthesis, young female patients in the MVr group showed a disappointing outcome in terms of repeated interventions, which was consistent with the results from most studies.22–24)
Echocardiography outcomes
According to the echo evaluations at different times, it could be concluded that all MV interventions could greatly mitigate mitral stenosis, which is marked by the shrinkages of left atrium, the reduction of MV mean gradient, and the enlargement of MVA right after interventions. While interestingly, the PBMV group was the only one where MV mean gradient elevated significantly during follow-up, and the mean MVA of this group was also the smallest. With above evidence, it could be suggested that restenosis of MV was more likely to happen among patients who experienced PBMV, and this finding was in accordance with Song et al.’ s study.9) Additionally, as the disease still progressed out of control after PBMV, the leaflet, chordae, and papillary muscle got damaged successively or simultaneously, contributing to the aggravation of MR. Given this perspective, the persistently high left atrial pressure and the progressive destruction of MV device resulted in the poor performance of PBMV, including sinus rhythm restoring and avoidance of reoperations. Similarly, the same destructions existed persistently even they had gotten repaired already, which may explain why a substantial portion of such patients suffered from MR again and why the durability of repair was not nearly so as MR. More studies on MVr in the young RMS patients are needed in the future.
Childbearing performances after MV interventions
It has been confirmed that women with cardiac diseases could have better reproductive outcomes because of the mature management of cardiac diseases during pregnancy.6,25) The subsequent childbearing performance of women after cardiac surgeries was first noticed by Nunley et al.,26) who suggested that cardiac surgeries requiring cardiopulmonary bypass were more likely to discourage women from making childbearing decisions.
Current guidelines and studies have suggested that PBMV is a safe and effective treatment for RMVD, especially for women during pregnancy or with pregnancy plans.1,4,7,11,27,28) Unfortunately, few focuses were put on the subsequent childbearing after different MV interventions. Considering that anticoagulant therapy during pregnancy may lead to a series of adverse fetal–maternal outcomes,29,30) treatments including biological prosthesis, surgical repair, and PBMV were recommended to female patients. Most clinicians are also justified in feeling that avoiding anticoagulant therapy is the most important thing in the management of pregnant women. In this study, more childbearing attempts were indeed observed in PBMV and MVr groups than in those with mechanical prosthesis. However, the high incidences of cardiac medication uses and acute heart failure during these patients’ pregnancies cannot be ignored. Ayad et al. reported in their study that the complications during pregnancy were strongly associated with echo valve area.29) The restenosis or aggravation of MR is commonly observed in either surgical repair or BPMV. Due to volume overload during pregnancy, these problems may get aggravated in advance and then trigger the occurrence of cardiac complications. By contrast, patients with biologic prosthesis had good performances either in childbearing attempts or during pregnancy, which indicated that a thorough treatment for MV diseases and avoidance of anticoagulant are equally important for pregnant women.
Our study was mainly limited by the small sample size, which made it difficult to determine reliable rules among observed events through statistics. Additionally, considering the presence of subjective and other numerous confounding factors, only a cross-sectional study on patients’ childbearing performances was performed.
Conclusions
Though women with childbearing plans could get more encouragements from biological prosthesis and valve repair techniques, valve repair techniques including MVr or PBMV are not recommended for higher incidences of repeated MV interventions and potential cardiac complications during pregnancy. Safe and stable pregnancy is more likely to be present among patients with biological prosthesis.
Data Availability
All data included in this study are available upon request by contact with the corresponding author.
Statement of Ethics
The institutional review board at the Beijing Anzhen Hospital, Capital Medical University, has approved the study. All patients have given their written informed consent.
Funding Statement
This study was supported by the National Natural Science Foundation of China (Grant No. 81770320).
Disclosure Statement
None.
Supplementary Materials
References
- 1).Otto CM, Nishimura RA, Bonow RO, et al. ACC/AHA guideline for the management of patients with valvular heart disease: report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. J Am Coll Cardiol 2021; 77: e25–197. [DOI] [PubMed] [Google Scholar]
- 2).Zhimin W, Yubao Z, Lei S, et al. Prevalence of chronic rheumatic heart disease in Chinese adults. Int J Cardiol 2006; 107: 356–9. [DOI] [PubMed] [Google Scholar]
- 3).Mensah GA. The burden of valvular heart disease. In: Valvular Heart Disease: A Companion to Braunwald’s Heart Disease.; 2009.
- 4).van Hagen IM, Thorne SA, Taha N, et al. Pregnancy outcomes in women with rheumatic mitral valve disease: results from the registry of pregnancy and cardiac disease. Circulation 2018; 137: 806–16. [DOI] [PubMed] [Google Scholar]
- 5).Regitz-Zagrosek V, Roos-Hesselink JW, Bauersachs J, et al. 2018 ESC guidelines for the management of cardiovascular diseases during pregnancy. 2019; 77: 245–326. [DOI] [PubMed] [Google Scholar]
- 6).Ruys TPE, Cornette J, Roos-Hesselink JW. Pregnancy and delivery in cardiac disease. J Cardiol 2013; 61: 107–12. [DOI] [PubMed] [Google Scholar]
- 7).de Souza JAM, Martinez EE, Jr., Ambrose JA, et al. Percutaneous balloon mitral valvuloplasty in comparison with open mitral valve commissurotomy for mitral stenosis during pregnancy. J Am Coll Cardiol 2001; 37: 900–3. [DOI] [PubMed] [Google Scholar]
- 8).Nobuyoshi M, Arita T, Shirai S, et al. Percutaneous balloon mitral valvuloplasty: a review. Circulation 2009; 119: e211–9. [DOI] [PubMed] [Google Scholar]
- 9).Song JK, Kim MJ, Yun SC, et al. Long-term outcomes of percutaneous mitral balloon valvuloplasty versus open cardiac surgery. J Thorac Cardiovasc Surg 2010; 139: 103–10. [DOI] [PubMed] [Google Scholar]
- 10).Fu J, Li Y, Zhang H, et al. Outcomes of mitral valve repair compared with replacement for patients with rheumatic heart disease. J Thorac Cardiovasc Surg 2021; 162: 72–82.E7. [DOI] [PubMed] [Google Scholar]
- 11).Baumgartner H, Falk V, Bax JJ, et al. 2017 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J 2017; 38: 2739–91. [DOI] [PubMed] [Google Scholar]
- 12).Hirsch R. Should we offer a bioprosthetic valve to women of child-bearing age who need valve replacement? Interv Cardiol 2014; 6: 425–31. [Google Scholar]
- 13).Wang J, Li S, Ye Q, et al. Catheter ablation or surgical therapy in moderate-severe tricuspid regurgitation caused by long-standing persistent atrial fibrillation. Propensity score analysis. J Cardiothorac Surg 2020; 15: 277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).Nunes MCP, Levine RA, Braulio R, et al. Mitral regurgitation after percutaneous mitral valvuloplasty: insights into mechanisms and impact on clinical outcomes. JACC Cardiovasc Imaging 2020; 13: 2513–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Zoghbi WA, Adams D, Bonow RO, et al. Recommendations for noninvasive evaluation of native valvular regurgitation: a report from the American Society of Echocardiography developed in collaboration with the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr 2017; 30: 303–71. [DOI] [PubMed] [Google Scholar]
- 16).Burgette L, Griffin B, McCaffrey D. Propensity scores for repeated treatments: a tutorial for the iptw function in the TWANG Package. Propensity Scores Repeated Treat A Tutor iptw Funct TWANG Packag. 2017; 015697: 1–22. [Google Scholar]
- 17).Keller B, Tipton E. Propensity score analysis in R: a software review. J Educ Behav Stat 2016; 41: 326–48. [Google Scholar]
- 18).Cohen J. Statistical Power Analysis for the Behavioural Science (2nd Edition); 1988.
- 19).Moons P. Propensity weighting: how to minimise comparative bias in non-randomised studies? Eur J Cardiovasc Nurs 2020; 19: 83–8. [DOI] [PubMed] [Google Scholar]
- 20).Nishimura RA, Vahanian A, Eleid MF, et al. Mitral valve disease – current management and future challenges. Lancet 2016; 387: 1324–34. [DOI] [PubMed] [Google Scholar]
- 21).Ambari AM, Setianto B, Santoso A, et al. Survival analysis of patients with rheumatic MS after PBMV compared with MVS in a low-to-middle-income country. Neth Heart J 2019; 27: 559–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22).Wijesurendra RS, Casadei B. Mechanisms of atrial fibrillation. Heart 2019; 105: 1860–7. [DOI] [PubMed] [Google Scholar]
- 23).Russell EA, Walsh WF, Reid CM, et al. Outcomes after mitral valve surgery for rheumatic heart disease. Heart Asia 2017; 9: e010916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24).Chen SW, Chen CY, Chien-Chia Wu V, et al. Mitral valve repair versus replacement in patients with rheumatic heart disease. J Thorac Cardiovasc Surg 2022; 164: 57–67.E11. [DOI] [PubMed] [Google Scholar]
- 25).Regitz-Zagrosek V, Roos-Hesselink JW, Bauersachs J, et al. 2018 ESC guidelines for the management of cardiovascular diseases during pregnancy. Eur Heart J 2018; 39: 3165–241. [DOI] [PubMed] [Google Scholar]
- 26).Nunley WC, Jr., Kolp LA, Dabinett LN, et al. Subsequent fertility in women who undergo cardiac surgery. Am J Obstet Gynecol 1989; 161: 573–6. [DOI] [PubMed] [Google Scholar]
- 27).Sreerama D, Surana M, Moolchandani K, et al. Percutaneous balloon mitral valvotomy during pregnancy: a systematic review and meta-analysis. Acta Obstet Gynecol Scand 2021; 100: 666–75. [DOI] [PubMed] [Google Scholar]
- 28).Sharma JB, Yadav V, Mishra S, et al. Comparative study on maternal and fetal outcome in pregnant women with rheumatic heart disease and severe mitral stenosis undergoing percutaneous balloon mitral valvotomy before or during pregnancy. Indian Heart J 2018; 70: 685–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29).Ayad SW, Hassanein MM, Mohamed EA, et al. Maternal and fetal outcomes in pregnant women with a prosthetic mechanical heart valve. Clin Med Insights Cardiol 2016; 10: CMC.S36740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30).Verhamme P, Herregods MC, Van Dewerf F. Anticoagulation of pregnant women with mechanical heart valves: protecting mother or child? Eur Heart J 2017; 38: 1517–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data included in this study are available upon request by contact with the corresponding author.


