Abstract
Background and aims
Hepatitis B virus (HBV) is a common cause of hepatocellular carcinoma (HCC) in China, and this study aimed to identify high-risk factors for overall survival and develop a nomogram prediction model.
Methods
In the present retrospective cohort study, patients with HBV-associated HCC diagnosed from January 2009 to December 2018 were enrolled. Their clinical characteristics and time-to-event information were retrieved from electronic medical records. The zero time was the date of HCC diagnosis, and the endpoint was death or liver transplantation. Multivariable COX proportional hazard regression was used to screen independent risk factors for overall survival; then a nomogram model was developed to predict the survival probability of HCC patients.
Results
A total of 1723 patients were enrolled, with 82.7 % male and a median age of 54.0 years. During a median follow-up time of 41.3 months, 672 cases (39.0 %) died. Age ≥60 years (HR = 1.209), Male (HR = 1.293), ALB <35 g/L (HR = 1.491), AST ≥80 U/L (HR = 1.818); AFP 20–400 ng/mL (HR = 2.284), AFP ≥400 ng/mL (HR = 2.746); LSM 9–22 kPa (HR = 2.266), LSM ≥22 kPa (HR = 4.326); BCLC stage B/C (HR = 4.079) and BCLC stage D (HR = 16.830) were the independent high-risk factors associated with HCC survival. A prognostic nomogram with a consistency index of 0.842 (95 % CI: 0.827–0.858) was developed. The calibration curve for long-term survival rate fitted well.
Conclusions
This study identified independent risk factors affecting the survival of patients with HBV-associated HCC and constructed a predictive nomogram model, which can individually predict the overall survival and has good clinical application value.
Keywords: Prediction, The time-to-event analysis, Hepatitis B virus, Hepatocellular carcinoma, Prognosis
1. Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignant tumors in the world, accounting for the third place in cancer deaths [1,2], and chronic hepatitis B virus (HBV) infection is the leading cause of HCC incidence in China [3]. There have been many examinations aiming at screening individuals at high risk of HCC, and the therapeutic effect is increasing. Models such as REACH-B, mPAGE-B, THRI, etc. [[4], [5], [6]], which apply to patients with chronic HBV infection, may allow screening high-risk HCC populations. It is clinically essential to clarify the prognostic factors of HCC patients and to individualize the assessment of survival. However, a survival prediction model for patients with HBV-associated HCC is currently lacking. The nomogram, which creates a simple graphical representation of a statistical predictive model that generates a numerical probability of a clinical event, is often used in the identification and stratification of patients due to its user-friendly interfaces [7]. Previous studies proved that nomograms could be used as a statistical tool for predicting the prognosis of liver cancer. For example, He et al. plotted and validated the nomogram, which predicted the survival of liver cancer patients after recurrence with a c-index as high as 0.797 [8]. Shim et al. established a predictive nomogram with a c-index of 0.69 based on data from 1085 patients with early-stage HCC who underwent curative resection at the Asan Liver Center [9]. Kong et al. predicted the prognosis of young adults with HCC using a nomogram-validated model, and the c-index was 0.786 (95 % CI: 0.759–0.813) [10]. In the present study, we retrospectively collected the medical records of patients with HBV-associated HCC and performed time-to-event analysis, aiming to explore the risk factors for overall survival (OS) and to construct a nomogram predicting patients' 1-, 3-, and 5-year OS, which could help to individualize the assessment of clinical outcomes of HCC.
2. Methods
2.1. Study design and patients
In this retrospective noninterventional cohort study, we enrolled the hospitalized patients admitted to the Fifth Medical Center of Chinese PLA General Hospital from January 2009 to December 2018 with HBV-associated HCC according to electronic medical records. The inclusion criteria were: (1) aged over 18 years old; (2) diagnosed as HBV-associated HCC according to the guidelines for the diagnosis and treatment of chronic hepatitis B (CHB) [11] and primary liver cancer in China (2010 Edition) [12]; (3) received nucleos(t)ide analogues (NAs) treatment. Patients were excluded if they had any of the following conditions: (1) did not achieve sustained virological response (SVR) defined as HBV DNA <20 IU/mL after NAs treatment; (2) with other etiologies (e.g., other viral hepatitis, autoimmune liver disease, alcoholic liver disease or fatty liver disease, etc.); (3) secondary liver cancer or other malignant tumors; (4) incomplete follow-up information. Using the time of HCC diagnosis as the starting point and death or liver transplantation as the endpoint events, the follow-up cutoff was January 1, 2021. All enrolled patients were randomly assigned to either the training cohort for nomogram development or the validation cohort to confirm the model's performance at a ratio of 3 to 1. This study was approved by the Ethics Committee of the Fifth Medical Center of the Chinese PLA General Hospital (No. 2020055D).
2.2. Clinical characteristics
The demographic features (age, gender, family history, etc.), laboratory characteristics (alpha-fetoprotein [AFP], total bilirubin [TBIL], serum alanine aminotransferase [ALT], aspartate aminotransferase [AST], albumin [ALB], platelet [PLT], hepatitis B serological markers), and liver stiffness measurement (LSM) values at the time of HCC diagnosis were collected as baseline data. The LSM values were categorized into <9, 9–22, and ≥22 kPa groups [11]. The corresponding treatment was performed according to Barcelona Clinic Liver Cancer classification (BCLC) [13], and all patients were followed up until the endpoint event occurred or the follow-up time expired.
2.3. Statistical analysis
Statistical software R (4.0.4) was used for data processing and analysis. Continuous variables were expressed as mean ± standard deviation (SD) or median with the corresponding interquartile range (IQR) and were compared using either Student's t-test or the Mann-Whitney U test if appropriate. Categorical variables were compared using the chi-squared test or Fisher's exact test. Survival analysis was performed by Kaplan-Meier curve and compared by log-rank test. Variables of significance in univariate analysis were included in multivariable COX regression to screen the risk factors associated with survival with its hazard ratio (HR) and 95 % confidence interval (CI). A P-value of <0.05 was considered statistically significant.
Then, a prognostic nomogram was established using the RMS package in R through the training cohort. The predictive performance of the nomogram was measured by the concordance index (C-index) and calibration with 1000-time bootstrapping to decrease the overfit bias using the rms package in R through the validation cohort.
Finally, the performance of the nomogram model was assessed using receiver operating characteristic (ROC) curve, which are a plot of sensitivity versus 1-specificity for all possible cutoff values. The area under the ROC curve (AUROC) were verified again by 200-time 10-fold cross-validation and time-dependent ROC analysis.
3. Result
3.1. Baseline characteristics
A total of 2798 patients diagnosed with liver cancer were screened, 238 (8.5 %) with other etiologies, 127 (4.5 %) with non-HCC cancer, 256 (9.1 %) not achieving SVR, 169 (6.1 %) not receiving NAs treatment due to undetectable HBV DNA at HCC diagnosis, and 285 (10.2 %) with incomplete data or lost were excluded. Therefore, 1723 patients were enrolled in this study with a median age of 54.0 years (Fig. 1). Among them, 1425 (82.7 %) were male, 211 patients (12.2 %) had diabetes mellitus, and 835 (48.5 %) had positive HBeAg. BCLC stages were as follows: 827 (48.0 %) in stage 0/A, 835 (48.5 %) in stage B/C, and 61 (3.5 %) in stage D (Table 1).
Fig. 1.
Inclusion and exclusion diagram of the study population. HCC, Hepatocellular carcinoma; NAs, nucleos(t)ide analogues; BCLC, Barcelona Clinic Liver Cancer; SVR, sustained virological response defined as HBV DNA <20 IU/mL.
Table 1.
Baseline clinical characteristics of the enrolled patients with HBV-associated HCC.
| Entire patients |
||||
|---|---|---|---|---|
| Overall (n = 1723) | Survival (n = 1051) | Death/LT (n = 672) | P | |
| Age (years) | 54.0 (47.0, 61.0) | 53.0 (46.0, 60.0) | 54.0 (47.8, 62.0) | 0.038 |
| Age ≥60 years † | 503 (29.2) | 282 (26.8) | 221 (32.9) | 0.008 |
| Male † | 1425 (82.7) | 838 (79.7) | 587 (87.4) | <0.001 |
| DM † | 211 (12.2) | 121 (11.5) | 90 (13.4) | 0.319 |
| HBsAg (IU/ml) | 5663 (3382, 6983) | 5658 (3431, 6923) | 5676 (3256, 7088) | 0.246 |
| HBeAg positive † | 835 (48.5) | 530 (50.4) | 305 (45.4) | 0.058 |
| ALT (U/L) ≥ 80 U/L † | 293 (17.0) | 165 (15.7) | 128 (19.0) | 0.082 |
| AST ≥80 U/L † | 397 (23.0) | 146 (13.9) | 251 (37.4) | <0.001 |
| ALB ≥35 g/L † | 1024 (59.4) | 732 (69.6) | 292 (43.5) | <0.001 |
| TBIL ≥34 μmol/L † | 291 (16.9) | 100 (9.5) | 191 (28.4) | <0.001 |
| PLT ≥100 × 109/L † | 1013 (58.8) | 699 (66.5) | 314 (46.7) | <0.001 |
| LSM † | <0.001 | |||
| <9 kPa | 417 (24.2) | 365 (34.7) | 52 (7.8) | |
| 9–22 kPa | 887 (51.5) | 591 (56.2) | 296 (44.0) | |
| ≥22 kPa | 419 (24.3) | 95 (9.1) | 324 (48.2) | |
| AFP † | <0.001 | |||
| <20 ng/mL | 636 (36.9) | 521 (49.6) | 115 (17.1) | |
| 20–400 ng/mL | 606 (35.2) | 309 (29.4) | 297 (44.2) | |
| ≥400 ng/mL | 481 (27.9) | 221 (21.0) | 260 (38.7) | |
| BCLC † | <0.001 | |||
| 0/A | 827 (48.0) | 674 (64.1) | 153 (22.8) | |
| B/C | 835 (48.5) | 373 (35.5) | 462 (68.7) | |
| D | 61 (3.5) | 4 (0.4) | 57 (8.5) | |
DM, diabetes mellitus; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALB albumin; TBIL, total bilirubin; PLT, platelet; LSM, liver stiffness measurement; AFP, alpha-fetoprotein; BCLC, Barcelona Clinic Liver Cancer; LT, liver transplantation.
†n, (%).
3.2. Risk factors influencing the prognosis of chronic HBV-associated HCC
During a median follow-up time of 41.3 months, 672 cases (39.0 %) died and 1051 (61.0 %) survived. The multivariable COX regression identified that Age ≥60 years (HR = 1.209, 95 % CI: 1.027–1.424), Male (HR = 1.293, 95 % CI: 1.025–1.631), ALB <35 g/L (HR = 1.491, 95 % CI: 1.248–1.780), AST ≥80 U/L (HR = 1.818, 95 % CI: 1.517–2.177); AFP 20–400 ng/mL (HR = 2.284, 95 % CI: 1.830–2.852), AFP ≥400 ng/mL (HR = 2.746, 95 % CI: 2.168–3.479); LSM 9–22 kPa (HR = 2.266, 95 % CI: 1.684–3.049), LSM ≥22 kPa (HR = 4.326, 95 % CI: 3.201–5.847); BCLC stage B/C (HR = 4.079, 95 % CI: 3.348–4.971) and BCLC stage D (HR = 16.830, 95 % CI: 12.048–23.509) were the independent risk factors associated with HCC survival (p < 0.05) (Fig. 2 and supplementary Table 1).
Fig. 2.
High-risk factors for the overall survival of HBV-associated HCC. Each square represents the estimated HR, the horizontal lines represent the 95 % CIs. References for various factors (HR = 1): PLT ≥100 × 109/L; TBIL ≤34 μmol/L; Female; ALB ≥35 g/L; AST ≤80 U/L; AFP ≤20 ng/mL; LSM ≤9.0 kPa; BCLC stage 0/A. ALB albumin; AST, aspartate aminotransferase; AFP, alpha-fetoprotein; BCLC, Barcelona Clinic Liver Cancer; LSM, liver stiffness measurement; PLT, platelet; TBIL, total bilirubin; HR, hazard ratio; CI, confidence interval.
3.3. Survival analysis
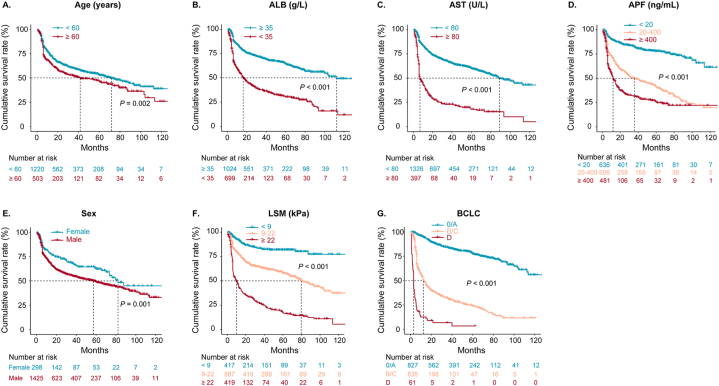
The Kaplan-Meier curve was plotted using the independent risk factors mentioned above based on the entire enrolled patients. The results showed the median survival time (months) of age ≥60 years subgroup (41.6 months) was significantly shorter than its counterpart subgroup (71.9 months); that of male (57.4 months) was significantly shorter than its counterpart subgroup (82.1 months); that of ALB <35 g/L (112.4 months) was significantly shorter than its counterpart subgroup (16.2 months); that of AST ≥80 U/L (6.9 months) was significantly shorter than its counterpart subgroup (89.0 months). As AFP levels increased, the median survival time gradually shortened (that of AFP <20 ng/mL did not reach, that of AFP between 20 and 400 ng/mL was 35.8 months, that of AFP ≥400 ng/mL was 12.0 months). The higher the LSM value, the shorter the median survival period (that of LSM ≤9.0 kPa did not reach, that of LSM between 9 and 22 kPa was 79.7 months, and that of LSM ≥22 kPa was 10.2 months). Regarding BCLC stages, the median survival time of stage 0/A did not reach, stage B/C was 12.5 months, and stage D was 3.0 months (Fig. 3).
Fig. 3.
Kaplan-Meier curve analysis of independent risk factors for overall survival. P values were estimated by log-rank test. Groups were compared using the log-rank test. 0 in the X-axis indicates the time when HCC was diagnosed.
ALB albumin; AST, aspartate aminotransferase; AFP, alpha-fetoprotein; BCLC, Barcelona Clinic Liver Cancer; LSM, liver stiffness measurement.
3.4. Nomogram construction and validation
All enrolled patients were randomly assigned to either the training cohort or validation cohort at a ratio of 3 to 1, there was no significant difference between these two groups (Table 2). The univariable and multivariable COX regression were performed to investigate the high-risk factors through the training cohort (supplementary Table 2), which were then incorporated into a nomogram to establish a prognostic prediction model with the C-index of 0.842 (95 % CI: 0.827–0.858) (Fig. 4A). Furthermore, the calibration curve was shown to be close to the ideal curve (diagonal) in both training cohort and validation cohort, indicating that the predicted values of the model were consistent with the actual observed values and also indicating that the accuracy of the nomogram model prediction was good (Fig. 4B and C).
Table 2.
The comparison between the training and validation cohort.
| Training cohort |
Validation cohort |
|||||
|---|---|---|---|---|---|---|
| Total (n = 1292) | Survival (n = 784) | Death/LT (n = 508) | P | Total (n = 431) | P‡ | |
| Age (years) | 54.0 (47.0, 61.0) | 53.0 (46.0, 60.0) | 54.0 (48.0, 62.0) | 0.060 | 53.0 (46.5, 60.0) | 0.379 |
| Age ≥60 years † | 384 (29.7) | 213 (27.2) | 171 (33.7) | 0.015 | 119 (27.6) | 0.439 |
| Male † | 1061 (82.1) | 620 (79.1) | 441 (86.8) | 0.001 | 364 (84.5) | 0.300 |
| DM † | 165 (12.8) | 96 (12.2) | 69 (13.6) | 0.583 | 46 (10.7) | 0.305 |
| HBsAg (IU/ml) | 5672 (3330, 7004) | 5662 (3258, 6940) | 5681 (3395, 7096) | 0.285 | 5656 (3411, 6907) | 0.588 |
| HBeAg positive † | 641 (49.6) | 407 (51.9) | 234 (46.1) | 0.068 | 194 (45.0) | 0.165 |
| ALT (U/L) ≥ 80 U/L † | 217 (16.8) | 125 (15.9) | 92 (18.1) | 0.347 | 76 (17.6) | 0.744 |
| AST ≥80 U/L † | 304 (23.5) | 110 (14.0) | 194 (38.2) | <0.001 | 93 (21.6) | 0.443 |
| ALB ≥35 g/L † | 759 (58.7) | 541 (69.0) | 218 (42.9) | <0.001 | 265 (61.5) | 0.344 |
| TBIL ≥34 μmol/L † | 215 (16.6) | 69 (8.8) | 146 (28.7) | <0.001 | 76 (17.6) | 0.688 |
| PLT ≥100 × 109/L † | 763 (59.1) | 520 (66.3) | 243 (47.8) | <0.001 | 250 (58.0) | 0.743 |
| LSM † | <0.001 | 0.790 | ||||
| <9 kPa | 308 (23.8) | 265 (33.8) | 43 (8.5) | 109 (25.3) | ||
| 9–22 kPa | 666 (51.6) | 438 (55.9) | 228 (44.9) | 221 (51.3) | ||
| ≥22 kPa | 318 (24.6) | 81 (10.3) | 237 (46.6) | 101 (23.4) | ||
| AFP † | <0.001 | 0.728 | ||||
| <20 ng/mL | 470 (36.4) | 386 (49.2) | 84 (16.5) | 166 (38.5) | ||
| 20–400 ng/mL | 458 (35.4) | 234 (29.9) | 224 (44.1) | 148 (34.3) | ||
| ≥400 ng/mL | 364 (28.2) | 164 (20.9) | 200 (39.4) | 117 (27.2) | ||
| BCLC † | <0.001 | 0.872 | ||||
| 0/A | 621 (48.1) | 506 (64.5) | 115 (22.6) | 206 (47.8) | ||
| B/C | 627 (48.5) | 276 (35.2) | 351 (69.1) | 208 (48.3) | ||
| D | 44 (3.4) | 2 (0.3) | 42 (8.3) | 17 (3.9) | ||
DM, diabetes mellitus; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALB albumin; TBIL, total bilirubin; PLT, platelet; LSM, liver stiffness measurement; AFP, alpha-fetoprotein; BCLC, Barcelona Clinic Liver Cancer.
†n, (%); ‡ Comparison between the training and validation group.
Supplementary Table 2.
High-risk factors for the survival of HBV-related HCC on the training cohort
| Univariable |
Multivariable |
|||
|---|---|---|---|---|
| HR (95 % CI) | P | HR (95 % CI) | P | |
| AGE ≥60 years | 1.270 (1.064–1.537) | 0.009 | 1.324 (1.097–1.579) | 0.003 |
| Male | 1.489 (1.151–1.925) | 0.002 | 1.317 (1.012–1.714) | 0.040 |
| ALB <35 g/L | 2.867 (2.401–3.423) | <0.001 | 1.512 (1.227–1.864) | <0.001 |
| AST ≥80 U/L | 4.016 (3.342–4.827) | <0.001 | 1.812 (1.469–2.235) | <0.001 |
| TBIL ≥34 μmol/L | 3.122 (2.573–3.788) | <0.001 | 1.203 (0.956–1.514) | 0.114 |
| PLT <100 × 109/L | 1.605 (1.349–1.911) | <0.001 | 1.055 (0.874–1.273) | 0.578 |
| AFP (take <20 ng/mL as a reference) | ||||
| 20–400 ng/mL | 3.664 (2.849–4.712) | <0.001 | 2.414 (1.864–3.125) | <0.001 |
| ≥400 ng/mL | 5.969 (4.610–7.728) | <0.001 | 2.805 (2.125–3.702) | <0.001 |
| LSM (take <9 kPa as a reference) | ||||
| 9–22 kPa | 2.578 (1.861–3.571) | <0.001 | 2.040 (1.496–2.833) | <0.001 |
| ≥22 kPa | 7.092 (5.121–9.821) | <0.001 | 3.584 (2.563–5.012) | <0.001 |
| BCLC (take 0/A as a reference) | ||||
| B/C | 6.495 (5.231–8.064) | <0.001 | 4.175 (3.325–5.243) | <0.001 |
| D | 35.285 (24.387–51.053) | <0.001 | 15.456 (10.389–22.995) | <0.001 |
Note: ALB albumin; AST, aspartate aminotransferase; TBIL, total bilirubin; PLT, platelet; AFP, alpha-fetoprotein; LSM, liver stiffness measurement; BCLC, Barcelona Clinic Liver Cancer; HR, hazard ratio; CI, confidence interval.
Fig. 4.
Discrimination and calibration of the nomogram model to predict the survival probability of HBV-related HCC. (A) The Discrimination of established nomogram model. The value of each variable is given a certain score on a point scale from 0 to 100, to use the nomogram, find the position of each variable on the corresponding axis, draw a line to the points axis for the number of points, add the points from all of the variables, and project the total points to the lower risk lines to determine the 1-, 3-, or 5- year survival probabilities. (B) Calibration of the predictive performance of the nomogram in the training cohort by 1000-time bootstrapping; (C) Calibration of the predictive performance of the nomogram in the validation cohort by 1000-time bootstrapping.
ALB albumin; AST, aspartate aminotransferase; AFP, alpha-fetoprotein; BCLC, Barcelona Clinic Liver Cancer; LSM, liver stiffness measurement.
In addition, the individual probability for survival was calculated according to the established nomogram model, and was assessed by time-depended ROC analysis (Fig. 5A–B). The 200-time 10-fold cross-validation showed that the 1-, 3-, and 5-year average AUROC values were 0.896 (IQR: 0.874–0.915), 0.893 (IQR: 0.867–0.916), and 0.904 (IQR: 0.877–0.927) in the training cohort (Fig. 5C), and 0.905 (IQR: 0.864–0.940), 0.893 (IQR: 0.847–0.934), 0.904 (IQR: 0.850–0.920) in the validation cohort, respectively, indicating the strong repeatability of the nomogram model (Fig. 5D).
Fig. 5.
Prediction performance of the nomogram model by ROC analyses. (A) Time-dependent ROC in the training cohort; (B) Time-dependent ROC in the validation cohort; (C) The 200-time 10-fold cross-validation in the training cohort; (D) The 200-time 10-fold cross-validation in the validation cohort.
The histogram represents the AUROC distribution after 2000 validation calculations (200-time 10-fold). AUROC: Area Under the Receiver Operating Curve.
4. Discussion
The present retrospective study identified that age, male, ALB, AST, AFP, LSM, and BCLC stage were the risk factors for the prognosis of HCC, then established a prognosis prediction nomogram.
Age and sex play an essential role in studying the risk of HCC. The commonly used risk stratification models of HCC, such as REACH-B, mPAGE-B, THRI, etc., all contain age factors [14,15]. A previous study including 1110 HCC patients revealed male had significantly worse prognosis than female independent of tumor stage or liver disease severity regarding OS and treatment response [16]. These results were in accord with the ours.
ALB reflects the biosynthetic ability of the liver. It was initially included in the Child-Pugh score to assess liver function and predict the prognosis of liver cancer surgery [17]. In recent years, it has been included in the Albumin Bilirubin score to predict postoperative liver failure (PHLF) and OS in patients with liver cancer surgery [18]. The present study found that ALB is an independent prognostic risk factor for HBV-associated HCC, ALB below 35 g/L can significantly decrease the OS rate of HCC.
AFP is the most commonly used biomarker in diagnosing HCC and is associated with poor patient outcomes [19,20], consistent with our result. A previous study revealed that AFP exerted its growth-promoting effect on HCC by suppressing the Fas/FADD-mediated extrinsic apoptotic pathway, and suggested that blockade of AFP might be a promising strategy to treat advanced HCC [21]. On the other hand, AFP levels remain normal in 15–30 % advanced HCC, and its elevation can also occur in benign liver diseases, such as hepatitis and cirrhosis [22]. Therefore, it is necessary to combine AFP with other parameters for early detection and the prognosis of HCC, which is the meaningfulness of the present study.
AST is a reliable and sensitive biochemical marker of liver injury [23]. Damaged mitochondria can lead to a significant release of AST; at the same time, liver fibrosis or cirrhosis can also reduce the clearance rate of AST, which indicates that AST is always associated with liver fibrosis. In addition, previous studies found that HCC patients with high AST levels had lower survival rates, possibly because the invasion of cancer cells leads to mass necrosis of normal liver cells and fibrosis progression [[24], [25], [26]].
LSM can reflect the level of liver fibrosis. In recent years, the value of LSM has gradually emerged in the risk stratification of HCC. Previous studies have shown that the incidence of HCC is related to portal vein pressure or LSM [27]. The higher the LSM, the higher the incidence of HCC [[28], [29], [30], [31]]. Our research also demonstrated that LSM can be used to evaluate the long-term prognosis of patients with HCC.
The BCLC staging is currently a better system that combines tumor staging, treatment regimen, and expected survival and has an excellent guiding significance for the treatment of HCC. Some studies have shown that the overall 5-year survival rate of patients with early BCLC HCC can be as high as 69.0–86.2 % [32]. In the present study BCLC staging combined with above-mentioned factors can further improve its prognostic ability.
Nomograms are widely used for cancer prognosis, primarily because of their ability to reduce statistical predictive models into a single numerical estimate of the probability of an event, such as death or recurrence, tailored to the profile of an individual patient. User-friendly graphical interfaces for generating these estimates facilitate the use of nomograms during clinical encounters to inform clinical decision-making. The now-established nomogram model is of particular importance regarding HCC individual stratification management, for example, the option of treatment strategy, the timely administration of tyrosine kinase inhibitors (TKIs) or immune checkpoint inhibitors (ICIs), and follow-up intervals of surveillance, etc.
There are still some limitations in this study. First and foremost, this was a single-center retrospective analysis. Due to the inherent characteristics of retrospective study, selection bias might exist, and further studies are warranted to verify the reliability of the nomogram model. In fact, the present study contained a large sample size with a long follow-up duration and adopted multiple statistical methods to test the discrimination and calibration of the established model, which might overcome the above shortage somehow and therefore provide robust results. Last but not least, the impact of different treatment regimens on prognosis has not been completely evaluated, although the enrolled patients received standardized treatment according to the BCLC stage. Nevertheless, in a real-world setting, the treatment strategies for advanced HCC were more complex than expected and hard to classify to a certain treatment group. For example, patients might receive ablation followed by TACE and the combination therapy of TKIs and ICIs during their prolonged life span. The purpose of the study is just to establish a prognostic prediction model to guide the subsequent treatment after the first diagnosis of HCC.
In summary, this study identified independent risk factors affecting the overall survival of patients with HBV-associated HCC, such as age, sex, ALB, AST, AFP, LSM, and BCLC staging. Based on the above factors, a predictive nomogram model was constructed, which can individually predict the long-term survival of such patients and has good clinical application value.
Funding
This study was supported by Beijing Natural Science Foundation, China (No. 7222173).
Ethical statement
The Ethics Review Committee of the Fifth Medical Center of the Chinese PLA General Hospital approved the research plan (No. 2020055D).
Data availability statement
Data associated with the present study has not been deposited into a publicly available repository, and will be made available on request.
Supplementary Table 1High-risk factors for the survival of HBV-related HCC on entire enrolled patients
| Univariable |
Multivariable |
|||
|---|---|---|---|---|
| HR (95%CI) | P | HR (95%CI) | P | |
| Age ≥60 years | 1.292 (1.100–1.518) | 0.002 | 1.209 (1.027–1.424) | 0.023 |
| Male | 1.470 (1.171–1.845) | 0.001 | 1.293 (1.025–1.631) | 0.030 |
| ALB <35 g/L | 2.826 (2.424–3.295) | <0.001 | 1.491 (1.248–1.780) | <0.001 |
| AST ≥80 U/L | 3.863 (3.292–4.532) | <0.001 | 1.818 (1.517–2.177) | <0.001 |
| TBIL ≥34 μmol/L | 2.975 (2.514–3.521) | <0.001 | 1.174 (0.964–1.430) | 0.111 |
| PLT <100 × 109/L | 1.669 (1.434–1.942) | <0.001 | 1.114 (0.946–1.313) | 0.196 |
| AFP (take <20 ng/mL as a reference) | ||||
| 20–400 ng/mL | 3.505 (2.825–4.349) | <0.001 | 2.284 (1.830–2.852) | <0.001 |
| ≥400 ng/mL | 5.724 (4.583–7.149) | <0.001 | 2.746 (2.168–3.479) | <0.001 |
| LSM (take <9 kPa as a reference) | ||||
| 9–22 kPa | 2.846 (2.120–3.822) | <0.001 | 2.266 (1.684–3.049) | <0.001 |
| ≥22 kPa | 8.333 (6.213–11.176) | <0.001 | 4.326 (3.201–5.847) | <0.001 |
| BCLC (take 0/A as a reference) | ||||
| B/C | 6.386 (5.292–7.706) | <0.001 | 4.079 (3.348–4.971) | <0.001 |
| D | 27.574 (20.173–37.690) | <0.001 | 16.830 (12.048–23.509) | <0.001 |
Note: ALB albumin; AST, aspartate aminotransferase; TBIL, total bilirubin; PLT, platelet; AFP, alpha-fetoprotein; LSM, liver stiffness measurement; BCLC, Barcelona Clinic Liver Cancer; HR, hazard ratio; CI, confidence interval.
CRediT authorship contribution statement
Jianjun Wang: Methodology, Writing – original draft. Kexin Wang: Methodology, Writing – original draft. Chun Chen: Investigation, Resources. Yuting Xiong: Methodology, Writing – original draft. Chang Guo: Investigation, Writing – review & editing. Chunyan Wang: Investigation. Wucai Yang: Investigation. Yiming Fu: Investigation. Min Su: Investigation. Shuyao Li: Investigation. Dong Ji: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank the investigators, site personnel, and patients who participated in the study.
References
- 1.Rumgay H., Arnold M., Ferlay J., Lesi O., Cabasag C.J., Vignat J., et al. Global burden of primary liver cancer in 2020 and predictions to 2040. J. Hepatol. 2022;77(6):1598–1606. doi: 10.1016/j.jhep.2022.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Llovet J.M., Kelley R.K., Villanueva A., Singal A.G., Pikarsky E., Roayaie S., et al. Hepatocellular carcinoma. Nat. Rev. Dis. Prim. 2021;7(1):6. doi: 10.1038/s41572-020-00240-3. [DOI] [PubMed] [Google Scholar]
- 3.Jeng W.J., Papatheodoridis G.V., Lok A.S.F., Hepatitis B. Lancet. 2023;401(10381):1039–1052. doi: 10.1016/S0140-6736(22)01468-4. [DOI] [PubMed] [Google Scholar]
- 4.Sharma S.A., Kowgier M., Hansen B.E., Brouwer W.P., Maan R., Wong D., et al. Toronto HCC risk index: a validated scoring system to predict 10-year risk of HCC in patients with cirrhosis. J. Hepatol. 2017;(17):32248. doi: 10.1016/j.jhep.2017.07.033. S0168–8278. 1. [DOI] [PubMed] [Google Scholar]
- 5.Papatheodoridis G., Dalekos G., Sypsa V., Yurdaydin C., Buti M., Goulis J., et al. PAGE-B predicts the risk of developing hepatocellular carcinoma in Caucasians with chronic hepatitis B on 5-year antiviral therapy. J. Hepatol. 2016;64(4):800–806. doi: 10.1016/j.jhep.2015.11.035. [DOI] [PubMed] [Google Scholar]
- 6.Voulgaris T., Papatheodoridi M., Lampertico P., Papatheodoridis G.V. Clinical utility of hepatocellular carcinoma risk scores in chronic hepatitis B. Liver Int. 2020;40(3):484–495. doi: 10.1111/liv.14334. [DOI] [PubMed] [Google Scholar]
- 7.Iasonos A., Schrag D., Raj G.V., Panageas K.S. How to build and interpret a nomogram for cancer prognosis. J. Clin. Oncol. 2008;26(8):1364–1370. doi: 10.1200/JCO.2007.12.9791. [DOI] [PubMed] [Google Scholar]
- 8.He W., Peng B., Tang Y., Yang J., Zheng Y., Qiu J., et al. Nomogram to predict survival of patients with recurrence of hepatocellular carcinoma after surgery. Clin. Gastroenterol. Hepatol. 2018;16(5):756–764.e10. doi: 10.1016/j.cgh.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 9.Shim J.H., Jun M.J., Han S., Lee Y.J., Lee S.G., Kim K.M., et al. Prognostic nomograms for prediction of recurrence and survival after curative liver resection for hepatocellular carcinoma. Ann. Surg. 2015;261(5):939–946. doi: 10.1097/SLA.0000000000000747. [DOI] [PubMed] [Google Scholar]
- 10.Kong J., Wang T., Shen S., Zhang Z., Wang W. A nomogram predicting the prognosis of young adult patients diagnosed with hepatocellular carcinoma: a population-based analysis. PLoS One. 2019;14(7) doi: 10.1371/journal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chinese Society of Hepatology and Chinese Society of Infectious Diseases Chinese Medical Association, [The guideline of prevention and treatment for chronic hepatitis B (2010 version)] Zhonghua Liuxingbingxue Zazhi. 2011;32(4):405–415. [PubMed] [Google Scholar]
- 12.Ye S.L. [Expert consensus on standardization of the management of primary liver cancer] Zhonghua Gan Zang Bing Za Zhi. 2009;17(6):403–410. [PubMed] [Google Scholar]
- 13.Llovet J.M., Brú C., Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin. Liver Dis. 1999;19(3):329–338. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- 14.Tsilimigras D.I., Bagante F., Sahara K., Moris D., Hyer J.M., Wu L., et al. Prognosis after resection of Barcelona clinic liver cancer (BCLC) stage 0, A, and B hepatocellular carcinoma: a comprehensive assessment of the current BCLC classification. Ann. Surg Oncol. 2019;26(11):3693–3700. doi: 10.1245/s10434-019-07580-9. [DOI] [PubMed] [Google Scholar]
- 15.Papatheodoridis G.V., Sypsa V., Dalekos G.N., Yurdaydin C., Van Boemmel F., Buti M., et al. Hepatocellular carcinoma prediction beyond year 5 of oral therapy in a large cohort of Caucasian patients with chronic hepatitis B. J. Hepatol. 2020;72(6):1088–1096. doi: 10.1016/j.jhep.2020.01.007. [DOI] [PubMed] [Google Scholar]
- 16.Rich N.E., Murphy C.C., Yopp A.C., Tiro J., Marrero J.A., Singal A.G. Sex disparities in presentation and prognosis of 1110 patients with hepatocellular carcinoma. Aliment. Pharmacol. Ther. 2020;52(4):701–709. doi: 10.1111/apt.15917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y.Y., Zhong J.H., Su Z.Y., Huang J.F., Lu S.D., Xiang B.D., et al. Albumin-bilirubin versus Child-Pugh score as a predictor of outcome after liver resection for hepatocellular carcinoma. Br. J. Surg. 2016;103(6):725–734. doi: 10.1002/bjs.10095. [DOI] [PubMed] [Google Scholar]
- 18.Ho S.Y., Liu P.H., Hsu C.Y., Hsia C.Y., Su C.W., Lee Y.H., et al. Comparison of twelve liver functional reserve models for outcome prediction in patients with hepatocellular carcinoma undergoing surgical resection. Sci. Rep. 2018;8(1):4773. doi: 10.1038/s41598-018-22923-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seino S., Tsuchiya A., Watanabe Y., Kawata Y., Kojima Y., Ikarashi S., et al. Clinical outcome of hepatocellular carcinoma can be predicted by the expression of hepatic progenitor cell markers and serum tumour markers. Oncotarget. 2018;9(31):21844–21860. doi: 10.18632/oncotarget.25074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galle P.R., Foerster F., Kudo M., Chan S.L., Llovet J.M., Qin S., et al. Biology and significance of alpha-fetoprotein in hepatocellular carcinoma. Liver Int. 2019;39(12):2214–2229. doi: 10.1111/liv.14223. [DOI] [PubMed] [Google Scholar]
- 21.Chen T., Dai X., Dai J., Ding C., Zhang Z., Lin Z., et al. AFP promotes HCC progression by suppressing the HuR-mediated Fas/FADD apoptotic pathway. Cell Death Dis. 2020;11(10):822. doi: 10.1038/s41419-020-03030-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luo P., Wu S., Yu Y., Ming X., Li S., Zuo X., et al. Current status and perspective biomarkers in AFP negative HCC: towards screening for and diagnosing hepatocellular carcinoma at an earlier stage. Pathol. Oncol. Res. 2020;26(2):599–603. doi: 10.1007/s12253-019-00585-5. [DOI] [PubMed] [Google Scholar]
- 23.He C., Peng W., Li C., Wen T.F. Postoperative aspartate aminotransferase to lymphocyte ratio index change is an independent predictor of survival in patients with small hepatocellular carcinoma. Medicine (Baltim.) 2017;96(45) doi: 10.1097/MD.0000000000008540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaewdech A., Sripongpun P., Assawasuwannakit S., Wetwittayakhlang P., Jandee S., Chamroonkul N., et al. FAIL-T (AFP, AST, tumor sIze, ALT, and Tumor number): a model to predict intermediate-stage HCC patients who are not good candidates for TACE. Front. Med. 2023;10 doi: 10.3389/fmed.2023.1077842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang L.X., Luo P.Q., Chen L., Song D.D., Xu A.M., Xu P., et al. Model to predict overall survival in patients with hepatocellular carcinoma after curative hepatectomy. Front. Oncol. 2021;10 doi: 10.3389/fonc.2020.537526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ding Y., Liu K., Xu Y., Zhao Q., Lou S., Xiang X., et al. Combination of inflammatory score/liver function and AFP improves the diagnostic accuracy of HBV-related hepatocellular carcinoma. Cancer Med. 2020;9(9):3057–3069. doi: 10.1002/cam4.2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim B.S., Seo Y.S., Kim Y.S., Lee C.H., Lee H.A., Um S.H., et al. Reduced risk of hepatocellular carcinoma by achieving a subcirrhotic liver stiffness through antiviral agents in hepatitis B virus-related advanced fibrosis or cirrhosis. J. Gastroenterol. Hepatol. 2018;33(2):503–510. doi: 10.1111/jgh.13854. [DOI] [PubMed] [Google Scholar]
- 28.de Martel C., Georges D., Bray F., Ferlay J., Clifford G.M. Global burden of cancer attributable to infections in 2018: a worldwide incidence analysis. Lancet Global Health. 2020;8(2):e180–e190. doi: 10.1016/S2214-109X(19)30488-7. [DOI] [PubMed] [Google Scholar]
- 29.Wang H.M., Hung C.H., Lu S.N., Chen C.H., Lee C.M., Hu T.H., et al. Liver stiffness measurement as an alternative to fibrotic stage in risk assessment of hepatocellular carcinoma incidence for chronic hepatitis C patients. Liver Int. 2013;33(5):756–761. doi: 10.1111/liv.12118. [DOI] [PubMed] [Google Scholar]
- 30.Ji D., Chen Y., Shang Q., Liu H., Tan L., Wang J., et al. Unreliable estimation of fibrosis regression during treatment by liver stiffness measurement in patients with chronic hepatitis B. Am. J. Gastroenterol. 2021;116(8):1676–1685. doi: 10.14309/ajg.0000000000001239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ji D., Chen Y., Bi J., Shang Q., Liu H., Wang J.B., et al. Entecavir plus Biejia-Ruangan compound reduces the risk of hepatocellular carcinoma in Chinese patients with chronic hepatitis B. J. Hepatol. 2022;77(6):1515–1524. doi: 10.1016/j.jhep.2022.07.018. [DOI] [PubMed] [Google Scholar]
- 32.Tsilimigras D.I., Bagante F., Sahara K., Moris D., Hyer J.M., Wu L., et al. Prognosis after resection of Barcelona clinic liver cancer (BCLC) stage 0, A, and B hepatocellular carcinoma: a comprehensive assessment of the current BCLC classification. Ann. Surg Oncol. 2019;26(11):3693–3700. doi: 10.1245/s10434-019-07580-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data associated with the present study has not been deposited into a publicly available repository, and will be made available on request.
Supplementary Table 1High-risk factors for the survival of HBV-related HCC on entire enrolled patients
| Univariable |
Multivariable |
|||
|---|---|---|---|---|
| HR (95%CI) | P | HR (95%CI) | P | |
| Age ≥60 years | 1.292 (1.100–1.518) | 0.002 | 1.209 (1.027–1.424) | 0.023 |
| Male | 1.470 (1.171–1.845) | 0.001 | 1.293 (1.025–1.631) | 0.030 |
| ALB <35 g/L | 2.826 (2.424–3.295) | <0.001 | 1.491 (1.248–1.780) | <0.001 |
| AST ≥80 U/L | 3.863 (3.292–4.532) | <0.001 | 1.818 (1.517–2.177) | <0.001 |
| TBIL ≥34 μmol/L | 2.975 (2.514–3.521) | <0.001 | 1.174 (0.964–1.430) | 0.111 |
| PLT <100 × 109/L | 1.669 (1.434–1.942) | <0.001 | 1.114 (0.946–1.313) | 0.196 |
| AFP (take <20 ng/mL as a reference) | ||||
| 20–400 ng/mL | 3.505 (2.825–4.349) | <0.001 | 2.284 (1.830–2.852) | <0.001 |
| ≥400 ng/mL | 5.724 (4.583–7.149) | <0.001 | 2.746 (2.168–3.479) | <0.001 |
| LSM (take <9 kPa as a reference) | ||||
| 9–22 kPa | 2.846 (2.120–3.822) | <0.001 | 2.266 (1.684–3.049) | <0.001 |
| ≥22 kPa | 8.333 (6.213–11.176) | <0.001 | 4.326 (3.201–5.847) | <0.001 |
| BCLC (take 0/A as a reference) | ||||
| B/C | 6.386 (5.292–7.706) | <0.001 | 4.079 (3.348–4.971) | <0.001 |
| D | 27.574 (20.173–37.690) | <0.001 | 16.830 (12.048–23.509) | <0.001 |
Note: ALB albumin; AST, aspartate aminotransferase; TBIL, total bilirubin; PLT, platelet; AFP, alpha-fetoprotein; LSM, liver stiffness measurement; BCLC, Barcelona Clinic Liver Cancer; HR, hazard ratio; CI, confidence interval.