Abstract
Background
Previous studies have discovered an association between dietary factors and breast cancer. However, few studies have used Mendelian randomization (MR) to assess the potential causal relationship between dietary factors and breast cancer.
Methods
The exposure datasets for fresh fruit intake, dried fruit intake, salad/raw vegetable intake, cooked vegetable intake, oily fish intake, non-oily fish intake, cheese intake, and bread intake were obtained from the UK Biobank. The outcome dataset was extracted from the Breast Cancer Association Consortium (BCAC). We used the inverse variance weighted (IVW) method as the primary approach for the two-sample MR analysis. To ensure the accuracy of the results, we conducted heterogeneity and horizontal pleiotropy analyses. Additionally, multivariable MR analysis was conducted to ensure the stability of the results.
Results
Dried fruit intake was found to be a protective factor for overall breast cancer (outliers excluded: OR: 0.549; 95 % CI: 0.429–0.702; p = 1.75 × 10−6). Subtype analyses showed that dried fruit intake was inversely associated with both estrogen receptor-positive (ER+) breast cancer (outliers excluded: OR: 0.669; 95 % CI: 0.512–0.875; p = 0.003) and ER-negative (ER−) breast cancer (OR: 0.559; 95 % CI: 0.379–0.827; p = 0.004), while fresh fruit intake was inversely associated with ER− breast cancer (excluded outliers: OR: 0.510; 95 % CI: 0.308–0.846; p = 0.009). No significant causal relationship was found between other dietary intakes and breast cancer. After adjusting for the effects of possible confounders, the causal relationships found by the two-sample MR analysis remained.
Conclusion
Our study provides evidence that dried fruit intake may reduce the risk of both ER+ and ER− breast cancer, and fresh fruit intake may reduce the risk of ER− breast cancer. Other factors included in this study were not linked to breast cancer.
Keywords: Breast cancer, Dietary factors, Mendelian randomization, Causal relationship, Fruit intake
1. Introduction
Breast cancer is the most common cancer globally and represents a leading public health burden, with 2,261,419 new cases reported in 2020 [[1], [2], [3], [4]]. As a multifactorial disease, identifying modifiable risk factors for breast cancer, such as dietary factors, lifestyle, and environmental factors, is crucial for preventing the disease and reducing its burden [[5], [6], [7], [8]].
Several observational studies have suggested that fruit intake [9,10], vegetable intake [10], fish intake [11], and cheese intake [9,12] may reduce the risk of breast cancer, while bread intake may increase the risk of breast cancer [13,14]. However, other studies have not consistently found these associations [[15], [16], [17]]. Importantly, residual confounding is a common issue encountered in all observational studies, and these statistical correlations cannot infer causality [9,10,18]. Therefore, whether there is a causal relationship between these dietary factors and breast cancer remains unclear.
Mendelian randomization (MR) study is an increasingly popular method for assessing causal relationships between exposures and outcomes using genetic variants as instrumental variables (IVs) [19,20]. By exploiting the random assignment of genetic variants at the time of conception, MR studies can reduce confounding factors and reverse causation, which are common limitations in observational studies [[21], [22], [23]]. This approach has been widely used to study the causal effects of various risk factors on disease outcomes [[24], [25], [26]].
Thus, we aimed to estimate the potential causal association between dietary factors and breast cancer risk by MR analysis.
2. Materials and methods
2.1. Study design
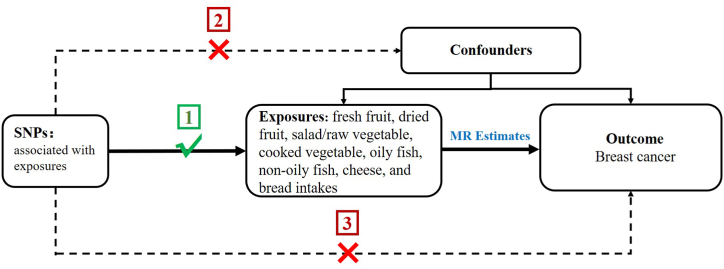
A two-sample MR study was carried out to determine the causal association between factors and breast cancer. The single nucleotide polymorphisms (SNPs) selected as IVs were supposed to comply with the three key assumptions: (1) SNPs must be intensely linked to the exposure factors, (2) SNPs must not be linked to any confounding factor, and (3) SNPs not directly be linked to breast cancer (Fig. 1).
Fig. 1.
Schematic diagram of the MR analysis design.
2.2. Data source
The exposure factors included in this study were fresh fruit intake, dried fruit intake, salad/raw vegetable intake, cooked vegetable intake, oily fish intake, non-oily fish intake, cheese intake, and bread intake. These exposure datasets were obtained from the UK Biobank through the IEU Open GWAS project (https://gwas.mrcieu.ac.uk/). See Table 1 and Supplementary Table 1 for more information about exposures. We obtained the GWAS summary data for breast cancer from the Breast Cancer Association Consortium (BCAC). The dataset included 122,977 cases (69,501 estrogen receptor (ER) + breast cancer; 21,468 ER− breast cancer) and 105,974 controls of European ancestry [27] (Table 2).
Table 1.
Information on the datasets for exposures.
| IEU GWAS id | Exposure | Used SNPs | Sample size | Ancestry |
|---|---|---|---|---|
| ukb-b-3881 | Fresh fruit intake | 53 | 446,462 | European |
| ukb-b-16576 | Dried fruit intake | 40 | 421,764 | European |
| ukb-b-1996 | Salad/raw vegetable intake | 18 | 435,435 | European |
| ukb-b-8089 | Cooked vegetable intake | 16 | 448,651 | European |
| ukb-b-2209 | Oily fish intake | 57 | 460,443 | European |
| ukb-b-17627 | Non-oily fish intake | 9 | 460,880 | European |
| ukb-b-1489 | Cheese intake | 58 | 451,486 | European |
| ukb-b-11348 | Bread intake | 27 | 452,236 | European |
IEU—Integrative Epidemiology Unit; GWAS—genome-wide association study; SNPs—single.
nucleotide polymorphisms.
Table 2.
Information on the dataset for breast cancer.
| IEU GWAS id | Outcomes | ncase | ncontrol | Sample size | Consortium | Ancestry |
|---|---|---|---|---|---|---|
| ieu-a-1126 | Overall Breast cancer | 122,977 | 105,974 | 228,951 | BCAC | European |
| ieu-a-1127 | ER + Breast cancer | 69,501 | 105,974 | 175,475 | BCAC | European |
| ieu-a-1128 | ER− Breast cancer | 21,468 | 105,974 | 127,442 | BCAC | European |
BCAC—Breast Cancer Association Consortium.
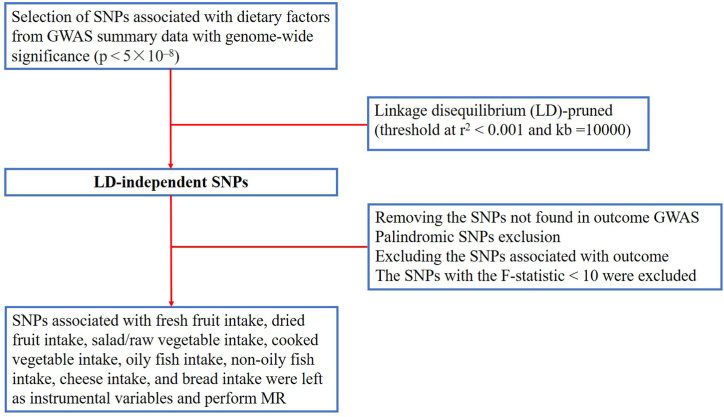
2.3. Selection of IVs
Firstly, we screened for SNPs that were strongly associated with exposure at a genome-wide significance level (p < 5 × 10−8). Subsequently, a threshold (r2 < 0.001, kb = 10000) was set to obtain linkage disequilibrium (LD)-independent SNPs. Next, we removed SNPs that were not found in the outcome GWAS dataset, as well as palindromic SNPs that could lead to bias [28]. SNPs strongly correlated with the outcome (p < 5 × 10−8) were also removed because they did not conform to the key assumptions of IVs. Finally, F-statistics were calculated to exclude weak instrumental variable bias [29] (Fig. 2). The F-statistic was calculated as F R2 (n-k-1)/[k (1-R2)], and R2 = 2 × (1-MAF) × MAF × (β/SD) 2, where n was the sample size, k was the number of SNPs, MAF was the secondary allele frequency, a value equivalent to EAF in calculating R2, β was the allele effect size, and SD was the standard deviation [30].
Fig. 2.
Flowsheet of SNPs selection in this study.
2.4. Statistical analysis
We employed three distinct methods, namely inverse variance weighted (IVW), weighted median, and MR Egger, to explore the potential causal relationship between dietary factors and breast cancer. Among these methods, we selected IVW as our primary approach for two-sample MR analysis due to its demonstrated superior ability to determine causality [31]. Cochran's Q test was applied to assess heterogeneity (p < 0.05 indicating heterogeneity) [32]. However, the presence of heterogeneity does not necessarily imply the invalidity of the IVW method. MR-Egger regression test was utilized to detect horizontal pleiotropy, with a zero intercept indicating the absence of horizontal pleiotropy (p > 0.05) [33]. Leave-one-out analysis was conducted to determine whether the results were significantly affected after removing a single SNP [34]. Outliers were detected using the MR-PRESSO method and subsequently removed [35]. The MR analysis was then repeated with the exclusion of these outliers. We used odds ratios (ORs) to express the effects of dietary factors on breast cancer risk. To determine the observed effects of dietary factors on breast cancer were stable, we performed multivariable MR (MVMR) analysis that accounted for possible confounders between dietary factors and breast cancer [36]. All analyses were conducted using the “TwoSampleMR”, “MRPRESSO”, and “MendelianRandomization” packages in R software (version 4.2.3) [37].
3. Results
3.1. IVs selection
According to the screening process described in the Methods section, 35 SNPs, 34 SNPs, and 30 SNPs were removed from the analyses of overall, ER+, and ER− breast cancer, respectively (Supplementary Tables 2 and 3). After removing these SNPs, the number of SNPs screened as exposures ranged from 9 to 58, and the range after removal of outliers by MR-PRESSO was between 8 and 57 (Supplementary Tables 4–6). The F-statistics for all SNPs included in the analysis were greater than 10, ensuring a strong correlation between the exposure and IVs [38]. Supplementary sheet presents details of the SNPs.
3.2. Relationships between dietary intakes and overall breast cancer
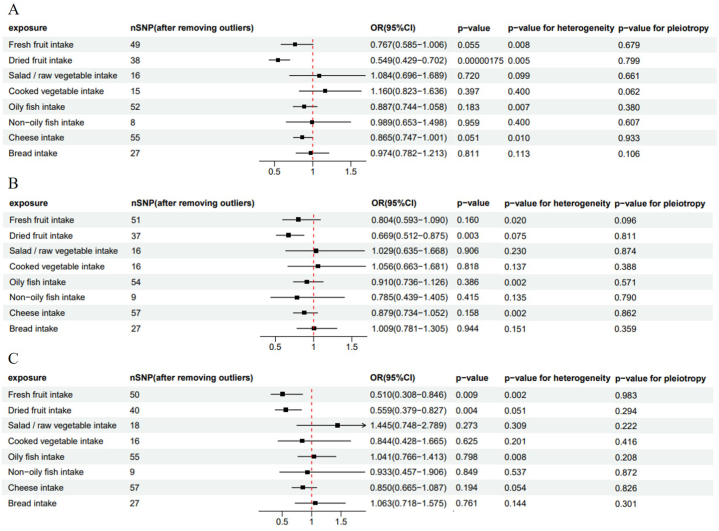
As shown in Fig. 3A, based on the IVW method, overall breast cancer risk was reduced by 45.1 % (outliers excluded: OR: 0.549; 95%CI: 0.429–0.702; P = 1.75 × 10−6) for every increase in the intake of dried fruits by one standard deviation (SD). The protective effect of dried fruit intake was also observed in the weighted median method (outliers excluded: OR: 0.591; 95%CI: 0.441–0.792; P = 0.000431). However, no significant result was available in the MR-Egger method (p > 0.05). Fresh fruit intake indicated a positive value before the removal of outliers, while the positive value disappeared after the removal of outliers (P = 0.033 vs 0.055). This study also discovered that salad/raw vegetable intake, cooked vegetable intake, oily fish intake, non-oily fish intake, cheese intake, and bread intake were not linked to overall breast cancer before and after the removal of outliers. More results of two-sample MR analysis are presented in Supplementary Table 4.
Fig. 3.
Forest plot of relationships between dietary intakes and breast cancer risk (after removing outliers). (A) Overall breast cancer; (B) ER + breast cancer; (C) ER− breast cancer. OR, odds ratio; CI, confidence interval.
3.3. The effects of dietary intakes on ER+ and ER− breast cancer
Using the GWAS dataset of ER+ and ER− breast cancer, we conducted two-sample MR analysis to evaluate the potential association between dietary intakes and different subtypes of breast cancer. Our study revealed that per SD increase in dried fruit intake, the risk of ER + breast cancer was reduced by 33.1 % (outliers excluded: OR: 0.669; 95 % CI: 0.512–0.875; p = 0.003), and the risk of ER− breast cancer was reduced by 44.1 % (OR: 0.559; 95 % CI: 0.379–0.827; p = 0.004) based on the IVW method. Additionally, a significant 49 % reduction in the risk of ER− breast cancer (excluded outliers: OR: 0.510; 95 % CI: 0.308–0.846; p = 0.009) was observed for each SD increase in fresh fruit intake, but no significant association was found for ER + breast cancer. The MR analysis further indicated that salad/raw vegetable intake, cooked vegetable intake, oily fish intake, non-oily fish intake, cheese intake, and bread intake did not affect the risk of ER + or ER− breast cancer. (Fig. 3B and C and Supplementary Tables 5 and 6).
3.4. Sensitivity analysis
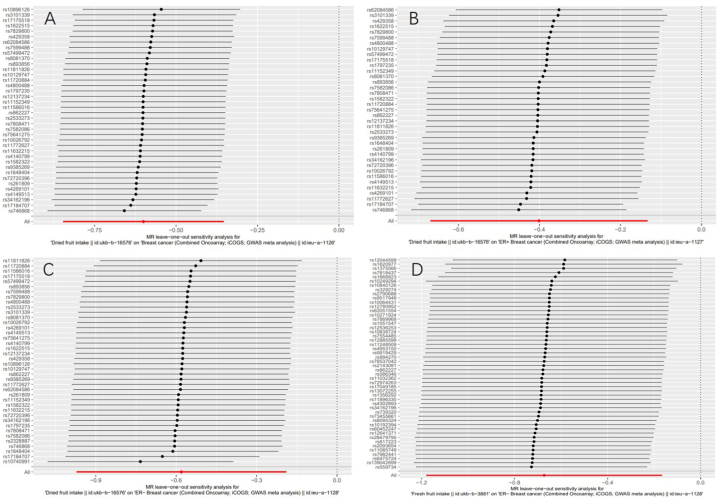
Although heterogeneity was found in several exposures (Cochrane's Q test P < 0.05), MR-Egger regression tests did not discover the existence of horizontal pleiotropy (Fig. 3A–C). Leave-one-out analyses showed no significant effects on the positive results after removing a single SNP, suggesting that the causalities of the results were very robust (Fig. 4).
Fig. 4.
The results of Leave-one-out analyses (after removing outliers) (A) between dried fruit intake and overall breast cancer (B) between dried fruit intake and ER + breast cancer (C) between dried fruit intake and ER− breast cancer (D) between fresh fruit intake and ER− breast cancer.
3.5. Analysis using the MVMR
To determine the effects of dried fruit intake and fresh fruit intake on breast cancer observed in the two-sample MR analysis, we further carried out MVMR analyses. After adjusting for body mass index (BMI), vigorous physical activity, and age at menopause, dried fruit intake remained a causal association with overall, ER+, and ER− breast cancer; and fresh fruit intake remained a causal relationship with ER− breast cancer (Table 3). Besides, no potential horizontal pleiotropy was detected for the MR-Egger intercept.
Table 3.
Assessing the effects of dried fruit intake and fresh fruit intake on breast cancer using IVW multivariable MR.
| Expose | Outcome | OR (95%CI) | P | Egger-Intercept | Int.p |
|---|---|---|---|---|---|
| Dried fruit intake | Overall breast cancer | 0.405 (0.302–0.542) | 1.218 × 10−9 | <0.001 | 0.597 |
| Dried fruit intake | ER + breast cancer | 0.378 (0.272–0.526) | 7.470 × 10−9 | <0.001 | 0.993 |
| Dried fruit intake | ER− breast cancer | 0.406 (0.262–0.629) | 5.444 × 10−5 | −0.002 | 0.062 |
| Fresh fruit intake | ER− breast cancer | 0.392 (0.225–0.684) | 0.001 | −0.002 | 0.133 |
Int.p refers to the p-value derived from the Egger-intercept. IVW, inverse-variance-weighted.
4. Discussion
Our study explored the causal relationship between dietary factors and breast cancer risk using MR analysis. In this study, if the MR-PRESSO method detected outliers, the MR analysis was repeated after excluding the outliers, and the new results of the IVW method were used as a basis for assessing causality. The two-sample MR analysis showed that dried fruit intake significantly reduced overall breast cancer risk. As the pathogenesis differs for each pathological state, we performed subtype analyses based on the ER status of breast cancer. The findings revealed that dried fruit intake reduced the risks of both ER+ and ER− breast cancer, and fresh fruit intake reduced the risk of ER− breast cancer. After adjusting for possible confounders, MVMR further confirmed stable effects of dried fruit intake and fresh fruit intake on breast cancer. In addition, this study found that salad/raw vegetable intake, cooked vegetable intake, oily fish intake, non-oily fish intake, cheese intake, and bread intake were not associated with breast cancer. These results revealed the causal role of dietary factors in breast cancer risk.
In this study, subtype analyses discovered a significant inverse association between fresh fruit intake and ER− breast cancer risk, but no association with ER + breast cancer. Similar results were reported in a previous study, suggesting that each serving of fruit intake was associated with a 12 % reduction in ER− breast cancer, whereas no association was discovered for overall breast cancer or ER + breast cancer [39]. This is possibly due to the leading role of hormone-related factors in the etiology of ER + breast cancer [40,41]. Fung et al. reported that ER− breast cancer may be more strongly related to diet than ER + breast cancer [42]. Furthermore, one study involving 75,929 women found that higher intake of peaches and berries was associated with lower risk of ER− breast cancer [43].
There are several possible factors that may underlie the inverse association of fresh and dried fruit intakes with breast cancer. Fruits contain rich potential anti-carcinogenic nutrients, including fiber, carotenoids, and other bioactive substances [[44], [45], [46]]. In particular, carotenoids have potent cytotoxic and antiproliferative effects on cancer cells [47]. This is consistent with previous studies showing an inverse relationship between carotenoids and breast cancer, especially ER− breast cancer [44,48]. Some studies also discovered that bioactive compounds in grapes can modulate breast cancer cell signaling, apoptosis, and metastasis [49,50]. Another study has showed that ursolic acid, which is widely distributed in different fruits, can induce apoptosis in breast cancer cells [51].
Notably, dried fruits are obtained by dehydrating fresh fruits, resulting in a more concentrated nutrient profile [52]. Particularly, drying concentrates the content of phytonutrients, including polyphenols, thereby increasing their antioxidant activity [53]. Polyphenols were found to inhibit breast cancer by modulating DNA methylation and histone modifications, restoring the expression of tumor suppressor genes, and inhibiting cancer cell proliferation and metastasis [54,55]. A cohort study in the UK reported that women who ate dried fruit regularly had a 40 % lower risk of developing breast cancer [56]. Consistent with our findings, a recent MR study on the association of dried fruit intake with the risk of 11 types of cancer showed that dried fruit was a protective factor for breast cancer [57]. Compared to previous studies, our MR study used an updated and more comprehensive GWAS database of breast cancer and conducted subtype analyses based on ER status.
The findings of our study have practical significance for clinicians to enhance health education for breast cancer patients. Encouraging high fruit intake could reduce the risk of developing breast cancer in those at high risk of the disease. However, further research is needed to elucidate the underlying biological mechanisms and confirm the clinical significance. Future studies may explore specific molecular pathways involved in the observed association between dietary factors and breast cancer. In addition, randomized controlled trials may be necessary to validate the preventive effects of dried and fresh fruit intakes on breast cancer. These future research efforts will contribute to breast cancer prevention and have important implications for public health.
Traditional observational studies have shown associations between intakes of vegetables, fish, cheese, and bread and breast cancer. A study using data from two large prospective studies demonstrated that higher intake of vegetables may decrease the risk of breast cancer [41]. In 2020, a case-control study involving the Polish population found that eliminating fish from the diet increased the risk of breast cancer [58]. There has been increasing reporting on the association between cheese intake and breast cancer risk during recent years [59,60]. Additionally, a case-control study that included 2569 women with breast cancer and 2588 controls indicated a positive link between bread intake and breast cancer risk [13]. However, the MR analysis failed to detect a significant association of vegetable, fish, cheese, and bread intakes with breast cancer (p > 0.05). Although traditional observational studies can provide some initial insight into the relationship between dietary factors and breast cancer, their results may be affected by various confounding factors [61,62]. Moreover, our results differed from previous observation studies, possibly due to the sample size of the GWAS dataset was not large enough to generate statistical differences. Therefore, randomized controlled trials remain the most accurate method for determining the link between dietary factors and breast cancer.
This study has several strengths. First, this study explored the causal associations between a range of dietary factors and breast cancer through MR analysis, which can avoid the influence of confounding factors. Second, SNPs used for IV were strongly correlated with dietary intake, excluding potential weak instrumental bias. Additionally, the MR-PRESSO method was applied to identify and correct for bias caused by outliers.
However, there are some limitations in this study. First, the participants included in the exposure and outcome were all of European ancestry, thus, whether our findings can be generalized to other ethnic groups needs further study. Furthermore, there may be some participants overlap in our study, which may cause the results to be overestimated. In addition, we could not determine whether there was a dose-response relationship between dietary factors and breast cancer. Finally, we cannot stratify analyses by sex due to the lack of sex-specific summary-level GWAS data. Future GWAS studies on dietary factors need to differentiate between male and female subjects.
5. Conclusion
Our study provides evidence that dried fruit intake may reduce the risk of both ER+ and ER− breast cancer, and fresh fruit intake may reduce the risk of ER− breast cancer. This study also found that salad/raw vegetable intake, cooked vegetable intake, oily fish intake, non-oily fish intake, cheese intake, and bread intake were not causally associated with breast cancer. These results provide important insights into the potential risk and protective factors for breast cancer. Therefore, appropriate dietary modification may help to prevent breast cancer.
Data availability statement
Data associated with this study were obtained from the IEU Open GWAS project (https://gwas.mrcieu.ac.uk/).
Funding
Supported by Science and Technology Department of Jiangxi Province Project (20224BAB206080), Health Commission of Jiangxi Province Project (202211009), and Jiangxi Postgraduate Innovation Fund (YC2022-s206).
CRediT authorship contribution statement
Chengdong Yu: Conceptualization, Data curation, Writing – original draft, Writing – review & editing, Methodology, Software. Jiawei Xu: Data curation, Funding acquisition, Resources, Supervision, Writing – review & editing. Siyi Xu: Data curation, Formal analysis, Methodology, Validation, Writing – review & editing. Huoping Peng: Data curation, Resources. Lei Tang: Data curation, Resources. Zhengkui Sun: Conceptualization, Validation, Writing – review & editing. Wen Chen: Funding acquisition, Validation, Writing – original draft, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Thanks to the MRC Integrative Epidemiology Unit (IEU) at the University of Bristol for developing the IEU open GWAS project. Thank them for deriving relevant summary-level GWAS data from UK Biobank and BCAC.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e20980.
Contributor Information
Zhengkui Sun, Email: sunzhengkui2023@163.com.
Wen Chen, Email: chenwen2066@163.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
References
- 1.Sung H., Ferlay J., Siegel R.L., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries [J] CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Loibl S., Poortmans P., Morrow M., et al. Breast cancer [J] Lancet. 2021;397(10286):1750–1769. doi: 10.1016/S0140-6736(20)32381-3. [DOI] [PubMed] [Google Scholar]
- 3.Britt K.L., Cuzick J., Phillips K.A. Key steps for effective breast cancer prevention [J] Nat. Rev. Cancer. 2020;20(8):417–436. doi: 10.1038/s41568-020-0266-x. [DOI] [PubMed] [Google Scholar]
- 4.Arnold M., Morgan E., Rumgay H., et al. Current and future burden of breast cancer: global statistics for 2020 and 2040 [J] Breast. 2022;66:15–23. doi: 10.1016/j.breast.2022.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Toledo E., Salas-Salvado J., Donat-Vargas C., et al. Mediterranean diet and invasive breast cancer risk among women at high cardiovascular risk in the predimed trial: a randomized clinical trial [J] JAMA Intern. Med. 2015;175(11):1752–1760. doi: 10.1001/jamainternmed.2015.4838. [DOI] [PubMed] [Google Scholar]
- 6.Hiatt R.A., Brody J.G. Environmental determinants of breast cancer [J] Annu Rev Public Health. 2018;39:113–133. doi: 10.1146/annurev-publhealth-040617-014101. [DOI] [PubMed] [Google Scholar]
- 7.Campbell N.J., Barton C., Cutress R.I., et al. Impact of obesity, lifestyle factors and health interventions on breast cancer survivors [J] Proc. Nutr. Soc. 2023;82(1):47–57. doi: 10.1017/S0029665122002816. [DOI] [PubMed] [Google Scholar]
- 8.Papadimitriou N., Markozannes G., Kanellopoulou A., et al. An umbrella review of the evidence associating diet and cancer risk at 11 anatomical sites [J] Nat. Commun. 2021;12(1):4579. doi: 10.1038/s41467-021-24861-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kazemi A., Barati-Boldaji R., Soltani S., et al. Intake of various food groups and risk of breast cancer: a systematic review and dose-response meta-analysis of prospective studies [J] Adv. Nutr. 2021;12(3):809–849. doi: 10.1093/advances/nmaa147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farvid M.S., Barnett J.B., Spence N.D. Fruit and vegetable consumption and incident breast cancer: a systematic review and meta-analysis of prospective studies [J] Br. J. Cancer. 2021;125(2):284–298. doi: 10.1038/s41416-021-01373-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng J.S., Hu X.J., Zhao Y.M., et al. Intake of fish and marine n-3 polyunsaturated fatty acids and risk of breast cancer: meta-analysis of data from 21 independent prospective cohort studies [J] BMJ. 2013;346:f3706. doi: 10.1136/bmj.f3706. [DOI] [PubMed] [Google Scholar]
- 12.Wu Y., Huang R., Wang M., et al. Dairy foods, calcium, and risk of breast cancer overall and for subtypes defined by estrogen receptor status: a pooled analysis of 21 cohort studies [J] Am. J. Clin. Nutr. 2021;114(2):450–461. doi: 10.1093/ajcn/nqab097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Augustin L.S., Malerba S., Lugo A., et al. Associations of bread and pasta with the risk of cancer of the breast and colorectum [J] Ann. Oncol. 2013;24(12):3094–3099. doi: 10.1093/annonc/mdt383. [DOI] [PubMed] [Google Scholar]
- 14.Bellicha A., Wendeu-Foyet G., Coumoul X., et al. Dietary exposure to acrylamide and breast cancer risk: results from the NutriNet-Sante cohort [J] Am. J. Clin. Nutr. 2022;116(4):911–919. doi: 10.1093/ajcn/nqac167. [DOI] [PubMed] [Google Scholar]
- 15.Watling C.Z., Schmidt J.A., Dunneram Y., et al. Risk of cancer in regular and low meat-eaters, fish-eaters, and vegetarians: a prospective analysis of UK Biobank participants [J] BMC Med. 2022;20(1):73. doi: 10.1186/s12916-022-02256-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fraser G.E., Jaceldo-Siegl K., Orlich M., et al. Dairy, soy, and risk of breast cancer: those confounded milks [J] Int. J. Epidemiol. 2020;49(5):1526–1537. doi: 10.1093/ije/dyaa007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Key T.J., Appleby P.N., Crowe F.L., et al. Cancer in British vegetarians: updated analyses of 4998 incident cancers in a cohort of 32,491 meat eaters, 8612 fish eaters. 18,298 vegetarians, and 2246 vegans [J]. Am J Clin Nutr. 2014;100(Suppl 1):378S. doi: 10.3945/ajcn.113.071266. 1. 85S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Key T.J., Balkwill A., Bradbury K.E., et al. Foods, macronutrients and breast cancer risk in postmenopausal women: a large UK cohort [J] Int. J. Epidemiol. 2019;48(2):489–500. doi: 10.1093/ije/dyy238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zuber V., Grinberg N.F., Gill D., et al. Combining evidence from Mendelian randomization and colocalization: review and comparison of approaches [J] Am. J. Hum. Genet. 2022;109(5):767–782. doi: 10.1016/j.ajhg.2022.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yuan Z., Liu L., Guo P., et al. Likelihood-based Mendelian randomization analysis with automated instrument selection and horizontal pleiotropic modeling [J] Sci. Adv. 2022;8(9) doi: 10.1126/sciadv.abl5744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yarmolinsky J., Wade K.H., Richmond R.C., et al. Causal inference in cancer Epidemiology: what is the role of mendelian randomization? [J] Cancer Epidemiol. Biomarkers Prev. 2018;27(9):995–1010. doi: 10.1158/1055-9965.EPI-17-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sekula P., Del Greco M.F., Pattaro C., et al. Mendelian randomization as an approach to assess causality using observational data [J] J. Am. Soc. Nephrol. 2016;27(11):3253–3265. doi: 10.1681/ASN.2016010098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Emdin C.A., Khera A.V., Kathiresan S. Mendelian randomization [J] JAMA. 2017;318(19):1925–1926. doi: 10.1001/jama.2017.17219. [DOI] [PubMed] [Google Scholar]
- 24.Davies N.M., Holmes M.V., Davey Smith G. Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians [J] BMJ. 2018;362:k601. doi: 10.1136/bmj.k601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harrison S., Davies A.R., Dickson M., et al. The causal effects of health conditions and risk factors on social and socioeconomic outcomes: Mendelian randomization in UK Biobank [J] Int. J. Epidemiol. 2020;49(5):1661–1681. doi: 10.1093/ije/dyaa114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spiga F., Gibson M., Dawson S., et al. Tools for assessing quality and risk of bias in Mendelian randomization studies: a systematic review [J] Int. J. Epidemiol. 2023;52(1):227–249. doi: 10.1093/ije/dyac149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Michailidou K., Lindstrom S., Dennis J., et al. Association analysis identifies 65 new breast cancer risk loci [J] Nature. 2017;551(7678):92–94. doi: 10.1038/nature24284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Connor L.J., Price A.L. Distinguishing genetic correlation from causation across 52 diseases and complex traits [J] Nat. Genet. 2018;50(12):1728–1734. doi: 10.1038/s41588-018-0255-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burgess S., Thompson S.G. Use of allele scores as instrumental variables for Mendelian randomization [J] Int. J. Epidemiol. 2013;42(4):1134–1144. doi: 10.1093/ije/dyt093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burgess S., Thompson S.G., Collaboration C.C.G. Avoiding bias from weak instruments in Mendelian randomization studies [J] Int. J. Epidemiol. 2011;40(3):755–764. doi: 10.1093/ije/dyr036. [DOI] [PubMed] [Google Scholar]
- 31.Hartwig F.P., Davey Smith G., Bowden J. Robust inference in summary data Mendelian randomization via the zero modal pleiotropy assumption [J] Int. J. Epidemiol. 2017;46(6):1985–1998. doi: 10.1093/ije/dyx102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cohen J.F., Chalumeau M., Cohen R., et al. Cochran's Q test was useful to assess heterogeneity in likelihood ratios in studies of diagnostic accuracy [J] J. Clin. Epidemiol. 2015;68(3):299–306. doi: 10.1016/j.jclinepi.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 33.Bowden J., Davey Smith G., Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression [J] Int. J. Epidemiol. 2015;44(2):512–525. doi: 10.1093/ije/dyv080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hemani G., Bowden J., Davey Smith G. Evaluating the potential role of pleiotropy in Mendelian randomization studies [J] Hum. Mol. Genet. 2018;27(R2):R195–R208. doi: 10.1093/hmg/ddy163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Verbanck M., Chen C.Y., Neale B., et al. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases [J] Nat. Genet. 2018;50(5):693–698. doi: 10.1038/s41588-018-0099-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sanderson E. Multivariable mendelian randomization and mediation [J] Cold Spring Harb Perspect Med. 2021;11(2) doi: 10.1101/cshperspect.a038984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yavorska O.O., Burgess S. MendelianRandomization: an R package for performing Mendelian randomization analyses using summarized data [J] Int. J. Epidemiol. 2017;46(6):1734–1739. doi: 10.1093/ije/dyx034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pierce B.L., Ahsan H., Vanderweele T.J. Power and instrument strength requirements for Mendelian randomization studies using multiple genetic variants [J] Int. J. Epidemiol. 2011;40(3):740–752. doi: 10.1093/ije/dyq151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fung T.T., Hu F.B., Holmes M.D., et al. Dietary patterns and the risk of postmenopausal breast cancer [J] Int. J. Cancer. 2005;116(1):116–121. doi: 10.1002/ijc.20999. [DOI] [PubMed] [Google Scholar]
- 40.Jung S., Spiegelman D., Baglietto L., et al. Fruit and vegetable intake and risk of breast cancer by hormone receptor status [J] J Natl Cancer Inst. 2013;105(3):219–236. doi: 10.1093/jnci/djs635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Farvid M.S., Chen W.Y., Rosner B.A., et al. Fruit and vegetable consumption and breast cancer incidence: repeated measures over 30 years of follow-up [J] Int. J. Cancer. 2019;144(7):1496–1510. doi: 10.1002/ijc.31653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fung T.T., Hu F.B., Mccullough M.L., et al. Diet quality is associated with the risk of estrogen receptor-negative breast cancer in postmenopausal women [J] J. Nutr. 2006;136(2):466–472. doi: 10.1093/jn/136.2.466. [DOI] [PubMed] [Google Scholar]
- 43.Fung T.T., Chiuve S.E., Willett W.C., et al. Intake of specific fruits and vegetables in relation to risk of estrogen receptor-negative breast cancer among postmenopausal women [J] Breast Cancer Res. Treat. 2013;138(3):925–930. doi: 10.1007/s10549-013-2484-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eliassen A.H., Hendrickson S.J., Brinton L.A., et al. Circulating carotenoids and risk of breast cancer: pooled analysis of eight prospective studies [J] J Natl Cancer Inst. 2012;104(24):1905–1916. doi: 10.1093/jnci/djs461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Farvid M.S., Eliassen A.H., Cho E., et al. Dietary fiber intake in young adults and breast cancer risk [J] Pediatrics. 2016;137(3) doi: 10.1542/peds.2015-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Farvid M.S., Holmes M.D., Chen W.Y., et al. Postdiagnostic fruit and vegetable consumption and breast cancer survival: prospective analyses in the nurses' health studies [J] Cancer Res. 2020;80(22):5134–5143. doi: 10.1158/0008-5472.CAN-18-3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saini R.K., Keum Y.S., Daglia M., et al. Dietary carotenoids in cancer chemoprevention and chemotherapy: a review of emerging evidence [J] Pharmacol. Res. 2020;157 doi: 10.1016/j.phrs.2020.104830. [DOI] [PubMed] [Google Scholar]
- 48.Bakker M.F., Peeters P.H., Klaasen V.M., et al. Plasma carotenoids, vitamin C, tocopherols, and retinol and the risk of breast cancer in the European Prospective Investigation into Cancer and Nutrition cohort [J] Am. J. Clin. Nutr. 2016;103(2):454–464. doi: 10.3945/ajcn.114.101659. [DOI] [PubMed] [Google Scholar]
- 49.Ferraz Da Costa D.C., Pereira Rangel l, Quarti J., et al. Bioactive compounds and metabolites from grapes and red wine in breast cancer chemoprevention and therapy [J] Molecules. 2020;25(15) doi: 10.3390/molecules25153531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grimes K.L., Stuart C.M., Mccarthy J.J., et al. Enhancing the cancer cell growth inhibitory effects of table grape anthocyanins [J] J. Food Sci. 2018;83(9):2369–2374. doi: 10.1111/1750-3841.14294. [DOI] [PubMed] [Google Scholar]
- 51.Yin R., Li T., Tian J.X., et al. Ursolic acid, a potential anticancer compound for breast cancer therapy [J] Crit. Rev. Food Sci. Nutr. 2018;58(4):568–574. doi: 10.1080/10408398.2016.1203755. [DOI] [PubMed] [Google Scholar]
- 52.Alasalvar C., Salvado J.S., Ros E. Bioactives and health benefits of nuts and dried fruits [J] Food Chem. 2020;314 doi: 10.1016/j.foodchem.2020.126192. [DOI] [PubMed] [Google Scholar]
- 53.Sadler M.J., Gibson S., Whelan K., et al. Dried fruit and public health - what does the evidence tell us? [J] Int. J. Food Sci. Nutr. 2019;70(6):675–687. doi: 10.1080/09637486.2019.1568398. [DOI] [PubMed] [Google Scholar]
- 54.Selvakumar P., Badgeley A., Murphy P., et al. Flavonoids and other polyphenols act as epigenetic modifiers in breast cancer [J] Nutrients. 2020;12(3) doi: 10.3390/nu12030761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Briguglio G., Costa C., Pollicino M., et al. Polyphenols in cancer prevention: new insights (Review) [J] Int J Funct Nutr. 2020;1(2):9. [Google Scholar]
- 56.Dunneram Y., Greenwood D.C., Cade J.E. Diet and risk of breast, endometrial and ovarian cancer: UK Women's Cohort Study [J] Br. J. Nutr. 2019;122(5):564–574. doi: 10.1017/S0007114518003665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jin C., Li R., Deng T., et al. Association between dried fruit intake and pan-cancers incidence risk: a two-sample Mendelian randomization study [J] Front. Nutr. 2022;9 doi: 10.3389/fnut.2022.899137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dydjow-Bendek D., Zagozdzon P. Total dietary fats, fatty acids, and omega-3/omega-6 ratio as risk factors of breast cancer in the polish population - a case-control study. J]. In Vivo. 2020;34(1):423–431. doi: 10.21873/invivo.11791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nilsson L.M., Winkvist A., Esberg A., et al. Dairy products and cancer risk in a northern Sweden population [J] Nutr. Cancer. 2020;72(3):409–420. doi: 10.1080/01635581.2019.1637441. [DOI] [PubMed] [Google Scholar]
- 60.Wajszczyk B., Charzewska J., Godlewski D., et al. Consumption of dairy products and the risk of developing breast cancer in polish women [J] Nutrients. 2021;13(12) doi: 10.3390/nu13124420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang M.T., Bolland M.J., Grey A. Reporting of limitations of observational research [J] JAMA Intern. Med. 2015;175(9):1571–1572. doi: 10.1001/jamainternmed.2015.2147. [DOI] [PubMed] [Google Scholar]
- 62.Nguyen V.T., Engleton M., Davison M., et al. Risk of bias in observational studies using routinely collected data of comparative effectiveness research: a meta-research study [J] BMC Med. 2021;19(1):279. doi: 10.1186/s12916-021-02151-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data associated with this study were obtained from the IEU Open GWAS project (https://gwas.mrcieu.ac.uk/).