Abstract
Herein, we reported carbon paste electrode modified with graphitic carbon nitride (g–C3N4–CPE) to determine of tryptophan (Trp) using voltametric techniques. Various spectroscopic and electrochemical techniques were used to characterize the as-synthesized g-C3N4 and the assembled electrodes. The transfer coefficient, rate constant and the diffusion coefficient of Trp in this system were found to be 0.28, 1.9 × 104 M−1s−1 and 3.2 × 10−5 cm2s−1, respectively. The linear range was obtained for the detection of Trp using LSV is from 0.1 μM to 120 μM at pH 5. The limit of detection (LOD) (3σ/m) was 0.085 μM. The demonstrated modified CPE was also effectively used for the detection of Trp in milk with percentage recovery of 98 %–105.2 %. Furthermore, the modified CPE exhibited good repeatability, reproducibility and appropriate selectivity.
Keywords: Electrochemical detection, Tryptophan, Graphitic carbon nitride modified carbon paste electrode, Cyclic voltammetry, Linear sweep voltammetry
Graphical abstract

Highlights
-
•
g–C3N4–CPE were prepared for voltammetric determination of Trp.
-
•
g-C3N4-CPE showed superior electrochemical characteristics and increased anodic peak current of Trp than bare CPE.
-
•
The higher signal at g-C3N4-CPE is due to the higher efficiency of electron transfer due to the presence of g-C3N4.
1. Introduction
Tryptophan (Trp) is an essential amino acid with biochemical, nutritional, and medical significance in humans [1]. It is useful for various important issues, such as for nitrogen balance in human development, production of niacin, which is very essential for secretion of serotonin and melatonin neurotransmitters [2,3]. Insufficient amount of Trp in human body results in less amount of these neurotransmitters thus, sleep disorders and depression may be occurred [4,5]. Hence, it is of great importance to develop accurate and economical method to detect Trp in pharmaceutical, food products and biological samples.
Until now, numerous analytical detection methods have been established for the Trp sensing such as chromatography [6], chemiluminescence [7], capillary electrophoresis [8], spectroscopic detection [9]. As Trp is electroactive, electrochemical methods with high sensitivity, selectivity, fast response and easy operation gained more attention. However, it is still difficult to measure Trp directly at unmodified electrodes due to the slowness of the redox process leads to high overpotential at the electrode [10,11].
Carbon nitrides are a type of polymeric substances primarily made up of carbon and nitrogen. One of the carbon nitride allotropes with interesting characteristics is graphitic carbon nitride (g-C3N4), which has a high surface area and superior electrocatalytic activity. In addition, its high thermal and chemical stability allows it to function well in various chemical environments and at high temperatures [12,13]. g-C3N4 has been widely utilized for a variety of sensing applications. g-C3N4 nano-sheet modified carbon paste electrode (CPE) for methotrexate sensing was recently described by Saleh et al. [14]. Balasubramanian and co-workers successful synthesized and used g-C3N4 for voltammetric determination the antibiotic sulfamethoxazole [15]. A rapid voltammetric sensor for the detection of sulfathiazole and sulfapyridine was demonstrated by Javad et al. using CPE modified with g-C3N4 and manganese oxide [16]. Ilager and his co-workers developed cetyltrimethylammonium bromide and g-C3N4 modified CPE to determine trace amounts of the herbicides [17].
Adams was the first to introduce the carbon paste electrode (CPE), one of the widely used electrodes [18]. CPEs are made up of graphite powder and liquid binder assembled into a properly prepared electrode body [19]. Due to its unique features, such as low cost, superior electrical characteristics, large potential windows, and easy surface renewability, CPEs have been used extensively as voltametric transducers for the determination of important electroactive substances [[20], [21], [22]]. Other than the interesting properties listed before, the possibility of modification of CPE by incorporating various substances during preparation has become widely employed to enhance electron transfer process for sensitive determination of electroactive species [23]. For instance modified CPEs have been commonly used for sensitive analysis of trace heavy metals [24,25], biologically important substances [26], drugs [27], food [28] and so on. In this paper, we showed how to easily create a g–C3N4–CPE for sensitive electrochemical detection for Trp. In comparison to bare CPE, the g–C3N4–CPE showed superior electrochemical catalytic characteristics, less overpotential and increased anodic peak current of Trp. Furthermore, the suggested electrode showed excellent repeatability, reproducibility, and a suitable detection limit. Trp in a milk sample was successfully determined using the produced g–C3N4–CPE. The approach used in this study can also be used to other electroactive compounds.
2. Materials and methods
2.1. Chemicals
Trp (C11H12N2O2, 99.9 %) (Sinopharm Chemical Reagent, China), urea (CH4N2O, 99 % (Alfa Aesar Chemical Reagent, China), phosphoric acid (H3PO4, 85 %, (Riedel de Haen, Germany), sodium hydroxide (NaOH, 98 %, Loba Chemie, India), potassium chloride (KCl, 99.8 %) (Aladdin Reagents, China), graphite powder (98 %) (BDH, England), potassium dihydrogen phosphate (KH2PO4, 99.5 %) (Aladdin Reagents, China), Potassium phosphate dibasic (K2HPO4, 99 %) (Aladdin Reagents, China), paraffin oil (light, density:0.84–0.86) (Aladdin Reagents, China) were used. All the aqueous solutions were prepared with double distilled water.
2.2. Instrumentations
The surface structure of the prepared g-C3N4 was examined using scanning electron microscopy (JCM-6000 plus). The X-ray diffraction (XRD) (XPERT-PRO, The Netherlands) spectroscopy was used to depict the crystal structure of g-C3N4. Fourier transform infrared spectroscopy (FT-IR) (JASCO 4600LE spectrometer) was used to analyze the functional groups. Epsilon (Bioanalytical System, USA) was used for investigate CV and LSV experiments. As a working, reference, and auxiliary electrode, respectively, we used g–C3N4–CPE, Ag/AgCl (saturated KCl), and platinum wire.
2.3. Preparation of g-C3N4
In a muffle furnace, 10 g of urea was heated to 450 °C, 500 °C, 550 °C, and 600 °C at a rate of 5 °C min−1 and kept at each temperature for 2 h under air conditions. Yellow-colored g-C3N4 was found after cooling to room temperature. Then, it was crushed and preserved until used [29].
2.4. Sample preparation
10 mM Trp was prepared as a stock solution using double distilled water and working solution of Trp was prepared by diluting a required volume of stock solution of Trp in 0.1 M PBS of pH 5.0 at the day of the experiment. As a supporting electrolyte, 0.1 M phosphate buffer solutions (PBS), pH 5.0, were prepared using 0.1 M KH2PO4 and Na2HPO4.
2.5. Preparation of bare CPE
To prepare CPE, paraffin oil (20 % (w/w)) and graphite powder (80 % (w/w)) were homogenized in a mortar and pestle for 1 h. A Teflon tube (3 mm in diameter) was filled with the resulting paste. At the opposite end of the Teflon tube copper wire was inserted for electrical contact. Finally, a clean, smooth sheet of weighing paper was used to smooth the surface of the assembled CPE.
2.6. Preparation of g–C3N4–CPE
The g–C3N4–CPE was prepared by mixing various compositions of g-C3N4 and graphite powder as shown in Table 1. The graphite powder, g-C3N4 and paraffin liquid were mixed uniformly for 1 h. Then the homogenized paste was filled in a Teflon tube in the same manner as unmodified CPE was prepared.
Table 1.
The Compositions of g–C3N4–CPE tested.
| No | Composition (%w/w) |
||
|---|---|---|---|
| g-C3N4 | Graphite powder | Paraffin oil | |
| 1 | 10 | 70 | 20 |
| 2 | 15 | 65 | 20 |
| 3 | 20 | 60 | 20 |
| 4 | 25 | 55 | 20 |
| 5 | 30 | 50 | 20 |
| 6 | 35 | 45 | 20 |
2.7. Real sample analysis
For real samples analysis, the percentage recoveries were done by spiking different standard solution of Trp. Fresh milk was obtained from a market, in Jimma, Ethiopia. Firstly, 1 mL of 0.01 M HCl was mixed with 5.0 mL of the purchased milk and agitated for 10 min [30]. Then, the supernatant was taken after the resulting solution was centrifuged for 20 min at 8000 rpm. Then, Trp was detected to prove the capability of the proposed modified CPE to sense Trp in milk sample. Determination of Trp in milk samples was taken place under the optimum experimental conditions.
3. Results and discussion
3.1. Characterization of g-C3N4
Fig. 1A shows the results of the XRD analysis of the crystal structure of the prepared g-C3N4. The polymerization temperatures used to produce the g-C3N4 ranged from 450 to 600 °C. Two distinct peaks in the XDR pattern, at 13.2° and 27.6°, respectively, are attributed to the planes of the (100) and (002) crystal planes of g-C3N4 (JPCDS No. 87–1526) [31]. The (002) reflection of an aromatic graphitic structure is responsible for the high peak found at 27.6°, and a weak diffraction peak at 13.2° is due to the heptazine network [32].
Fig. 1.
XRD patterns (A), FT-IR spectra (B), C 1s (C) and N 1s (D) XPS spectra of g-C3N4 synthesized at different temperatures.
Fig. 1B, displays the FT-IR spectra of the g-C3N4 synthesized at different polymerization temperatures. The absorption peak at 810 cm−1 denotes the formation of g-C3N4 with triazine or heptazine rings. The peak between 1200 and 1650 cm−1 are given to the typical stretching modes of carbon nitride heterocycles [33], the peak seen in the region of 3000–3500 cm−1 was due to N–H stretchings [34].
The chemical states of nitrogen and carbon in the synthesized g-C3N4 were also examined using XPS. The C 1s spectra of the g-C3N4 produced at 450, 500, 550, and 600 °C are shown in Fig. 1C. The peaks identified as C–C/C–C, C-NHx, and N–C–N at 284.7, 286.5, and 288.7 eV, respectively. The N 1s spectra for g-C3N4 at different temperatures were obtained at approximately 398.7, 399.9, and 400.9 eV (Fig. 1D), and they can be referred to the N–C–N (N(C)2), N–C–N (N(C)3), and N-Hx bonds, respectively [35,36].
Using the SEM technique, the surface morphology of the g-C3N4 was examined. SEM images show the prepared g-C3N4 are composed of loaded layered structure (Fig. 2A–D) [37].
Fig. 2.
Images taken using SEM for g-C3N4 at 450 °C (A), 500 °C (B), 550 °C (C), and 600 °C (D).
3.2. CV and EIS characterization of bare CPE and g–C3N4–CPE
Using CV and EIS, the electrical response of bare and modified CPE was studied in 0.1 mM [Fe(CN)6]3-/4- containing 0.1 M KCl. Both bare CPE and g–C3N4–CPE exhibited a pair of redox peaks, as shown in Fig. 3A. However, compared to bare CPE, g–C3N4–CPE displayed stronger redox peak currents. This is because g-C3N4 enhanced the conductivity of the modified CPE which enables rapid transfer of electron at the electrode-solution interface [38]. EIS is also a vital tool for analyzing the interfacial electrochemical behavior of electrodes [39]. For the purpose of evaluating the impedance of the bare CPE and g–C3N4–CPE, EIS experiments were conducted in [Fe(CN)6]3-/4- solution. The charge transfer resistance (Rct) at the electrode surface correlates to the diameter of the semicircle in the Nyquist plot. As shown in Fig. 3B, g–C3N4–CPE (curve b) exhibits a smaller semicircle than bare CPE (curve a), corresponding to a lower Rct which is consistent with the CV results.
Fig. 3.
(A) CVs and (B) the Impedance spectra ((a) bare CPE and (b) g–C3N4–CPE) in 0.1 M KCl containing 0.1 mM [Fe(CN)6]3−/4−; (C) CVs of the g–C3N4–CPE in [Fe(CN)6]3- at different scan rates for EASA calculation (The inset: I vs. ν1/2 plot); (D) CVs of bare CPE in blank (a) & in 10 μM Trp (c) and g–C3N4–CPE in blank (b) & in 10 μM Trp (d) in 0.1 M pH 5.0 PBS at 100 mV/s scan rate.
For the purpose of determining electrochemical active surface area (EASA), the CVs were measured in [Fe(CN)6]3-/4- solution at different scan rates (10–100 mV/s) on both bare CPE and g–C3N4–CPE (Fig. 3C). Using the Randles-Sevcik equation, the EASA of the electrode was calculated. The following equation (Eq. (1)) describes the peak current (Ip) [40].
| (1) |
where n denotes the electron number (1 for [Fe(CN)6]3-), A denotes the surface area, D denotes the diffusion coefficient (7.6 × 10−6 cm2s−1), C is the [Fe(CN)6]3−concentration (1 × 10−4 molcm−3) and v denotes the scan rate (Vs−1). The EASA of bare CPE and g–C3N4–CPE was found to be 0.34 and 0.72 cm2, respectively.
3.3. Cyclic voltammetric study of Trp at bare CPE and g–C3N4–CPE
Fig. 3D shows the voltammograms of bare CPE and g–C3N4–CPE in the blank PBS and in the presence of Trp. In blank PBS, both bare CPE and g–C3N4–CPE (curves a and b) shows no redox peaks. In 10 μM Trp solution, both electrodes showed anodic peaks (curves c and d). No cathodic peaks were observed for Trp in backward scan, thus confirming the redox process of Trp is irreversible at bare CPE and g–C3N4–CPE. Furthermore, the oxidation peak for Trp was observed at an anodic peak potential (Epa) of 1.06 V for bare CPE with a anodic peak current (Ipa) value of 39.1 μA (curve c). For g–C3N4–CPE the Epa was observed at 1.02 V and Ipa of 268 μA (curve d). The higher oxidation peak current at g–C3N4–CPE is ascribed to the higher efficiency of electron transfer due to the presence of g-C3N4.
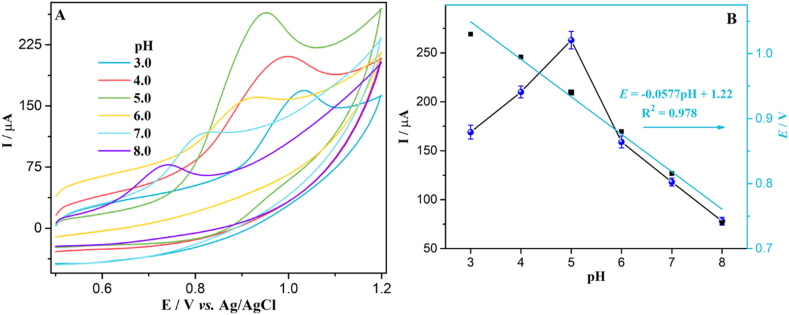
3.4. Effects of pH
As illustrated in Fig. 4, the pH of buffer on the anodic process of Trp at g–C3N4–CPE was examined by recording CVs of Trp in the range from 3.0 to 8.0 (0.1 M PBS). It is clearly seen that the anodic peak currents were changed with the change in the pH of buffer and highest anodic current for Trp was obtained at pH 5.0 (Fig. 4 A and B). Thus, PBS with pH 5.0 was chosen as optimum pH for detection of Trp in this work. Moreover, the oxidation potential was also affected by a change in pH. The anodic potential of Trp was shifted to a lower potential with rise in pH. This confirms the involvement of the proton in the anodic process. The oxidation potential of Trp is nearly directly related to pH with an equation of E(V) = −0.0577 pH + 1.22, R2 = 0.978 (Fig. 4B). The acquired slope value was then applied to the Nernst Eq. (2) [41].
| (2) |
where, n is the number of electron and m is the number of proton. Using Eq. (2) the value of m/n for Trp was found to be 0.976. This value suggests that the oxidation reaction of Trp at g–C3N4–CPE involves an identical number of protons and electrons. Scheme 1 illustrates the Trp oxidation mechanism at g–C3N4–CPE. Based on the obtained data, the anodic process of Trp at the g–C3N4–CPE was irreversible, two protons and two electrons reaction takes place at the amine in the indole ring and methyl in the side chain.
Fig. 4.
(A) Effect of pH on anodic process of 10 μM Trp at g–C3N4–CPE (B) Plot of oxidation current and potential of Trp as a function of pH.
Scheme 1.

Anodic reaction mechanism of Trp at g–C3N4–CPE.
3.5. Effects of scan rate
The electrode process of Trp at g–C3N4–CPE was explored by studying the relation between oxidation current and scan rate. The effect of scan rate on the anodic peak current of Trp at g–C3N4–CPE is shown in Fig. 5. The anodic peak currents (Ipa) increased when the scan rate increased from 10 to 200 mV/s (Fig. 5A). As shown in Fig. 5B and C, the values of peak current are linearly related with the scan rates (with a regression equation Ipa (μA) = 2.71v + 28.9 (R2 = 0.991)) than the square root of scan rates (with a regression equation Ipa (μA) = 35.97v2 – 63 (R2 = 0.942). This shows that the anodic reaction of Trp at the g–C3N4–CPE is an adsorption-controlled process. Fig. 5D displays a Tafel plot that was obtained using data from the Tafel region of the CV and detail information regarding the rate-determining step was obtained. The points of the Tafel region of the CV of Trp solution at the g–C3N4–CPE surface yield a 0.28 electron transfer coefficient (α), assuming that Trp undergo one electron transfer during the rate-determining phase [42].
Fig. 5.
(A) CVs for Trp in PBS (pH = 5.0) at g–C3N4–CPE with different scan rates: 10 to 200 mVs−1; (B) and (C) the plots of the linear relationship between I vs. v and I vs. v1/2; (D) CV of 10 μM Trp at 10 mVs−1 scan rate in 0.1 M PBS (pH 5.0) used for the Tafel plot (Inset: The Tafel plot).
3.6. Reaction kinetics for oxidation of Trp at g–C3N4–CPE
Chronoamperometry (CA) was employed to study the reaction kinetics of the g–C3N4–CPE system. Fig. 6 shows the CA response of the g–C3N4–CPE in blank and Trp solution in 0.1 M PBS (pH 5.0) at 1.2 V. The CA current response of Trp at g–C3N4–CPE (Ip) is significantly greater than the current response g–C3N4–CPE in blank (Ib). The kinetic rate constant (Kcat) can be calculated using Eq. (3) [43,44]:
| (3) |
where Ip is current response of g–C3N4–CPE in Trp and Ib the current response g–C3N4–CPE in blank, C is the concentration of Trp (mol cm−3), t is time (seconds), and reaction rate constant is represented as K. Accordingly, the value of Kcat is calculated as 1.9 × 104 M−1s−1 from the Ip/Ib vs. t1/2 plot presented in Fig. 6 (inset). Furthermore, the Cottrell equation can be used to compute the diffusion coefficient (D) of Trp (Eq. (4)) [43,44]:
| (4) |
Fig. 6.
CA response of g–C3N4–CPE in blank and in the presence of Trp in 0.1 M PBS pH 5 (Inset: plot of Ip/Ibvs. t1/2).
Ip stands for peak current (A), n for the number of electrons, F for the Faraday constant (96,500C mol−1), A for the electrode's surface area (cm2), C for the analyte's bulk concentration (mol cm−3), and t for the time (seconds). As a result, using Eq. (4), Trp has a value of D = 3.2 × 10−5 cm2s−1.
3.7. Effects of modifier composition
By varying the amounts of g-C3N4, paraffin oil, and graphite powder, the effect of g-C3N4 on the voltammetric response of Trp was investigated. As presented in Fig. 7A, the anodic current rises with increasing the amount of g-C3N4 from 10 % up to 30 % (w/w). Higher than 30 % (w/w) of g-C3N4 the peak currents decline considerably. This may be due to fewer amounts of graphite powder in the paste and thus decrease in the conductivity of the modified electrode. Therefore, 30 %, 50 % and 20 % (w/w) of g-C3N4, graphite powder and paraffin oil, respectively was chosen as the best composition for the modified CPE preparation.
Fig. 7.
(A) CVs showing effect of composition of g-C3N4 (a) 10 %, (b) 15 %, (c) 20 % (d) 25 %, (e) 30 %, and (f) 35 % on the oxidation current of Trp (Inset of Fig. 6A: Bar graph); (B) CVs showing effect of polymerization temperature of g-C3N4 (Inset of Fig. 6B: bar graph).
3.8. Effect of temperature
The dependency of the oxidation current of Trp on the temperature at which the g-C3N4 was synthesized was examined. By raising the polymerization temperature up to 550 °C the oxidation current of Trp was improved, but, the anodic current decreased when the temperature increases to 600 °C (Fig. 7B). Therefore, polymerization temperature of 550 °C was selected as an optimum temperature.
3.9. Calibration plot of Trp
Fig. 8 shows LSVs of different concentration of Trp at g–C3N4–CPE recorded at optimal experimental parameters. The anodic current of Trp was rises with increasing the concentration of Trp (Fig. 8A). The anodic current of Trp at g–C3N4–CPE is directly related to the Trp concentration in the range of 0.1 μM–120 μM (Fig. 8B), with regression equation:
Fig. 8.
(A) The LSV response for different concentration of Trp at g–C3N4–CPE; (B) Plot of linear calibration curve of Trp at g–C3N4–CPE at a pH of 5.0.
The calculated limit of detection (LOD) is about 0.085 μM (3σ/m) where σ is the standard deviation of the blank and m is the slope of the calibration equation. The dynamic linear range, the type of modifier used and LOD of the present work was compared with other related voltammetric techniques reported earlier for the detection of Trp and summarized in Table 2. We can confirm that the g–C3N4–CPE attained a comparable linear range, the type of modifier used and LOD with the previously reported electrochemical methods.
Table 2.
Comparison of the LOD and the linear range of Trp determined at various electrodes.
| Electrode | LOD (μM) | Linear range (μM) | Method | Ref. |
|---|---|---|---|---|
| Poly(glycine) modified carbon nanotube paste electrode | 0.42 | 20–100 | DPV | [45] |
| Nitrogen-doped ordered mesoporous carbon/GCE | 0.035 | 0.5–70 & 70–200 |
CV | [46] |
| Nanoporous carbon/GCE | 0.03 | 1–103 | Amperometry | [47] |
| NiO/carbon nanotube (CNT)/poly(3,4-ethylenedioxythiophene)/GCE | 0.21 | 1–41 | DPV | [48] |
| Polyvinylpyrrolidone coated gold nanoparticles modified GCE | 0.3 | 1–50 & 50–350 |
LSV | [49] |
| Co3O4 nanosheets/rGO composites/GCE | 0.26 | 1–800 | [50] | |
| Graphite electrode from waste batteries | 1.73 | 5–150 | DPV | [51] |
| Reduced graphene oxide with SnO2 nanoparticles/GCE | 0.04 | 1–100 | DPV | [52] |
| Graphene-Modified Electrode | 0.3 | 0.1–100 | LSV | [53] |
| g-C3N4-CPE | 0.085 | 0.1–120 | LSV | This work |
SWV - Square wave voltammetry; DPV - Differential wave Voltammetry.
3.10. Repeatability and reproducibility
The repeatability of g–C3N4–CPE was obtained by recording the oxidation current values for 10 μM Trp for 9 repeated measurements, thus, the RSD was obtained at 1.9 % (Fig. 9A). CA study was also checked for long time with presence and absence of Trp for the stability test and shows good stability (Inset of Fig. 9A). For reproducibility test, three different g–C3N4–CPEs, which were assembled separately by following the same assembly procedure were tested and RSD of 5.6 % for the determination of 10 μM Trp was attained (Fig. 9B). Therefore, we can conclude that acceptable repeatability and reproducibility was obtained for the determination of Trp at g–C3N4–CPE. Furthermore, the stability of the anodic current response of 10 μM Trp was tested at g–C3N4–CPE after the electrode was reserved at room temperature for one month. The observed result indicates that 92.5 % of the initial oxidation current value was obtained, signifying excellent stability.
Fig. 9.
(A) LSV response nine repeated measurement of 10 μM Trp at g–C3N4–CPE (Inset: Chronoamperogram curves of g–C3N4–CPE in blank and presence of Trp as stability test); (B) Reproducibility study of the g–C3N4–CPE for the detection of 10 μM Trp at three different modified electrodes.
3.11. Real sample analysis
The practical use of the demonstrated modified CPE for the sensing Trp was applied to detect Trp in milk sample by spiking a standard Trp (5, 10, 20 and 30 μM) and the %recovery was calculated. As summarized in Table 3, the recovery values of Trp in the milk sample were obtained from 98 % to 105.2 %.
Table 3.
The application of g–C3N4–CPE for determination of Trp in milk (n = 5).
| Sample | Spiked | Found | Recovery % |
|---|---|---|---|
| Milk | 0 | ND | – |
| 5 | 5.1 | 98 | |
| 10 | 9.8 | 102 | |
| 20 | 19.8 | 101 | |
| 30 | 28.5 | 105.2 |
3.12. Interference study
To evaluate the effect of selected interfering substances such as, ascorbic acid (AA), dopamine (DA), uric acid (UA), l-cysteine (L-cys), l-arginine (L-arg), l-alanine (L-ala), and methionine (Meth) were used as depicted in Fig. 10. It was found that 10 fold higher concentrations of the selected biological substances did not interfere in the analysis of Trp. This might be because the different redox potentials of the selected substances. Thus demonstrated modified CPE has outstanding selectivity towards Trp.
Fig. 10.
Effect of Interferences on a determination of Trp at g–C3N4–CPE.
4. Conclusion
In summary, in this work, g–C3N4–CPE was proposed and used for the voltammetric detection of Trp. The experimental result showed that g–C3N4–CPE was sensitive and its performance is comparable with other CPE based detection of Trp. The modified CPE exhibited lower detection limit, good repeatability, reproducibility and appropriate selectivity. In addition, it was effectively employed for the detection of Trp in the milk sample.
Data availability statement
Data will be made available on request.
CRediT authorship contribution statement
Habtamu Adefris Abebe: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. Abebe Diro: Investigation, Supervision, Validation. Shimeles Addisu Kitte: Conceptualization, Project administration, Resources, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors would like to acknowledge Jimma University School of Graduate Studies and Department of Chemistry for funding and material support.
References
- 1.Fiorucci A.R., Cavalheiro É.T.G. The use of carbon paste electrode in the direct voltammetric determination of tryptophan in pharmaceutical formulations. J. Pharmaceut. Biomed. Anal. 2002;28(5):909–915. doi: 10.1016/s0731-7085(01)00711-7. [DOI] [PubMed] [Google Scholar]
- 2.Young S.N., Leyton M. The role of serotonin in human mood and social interaction: insight from altered tryptophan levels. Pharmacol. Biochem. Behav. 2002;71(4):857–865. doi: 10.1016/s0091-3057(01)00670-0. [DOI] [PubMed] [Google Scholar]
- 3.Schaechter J.D., Wurtman R.J. Serotonin release varies with brain tryptophan levels. Brain Res. 1990;532(1):203–210. doi: 10.1016/0006-8993(90)91761-5. [DOI] [PubMed] [Google Scholar]
- 4.Benevenga N.J., Steele R.D. Adverse effects of excessive consumption of amino acids. Annu. Rev. Nutr. 1984;4(1):157–181. doi: 10.1146/annurev.nu.04.070184.001105. [DOI] [PubMed] [Google Scholar]
- 5.Yamashita M., Yamamoto T. Tryptophan and kynurenic acid may produce an amplified effect in central fatigue induced by chronic sleep disorder. Int. J. Tryptophan Res. 2014;7 doi: 10.4137/IJTR.S14084. IJTR.S14084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ravindran G., Bryden W.L. Tryptophan determination in proteins and feedstuffs by ion exchange chromatography. Food Chem. 2005;89(2):309–314. [Google Scholar]
- 7.Alwarthan A.A. Chemiluminescent determination of tryptophan in a flow injection system. Anal. Chim. Acta. 1995;317(1):233–237. [Google Scholar]
- 8.Altria K.D., Harkin P., Hindson M.G. Quantitative determination of tryptophan enantiomers by capillary electrophoresis. J. Chromatogr. B Biomed. Sci. Appl. 1996;686(1):103–110. doi: 10.1016/s0378-4347(96)00037-0. [DOI] [PubMed] [Google Scholar]
- 9.Ren J., Zhao M., Wang J., Cui C., Yang B. Spectrophotometric method for determination of tryptophan in protein hydrolysates. Food Technol. Biotechnol. 2007;45 [Google Scholar]
- 10.Gautam J., Raj M., Goyal R. Determination of tryptophan at carbon nanomaterials modified glassy carbon sensors: a comparison. J. Electrochem. Soc. 2020;167 [Google Scholar]
- 11.Huang K.-J., Xu C.-X., Xie W.-Z., Wang W. Electrochemical behavior and voltammetric determination of tryptophan based on 4-aminobenzoic acid polymer film modified glassy carbon electrode. Colloids Surf. B Biointerfaces. 2009;74(1):167–171. doi: 10.1016/j.colsurfb.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 12.Zhu J., Xiao P., Li H., Carabineiro S.A.C. Graphitic carbon nitride: synthesis, properties, and applications in catalysis. ACS Appl. Mater. Interfaces. 2014;6:16449–16465. doi: 10.1021/am502925j. [DOI] [PubMed] [Google Scholar]
- 13.Wang L., Wang K., He T., Zhao Y., Song H., Wang H. Graphitic carbon nitride-based photocatalytic materials: preparation strategy and application. ACS Sustain. Chem. Eng. 2020;8:16048–16085. [Google Scholar]
- 14.Elfarargy R.G., Saleh M.A., Abodouh M.M., Hamza M.A., Allam N.K. Graphitic carbon nitride nanoheterostructures as novel platforms for the electrochemical sensing of the chemotherapeutic and immunomodulator agent MTX. Biosensors. 2023;13(1):51. doi: 10.3390/bios13010051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Balasubramanian P., Settu R., Chen S.-M., Chen T.-W. Voltammetric sensing of sulfamethoxazole using a glassy carbon electrode modified with a graphitic carbon nitride and zinc oxide nanocomposite. Microchim. Acta. 2018;185(8):396. doi: 10.1007/s00604-018-2934-z. [DOI] [PubMed] [Google Scholar]
- 16.Javanshiri-Ghasemabadi J., Sadeghi S. Facile fabrication of an electrochemical sensor for the determination of two sulfonamide antibiotics in milk, honey and water samples using the effective modification of carbon paste electrode with graphitic carbon nitride and manganese oxide nanostructures. J. Food Compos. Anal. 2023;120 [Google Scholar]
- 17.Ilager D., Shetti N.P., Reddy K.R., Tuwar S.M., Aminabhavi T.M. Nanostructured graphitic carbon nitride (g-C3N4)-CTAB modified electrode for the highly sensitive detection of amino-triazole and linuron herbicides. Environ. Res. 2022;204 doi: 10.1016/j.envres.2021.111856. [DOI] [PubMed] [Google Scholar]
- 18.Adams R.N. Carbon paste electrodes. Anal. Chem. 1958;30 1576-1576. [Google Scholar]
- 19.Ghoreishi S.M., Behpour M., Ghoreishi F.S., Mousavi S. Voltammetric determination of tryptophan in the presence of uric acid and dopamine using carbon paste electrode modified with multi-walled carbon nanotubes. Arab. J. Chem. 2017;10:S1546–S1552. [Google Scholar]
- 20.Ghoreishi S.M., Behpour M., Jafari N., Khoobi A. Determination of tyrosine in the presence of sodium dodecyl sulfate using a gold nanoparticle modified carbon paste electrode. Anal. Lett. 2013;46:299–311. [Google Scholar]
- 21.Ganesh P.-S., Teradale A.B., Kim S.-Y., Ko H.-U., Ebenso E.E. Electrochemical sensing of anti-inflammatory drug mesalazine in pharmaceutical samples at polymerized-Congo red modified carbon paste electrode. Chem. Phys. Lett. 2022;806 [Google Scholar]
- 22.Ganesh P.-S., Kim S.-Y., Kaya S., Salim R. An experimental and theoretical approach to electrochemical sensing of environmentally hazardous dihydroxy benzene isomers at polysorbate modified carbon paste electrode. Sci. Rep. 2022;12(1):2149. doi: 10.1038/s41598-022-06207-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tashkhourian J., Nezhad M.R.H., Khodavesi J., Javadi S. Silver nanoparticles modified carbon nanotube paste electrode for simultaneous determination of dopamine and ascorbic acid. J. Electroanal. Chem. 2009;633:85–91. [Google Scholar]
- 24.Estrada-Aldrete J., Hernández-López J.M., García-León A.M., Peralta-Hernández J.M., Cerino-Córdova F.J. Electroanalytical determination of heavy metals in aqueous solutions by using a carbon paste electrode modified with spent coffee grounds. J. Electroanal. Chem. 2020;857 [Google Scholar]
- 25.Niu B., Yao B., Zhu M., Guo H., Ying S., Chen Z. Carbon paste electrode modified with fern leave-like MIL-47(as) for electrochemical simultaneous detection of Pb(II), Cu(II) and Hg(II) J. Electroanal. Chem. 2021;886 [Google Scholar]
- 26.Ashoka N.B., Swamy B.E.K., Jayadevappa H., Sharma S.C. Simultaneous electroanalysis of dopamine, paracetamol and folic acid using TiO2-WO3 nanoparticle modified carbon paste electrode. J. Electroanal. Chem. 2020;859 [Google Scholar]
- 27.Deepa S., Swamy B.E.K., Pai K.V. A surfactant SDS modified carbon paste electrode as an enhanced and effective electrochemical sensor for the determination of doxorubicin and dacarbazine its applications: a voltammetric study. J. Electroanal. Chem. 2020;879 [Google Scholar]
- 28.Penagos-Llanos J., García-Beltrán O., Calderón J.A., Hurtado-Murillo J.J., Nagles E., Hurtado J.J. Simultaneous determination of tartrazine, sunset yellow and allura red in foods using a new cobalt-decorated carbon paste electrode. J. Electroanal. Chem. 2019;852 [Google Scholar]
- 29.Melak F., Redi M., Tessema M., Alemayehu E. Electrochemical determination of catechol in tea samples using anthraquinone modified carbon paste electrode. Nat. Sci. 2013;5:888–894. [Google Scholar]
- 30.Wang X., Maeda K., Thomas A., Takanabe K., Xin G., Carlsson J.M., Domen K., Antonietti M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009;8(1):76–80. doi: 10.1038/nmat2317. [DOI] [PubMed] [Google Scholar]
- 31.Liu D., Zhang Z., Luo F., Wu J. Elemental mercury capture from simulated flue gas by graphite-phase carbon nitride. Energy & Fuels. 2020;34(6):6851–6861. [Google Scholar]
- 32.Fang H.-B., Luo Y., Zheng Y.-Z., Ma W., Tao X. Facile large-scale synthesis of urea-derived porous graphitic carbon nitride with extraordinary visible-light spectrum photodegradation. Ind. Eng. Chem. Res. 2016;55(16):4506–4514. [Google Scholar]
- 33.Xu J., Li Y., Peng S., Lu G., Li S. Eosin Y-sensitized graphitic carbon nitride fabricated by heating urea for visible light photocatalytic hydrogen evolution: the effect of the pyrolysis temperature of urea. Phys. Chem. Chem. Phys. 2013;15(20):7657–7665. doi: 10.1039/c3cp44687e. [DOI] [PubMed] [Google Scholar]
- 34.He Q., Tian Y., Wu Y., Liu J., Li G., Deng P., Chen D. Electrochemical sensor for rapid and sensitive detection of tryptophan by a Cu2O nanoparticles-coated reduced graphene oxide nanocomposite. Biomolecules. 2019;9(5):176. doi: 10.3390/biom9050176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chamorro-Posada P., Dante R.C., Vázquez-Cabo J., Dante D.G., Martín-Ramos P., Rubiños-López Ó., Sánchez-Arévalo F.M. From urea to melamine cyanurate: study of a class of thermal condensation routes for the preparation of graphitic carbon nitride. J. Solid State Chem. 2022;310 [Google Scholar]
- 36.Li C., Yang X., Yang B., Yan Y., Qian Y. Synthesis and characterization of nitrogen-rich graphitic carbon nitride. Mater. Chem. Phys. 2007;103(2):427–432. [Google Scholar]
- 37.Paul D.R., Sharma R., Nehra S.P., Sharma A. Effect of calcination temperature, pH and catalyst loading on photodegradation efficiency of urea derived graphitic carbon nitride towards methylene blue dye solution. RSC Adv. 2019;9(27):15381–15391. doi: 10.1039/c9ra02201e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu J., Xiao P., Li H., Carabineiro S.A.C. Graphitic carbon nitride: synthesis, properties, and applications in catalysis. ACS Appl. Mater. Interfaces. 2014;6(19):16449–16465. doi: 10.1021/am502925j. [DOI] [PubMed] [Google Scholar]
- 39.Magar H.S., Hassan R.Y.A., Mulchandani A. Electrochemical impedance spectroscopy (EIS): principles, construction, and biosensing applications. Sensors. 2021;21(19) doi: 10.3390/s21196578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bushira F.A., Kitte S.A., Wang Y., Li H., Wang P., Jin Y. Plasmon-boosted Cu-doped TiO2 oxygen vacancy-rich luminol electrochemiluminescence for highly sensitive detection of alkaline phosphatase. Anal. Chem. 2021;93(45):15183–15191. doi: 10.1021/acs.analchem.1c03842. [DOI] [PubMed] [Google Scholar]
- 41.Sundaresan R., Mariyappan V., Chen S.-M., Keerthi M., Ramachandran R. Electrochemical sensor for detection of tryptophan in the milk sample based on MnWO4 nanoplates encapsulated RGO nanocomposite. Colloids Surf. A Physicochem. Eng. Asp. 2021;625 [Google Scholar]
- 42.Beitollahi H., Garkani-Nejad F., Tajik S., Ganjali M.R. Voltammetric determination of acetaminophen and tryptophan using a graphite screen printed electrode modified with functionalized graphene oxide nanosheets within a Fe(3)O(4)@SiO(2) nanocomposite. Iran. J. Pharm. Res. (IJPR) 2019;18(1):80–90. [PMC free article] [PubMed] [Google Scholar]
- 43.Manavalan S., Ganesamurthi J., Chen S.-M., Veerakumar P., Murugan K. A robust Mn@FeNi-S/graphene oxide nanocomposite as a high-efficiency catalyst for the non-enzymatic electrochemical detection of hydrogen peroxide. Nanoscale. 2020;12(10):5961–5972. doi: 10.1039/c9nr09148c. [DOI] [PubMed] [Google Scholar]
- 44.Rajkumar C., Thirumalraj B., Chen S.-M., Veerakumar P., Liu S.-B. Ruthenium nanoparticles decorated tungsten oxide as a bifunctional catalyst for electrocatalytic and catalytic applications. ACS Appl. Mater. Interfaces. 2017;9(37):31794–31805. doi: 10.1021/acsami.7b07645. [DOI] [PubMed] [Google Scholar]
- 45.Prinith N.S., Manjunatha J.G. Electrochemical analysis of L-Tryptophan at highly sensitive poly(Glycine) modified carbon nanotube paste sensor. Mater. Res. Innovat. 2022;26(3):134–143. [Google Scholar]
- 46.Zhang Y., Waterhouse G.I.N., Xiang Z.-p., Che J., Chen C., Sun W. A highly sensitive electrochemical sensor containing nitrogen-doped ordered mesoporous carbon (NOMC) for voltammetric determination of l-tryptophan. Food Chem. 2020;326 doi: 10.1016/j.foodchem.2020.126976. [DOI] [PubMed] [Google Scholar]
- 47.Han J., Zhao J., Li Z., Zhang H., Yan Y., Cao D., Wang G. Nanoporous carbon derived from dandelion pappus as an enhanced electrode material with low cost for amperometric detection of tryptophan. J. Electroanal. Chem. 2018;818:149–156. [Google Scholar]
- 48.Sun D., Li H., Li M., Li C., Dai H., Sun D., Yang B. Electrodeposition synthesis of a NiO/CNT/PEDOT composite for simultaneous detection of dopamine, serotonin, and tryptophan. Sensor. Actuator. B Chem. 2018;259:433–442. [Google Scholar]
- 49.Rezaei F., Ashraf N., Zohuri G.H. A smart electrochemical sensor based upon hydrophilic core–shell molecularly imprinted polymer for determination of L-tryptophan. Microchem. J. 2023;185 [Google Scholar]
- 50.Zhang S., Ling P., Chen Y., Liu J., Yang C. 2D/2D porous Co3O4/rGO nanosheets act as an electrochemical sensor for voltammetric tryptophan detection. Diam. Relat. Mater. 2023;135 [Google Scholar]
- 51.Tasić Ž.Z., Mihajlović M.B.P., Radovanović M.B., Simonović A.T., Medić D.V., Antonijević M.M. Electrochemical determination of L-tryptophan in food samples on graphite electrode prepared from waste batteries. Sci. Rep. 2022;12(1):5469. doi: 10.1038/s41598-022-09472-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Haldorai Y., Yeon S.-H., Huh Y.S., Han Y.-K. Electrochemical determination of tryptophan using a glassy carbon electrode modified with flower-like structured nanocomposite consisting of reduced graphene oxide and SnO2. Sensor. Actuator. B Chem. 2017;239:1221–1230. [Google Scholar]
- 53.Pogacean F., Varodi C., Coros M., Kacso I., Radu T., Cozar B.I., Mirel V., Pruneanu S. Investigation of L-tryptophan electrochemical oxidation with a graphene-modified electrode. Biosensors. 2021;11(2):36. doi: 10.3390/bios11020036. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.