Abstract
Osteopontin (OPN) is a multifunctional protein secreted intracellularly and extracellularly by various cell types, including NK cells, macrophages, osteoblasts, T cells, and cancer cells. Owing to its diverse distribution, OPN plays a role in cell proliferation, stem-cell-like properties, epithelial–mesenchymal transformation, glycolysis, angiogenesis, fibrosis, invasion, and metastasis. In this review, we discuss recent findings, interpret representative studies on OPN expression in cancer, clarify that elevated OPN levels are observed in multiple cancer types (including colorectal, breast, lung, and liver cancer), and explore how OPN-macrophage interactions shape the tumor microenvironment. We also summarize progress in OPN research with regard to tumor therapy, which can facilitate the development of novel anti-tumor treatment strategies.
Keywords: Osteopontin, Colorectal cancer, Tumor therapy, Tumor microenvironment, Macrophages
1. Introduction
The review investigates the structure, function, and variants of osteopontin (OPN), also known as secreted phosphoprotein 1 (SPP1).
1.1. Structure and function of OPN
OPN also known as SPP1, is the main salivary protein that regulates bone formation and remodeling in bone tissue [1,2]. OPN is closely associated with early T lymphocytes activated protein 1 (ETA1) and bone sialoprotein 1(BSP1) [3]. The gene locus of OPN contains the arginine-glycine-aspartic acid (RGD) sequence, which can bind to various integrins [4,5], including αvβ1, αvβ3, αvβ5, αvβ6, and others. OPN interacts with integrin αvβ1 and regulates the expression of C/EBPs, inhibiting adipogenic differentiation while promoting osteogenic differentiation of mesenchymal stem cells (MSCs) [6]. When combined with αvβ3, OPN activates the PI3K/pAkt/NF-κB pathway, regulates the NF-κB/ZEB-dependent epithelial–mesenchymal transition (EMT) signal, and promotes tumor development [7]. Additionally, the SVVYGLR domain (non-RGD domain) of OPN can bind to α9β1, α4β1, and γ4β7. These interactions between OPN and α9β1 integrin activate the ERK and p38 signaling pathways, stimulate COX-2 expression in macrophages, and induce angiogenesis [8]. Furthermore, OPN promotes leukocyte adhesion through α4β1 integrin [9]. OPN binds to various forms of CD44, such as the CD44 standard type (CD44s) and CD44v [10]. CD44v6 and CD44v are considered important structures of OPN that promote cancer invasion. They have been identified as protein markers for metastatic behavior in colorectal, gastric, hepatocellular, and breast cancers [[11], [12], [13], [14]].
1.2. Variants of OPN
The SPP1 gene, located on chromosome 4 (4q13), encodes OPN [15]. Owing to the alternative splicing of SPP1mRNA, OPN undergoes post-translational modifications, such as proteolysis, glycosylation, tyrosine sulfation, and serine/threonine phosphorylation, resulting in several types of OPN variants [16]. OPN in cells (iOPN) lacks an N-terminal signal sequence, causing it to persist in the cytoplasm. iOPN negatively regulates toll-like receptor-mediated immune reactions, reducing the production of pro-inflammatory cytokines and thereby hindering the development of liver cancer [17]. In fibroblasts, iOPN co-localizes with hyaluronate–CD44–ERM complexes in the perimembrane region, contributing to cell migration [18]. Within the nuclei of human renal epithelial cells (293 cells), iOPN co-localizes with polo-like kinase 1 and participates in cell replication [19]. iOPN also plays a role in mesenchymal-to-epithelial transformation (MET), which involves the reversal of EMT. This transformation allows highly expressed disseminated tumor cells to revert to normal epithelial phenotypes during the later stages of metastatic dissemination [20]. iOPN represents an unidentified intermediate of the IL-15 signaling pathway, which ensures the steady-state expansion of NK cells. It holds potential as an immunotherapeutic agent for treating infectious diseases or cancer [21]. Secretory OPN (sOPN) includes the full-length isoform OPN-A and two mutually exclusive splice variants lacking exons 5 and 4, known as OPN-B and OPN-C, respectively [22]. OPN-A and OPN-C may synergistically contribute to tumor progression, but OPN-C is presumably more capable of promoting cell invasion [23,24]. Walaszek et al. demonstrated that OPN-C can be used as an indicator of breast cancer precancerous lesion rate and survival rate [25]. The expression levels of the three variants of OPN were increased in gastric cancer (GC) tissue. OPN-B may promote the survival of GC cells by regulating the expression of CD44v and Bcl-2 family proteins. In addition, OPN-C effectively stimulated GC transfer activity by augmenting the secretion of uPa, MMP-2, and IL-8 [26] (Fig. 1).
Fig. 1.
OPN interacts with integrin and CD44 receptors to mediate the development of tumor cells.
OPN interacts with integrin and CD44 receptors to mediate the occurrence and development of tumor cells. OPN increases tumor angiogenesis by inducing CD44 receptor and αvβ3 integrin to activate PI3K/Akt, regulate NF-κB/dependent epithelial mesenchymal transition (EMT) signaling, and regulate HIF1α dependent VEGF expression. OPN and αvβ3 integrin can also mediate the expression of metalloproteinases MMP2 and MMP9 through JNK signaling pathway and promote tumor invasion. OPN inhibits adipogenic differentiation and promotes osteogenic differentiation of MSCS by interacting with integrin αvβ1 and regulating the expression of C/EBPs. OPN induces COX-2 secretion through α9β1 integrin activation of ERK and p38, thereby enhancing tumor cell motility and angiogenesis. OPN binds to its receptor α4β1 integrin and induces relapse through phosphorylation of IKKβ, which induces the expression of pro-survival genes of NF-κB and also enhances adhesion between leukocytes.
2. Method
The PubMed database was used to explore eligible studies. The time frame considered for this review was from the establishment of the database to July 19, 2023. Only the studies that were published in the English language were included. The search was conducted using keywords (“OPN” or “SPP1”) and “Cancer”, (“OPN” or “SPP1”) and “Macrophages”, (“OPN” or “SPP1”) and “Treatment”. Retrospective studies, preclinical studies, case reports and studies that were not majorly co-related with the subject of this review were excluded. A total of 117 English articles were included in the present study (Table 1).
Table 1.
The search strategies.
| Items | Specification |
|---|---|
| Date of search | July19,2023 |
| Databases and other sources searched | PubMed |
| Search formula used | (“OPN” OR “SPP1”) AND “Cancer” (“OPN” OR “SPP1”) AND “Macrophages” (“OPN” OR “SPP1”) AND “Treatment” |
| Timeframe | From January 1997 to July 2023 |
| Inclusion and exclusion criteria | Retrospective study, preclinical studies, case report, studies not written in English, The correlation with the study content and the quality of the literature were Excluded |
| Selection process | Two co-first authors conducted initial screening by title and abstract. The included articles were read in full text. Eventually, all the authors participated in the discussion and received the same opinion |
3. Results and discussion
3.1. Role of OPN in various types of cancer
3.1.1. Role of OPN in colorectal cancer
Colorectal cancer (CRC) is the most common cancer after lung and breast cancer [27] and ranks fourth in cancer-related mortality [28]. Notably, the incidence of CRC in China is increasing, and the age of onset is decreasing [29]. Although surgical treatment, chemotherapy, drug therapy, and immunotherapy have improved patient outcomes, the mortality and recurrence rate in patients with CRC remains very high. Currently, the mechanism by which OPN promotes the occurrence and development of CRC remains unclear. However, studies have shown that OPN increases the migration and invasion of CRC cells in a concentration-dependent manner [30]. Additionally, OPN induces the activation of the downstream PI3K-AKT-GSK/3b-b/catenin pathway in CRC cells. Notably, the knockout of highly expressed OPN in CRC significantly inhibits cell proliferation and migration [25]. Furthermore, Chang et al. reported that tumor cell debris in CRC accelerates tumor growth by stimulating OPN production by macrophages in tumor cells and the host microenvironment [31]. Amilca-Seba et al. demonstrated that Slug can directly regulate OPNs at the promoter level, suggesting that OPN can serve as a biomarker to evaluate the invasive phenotype of CRC [32]. Lastly, OPN inhibits autophagy in CRC cells by activating the p38 MAPK signaling pathway [33].
3.1.2. Role of OPN in breast carcinoma
Breast cancer (BRC) is the most common type of cancer and the leading cause of death among women worldwide [34]. China has the highest number of patients with BRC worldwide [35,36]. Studies have shown that OPN expression is increased in BRC. The increased concentration of OPN is associated with tumor invasiveness, disease progression, and reduced survival [37,38]. OPN, when combined with the receptors CD44 and avb3, activates breast fibroblast inflammation and promotes tumor growth [39]. Pio et al. reported that SPP1 may promote breast cancer cell migration and stem-like behavior [“stem-like” cells also known as “cancer stem cells” (CSCs)] by activating the WNK-1 and PRAS40-related pathways [40]. Sample stem cell behavior in vitro shows proliferation, migration, adhesion, and invasion, indicating their potential to transfer into the body [41]. Similarly, OPN promotes BRC metastasis by activating the JNK signaling pathway. Furthermore, OPN can activate the extracellular signal-regulated kinases 1 and 2 (ERK1/2) and the phosphatidylinositol 3-kinase/protein kinase B (PI3K/AKT) pathways, leading to VEGF secretion in endothelial cells and promoting angiogenesis [42,43]. Raineri et al. demonstrated that OPN-triggered ICOSL (B7–H2, CD275, belonging to the B7 family) could induce BRC cell migration and increase angiogenesis both in vivo and in vitro [44].
3.1.3. Role of OPN in non-small cell lung cancer
Non-small cell lung cancer (NSCLC) is the main type of lung cancer and the leading cause of cancer-related mortality globally [45]. Despite recent advances in the treatment of NSCLC, the mortality rate from NSCLC remains high [46]. Studies have shown that OPN is highly expressed in NSCLC and possesses significant metastasis and invasion potential [47,48]. Zhang et al. found that the combination of lipopolysaccharide (LPS) and lipoteichoic acid (LTA) could significantly activate SPP1 and up-regulate the expression of αVβ3 [49]. Activation of the downstream ERK and FAK/AKT signaling pathways promotes NSCLC [50]. Additionally, OPN activates the RON signaling pathway, thereby facilitating migration and invasion of NSCLC cells [51]. Moreover, it promotes the progression of NSCLC cells and mediates drug resistance via the MAPK signaling pathway [52]. SPP1+ tumor-associated macrophages (TAMs) are closely related to the endothelial cells and fibroblasts associated with NSCLC, thereby regulating the tumor microenvironment (TME) [53]. OPN induces the accumulation of VEGF and stimulates neovascularization through autocrine and paracrine mechanisms, thereby promoting NSCLC growth [54]. SPP1 acts as a promoter of EMT, which leads to cell migration and invasion in lung adenocarcinoma (a common subtype of NSCLC) through the upregulation of COL1A1 [55].
3.1.4. Role of OPN in liver cancer
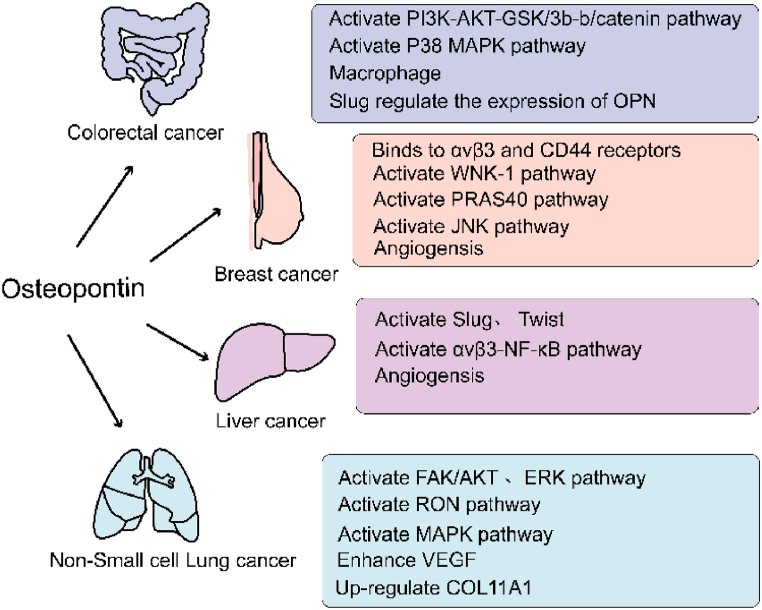
Hepatocellular carcinoma (HCC) is the most common type of primary liver cancer. It is responsible for 790,000 deaths globally annually [56]. Owing to its high recurrence and intrahepatic metastasis rates after surgical resection and poor prognosis, understanding the mechanism behind HCC progression holds high clinical significance [57,58]. In patients with HCC, elevated levels of OPN are closely associated with liver function deterioration and positively correlated with tumor stage. Therefore, OPN levels are effective diagnostic biomarkers [59]. Evidence suggests that the detection performance of OPN in HCC is superior to that of alpha-fetoprotein (AFP) in the preclinical stage; however, diagnostic tests involving OPN and AFP significantly improve clinical diagnostic accuracy [60]. The combination of OPN and CD44 induces the expression of Twist (a major regulator of EMT, which is crucial for tumor metastasis). This activation occurs through the PI3K/AKT signaling pathway, promoting EMT and HCC metastasis [61,62]. In HCC cells, OPN plays a central role in angiogenesis and supports the formation of the TME [63]. OPN promotes glycolysis of HCC by activating αvβ3-NF-κB signaling [64]. The acidic TME derived from glycolysis is closely associated with tumor metastasis and immune response [65]. Additionally, OPN promotes the progression and metastasis of HCC by activating CCR1 expression, while miR-196a reduces downstream OPN by targeting Runx2 expression. Thus, EMT regulatory factors, such as Slug and Twist, are activated, further driving the aggressiveness of HCC [66,67] (Fig. 2).
Fig. 2.
The role of OPN in cancer.
The role of OPN in cancer. OPN plays an important role in the occurrence and development of tumors through different mechanisms. In colorectal cancer, OPN stimulates macrophages to maintain M2 phenotype by activating PI3K-AKT-GSK/3b-b/catenin and P38 MAPK signaling pathways, and the expression of SLUG is closely related to OPN. OPN can promote angiogenesis in breast cancer by activating WNK-1, PRAS40, JNK and other signaling pathways and binding to integrin and CD44 receptors. It can promote tumor angiogenesis by activating ανβ3-NF-κB signaling pathway, Slug and Twist in liver cancer. In non-small cell lung cancer, VEGF, COL11A1 and other angiogenic factors are highly expressed by activating FAK/AKT, ERK, RON, MAPK signaling pathways.
3.2. OPN and macrophages
The TME comprises a structural framework of tumor tissue consisting of stromal cells, such as connective tissue cells, vascular components, and immune cells. These components play a critical role in tumor metastasis and progression. Immune cells present in the microenvironment include macrophages (Mφ), lymphocytes, monocytes, and dendritic cells (DC), along with immune checkpoint molecules, such as programmed cell death-1 (PD-1) and programmed cell death ligand 1 (PD-L1) [68]. TAMs are the predominant inflammatory cells infiltrating the TME [69], and their high infiltration is closely related to various tumors [70]. In recent years, inflammation in the TME has gained recognition as a cancer marker [26]. OPN is a pro-inflammatory molecule that regulates the function of all types of immune cells within the TME [71]. In addition, OPN can be activated by matrix metalloproteinases (MMPs), resulting in the formation of smaller pro-inflammatory molecules. OPN production is closely related to the enzyme cyclooxygenase 2 (COX2), which induces inflammation [72].
3.2.1. OPN maintains the M2 phenotype of macrophages and promotes tumor-related processes
TAMs can be categorized into anti-tumor M1 and pro-tumor M2 phenotypes. Once TAMs originating from peripheral blood mononuclear cells are recruited into the TME by tumor-secreted attractants, they experience M1-like or M2-like activation in response to diversified stimulation [ [73,74]]. Wei et al. proposed that OPN is a potent chemotactic factor for Mφ, and blocking OPN markedly weakens the ability of glioma cells to recruit Mφ [75]. In lung cancer, Zhang et al. found that SPP1 plays a vital role in the crosstalk between NSCLC cells and TAMs and that the M2 phenotype promotes the migration of NSCLC cells [76]. Additionally, OPN can activate PD-L1 expression in HCC Mφ through the cSF1-cSF1r pathway [77]. Tumor-derived SPP1 can induce M2 reprogramming through the integrin and protein tyrosine kinase 2 (PTK2)-Akt signaling pathways [78].
Interestingly, OPN recruits monocytes, which subsequently differentiate into TAMs [68]. Recent studies have shown that SPP1 (OPN) can bind to CD44 on macrophages, leading to the polarization of TAMs into the M2 phenotype in HCC cells [79]. A distinct TAM subtype, known as SPP1+ macrophages, was recently reported as a potential target against tumor growth and metastasis owing to its immunosuppressive properties and positive correlation with EMT markers [80]. Jiang et al. found that SPP1+ macrophages interact with endothelial cells through VEGFA-VEGFR1/Vegfr2 and promote angiogenesis [81]. LIU et al. proposed that the spatial structure of the tumor immune barrier (TIB), composed of SPP1+ macrophages and cancer-associated fibroblasts (CAF), is associated with blocking immune checkpoints, thus limiting immune infiltration of the tumor core [82]. Qi et al. also suggested that cancer-associated fibroblast subtypes (FAP + fibroblasts) and SPP1+ macrophages contribute to extracellular matrix(ECM) remodeling and cooperate to form a desmoplastic microenvironment [83]. SPP1+ TAMs are mainly present in liver metastases(MetS) and show high pro-angiogenic ability [84]. SPP1+ TAMs are closely associated with tumor-associated endothelial cells and fibroblasts, thereby regulating the TME [85,86]. Li et al. observed that SPP1+ TAMs exhibited overall pro-tumor features, including reduced inflammation, phagocytosis, and increased angiogenesis [87].
3.2.2. Effect of OPN-CD44 axis on macrophages in tumor development
In the analysis of cell–cell interaction, the presence of SPP1–CD44 was identified within the SPP1-Mφ cluster [53]. The binding of SPP1 (OPN) to the CD44 receptor on macrophages forms the SPP1–OPN axis, which is considered a unique interaction between macrophages and HCC malignancy [88]. The activation of H3K4me3 may promote the immune evasion of pancreatic cancer by activating the OPN–CD44 axis, thereby accelerating the growth and progression of pancreatic cancer [89]. The activation of OPN/CD44 signaling is closely associated with CD8+T cell dysfunction, initiation of metastasis, and promotion of tumor growth [ [87,90,91]]. OPN inhibits CD8+T cells from producing IFN-γ and promotes tumor immune tolerance and evasion in colorectal cancer [92] (Fig. 3).
Fig. 3.
OPN interactions with macrophages in the tumor microenvironment.
OPN in the tumor microenvironment interacts with macrophages. OPN is a potent chemotactic factor for macrophages, and Tams derived from peripheral blood monocytes are recruited by OPN to the TME and undergo M2-like activation. A unique interaction formed between the OPN-CD44 axis of macrophages and malignancy. OPN can also inhibit the production of IFN-γ by CD8+T cells and promote tumor immune escape.
3.3. Treatment
Numerous therapies target OPN, including targeted inhibition of OPN expression at the transcriptional and protein levels, blockade of its receptor and upstream and downstream pathways, OPN inhibitors, and immune checkpoint blockade. However, further studies are needed to bridge the gap between experimental findings and clinical practice (Fig. 4).
Fig. 4.
New strategies for the treatment of OPN.
3.3.1. Immunotherapy
PD-1 receptor and PD-L1 are two astrocytic molecules responsible for T cell-mediated anti-tumor immune responses. Although PD-1/PD-L1 immune checkpoint blocking has been clinically successful, less than a quarter of the treated patients achieve a lasting response, suggesting that the inefficacy may be closely related to immunosuppression in infiltrating cells [ [90,93]]. OPN also acts as an immune checkpoint. In colorectal cancer, OPN induces T cell suppression and inhibits CD8+T cells to produce IFN-γ, thereby promoting host tumor immune tolerance and tumor immune avoidance [92]. Lu et al. showed that the gene SPP1, encoding the OPN protein and its receptor, is highly enriched in H3K4me3 in the pancreatic cancer genome, which promotes immune evasion and anti-PD-1 immunotherapy in pancreatic cancer [89]. Qi et al. found that patients with high SPP1 expression or FAP were treated only with anti-PD-L1, and the therapeutic effect was not ideal. Therefore, improving immunotherapy by disrupting the interactions between SPP1+ macrophages and FAP + fibroblasts is a potential therapeutic strategy [83].
3.3.2. OPN neutralizing antibody therapy
OPN neutralizing antibody or synthetic peptide directly targets OPN and its receptor CD44, as well as the interaction between αvβ3 integrin and OPN with high safety [94]. In vivo, the anti-OPN antibody exhibits anti-angiogenic effects, suggesting that OPN could serve as a potential target for the development of novel anti-angiogenesis therapies in cancer treatment [43]. These OPN-neutralizing antibodies may neutralize some of the tumor-promoting effects of Slug in patients with advanced CRC [33]. In BRC, the use of osteoclast precursors and OPN-neutralizing antibodies reduces osteoclast differentiation and bone metastasis [95]. The OPN-neutralizing monoclonal antibodies 100D3 and 103D6 inhibit colonic neoplasm growth and hold great potential in cancers treated with anti-PD-1 immunotherapy [96]. AOM1 is a type of anti-OPN monoclonal antibody that blocks the integrin αvβ3 connection site and thrombin cracking site on OPN. AOM1 treatment effectively inhibits the expression of αvβ3 and suppresses tumor cell migration [96]. Moreover, anti-OPN monoclonal antibody combined with anti-PD1 was more effective than anti-PD1 immunotherapy alone in inhibiting tumor growth [97].
3.3.3. Epigenetic therapy
Therapeutic approaches based on epigenetic modulators, such as small interfering RNA (siRNAs) and microRNAs, hold great promise for effectively treating various cancers. These modulators bind to their targets and effectively silence genes. Currently, there are ongoing clinical tests to assess their efficacy [98]. miR-181c plays a role in regulating the sensitivity of breast carcinoma cells to adriamycin by downregulating OPN expression [99]. In experiments where miR-196a was knocked down, OPN expression decreased, leading to a marked inhibition of lung metastasis in HCC [67]. Moreover, intra-tumoral injection of siRNA against OPN inhibits breast tumor growth and angiogenesis. In a study by Cho et al. PSOT, a novel gene vector delivering siRNA, effectively silenced OPN expression, leading to the inhibition of NSCLC growth. This finding suggests that PSOT may have a potential in anti-lung cancer therapy [100]. Therefore, combining epigenetic modulators that inhibit OPN splicing or other tumor-specific splicing pathways with conventional chemotherapy could be an effective strategy to prevent tumor progression and recurrence [98].
3.3.4. Small molecule inhibitors targeting OPN
Small-molecule inhibitors play a significant role in specifically targeting certain signaling pathways associated with cancer progression. Their small volume and easy accessibility to the tumor site contribute to their importance. For example, luteolin can inhibit OPN targets and induce the apoptosis of cancer cells through a caspase-dependent pathway, which has anticancer effects [101]. In prostate cancer, curcumin regulates VEGF expression through the OPN/αvβ3 pathway, which possesses anti-angiogenic and anti-tumor invasion properties [72]. In breast cancer, andrographolide (Andro) decreases the expression of OPN and inhibits the interaction between endothelial cells and the tumor by suppressing the expression of c-jun and activating PI3K/Akt [102]. In addition, the binding of OPN to the αvβ3 receptor induces the activation of Rho GTPase, which is inhibited by bisphosphonates (BPs), attenuates the CD44/MMP-9 interaction on the cell surface, and suppresses the migration of prostate cancer cells [103]. Blocking NR4A2 and Wnt signaling by downregulating OPN with a parecoxistep reduces colon cancer risk [104]. Moreover, IL-33 may play an anti-tumor role during early cetuximab treatment by inhibiting OPN expression [105]. Simvastatin can reduce OPN expression by inhibiting the IL-13-activated STAT6 pathway [106].
3.3.5. Targeting CD44 and integrin receptor therapy
MI et al. showed that targeting CD44 resulted in an OPN-mediated reduction in tumor growth. Additionally, blocking the interaction between OPN and integrin αvβ3 resulted in a decreased expression of ILK, urinary plasminogen activator, and MMP-2 in mouse mammary gland epithelial cancer cells [107]. In a study conducted by Robertson et al. it was found that OPN binds to CD44 or αvβ3 receptors in PC3 cells via the Akt pathway, resulting in differential effects on the proliferation and survival of prostate cancer cells [108]. By functioning as an aptamer of OPN RNA, OPN-R3 can reduce the combination of OPN, αvβ3 and CD44 on the surface of human mammary carcinoma cells. This inhibition ultimately reduces local invasion and distant metastasis in a breast cancer xenotransplantation model. The mechanism behind this effect involves the induction of JNK1/2, Src, and PI3K-Akt signaling by OPN-R3 [109].
3.3.6. Angiogenesis and therapy
Inhibition of angiogenesis is one of the major treatment strategies for cancer. A new strategy for personalized cancer treatment involves combining VEGF-targeted antiangiogenic therapy with the blocking of other pro-angiogenic factors [110]. Angiogenesis involves the activation, proliferation, and migration of ECs. Studies have shown the significant role of the integrin family in tumor angiogenesis [111]. The first integrin to be discovered as a regulator of angiogenesis was αvβ3, which exhibits high expression levels associated with tumor angiogenesis [112]. In response to stimulation by tumor-derived angiogenic factors, αvβ3 expression is elevated and interacts with multiple ECM proteins, such as OPN, to promote angiogenesis and metastasis [113]. OPN activates the I-Kappa-B kinase (IKK)/NF-Kb signal cascade to induce COX-2 expression, which regulates the production of prostaglandin E2 (PGE2) and leads to COX-2/PGE2-stimulated angiogenesis. VEGF is a marker of hypoxia and angiogenesis [114]. Vergis R. et al. discovered that OPN expression was closely correlated with the expression of hypoxia-inducible factor-1α (HIF-1α) and VEGF [115]. OPN can induce integrin kinase (ILK)/AKT-mediated NF-kB activation under hypoxic conditions, leading to HIF1α-dependent VEGF expression and angiogenesis in human BRC specimens [116]. TAMs are key inducers of angiogenic switching in animal tumor models. OPN can directly or indirectly activate tumor angiogenesis in vivo and in vitro in mouse tumor models [117]. OPN-induced VEGF enhanced VEGFR-2 phosphorylation and angiogenesis in endothelial cells [54].
New strategies for the treatment of OPN. Small molecule inhibitors, siRNA, miRNA, and OPN neutralizing antibodies were used to target OPN and disrupt the interaction of OPN with integrin and CD44 and inhibit angiogenesis.
4. Conclusion
The findings suggest that OPN is a potential therapeutic target for various cancers and may serve as a valuable diagnostic or prognostic marker for specific cancer types. However, further research is needed to fully understand the underlying mechanisms of OPN in cancer development and progression. In macrophages, OPN can maintain the M2 phenotype and promote its formation. In recent years, the SPP1 (OPN) + TAM model has provided new perspectives in cancer immunotherapy. OPN can be used as a new immune checkpoint to supplement the limitations of PD-L, PD-L1, and other immune checkpoints as it has been noted that a combination of an anti-OPN monoclonal antibody and anti-PD1 is more effective in inhibiting tumor growth. However, application of OPN in cancer therapy still remains at a nascent stage because of the lack of research in this field Therefore, extensive research is needed to establish OPN as a new target for cancer treatment.
Funding statement
This work was supported by the College Students' Innovation and Entrepreneurship Training Program (No: 202213705030, S202213705062) and Sichuan Provincial Administration of Traditional Chinese Medicine (no. 2021MS101, 2021MS508).
Data availability statement
No data was used for the research described in the article.
CRediT authorship contribution statement
Zhihua Yan: Investigation, Methodology, Visualization, Writing – original draft. Xue Hu: Software, Writing – original draft, Writing – review & editing. Bin Tang: Funding acquisition, Writing – review & editing. Fengmei Deng: Funding acquisition, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Prince C.W., Oosawa T., Butler W.T., Tomana M., Bhown A.S., Bhown M., Schrohenloher R.E. Isolation, characterization, and biosynthesis of a phosphorylated glycoprotein from rat bone. J. Biol. Chem. 1987;262:2900–2907. doi: 10.1016/S0021-9258(18)61592-3. [DOI] [PubMed] [Google Scholar]
- 2.Senger D.R., Wirth D.F., Hynes R.O. Transformed mammalian cells secrete specific proteins and phosphoproteins. Cell. 1979;16:885–893. doi: 10.1016/0092-8674(79)90103-x. [DOI] [PubMed] [Google Scholar]
- 3.Pagel C.N., Wasgewatte Wijesinghe D.K., Taghavi Esfandouni N., Mackie E.J. Osteopontin, inflammation and myogenesis: influencing regeneration, fibrosis and size of skeletal muscle. J. Cell Commun. Signal. 2014;8:95–103. doi: 10.1007/s12079-013-0217-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wei R., Wong J.P.C., Kwok H.F. Osteopontin -- a promising biomarker for cancer therapy. J. Cancer. 2017;8:2173–2183. doi: 10.7150/jca.20480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prochazka L., Tesarik R., Turanek J. Regulation of alternative splicing of CD44 in cancer, Cell. Signal. 2014;26:2234–2239. doi: 10.1016/j.cellsig.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 6.Chen Q., Shou P., Zheng C., Jiang M., Cao G., Yang Q., Cao J., Xie N., Velletri T., Zhang X., Xu C., Zhang L., Yang H., Hou J., Wang Y., Shi Y. Fate decision of mesenchymal stem cells: adipocytes or osteoblasts? Cell Death Differ. 2016;23:1128–1139. doi: 10.1038/cdd.2015.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Urtasun R., Lopategi A., George J., Leung T.-M., Lu Y., Wang X., Ge X., Fiel M.I., Nieto N. Osteopontin, an oxidant stress sensitive cytokine, up-regulates collagen-I via integrin α(V)β(3) engagement and PI3K/pAkt/NFκB signaling. Hepatol. Baltim. Md. 2012;55:594–608. doi: 10.1002/hep.24701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kale S., Raja R., Thorat D., Soundararajan G., Patil T.V., Kundu G.C. Osteopontin signaling upregulates cyclooxygenase-2 expression in tumor-associated macrophages leading to enhanced angiogenesis and melanoma growth via α9β1 integrin. Oncogene. 2014;33:2295–2306. doi: 10.1038/onc.2013.184. [DOI] [PubMed] [Google Scholar]
- 9.Bayless K.J., Meininger G.A., Scholtz J.M., Davis G.E. Osteopontin is a ligand for the alpha4beta1 integrin. J. Cell Sci. 1998;111(Pt 9):1165–1174. doi: 10.1242/jcs.111.9.1165. [DOI] [PubMed] [Google Scholar]
- 10.Kahles F., Findeisen H.M., Bruemmer D. Osteopontin: a novel regulator at the cross roads of inflammation, obesity and diabetes. Mol. Metab. 2014;3:384–393. doi: 10.1016/j.molmet.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu J., Li G., Zhang P., Zhuang X., Hu G. A CD44v+ subpopulation of breast cancer stem-like cells with enhanced lung metastasis capacity. Cell Death Dis. 2017;8:e2679. doi: 10.1038/cddis.2017.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goodison S., Urquidi V., Tarin D. CD44 cell adhesion molecules. Mol. Pathol. MP. 1999;52:189–196. doi: 10.1136/mp.52.4.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rudzki Z., Jothy S. CD44 and the adhesion of neoplastic cells. Mol. Pathol. MP. 1997;50:57–71. doi: 10.1136/mp.50.2.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ponta H., Sherman L., Herrlich P.A. CD44: from adhesion molecules to signalling regulators. Nat. Rev. Mol. Cell Biol. 2003;4:33–45. doi: 10.1038/nrm1004. [DOI] [PubMed] [Google Scholar]
- 15.Fatherazi S., Matsa-Dunn D., Foster B.L., Rutherford R.B., Somerman M.J., Presland R.B. Phosphate regulates osteopontin gene transcription. J. Dent. Res. 2009;88:39–44. doi: 10.1177/0022034508328072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Castello L.M., Raineri D., Salmi L., Clemente N., Vaschetto R., Quaglia M., Garzaro M., Gentilli S., Navalesi P., Cantaluppi V., Dianzani U., Aspesi A., Chiocchetti A. Osteopontin at the crossroads of inflammation and tumor progression. Mediat. Inflamm. 2017 doi: 10.1155/2017/4049098. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yi X., Luo L., Zhu Y., Deng H., Liao H., Shen Y., Zheng Y. SPP1 facilitates cell migration and invasion by targeting COL11A1 in lung adenocarcinoma. Cancer Cell Int. 2022;22:324. doi: 10.1186/s12935-022-02749-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zohar R., Suzuki N., Suzuki K., Arora P., Glogauer M., McCulloch C.A., Sodek J. Intracellular osteopontin is an integral component of the CD44-ERM complex involved in cell migration. J. Cell. Physiol. 2000;184:118–130. doi: 10.1002/(SICI)1097-4652. (200007)184:1<118::AID-JCP13>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 19.Junaid A., Moon M.C., Harding G.E.J., Zahradka P. Osteopontin localizes to the nucleus of 293 cells and associates with polo-like kinase-1. Am. J. Physiol. Cell Physiol. 2007;292:C919–C926. doi: 10.1152/ajpcell.00477.2006. [DOI] [PubMed] [Google Scholar]
- 20.Jia R., Liang Y., Chen R., Liu G., Wang H., Tang M., Zhou X., Wang H., Yang Y., Wei H., Li B., Song Y., Zhao J. Osteopontin facilitates tumor metastasis by regulating epithelial-mesenchymal plasticity. Cell Death Dis. 2016;7 doi: 10.1038/cddis.2016.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leavenworth J.W., Verbinnen B., Wang Q., Shen E., Cantor H. Intracellular osteopontin regulates homeostasis and function of natural killer cells. Proc. Natl. Acad. Sci. U. S. A. 2015;112:494–499. doi: 10.1073/pnas.1423011112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gimba E.R., Tilli T.M. Human osteopontin splicing isoforms: known roles, potential clinical applications and activated signaling pathways. Cancer Lett. 2013;331:11–17. doi: 10.1016/j.canlet.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 23.Briones-Orta M.A., Avendaño-Vázquez S.E., Aparicio-Bautista D.I., Coombes J.D., Weber G.F., Syn W.-K. Osteopontin splice variants and polymorphisms in cancer progression and prognosis. Biochim. Biophys. Acta Rev. Cancer. 2017;1868:93–108. doi: 10.1016/j.bbcan.2017.02.005. A. [DOI] [PubMed] [Google Scholar]
- 24.Weber G.F. Metabolism in cancer metastasis. Int. J. Cancer. 2016;138:2061–2066. doi: 10.1002/ijc.29839. [DOI] [PubMed] [Google Scholar]
- 25.He B., Mirza M., Weber G.F. An osteopontin splice variant induces anchorage independence in human breast cancer cells. Oncogene. 2006;25:2192–2202. doi: 10.1038/sj.onc.1209248. [DOI] [PubMed] [Google Scholar]
- 26.Tang X., Li J., Yu B., Su L., Yu Y., Yan M., Liu B., Zhu Z. Osteopontin splice variants differentially exert clinicopathological features and biological functions in gastric cancer. Int. J. Biol. Sci. 2013;9:55–66. doi: 10.7150/ijbs.5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ganesh K., Stadler Z.K., Cercek A., Mendelsohn R.B., Shia J., Segal N.H., Diaz L.A. Immunotherapy in colorectal cancer: rationale, challenges and potential. Nat. Rev. Gastroenterol. Hepatol. 2019;16:361–375. doi: 10.1038/s41575-019-0126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bever K.M., Le D.T. An expanding role for immunotherapy in colorectal cancer. J. Natl. Compr. Cancer Netw. JNCCN. 2017;15:401–410. doi: 10.6004/jnccn.2017.0037. [DOI] [PubMed] [Google Scholar]
- 29.Bode A.M., Dong Z., Wang H. Cancer prevention and control: alarming challenges in China. Natl. Sci. Rev. 2016;3:117–127. doi: 10.1093/nsr/nwv054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang G., Zhang S., Gao F., Liu Z., Lu M., Peng S., Zhang T., Zhang F. Osteopontin enhances the expression of HOTAIR in cancer cells via IRF1. Biochim. Biophys. Acta. 2014;1839:837–848. doi: 10.1016/j.bbagrm.2014.06.020. [DOI] [PubMed] [Google Scholar]
- 31.Chang J., Bhasin S.S., Bielenberg D.R., Sukhatme V.P., Bhasin M., Huang S., Kieran M.W., Panigrahy D. Chemotherapy-generated cell debris stimulates colon carcinoma tumor growth via osteopontin. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2019;33:114–125. doi: 10.1096/fj.201800019RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Amilca-Seba K., Tan T.Z., Thiery J.-P., Louadj L., Thouroude S., Bouygues A., Sabbah M., Larsen A.K., Denis J.A. Osteopontin (OPN/SPP1), a mediator of tumor progression, is regulated by the mesenchymal transcription factor slug/SNAI2 in colorectal cancer (CRC) Cells. 2022;11 doi: 10.3390/cells11111808. 1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang R.-H., Quan Y.-J., Chen J.-H., Wang T.-F., Xu M., Ye M., Yuan H., Zhang C.-J., Liu X.-J., Min Z.-J. Osteopontin promotes cell migration and invasion, and inhibits apoptosis and autophagy in colorectal cancer by activating the p38 MAPK signaling pathway. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2017;41:1851–1864. doi: 10.1159/000471933. [DOI] [PubMed] [Google Scholar]
- 34.Harbeck N., Gnant M. Breast cancer. Lancet Lond. Engl. 2017;389:1134–1150. doi: 10.1016/S0140-6736(16)31891-8. [DOI] [PubMed] [Google Scholar]
- 35.Li H., Zheng R.S., Zhang S.W., Zeng H.M., Sun K.X., Xia C.F., Yang Z.X., Chen W.Q., He J. [Incidence and mortality of female breast cancer in China, 2014] Zhonghua Zhongliu Zazhi. 2018;40:166–171. doi: 10.3760/cma.j.issn.0253-3766.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 36.Sun K.X., Zheng R.S., Gu X.Y., Zhang S.W., Zeng H.M., Zou X.N., Xia C.F., Yang Z.X., Li H., Chen W.Q., He J. [Incidence trend and change in the age distribution of female breast cancer in cancer registration areas of China from 2000 to 2014] Zhonghua Yufang Yixue Zazhi. 2018;52:567–572. doi: 10.3760/cma.j.issn.0253-9624.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 37.Lindahl G., Rzepecka A., Dabrosin C. Increased extracellular osteopontin levels in normal human breast tissue at high risk of developing cancer and its association with inflammatory biomarkers in situ. Front. Oncol. 2019;9:746. doi: 10.3389/fonc.2019.00746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singhal H., Bautista D.S., Tonkin K.S., O'Malley F.P., Tuck A.B., Chambers A.F., Harris J.F. Elevated plasma osteopontin in metastatic breast cancer associated with increased tumor burden and decreased survival. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 1997;3:605–611. [PubMed] [Google Scholar]
- 39.Sharon Y., Raz Y., Cohen N., Ben-Shmuel A., Schwartz H., Geiger T., Erez N. Tumor-derived osteopontin reprograms normal mammary fibroblasts to promote inflammation and tumor growth in breast cancer. Cancer Res. 2015;75:963–973. doi: 10.1158/0008-5472.CAN-14-1990. [DOI] [PubMed] [Google Scholar]
- 40.Pio G.M., Xia Y., Piaseczny M.M., Chu J.E., Allan A.L. Soluble bone-derived osteopontin promotes migration and stem-like behavior of breast cancer cells. PLoS One. 2017;12 doi: 10.1371/journal.pone.0177640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Croker A.K., Goodale D., Chu J., Postenka C., Hedley B.D., Hess D.A., Allan A.L. High aldehyde dehydrogenase and expression of cancer stem cell markers selects for breast cancer cells with enhanced malignant and metastatic ability. J. Cell Mol. Med. 2009;13:2236–2252. doi: 10.1111/j.1582-4934.2008.00455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Insua-Rodríguez J., Pein M., Hongu T., Meier J., Descot A., Lowy C.M., De Braekeleer E., Sinn H.-P., Spaich S., Sütterlin M., Schneeweiss A., Oskarsson T. Stress signaling in breast cancer cells induces matrix components that promote chemoresistant metastasis. EMBO Mol. Med. 2018;10 doi: 10.15252/emmm.201809003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dai J., Peng L., Fan K., Wang H., Wei R., Ji G., Cai J., Lu B., Li B., Zhang D., Kang Y., Tan M., Qian W., Guo Y. Osteopontin induces angiogenesis through activation of PI3K/AKT and ERK1/2 in endothelial cells. Oncogene. 2009;28:3412–3422. doi: 10.1038/onc.2009.189. [DOI] [PubMed] [Google Scholar]
- 44.Raineri D., Dianzani C., Cappellano G., Maione F., Baldanzi G., Iacobucci I., Clemente N., Baldone G., Boggio E., Gigliotti C.L., Boldorini R., Rojo J.M., Monti M., Birolo L., Dianzani U., Chiocchetti A. Osteopontin binds ICOSL promoting tumor metastasis. Commun. Biol. 2020;3:615. doi: 10.1038/s42003-020-01333-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Torre L.A., Siegel R.L., Jemal A. Lung cancer statistics. Adv. Exp. Med. Biol. 2016;893:1–19. doi: 10.1007/978-3-319-24223-1_1. [DOI] [PubMed] [Google Scholar]
- 46.Jemal A., Siegel R., Ward E., Hao Y., Xu J., Thun M.J. Cancer statistics. CA. Cancer J. Clin. 2009;59(2009):225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 47.Hu Z., Lin D., Yuan J., Xiao T., Zhang H., Sun W., Han N., Ma Y., Di X., Gao M., Ma J., Zhang J., Cheng S., Gao Y. Overexpression of osteopontin is associated with more aggressive phenotypes in human non-small cell lung cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2005;11:4646–4652. doi: 10.1158/1078-0432.CCR-04-2013. [DOI] [PubMed] [Google Scholar]
- 48.Wang X.-M., Li J., Yan M.-X., Liu L., Jia D.-S., Geng Q., Lin H.-C., He X.-H., Li J.-J., Yao M. Integrative analyses identify osteopontin, LAMB3 and ITGB1 as critical pro-metastatic genes for lung cancer. PLoS One. 2013;8 doi: 10.1371/journal.pone.0055714. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 49.Zhang M., Sun Y., Zhang Y., Wang Z., Wang Z.-Y., Ming X.-Y., Guo Z.-D. Lipopolysaccharide and lipoteichoic acid regulate the PI3K/AKT pathway through osteopontin/integrin β3 to promote malignant progression of non-small cell lung cancer. J. Thorac. Dis. 2023;15:168–185. doi: 10.21037/jtd-22-1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fu Y., Zhang Y., Lei Z., Liu T., Cai T., Wang A., Du W., Zeng Y., Zhu J., Liu Z., Huang J.-A. Abnormally activated OPN/integrin αVβ3/FAK signalling is responsible for EGFR-TKI resistance in EGFR mutant non-small-cell lung cancer. J. Hematol. Oncol.J Hematol Oncol. 2020;13:169. doi: 10.1186/s13045-020-01009-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hao C., Cui Y., Chang S., Huang J., Birkin E., Hu M., Zhi X., Li W., Zhang L., Cheng S., Jiang W.G. OPN promotes the aggressiveness of non-small-cell lung cancer cells through the activation of the RON tyrosine kinase. Sci. Rep. 2019;9 doi: 10.1038/s41598-019-54843-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cui J., Wang J., Lin C., Liu J., Zuo W. Osteopontin mediates cetuximab resistance via the MAPK pathway in NSCLC cells. OncoTargets Ther. 2019;12:10177–10185. doi: 10.2147/OTT.S228437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang C., Yu Q., Song T., Wang Z., Song L., Yang Y., Shao J., Li J., Ni Y., Chao N., Zhang L., Li W. The heterogeneous immune landscape between lung adenocarcinoma and squamous carcinoma revealed by single-cell RNA sequencing. Signal Transduct. Targeted Ther. 2022;7:289. doi: 10.1038/s41392-022-01130-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chakraborty G., Jain S., Kundu G.C. Osteopontin promotes vascular endothelial growth factor-dependent breast tumor growth and angiogenesis via autocrine and paracrine mechanisms. Cancer Res. 2008;68:152–161. doi: 10.1158/0008-5472.CAN-07-2126. [DOI] [PubMed] [Google Scholar]
- 55.Ettinger D.S., Wood D.E., Akerley W., Bazhenova L.A., Borghaei H., Camidge D.R., Cheney R.T., Chirieac L.R., D'Amico T.A., Demmy T.L., Dilling T.J., Govindan R., Grannis F.W., Horn L., Jahan T.M., Komaki R., Kris M.G., Krug L.M., Lackner R.P., Lanuti M., Lilenbaum R., Lin J., Loo B.W., Martins R., Otterson G.A., Patel J.D., Pisters K.M., Reckamp K., Riely G.J., Rohren E., Schild S., Shapiro T.A., Swanson S.J., Tauer K., Yang S.C., Gregory K., Hughes M. Non-small cell lung cancer, version 1.2015. J. Natl. Compr. Cancer Netw. JNCCN. 2014;12:1738–1761. doi: 10.6004/jnccn.2014.0176. [DOI] [PubMed] [Google Scholar]
- 56.Moon A.M., Singal A.G., Tapper E.B. Contemporary epidemiology of chronic liver disease and cirrhosis. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2020;18:2650–2666. doi: 10.1016/j.cgh.2019.07.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kulik L., El-Serag H.B. Epidemiology and management of hepatocellular carcinoma. Gastroenterology. 2019;156:477–491.e1. doi: 10.1053/j.gastro.2018.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Braillon A. Hepatocellular carcinoma screening: seeking robust evidence. Gastroenterology. 2019;156:288–289. doi: 10.1053/j.gastro.2018.08.064. [DOI] [PubMed] [Google Scholar]
- 59.Kim J., Ki S.S., Lee S.D., Han C.J., Kim Y.C., Park S.H., Cho S.Y., Hong Y.-J., Park H.Y., Lee M., Jung H.H., Lee K.H., Jeong S.-H. Elevated plasma osteopontin levels in patients with hepatocellular carcinoma. Am. J. Gastroenterol. 2006;101:2051–2059. doi: 10.1111/j.1572-0241.2006.00679.x. [DOI] [PubMed] [Google Scholar]
- 60.Khan I.M., Gjuka D., Jiao J., Song X., Wang Y., Wang J., Wei P., El-Serag H.B., Marrero J.A., Beretta L. A novel biomarker panel for the early detection and risk assessment of hepatocellular carcinoma in patients with cirrhosis. Cancer Prev. Res. Phila. Pa. 2021;14:667–674. doi: 10.1158/1940-6207.CAPR-20-0600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yu X., Zheng Y., Zhu X., Gao X., Wang C., Sheng Y., Cheng W., Qin L., Ren N., Jia H., Dong Q. Osteopontin promotes hepatocellular carcinoma progression via the PI3K/AKT/Twist signaling pathway. Oncol. Lett. 2018;16:5299–5308. doi: 10.3892/ol.2018.9281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang J., Mani S.A., Donaher J.L., Ramaswamy S., Itzykson R.A., Come C., Savagner P., Gitelman I., Richardson A., Weinberg R.A. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927–939. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 63.Thomann S., Weiler S.M.E., Marquard S., Rose F., Ball C.R., Tóth M., Wei T., Sticht C., Fritzsche S., Roessler S., De La Torre C., Ryschich E., Ermakova O., Mogler C., Kazdal D., Gretz N., Glimm H., Rempel E., Schirmacher P., Breuhahn K. YAP orchestrates heterotypic endothelial cell communication via HGF/c-MET signaling in liver tumorigenesis. Cancer Res. 2020;80:5502–5514. doi: 10.1158/0008-5472.CAN-20-0242. [DOI] [PubMed] [Google Scholar]
- 64.Lu C., Fang S., Weng Q., Lv X., Meng M., Zhu J., Zheng L., Hu Y., Gao Y., Wu X., Mao J., Tang B., Zhao Z., Huang L., Ji J. Integrated analysis reveals critical glycolytic regulators in hepatocellular carcinoma. Cell Commun. Signal. CCS. 2020;18:97. doi: 10.1186/s12964-020-00539-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Corbet C., Feron O. Tumour acidosis: from the passenger to the driver's seat. Nat. Rev. Cancer. 2017;17:577–593. doi: 10.1038/nrc.2017.77. [DOI] [PubMed] [Google Scholar]
- 66.Zhu Y., Gao X.-M., Yang J., Xu D., Zhang Y., Lu M., Zhang Z., Sheng Y.-Y., Li J.-H., Yu X.-X., Zheng Y., Dong Q.-Z., Qin L.-X. C-C chemokine receptor type 1 mediates osteopontin-promoted metastasis in hepatocellular carcinoma. Cancer Sci. 2018;109:710–723. doi: 10.1111/cas.13487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang S.-Y., Chen C.-L., Hu Y.-C., Chi Y., Huang Y.-H., Su C.-W., Jeng W.-J., Liang Y.-J., Wu J.-C. High expression of MicroRNA-196a is associated with progression of hepatocellular carcinoma in younger patients. Cancers. 2019;11:1549. doi: 10.3390/cancers11101549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ribas A., Wolchok J.D. Cancer immunotherapy using checkpoint blockade. Science. 2018;359:1350–1355. doi: 10.1126/science.aar4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mantovani A., Sica A. Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Curr. Opin. Immunol. 2010;22:231–237. doi: 10.1016/j.coi.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 70.Murray P.J., Allen J.E., Biswas S.K., Fisher E.A., Gilroy D.W., Goerdt S., Gordon S., Hamilton J.A., Ivashkiv L.B., Lawrence T., Locati M., Mantovani A., Martinez F.O., Mege J.-L., Mosser D.M., Natoli G., Saeij J.P., Schultze J.L., Shirey K.A., Sica A., Suttles J., Udalova I., van Ginderachter J.A., Vogel S.N., Wynn T.A. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Iida T., Wagatsuma K., Hirayama D., Nakase H. Is osteopontin a friend or foe of cell apoptosis in inflammatory gastrointestinal and liver diseases? Int. J. Mol. Sci. 2017;19:7. doi: 10.3390/ijms19010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zagani R., Hamzaoui N., Cacheux W., de Reyniès A., Terris B., Chaussade S., Romagnolo B., Perret C., Lamarque D. Cyclooxygenase-2 inhibitors down-regulate osteopontin and Nr4A2-new therapeutic targets for colorectal cancers. Gastroenterology. 2009;137:1358–1366. doi: 10.1053/j.gastro.2009.06.039. e1–3. [DOI] [PubMed] [Google Scholar]
- 73.Mosser D.M., Edwards J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Guiducci C., Vicari A.P., Sangaletti S., Trinchieri G., Colombo M.P. Redirecting in vivo elicited tumor infiltrating macrophages and dendritic cells towards tumor rejection. Cancer Res. 2005;65:3437–3446. doi: 10.1158/0008-5472.CAN-04-4262. [DOI] [PubMed] [Google Scholar]
- 75.Wei J., Marisetty A., Schrand B., Gabrusiewicz K., Hashimoto Y., Ott M., Grami Z., Kong L.-Y., Ling X., Caruso H., Zhou S., Wang Y.A., Fuller G.N., Huse J., Gilboa E., Kang N., Huang X., Verhaak R., Li S., Heimberger A.B. Osteopontin mediates glioblastoma-associated macrophage infiltration and is a potential therapeutic target. J. Clin. Invest. 2019;129:137–149. doi: 10.1172/JCI121266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang Y., Du W., Chen Z., Xiang C. Upregulation of PD-L1 by SPP1 mediates macrophage polarization and facilitates immune escape in lung adenocarcinoma. Exp. Cell Res. 2017;359:449–457. doi: 10.1016/j.yexcr.2017.08.028. [DOI] [PubMed] [Google Scholar]
- 77.Zhu Y., Yang J., Xu D., Gao X.-M., Zhang Z., Hsu J.L., Li C.-W., Lim S.-O., Sheng Y.-Y., Zhang Y., Li J.-H., Luo Q., Zheng Y., Zhao Y., Lu L., Jia H.-L., Hung M.-C., Dong Q.-Z., Qin L.-X. Disruption of tumour-associated macrophage trafficking by the osteopontin-induced colony-stimulating factor-1 signalling sensitises hepatocellular carcinoma to anti-PD-L1 blockade. Gut. 2019;68:1653–1666. doi: 10.1136/gutjnl-2019-318419. [DOI] [PubMed] [Google Scholar]
- 78.Ellert-Miklaszewska A., Wisniewski P., Kijewska M., Gajdanowicz P., Pszczolkowska D., Przanowski P., Dabrowski M., Maleszewska M., Kaminska B. Tumour-processed osteopontin and lactadherin drive the protumorigenic reprogramming of microglia and glioma progression. Oncogene. 2016;35:6366–6377. doi: 10.1038/onc.2016.55. [DOI] [PubMed] [Google Scholar]
- 79.Rao G., Wang H., Li B., Huang L., Xue D., Wang X., Jin H., Wang J., Zhu Y., Lu Y., Du L., Chen Q. Reciprocal interactions between tumor-associated macrophages and CD44-positive cancer cells via osteopontin/CD44 promote tumorigenicity in colorectal cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2013;19:785–797. doi: 10.1158/1078-0432.CCR-12-2788. [DOI] [PubMed] [Google Scholar]
- 80.Georgoudaki A.-M., Prokopec K.E., Boura V.F., Hellqvist E., Sohn S., Östling J., Dahan R., Harris R.A., Rantalainen M., Klevebring D., Sund M., Brage S.E., Fuxe J., Rolny C., Li F., Ravetch J.V., Karlsson M.C.I. Reprogramming tumor-associated macrophages by antibody targeting inhibits cancer progression and metastasis. Cell Rep. 2016;15:2000–2011. doi: 10.1016/j.celrep.2016.04.084. [DOI] [PubMed] [Google Scholar]
- 81.Jiang X., Zhang X., Jiang N., Sun Y., Li T., Zhang J., Shen Y., Cao J. The single-cell landscape of cystic echinococcosis in different stages provided insights into endothelial and immune cell heterogeneity. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.1067338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu Y., Xun Z., Ma K., Liang S., Li X., Zhou S., Sun L., Liu Y., Du Y., Guo X., Cui T., Zhou H., Wang J., Yin D., Song R., Zhang S., Cai W., Meng F., Guo H., Zhang B., Yang D., Bao R., Hu Q., Wang J., Ye Y., Liu L. Identification of a tumour immune barrier in the HCC microenvironment that determines the efficacy of immunotherapy. J. Hepatol. 2023;78:770–782. doi: 10.1016/j.jhep.2023.01.011. [DOI] [PubMed] [Google Scholar]
- 83.Qi J., Sun H., Zhang Y., Wang Z., Xun Z., Li Z., Ding X., Bao R., Hong L., Jia W., Fang F., Liu H., Chen L., Zhong J., Zou D., Liu L., Han L., Ginhoux F., Liu Y., Ye Y., Su B. Single-cell and spatial analysis reveal interaction of FAP+ fibroblasts and SPP1+ macrophages in colorectal cancer. Nat. Commun. 2022;13:1742. doi: 10.1038/s41467-022-29366-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu Y., Zhang Q., Xing B., Luo N., Gao R., Yu K., Hu X., Bu Z., Peng J., Ren X., Zhang Z. Immune phenotypic linkage between colorectal cancer and liver metastasis. Cancer Cell. 2022;40:424–437.e5. doi: 10.1016/j.ccell.2022.02.013. [DOI] [PubMed] [Google Scholar]
- 85.Casanova-Acebes M., Dalla E., Leader A.M., LeBerichel J., Nikolic J., Morales B.M., Brown M., Chang C., Troncoso L., Chen S.T., Sastre-Perona A., Park M.D., Tabachnikova A., Dhainaut M., Hamon P., Maier B., Sawai C.M., Agulló-Pascual E., Schober M., Brown B.D., Reizis B., Marron T., Kenigsberg E., Moussion C., Benaroch P., Aguirre-Ghiso J.A., Merad M. Tissue-resident macrophages provide a pro-tumorigenic niche to early NSCLC cells. Nature. 2021;595:578–584. doi: 10.1038/s41586-021-03651-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ren X., Zhang L., Zhang Y., Li Z., Siemers N., Zhang Z. Insights gained from single-cell analysis of immune cells in the tumor microenvironment. Annu. Rev. Immunol. 2021;39:583–609. doi: 10.1146/annurev-immunol-110519-071134. [DOI] [PubMed] [Google Scholar]
- 87.Li X., Zhao S., Bian X., Zhang L., Lu L., Pei S., Dong L., Shi W., Huang L., Zhang X., Chen M., Chen X., Yin M. Signatures of EMT, immunosuppression, and inflammation in primary and recurrent human cutaneous squamous cell carcinoma at single-cell resolution. Theranostics. 2022;12:7532–7549. doi: 10.7150/thno.77528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu Y., Zhang L., Ju X., Wang S., Qie J. Single-cell transcriptomic analysis reveals macrophage-tumor crosstalk in hepatocellular carcinoma. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.955390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lu C., Liu Z., Klement J.D., Yang D., Merting A.D., Poschel D., Albers T., Waller J.L., Shi H., Liu K. WDR5-H3K4me3 epigenetic axis regulates OPN expression to compensate PD-L1 function to promote pancreatic cancer immune escape. J. Immunother. Cancer. 2021;9 doi: 10.1136/jitc-2021-002624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen L., Han X. Anti-PD-1/PD-L1 therapy of human cancer: past, present, and future. J. Clin. Invest. 2015;125:3384–3391. doi: 10.1172/JCI80011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pietras A., Katz A.M., Ekström E.J., Wee B., Halliday J.J., Pitter K.L., Werbeck J.L., Amankulor N.M., Huse J.T., Holland E.C. Osteopontin-CD44 signaling in the glioma perivascular niche enhances cancer stem cell phenotypes and promotes aggressive tumor growth. Cell Stem Cell. 2014;14:357–369. doi: 10.1016/j.stem.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Klement J.D., Paschall A.V., Redd P.S., Ibrahim M.L., Lu C., Yang D., Celis E., Abrams S.I., Ozato K., Liu K. An osteopontin/CD44 immune checkpoint controls CD8+ T cell activation and tumor immune evasion. J. Clin. Invest. 2018;128:5549–5560. doi: 10.1172/JCI123360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pitt J.M., Vétizou M., Daillère R., Roberti M.P., Yamazaki T., Routy B., Lepage P., Boneca I.G., Chamaillard M., Kroemer G., Zitvogel L. Resistance mechanisms to immune-checkpoint blockade in cancer: tumor-intrinsic and -extrinsic factors. Immunity. 2016;44:1255–1269. doi: 10.1016/j.immuni.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 94.Boumans M.J.H., Houbiers J.G.A., Verschueren P., Ishikura H., Westhovens R., Brouwer E., Rojkovich B., Kelly S., den Adel M., Isaacs J., Jacobs H., Gomez-Reino J., Holtkamp G.M., Hastings A., Gerlag D.M., Tak P.P. Safety, tolerability, pharmacokinetics, pharmacodynamics and efficacy of the monoclonal antibody ASK8007 blocking osteopontin in patients with rheumatoid arthritis: a randomised, placebo controlled, proof-of-concept study. Ann. Rheum. Dis. 2012;71:180–185. doi: 10.1136/annrheumdis-2011-200298. [DOI] [PubMed] [Google Scholar]
- 95.Zuo H., Yang D., Wan Y. Fam20C regulates bone resorption and breast cancer bone metastasis through osteopontin and BMP4. Cancer Res. 2021;81:5242–5254. doi: 10.1158/0008-5472.CAN-20-3328. [DOI] [PubMed] [Google Scholar]
- 96.Klement J.D., Poschel D.B., Lu C., Merting A.D., Yang D., Redd P.S., Liu K. Osteopontin blockade immunotherapy increases cytotoxic T lymphocyte lytic activity and suppresses colon tumor progression. Cancers. 2021;13:1006. doi: 10.3390/cancers13051006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Osteopontin induces growth of metastatic tumors in a preclinical model of non-small lung cancer - PubMed, (n.d.) https://pubmed.ncbi.nlm.nih.gov/22444159/ [DOI] [PMC free article] [PubMed]
- 98.Chang S., Huang J., Niu H., Wang J., Si Y., Bai Z., Cheng S., Ding W. Epigenetic regulation of osteopontin splicing isoform c defines its role as a microenvironmental factor to promote the survival of colon cancer cells from 5-FU treatment. Cancer Cell Int. 2020;20:452. doi: 10.1186/s12935-020-01541-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Han B., Huang J., Han Y., Hao J., Wu X., Song H., Chen X., Shen Q., Dong X., Pang H., Cai L. The microRNA miR-181c enhances chemosensitivity and reduces chemoresistance in breast cancer cells via down-regulating osteopontin. Int. J. Biol. Macromol. 2019;125:544–556. doi: 10.1016/j.ijbiomac.2018.12.075. [DOI] [PubMed] [Google Scholar]
- 100.Cho W.-Y., Hong S.-H., Singh B., Islam M.A., Lee S., Lee A.Y., Gankhuyag N., Kim J.-E., Yu K.-N., Kim K.-H., Park Y.-C., Cho C.-S., Cho M.-H. Suppression of tumor growth in lung cancer xenograft model mice by poly(sorbitol-co-PEI)-mediated delivery of osteopontin siRNA. Eur. J. Pharm. Biopharm. Off. J. Arbeitsgemeinschaft Pharm. Verfahrenstechnik EV. 2015;94:450–462. doi: 10.1016/j.ejpb.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 101.Gupta A., Zhou C.Q., Chellaiah M.A. Osteopontin and MMP9: associations with VEGF expression/secretion and angiogenesis in PC3 prostate cancer cells. Cancers. 2013;5:617–638. doi: 10.3390/cancers5020617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kanda R., Kawahara A., Watari K., Murakami Y., Sonoda K., Maeda M., Fujita H., Kage M., Uramoto H., Costa C., Kuwano M., Ono M. Erlotinib resistance in lung cancer cells mediated by integrin β1/Src/Akt-driven bypass signaling. Cancer Res. 2013;73:6243–6253. doi: 10.1158/0008-5472.CAN-12-4502. [DOI] [PubMed] [Google Scholar]
- 103.Kumar S., Patil H.S., Sharma P., Kumar D., Dasari S., Puranik V.G., Thulasiram H.V., Kundu G.C. Andrographolide inhibits osteopontin expression and breast tumor growth through down regulation of PI3 kinase/Akt signaling pathway. Curr. Mol. Med. 2012;12:952–966. doi: 10.2174/156652412802480826. [DOI] [PubMed] [Google Scholar]
- 104.Desai B., Rogers M.J., Chellaiah M.A. Mechanisms of osteopontin and CD44 as metastatic principles in prostate cancer cells. Mol. Cancer. 2007;6:18. doi: 10.1186/1476-4598-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhang X., Bi K., Tu X., Zhang Q., Cao Q., Liang Y., Zeng P., Wang L., Liu T., Fang W., Diao H. Interleukin-33 as an early predictor of cetuximab treatment efficacy in patients with colorectal cancer. Cancer Med. 2021;10:8338–8351. doi: 10.1002/cam4.4331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Maneechotesuwan K., Kasetsinsombat K., Wongkajornsilp A., Barnes P.J. Simvastatin up-regulates adenosine deaminase and suppresses osteopontin expression in COPD patients through an IL-13-dependent mechanism. Respir. Res. 2016;17:104. doi: 10.1186/s12931-016-0424-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mi Z., Bhattacharya S.D., Kim V.M., Guo H., Talbot L.J., Kuo P.C. Osteopontin promotes CCL5-mesenchymal stromal cell-mediated breast cancer metastasis. Carcinogenesis. 2011;32:477–487. doi: 10.1093/carcin/bgr009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Robertson B.W., Bonsal L., Chellaiah M.A. Regulation of Erk1/2 activation by osteopontin in PC3 human prostate cancer cells. Mol. Cancer. 2010;9:260. doi: 10.1186/1476-4598-9-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mi Z., Guo H., Russell M.B., Liu Y., Sullenger B.A., Kuo P.C. RNA aptamer blockade of osteopontin inhibits growth and metastasis of MDA-MB231 breast cancer cells. Mol. Ther. J. Am. Soc. Gene Ther. 2009;17:153–161. doi: 10.1038/mt.2008.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dost Gunay F.S., Kırmızı B.A., Ensari A., İcli F., Akbulut H. Tumor-associated macrophages and neuroendocrine differentiation decrease the efficacy of bevacizumab plus chemotherapy in patients with advanced colorectal cancer. Clin. Colorectal Cancer. 2019;18:e244. doi: 10.1016/j.clcc.2018.12.004. –e250. [DOI] [PubMed] [Google Scholar]
- 111.Larionova I., Kazakova E., Gerashchenko T., Kzhyshkowska J. New angiogenic regulators produced by TAMs: perspective for targeting tumor angiogenesis. Cancers. 2021;13:3253. doi: 10.3390/cancers13133253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Li X., Carmeliet P. Targeting angiogenic metabolism in disease. Science. 2018;359:1335–1336. doi: 10.1126/science.aar5557. [DOI] [PubMed] [Google Scholar]
- 113.Wang Y., Yan W., Lu X., Qian C., Zhang J., Li P., Shi L., Zhao P., Fu Z., Pu P., Kang C., Jiang T., Liu N., You Y. Overexpression of osteopontin induces angiogenesis of endothelial progenitor cells via the avβ3/PI3K/AKT/eNOS/NO signaling pathway in glioma cells. Eur. J. Cell Biol. 2011;90:642–648. doi: 10.1016/j.ejcb.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 114.Jain S., Chakraborty G., Kundu G.C. The crucial role of cyclooxygenase-2 in osteopontin-induced protein kinase C alpha/c-Src/IkappaB kinase alpha/beta-dependent prostate tumor progression and angiogenesis. Cancer Res. 2006;66:6638–6648. doi: 10.1158/0008-5472.CAN-06-0661. [DOI] [PubMed] [Google Scholar]
- 115.Vergis R., Corbishley C.M., Norman A.R., Bartlett J., Jhavar S., Borre M., Heeboll S., Horwich A., Huddart R., Khoo V., Eeles R., Cooper C., Sydes M., Dearnaley D., Parker C. Intrinsic markers of tumour hypoxia and angiogenesis in localised prostate cancer and outcome of radical treatment: a retrospective analysis of two randomised radiotherapy trials and one surgical cohort study. Lancet Oncol. 2008;9:342–351. doi: 10.1016/S1470-2045(08)70076-7. [DOI] [PubMed] [Google Scholar]
- 116.Raja R., Kale S., Thorat D., Soundararajan G., Lohite K., Mane A., Karnik S., Kundu G.C. Hypoxia-driven osteopontin contributes to breast tumor growth through modulation of HIF1α-mediated VEGF-dependent angiogenesis. Oncogene. 2014;33:2053–2064. doi: 10.1038/onc.2013.171. [DOI] [PubMed] [Google Scholar]
- 117.Psallidas I., Stathopoulos G.T., Maniatis N.A., Magkouta S., Moschos C., Karabela S.P., Kollintza A., Simoes D.C.M., Kardara M., Vassiliou S., Papiris S.A., Roussos C., Kalomenidis I. Secreted phosphoprotein-1 directly provokes vascular leakage to foster malignant pleural effusion. Oncogene. 2013;32:528–535. doi: 10.1038/onc.2012.57. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.