Abstract
Neuroblastoma is the most common extra-cranial solid tumor in children and is known for its clinical heterogeneity. A greater understanding of the biology of this disease has led to both improved risk stratification and new approaches to therapy. Outcomes for children with low and intermediate risk disease are excellent overall, and efforts to decrease therapy for such patients have been largely successful. Although survival has improved over time for patients with high-risk disease and treatments evaluated in the relapse setting are now being moved into earlier phases of treatment, much work remains to improve survival and decrease therapy-related toxicities. Studies of highly annotated biobanked samples continue to lead to important insights regarding neuroblastoma biology. Such studies, along with correlative biology studies incorporated into therapeutic trials, are expected to continue to provide insights that lead to new and more effective therapies. A focus on translational science is accompanied by an emphasis on new agent development, optimized risk stratification, and international collaboration to address questions relevant to molecularly defined subsets of patients. In addition, the COG Neuroblastoma Committee is committed to addressing the patient/family experience, mitigating late effects of therapy, and studying social determinants of health in patients with neuroblastoma.
OVERVIEW
Neuroblastoma arises from embryonic neural crest cells of the sympathetic nervous system. It is the most common extra-cranial solid tumor in children and is known for its biologic and clinical heterogeneity. Some infants with neuroblastoma experience complete tumor regression without therapy, while other children have relentless disease progression despite modern multi-modality therapy. Nearly 550 newly-diagnosed patients with neuroblastoma per year are enrolled on the Children’s Oncology Group (COG) Neuroblastoma Biology Study, ANBL00B1, ~45% of whom are <18 months of age.1 The majority of patients are diagnosed before the age of 5 years.
STAGING, RISK STRATIFICATION AND CURRENT OUTCOMES
The International Neuroblastoma Risk Group (INRG) Staging System makes a distinction between locoregional (L) and metastatic (M) disease. For locoregional disease, imaging features known as image-defined risk factors (IDRFs) are critical for disease stage assignment.2 Locoregional tumors that lack IDRFs and are confined to one body compartment are designated as L1, and those with at least one IDRF are L2. Stage M patients have disease dissemination to distant lymph nodes, bone marrow, cortical bone, liver, and/or other organs, unless criteria for metastatic disease with a specific pattern (MS) are met. Infants <18 months with metastases confined to liver, skin, and limited (<10%) bone marrow involvement without bone metastasis have Stage MS.2
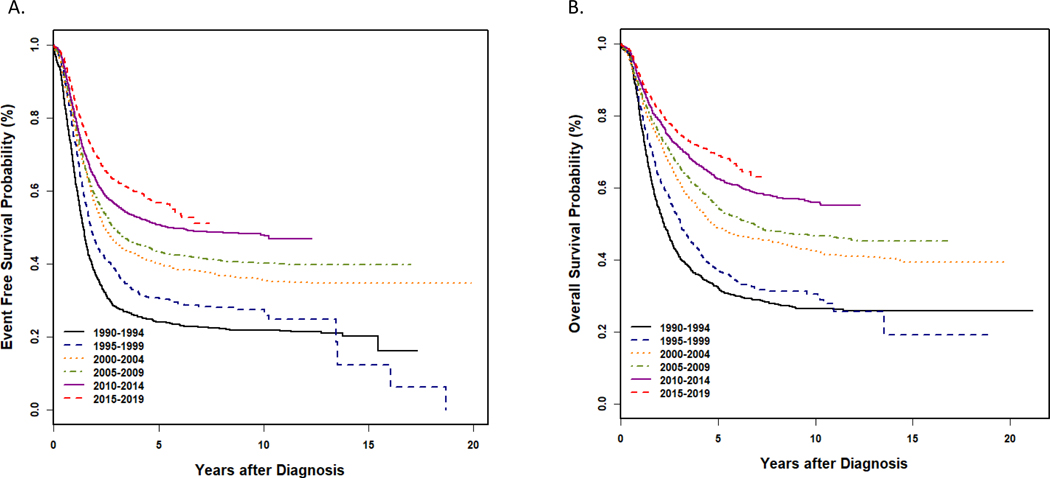
Analysis of data from ANBL00B1 has facilitated integration of INRG staging into the current COG risk classification schema.1 The revised risk classifier also incorporates additional prognostic variables. Using data from 4,832 patients, key clinical and biologic factors were associated with event-free and overall survival (EFS, OS) to form risk groups defined by current era outcomes.1 This classifier also incorporates information regarding the intensity of therapy delivered on or according to COG trials, as well as the likelihood of successful salvage following relapse or progression. The current approach to risk classification is summarized in Table 1. Low risk patients are those with an expected OS ≥95%. This group includes patients of any age with INRG Stage L1 tumors, except for those with incompletely resected MYCN amplified (MYCN-A) tumors, and infants <12 months of age with INRG MS disease who are asymptomatic and whose tumors have all favorable features. Patients classified as intermediate-risk also have excellent outcomes following moderate intensity therapy, with expected OS ≥85–90%. Patients with INRG Stage L2, MYCN non-amplified (MYCN-NA) neuroblastoma diagnosed prior to 18 months are designated as intermediate-risk, as are children >18 months with MYCN-NA L2 tumors with favorable histologic features. Symptomatic infants with INRG MS disease and asymptomatic infants whose MS tumors are MYCN-NA but have ≥1 unfavorable feature (unfavorable histology, diploidy, segmental chromosomal aberrations (SCAs)) are classified as intermediate-risk. Additional intermediate-risk patients are those <12 months with INRG Stage M MYCN-NA disease and 12–18 month-old stage M toddlers whose tumors have all favorable features. Children >18 months with INRG M and patients with INRG L2, MS and M disease with MYCN-A are classified high-risk, as are rare patients with incompletely resected L1, MYCN-A tumors. While survival of patients with high-risk disease has improved over time as treatment has evolved (Figure 1), 3-year EFS remains just over 50%.3 Survival rates of patients with L2, MYCN non-amplified tumors with unfavorable histology are superior to those of other high-risk patients, however they are still classified as high-risk because outcomes remain unsatisfactory, particularly among patients whose tumors harbor an SCA at 11q .4
Table 1.
Risk Groups according to Revised COG Risk Classifier, 20211: Shown are estimated survival ranges for risk groups, patient cohorts (by age, stage, and tumor biology) classified as low, intermediate and high risk, and general treatment approaches.
| Risk Group | Low-Risk | Intermediate-Risk | High-Risk |
|---|---|---|---|
| Survival | OS: ≥95% | OS: ≥85% | EFS: 50 - <80% |
| Patient/Tumor Characteristics | • L1 (except MYCN-A incompletely resected) • MS <12 mo MYCN-NA, INPC-fav, No SCA, no symptoms |
• L2 <18mo MYCN-NA • L2 >18 mo MYCN-NA, INPC-fav • M <12mo MYCN-NA • M or MS, 12–18 mo MYCN-NA, INPC-fav, No SCA • MS <12mo MYCN-NA, INPC-unfav OR SCA+ OR diploid |
• L2, M, MS - MYCN-A (any age) • M or MS, 12–18 mo- MYCN-NA AND INPC-unfav OR SCA+ OR diploid • M >18mo (any biology) |
| Typical Treatment Approaches | Observation OR Surgery Chemotherapy only for MS-related symptoms, cord compression |
2–8 cycles lower intensity chemotherapy (based on stage, tumor biology) +/− surgery |
Induction: Chemo, Surgery Consolidation: ASCT(s)*, radiation therapy Post Consolidation: Anti-GD2 immunotherapy |
MYCN-NA, MYCN non-amplified: MYCN-A, MYCN amplified: INPC, International Neuroblastoma Pathology Classification; fav, favorable; unfav, unfavorable; SCAs, segmental chromosome aberrations
Note: not all high-risk patients are eligible for tandem transplant on recent protocols
Figure 1 – Survival among North American patients with high-risk neuroblastoma based on year of diagnosis.
Kaplan-Meier curves of EFS and OS for patients diagnosed with high-risk neuroblastoma and enrolled on Children’s Oncology Group ANBL00B1 (2001 and thereafter) and predecessor cooperative group biology studies (prior to 2001) between 1990 and 2019 shown in 5-year intervals. A. Event-free survival; B. Overall survival. Data are courtesy of Arlene Naranjo, Children’s Oncology Group Statistics and Data Center.
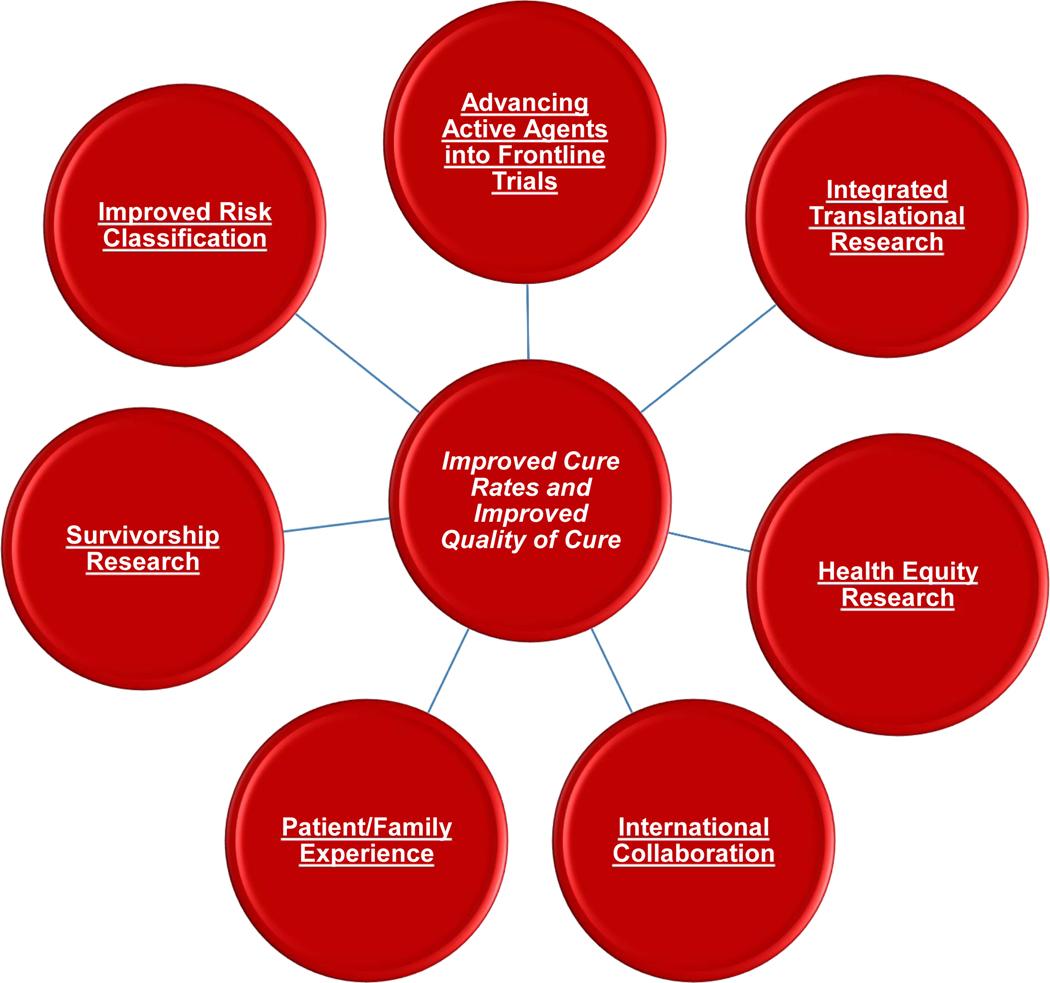
Figure 3 – Overall Strategy of the COG Neuroblastoma Committee.
The mission of the COG Neuroblastoma Committee is to conduct practice-change research aimed to improve outcomes for children with neuroblastoma. The committee emphasizes translational research and the development and implementation of clinical trials while working to improve risk classification and new agent development. In addition, the committee emphasizes international collaboration to address key questions in the field as well as research that addresses the patient/family experience in the context of a neuroblastoma diagnosis. Research related to social determinants of health and survivorship are also being prioritized.
STATE OF THE DISEASE
Biology and Molecular Characterization
Studies of germline samples have identified autosomal dominant missense mutations in PHOX2B and ALK that account for neuroblastoma predisposition in 1–2% of patients.5 Less commonly, other cancer predisposition syndromes can be associated with neuroblastoma including RAS-opathies, Li-Fraumeni and Beckwith-Weideman syndromes, and DNA repair disorders.6 Genome-wide association studies (GWAS) have identified common variants in multiple genes including BARD1, LMO1, and LIN28B that are associated with neuroblastoma risk.5 Combinations of these and other host genetic variants may predispose patients to sporadic neuroblastoma and more or less aggressive tumor phenotypes.
Genomic and molecular features have been used for neuroblastoma risk classification for decades.7 Amplification of the MYCN oncogene (MYCN-A) remains the strongest prognostic biomarker identified to date, predicting poor outcome independent of age, stage and other risk factors.8–10 High MYCN expression, and in some cases C-MYC,11 results in additional gene expression pattern changes via MYCN regulation of the core transcriptional regulatory circuitry.12 Thus pre-clinical studies targeting MYCN activity via bromodomain (eg BRD4) or CDK7 inhibitors13,14 and destabilization of MYC with Aurora kinase inhibitors15 suggest that these approaches are promising.
In addition to MYCN-A, other copy number alterations (CNAs) are important biomarkers in neuroblastoma.16,17 Tumors with aneuploid or hyperdiploid DNA content and whole chromosome aberrations are associated with favorable outcomes and are more common in younger patients and those with lower stage disease. In contrast, tumors with SCAs and diploidy are often associated with poor outcome. SCAs include partial gains and losses of chromosomal regions predicted to encode oncogenes and tumor suppressors, respectively. Most copy number analyses performed using COG specimens have focused on loss of heterozygosity (LOH) of 1p and 11q. 1p LOH is detected in 23–32% of tumors, predicts poor outcome, and often correlates with MYCN-A.18,19 In contrast, 11q LOH, detected in 30–45% of tumors18,20, is rarely associated with MYCN-A and therefore independently predicts poor outcome in some subsets. Analyses of data from ANBL00B1 indicate that 1p and/or 11q SCAs can identify groups of patients with inferior outcomes.1 SCA status was informative in 12–18 month olds with Stage M disease with MYCN-NA tumors and those ≥18 months with Stage L2 disease, a finding recently validated through analyses of data from high-risk trials (A3973, ANBL12P1, ANBL0532).4 Retrospective studies have supported the prognostic strength of SCAs including loss of 1p and 11q as well as loss of 3p and 4p and gain of 1q, 2p, and 17q.21,22 These SCAs are especially important in subgroups with otherwise favorable features.23,24 For this reason, the updated COG risk classifier designates a tumor as harboring an SCA if an aberration is detected at ≥1 of these seven loci.1
Next generation sequencing (NGS) studies of well-annotated cohorts have revealed that activating missense mutations in the tyrosine kinase domain of ALK are the most common recurrent single nucleotide variants, detected in ~10% of tumors.25 A higher incidence of ALK mutations has been reported at relapse.26,27 Tumors from an additional 4% of newly diagnosed patients demonstrate high level ALK amplification.25,28 ALK alterations are associated with inferior outcome in subsets of patients,28,29 and have provided an opportunity for precision trials. These studies have been conducted primarily in patients with relapsed/refractory disease, and have included trials of the tyrosine kinase inhibitors crizotinib30, ceritinib31, and lorlatinib.32 Other alterations detected at diagnosis and at relapse include genes in the RAS-MAPK pathway and others involved in neuritogenesis and chromatin remodeling.25,26
Aberrant telomere lengthening mechanisms (TLM), including high levels of TERT expression, TERT fusions, mutations in ATRX, and presence of alternative lengthening of telomeres (ALT) have been shown to be prognostic in subsets of neuroblastoma patients33–36 and novel therapies that target these alterations are under development. Retrospective studies have demonstrated that patients currently classified as non-high-risk whose tumors have TLM alterations are at higher risk for recurrence.33,36 Among those with high-risk disease, certain TLM alterations together with mutations in RAS-MAPK and p53 pathways may identify patients in the most unfavorable group.33 In retrospective studies of COG high-risk cohorts, patients with tumors with low TERT and absence of ALT have superior survival.35 TLM analyses using banked samples are ongoing, and current trials are evaluating TLMs prospectively.
Studies of the tumor microenvironment (including immune infiltrates) and serum immunocorrelative studies have been undertaken to examine prognostic implications, and to predict response to anti-GD2 immunotherapy regimens.37–40 With the exception of host polymorphisms of KIR/KIR-ligand genes,39,41 no other predictive biomarkers have been identified. However, additional correlative studies, including monitoring GD2 expression, are ongoing using samples from COG trials.
Finally, several groups have reported that neuroblastoma tumors contain distinct lineage-committed mesenchymal (MES) and adrenergic (ADR) cells.42,43 These MES and ADR cell types are genetically identical but defined by unique super enhancer transcriptional networks. Early data suggest that these cell types may respond differently to chemotherapy, immunotherapies, and some targeted agents.43,44 It is expected that future studies including many of these biomarkers will facilitate further optimization and precision in risk classification systems and treatments.
Non-High-Risk Neuroblastoma
Among patients with non-high-risk disease, outcomes remain excellent. Observation alone for infants ≤6 months of age with small adrenal masses is considered standard, as EFS and OS were outstanding on COG ANBL00P2.45 ANBL1232 is evaluating expectant observation in additional patients, including infants ≤12 months with larger tumors and non-adrenal primaries, as well as symptomatic children <18 months of age with L2 tumors and all favorable features. The A3961 trial46 and trials in Europe47,48 demonstrated that excellent outcomes were maintained with reductions in therapy for subsets of patients with intermediate-risk disease. Systemic therapy was also successfully reduced for additional subsets of non-high-risk patients enrolled on ANBL0531.24 Patients were assigned to receive 2, 4 or 8 chemotherapy cycles followed by primary tumor resection in those with less than partial response (PR). Importantly, this study showed that in patients with localized intermediate-risk disease, PR is an acceptable therapy endpoint, and ongoing chemotherapy and/or surgery to achieve a complete response (CR) is not required. OS among eligible patients on ANBL0531 approached 95%, and OS for those with localized disease was 100%. EFS for infants with Stage M disease with favorable biologic features exceeded 85%. EFS of Stage M infants with unfavorable features (diploidy or presence of 1p and/or 11q SCAs) was <70%. Studies of tumor biology are being undertaken with the goal of developing specific therapies for this subset of patients.
High-Risk Neuroblastoma
Current therapy for children with newly diagnosed high-risk disease typically consists of three phases: Induction, Consolidation, and Post-Consolidation. In Induction, intensive multiagent chemotherapy and surgical resection are used to reduce disease burden. An analysis from recent COG trials demonstrated that ~80% of patients achieve ≥ PR at end of Induction, with only 20% achieving a CR.49 Moreover, patients with less favorable induction response have inferior outcomes, highlighting the need to improve induction response to impact longer-term outcomes.
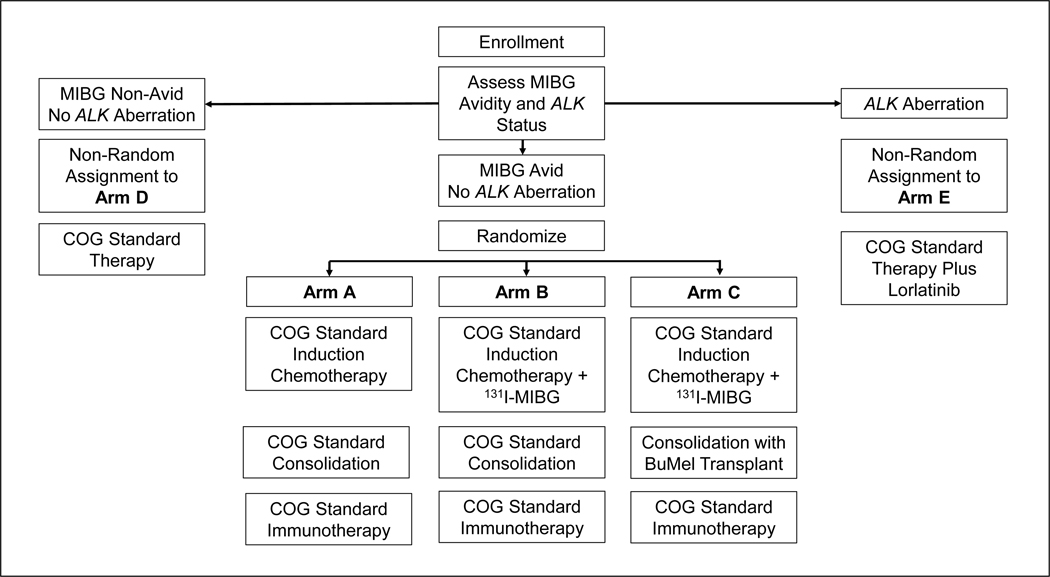
The COG Neuroblastoma Committee has embarked on two clinical trials targeting Induction: ANBL1531 and ANBL17P1. ANBL1531 was designed to determine if the addition of the targeted radiopharmaceutical 131I-metaiodobenzylguanidine (131I-MIBG) during Induction improves EFS in patients with MIBG-avid, ALK wildtype disease. Patients are randomized to receive COG standard therapy with or without 131I-MIBG during Induction (Figure 2). A second objective is to determine if the addition of the ALK inhibitor lorlatinib32 improves EFS in patients with ALK aberrant tumors. EFS and OS will be compared both to ANBL1531 patients with ALK wild-type tumors treated with standard therapy, and separately to patients with ALK aberrant disease treated on COG trial ANBL0532 (see below).
Figure 2 – Clinical trial schema for Children’s Oncology Group study ANBL1531, A Phase 3 Study of 131I-Metaiodobenzylguanidine (131I-MIBG) or Lorlatinib Added to Intensive Therapy for Children with Newly Diagnosed High-Risk Neuroblastoma.
Enrolled patients receive an initial cycle of Induction therapy while tumors are tested for ALK status and central review of diagnostic MIBG scans is performed. Patients with tumors harboring an activating mutation in or amplification of the ALK gene are assigned to receive intensive, multi-modality therapy along with the ALK inhibitor lorlatinib (Arm E). Patients with ALK wild type, MIBG non-avid disease receive COG standard therapy (Arm D). Patients with ALK wild type, MIBG avid disease are randomized to receive standard therapy with (Arm B) or without (Arm A) the addition of 131I-MIBG during Induction. Patients on Arms A, B, D and E undergo tandem cycles of high dose chemotherapy during consolidation before proceeding to radiation therapy and immunotherapy. Patients on Arm E continue therapy with single agent lorlatinib for one year after completion of post-Consolidation immunotherapy. Randomized trial Arm C includes 131I-MIBG therapy during Induction followed by a Consolidation containing a single cycle of high dose chemotherapy with autologous stem cell rescue, radiation and immunotherapy.
ANBL17P1 leveraged the results of a single institution study demonstrating feasibility and activity of anti-GD2 monoclonal antibody added to Induction.50 In the multi-center ANBL17P1 trial, dinutuximab was added to Induction cycles 3–5. Preliminary results demonstrated feasibility and tolerability,51 paving the way for a planned randomized phase 3 trial (see Key Clinical Trials to be Pursued).
In the Consolidation phase, patients receive high-dose chemotherapy with autologous peripheral blood stem cell rescue followed by radiotherapy to the primary tumor bed and residual metastatic sites detected at the end of Induction. COG ANBL0532 randomized patients at the end of Induction to a single round of high-dose chemotherapy or to two back-to-back rounds (“tandem transplant”).3 Patients randomized to tandem transplant had significantly higher EFS at 3-years post-randomization, establishing this approach as a new standard for most patients with high-risk disease. ANBL0532 also evaluated the role of radiation boosts for patients with incomplete surgical resection, and demonstrated no improvement in local control rates.52 Current open questions in the field are optimal selection criteria to proceed to transplant and whether chemoimmunotherapy might ultimately replace high-dose chemotherapy approaches (see Key Clinical Trials to be Pursued).
In the Post-Consolidation phase, patients receive therapies directed against minimal residual disease (MRD). The current approach was informed by the landmark ANBL0032 trial demonstrating significantly higher EFS for patients randomized to receive the anti-GD2 monoclonal antibody dinutuximab together with cytokines and isotretinoin compared to patients assigned to isotretinoin alone.53 The feasibility of adding irinotecan and temozolomide to dinutuximab during post-consolidation is being evaluated in the ANBL19P1 pilot study. Accrual is complete, and results are pending.
Relapsed High-Risk Neuroblastoma
Outcomes for most relapsed high-risk neuroblastoma patients are poor, with few long-term survivors. The treatment approach is highly individualized and depends upon multiple clinical and biological factors. The results of ANBL1221 have informed an approach now commonly used at first relapse. Patients on ANBL1221 were randomized to irinotecan/temozolomide with either temsirolimus or dinutuximab. The response rate for patients randomized to chemoimmunotherapy with dinutuximab was substantially higher (53% vs. 6%).54 These results led to widespread incorporation of chemo-immunotherapy in the relapse setting and also led to ANBL17P1 and ANBL19P1 trials described earlier.
The ANBL1221 experience also informed the development of an ongoing randomized phase 2 trial, ANBL1821. In this trial, patients with first relapsed/refractory disease are randomized to a standard arm with chemoimmunotherapy according to ANBL1221 or to that therapy plus the ornithine decarboxylase inhibitor difluoromethylornithine (DFMO). DFMO inhibits polyamine synthesis, which is predicted to target tumors dependent upon MYC family proteins.55 DFMO has also been shown to target immunosuppressive macrophages in the tumor microenvironment,56 supporting its combination with chemoimmunotherapy. ANBL1821 is expected to complete accrual in 2023.
RESEARCH STRATEGY
Translational Research Strategy
The Neuroblastoma Committee is committed to continuing to facilitate studies of clinically annotated biobanked samples and supports the development and sharing of cell and patient-derived xenograft models generated from ANBL00B1 specimens (https://www.cccells.org). The Committee integrates critical biocorrelates within therapeutic trials to enhance clinical annotation of biospecimens. NGS approaches to better characterize tumor cell heterogeneity and evolution are also being prioritized. It is expected that for subsets of patients, specimen analysis through the Molecular Characterization Initiative (MCI) will permit profiling based on current integral biomarkers and promote unprecedented opportunities for discovery. Additional newer sequencing approaches are being considered for correlative studies on upcoming trials, including prospective evaluation of TLMs and single cell RNA sequencing. Future biocorrelative studies will focus on the tumor microenvironment and host and tumor determinants of response to immunotherapy regimens, including studies of immune-cell infiltrates and changes in GD2 cell-surface expression.
To further interrogate the heterogeneous tumor landscape at diagnosis and during therapy, plasma is being collected at serial timepoints on high-risk trials to facilitate circulating tumor DNA (ctDNA) studies. Current approaches to these analyses include determination of total ctDNA content, ultra-low passage assessment of CNAs, 5-hydroxymethylcytosine profiles, and deeper sequencing to detect subclonal genetic alterations. For example, deep sequencing is being performed to detect low level ALK variants and additional genes predicted to impact drug sensitivity/resistance for patients being treated with lorlatinib on ANBL1531. It is expected that ctDNA analyses may ultimately replace older MRD assessment approaches and may be used to determine clinical trial eligibility.
Additional Aspects of Overall Strategy
As our understanding of neuroblastoma biology expands, so too does our appreciation for the heterogeneous nature of the disease. To better understand the natural history and response to therapy among molecularly and clinically defined subsets, and to evaluate the efficacy of targeted therapies, trials that subdivide patients into smaller groups will be required. International collaboration is necessary to conduct trials for these small cohorts in the precision medicine era. The track record for collaboration among neuroblastoma investigators around the world is long, and tools built through international consensus will be critically important as we design and conduct trials across continents.2,10,57–60 International collaborative efforts to study the addition of an ALK inhibitor to standard multi-modality high-risk therapy are ongoing, as are efforts to develop trials for other rare subsets of patients with intermediate and high-risk disease.
As this work proceeds, the many challenges facing children diagnosed with and treated for neuroblastoma must be recognized. The Neuroblastoma Committee is expanding the scope of its research to address questions that both investigators and patient advocates highlight as important to patients and their families (Figure 3). More than a decade ago, it was shown that EFS among Native American and Black children with neuroblastoma was lower than the EFS for White children, though survival outcomes were similar across races and ethnicities when within-risk group outcomes were evaluated.61 More recently, impact of poverty on outcomes among children enrolled on COG post-consolidation trials of anti-GD2 antibody therapy was evaluated. In children exposed to both neighborhood and household poverty, statistically significant increases in the risk of relapse and death were observed.62 To better understand and address health disparities, objectives regarding social determinants of health are now being systematically incorporated into COG Neuroblastoma Committee trials.
Additionally, as a greater percentage of high-risk patients survive, late effects questions are becoming important to more patients and their families. Recognizing high rates of chronic health conditions and neuropsychological impairments, and the incidence of second malignancies among survivors of intensive therapy for high-risk disease63–65 led to rigorous evaluations of multiple health conditions in survivors through the COG Late Effects After High-Risk Neuroblastoma Study (LEAHRN; ALTE15N2)66 and systematic incorporation of survivorship questions into frontline trials. Due to the >60% rate of clinically significant hearing loss in the LEAHRN cohort, a task force has been established to evaluate approaches to mitigate ototoxicity in patients receiving high-risk therapy. LEAHRN successor studies are in development, to learn more about late effects and potential biomarkers following modern-era therapy that increasingly includes targeted therapies.
Inclusion of patient advocates has been important for this work and has helped the Neuroblastoma Committee appreciate the breadth of patient and parent concerns. An advocate who is a parent of an intensively-treated high-risk neuroblastoma survivor highlights patient/family perspectives and is a key a member of the Neuroblastoma Executive Committee, task forces, and study committees.
Key Clinical Trials to be Pursued
The Neuroblastoma Committee has plans for new trials for different risk groups. In the non-high-risk population, there remain subsets of patients with unsatisfactory outcomes. Patients with INRG L2 MYCN non-amplified tumors with unfavorable histology have historically been treated with high-risk therapy with single transplant in North America. In Europe, these patients are treated according to an intermediate-risk paradigm. The finding that 11q loss may help to risk-stratify this group of patients4 has led to plans to include 11q status among integral biomarkers in a trial under development that will assign patients at lower recurrence risk to less toxic therapy, while assigning those at greater risk to more intensive treatment. This approach aligns with our overall goal to improve cure rates for higher risk patients, while avoiding overtreatment for patients with lower risk disease.
For patients with newly diagnosed high-risk disease, the Phase 3 trial ANBL2131 will assess the role of chemoimmunotherapy during Induction. Patients will be randomized to either COG standard therapy described above or to this therapy with the addition of dinutuximab during Induction cycles 2–5. Patients on the standard arm who have a poor end-Induction response will be assigned to receive bridging therapy with dinutuximab/irinotecan/temozolomide to improve remission status prior to Consolidation. The ANBL2131 trial will test whether early administration of chemoimmunotherapy improves EFS or whether reserving chemoimmunotherapy for patients with poor response to standard Induction yields similar outcomes. This trial is expected to open in Fall 2023. Embedded correlative studies will seek to identify predictors of response to early chemoimmunotherapy.
The success of chemoimmunotherapy has also raised the question as to whether standard Consolidation with tandem transplant is required for all high-risk patients or whether a chemoimmunotherapy strategy might substitute for high-dose chemotherapy. This approach would help to mitigate some toxicity associated with current standard Consolidation, including risks of sinusoidal obstruction syndrome and transplant-associated microangiopathy. Prompted in part by concerns expressed by patients, families and advocates,67 a task force has begun designing a phase 3 trial to follow ANBL2131 that will evaluate a chemoimmunotherapy Consolidation.
The committee has a strategic goal to conduct groupwide trials available for patients with first recurrent high-risk disease. Such trials have informed new strategies to be studied in the frontline setting and have increased biospecimen collection from relapsed patients. As GD2-directed chemoimmunotherapy is used more commonly in the frontline setting, a task force is evaluating potential approaches for patients who have relapsed after prior chemoimmunotherapy. These strategies include: 1) approaches to evaluate therapies that have potential to augment GD2-directed chemoimmunotherapy activity or 2) novel targeted therapies for those who demonstrate resistance to GD2-directed therapies.
CONCLUSION
The COG Neuroblastoma Committee has built on the success of prior trials and is committed to conducting practice-changing research to improve neuroblastoma outcomes. A strategy of engagement promotes collaborations with investigators with a wide range of expertise from across COG member sites and around the world, and also promotes incorporation of patient/family perspectives throughout the research process. Broadening and deepening our understanding of neuroblastoma biology remains central to our work. In addition, the scope of our research has broadened to address social determinants of health and survivorship issues.
Funding support:
NCTN Operations Center Grant U10CA180886, NCTN Statistics & Data Center Grant U10CA180899, R01CA14912 (RB, SGD), DOD W81XWH2010815 (RB, SGD)
Table of Abbreviations
- COG
Children’s Oncology Group
- EFS
Event-free survival
- IDRF, IDRFs
image-defined risk factor(s)
- INRG
International Neuroblastoma Risk Group
- OS
Overall survival
- SCAs
Segmental chromosomal aberrations
- GWAS
Genome-wide association studies
- CNAs
Copy number alterations
- LOH
Loss of heterozygosity
- MYCN-NA
MYCN non-amplified
- NGS
Next generation sequencing
- TLM
Telomere lengthening mechanisms
- ALT
Alternative lengthening of telomeres
- MES
Mesenchymal cells
- ADR
Adrenergic cells
- PR
Partial response
- CR
Complete response
- I-MIBG
I-Metaiodobenzylguanidine
- MRD
Minimal residual disease
- DFMO
Difluoromethylornithine
- MCI
Molecular Characterization Initiative
- ctDNA
Circulating tumor DNA
Footnotes
Disclosures: SGD reports consulting fees from Amgen, Bayer, and Jazz and travel expenses from Loxo, Roche, and Salarius. AN reports consulting fees from Novartis.
Contributor Information
Rochelle Bagatell, Department of Pediatrics, The Children’s Hospital of Philadelphia, Philadelphia, Pennsylvania, USA.
Steven G. DuBois, Department of Pediatrics, Dana-Farber/Boston Children’s Cancer and Blood Disorders Center, Boston, Massachusetts, USA
Arlene Naranjo, Department of Pediatrics, University of Florida, Gainesville, Florida, USA.
Jen Belle, Children’s Oncology Group, Monrovia, California, USA.
Kelly C. Goldsmith, Department of Pediatrics, Children’s Healthcare of Atlanta Inc Aflac Cancer and Blood Disorders Center, Atlanta, Georgia, USA
Julie R. Park, Department of Oncology, St Jude Children’s Research Hospital Department of Oncology, Memphis, Tennessee, USA
Meredith S. Irwin, Department of Pediatrics, The Hospital for Sick Children, Toronto, Canada
COG Neuroblastoma Committee, Department of Pediatrics, The Hospital for Sick Children, Toronto, Canada; Department of Pediatrics, The Children’s Hospital of Philadelphia, Philadelphia, Pennsylvania, USA.
REFERENCES
- 1.Irwin MS, Naranjo A, Zhang FF, et al. Revised Neuroblastoma Risk Classification System: A Report From the Children’s Oncology Group. J Clin Oncol. 2021;39(29):3229–3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Monclair T, Brodeur GM, Ambros PF, et al. The International Neuroblastoma Risk Group (INRG) staging system: an INRG Task Force report. J Clin Oncol. 2009;27(2):298–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park JR, Kreissman SG, London WB, et al. Effect of Tandem Autologous Stem Cell Transplant vs Single Transplant on Event-Free Survival in Patients With High-Risk Neuroblastoma: A Randomized Clinical Trial. JAMA. 2019;322(8):746–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pinto N, Naranjo A, Ding X, et al. Impact of Genomic and Clinical Factors on Outcome of Children >/=18 Months of Age with Stage 3 Neuroblastoma with Unfavorable Histology without MYCN Amplification. Clin Cancer Res. 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ritenour LE, Randall MP, Bosse KR, Diskin SJ. Genetic susceptibility to neuroblastoma: current knowledge and future directions. Cell Tissue Res. 2018;372(2):287–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kamihara J, Bourdeaut F, Foulkes WD, et al. Retinoblastoma and Neuroblastoma Predisposition and Surveillance. Clin Cancer Res. 2017;23(13):e98–e106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liang WH, Federico SM, London WB, et al. Tailoring Therapy for Children With Neuroblastoma on the Basis of Risk Group Classification: Past, Present, and Future. JCO Clin Cancer Inform. 2020;4:895–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brodeur GM, Seeger RC, Schwab M, Varmus HE, Bishop JM. Amplification of N-myc in untreated human neuroblastomas correlates with advanced disease stage. Science. 1984;224(4653):1121–1124. [DOI] [PubMed] [Google Scholar]
- 9.Seeger RC, Brodeur GM, Sather H, et al. Association of multiple copies of the N-myc oncogene with rapid progression of neuroblastomas. N Engl J Med. 1985;313(18):1111–1116. [DOI] [PubMed] [Google Scholar]
- 10.Cohn SL, Pearson AD, London WB, et al. The International Neuroblastoma Risk Group (INRG) classification system: an INRG Task Force report. J Clin Oncol. 2009;27(2):289–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang LL, Teshiba R, Ikegaki N, et al. Augmented expression of MYC and/or MYCN protein defines highly aggressive MYC-driven neuroblastoma: a Children’s Oncology Group study. Br J Cancer. 2015;113(1):57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Durbin AD, Zimmerman MW, Dharia NV, et al. Selective gene dependencies in MYCN-amplified neuroblastoma include the core transcriptional regulatory circuitry. Nat Genet. 2018;50(9):1240–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Puissant A, Frumm SM, Alexe G, et al. Targeting MYCN in neuroblastoma by BET bromodomain inhibition. Cancer Discov. 2013;3(3):308–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chipumuro E, Marco E, Christensen CL, et al. CDK7 inhibition suppresses super-enhancer-linked oncogenic transcription in MYCN-driven cancer. Cell. 2014;159(5):1126–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brockmann M, Poon E, Berry T, et al. Small molecule inhibitors of aurora-a induce proteasomal degradation of N-myc in childhood neuroblastoma. Cancer Cell. 2013;24(1):75–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schleiermacher G, Janoueix-Lerosey I, Ribeiro A, et al. Accumulation of segmental alterations determines progression in neuroblastoma. J Clin Oncol. 2010;28(19):3122–3130. [DOI] [PubMed] [Google Scholar]
- 17.Janoueix-Lerosey I, Schleiermacher G, Michels E, et al. Overall genomic pattern is a predictor of outcome in neuroblastoma. J Clin Oncol. 2009;27(7):1026–1033. [DOI] [PubMed] [Google Scholar]
- 18.Attiyeh EF, London WB, Mosse YP, et al. Chromosome 1p and 11q deletions and outcome in neuroblastoma. N Engl J Med. 2005;353(21):2243–2253. [DOI] [PubMed] [Google Scholar]
- 19.Maris JM, Weiss MJ, Guo C, et al. Loss of heterozygosity at 1p36 independently predicts for disease progression but not decreased overall survival probability in neuroblastoma patients: a Children’s Cancer Group study. J Clin Oncol. 2000;18(9):1888–1899. [DOI] [PubMed] [Google Scholar]
- 20.Guo C, White PS, Weiss MJ, et al. Allelic deletion at 11q23 is common in MYCN single copy neuroblastomas. Oncogene. 1999;18(35):4948–4957. [DOI] [PubMed] [Google Scholar]
- 21.Schleiermacher G, Mosseri V, London WB, et al. Segmental chromosomal alterations have prognostic impact in neuroblastoma: a report from the INRG project. Br J Cancer. 2012;107(8):1418–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caren H, Kryh H, Nethander M, et al. High-risk neuroblastoma tumors with 11q-deletion display a poor prognostic, chromosome instability phenotype with later onset. Proc Natl Acad Sci U S A. 2010;107(9):4323–4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schleiermacher G, Michon J, Ribeiro A, et al. Segmental chromosomal alterations lead to a higher risk of relapse in infants with MYCN-non-amplified localised unresectable/disseminated neuroblastoma (a SIOPEN collaborative study). Br J Cancer. 2011;105(12):1940–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Twist CJ, Schmidt ML, Naranjo A, et al. Maintaining Outstanding Outcomes Using Response- and Biology-Based Therapy for Intermediate-Risk Neuroblastoma: A Report From the Children’s Oncology Group Study ANBL0531. J Clin Oncol. 2019;37(34):3243–3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pugh TJ, Morozova O, Attiyeh EF, et al. The genetic landscape of high-risk neuroblastoma. Nat Genet. 2013;45(3):279–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eleveld TF, Oldridge DA, Bernard V, et al. Relapsed neuroblastomas show frequent RAS-MAPK pathway mutations. Nat Genet. 2015;47(8):864–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schleiermacher G, Javanmardi N, Bernard V, et al. Emergence of new ALK mutations at relapse of neuroblastoma. J Clin Oncol. 2014;32(25):2727–2734. [DOI] [PubMed] [Google Scholar]
- 28.Bresler SC, Weiser DA, Huwe PJ, et al. ALK mutations confer differential oncogenic activation and sensitivity to ALK inhibition therapy in neuroblastoma. Cancer Cell. 2014;26(5):682–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bellini A, Potschger U, Bernard V, et al. Frequency and Prognostic Impact of ALK Amplifications and Mutations in the European Neuroblastoma Study Group (SIOPEN) High-Risk Neuroblastoma Trial (HR-NBL1). J Clin Oncol. 2021;39(30):3377–3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Foster JH, Voss SD, Hall DC, et al. Activity of Crizotinib in Patients with ALK-Aberrant Relapsed/Refractory Neuroblastoma: A Children’s Oncology Group Study (ADVL0912). Clin Cancer Res. 2021;27(13):3543–3548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fischer M, Moreno L, Ziegler DS, et al. Ceritinib in paediatric patients with anaplastic lymphoma kinase-positive malignancies: an open-label, multicentre, phase 1, dose-escalation and dose-expansion study. Lancet Oncol. 2021;22(12):1764–1776. [DOI] [PubMed] [Google Scholar]
- 32.Goldsmith KC, Park JR, Kayser K, et al. Lorlatinib with or without chemotherapy in ALK-driven refractory/relapsed neuroblastoma: phase 1 trial results. Nat Med. 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ackermann S, Cartolano M, Hero B, et al. A mechanistic classification of clinical phenotypes in neuroblastoma. Science. 2018;362(6419):1165–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hartlieb SA, Sieverling L, Nadler-Holly M, et al. Alternative lengthening of telomeres in childhood neuroblastoma from genome to proteome. Nat Commun. 2021;12(1):1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koneru B, Lopez G, Farooqi A, et al. Telomere Maintenance Mechanisms Define Clinical Outcome in High-Risk Neuroblastoma. Cancer Res. 2020;80(12):2663–2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peifer M, Hertwig F, Roels F, et al. Telomerase activation by genomic rearrangements in high-risk neuroblastoma. Nature. 2015;526(7575):700–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Majzner RG, Simon JS, Grosso JF, et al. Assessment of programmed death-ligand 1 expression and tumor-associated immune cells in pediatric cancer tissues. Cancer. 2017;123(19):3807–3815. [DOI] [PubMed] [Google Scholar]
- 38.Wei JS, Kuznetsov IB, Zhang S, et al. Clinically Relevant Cytotoxic Immune Cell Signatures and Clonal Expansion of T-Cell Receptors in High-Risk MYCN-Not-Amplified Human Neuroblastoma. Clin Cancer Res. 2018;24(22):5673–5684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Erbe AK, Diccianni MB, Mody R, et al. KIR/KIR-ligand genotypes and clinical outcomes following chemoimmunotherapy in patients with relapsed or refractory neuroblastoma: a report from the Children’s Oncology Group. J Immunother Cancer. 2023;11(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Verhoeven BM, Mei S, Olsen TK, et al. The immune cell atlas of human neuroblastoma. Cell Rep Med. 2022;3(6):100657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Erbe AK, Wang W, Carmichael L, et al. Neuroblastoma Patients’ KIR and KIR-Ligand Genotypes Influence Clinical Outcome for Dinutuximab-based Immunotherapy: A Report from the Children’s Oncology Group. Clin Cancer Res. 2018;24(1):189–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boeva V, Louis-Brennetot C, Peltier A, et al. Heterogeneity of neuroblastoma cell identity defined by transcriptional circuitries. Nat Genet. 2017;49(9):1408–1413. [DOI] [PubMed] [Google Scholar]
- 43.van Groningen T, Koster J, Valentijn LJ, et al. Neuroblastoma is composed of two super-enhancer-associated differentiation states. Nat Genet. 2017;49(8):1261–1266. [DOI] [PubMed] [Google Scholar]
- 44.Sengupta S, Das S, Crespo AC, et al. Mesenchymal and adrenergic cell lineage states in neuroblastoma possess distinct immunogenic phenotypes. Nat Cancer. 2022;3(10):1228–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nuchtern JG, London WB, Barnewolt CE, et al. A prospective study of expectant observation as primary therapy for neuroblastoma in young infants: a Children’s Oncology Group study. Ann Surg. 2012;256(4):573–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baker DL, Schmidt ML, Cohn SL, et al. Outcome after reduced chemotherapy for intermediate-risk neuroblastoma. N Engl J Med. 2010;363(14):1313–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rubie H, De Bernardi B, Gerrard M, et al. Excellent outcome with reduced treatment in infants with nonmetastatic and unresectable neuroblastoma without MYCN amplification: results of the prospective INES 99.1. J Clin Oncol. 2011;29(4):449–455. [DOI] [PubMed] [Google Scholar]
- 48.De Bernardi B, Gerrard M, Boni L, et al. Excellent outcome with reduced treatment for infants with disseminated neuroblastoma without MYCN gene amplification. J Clin Oncol. 2009;27(7):1034–1040. [DOI] [PubMed] [Google Scholar]
- 49.Pinto N, Naranjo A, Hibbitts E, et al. Predictors of differential response to induction therapy in high-risk neuroblastoma: A report from the Children’s Oncology Group (COG). Eur J Cancer. 2019;112:66–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Furman WL, McCarville B, Shulkin BL, et al. Improved Outcome in Children With Newly Diagnosed High-Risk Neuroblastoma Treated With Chemoimmunotherapy: Updated Results of a Phase II Study Using hu14.18K322A. J Clin Oncol. 2022;40(4):335–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Federico SM, Naranjo A, Zhang FF, et al. A pilot induction regimen incorporating dinutuximab and sargramostim for the treatment of newly diagnosed high-risk neuroblastoma: A report from the Children’s Oncology Group. June 2, 2022, 2022; American Society of Clinical Oncology Annual Meeting. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu KX, Naranjo A, Zhang FF, et al. Prospective Evaluation of Radiation Dose Escalation in Patients With High-Risk Neuroblastoma and Gross Residual Disease After Surgery: A Report From the Children’s Oncology Group ANBL0532 Study. J Clin Oncol. 2020;38(24):2741–2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yu AL, Gilman AL, Ozkaynak MF, et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med. 2010;363(14):1324–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mody R, Naranjo A, Van Ryn C, et al. Irinotecan-temozolomide with temsirolimus or dinutuximab in children with refractory or relapsed neuroblastoma (COG ANBL1221): an open-label, randomised, phase 2 trial. Lancet Oncol. 2017;18(7):946–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Evageliou NF, Haber M, Vu A, et al. Polyamine Antagonist Therapies Inhibit Neuroblastoma Initiation and Progression. Clin Cancer Res. 2016;22(17):4391–4404. [DOI] [PubMed] [Google Scholar]
- 56.Hayes CS, Burns MR, Gilmour SK. Polyamine blockade promotes antitumor immunity. Oncoimmunology. 2014;3(1):e27360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brodeur GM, Pritchard J, Berthold F, et al. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J Clin Oncol. 1993;11(8):1466–1477. [DOI] [PubMed] [Google Scholar]
- 58.Park JR, Bagatell R, Cohn SL, et al. Revisions to the International Neuroblastoma Response Criteria: A Consensus Statement From the National Cancer Institute Clinical Trials Planning Meeting. J Clin Oncol. 2017;35(22):2580–2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shimada H, Ambros IM, Dehner LP, et al. The International Neuroblastoma Pathology Classification (the Shimada system). Cancer. 1999;86(2):364–372. [PubMed] [Google Scholar]
- 60.Matthyssens LE, Nuchtern JG, Van De Ven CP, et al. A Novel Standard for Systematic Reporting of Neuroblastoma Surgery: The International Neuroblastoma Surgical Report Form (INSRF): A Joint Initiative by the Pediatric Oncological Cooperative Groups SIOPEN *, COG * *, and GPOH * * *. Ann Surg. 2022;275(3):e575–e585. [DOI] [PubMed] [Google Scholar]
- 61.Henderson TO, Bhatia S, Pinto N, et al. Racial and ethnic disparities in risk and survival in children with neuroblastoma: a Children’s Oncology Group study. J Clin Oncol. 2011;29(1):76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bona K, Li Y, Winestone LE, et al. Poverty and Targeted Immunotherapy: Survival in Children’s Oncology Group Clinical Trials for High-Risk Neuroblastoma. J Natl Cancer Inst. 2021;113(3):282–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Laverdiere C, Liu Q, Yasui Y, et al. Long-term outcomes in survivors of neuroblastoma: a report from the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2009;101(16):1131–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Applebaum MA, Vaksman Z, Lee SM, et al. Neuroblastoma survivors are at increased risk for second malignancies: A report from the International Neuroblastoma Risk Group Project. Eur J Cancer. 2017;72:177–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zheng DJ, Krull KR, Chen Y, et al. Long-term psychological and educational outcomes for survivors of neuroblastoma: A report from the Childhood Cancer Survivor Study. Cancer. 2018;124(15):3220–3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Diller L Surviving High-Risk Neuroblastoma: A Preliminary Descriptive Report from Project LEAHRN. Advance in Neuroblastoma Research; January 25, 2021, 2021; Virtual Meeting, 2021. [Google Scholar]
- 67.Bird N, Scobie N, Palmer A, Ludwinski D. To transplant, or not to transplant? That is the question. A patient advocate evaluation of autologous stem cell transplant in neuroblastoma. Pediatr Blood Cancer. 2022;69(8):e29663. [DOI] [PubMed] [Google Scholar]