Abstract
Thoracic hernias are characterized by the protrusion of the thoracic contents outside their normal anatomical confines. This case involves a left pleural effusion secondary to a spontaneous lung intercostal hernia (SLIH) in a 52-year-old male. Imaging revealed herniated pleural fluid in the intercostal space. Intra-operatively, there was herniation of the lung parenchyma into an intercostal defect. Pleural effusion secondary to a SLIH is an indication for surgical repair.
Keywords: Pleural effusion, Lung hernia, Intercostal hernia, COPD, Obesity
1. Introduction
Thoracic hernias are characterized by the protrusion of the thoracic contents outside their normal anatomical confines [1]. Lung hernias, a subtype of thoracic hernias, are rare conditions that occur when the lung parenchyma along with pleural membranes protrude through a defect in the chest wall [1]. Morel-Lavallée classified lung hernias defined by location (cervical, diaphragmatic and thoracic) and aetiology (congenital or acquired) [2]. He further divided the acquired cases into traumatic, spontaneous and pathological. The intercostal subtype, where the lung protrudes through a defect in the intercostal muscles between adjacent ribs, accounts for approximately 60–80 % of all lung hernia cases [3]. Spontaneous lung hernias account for 30 % of acquired cases [4]. Usually, spontaneous lung hernias are of the intercostal type [5]. SLIH is thought to occur secondary to an increase in intrathoracic pressure from movements such as coughing or straining. Risk factors for development include central obesity, male gender, history of smoking, chronic obstructive pulmonary disease (COPD) and steroid use [6,7]. There have been approximately 120 cases of SLIH documented in medical literature worldwide [8].
This case involves a 52-year-old male presenting with a left pleural effusion. Imaging revealed herniated pleural fluid in the intercostal space. Intra-operatively, there was herniation of the lung parenchyma into an intercostal defect. Pleural effusion secondary to a SLIH is an indication for surgical repair.
2. Case report
A 52 year old male presented to the emergency department (ED) with a one-week history of left-sided chest pain. His past medical history was significant for hypertension, hyperlipidemia, obesity (body mass index: 37.0), and a history of smoking (10 pack per year history). The patient had never been formally diagnosed with COPD. He had no history of chest wall trauma, surgical procedures in his thoracic area or chronic steroid use.
The chest pain began after a vigorous coughing episode, and the patient described a “tearing” sensation in his left hemithorax. The chest pain was exacerbated by movements that increased intra-thoracic pressure such as coughing, sneezing and deep inspiration. The pain was accompanied by shortness of breath and persistent dry cough.
On admission, vital signs were as follows: blood pressure 176/102, heart rate 107, respiratory rate 20, temperature 36.7 °C, oxygen saturation 96 % on room air.
On inspection of the chest wall, there was no ecchymosis, bruising or a visible chest wall bulge. On palpation, the left anterior and lateral chest wall areas were tender. There was splaying of the left eighth and ninth ribs. No crepitus or intercostal bulge could be detected. Valsalva manoeuvre was not performed. On auscultation of the lungs, there were decreased breath sounds at the left lung base.
Laboratory investigations revealed a white blood cell count of 12.9 (reference range; 5–10 k/uL), haemoglobin 14.1 (reference range; 14–18 g/dL), creatinine 0.79 (reference range; 0.6–1.3 mg/dL) and platelets 413 (reference range; 150–500 k/uL). Lactic acid was normal.
Chest X-ray (CXR) revealed left-sided blunting of the costophrenic angle (Fig. 1). Computed Tomography (CT) Chest showed a moderate left-sided pleural effusion with compressive atelectasis (Fig. 2). There were no rib fractures, lung or pleural masses. There was no reported evidence of rib separation or pleural fluid herniation between the intercostal spaces included in the initial radiology report.
Fig. 1.
Chest x-ray illustrating left lower lobe pleural effusion.
Fig. 2.
Axial and coronal sections of a chest CT showing a left-sided pleural effusion with herniation of pleural fluid between the left eighth and ninth rib spaces. Rib separation between the left eighth and ninth ribs is evident on the coronal image.
The patient was started on ceftriaxone and azithromycin for treatment of a suspected community-acquired pneumonia (CAP). There was concern for an underlying pulmonary malignancy given the patient's smoking history. He underwent left-sided thoracentesis with 1.5 L (L) of serosanguinous fluid removed. According to Light's criteria, the fluid was exudative [9]. Pleural fluid protein was 4.2 g/dL, lactate dehydrogenase (LDH) 606 U/L, pH 7.40 and glucose 107 mg/dL. WBC count was 5344 with 68 % neutrophils, 30 % lymphocytes and 2 % monocytes. The pleural fluid was negative for gram stain, culture and cytology was negative for malignant cells. After the thoracentesis, the patient had reduced chest pain and shortness of breath. Vital signs and laboratory work, including haemoglobin and white cell count, remained stable. He was discharged two days after admission with augmentin and azithromycin to complete treatment of a suspected CAP. As well, an outpatient CT scan was ordered to assess for continued resolution of the pleural effusion.
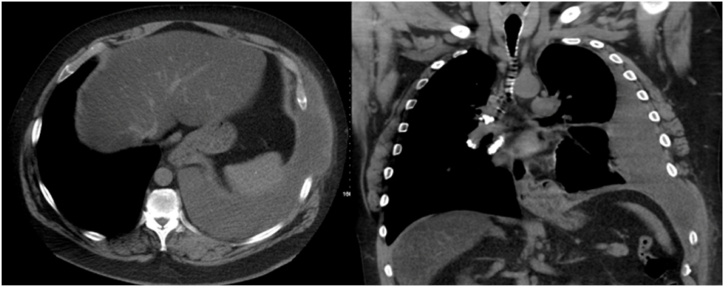
The outpatient CT scan was performed two weeks after discharge when the patient was able to obtain an appointment. It noted a large left pleural effusion, left eighth and ninth rib separation with herniation of pleural fluid in that intercostal space. There were no rib fractures, lung or pleural masses (Fig. 3).
Fig. 3.
Axial and coronal sections of a chest CT showing a large left-sided pleural effusion with herniation of pleural fluid between the left eighth and ninth rib spaces. Rib separation between the left eighth and ninth ribs is evident on the coronal image.
The patient was asked to come to the ED for further evaluation. He endorsed shortness of breath, left-sided chest pain and persistent dry cough. The chest pain continued to be exacerbated by coughing, sneezing and deep inspiration.
Upon re-admission to the ED, the patient was administered 2 L of oxygen via nasal cannula whereupon his oxygen saturation was 94 %. Vital signs were as follows: blood pressure 159/74, heart rate 119, respiratory rate 24, temperature 37.1 °C.
The left anterior and lateral chest wall were tender to palpation. There was splaying of the eighth and ninth ribs. A bulge was palpated through the left anterior eighth and ninth ribs on cough impulse. On auscultation of the lungs, decreased breath sounds at the left lung base were observed.
Laboratory investigations revealed a white blood cell count of 14.0 (reference range; 5–10 k/uL), haemoglobin 12.8 (reference range; 14–18 g/dL), creatinine 0.71 (reference range; 0.8–1.3 mg/dL) and platelets 398 (reference range; 150–500 k/uL). The lactic acid level was normal.
Left thoracentesis was again performed and 2 L of serosanguinous fluid were removed. The fluid was exudative according to Light's criteria [9]. Pleural fluid protein was 4.2 g/dL, LDH 524 U/L, pH 7.42 and glucose 162 mg/dL. WBC count was 2568 with 19 % neutrophils, 35 % lymphocytes, 6 % monocytes and 40 % eosinophils. The pleural fluid was negative for gram stain, culture and cytology was negative for malignant cells. CT chest was performed post-thoracentesis (Fig. 4). Imaging noted a decrease in the size of the pleural effusion, left eighth and ninth rib separation with continued herniation of pleural fluid in that intercostal space.
Fig. 4.
Axial and coronal sections of a chest CT post-thoracentesis showing a small residual left-sided pleural effusion with herniation of pleural fluid between the left eighth and ninth rib spaces. Rib separation between the left eighth and ninth ribs is evident on the coronal image.
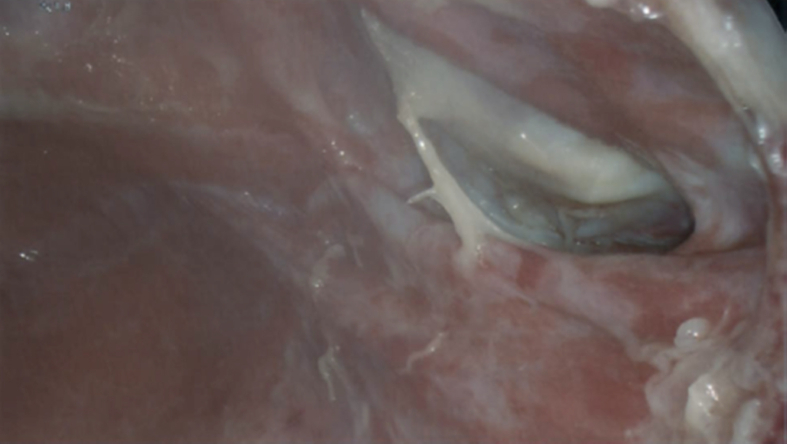
Thoracic surgery was consulted given the concern for SLIH. The patient was electively taken to the operating room to confirm the diagnosis of the SLIH as well as for surgical correction of the hernia. Initially, video-assisted thoracoscopic surgery (VATS) was performed to confirm diagnosis of the intercostal hernia. Upon entrance into the thoracic cavity, serosanguinous fluid was encountered which was sent for gram stain, culture and cytology. An intercostal muscle rupture between the antero-lateral left eighth and ninth ribs was visualized (Fig. 5). There was herniation of the lung parenchyma into the intercostal defect. Pleural biopsies were obtained to rule out pleural malignancy. The VATS was converted to an open thoracotomy for surgical correction of the hernia. A bio-prosthetic mesh was placed over the defect and secured with five figure-of-eight stitches. The pleural fluid was negative on gram stain, culture and cytology was negative for malignant cells. The pleural biopsy was negative for malignancy. The patient had an uncomplicated post-operative course. He was discharged on post-operative day five in good condition.
Fig. 5.
An intraoperative thoracoscopic image of the hernia defect between the left eighth and ninth ribs.
3. Discussion
3.1. Clinical discussion
This patient presented a diagnostic challenge in the setting of a pleural effusion. Initially, the patient was treated for a CAP with concern for an underlying malignancy.
The history played an important role in suspecting the diagnosis of SLIH. The patient was male, obese and had a history of smoking. He presented with a history of a vigorous coughing episode and an initial “tearing” sensation felt in his left hemithorax. His chest pain was worsened by coughing, sneezing or deep inspiration.
The physical exam played an important role in considering the diagnosis of SLIH. Chest wall tenderness and splaying of the left eighth and ninth ribs was detected on palpation of the chest wall. As well, upon cough impulse a bulge could be palpated between the left eighth and ninth rib spaces, corresponding to the herniating lung parenchyma.
3.2. Imaging discussion
The patient's CXR showed a left-sided pleural effusion. The radiologist did not mention evidence of rib separation or pleural fluid herniation between the intercostal spaces on the initial CT scan. However, upon retrospective review of the images completed after the diagnosis of SLIH had been confirmed, there was in fact left eighth and ninth rib separation and a small amount of pleural fluid herniated between the left eighth and ninth rib space. The imaging findings of rib separation with pleural fluid herniation prompted consideration of SLIH. Intra-operatively, direct visualization of the lung parenchyma herniating into the intercostal defect confirmed the diagnosis.
3.3. Pathology discussion
The pleural effusion was serosanguinous and exudative according to Light's criteria [9]. The differential of an exudative pleural effusion is broad and can include underlying infectious, malignant or post-traumatic causes [10]. The initial aetiology of the exudative effusion was thought to be secondary to CAP. No gross blood on thoracentesis ruled out a hemothorax. Normal pleural fluid pH and glucose, along with negative gram stain and culture ruled out infection. Negative cytology for malignant cells on both pleural fluid samples lowered the suspicion for malignancy and prompted consideration of SLIH. Intraoperative pleural fluid and pleural biopsy specimens were negative on gram stain, culture and cytology was negative for malignant cells. This further supported the diagnosis of SLIH as the aetiology of the pleural effusion.
3.4. Brief review of the literature
Diagnosis of SLIH is challenging, commonly misdiagnosed as CAP or COPD exacerbation [11]. The patient presented with a classic clinical history of SLIH [8,12]. He describes an acute chest wall pain after a vigorous coughing episode. He endorsed chest pain pleuritic in nature, worsened by movements that increased intra-thoracic pressure such as coughing and sneezing. The chest pain was accompanied by shortness of breath and persistent cough.
The patient was male and obese, with a history of smoking. Known risk factors for the development of SLIH include central obesity, male gender and tobacco use [13]. The risk of lung herniation of patients with a history of smoking is thought to be secondary to dynamic hyperinflation, respiratory muscle weakness, and frequent coughing leading to raised intrathoracic pressure. Anatomically, most cases of SLIH occur in the anterior thoracic wall between the eighth and ninth ribs where there is reduced muscular support [14]. The intercostal muscle rupture between the left eighth and ninth ribs in this patient was likely secondary to an acute and intense increase in intrathoracic pressure from a vigorous coughing episode.
The signs of lung herniation in this patient were subtle. They did not have the typical findings of a visible chest wall bulge, ecchymosis or bruising [13]. The Valsalva manoeuvre was necessary to elicit findings making appear a lump on palpation that corresponded to the herniated lung parenchyma [5].
On imaging, the patient was found to have a left-sided pleural effusion with intercostal herniation of pleural fluid between splayed left eighth and ninth ribs. This case is unique as detected on imaging, pleural fluid had herniated into the intercostal space. Intra-operative visualization of lung parenchyma herniating into an intercostal defect confirmed the diagnosis. In most cases of SLIH, imaging reveals both the lung parenchyma and pleural membrane without pleural fluid herniating outside of the chest wall cavity [8]. Herniation of pleural fluid outside the chest wall cavity should raise suspicion for a chest wall defect [15,16]. It is possible that the lung parenchyma was transiently protruding through the intercostal defect during changes in intra-thoracic pressure. This could explain the discrepancy between the imaging and intra-operative findings. Asking the patient to perform the Valsalva manoeuvre during the imaging study may cause the lung to further protrude into the chest wall defect, making the hernia more apparent [14].
The effusion was exudative according to Light's criteria [9]. The rupture of the intercostal muscle likely led to an intense inflammatory response and continuous leakage of proteinaceous fluid into the pleural space.
SLIH can be managed conservatively or surgically. A gold standard treatment for lung hernias has not yet been established [8]. Common indications for surgical intervention include refractory pain, recurrent respiratory infections, haemoptysis or interference with daily activities of living [17]. Ugolini et al. recently proposed an algorithm for SLIH management. In this algorithm the presence of pleural effusion should prompt surgical intervention [8]. The patient was managed surgically given the recurrence of pleural effusion. He initially required VATS to visualize the protrusion of lung tissue into the intercostal defect, confirming the diagnosis. Ultimately, conversion to a thoracotomy and placement of a bio-prosthetic mesh over the hernia was performed.
4. Conclusion
-
-
Spontaneous lung intercostal hernia is rare but should be considered in the differential diagnosis in a patient with a history of a vigorous coughing episode and pleuritic chest pain.
-
-
On imaging, herniation of pleural fluid in the intercostal space should raise suspicion for a intercostal defect.
-
-
Spontaneous lung intercostal hernias can be managed conservatively or surgically. A pleural effusion secondary to a spontaneous lung intercostal hernia is an indication for surgical repair.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
There are no acknowledgements. There was no financial support for this project.
References
- 1.Chaturvedi A., Rajiah P., Croake A., Saboo S., Chaturvedi A. Imaging of thoracic hernias: types and complications. Insights into Imaging. 2018;9(6):989–1005. doi: 10.1007/s13244-018-0670-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morel-Lavallee Morel-Lavallee Bull Soc Chir Paris. 1847;1(195):75. Hernies du poumon (Article in French) p. 195. [Google Scholar]
- 3.Rao E.M. Intercostal Lung hernia: a case for conservative treatment. Clin. Med. Rev. Case Rep. 2015;2(6) doi: 10.23937/2378-3656/1410038. [DOI] [Google Scholar]
- 4.Tack D., Wattiez A., Schtickzelle J.-C., Delcour C. Spontaneous lung herniation after a single cough. Eur. Radiol. 2000;10(3):500–502. doi: 10.1007/s003300050084. [DOI] [PubMed] [Google Scholar]
- 5.Weissberg D., Refaely Y. Hernia of the lung. Ann. Thorac. Surg. 2002;74(6):1963–1966. doi: 10.1016/s0003-4975(02)04077-8. [DOI] [PubMed] [Google Scholar]
- 6.Seder C.W., Allen M.S., Nichols F.C., et al. Primary and prosthetic repair of acquired Chest Wall Hernias: a 20-Year experience. Ann. Thorac. Surg. 2014;98(2):484–489. doi: 10.1016/j.athoracsur.2014.03.021. [DOI] [PubMed] [Google Scholar]
- 7.Kollipara V., Lutchmedial S., Patel B., Ie S., Rubio E. Spontaneous posterior lung herniation: a case report and literature review. Lung India. 2021;38(5):481. doi: 10.4103/lungindia.lungindia_540_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ugolini S., Abdelghafar M., Vokkri E., et al. Case report: spontaneous lung intercostal hernia series and literature review. Frontiers in Surgery. 2023;9 doi: 10.3389/fsurg.2022.1091727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Light R.W. Pleural effusions: the diagnostic separation of transudates and exudates. Ann. Intern. Med. 1972;77(4):507. doi: 10.7326/0003-4819-77-4-507. [DOI] [PubMed] [Google Scholar]
- 10.Karkhanis V., Joshi J. Pleural effusion: diagnosis, treatment, and management. Open access emergency medicine. Published online. 2012:31. doi: 10.2147/oaem.s29942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cox M., Thota D., Trevino R. Spontaneous lung herniation through the chest wall. Mil. Med. 2017;183(3–4) doi: 10.1093/milmed/usx063. [DOI] [PubMed] [Google Scholar]
- 12.O’ Mahony A.M., Murphy K.M., O'Connor T.M., Curran D.R. Spontaneous pulmonary hernia secondary to intercostal muscle tear. BMJ Case Rep. 2019;12(10) doi: 10.1136/bcr-2019-231706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brock M.V., Heitmiller R.F. Spontaneous anterior thoracic lung hernias. J. Thorac. Cardiovasc. Surg. 2000;119(5):1046–1047. doi: 10.1016/s0022-5223(00)70103-6. [DOI] [PubMed] [Google Scholar]
- 14.Zia Z., Bashir O., Ramjas G.E., Kumaran M., Pollock J.G., Pointon K. Intercostal lung hernia: radiographic and MDCT findings. Clin. Radiol. 2013;68(7) doi: 10.1016/j.crad.2012.11.024. [DOI] [PubMed] [Google Scholar]
- 15.Newman T.A., Brady A.K., Shriki J.E., Takasugi J.E., Adamson R. Intercostal herniation of pleural fluid. Am. J. Respir. Crit. Care Med. 2020;201(2) doi: 10.1164/rccm.201812-2265im. [DOI] [PubMed] [Google Scholar]
- 16.Romero C., Varon D.S., Surani S., Varon J. Thoracic herniation secondary to pleural effusion. Respiratory Medicine Case Reports. 2018;23:96–97. doi: 10.1016/j.rmcr.2018.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bangalore Lakshminarayana U., Cowen M., Kastelik J.A., Morjaria J.B. Intermittent swelling in the chest; a case of spontaneous intermittent lung herniation. Case Reports. 2013;2013(oct31 1) doi: 10.1136/bcr-2013-2Respiratory.Medicine.Case.Reports01380. [DOI] [PMC free article] [PubMed] [Google Scholar]