Abstract
Viral replication was inhibited in a dose-dependent manner after administration of the phosphorothioate oligonucleotide TTGGGGTT (ISIS 5320) to human immunodeficiency virus type 1 (HIV-1)-infected SCID-hu Thy/Liv mice. Potent in vivo antiviral activity was observed against the T-cell-tropic molecular clone NL4-3; the agent was found to have weak activity against one primary HIV-1 isolate, and the agent was inactive against a second primary isolate.
The phosphorothioate oligonucleotide TTGGGGTT (ISIS 5320) forms a parallel-stranded, tetrameric guanosine quartet (G-quartet) structure that binds to the human immunodeficiency virus type 1 (HIV-1) envelope glycoprotein (gp120) and inhibits cell-to-cell and virus-to-cell infection in vitro (3, 10). In this study, the antiviral activity of this compound was assessed both in phytohemagglutinin (PHA)-activated human peripheral blood mononuclear cells (PBMCs) and in the SCID-hu Thy/Liv mouse model of HIV-1 infection (5, 7).
ISIS 5320 and a non-tetrad-forming negative control, phosphorothioate GTGTGTGT (ISIS 9477), were tested against the infectious molecular clone NL4-3 and the primary HIV-1 isolates EW and JD in PHA-activated human PBMCs after preincubation of virus with serially diluted test agent for 30 min at 37°C. The cells were lysed 7 days after inoculation, and the amount of p24 antigen in treated and untreated cultures was determined by an HIV-1 gag p24 enzyme-linked immunosorbent assay (ELISA). The 50% inhibitory concentrations (IC50s) of ISIS 5320 ranged from 2.0 to 6.4 μM for the three isolates (Table 1), and similar IC50s were obtained whether the viral input was 5 or 50 50% tissue culture infectious doses (TCID50s) per well (data not shown). The IC50 of the control oligonucleotide ISIS 9477 was ≥30 μM for all three isolates. Parallel cellular toxicity determinations with treated uninfected PBMCs incubated with (4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrasodium bromide (MTT) (6) on day 7 yielded mean 50% cytotoxic concentrations (CC50s) of 6.6 μM (range, 0.7 to 10 μM) for ISIS 5320 and approximately 30 μM for ISIS 9477 in three separate assays. The CC50s that we obtained for ISIS 5320 are considerably lower than those reported by Buckheit et al. (3), who initially found no detectable toxicity in PBMCs at 100 μM. Further work by Buckheit (2) on ISIS 5320 in PBMCs yielded CC50s of 20 to 46 μM in MTT, 3H-thymidine uptake, and cell proliferation assays, although no reduction in cell viability was detected by trypan blue dye exclusion at 100 μM.
TABLE 1.
In vitro inhibition of HIV-1 isolates and cytotoxicity of ISIS 5320, ISIS 9477, and AZT in PHA-stimulated PBMCs
| Drug | Expt no. | IC50 (μM)
|
CC50 (μM) | ||
|---|---|---|---|---|---|
| NL4-3 | JD | EW | |||
| ISIS 5320 | 1 | 2.0 | 2.0 | 10, 0.72a | |
| 2 | 6.0 | 6.4 | 9.0 | ||
| ISIS 9477 | 1 | >30 | >30 | >30b | |
| 2 | >30 | >30 | 25 | ||
| AZT | 1 | 0.16 | 0.013 | >0.37 | |
| 2 | 0.079 | 0.022 | >0.37 | ||
Results are for two independent determinations performed on the same day.
MTT assays yielded 46 and 29% inhibition with ISIS 9477 at 30 μM in two independent determinations performed on the same day.
The efficacy of ISIS 5320 against HIV isolates NL4-3, JD, and EW was tested in vivo in the SCID-hu Thy/Liv model. The human thymus implant in this model supports long-term (>11-month) differentiation of human and myeloid cells and has been used previously to study the pathogenic effects of HIV in vivo (1, 9). The model has also been standardized for the preclinical evaluation of antiviral compounds against HIV-1 (4, 8). By these previously described procedures, ISIS 5320 or ISIS 9477 was administered subcutaneously once a day at various doses to SCID-hu Thy/Liv mice starting 48 h before virus inoculation. One hour after administration of the third dose, mice were challenged with one of three HIV-1 isolates (NL4-3, JD, or EW) by direct inoculation of 1,000 TCID50s into each Thy/Liv implant. Each experiment was performed with mice whose implants were constructed with thymus and liver from the same donor (8), and each dosing group comprised four to seven mice. After continued once-daily dosing with the test agents, the Thy/Liv implants were harvested 12 or 14 days after virus inoculation. The implants were dispersed into single-cell suspensions, and the cells were lysed for quantitation of p24 by ELISA and were stained with antibodies to CD4 and CD8 for analysis by flow cytometry. Cell viability was determined by analysis of the forward- and side-scatter characteristics of implant thymocytes, and the number of live thymocytes was determined by gate exclusion of both dying and shrunken, dead cells. The Mann-Whitney U test was used for statistical analysis of implant p24 concentrations, and P values of ≤0.05 were considered statistically significant.
In an experiment performed to determine whether sufficient concentrations of ISIS 5320 could be achieved in vivo, plasma and tissue drug levels were measured by anion-exchange high-pressure liquid chromatography. Samples from uninfected SCID-hu Thy/Liv mice were obtained after the administration of five daily doses of either 25 or 50 mg/kg of body weight/day and were collected 1 h after the administration of the fifth dose. The concentrations of the intact 8-mer were 16 to 21 μM in the plasma and 8.2 to 10 μM in the implants of mice treated with 50 mg/kg/day (Table 2), and thus were higher than the in vitro IC50s for all three isolates.
TABLE 2.
Plasma and Thy/Liv implant ISIS 5320a concentrations after the administration of five daily subcutaneous doses
| Dosage (mg/kg/day) | Mouse no. | ISIS 5320 concn (μM)
|
|
|---|---|---|---|
| Plasma | Thy/Liv implant | ||
| 25 | 1 | 9.6 | 3.5 |
| 2 | 8.6 | NAb | |
| 3 | 11 | 5.3 | |
| 50 | 1 | 19 | NA |
| 2 | 21 | 8.2 | |
| 3 | 16 | 10 | |
Intact 8-mer oligonucleotide.
NA, not available.
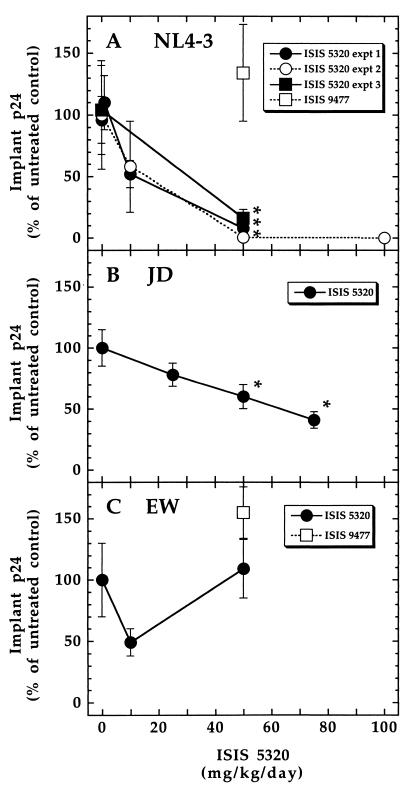
At 50 and 100 mg/kg/day, ISIS 5320 demonstrated dose-dependent antiviral activity against the HIV-1 molecular clone NL4-3 (Fig. 1A), causing statistically significant reductions in Thy/Liv implant p24 levels compared to those in untreated mice. At 50 mg/kg/day, ISIS 5320 reduced implant p24 levels to 9.3, 0.36, and 13% of those in untreated control implants in the three experiments, respectively, without apparent toxicity to implant thymocytes. At 100 mg/kg/day, ISIS 5320 reduced the implant p24 levels to undetectable levels, but this dose also caused toxicity to the Thy/Liv implants, reducing the proportion of viable cells in the lymphoid gate to 10% of that in implants from untreated mice. The activity of ISIS 5320 against the HIV-1 clinical isolate JD was less pronounced (Fig. 1B) than that against NL4-3, but the oligonucleotide did cause dose-dependent reductions in implant p24 levels that were statistically significant at 50 mg/kg/day (60% of that for the control) and 75 mg/kg/day (41% of that for the control). These moderate reductions in JD replication were observed in the experiment in which 50 mg of ISIS 5320 per kg/day reduced p24 levels to 13% of that for the control in NL4-3-infected implants. There was no activity of ISIS 5320 against the EW clinical isolate at 50 mg/kg/day (Fig. 1C) in the experiment in which the NL4-3 p24 level was reduced to 0.36% of that for the control when ISIS 5320 was used at the same dose, and the negative control oligonucleotide ISIS 9477 showed no activity at 50 mg/kg/day against either NL4-3 or EW (Fig. 1A and 1C). Because once-daily dosing produced peak ISIS 5320 concentrations in the Thy/Liv implants (Table 2) only slightly higher than the in vitro antiviral IC50s for JD and EW (Table 1), a more frequent dosing regimen may have resulted in greater in vivo antiviral efficacy against these two primary isolates.
FIG. 1.
In vivo inhibition of HIV-1 by ISIS 5320. SCID-hu Thy/Liv mice infected with HIV-1 isolate NL4-3, JD, or EW were administered various doses of ISIS 5320 and the control oligonucleotide ISIS 9477. Implant p24 levels (in picograms per 106 cells) were converted to a percentage of the level for the control by dividing the values for treated mice by the mean value for untreated mice, and the results are expressed as the mean ± standard error. Data for treated mice (four to seven mice per dosage group) were compared to those for untreated infected mice, and P values of ≤0.05 were considered statistically significant (indicated by an asterisk). Mean p24 values (in picograms per 106 cells) for untreated groups were as follows: 290 (experiment 1), 500 (experiment 2), and 170 (experiment 3) for NL4-3-infected implants, 93 for EW-infected implants, and 800 for JD-infected implants.
In summary, ISIS 5320 had demonstrable activity against the HIV-1 T-cell tropic molecular clone NL4-3, weaker activity against primary isolate JD, and no activity against primary isolate EW. In mice treated with 50, 75, and 100 mg of ISIS 5320 per kg/day, splenomegaly and hemorrhaging at the site of injection were observed. These effects were not seen after the administration of 50 mg of ISIS 9477 per kg/day.
These experiments suggest that the oligonucleotide TTGGGGTT (ISIS 5320) has activity in vivo in the SCID-hu Thy/Liv mouse model. However, this conclusion is tempered by the toxicity observed in treated, PHA-activated PBMCs and in the Thy/Liv implants when the drug is used at a dose of 100 mg/kg/day. Notably, treatment with ISIS 5320 at 50 mg/kg/day does result in statistically significant inhibition of both NL4-3 and JD replication in the absence of overt implant toxicity. Similar reductions in implant viral load were obtained in NL4-3-infected SCID-hu Thy/Liv mice after the oral administration of zidovudine (AZT) at 50 mg/kg/day and nevirapine at 25 mg/kg/day (8). Testing of the toxicity and efficacy of ISIS 5320 in human clinical trials appears to be warranted and will provide a more definitive estimate of the therapeutic index for this antiviral compound.
Acknowledgments
This work was supported by a contract from NIAID/NIH (N01-AI-65309 to J.M.M.).
Footnotes
This report is dedicated to the memory of Mara Hincenbergs.
REFERENCES
- 1.Bonyhadi M L, Rabin L, Salimi S, Brown D A, Kosek J, McCune J M, Kaneshima H. HIV induces thymus depletion in vivo. Nature. 1993;363:728–732. doi: 10.1038/363728a0. [DOI] [PubMed] [Google Scholar]
- 2.Buckheit, R. W., Jr. Unpublished data.
- 3.Buckheit R W, Jr, Roberson J L, Lackman-Smith C, Wyatt J R, Vickers T A, Ecker D J. Potent and specific inhibition of HIV envelope-mediated cell fusion and virus binding by G quartet-forming oligonucleotide (ISIS 5320) AIDS Res Hum Retroviruses. 1994;10:1497–1506. doi: 10.1089/aid.1994.10.1497. [DOI] [PubMed] [Google Scholar]
- 4.Datema R, Rabin L, Hincenbergs M, Moreno M B, Warren S, Linquist V, Rosenwirth B, Seifert J, McCune J M. Antiviral efficacy in vivo of the anti-human immunodeficiency virus bicyclam SDZ SID 791 (JM 3100), an inhibitor of infectious cell entry. Antimicrob Agents Chemother. 1996;40:750–754. doi: 10.1128/aac.40.3.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCune J M, Namikawa R, Kaneshima H, Shultz L D, Lieberman M, Weissman I L. The SCID-hu mouse: murine model for the analysis of human hematolymphoid differentiation and function. Science. 1988;241:1632–1639. doi: 10.1126/science.241.4873.1632. [DOI] [PubMed] [Google Scholar]
- 6.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 7.Namikawa R, Weilbaecher K N, Kaneshima H, Yee E J, McCune J M. Long-term human hematopoiesis in the SCID-hu mouse. J Exp Med. 1990;172:1055–1063. doi: 10.1084/jem.172.4.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rabin L, Hincenbergs M, Moreno M B, Warren S, Linquist V, Datema R, Charpiot B, Seifert J, Kaneshima H, McCune J M. Use of standardized SCID-hu Thy/Liv mouse model for preclinical efficacy testing of anti-human immunodeficiency virus type 1 compounds. Antimicrob Agents Chemother. 1996;40:755–762. doi: 10.1128/aac.40.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Su L, Kaneshima H, Bonyhadi M, Salimi S, Kraft D, Rabin L, McCune J M. HIV-1-induced thymocyte depletion is associated with indirect cytopathicity and infection of progenitor cells in vivo. Immunity. 1995;2:25–36. doi: 10.1016/1074-7613(95)90076-4. [DOI] [PubMed] [Google Scholar]
- 10.Wyatt J R, Vickers T A, Roberson J L, Buckheit R W, Jr, Klimkait T, DeBaets E, Davis P W, Rayner B, Imbach J L, Ecker D. G-quartet structure is a potent inhibitor of HIV envelope-mediated cell fusion. Proc Natl Acad Sci USA. 1994;91:1356–1360. doi: 10.1073/pnas.91.4.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]