Abstract
Topic
Though lipid and cholesterol dyshomeostasis is thought to contribute to the pathogenesis of age-related macular degeneration (AMD), there is no consensus regarding which elements of systemic lipid homeostasis are perturbed in AMD. In this systematic review and meta-analysis, an update to that performed by Wang et al in 2016, we characterized serum lipoprotein profiles in patients with AMD and its various stages.
Clinical Relevance
These findings may identify novel therapeutic approaches for AMD, a leading cause of blindness among older adults in the industrialized world.
Methods
We used MEDLINE, Embase, and Web of Science to identify articles from database inception to May 2022 that reported blood/serum levels of lipid subspecies (triglycerides [TGs], total cholesterol [TC], low-density lipoprotein [LDL], and high-density lipoprotein [HDL]) in patients with AMD compared with controls. We meta-analyzed the data by generating multilevel random-effects models using restricted maximum likelihood estimation.
Results
Our updated meta-analysis included 56 studies, almost 3 times as many studies as the 2016 meta-analysis with a total of 308 188 participants. There were no significant differences in serum TG, TC, LDL, or HDL between patients with AMD and non-AMD controls. Given significant heterogeneity, we performed subanalyses specifically in patients with early to intermediate nonexudative AMD, advanced nonexudative AMD, and advanced exudative AMD. Compared with non-AMD controls, patients with early to intermediate nonexudative AMD had significantly lower serum TG (standardized mean difference [SMD]: −0.03; 95% confidence interval [95% CI]: −0.06 to −0.01) and higher serum HDL (SMD: 0.07; 95% CI: 0.04–0.11). Patients with advanced exudative AMD had significantly higher serum LDL (SMD: 0.33; 95% CI: 0.04–0.62) compared with non-AMD controls. There were no other significant differences identified.
Conclusion
We found that there is significant heterogeneity in systemic lipoproteins in patients with AMD compared with non-AMD controls. The specific pattern of lipid dyshomeostasis appeared to be distinct based on AMD stage. These findings highlight both the underlying heterogeneity of AMD as well as the presence of distinct pathophysiological mechanisms involved at different stages or subtypes of AMD and may inform the development of novel therapeutic approaches.
Financial Disclosure(s)
Proprietary or commercial disclosure may be found in the Footnotes and Disclosures at the end of this article.
Keywords: Age-related macular degeneration, Lipids, Meta-analysis, Cholesterol
Age-related macular degeneration (AMD) is a leading cause of blindness in the industrialized world. Over 19 million Americans > 40 years of age have AMD, and of those, approximately 1.5 million Americans have vision-threatening forms of AMD.1 Though we have therapies for advanced exudative disease targeting VEGF and a newly approved drug targeting the complement pathway that may have some role for slowing growth of geographic atrophy lesions,2 there are no treatments available to prevent progression to advanced disease. As the disease burden of AMD will likely increase with the aging population, there is a need for further research to uncover the pathophysiology underlying AMD to aid in the development of novel therapeutic approaches.3
Numerous studies in preclinical models have suggested that lipid dyshomeostasis likely contributes to the pathophysiology of AMD, though the relationship between AMD and lipid homeostasis, as studied in humans, is incompletely understood.4, 5, 6, 7, 8, 9 Furthermore, there is no consensus regarding which elements of systemic lipid homeostasis are perturbed in AMD.6 We recently showed that high-dose statin therapy, which is known to lower levels of systemic low-density lipoprotein (LDL), may contribute to the regression of some high-risk features of AMD.10 Additionally, a meta-analysis in 2016 found that patients with elevated systemic triglycerides (TGs), total cholesterol (TC), and LDL had a lower prevalence of AMD, whereas patients with elevated high-density lipoprotein (HDL) had a higher prevalence of AMD.11 Given a large number of new studies investigating this question since 2016, the goal of this systematic review and meta-analysis was to provide an updated characterization of systemic lipid homeostasis in AMD. These findings will not only improve our understanding of the relationship between lipid dyshomeostasis and AMD pathogenesis but may also contribute to the development of novel therapeutic strategies.
Methods
Protocol and Registration
We designed and executed a systematic literature search guided by a medical librarian (D.G.), following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2020 guidelines.12 The review protocol was not registered prior to publication. This meta-analysis of published group-level data did not require institutional review board approval and informed consent was not applicable. The study adhered to the tenets of the Declaration of Helsinki.
Search Strategy and Information Sources
We performed a comprehensive literature search using Ovid MEDLINE (1946-), Embase.com (1947-), and Web of Science (Core Collection 1900-). Each search utilized a combination of controlled vocabulary and keywords focused on risk factors of AMD and dyslipidemia. The full search syntax used for each database is provided in Appendix A (available at https://www.ophthalmologyscience.org). The Cochrane filter for human studies was used for MEDLINE and Embase, and the English language database filters were used for all databases. We did not apply any restrictions based on study design, date of publication, or country of origin. The final searches were executed on May 31, 2022. This search identified a total of 9349 articles after duplicates were removed. We exported references into Endnote 7.8 for deduplication and then to Covidence for study screening, selection, and data extraction.
Eligibility Criteria, Study Selection, and Screening
The titles and abstracts for each of the manuscripts identified by our comprehensive search strategy were reviewed by one of 2 authors (B.L. or J.B.L.). Articles were omitted at this stage if they were clearly not relevant to the present study or if they were review articles. The full texts of the remaining 251 manuscripts were obtained and assessed for inclusion by 2 authors (B.L. and J.B.L.). We included all studies that quantitatively described the association between serum lipid species (TG, TC, HDL, or LDL) and AMD with an odds ratio (OR) or relative risk or provided group-level data (i.e., mean or median with standard deviation or interquartile range) comparing AMD and non-AMD groups. Studies were included if they investigated ≥ 1 serum lipid species. Studies that analyzed serum lipid species data in a categorical fashion (i.e., serum TG are normal [< 150 mg/dl], borderline high [150–199 mg/dl], or high [> 200 mg/dl]) rather than in a continuous fashion were excluded due to inability to meta-analyze this data. Conflicts were resolved by discussion at the end of each stage.
Data Extraction and Quality Assessment
We created a data extraction form to extract the study characteristics and outcomes from each of the included studies (Appendix B; available at https://www.ophthalmologyscience.org). We used a modified Newcastle–Ottawa scale to assess the quality of included studies (Appendix C; available at https://www.ophthalmologyscience.org).
Summary Measures and Synthesis of Results
We performed statistical analysis with R 4.2.1 and RStudio Desktop using the meta,13 metafor,14 effectsize,15 compute.es,16 tidyverse,17 and esc18 packages. For studies reporting group-level serum lipid values in patients with AMD versus control, we calculated standardized mean differences (SMDs) using Hedges’ g. For studies reporting ORs to describe the association between serum lipid levels and AMD, we converted ORs to SMD using Hedges’ g using the compute.es package.16 Four studies19, 20, 21, 22 reported a median and/or interquartile range due to nonnormal data, which we converted to an estimated mean/standard deviation using established formulas.23 With all data represented as SMD, we ran a multilevel (nested), random-effects model to allow for the inclusion of multiple SMDs from individual studies (e.g., if a study reported data separately for early/intermediate AMD, advanced dry AMD, and advanced wet AMD). For one study,24 the confidence interval (CI) of the OR was estimated to be OR = 1.00, 95% CI [0.996, 1.004] to account for likely rounding error in the published OR = 1.00, 95% CI [1.00, 1.00] since the corresponding authors (K.S.W. and P.S.J.) did not respond to our request for clarification. We also performed subgroup analysis based on AMD stage. Given the heterogeneity of AMD classification criteria used, we subdivided AMD into the following categories: early to intermediate nonexudative AMD (e.g., presence of medium to large drusen, pigmentary changes), advanced nonexudative AMD (i.e., presence of geographic atrophy), and advanced exudative AMD (i.e., presence of choroidal neovascularization). Studies that reported data for patients with “advanced AMD” but without specifying further details were unable to be included in subgroup analysis.
Assessment of Heterogeneity, Outliers, and Publication Bias
We assessed between-study heterogeneity using Higgin’s and Thompson’s I2. We defined outliers as those with 95% confidence intervals (95% CIs) of their SMD that did not overlap with the 95% CI of the pooled effect size. Because of the significant between-study heterogeneity in the “all AMD” models and the fact that no significant differences were found in these comparisons, we performed sensitivity analyses in the subgroup analyses only, rerunning these models after omitting outliers, and not in the overall “all AMD” models. For all these analyses, removal of outliers did not significantly change the pooled effect size (Table S1; available at https://www.ophthalmologyscience.org). Additionally, we reanalyzed data after omitting data from the study of lower quality20 based on the modified Newcastle–Ottawa scale and for the study24 where the OR was adjusted to account for rounding error and found that this did not significantly change the observed results. Finally, to ensure that conversion of data from ORs to SMD did not introduce bias, we performed subgroup analyses using a mixed-effects model and found that there were no significant differences between the pooled effect of studies that underwent data conversion and ones that did not.
We analyzed publication bias with a funnel plot and tested for asymmetry with the rank correlation test. Most models showed no evidence of significant publication bias. The exception was the LDL and all AMD models (τ = 0.4058, P < 0.0001), though this was unlikely to introduce problematic bias, as we would expect publication bias to bias us toward a significant result while we found a null result instead.
Results
Study Characteristics
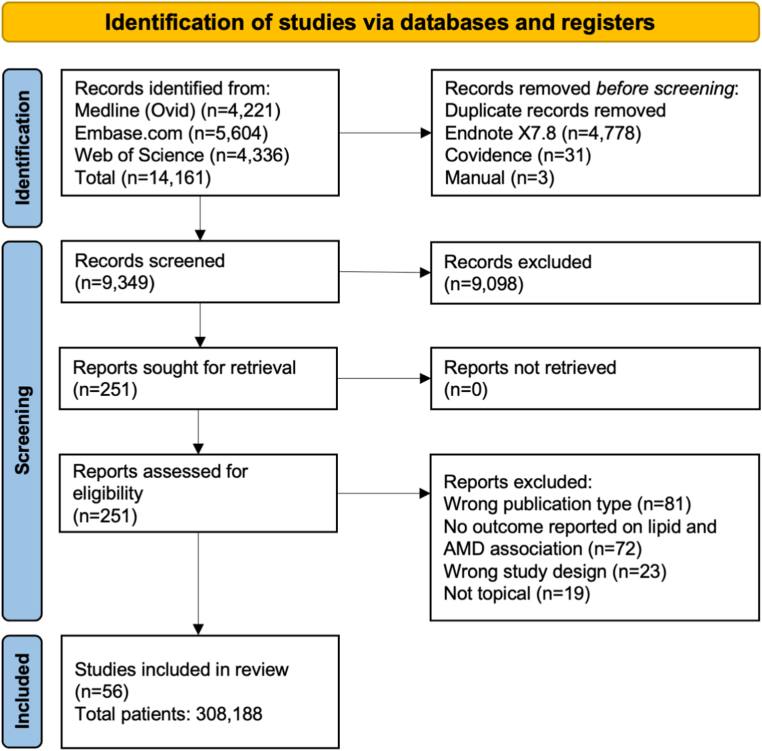
Our initial electronic search identified 9349 unique articles. After omitting articles that were clearly not relevant based on their title and abstract, we assessed the full texts of 251 articles. Of these, 81 (41%) were excluded for being the wrong publication type (e.g., conference abstracts), 72 (37%) were excluded since they did not quantify an association between lipids and AMD, 23 (12%) were excluded for being the wrong study design (e.g., inappropriate case definition), and 19 (10%) were excluded for not being relevant to this topic (e.g., not studying AMD). In total, this process yielded 56 articles for inclusion (Figure 1).
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram showing citations identified, screened, included, and excluded. AMD = age-related macular degeneration.
The meta-analysis included 308 188 patients, of which 265 477 were controls and 42 711 were patients with AMD. Of the patients with AMD, 13 060 were patients with early to intermediate nonexudative AMD, 146 were patients with advanced nonexudative AMD, and 319 were patients with advanced exudative AMD. The mean proportion of females was 54%, and the mean age was 66. The Wisconsin Age-Related Maculopathy Grading System25 was used in 18 studies, the International Classification and Grading System26 in 11 studies, the Age-Related Eye Disease Study27 scale in 5 studies, the Rotterdam classification system28 in 3 studies, the Three Continent Consortium29 method in 3 studies, the Beckman classification system30 in 2 studies, other nonstandardized classification criteria in 6 studies, and nonspecified methods in 8 studies. The most common countries in which the included studies were performed included 7 from the United States (13%),31, 32, 33, 34, 35, 36, 37 5 from Germany (9%),19,38, 39, 40, 41 and 4 from Australia (7%).42, 43, 44, 45 A summary of study characteristics is provided in Table 2.
Table 2.
Study Characteristics for Studies Included in This Meta-analysis
| Study; Country/Countries [reference] | Study Design | Mean Age | AMD Classification | Outcomes Data Type | Lipids Investigated | Number of Participants |
||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control | All AMD | Early/Int dry AMD | Adv Dry AMD | Adv Wet AMD | ||||||
| Abalain et al (2022); France46 | Case-control | 76.2 | WARMGS | Means | TG, TC, LDL, HDL | 62 | 84 | - | 33 | - |
| Ambreen et al (2014); Pakistan47 | Case-control | 63.7 | ICGS | Means | TG, TC, LDL, HDL | 100 | 90 | - | - | - |
| AnandBabu et al (2016); India48 | Case-control | 59.1 | AREDS | Means | TG, TC ,LDL, HDL | 30 | 48 | - | - | - |
| Arifoglu et al (2016); Turkey49 | Case-control | 63.8 | Other | Means | TG, TC, LDL, HDL | 24 | 20 | - | - | 20 |
| Baker et al (2009); US37 | Cross-sectional | 78.3 | WARMGS | Means | TG, TC, LDL, HDL | 1737 | 351 | - | - | - |
| Bikbov et al (2020); Russia50 | Cross-sectional | 58.5 | Beckman | OR | TG, TC, LDL, HDL | 4412 | 520 | - | - | - |
| Blumenkranz et al (1986); US36 | Case-control | 76.0 | Not specified | Means | TG, TC, LDL, HDL | 26 | 23 | - | - | - |
| Brandl et al (2016); Germany38 | Cross-sectional | 47.5 | AREDS | OR | LDL, HDL | 2208 | 343 | 270 | - | - |
| Brandl et al (2022) - KORA-FF4; Germany39 | Cohort | 61.9 | 3CC | OR | LDL, HDL | 172 | 122 | 122 | - | - |
| Brandl et al (2022) - KORA-Fit; Germany39 | Cohort | 44.6 | 3CC | OR | LDL, HDL | 172 | 122 | 122 | - | - |
| Cackett et al (2008); Singapore51 | Cross-sectional | 58.7 | WARMGS | OR | TG, TC, LDL, HDL | 3075 | 190 | 169 | - | - |
| Cezario et al (2015); Brazil52 | Case-control | 64.8 | Not specified | Means | TG, TC, LDL, HDL | 30 | 30 | - | - | - |
| Cheung et al (2007); US35 | Cross-sectional | 59.8 | WARMGS | Means | TG, TC | 9500 | 454 | 454 | - | - |
| Cheung et al (2012); Singapore53 | Cross-sectional | 53.7 | AREDS | Means | TC, LDL, HDL | 2961 | 211 | - | - | - |
| Cheung et al (2013) - Central Indians; Singapore and India54 | Cross-sectional | 57.8 | WARMGS | OR | TC, HDL | 3213 | 209 | 209 | - | - |
| Cheung et al (2013) - Singaporeans; Singapore and India54 | Cross-sectional | 57.8 | WARMGS | OR | TC, HDL | 3149 | 188 | 188 | - | - |
| Cheung et al (2014); Singapore55 | Cross-sectional | 59.7 | WARMGS | OR | TC | 3046 | 266 | - | - | - |
| Colak et al (2011); Serbia56 | Cross-sectional | 69.8 | AREDS | Means | TG, TC, LDL, HDL | 80 | 82 | - | - | - |
| Colijn et al (2019); France, Germany, Italy, Netherlands, Norway, Portugal, UK57 | Cross-sectional | 69.5 | Rotterdam | OR | TG, TC, LDL, HDL | 19 539 | 5374 | 3768 | - | - |
| Cougnard-Gregoire et al (2014); France58 | Cross-sectional | 80.2 | ICGS | OR | TG, TC, LDL, HDL | 630 | 308 | 247 | - | - |
| Davari et al (2013); Iran59 | Case-control | 75.0 | Other | Means | TG, TC, LDL, HDL | 32 | 32 | - | - | - |
| Delcourt et al (2001); France60 | Cross-sectional | 70.1 | ICGS | OR | TG, TC, HDL | 1415 | 768 | 730 | - | - |
| Erke et al (2014); Norway61 | Cohort | 72.3 | ICGS | OR | TG, TC, LDL, HDL | 2048 | 727 | 635 | - | - |
| Fan et al (2017); Several; US and non-US34 | Case-control | - | Other | OR | TG, LDL, HDL | 23 107 | 18 363 | - | - | - |
| Fauser et al (2011); Germany, Netherlands40 | Case-control | 74.9 | Other | Means | TG, TC, HDL | 521 | 792 | - | - | - |
| Fernandez et al (2012); US33 | Cohort | 62.0 | WARMGS | Means | TC, LDL | 5338 | 895 | 893 | - | - |
| Hashemi et al (2018); Iran62 | Case-control | - | Not specified | Means | TG, TC, LDL, HDL | 15 | 15 | - | - | - |
| Javadzadeh et al (2007); Iran63 | Case-control | 70.0 | Not specified | Means | TG, TC, LDL, HDL | 60 | 60 | - | - | 60 |
| Joachim et al (2015); Australia42 | Cohort | 62.2 | WARMGS | Means | TG, TC, HDL | 2425 | 534 | 534 | - | - |
| Jonasson et al (2014); Iceland64 | Cross-sectional | 74.7 | WARMGS | OR | TC, HDL | 2540 | 328 | - | - | - |
| Kawasaki et al (2008); Japan65 | Cross-sectional | 60.4 | WARMGS | Means | TC, HDL | 1559 | 66 | 58 | - | - |
| Klein et al (1993); US32 | Cross-sectional | 61.7 | WARMGS | OR | TC, HDL | 1746 | 773 | 745 | 11 | 17 |
| Klein et al (2003); US31 | Cohort | 78.5 | WARMGS | OR | TG, TC, LDL | 1995 | 366 | 366 | - | - |
| Koller et al (2022); Germany41 | Cohort | 78.2 | 3CC | Means | TG, TC, LDL, HDL | 1635 | 627 | - | - | - |
| Ludtke et al (2019); Germany19 | Cohort | - | Rotterdam | Means | TG, TC, LDL, HDL | 1088 | 753 | 745 | - | - |
| Mao et al (2019); China66 | Cohort | 51.0 | WARMGS | OR | TG, TC, LDL, HDL | 4823 | 214 | 214 | - | - |
| McCarty et al (2008); Australia43 | Cross-sectional | 73.9 | ICGS | Means | TG, TC, LDL, HDL | 160 | 160 | - | - | - |
| Neethu et al (2020); India20 | Cross-sectional | 62.0 | not specified | Means | TG, TC, LDL, HDL | 39 | 39 | - | - | - |
| Ngai et al (2011); UK67 | Cohort | 71.1 | ICGS | Means | TG, TC, LDL, HDL | 843 | 91 | - | - | - |
| Nordestgaard et al (2021); Denmark21 | Cohort | 58.0 | Not specified | Means | TG | 104 916 | 1787 | - | - | - |
| Ornek et al (2016); Turkey68 | Case-control | 73.0 | Not specified | Means | TG, TC, LDL, HDL | 78 | 98 | - | - | 47 |
| Park et al (2014); Korea24 | Cross-sectional | 55.1 | ICGS | OR | TG, TC, HDL | 12 667 | 1046 | 958 | 22 | 66 |
| Paunksnis et al (2005); Lithuania69 | Cross-sectional | - | ICGS | Means | TG, TC | 84 | 84 | 84 | - | - |
| Peiretti et al (2014); Italy70 | Cross-sectional | 76.2 | AREDS | OR | TC, HDL | 38 | 136 | 98 | - | - |
| Pertl et al (2016); Austria22 | Cross-sectional | 76.7 | Other | Means | TG, TC, LDL, HDL | 26 | 29 | - | - | 29 |
| Qin et al (2014); UK71 | Case-control | 58.0 | WARMGS | Means | TG, TC, LDL, HDL | 14 | 14 | - | - | - |
| Raman et al (2016); India72 | Cross-sectional | 72.0 | ICGS | OR | TC, LDL, HDL | 3805 | 986 | 893 | - | - |
| Roh et al (2008); Korea73 | Cross-sectional | 52.7 | Rotterdam | Means | TG, TC, LDL, HDL | 9082 | 235 | - | - | - |
| Semba et al (2019); Iceland74 | Case-control | 79.7 | Not specified | Means | TC, HDL | 80 | 160 | - | 80 | 80 |
| Smith et al (1998); Australia44 | Cross-sectional | 62.2 | WARMGS | OR | TG, TC, HDL | 3342 | 312 | 240 | - | - |
| Song et al (2009); Korea75 | Cross-sectional | 57.2 | ICGS | Means | TG, TC, HDL | 10 551 | 339 | 318 | - | - |
| Taniguchi et al (2013); Japan76 | Case-control | 71.3 | ICGS | Means | TG, TC, LDL, HDL | 40 | 88 | - | - | - |
| Tsang et al (1992); Australia45 | Case-control | 73.9 | Other | Means | TG, TC | 86 | 80 | - | - | - |
| Ulas et al (2013); Turkey77 | Cross-sectional | 71.0 | Not specified | Means | TG, TC, LDL, HDL | 141 | 142 | - | - | 142 |
| Vingerling et al (1995); Netherlands78 | Case-control | 71.0 | WARMGS | Means | TC, HDL | 1324 | 96 | - | - | - |
| Wang et al (2012); China79 | Cross-sectional | 60.4 | WARMGS | OR | TG, TC, LDL, HDL | 2865 | 161 | - | - | - |
| Xue et al (2021); China80 | Cross-sectional | 60.5 | Beckman | OR | TG, TC, LDL, HDL | 6112 | 1607 | - | - | - |
| Yip et al (2015); UK81 | Cohort | 67.4 | WARMGS | Means | TG, TC, LDL, HDL | 4671 | 673 | - | - | - |
3CC = Three Continent Consortium; Adv = advanced; AMD = age-related macular degeneration; AREDS = Age-Related Eye Disease Study; HDL = high-density lipoprotein; ICGS = International Classification and Grading System; Int = intermediate; LDL = low-density lipoprotein; OR = odds ratio; TC = total cholesterol; TG = triglyceride; UK = United Kingdom; US = United States; WARMGS = Wisconsin Age-Related Maculopathy Grading System.
Quality Assessment of Included Studies
We assessed the quality of included studies based on a modified Newcastle–Ottawa scale (Appendix C; available at https://www.ophthalmologyscience.org). One study20 had a score of 4, due to a lack of specific case definition, no information provided regarding the method used to obtain AMD diagnosis, and no measures performed to increase comparability between case and control groups. Otherwise, all other included studies scored ≥ 7, indicating acceptable study quality (Table S3; available at https://www.ophthalmologyscience.org). The removal of this low-scoring study from the models did not significantly impact any of the pooled effect sizes.
Systemic TGs in AMD
By meta-analyzing 53 effect sizes from 43 studies,19, 20, 21, 22,24,31,34, 35, 36, 37,40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 56, 57, 58, 59, 60, 61, 62, 63, 66, 67, 68, 69, 71, 73, 75, 76, 77, 79, 80, 81 we found that there was no significant difference between systemic TGs in patients with AMD versus controls (SMD: −0.03; 95% CI: −0.06 to 0.01) (Figure 2). Because there was significant interstudy heterogeneity (I2 = 99.3%), we also performed subgroup analysis based on AMD stage, though heterogeneity remained high in the early to intermediate dry AMD (I2 = 88.8%) and advanced wet AMD groups (I2 = 96.0%). With this caveat in mind, subgroup analysis revealed a statistically significantly lower level of systemic TGs in patients with early to intermediate AMD versus controls (SMD: −0.03; 95% CI: −0.06 to −0.01). No significant difference in systemic TGs was found for patients with advanced dry AMD (SMD: −0.03; 95% CI: −0.94 to 0.88) or for patients with advanced wet AMD (SMD: −0.19; 95% CI: −0.89 to 0.51).
Figure 2.
Forest plot for systemic triglycerides (TG) in patients with age-related macular degeneration (AMD) versus non-AMD controls. Blue diamonds denote the 95% confidence interval (95% CI); dashed lines denote the 95% prediction interval. SMD = standardized mean difference.
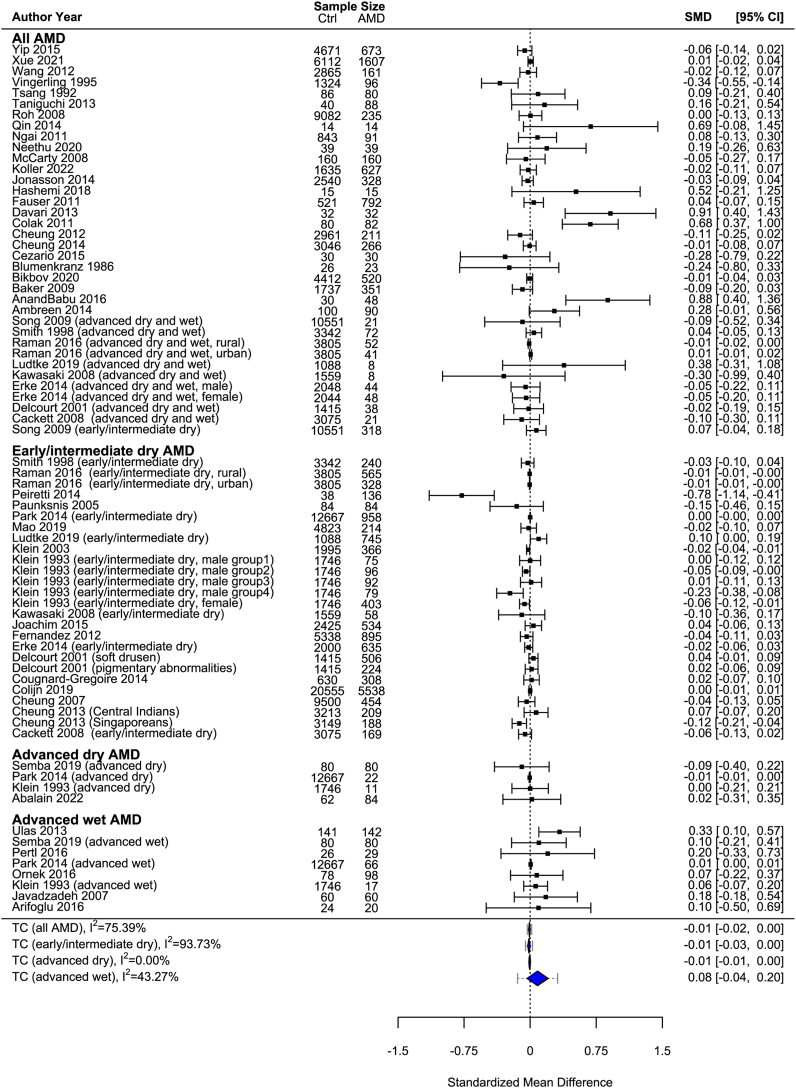
Systemic TC in AMD
By meta-analyzing 74 effect sizes from 52 studies,19,20,22,24,31, 32, 33,35, 36, 37,40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 56, 57, 58, 59, 60, 61, 62, 63, 66, 67, 68, 69, 71, 73, 75, 76, 77, 79, 80, 81, 54, 55, 53, 64, 65, 70, 72, 74, 78 we found that there was no significant difference in systemic TC levels in patients with AMD versus controls (SMD: −0.01; 95% CI: −0.02 to 0.00) (Figure 3). There was moderate to high heterogeneity (I2 = 75.4%) among meta-analyzed studies. Similarly, after subgroup analysis, there were no significant differences in systemic TC levels when examining patients with early to intermediate dry AMD (SMD: −0.01; 95% CI: −0.03 to 0.00), patients with advanced dry AMD (SMD: −0.01; 95% CI: −0.01 to 0.00), or patients with advanced wet AMD (SMD: 0.08; 95% CI: −0.04 to 0.20) when compared with controls.
Figure 3.
Forest plot for systemic total cholesterol (TC) in patients with age-related macular degeneration (AMD) versus non-AMD controls. Blue diamonds denote the 95% confidence interval (95% CI); dashed lines denote the 95% prediction interval. SMD = standardized mean difference.
Systemic LDL in AMD
By meta-analyzing 49 effect sizes from 38 studies,19,20,22,31,33,34,36, 37, 38, 39,41,43,46, 47, 48, 49, 50, 51, 52, 53, 56, 57, 58, 59,61, 62, 63, 66, 67, 68,71, 72, 73,76, 77, 79, 80, 81 we found no significant difference in systemic LDL in patients with AMD versus controls (SMD: −0.00; 95% CI: −0.01 to 0.00) (Figure 4). Again, because of significant interstudy heterogeneity (I2 = 84.9%), we also performed subgroup analysis based on AMD stage, which improved the extent of heterogeneity. This subgroup analysis revealed that there was significantly higher LDL among patients with advanced wet AMD versus control (SMD: 0.33; 95% CI: 0.04–0.62; I2 = 53.3%). In contrast, patients with early to intermediate dry AMD (SMD: −0.01; 95% CI: −0.02 to 0.00; I2 = 90.0%) or with advanced dry AMD (SMD: −0.10; 95% CI: −1.72 to 1.52; I2 = 0.0%) did not exhibit a significant difference in systemic LDL when compared with controls.
Figure 4.
Forest plot for systemic low-density lipoprotein (LDL) in patients with age-related macular degeneration (AMD) versus non-AMD controls. Blue diamonds denote the 95% confidence interval (95% CI); dashed lines denote the 95% prediction interval. SMD = standardized mean difference.
Systemic HDL in AMD
By meta-analyzing 69 effect sizes from 49 studies,19,20,22,24,32,34,36, 37, 38, 39, 40, 41, 42, 43, 44,46, 47, 48, 49, 50, 51, 52, 53, 64, 56, 57, 58, 59, 60, 61, 62, 63, 66, 67, 68, 69, 71, 73, 75, 76,70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81 we found no significant difference in systemic HDL in patients with AMD compared with controls (SMD: 0.19; 95% CI: −0.10 to 0.48) (Figure 5). There was high heterogeneity among meta-analyzed studies (I2 = 100.0%). Even after subgroup analysis, heterogeneity remained high in the early to intermediate dry AMD (I2 = 99.0%). With this caveat in mind, patients with early to intermediate dry AMD exhibited significantly higher systemic HDL compared with controls (SMD: 0.07; 95% CI: 0.04–0.11). There was no significant difference in systemic HDL when comparing patients with advanced dry AMD (SMD: 0.01; 95% CI: −0.04 to 0.07; I2 = 0.0%) or with advanced wet AMD (SMD: −0.06; 95% CI: −0.18 to 0.06; I2 = 23.6%) to controls.
Figure 5.
Forest plot for systemic high-density lipoprotein (HDL) in patients with age-related macular degeneration (AMD) versus non-AMD controls. Blue diamonds denote the 95% confidence interval (95% CI); dashed lines denote the 95% prediction interval. SMD = standardized mean difference.
Discussion
Here, we performed a comprehensive systematic review with guidance from an experienced medical librarian (D.G.) to update our understanding of the association between systemic lipoprotein profiles and AMD. Our meta-analysis included 58 studies and 308 188 patients, almost 3 times as many compared to the prior 2016 meta-analysis by Wang et al,11 which included 19 studies and 104 270 patients. When analyzing all patients with AMD regardless of stage of disease, we found no differences in systemic TGs, TC, LDL, or HDL compared to controls (Table 4). On the other hand, when analyzing patients with early to intermediate AMD separately, there were significantly lower levels of TGs and higher levels of HDL but no differences in the levels of TC or LDL. Patients with advanced dry AMD had no significant differences in TGs, TC, LDL, or HDL. Finally, patients with advanced wet AMD had significantly higher systemic LDL but no differences in TGs, TC, or HDL. Taken together, our results highlight the significant heterogeneity of AMD and suggest that the specific aspects of lipid homeostasis that are perturbed may depend on the underlying stage or subtype of disease.
Table 4.
Summary of SMDs with 95% Confidence Intervals and Measure of Heterogeneity (I2) in Triglycerides, Total Cholesterol, LDL, and HDL between Patients With AMD or Specific Stages of AMD Compared with Controls
| SMD [95% Confidence Interval]; I2 |
||||
|---|---|---|---|---|
| All AMD | Early/Intermediate Dry AMD | Advanced Dry AMD | Advanced Wet AMD | |
| Triglycerides | −0.03 [−0.06, 0.01]; 99.3% | −0.03 [−0.06, −0.01]; 88.8% | −0.03 [−0.94, 0.88]; 19.6% | −0.19 [−0.89, 0.51]; 96.0% |
| Total cholesterol | −0.01 [−0.02, 0.00]; 75.4% | −0.01 [−0.03, 0.00]; 93.7% | −0.01 [−0.01, 0.00]; 0.0% | 0.08 [−0.04, 0.20]; 43.3% |
| LDL | −0.00 [−0.01, 0.00]; 84.9% | −0.01 [−0.02, 0.00]; 90.0% | −0.10 [−1.72, 1.52]; 0.0% | 0.33 [0.04, 0.62]; 53.3% |
| HDL | 0.19 [−0.10, 0.48]; 100.0% | 0.07 [0.04, 0.11]; 99.0% | 0.01 [−0.04, 0.07]; 0.0% | −0.06 [−0.18, 0.06]; 23.6% |
AMD= age-related macular degeneration; HDL = high-density lipoprotein; LDL = low-density lipoprotein; SMD = standardized mean difference.
These findings may explain, in part, why there is a discrepancy in the literature published to date with respect to the specific facets of lipid dyshomeostasis that are associated with the pathophysiology of AMD.6 We speculate that among patients with AMD, there may be facets of lipid dyshomeostasis that are specific to certain stages of the disease process. In fact, recent studies have shown that patients with soft drusen are distinct from those with subretinal drusenoid deposits (SDDs) on the basis of their systemic associations, serum profiles, and genetic risk profiles and provided evidence that high HDL was associated with classic soft drusen, whereas low HDL was associated with SDDs.82,83 Interestingly, these authors also found an association between SDDs and the presence of coexisting systemic vascular disease (e.g., aortic stenosis, myocardial infarction) and suggest that both of these (i.e., low HDL and presence of SDDs) may be related to systematic atherosclerosis.
Further research is necessary to explore this heterogeneity within patients with AMD. It may be necessary to tailor therapy for these disparate underlying pathophysiologies. We have previously shown in a small pilot study that high-dose statin therapy may lead to reduction in progression to advanced exudative AMD in high-risk patients.10 Similarly, in a study of commercially insured patients, no patients taking very-high-dose lipophilic statins progressed to exudative AMD.84 These findings correspond well with the findings of this meta-analysis, as statins are most known for lowering systemic LDL, and elevated LDL was found to be most prominent in patients with advanced wet AMD. Lipid-based therapies may be efficacious if used in the appropriate patient population. Future studies investigating the association between systemic dyslipidemia and AMD should include careful characterization of patients with AMD. Some factors that would be important to consider are AMD stage (i.e., early/intermediate vs. late), intermediate AMD subtype (i.e., drusen vs. SDDs), demographic factors (e.g., age, sex, ethnicity), presence of coexisting systemic vascular disease, and genetic variants in genes related to lipid pathways such as cholesteryl ester transfer protein, apolipoprotein E, and hepatic lipase.85 These detailed studies may identify subtypes of AMD and thereby partially explain the heterogeneity found within the present study.
Our meta-analysis had several limitations. The most prominent limitation was the moderate to high heterogeneity of the meta-analyzed effect sizes (I2 > 90%), which affects the precision and reliability of the pooled effect size estimate. In many cases, heterogeneity remained high even after subgrouping the patients by AMD stage. This interstudy heterogeneity was likely related to underlying differences with respect to the studies’ country of origin, the AMD classification criteria used, the types of outcome data presented, and the study design, among other factors. However, they could also reflect the significant heterogeneity of AMD as a disease process and suggest that there is a need for improved, more precise AMD classification criteria based on our improving understanding of its underlying pathophysiological mechanisms at the cellular and molecular level.
Additionally, there were much less data from patients with advanced dry AMD (N = 146) or advanced wet AMD (N = 319) compared to those from patients with early to intermediate dry AMD (N = 13 060), leading to reduced statistical power in these associated meta-analyses. Finally, because studies differed in terms of how they reported their outcomes (e.g., reporting means of various groups vs. reporting ORs), we had to convert all of these outcomes to SMDs to allow for an estimate of the pooled effect size. Though we used validated mathematical formulas for these conversions, they could have introduced bias due to their underlying assumptions.
Overall, our findings support that there is systemic dyshomeostasis in patients with AMD compared with non-AMD controls and that the specific pattern of impaired lipid homeostasis appears to depend on AMD stage. We propose that these findings reflect the underlying heterogeneity of AMD, the presence of distinct pathophysiological mechanisms involved at different stages of AMD, and may even suggest the existence of distinct AMD subtypes that should be investigated further to inform novel therapeutic approaches.
Manuscript no. XOPS-D-23-00062.
Footnotes
Supplemental material available atwww.ophthalmologyscience.org.
Demetrios Vavvas, an editor of this journal, was recused from the peer-review process of this article and had no access to information regarding its peer-review.
Disclosures:
All authors have completed and submitted the ICMJE disclosures form.
The authors made the following disclosures:
J.B.L.: Supported by the VitreoRetinal Surgery Foundation and the Gragoudas-Folkman Award. D.G.V. was supported by the Monte J. Wallace Chair in Retina, the Solman and Libe Friedman OHMS Ophthalmology Chair, the Ines and Fred Yeatts Retina Research lab fund, the MLS Foundation, and the American Macular Degeneration Foundation. The sponsor or funding organization had no role in the design or conduct of this research. The remaining authors have no proprietary or commercial interest in any materials discussed in this article.
HUMAN SUBJECTS: Human subjects were included in this study. This meta-analysis of published group-level data did not require institutional review board approval and informed consent was not applicable. The study adhered to the tenets of the Declaration of Helsinki. No animal subjects were included in this study.
Author Contributions:
Conception and design: Li, Lin, Vavvas
Data Collection: Li, Goss, Lin
Analysis and interpretation: Li, Miller, Lin, Vavvas
Obtained funding: N/A; Study was performed as part of regular employment duties at Mass Eye and Ear/Harvard Medical School.
Overall responsibility: Li, Goss, Miller, Lin, Vavvas
Contributor Information
Jonathan B. Lin, Email: jonathan_lin@meei.harvard.edu.
Demetrios G. Vavvas, Email: demetrios_vavvas@meei.harvard.edu.
Supplementary Data
References
- 1.Rein D.B., Wittenborn J.S., Burke-Conte Z., et al. Prevalence of age-related macular degeneration in the US in 2019. JAMA Ophthalmol. 2022;140:1202–1208. doi: 10.1001/jamaophthalmol.2022.4401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liao D.S., Grossi F.V., El Mehdi D., et al. Complement C3 inhibitor pegcetacoplan for geographic atrophy secondary to age-related macular degeneration: a randomized phase 2 trial. Ophthalmology. 2020;127:186–195. doi: 10.1016/j.ophtha.2019.07.011. [DOI] [PubMed] [Google Scholar]
- 3.Wong W.L., Su X., Li X., et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Global Health. 2014;2:e106–e116. doi: 10.1016/S2214-109X(13)70145-1. [DOI] [PubMed] [Google Scholar]
- 4.Kishan A.U., Modjtahedi B.S., Martins E.N., et al. Lipids and age-related macular degeneration. Surv Ophthalmol. 2011;56:195–213. doi: 10.1016/j.survophthal.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 5.Vanderbeek B.L., Zacks D.N., Talwar N., et al. Role of statins in the development and progression of age-related macular degeneration. Retina. 2013;33:414. doi: 10.1097/IAE.0b013e318276e0cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin J.B., Halawa O.A., Husain D., et al. Dyslipidemia in age-related macular degeneration. Eye (Lond) 2022;36:312–318. doi: 10.1038/s41433-021-01780-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choudhary M., Ismail E.N., Yao P.-L., et al. LXRs regulate features of age-related macular degeneration and may be a potential therapeutic target. JCI Insight. 2020;5 doi: 10.1172/jci.insight.131928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ban N., Lee T.J., Sene A., et al. Impaired monocyte cholesterol clearance initiates age-related retinal degeneration and vision loss. JCI Insight. 2018;3 doi: 10.1172/jci.insight.120824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin J.B., Moolani H.V., Sene A., et al. Macrophage microRNA-150 promotes pathological angiogenesis as seen in age-related macular degeneration. JCI Insight. 2018;3 doi: 10.1172/jci.insight.120157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vavvas D.G., Daniels A.B., Kapsala Z.G., et al. Regression of some high-risk features of age-related macular degeneration (AMD) in patients receiving intensive statin treatment. EBioMedicine. 2016;5:198–203. doi: 10.1016/j.ebiom.2016.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Y., Wang M., Zhang X., et al. The association between the lipids levels in blood and risk of age-related macular degeneration. Nutrients. 2016;8:663. doi: 10.3390/nu8100663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Page M.J., McKenzie J.E., Bossuyt P.M., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372 doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balduzzi S., Rücker G., Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. 2019;22:153–160. doi: 10.1136/ebmental-2019-300117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36:1–48. [Google Scholar]
- 15.Ben-Shachar M.S., Lüdecke D., Makowski D. effectsize: estimation of effect size indices and standardized parameters. J Open Source Softw. 2020;5:2815. [Google Scholar]
- 16.Del Re A. Calculate effect sizes. https://www.acdelre.com/calculate-effect-sizes.html
- 17.Wickham H., Averick M., Bryan J., et al. Welcome to the tidyverse. J Open Source Softw. 2019;4:1686. [Google Scholar]
- 18.Lüdecke D. 2018. esc: Effect Size Computation for Meta Analysis. [Google Scholar]
- 19.Lüdtke L., Jürgens C., Ittermann T., et al. Age-related macular degeneration and associated risk factors in the population-based study of health in Pomerania (ship-trend) Med Sci Monit. 2019;25:6383–6390. doi: 10.12659/MSM.915493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neethu A., Jayashree K., Senthilkumar G.P., et al. Circulating adropin and vascular endothelial growth factor receptor-2 levels in age-related macular degeneration and T2DM patients-a cross-sectional study. J Fam Med Prim Care. 2020;9:4875–4879. doi: 10.4103/jfmpc.jfmpc_813_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nordestgaard L.T., Tybjærg-Hansen A., Frikke-Schmidt R., Nordestgaard B.G. Elevated apolipoprotein A1 and HDL cholesterol associated with age-related macular degeneration: 2 population cohorts. J Clin Endocrinol Metab. 2021;106:E2749–E2758. doi: 10.1210/clinem/dgab095. [DOI] [PubMed] [Google Scholar]
- 22.Pertl L., Kern S., Weger M., et al. High-density lipoprotein function in exudative age-related macular degeneration. PLoS One. 2016;11 doi: 10.1371/journal.pone.0154397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hozo S.P., Djulbegovic B., Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:1–10. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park S.J., Lee J.H., Kang S.W., et al. Age-related macular degeneration: prevalence and risk factors from Korean National Health and Nutrition Examination Survey, 2008 through 2011. Ophthalmology. 2014;121:1756–1765. doi: 10.1016/j.ophtha.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 25.Klein R., Davis M.D., Magli Y.L., et al. The Wisconsin age-related maculopathy grading system. Ophthalmology. 1991;98:1128–1134. doi: 10.1016/s0161-6420(91)32186-9. [DOI] [PubMed] [Google Scholar]
- 26.Bird A.C., Bressler N.M., Bressler S.B., et al. An international classification and grading system for age-related maculopathy and age-related macular degeneration. The International ARM Epidemiological Study Group. Surv Ophthalmol. 1995;39:367–374. doi: 10.1016/s0039-6257(05)80092-x. [DOI] [PubMed] [Google Scholar]
- 27.A simplified severity scale for age-related macular degeneration. Arch Ophthalmol. 2005;123:1570–1574. doi: 10.1001/archopht.123.11.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thee E.F., Meester-Smoor M.A., Luttikhuizen D.T., et al. Performance of classification systems for age-related macular degeneration in the Rotterdam study. Transl Vis Sci Technol. 2020;9:26. doi: 10.1167/tvst.9.2.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klein R., Myers C.E., Buitendijk G.H.S., et al. Lipids, lipid genes, and incident age-related macular degeneration: the three continent age-related macular degeneration consortium. Am J Ophthalmol. 2014;158:513–524.e3. doi: 10.1016/j.ajo.2014.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferris F.L., Wilkinson C.P., Bird A., et al. Clinical classification of age-related macular degeneration. Ophthalmology. 2013;120:844–851. doi: 10.1016/j.ophtha.2012.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klein R., Klein B.E.K., Marino E.K., et al. Early age-related maculopathy in the cardiovascular health study. Ophthalmology. 2003;110:25–33. doi: 10.1016/s0161-6420(02)01565-8. [DOI] [PubMed] [Google Scholar]
- 32.Klein R., Klein B.E.K., Franke T. The relationship of cardiovascular disease and its risk factors to age-related maculopathy: the Beaver Dam Eye study. Ophthalmology. 1993;100:406–414. doi: 10.1016/s0161-6420(93)31634-9. [DOI] [PubMed] [Google Scholar]
- 33.Fernandez A.B., Wong T.Y., Klein R., et al. Age-related macular degeneration and incident cardiovascular disease: the multi-ethnic study of atherosclerosis. Ophthalmology. 2012;119:765–770. doi: 10.1016/j.ophtha.2011.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fan Q., Maranville J.C., Fritsche L., et al. HDL-cholesterol levels and risk of age-related macular degeneration: a multiethnic genetic study using Mendelian randomization. Int J Epidemiol. 2017;46:1891–1902. doi: 10.1093/ije/dyx189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheung N., Liao D., Islam F.M.A., et al. Is early age-related macular degeneration related to carotid artery stiffness? The Atherosclerosis Risk in Communities study. Br J Ophthalmol. 2007;91:430–433. doi: 10.1136/bjo.2006.106054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blumenkranz M.S., Russell S.R., Robey M.G. Risk factors in age-related maculopathy complicated by choroidal neovascularization. Ophthalmology. 1986;93:552–558. doi: 10.1016/s0161-6420(86)33702-3. [DOI] [PubMed] [Google Scholar]
- 37.Baker M.L., Wang J.J., Rogers S., et al. Early age-related macular degeneration, cognitive function, and dementia: the cardiovascular health study. Arch Ophthalmol. 2009;127:667–673. doi: 10.1001/archophthalmol.2009.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brandl C., Breinlich V., Stark K.J., et al. Features of age-related macular degeneration in the general adults and their dependency on age, sex, and smoking: results from the German KORA study. PLoS One. 2016;11 doi: 10.1371/journal.pone.0167181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brandl C., Gunther F., Zimmermann M.E., et al. Incidence, progression and risk factors of age-related macular degeneration in 35-95-year-old individuals from three jointly designed German cohort studies. BMJ Open Ophthalmol. 2022;7 doi: 10.1136/bmjophth-2021-000912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fauser S., Smailhodzic D., Caramoy A., et al. Evaluation of serum lipid concentrations and genetic variants at high-density lipoprotein metabolism loci and TIMP3 in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2011;52:5525–5528. doi: 10.1167/iovs.10-6827. [DOI] [PubMed] [Google Scholar]
- 41.Koller A., Brandl C., Lamina C., et al. Relative telomere length is associated with age-related macular degeneration in women. Invest Ophthalmol Vis Sci. 2022;63:30. doi: 10.1167/iovs.63.5.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Joachim N.D.L., Mitchell P., Kifley A., Jinwang J. Incidence, progression, and associated risk factors of medium drusen in age-related macular degeneration findings from the 15-year follow-up of an Australian cohort. JAMA Ophthalmol. 2015;133:698–705. doi: 10.1001/jamaophthalmol.2015.0498. [DOI] [PubMed] [Google Scholar]
- 43.McCarty C.A., Dowrick A., Cameron J., et al. Novel measures of cardiovascular health and its association with prevalence and progression of age-related macular degeneration: the CHARM study. BMC Ophthalmol. 2008;8:25. doi: 10.1186/1471-2415-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith W., Mitchell P., Leeder S.R., Wang J.J. Plasma fibrinogen levels, other cardiovascular risk factors, and age-related maculopathy: the Blue Mountains Eye study. Arch Ophthalmol. 1998;116:583–587. doi: 10.1001/archopht.116.5.583. [DOI] [PubMed] [Google Scholar]
- 45.Tsang N.C.K., Penfold P.L., Snitch P.J., Billson F. Serum levels of antioxidants and age-related macular degeneration. Doc Ophthalmol. 1992;81:387–400. doi: 10.1007/BF00169100. [DOI] [PubMed] [Google Scholar]
- 46.Abalain J.H., Carre J.L., Leglise D., et al. Is age-related macular degeneration associated with serum lipoprotein and lipoparticle levels? Clin Chim Acta. 2002;326:97–104. doi: 10.1016/s0009-8981(02)00288-7. [DOI] [PubMed] [Google Scholar]
- 47.Ambreen F., Khan W.A., Qureshi N., Qureshi I.Z. Assessment of serum lipids in patients with age related macular degeneration from Pakistan. J Pak Med Assoc. 2014;64:664–669. [PubMed] [Google Scholar]
- 48.AnandBabu K., Bharathidevi S.R., Sripriya S., et al. Serum paraoxonase activity in relation to lipid profile in age-related macular degeneration patients. Exp Eye Res. 2016;152:100–112. doi: 10.1016/j.exer.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 49.Arifoglu H.B., Karatepe Hashas A.S., Atas M., et al. Systemic endothelial function in cases with wet-type age-related macular degeneration. Aging Clin Exp Res. 2016;28:853–856. doi: 10.1007/s40520-015-0377-5. [DOI] [PubMed] [Google Scholar]
- 50.Bikbov M.M., Zainullin R.M., Gilmanshin T.R., et al. Prevalence and associated factors of age-related macular degeneration in a Russian population: the Ural Eye and Medical study. Am J Ophthalmol. 2020;210:146–157. doi: 10.1016/j.ajo.2019.10.004. [DOI] [PubMed] [Google Scholar]
- 51.Cackett P., Wong T.Y., Aung T., et al. Smoking, cardiovascular risk factors, and age-related macular degeneration in Asians: the Singapore Malay Eye study. Am J Ophthalmol. 2008;146:960–967.e1. doi: 10.1016/j.ajo.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 52.Cezario S.M., Calastri M.C., Oliveira C.I., et al. Association of high-density lipoprotein and apolipoprotein E genetic variants with age-related macular degeneration. Arq Bras Oftalmol. 2015;78:85–88. doi: 10.5935/0004-2749.20150023. [DOI] [PubMed] [Google Scholar]
- 53.Cheung C.M.G., Tai E.S., Kawasaki R., et al. Prevalence of and risk factors for age-related macular degeneration in a multiethnic Asian cohort. Arch Ophthalmol. 2012;130:480–486. doi: 10.1001/archophthalmol.2011.376. [DOI] [PubMed] [Google Scholar]
- 54.Cheung C.M.G., Li X., Cheng C.Y., et al. Prevalence and risk factors for age-related macular degeneration in Indians: a comparative study in Singapore and India. Am J Ophthalmol. 2013;155:764–773. doi: 10.1016/j.ajo.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 55.Cheung C.M.G., Li X., Cheng C.Y., et al. Prevalence, racial variations, and risk factors of age-related macular degeneration in Singaporean Chinese, Indians, and Malays. Ophthalmology. 2014;121:1598–1603. doi: 10.1016/j.ophtha.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 56.Colak E.S., Majkic-Singh N.T., Stankovic S.S., et al. Gender associated lipid and apolipoprotein profile in patients with age-related macular degeneration. EJIFCC. 2011;22:16–23. [PMC free article] [PubMed] [Google Scholar]
- 57.Colijn J.M., Verzijden T., Meester-Smoor M.A., et al. Increased high-density lipoprotein levels associated with age-related macular degeneration: evidence from the EYE-RISK and European Eye Epidemiology Consortia. Ophthalmology. 2019;126:393–406. doi: 10.1016/j.ophtha.2018.09.045. [DOI] [PubMed] [Google Scholar]
- 58.Cougnard-Grégoire A., Delyfer M.N., Korobelnik J.F., et al. Elevated high-density lipoprotein cholesterol and age-related macular degeneration: the Alienor study. PLoS One. 2014;9 doi: 10.1371/journal.pone.0090973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Davari M.H., Gheitasi H., Yaghobi G., Heydari B. Correlation between serum lipids and age-related macular degeneration: a case-control study. J Res Health Sci. 2013;13:98–101. [PubMed] [Google Scholar]
- 60.Delcourt C., Michel F., Colvez A., et al. Associations of cardiovascular disease and its risk factors with age-related macular degeneration: the POLA study. Ophthalmic Epidemiol. 2001;8:237–249. doi: 10.1076/opep.8.4.237.1613. [DOI] [PubMed] [Google Scholar]
- 61.Erke M.G., Bertelsen G., Peto T., et al. Cardiovascular risk factors associated with age-related macular degeneration: the Tromsø study. Acta Ophthalmol. 2014;92:662–669. doi: 10.1111/aos.12346. [DOI] [PubMed] [Google Scholar]
- 62.Hashemi R., Bandarian M., Abedi-Taleb E., et al. The association between blood vitamins D and E with age-related macular degeneration: a pilot study. Interv Med Appl Sci. 2018;10:127–132. doi: 10.1556/1646.10.2018.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Javadzadeh A., Ghorbanihaghjo A., Rashtchizadeh N., et al. Enhanced susceptibility of low-density lipoprotein to oxidation in wet type age-related macular degeneration in male patients. Saudi Med J. 2007;28:221–224. [PubMed] [Google Scholar]
- 64.Jonasson F., Fisher D.E., Eiriksdottir G., et al. Five-year incidence, progression, and risk factors for age-related macular degeneration: the age, gene/environment susceptibility study. Ophthalmology. 2014;121:1766–1772. doi: 10.1016/j.ophtha.2014.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kawasaki R., Wang J.J., Ji G., et al. Prevalence and risk factors for age-related macular degeneration in an adult Japanese population. The Funagata study. Ophthalmology. 2008;115:1376–1381.e2. doi: 10.1016/j.ophtha.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 66.Mao F., Yang X., Yang K., et al. Six-year incidence and risk factors for age-related macular degeneration in a rural Chinese population: the Handan Eye study. Invest Ophthalmol Vis Sci. 2019;60:4966–4971. doi: 10.1167/iovs.19-27325. [DOI] [PubMed] [Google Scholar]
- 67.Ngai L.Y., Stocks N., Sparrow J.M., et al. The prevalence and analysis of risk factors for age-related macular degeneration: 18-year follow-up data from the Speedwell Eye study, United Kingdom. Eye. 2011;25:784–793. doi: 10.1038/eye.2011.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ornek N., Ornek K., Aydin S., et al. Serum vascular endothelial growth factor receptor-2 and adropin levels in age-related macular degeneration. Int J Ophthalmol. 2016;9:556–560. doi: 10.18240/ijo.2016.04.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Paunksnis A., Cimbalas A., Cerniauskiene L.R., et al. Early age-related maculopathy and risk factors of cardiovascular disease in middle-aged Lithuanian urban population. Eur J Ophthalmol. 2005;15:255–262. doi: 10.1177/112067210501500213. [DOI] [PubMed] [Google Scholar]
- 70.Peiretti E., Mandas A., Abete C., et al. Age-related macular degeneration and cognitive impairment show similarities in changes of neutral lipids in peripheral blood mononuclear cells. Exp Eye Res. 2014;124:11–16. doi: 10.1016/j.exer.2014.04.017. [DOI] [PubMed] [Google Scholar]
- 71.Qin L., Mroczkowska S.A., Ekart A., et al. Patients with early age-related macular degeneration exhibit signs of macro- and micro-vascular disease and abnormal blood glutathione levels. Graefes Arch Clin Exp Ophthalmol. 2014;252:23–30. doi: 10.1007/s00417-013-2418-0. [DOI] [PubMed] [Google Scholar]
- 72.Raman R., Pal S.S., Ganesan S., et al. The prevalence and risk factors for age-related macular degeneration in rural-urban India, Sankara Nethralaya Rural-Urban Age-related Macular Degeneration Study, Report No. 1. Eye (Lond) 2016;30:688–697. doi: 10.1038/eye.2016.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Roh M.I., Kim J.H., Byeou S.H., et al. Estimated prevalence and risk factor for age-related maculopathy. Yonsei Med J. 2008;49:931–941. doi: 10.3349/ymj.2008.49.6.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Semba R.D., Moaddel R., Cotch M.F., et al. Serum lipids in adults with late age-related macular degeneration: a case-control study. Lipids Health Dis. 2019;18:7. doi: 10.1186/s12944-018-0954-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Song S.J., Youm D.J., Chang Y., Yu H.G. Age-related macular degeneration in a screened South Korean population: prevalence, risk factors, and subtypes. Ophthalmic Epidemiol. 2009;16:304–310. [PubMed] [Google Scholar]
- 76.Taniguchi H., Shiba T., Takahashi M., et al. Cardio-ankle vascular index elevation in patients with exudative age-related macular degeneration. J Atheroscler Thromb. 2013;20:903–910. doi: 10.5551/jat.18796. [DOI] [PubMed] [Google Scholar]
- 77.Ulaş F., Balbaba M., Özmen S., et al. Association of dehydroepiandrosterone sulfate, serum lipids, C-reactive protein and body mass index with age-related macular degeneration. Int Ophthalmol. 2013;33:485–491. doi: 10.1007/s10792-013-9728-4. [DOI] [PubMed] [Google Scholar]
- 78.Vingerling J.R., Dielemans I., Bots M.L., et al. Age-related macular degeneration is associated with atherosclerosis - the Rotterdam study. Am J Epidemiol. 1995;142:404–409. doi: 10.1093/oxfordjournals.aje.a117648. [DOI] [PubMed] [Google Scholar]
- 79.Wang S., Xu L., Jonas J.B., et al. Dyslipidemia and eye diseases in the adult Chinese population: the Beijing Eye study. PLoS One. 2012;7 doi: 10.1371/journal.pone.0026871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xue C.C., Cui J., Gao L.Q., et al. Peripheral monocyte count and age-related macular degeneration. The Tongren Health Care study. Am J Ophthalmol. 2021;227:143–153. doi: 10.1016/j.ajo.2021.03.010. [DOI] [PubMed] [Google Scholar]
- 81.Yip J.L., Khawaja A.P., Chan M.P., et al. Cross sectional and longitudinal associations between cardiovascular risk factors and age related macular degeneration in the EPIC-Norfolk Eye study. PLoS One. 2015;10 doi: 10.1371/journal.pone.0132565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ledesma-Gil G., Otero-Marquez O., Alauddin S., et al. Subretinal drusenoid deposits are strongly associated with coexistent high-risk vascular diseases. BMJ Open Ophth. 2022;7 [Google Scholar]
- 83.Thomson R.J., Chazaro J., Otero-Marquez O., et al. Subretinal drusenoid deposits and soft drusen: are they markers for distinct retinal diseases? Retina. 2022;42:1311–1318. doi: 10.1097/IAE.0000000000003460. [DOI] [PubMed] [Google Scholar]
- 84.Ludwig C.A., Vail D., Rajeshuni N.A., et al. Statins and the progression of age-related macular degeneration in the United States. PLoS One. 2021;16 doi: 10.1371/journal.pone.0252878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lambert N.G., Singh M.K., ElShelmani H., et al. Risk factors and biomarkers of age-related macular degeneration. Prog Retin Eye Res. 2016;54:64–102. doi: 10.1016/j.preteyeres.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.