Abstract
Malignant solitary fibrous tumors in the retroperitoneum are rare, and their treatment strategies have not yet been established. A 61-year-old woman with dyspnea underwent laparotomy under a presumptive diagnosis of Meigs’ syndrome. She underwent both adnexectomy and retroperitoneal tumor excision. The histologic diagnosis was of a fibrothecoma of both ovaries and a retroperitoneal solitary fibrous tumor that was considered malignant based on its mitotic activity. Local recurrence was observed 9 months postoperatively; re-excision was performed, and radiation therapy was administered. Four months later, metastasis to the left lung was detected, and a thoracoscopic resection was performed. Although pazopanib was administered subsequently, it was discontinued after 11 months because of proteinuria. She complained of dysphagia 3 weeks after the withdrawal of the drug, and a metastatic tumor was observed at the cranial base. Radiotherapy was initiated; however, she died of the disease 35 months after the primary surgery. Medical guidelines should be established for malignant solitary fibrous tumors to improve patient prognosis.
Keywords: Computed tomography, Malignant solitary fibrous tumor, Meigs’ syndrome, Multidisciplinary treatment, Retroperitoneum
Introduction
Solitary fibrous tumors (SFTs) are rare fibroblastic tumors with an annual incidence of 0.1 per 100,000 persons. They were first identified in the pleura and have also been reported in various organs. Most SFTs involve the thoracic cavity (68%), and extra-thoracic SFTs arise in the abdomen (intraperitoneal/retroperitoneal/pelvic, 24%), head and neck region (6%), and extremities (2%) [1–4]. SFTs in the posterior bladder wall have been found in 37% patients with retroperitoneal SFTs [5].
Approximately 100 cases of retroperitoneal SFTs have been reported; among these, 10%-20% have involved malignant SFTs [6]. Resection is the first-line therapy for SFTs; achievement of tumor-negative margins contributes to a better prognosis [7]. Although several risk stratification models have been explored from clinical and histologic perspectives, optimal clinical management strategies for treating patients at high risk of SFT development have not yet been established [1].
Herein, we describe a case of a malignant retroperitoneal SFT that presented with Meigs’ syndrome. We have also reviewed the multidisciplinary treatments available for malignant SFTs.
Case report
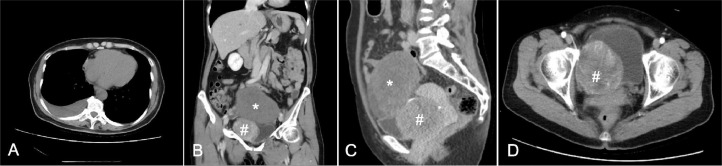
A 61-year-old Japanese woman with complaints of dyspnea was referred to our department for further examination of incidentally detected pelvic masses. The patient's medical and family histories were unremarkable. Pelvic examination revealed a tumor protruding from the right vaginal wall. Contrast-enhanced computed tomography (CT) revealed right pleural effusion and 2 solid pelvic masses. One mass (10 cm in size) was located on the superior aspect of the uterine body, while the other mass (7 cm in size) was circumscribed with vessels, heterogeneously enhanced, and located in front of the uterus (Fig. 1). These masses were suspected to be an ovarian tumor and a uterine leiomyoma, respectively. Drainage by thoracocentesis revealed a serous, yellowish exudate; bacterial cultures and cytological examinations were negative for infection and malignancy, respectively. The serum CA125 level was 321.8 U/mL (normal: <35.0 U/mL). We performed a laparotomy under a presumptive diagnosis of Meigs’ syndrome.
Fig. 1.
Contrast-enhanced computed tomography shows right pleural effusion (A) and 2 solid pelvic masses: a 10 cm mass located along the superior aspect of the uterine body (*), and a 7 cm mass located in front of the uterus (#) (B-D). The tumor (indicated by a hashtag [#]) is well-circumscribed and heterogeneously enhanced; it is eventually revealed to be a retroperitoneal solitary fibrous tumor.
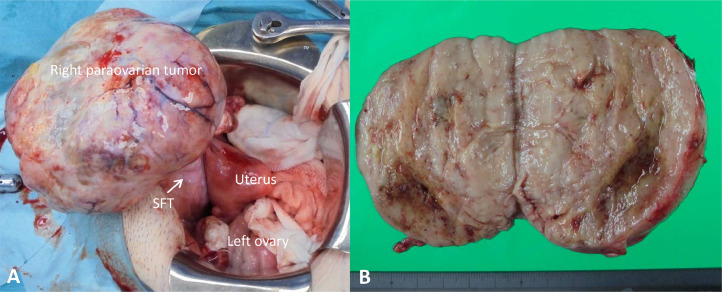
During surgery, we found a right paraovarian tumor and a retroperitoneal tumor attached to the posterior aspect of the bladder (Fig. 2). Right salpingo-oophorectomy was performed, and a frozen section diagnosis of a fibrothecoma was established. Subsequently, left salpingo-oophorectomy was performed, and the retroperitoneal tumor was excised with negative resection margins.
Fig. 2.
(A) Surgical view of the tumor in relation to the pelvic structures. (B) Gross appearance of the retroperitoneal solitary fibrous tumor. The resected specimen is solid, and the cut surface is yellowish-white in color. SFT, solitary fibrous tumor.
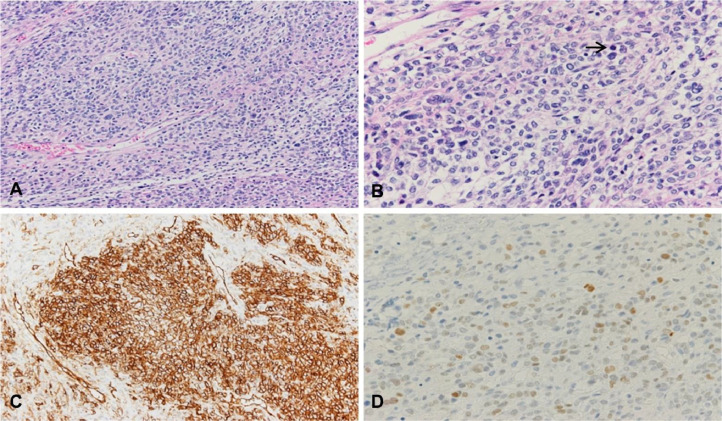
The results of the postoperative histologic analysis were consistent with fibrothecoma of both ovaries. The retroperitoneal tumor was 7.7 × 7.2 × 6.7 cm in size, and the cut surface was yellowish-white in color (Fig. 2). Histopathologic examination revealed mesenchymal cells with dense proliferation and thin-walled vessels. The retroperitoneal tumor cells showed nuclear atypia, and the mitotic count was > 10 per 10 high-power fields (HPF; Fig. 3). Immunohistochemical staining showed that these tumor cells were strongly positive for CD34, Bcl-2, and CD99 and diffusely positive for STAT6 (Fig. 3). Thus, the retroperitoneal tumor was diagnosed as a malignant SFT on the basis of the mitotic counts and nuclear atypia. The serum CA 125 level normalized, and the pleural effusion disappeared postoperatively.
Fig. 3.
Microscopic features. (A) The tumor consists of mesenchymal cells with dense proliferation and thin-walled vessels (hematoxylin and eosin [H&E] staining, × 200). (B) Mitosis (arrow) and nuclear atypia are noted (H&E staining, × 400). (C) Immunohistochemical staining is positive for CD44 (× 200). (D) Immunohistochemical staining is diffusely positive for STAT6 (× 400).
Nine months after the surgery, CT revealed another retroperitoneal lesion (5 cm in size); however, the serum CA125 level had been within the normal limit. The tumor was resected, and a histologic analysis confirmed recurrence. Postoperative magnetic resonance imaging (MRI) revealed a residual lesion; thus, intensity-modulated radiation therapy (RT; 60 Gy) was administered. Follow-up CT performed 4 months later revealed 2 masses in the left lung; thus, the patient underwent thoracoscopic wedge resections of the left upper and lower lobes. Findings from histologic analyses of the lung masses were indicative of malignant SFT metastasis. Although pazopanib was subsequently administered, it was discontinued after 11 months because of grade 4 proteinuria. She complained of dysphagia 3 weeks after the withdrawal of the drug, and a metastatic tumor was found at the cranial base. RT at a dose of 51 Gy was administrated again; however, further disease progression prevented her from continuing the therapy. Thus, she continued with RT at 21 Gy and succumbed to the disease 35 months after the primary surgery.
Discussion
To our knowledge, this is the first report concomitant SFT and Meigs’ syndrome in a patient. We could not diagnose SFT preoperatively because of the clinical features of Meigs’ syndrome; furthermore, the absence of credible indications prevented us from administering effective interventions involving RT.
According to the World Health Organization, SFTs have an intermediate or malignant potential, and their progression is gradual [3]. SFTs occur in patients older than 40 years of age, and no sex-based differences are observed in their occurrence. Many patients with SFTs are asymptomatic. In our case, dyspnea caused by Meigs’ syndrome led to the definitive diagnosis of an SFT. Meigs’ syndrome has not been reported as a complication of SFTs, and the most common benign tumors seen in patients with SFTs are uterine leiomyomas [8]. Furthermore, SFTs do not show definitive features on peripheral blood examination or CT and MRI examination [1,9]. The serum CA125 level in our case had normalized after the primary surgery and did not increase when the SFT progressed; this suggests that the elevation in the serum CA125 level was secondary to Meigs’ syndrome and not the SFT. Several characteristic CT features of SFTs have been indicated; these include a well-circumscribed and occasionally lobulated mass with heterogeneous enhancement due to circuitous vessels. The following tumors should be considered as differential diagnoses for retroperitoneal SFTs at imaging examinations: gastrointestinal stromal tumors, synovial sarcomas, malignant mesotheliomas, leiomyosarcomas, desmoid tumors, and neurogenic tumors and lymphomas [10]. Although CT findings of the solid pelvic mass in our case were compatible with those of an SFT, features such as pleural effusion and an ovarian solid mass were seemingly specific to Meigs’ syndrome; thus, we were unable to consider SFT as a differential diagnosis. The predictive value of imaging examinations for SFTs is reportedly 7.4% [2,10]. Histologically, SFTs are composed of spindled-to-ovoid cells that are arranged in a haphazard “patternless” pattern and intimately intertwined with collagen fibers of varying thickness; the tumors are associated with numerous dilated thin-walled blood vessels (in a so-called “staghorn pattern”). The morphological features of SFTs including high cellularity, high mitotic activity (>4 mitosis/10 HPF), pleomorphism, and necrosis have been described as criteria for malignancy [1,3]. In immunohistochemistry, the tumor cells are reportedly positive for CD34, vimentin, and Bcl-2. In recent years, it has become clear that the NAB2-STAT6 fusion gene is a driver of SFTs and diffuse and strong nuclear STAT6 immunostaining has been considered a surrogate marker for this fusion gene [1].
To our knowledge, the postoperative recurrence and metastatic rates for malignant SFTs are 11%-50% and 60%, respectively [11]. The lung/pleura, liver, and bones are common sites of metastasis. Brain metastasis occurs in approximately 3.0% cases of SFT [12]. However, only few reports have described multidisciplinary treatments for the recurrent or metastatic malignant SFTs. The mitotic counts are higher in non-curable patients with malignant SFTs than in cured patients (median, 7.0 vs. 4.0) [8]. Surgical resection is also recommended as the first-line therapy in cases of recurrence [9,13]. Regarding RT, Bishop et al. [11] administered adjuvant RT when malignant SFTs were >5 cm in size or when the surgical margins were inadequate; they reported 5-year and 10-year survival rates of 95% and 89%, respectively. For patients with locally advanced or metastatic malignant SFTs, the 5-year survival rates are reportedly 89.5% [14]. Therefore, combined adjuvant RT and RT alone are considered tumor suppression therapies for malignant SFTs. For eliminating large and hypervascular SFTs, intra-aortic balloons and preoperative transcatheter arterial embolization are considered. Although these procedures may not ameliorate intraoperative hemorrhaging, they may allow the complete resection of SFTs [15,16].
The efficacy of chemotherapy for malignant SFTs is difficult to predict. The median progression-free survival (PFS) after anthracycline-based chemotherapy or gemcitabine-based chemotherapy, which are common therapeutic candidates for patients with soft tissue sarcomas, ranges from 4 to 4.6 months [17,18]. Antiangiogenic agents have been used for metastatic or unresectable malignant SFTs; the median PFS with pazopanib, a multitarget tyrosine kinase inhibitor that blocks vascular endothelial growth factor and platelet-derived growth factor, was reported to be 5.57 months [19]. Rajeev et al. [7] reported that surgical resection, chemotherapy, and RT were performed in 2 of 6 patients who underwent multidisciplinary treatments; the median survival in their study was 69.2 months.
In this case, the survival time after recurrence was 26 months. No adjuvant treatment was provided following primary surgery because the tumor was completely resected, and the risk was moderate (based on the classification by Demicco et al. [12]). If the patient had undergone RT after primary surgery, considering the high mitotic value, the progression-free interval might have been prolonged. We administered pazopanib as systemic chemotherapy after thoracoscopic surgery. Although administration was eventually discontinued after 11 months because of proteinuria, the patient showed no signs of local tumor progression or metastasis at the 11 months’ follow-up. This indicates that pazopanib can be considered a candidate for postoperative adjuvant chemotherapy for advanced malignant SFTs.
In conclusion, we report a case of a retroperitoneal malignant SFT that presented along with Meigs’ syndrome. Retroperitoneal malignant SFTs are rare; therefore, establishing relevant medical guidelines for their management is difficult. Reporting of each case is expected to contribute to the selection of appropriate treatment strategies.
Patient consent
The patient provided permission to publish these features of her case, and the identity of the patient has been protected.
Footnotes
Acknowledgments: We would like to thank Editage (www.editage.com) for English language editing.
Competing Interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Davanzo B, Emerson RE, Lisy M, Koniaris LG, Kays JK. Solitary fibrous tumor. Transl Gastroenterol Hepatol. 2018;3:94. doi: 10.21037/tgh.2018.11.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schöffski P, Timmermans I, Hompes D, Stas M, Sinnaeve F, De Leyn P, et al. Clinical presentation, natural history, and therapeutic approach in patients with solitary fibrous tumor: a retrospective analysis. Sarcoma. 2020;2020 doi: 10.1155/2020/1385978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Demicco EG, Fritchie KJ, Han A. In: Soft tissue and bone tumours: WHO classification of tumours. 5th ed. The WHO Classification of Tumours Editorial Board, editor. IARC Press; Lyon, France: 2020. Solitary fibrous tumor; pp. 104–108. [Google Scholar]
- 4.Gold JS, Antonescu CR, Hajdu C, Ferrone CR, Hussain M, Lewis JJ, et al. Clinicopathologic correlates of solitary fibrous tumors. Cancer. 2002;94:1057–1068. [PubMed] [Google Scholar]
- 5.Hasegawa T, Matsuno Y, Shimoda T, Hasegawa F, Sano T, Hirohashi S. Extrathoracic solitary fibrous tumors: their histological variability and potentially aggressive behavior. Hum Pathol. 1999;30:1464–1473. doi: 10.1016/s0046-8177(99)90169-7. [DOI] [PubMed] [Google Scholar]
- 6.Zhou Y, Chu X, Yi Y, Tong L, Dai Y. Malignant solitary fibrous tumor in retroperitoneum: a case report and literature review. Medicine (Baltimore) 2017;96:e6373. doi: 10.1097/MD.0000000000006373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rajeev R, Patel M, Jayakrishnan TT, Johnston FM, Bedi M, Charlson J, et al. Retroperitoneal solitary fibrous tumor: surgery as first line therapy. Clin Sarcoma Res. 2015;5:19. doi: 10.1186/s13569-015-0034-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.England DM, Hochholzer L, McCarthy MJ. Localized benign and malignant fibrous tumors of the pleura. A clinicopathologic review of 223 cases. Am J Surg Pathol. 1989;13:640–658. doi: 10.1097/00000478-198908000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Yamada K, Abiko K, Kido A, Minamiguchi S, Horie A, Mandai M. Solitary fibrous tumor arising from pelvic retroperitoneum: a report of two cases and a review of the literature. J Obstet Gynaecol Res. 2019;45:1391–1397. doi: 10.1111/jog.13965. [DOI] [PubMed] [Google Scholar]
- 10.Ahmed TM, Blanco A, Weisberg EM, Fishman EK. CT of retroperitoneal solitary fibrous tumor. Radiol Case Rep. 2023;18:2241–2244. doi: 10.1016/j.radcr.2023.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bishop AJ, Zagars GK, Demicco EG, Wang WL, Feig BW, Guadagnolo BA. Soft tissue solitary fibrous tumor: combined surgery and radiation therapy results in excellent local control. Am J Clin Oncol. 2018;41:81–85. doi: 10.1097/COC.0000000000000218. [DOI] [PubMed] [Google Scholar]
- 12.Demicco EG, Park MS, Araujo DM, Fox PS, Bassett RL, Pollock RE, et al. Solitary fibrous tumor: a clinicopathological study of 110 cases and proposed risk assessment model. Mod Pathol. 2012;25:1298–1306. doi: 10.1038/modpathol.2012.83. [DOI] [PubMed] [Google Scholar]
- 13.Baldi GG, Stacchiotti S, Mauro V, Dei Tos AP, Gronchi A, Pastorino U, et al. Solitary fibrous tumor of all sites: outcome of late recurrences in 14 patients. Clin Sarcoma Res. 2013;3:4. doi: 10.1186/2045-3329-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haas RL, Walraven I, Lecointe-Artzner E, Scholten AN, van Houdt WJ, Griffin AM, et al. Radiation therapy as sole management for solitary fibrous tumors (SFT): A retrospective study from the global SFT initiative in collaboration with the sarcoma patients EuroNet. Int J Radiat Oncol Biol Phys. 2018;101:1226–1233. doi: 10.1016/j.ijrobp.2018.04.024. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka M, Hirayama T, Fujihara R, Fujino K, Terao Y, Itakura A. A case of a large solitary fibrous tumor arising from the retroperitoneum resected completely using an intra-aortic balloon. J Obstet Gynaecol Res. 2022;48:2647–2651. doi: 10.1111/jog.15353. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y, Wei R, Ji T, Chen Z, Guo W. Surgical treatment of primary solitary fibrous tumors involving the pelvic ring. PLoS One. 2018;13 doi: 10.1371/journal.pone.0207581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stacchiotti S, Libertini M, Negri T, Palassini E, Gronchi A, Fatigoni S, et al. Resposnse to chemotherapy of solitary fibrous tumour: a retrospective study. Eur J Cancer. 2013;49:2376–2383. doi: 10.1016/j.ejca.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 18.Park MS, Ravi V, Conley A, Patel SR, Trent JC, Lev DC, et al. The role of chemotherapy in advanced solitary fibrous tumors: a retrospective analysis. Clin Sarcoma Res. 2013;3:7. doi: 10.1186/2045-3329-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin-Broto J, Stacchiotti S, Lopez-Pousa A, Redondo A, Bernabeu D, de Alava E, et al. Pazopanib for treatment of advanced malignant and dedifferentiated solitary fibrous tumour: a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2019;20:134–144. doi: 10.1016/S1470-2045(18)30676-4. [DOI] [PubMed] [Google Scholar]